The Expression Regulation and Cancer-Promoting Roles of RACGAP1
Abstract
1. Introduction
2. RACGAP1 Overexpression in Multiple Visceral Cancers
3. Expression Regulation of RACGAP1
3.1. Upregulation of RACGAP1 in Cancers
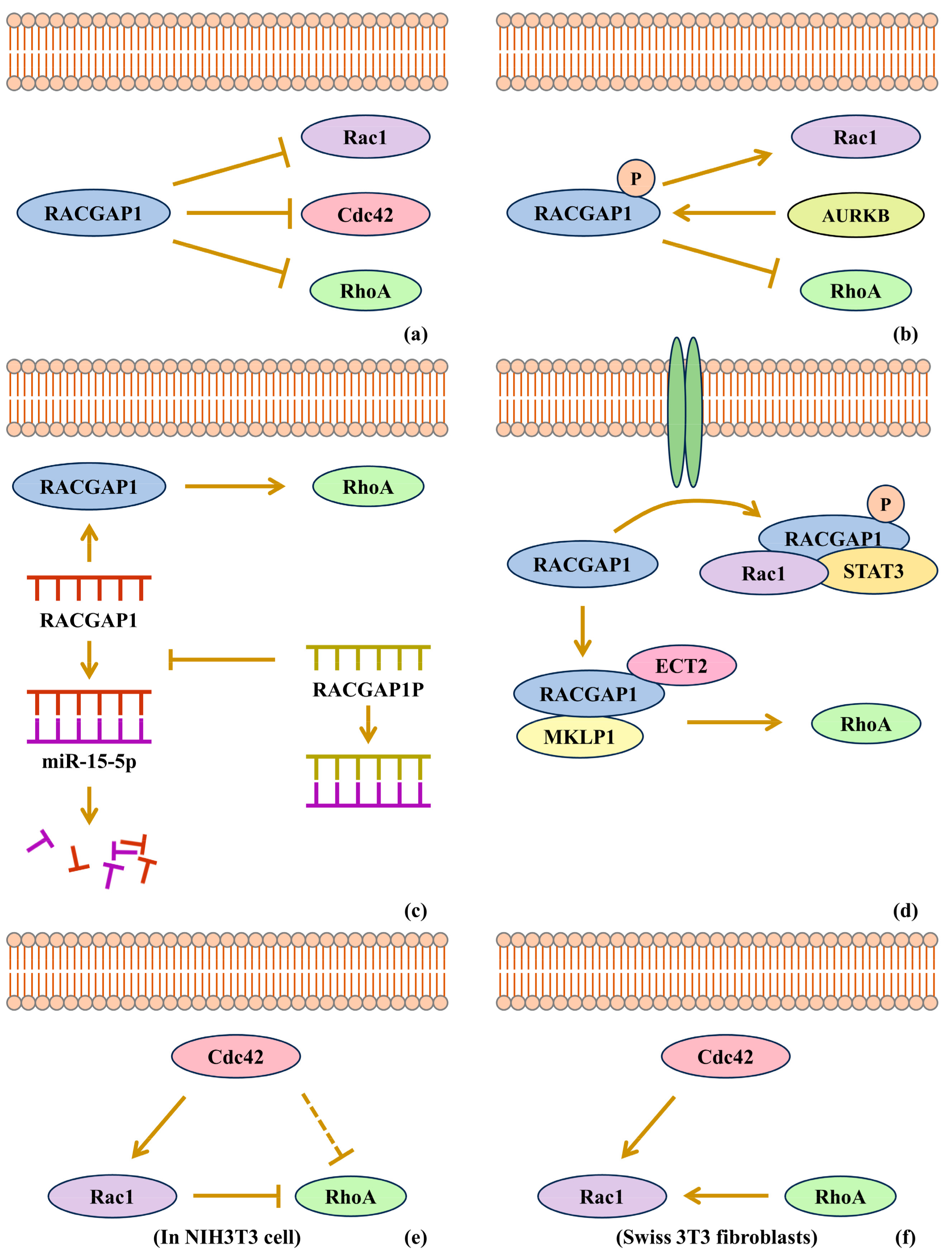
3.2. Feedback Regulation of RACGAP1
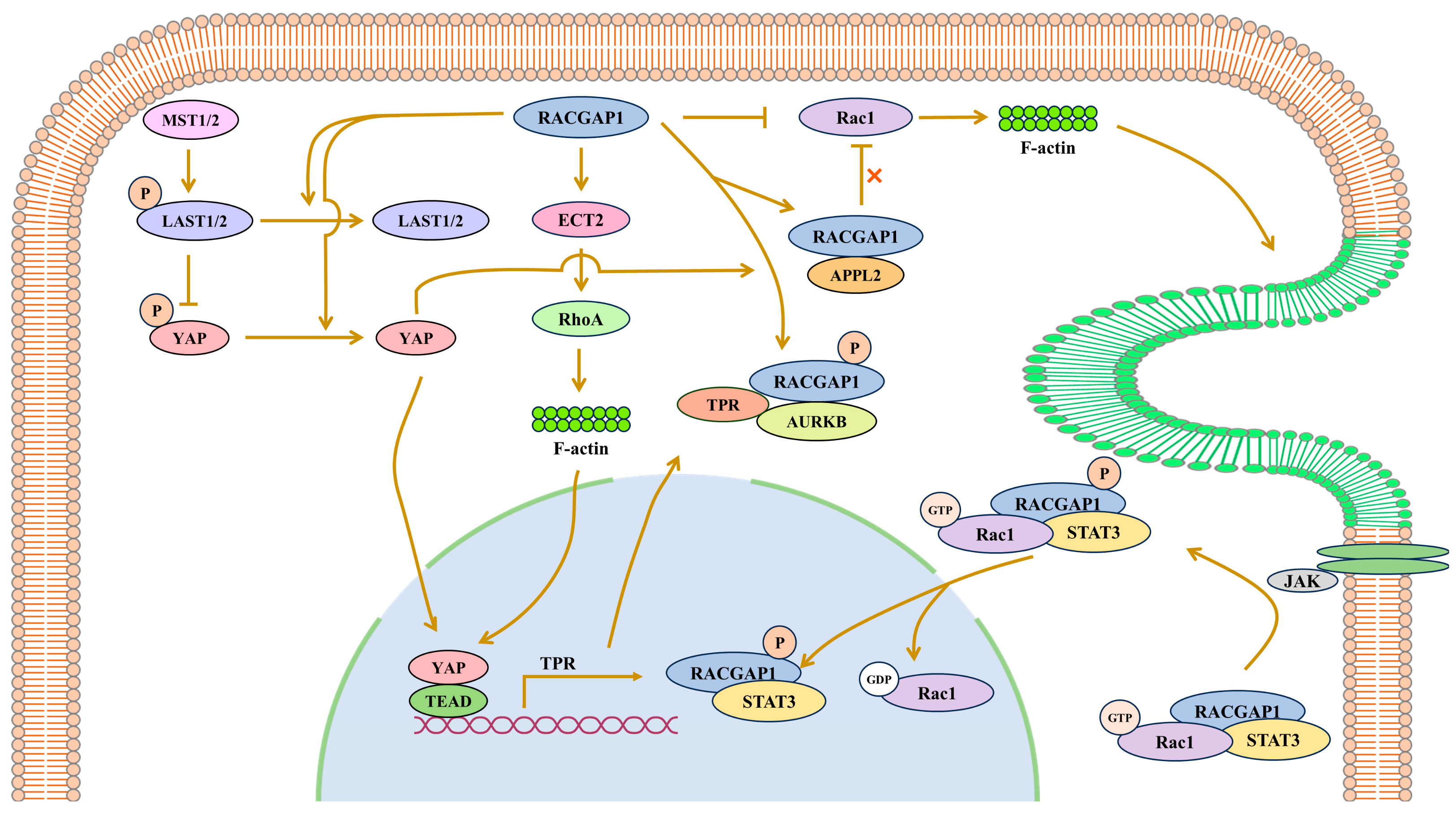
4. Cancer-Promoting Functions
4.1. RACGAP1 Promotes Cancers by Regulating Oncogenic Gene Expression in Signal Pathways
4.1.1. RACGAP1 Promotes TPR Expression by Activating YAP
4.1.2. RACGAP1 Promotes Survivin Expression by Phosphorylating STAT3
4.2. RACGAP1 Promotes Cancers by Rho-GTPase Activation
4.3. RACGAP1 Promotes Cancers in Coordination with GEF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Touré, A.; Dorseuil, O.; Morin, L.; Timmons, P.; Jégou, B.; Reibel, L.; Gacon, G. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J. Biol. Chem. 1998, 273, 6019–6023. [Google Scholar] [CrossRef]
- Sahai, E.; Marshall, C.J. RHO-GTPases and cancer. Nat. Rev. Cancer 2002, 2, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Cao, X.Y.; He, P.; Li, J.; Feng, M.X.; Zhang, Y.L.; Zhang, X.L.; Wang, Y.H.; Yang, Q.; Zhu, L.; et al. Overexpression of Rac GTPase Activating Protein 1 Contributes to Proliferation of Cancer Cells by Reducing Hippo Signaling to Promote Cytokinesis. Gastroenterology 2018, 155, 1233–1249.e22. [Google Scholar] [CrossRef]
- Kawashima, T.; Bao, Y.C.; Minoshima, Y.; Nomura, Y.; Hatori, T.; Hori, T.; Fukagawa, T.; Fukada, T.; Takahashi, N.; Nosaka, T.; et al. A Rac GTPase-activating protein, MgcRacGAP, is a nuclear localizing signal-containing nuclear chaperone in the activation of STAT transcription factors. Mol. Cell Biol. 2009, 29, 1796–1813. [Google Scholar] [CrossRef]
- Eid, R.A.; Soltan, M.A.; Eldeen, M.A.; Shati, A.A.; Dawood, S.A.; Eissa, M.; Zaki, M.S.A.; Algahtani, M.; Theyab, A.; Abdel-Daim, M.M.; et al. Assessment of RACGAP1 as a Prognostic and Immunological Biomarker in Multiple Human Tumors: A Multiomics Analysis. Int. J. Mol. Sci. 2022, 23, 14102. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Lu, M.; Ju, L.; Qian, K.; Wang, G.; Wu, C.L.; Liu, X.; Xiao, Y.; Wang, X. miR-4324-RACGAP1-STAT3-ESR1 feedback loop inhibits proliferation and metastasis of bladder cancer. Int. J. Cancer 2019, 144, 3043–3055. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, J.; Lu, L.; Hu, Z.; Li, X.; Huang, L.; Zhang, X.; Chen, M.; Qin, X.; Xie, L. Identification of Hub Genes Associated With Immune Infiltration and Predict Prognosis in Hepatocellular Carcinoma via Bioinformatics Approaches. Front. Genet. 