The Change of Skeletal Muscle Caused by Inflammation in Obesity as the Key Path to Fibrosis: Thoughts on Mechanisms and Intervention Strategies
Abstract
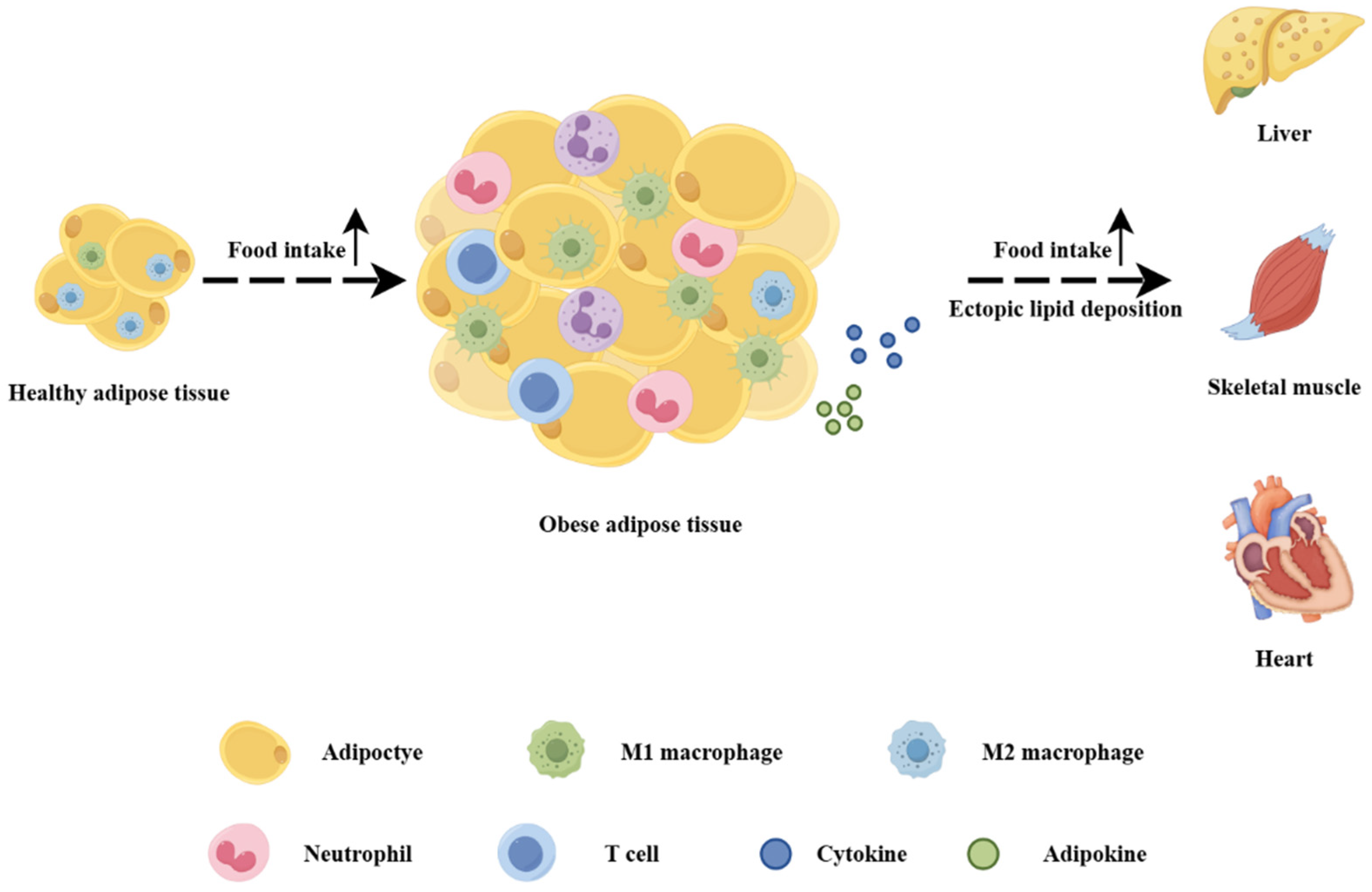
1. Introduction
2. The Development of Skeletal Muscle Inflammation Caused by Obesity
2.1. Lipid Deposition
2.2. Mitochondrial Dysfunction
2.3. Insulin Resistance
2.4. Other Metabolic Alterations
3. The Development of Skeletal Muscle Fibrosis Caused by Obesity
3.1. Pathological Changes
3.2. Impaired Regeneration
3.3. Mitochondrial Dysfunction
3.4. Changes in Different Types of Skeletal Muscle
3.5. Closely Related to Metabolic Diseases
4. The Association Between Inflammation and Fibrosis in Skeletal Muscle Caused by Obesity
4.1. Changes in Inflammatory Factors: The Close Association with the Occurrence of Fibrosis
4.2. Co-Regulation of Adipose–Liver–Skeletal Muscle Tissue Network
5. Intervention Strategies
5.1. Aerobic Exercise
5.2. Dietary Modification
5.3. Pharmacotherapy
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Varra, F.N.; Varras, M.; Varra, V.K.; Theodosis-Nobelos, P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options (Review). Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Tokarz, V.L.; Richter, E.A.; Klip, A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal muscle energy metabolism in obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- D’Souza, D.; Roubos, S.; Larkin, J.; Lloyd, J.; Emmons, R.; Chen, H.; De Lisio, M. The Late Effects of Radiation Therapy on Skeletal Muscle Morphology and Progenitor Cell Content are Influenced by Diet-Induced Obesity and Exercise Training in Male Mice. Sci. Rep. 2019, 9, 6691. [Google Scholar] [CrossRef]
- Bielawiec, P.; Harasim-Symbor, E.; Sztolsztener, K.; Konstantynowicz-Nowicka, K.; Chabowski, A. Attenuation of Oxidative Stress and Inflammatory Response by Chronic Cannabidiol Administration Is Associated with Improved n-6/n-3 PUFA Ratio in the White and Red Skeletal Muscle in a Rat Model of High-Fat Diet-Induced Obesity. Nutrients 2021, 13, 1603. [Google Scholar] [CrossRef]
- van Herpen, N.A.; Schrauwen-Hinderling, V.B. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Kang, C.; Goh, J.; Chae, S.I.; Kim, H.C.; Lee, T.J.; Abd El-Aty, A.M.; Jeong, J.H. WISP1 promotes non-alcoholic fatty liver disease and skeletal muscle insulin resistance via TLR4/JNK signaling. J. Cell Physiol. 2018, 233, 6077–6087. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Shin, S.K.; Kwon, E.Y. Luteolin Protects Against Obese Sarcopenia in Mice with High-Fat Diet-Induced Obesity by Ameliorating Inflammation and Protein Degradation in Muscles. Mol. Nutr. Food Res. 2023, 67, e2200729. [Google Scholar] [CrossRef] [PubMed]