2020, 11, 575762. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, R.; Ye, J.; Li, Q.; Liu, Z.; Gao, X.; Piao, X.; Mai, R.; Zou, D.; Ge, L. A twenty gene-based gene set variation score reflects the pathological progression from cirrhosis to hepatocellular carcinoma. Aging 2019, 11, 11157–11169. [Google Scholar] [CrossRef]
- Son, J.A.; Ahn, H.R.; You, D.; Baek, G.O.; Yoon, M.G.; Yoon, J.H.; Cho, H.J.; Kim, S.S.; Nam, S.W.; Eun, J.W.; et al. Novel Gene Signatures as Prognostic Biomarkers for Predicting the Recurrence of Hepatocellular Carcinoma. Cancers 2022, 14, 865. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Liu, X.; Zhang, Z. Role of TOP2A and CDC6 in liver cancer. Medicine 2023, 102, e35604. [Google Scholar] [CrossRef]
- Wang, S.M.; Ooi, L.L.; Hui, K.M. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin. Cancer Res. 2011, 17, 6040–6051. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pu, K.; Wu, X. Identifying novel biomarkers in hepatocellular carcinoma by weighted gene co-expression network analysis. J. Cell Biochem. 2019, 120, 11418–11431. [Google Scholar] [CrossRef]
- Liao, S.; Wang, K.; Zhang, L.; Shi, G.; Wang, Z.; Chen, Z.; Zhu, P.; He, Q. PRC1 and RACGAP1 are Diagnostic Biomarkers of Early HCC and PRC1 Drives Self-Renewal of Liver Cancer Stem Cells. Front. Cell Dev. Biol. 2022, 10, 864051. [Google Scholar] [CrossRef]
- Bian, R.; Dang, W.; Song, X.; Liu, L.; Jiang, C.; Yang, Y.; Li, Y.; Li, L.; Li, X.; Hu, Y.; et al. Rac GTPase activating protein 1 promotes gallbladder cancer via binding DNA ligase 3 to reduce apoptosis. Int. J. Biol. Sci. 2021, 17, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wang, C. KNTC1 and MCM2 are the molecular targets of gallbladder cancer. Aging 2023, 15, 7008–7022. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, S.; Zhou, B.; Cheng, B.; Tong, H.; Su, D.; Li, X.; Chen, Y.; Zhang, G. Telomere-related prognostic biomarkers for survival assessments in pancreatic cancer. Sci. Rep. 2023, 13, 10586. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Jiang, F.; Shen, Y.; Li, X.; Hu, X.; Wei, P.; Shen, X. Prognostic Prediction Using a Stemness Index-Related Signature in a Cohort of Gastric Cancer. Front. Mol. Biosci. 2020, 7, 570702. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, S.; Tanaka, K.; Mohri, Y.; Ohi, M.; Shimura, T.; Kitajima, T.; Kondo, S.; Okugawa, Y.; Toiyama, Y.; Inoue, Y.; et al. Clinical significance of RacGAP1 expression at the invasive front of gastric cancer. Gastric Cancer 2015, 18, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Lu, G.; Shang, R.; Hu, J.; Zhu, C.; Jin, L. RACGAP1 drives proliferation, migration and invasion and suppresses autophagy of gastric cancer cells via inhibiting SIRT1/Mfn2. Physiol. Int. 2024, 111, 35–46. [Google Scholar] [CrossRef]