- Pagel-Langenickel, I.; Bao, J.; Joseph, J.J.; Schwartz, D.R.; Mantell, B.S.; Xu, X.; Raghavachari, N.; Sack, M.N. PGC-1alpha integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J. Biol. Chem. 2008, 283, 22464–22472. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, E.; Saris, W.H.; Blaak, E.E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: Effects of lifestyle. Obes. Rev. 2009, 10, 178–193. [Google Scholar] [CrossRef]
- Jorgensen, W.; Rud, K.A.; Mortensen, O.H.; Frandsen, L.; Grunnet, N.; Quistorff, B. Your mitochondria are what you eat: A high-fat or a high-sucrose diet eliminates metabolic flexibility in isolated mitochondria from rat skeletal muscle. Physiol. Rep. 2017, 5, e13207. [Google Scholar] [CrossRef]
- Kunz, H.E.; Hart, C.R.; Gries, K.J.; Parvizi, M.; Laurenti, M.; Dalla Man, C.; Moore, N.; Zhang, X.; Ryan, Z.; Polley, E.C.; et al. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E105–E121. [Google Scholar] [CrossRef]
- Fletcher, E.; Wiggs, M.; Greathouse, K.L.; Morgan, G.; Gordon, P.M. Impaired proteostasis in obese skeletal muscle relates to altered immunoproteasome activity. Appl. Physiol. Nutr. Metab. 2022, 47, 555–564. [Google Scholar] [CrossRef]
- Jani, S.; Da Eira, D.; Hadday, I.; Bikopoulos, G.; Mohasses, A.; de Pinho, R.A.; Ceddia, R.B. Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci. Rep. 2021, 11, 19160. [Google Scholar] [CrossRef]
- Rowland, A.F.; Fazakerley, D.J.; James, D.E. Mapping insulin/GLUT4 circuitry. Traffic 2011, 12, 672–681. [Google Scholar] [CrossRef]
- Alvim, R.O.; Cheuhen, M.R.; Machado, S.R.; Sousa, A.G.; Santos, P.C. General aspects of muscle glucose uptake. An. Acad. Bras. Cienc. 2015, 87, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Teng, S.; Huang, P. The effect of type 2 diabetes mellitus and obesity on muscle progenitor cell function. Stem Cell Res. Ther. 2019, 10, 103. [Google Scholar] [CrossRef]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle—A focus on the molecular mechanisms of insulin resistance. Mol. Cell Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.Y.; Jun, H.S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-kappaB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Reyna, S.M.; Ghosh, S.; Tantiwong, P.; Meka, C.S.; Eagan, P.; Jenkinson, C.P.; Cersosimo, E.; Defronzo, R.A.; Coletta, D.K.; Sriwijitkamol, A.; et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 2008, 57, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef]
- Li, N.; Shi, H.; Guo, Q.; Gan, Y.; Zhang, Y.; Jia, J.; Zhang, L.; Zhou, Y. Aerobic Exercise Prevents Chronic Inflammation and Insulin Resistance in Skeletal Muscle of High-Fat Diet Mice. Nutrients 2022, 14, 3730. [Google Scholar] [CrossRef]
- Brondani, L.A.; Assmann, T.S.; Duarte, G.C.; Gross, J.L.; Canani, L.H.; Crispim, D. The role of the uncoupling protein 1 (UCP1) on the development of obesity and type 2 diabetes mellitus. Arq. Bras. Endocrinol. Metabol. 2012, 56, 215–225. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Torre-Villalvazo, I.; Noriega, L.G.; Rodriguez-Lopez, L.A.; Aleman, G.; Torre-Anaya, E.A.; Carino-Cervantes, Y.Y.; Palacios-Gonzalez, B.; Furuzawa-Carballeda, J.; Tovar, A.R.; et al. Pecans and Its Polyphenols Prevent Obesity, Hepatic Steatosis and Diabetes by Reducing Dysbiosis, Inflammation, and Increasing Energy Expenditure in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 2591. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.A. Skeletal muscle fibrosis: An overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Fry, C.S.; Lee, J.D.; Mula, J.; Kirby, T.J.; Jackson, J.R.; Liu, F.; Yang, L.; Mendias, C.L.; Dupont-Versteegden, E.E.; McCarthy, J.J.; et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2015, 21, 76–80. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef]
- Hernandez Bautista, R.J.; Mahmoud, A.M.; Konigsberg, M.; Lopez Diaz Guerrero, N.E. Obesity: Pathophysiology, monosodium glutamate-induced model and anti-obesity medicinal plants. Biomed. Pharmacother. 2019, 111, 503–516. [Google Scholar] [CrossRef]
- Zazula, M.F.; Saraiva, D.F.; Theodoro, J.L.; Maciel, M.; Sepulveda, E.; Zanardini de Andrade, B.; Boaretto, M.L.; Maciel, J.; Bronczek, G.A.; Soares, G.M.; et al. An Early and Sustained Inflammatory State Induces Muscle Changes and Establishes Obesogenic Characteristics in Wistar Rats Exposed to the MSG-Induced Obesity Model. Int. J. Mol. Sci. 2023, 24, 4730. [Google Scholar] [CrossRef]
- Zhao, L.; Son, J.S.; Wang, B.; Tian, Q.; Chen, Y.; Liu, X.; de Avila, J.M.; Zhu, M.J.; Du, M. Retinoic acid signalling in fibro/adipogenic progenitors robustly enhances muscle regeneration. EBioMedicine 2020, 60, 103020. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Dabur, R. Role of Pro-inflammatory Cytokines in Regulation of Skeletal Muscle Metabolism: A Systematic Review. Curr. Med. Chem. 2020, 27, 2161–2188. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, O.; Wang, J.; Ayoub, M.B.; Leduc-Gaudet, J.P.; Mayaki, D.; Dulac, M.; Hussain, S.N.A.; Bergeron, R.; Gouspillou, G. The impact of high-fat feeding and parkin overexpression on skeletal muscle mass, mitochondrial respiration, and H2O2 emission. Am. J. Physiol. Cell Physiol. 2023, 324, C366–C376. [Google Scholar] [CrossRef] [PubMed]
- Nisr, R.B.; Shah, D.S.; Ganley, I.G.; Hundal, H.S. Proinflammatory NFkB signalling promotes mitochondrial dysfunction in skeletal muscle in response to cellular fuel overloading. Cell Mol. Life Sci. 2019, 76, 4887–4904. [Google Scholar] [CrossRef]
- Cai, L.; Shi, L.; Peng, Z.; Sun, Y.; Chen, J. Ageing of skeletal muscle extracellular matrix and mitochondria: Finding a potential link. Ann. Med. 2023, 55, 2240707. [Google Scholar] [CrossRef]