- Bornschein, J.; Nielitz, J.; Drozdov, I.; Selgrad, M.; Wex, T.; Jechorek, D.; Link, A.; Vieth, M.; Malfertheiner, P. Expression of aurora kinase A correlates with the Wnt-modulator RACGAP1 in gastric cancer. Cancer Med. 2016, 5, 516–526. [Google Scholar] [CrossRef]
- Yin, C.; Toiyama, Y.; Okugawa, Y.; Shigemori, T.; Yamamoto, A.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Okita, Y.; et al. Rac GTPase-Activating Protein 1 (RACGAP1) as an Oncogenic Enhancer in Esophageal Carcinoma. Oncology 2019, 97, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, H.; Toiyama, Y.; Saigusa, S.; Kawamura, M.; Kawamoto, A.; Okugawa, Y.; Hiro, J.; Tanaka, K.; Inoue, Y.; Mohri, Y.; et al. RacGAP1 expression, increasing tumor malignant potential, as a predictive biomarker for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis 2015, 36, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yang, N.; Liu, Y. RRP9 and DDX21 as new biomarkers of colorectal cancer. Medicine 2023, 102, e34384. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Li, T.; Qin, S.; Yu, S.; Chu, Q.; Li, A.; Wu, K. Identifying Tumorigenesis and Prognosis-Related Genes of Lung Adenocarcinoma: Based on Weighted Gene Coexpression Network Analysis. BioMed Res. Int. 2020, 2020, 4169691. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lei, Y.; Li, S.; Li, F.; Lei, J. LncRNA PART1 Stimulates the Development of Ovarian Cancer by Up-regulating RACGAP1 and RRM2. Reprod. Sci. 2022, 29, 2224–2235. [Google Scholar] [CrossRef]
- Liu, Y.; Han, T.; Miao, R.; Zhou, J.; Guo, J.; Xu, Z.; Xing, Y.; Bai, Y.; Wu, J.; Hu, D. RACGAP1 promotes the progression and poor prognosis of lung adenocarcinoma through its effects on the cell cycle and tumor stemness. BMC Cancer 2024, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-S.; Huang, W.-M.; Xia, H.-M.; Mi, J.-L.; Li, Y.-Q.; Liang, H.-Q.; Zhou, L.; Lu, Z.-X.; Wu, F. Oncogenic and immunological roles of RACGAP1 in pan-cancer and its potential value in nasopharyngeal carcinoma. Apoptosis Int. J. Program. Cell Death 2024, 29, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Martini, T.; Heinkele, J.; Mayr, R.; Weis, C.A.; Wezel, F.; Wahby, S.; Eckstein, M.; Schnoller, T.; Breyer, J.; Wirtz, R.; et al. Predictive value of lymphangiogenesis and proliferation markers on mRNA level in urothelial carcinoma of the bladder after radical cystectomy. Urol. Oncol. 2018, 36, 530.e19–530.e27. [Google Scholar] [CrossRef]