- Fletcher, E.; Miserlis, D.; Sorokolet, K.; Wilburn, D.; Bradley, C.; Papoutsi, E.; Wilkinson, T.; Ring, A.; Ferrer, L.; Haynatzki, G.; et al. Diet-induced obesity augments ischemic myopathy and functional decline in a murine model of peripheral artery disease. Transl. Res. 2023, 260, 17–31. [Google Scholar] [CrossRef]
- Valle-Tenney, R.; Rebolledo, D.; Acuna, M.J.; Brandan, E. HIF-hypoxia signaling in skeletal muscle physiology and fibrosis. J. Cell Commun. Signal 2020, 14, 147–158. [Google Scholar] [CrossRef]
- Valle-Tenney, R.; Rebolledo, D.L.; Lipson, K.E.; Brandan, E. Role of hypoxia in skeletal muscle fibrosis: Synergism between hypoxia and TGF-beta signaling upregulates CCN2/CTGF expression specifically in muscle fibers. Matrix Biol. 2020, 87, 48–65. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Pataky, M.W.; Yu, C.S.; Nie, Y.; Arias, E.B.; Singh, M.; Mendias, C.L.; Ploutz-Snyder, R.J.; Cartee, G.D. Skeletal muscle fiber type-selective effects of acute exercise on insulin-stimulated glucose uptake in insulin-resistant, high-fat-fed rats. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E695–E706. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, N.; Zhang, Z.; Song, Y.; Li, J.; Wang, Z.; Bo, H.; Zhang, Y. The specific mitochondrial unfolded protein response in fast- and slow-twitch muscles of high-fat diet-induced insulin-resistant rats. Front. Endocrinol. 2023, 14, 1127524. [Google Scholar] [CrossRef] [PubMed]
- Umek, N.; Horvat, S.; Cvetko, E. Skeletal muscle and fiber type-specific intramyocellular lipid accumulation in obese mice. Bosn. J. Basic. Med. Sci. 2021, 21, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.M.; Wu, H.F.; Wang, K.F.; Yu, D.Y.; Zhang, X.Z.; Ren, Q.; Chen, W.Z.; Lin, F.; Yu, Z.; Zhuang, C.L. Transcriptome profiling of fast/glycolytic and slow/oxidative muscle fibers in aging and obesity. Cell Death Dis. 2024, 15, 459. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, X.; Cheng, D.; Liang, X.; Miao, M.; Li, X.; Lu, J.; Xu, N.; Hu, S.; Zhang, Q. Muscle Fat Content Is Associated with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in Chinese Adults. J. Nutr. Health Aging 2023, 27, 960–965. [Google Scholar] [CrossRef]
- Han, E.; Kim, M.K.; Lee, H.W.; Ryu, S.; Kim, H.S.; Jang, B.K.; Suh, Y. Muscle fat contents rather than muscle mass determines nonalcoholic steatohepatitis and liver fibrosis in patients with severe obesity. Obesity 2022, 30, 2440–2449. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Choi, H.; Baik, S.H.; et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin. Endocrinol. 2013, 78, 525–532. [Google Scholar] [CrossRef]
- Angulo, P.; Alba, L.M.; Petrovic, L.M.; Adams, L.A.; Lindor, K.D.; Jensen, M.D. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J. Hepatol. 2004, 41, 943–949. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Morselli-Labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Res. Clin. Pract. 2011, 93, 285–291. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, D.; Liu, J.; Fang, L.; Li, Q. Skeletal muscle mass to visceral fat area ratio is an important determinant associated with type 2 diabetes and metabolic syndrome. Diabetes Metab. Syndr. Obes. 2019, 12, 1399–1407. [Google Scholar] [CrossRef]
- Cho, Y.; Chang, Y.; Ryu, S.; Jung, H.S.; Kim, C.W.; Oh, H.; Kim, M.K.; Sohn, W.; Shin, H.; Wild, S.H.; et al. Skeletal muscle mass to visceral fat area ratio as a predictor of NAFLD in lean and overweight men and women with effect modification by sex. Hepatol. Commun. 2022, 6, 2238–2252. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Paul, H.A.; Hart, D.A.; Reimer, R.A.; Smith, I.C.; Rios, J.L.; Seerattan, R.A.; Herzog, W. A High-Fat High-Sucrose Diet Rapidly Alters Muscle Integrity, Inflammation and Gut Microbiota in Male Rats. Sci. Rep. 2016, 6, 37278. [Google Scholar] [CrossRef] [PubMed]
- Wahlin-Larsson, B.; Carnac, G.; Kadi, F. The influence of systemic inflammation on skeletal muscle in physically active elderly women. Age 2014, 36, 9718. [Google Scholar] [CrossRef] [PubMed]
- Chasapi, A.; Balampanis, K.; Kourea, E.; Kalfarentzos, F.; Lambadiari, V.; Lambrou, G.I.; Melachrinou, M.; Sotiropoulou-Bonikou, G. Can obesity-induced inflammation in skeletal muscle and intramuscular adipose tissue accurately detect liver fibrosis? J. Musculoskelet. Neuronal Interact. 2018, 18, 509–524. [Google Scholar] [PubMed]
- Khan, I.M.; Perrard, X.Y.; Brunner, G.; Lui, H.; Sparks, L.M.; Smith, S.R.; Wang, X.; Shi, Z.Z.; Lewis, D.E.; Wu, H.; et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int. J. Obes. 2015, 39, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Mendez-Sanchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Reeves, H.L.; Friedman, S.L. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front. Biosci. 2002, 7, d808–d826. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, Y.; Deng, Y. Metabolic associated fatty liver disease and sarcopenia additively increase mortality: A real-world study. Nutr. Diabetes 2023, 13, 21. [Google Scholar] [CrossRef]
- Pedraza-Vazquez, G.; Mena-Montes, B.; Hernandez-Alvarez, D.; Gomez-Verjan, J.C.; Toledo-Perez, R.; Lopez-Teros, M.T.; Konigsberg, M.; Gomez-Quiroz, L.E.; Luna-Lopez, A. A low-intensity lifelong exercise routine changes miRNA expression in aging and prevents osteosarcopenic obesity by modulating inflammation. Arch. Gerontol. Geriatr. 2023, 105, 104856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Jiao, R.Q.; Kong, L.D. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Krawczyk, S.A.; Doridot, L.; Fowler, A.J.; Wang, J.X.; Trauger, S.A.; Noh, H.L.; Kang, H.J.; Meissen, J.K.; Blatnik, M.; et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J. Clin. Investig. 2016, 126, 4372–4386. [Google Scholar] [CrossRef] [PubMed]