- Pan, S.; Zhan, Y.; Chen, X.; Wu, B.; Liu, B. Identification of Biomarkers for Controlling Cancer Stem Cell Characteristics in Bladder Cancer by Network Analysis of Transcriptome Data Stemness Indices. Front. Oncol. 2019, 9, 613. [Google Scholar] [CrossRef]
- Stone, R., 2nd; Sabichi, A.L.; Gill, J.; Lee, I.L.; Adegboyega, P.; Dai, M.S.; Loganantharaj, R.; Trutschl, M.; Cvek, U.; Clifford, J.L. Identification of genes correlated with early-stage bladder cancer progression. Cancer Prev. Res. 2010, 3, 776–786. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.; Zhuo, L.; Gao, H.; Yang, Y.; Li, W. RACGAP1 is a pivotal gene in lung adenocarcinoma-associated membranous nephropathy: Based on comprehensive bioinformatics analysis and machine learning. Int. Immunopharmacol. 2024, 139, 112783. [Google Scholar] [CrossRef] [PubMed]
- Amjad, E.; Asnaashari, S.; Sokouti, B.; Dastmalchi, S. Systems biology comprehensive analysis on breast cancer for identification of key gene modules and genes associated with TNM-based clinical stages. Sci. Rep. 2020, 10, 10816. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Ferreira, S.; Morin, M.; Guichaoua, G.; Moindjie, H.; Haykal, M.M.; Collier, O.; Stoven, V.; Nahmias, C. A Network of 17 Microtubule-Related Genes Highlights Functional Deregulations in Breast Cancer. Cancers 2023, 15, 4870. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, R.; Gao, C.; Zhang, T.; Qi, L.; Liu, G.; Zhang, W.; Wang, X.; Li, J.; Li, J.; et al. Identification of prognostic biomarkers for breast cancer based on miRNA and mRNA co-expression network. J. Cell Biochem. 2019, 120, 15378–15388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Yu, Z.; Zhou, Q.; Sun, F.; Han, J.; Gao, L.; Dou, B.; Zhang, H.; Fu, J.; et al. Reciprocal regulation between RACGAP1 and AR contributes to endocrine therapy resistance in prostate cancer. Cell Commun. Signal 2024, 22, 339. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, X.; Xie, J.-X.; Hou, Y.; Fan, W.-M.; Wang, X.-Q.; Zhang, L.-W.; Yang, X.-M.; Li, J.; Fei, H. RACGAP1 knockdown synergizes and enhances the effects of chemotherapeutics on ovarian cancer. Am. J. Transl. Res. 2024, 16, 2132–2146. [Google Scholar] [CrossRef] [PubMed]
- Anurogo, D.; Liu, C.-L.; Chang, Y.-C.; Chang, Y.-H.; Qiu, J.T. Discovery of differentially expressed proteins for CAR-T therapy of ovarian cancers with a bioinformatics analysis. Aging 2024, 16, 11409–11433. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, J.; Lin, J.; Cai, Y.; Zhao, Z. Constructing lactylation-related genes prognostic model to effectively predict the disease-free survival and treatment responsiveness in prostate cancer based on machine learning. Front. Genet. 2024, 15, 1343140. [Google Scholar] [CrossRef]
- Cai, K.T.; Liu, A.G.; Wang, Z.F.; Jiang, H.W.; Zeng, J.J.; He, R.Q.; Ma, J.; Chen, G.; Zhong, J.C. Expression and potential molecular mechanisms of miR2045p in breast cancer, based on bioinformatics and a metaanalysis of 2,306 cases. Mol. Med. Rep. 2019, 19, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Idichi, T.; Seki, N.; Wada, M.; Yamada, Y.; Fukuhisa, H.; Toda, H.; Kita, Y.; Kawasaki, Y.; Tanoue, K.; et al. Gene Regulation by Antitumor miR-204-5p in Pancreatic Ductal Adenocarcinoma: The Clinical Significance of Direct RACGAP1 Regulation. Cancers 2019, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Wang, J.; Wei, H.; Lu, T.; Wu, X.; Wu, Y.; Shao, Z.; Luo, C.; Lu, Y. lncRNA MAGI2-AS3 Prevents the Development of HCC via Recruiting KDM1A and Promoting H3K4me2 Demethylation of the RACGAP1 Promoter. Mol. Ther. Nucleic Acids 2019, 18, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Chen, D.P.; Qi, B.; Li, M.Y.; Zhu, Y.Y.; Yin, W.J.; He, L.; Yu, Y.; Li, Z.Y.; Lin, L.; et al. Pseudogene RACGAP1P activates RACGAP1/Rho/ERK signalling axis as a competing endogenous RNA to promote hepatocellular carcinoma early recurrence. Cell Death Dis. 2019, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.D.; Feng, Z.B.; Lai, Z.F.; Qin, Y.; Liu, L.M.; Fu, H.X.; He, R.Q.; Wu, H.Y.; Dang, Y.W.; Chen, G.; et al. High throughput circRNA sequencing analysis reveals novel insights into the mechanism of nitidine chloride against hepatocellular carcinoma. Cell Death Dis. 2019, 10, 658. [Google Scholar] [CrossRef]
- Mollanoori, H.; Ghelmani, Y.; Hassani, B.; Dehghani, M. Integrated whole transcriptome profiling revealed a convoluted circular RNA-based competing endogenous RNAs regulatory network in colorectal cancer. Sci. Rep. 2024, 14, 91. [Google Scholar] [CrossRef]
- Mahmoodi Chalbatani, G.; Gharagouzloo, E.; Malekraeisi, M.A.; Azizi, P.; Ebrahimi, A.; Hamblin, M.R.; Mahmoodzadeh, H.; Elkord, E.; Miri, S.R.; Sanati, M.H.; et al. The integrative multi-omics approach identifies the novel competing endogenous RNA (ceRNA) network in colorectal cancer. Sci. Rep. 2023, 13, 19454. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Mu, Q.; Liu, S.; Huang, K.; Tang, Y.; Zhang, P.; Zhao, J.; Shu, C. m6A-modified circASXL1 promotes proliferation and migration of ovarian cancer through the miR-320d/RACGAP1 axis. Carcinogenesis 2023, 44, 859–870. [Google Scholar] [CrossRef]
- Reyes, M.E.; Ma, J.; Grove, M.L.; Ater, J.L.; Morrison, A.C.; Hildebrandt, M.A.T. RNA sequence analysis of inducible pluripotent stem cell-derived cardiomyocytes reveals altered expression of DNA damage and cell cycle genes in response to doxorubicin. Toxicol. Appl. Pharmacol. 2018, 356, 44–53. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Wang, C.; Liu, Y.; Liu, H.; Luo, S. RACGAP1 is transcriptionally regulated by E2F3, and its depletion leads to mitotic catastrophe in esophageal squamous cell carcinoma. Ann. Transl. Med. 2020, 8, 950. [Google Scholar] [CrossRef]
- Hazar-Rethinam, M.; de Long, L.M.; Gannon, O.M.; Boros, S.; Vargas, A.C.; Dzienis, M.; Mukhopadhyay, P.; Saenz-Ponce, N.; Dantzic, D.D.; Simpson, F.; et al. RacGAP1 Is a Novel Downstream Effector of E2F7-Dependent Resistance to Doxorubicin and Is Prognostic for Overall Survival in Squamous Cell Carcinoma. Mol. Cancer Ther. 2015, 14, 1939–1950. [Google Scholar] [CrossRef]
- Song, Z.; Cao, Q.; Guo, B.; Zhao, Y.; Li, X.; Lou, N.; Zhu, C.; Luo, G.; Peng, S.; Li, G.; et al. Overexpression of RACGAP1 by E2F1 Promotes Neuroendocrine Differentiation of Prostate Cancer by Stabilizing EZH2 Expression. Aging Dis. 2023, 14, 1757–1774. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Jiang, Y.; Huan, Y.; Han, Y.; Liu, W.; Liu, X.; Wang, Y.; He, L.; Cao, Z.; He, X.; et al. Rac GTPase activating protein 1 promotes the glioma growth by regulating the expression of MCM3. Transl. Oncol. 2023, 37, 101756. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, B.; Guo, D.; Pan, L.; Luo, X.; Tang, J.; Yang, W.; Zhang, Y.; Zhang, L.; Huang, J.; et al. Up-Regulation of RACGAP1 Promotes Progressions of Hepatocellular Carcinoma Regulated by GABPA via PI3K/AKT Pathway. Oxid. Med. Cell Longev. 2022, 2022, 3034150. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, J.; Zhou, D.; Pan, X.; Chu, Y.; Yin, J. FOXM1 transcriptional regulation of RacGAP1 activates the PI3K/AKT signaling pathway to promote the proliferation, migration, and invasion of cervical cancer cells. Int. J. Clin. Oncol. 2024, 29, 333–344. [Google Scholar] [CrossRef]
- Wilson, B.D.; Ricks-