- Lizak, B.; Szarka, A.; Kim, Y.; Choi, K.S.; Nemeth, C.E.; Marcolongo, P.; Benedetti, A.; Banhegyi, G.; Margittai, E. Glucose Transport and Transporters in the Endomembranes. Int. J. Mol. Sci. 2019, 20, 5898. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Perez-Felpete, N.; Fernandez-Fernandez, C.; Donapetry-Garcia, C.; Pazos-Garcia, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef]
- Xie, X.W. Liquiritigenin attenuates cardiac injury induced by high fructose-feeding through fibrosis and inflammation suppression. Biomed. Pharmacother. 2017, 86, 694–704. [Google Scholar] [CrossRef]
- Collins, K.H.; Hart, D.A.; Smith, I.C.; Issler, A.M.; Reimer, R.A.; Seerattan, R.A.; Rios, J.L.; Herzog, W. Acute and chronic changes in rat soleus muscle after high-fat high-sucrose diet. Physiol. Rep. 2017, 5, e13270. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Chang, P.F.; Acevedo, D.; Mandarino, L.J.; Reyna, S.M. Triterpenoid CDDO-EA inhibits lipopolysaccharide-induced inflammatory responses in skeletal muscle cells through suppression of NF-kappaB. Exp. Biol. Med. 2023, 248, 175–185. [Google Scholar] [CrossRef]
- Kim, M.B.; Bae, M.; Lee, Y.; Kang, H.; Hu, S.; Pham, T.X.; Park, Y.K.; Lee, J.Y. Consumption of Low Dose Fucoxanthin Does Not Prevent Hepatic and Adipose Inflammation and Fibrosis in Mouse Models of Diet-Induced Obesity. Nutrients 2022, 14, 2280. [Google Scholar] [CrossRef] [PubMed]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Sastre, J.; Bellanti, F.; Vina, J.; Vendemiale, G.; Altomare, E. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol. Asp. Med. 2008, 29, 22–35. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Guo, W.; Li, H.; Wang, Y.; Liu, X.; Kong, W. The Change of Skeletal Muscle Caused by Inflammation in Obesity as the Key Path to Fibrosis: Thoughts on Mechanisms and Intervention Strategies. Biomolecules 2025, 15, 20. https://doi.org/10.3390/biom15010020
Li Y, Guo W, Li H, Wang Y, Liu X, Kong W. The Change of Skeletal Muscle Caused by Inflammation in Obesity as the Key Path to Fibrosis: Thoughts on Mechanisms and Intervention Strategies. Biomolecules. 2025; 15(1):20. https://doi.org/10.3390/biom15010020
Chicago/Turabian StyleLi, Yixuan, Wenwen Guo, Han Li, Yuhao Wang, Xinwei Liu, and Wen Kong. 2025. "The Change of Skeletal Muscle Caused by Inflammation in Obesity as the Key Path to Fibrosis: Thoughts on Mechanisms and Intervention Strategies" Biomolecules 15, no. 1: 20. https://doi.org/10.3390/biom15010020
APA StyleLi, Y., Guo, W., Li, H., Wang, Y., Liu, X., & Kong, W. (2025). The Change of Skeletal Muscle Caused by Inflammation in Obesity as the Key Path to Fibrosis: Thoughts on Mechanisms and Intervention Strategies. Biomolecules, 15(1), 20. https://doi.org/10.3390/biom15010020





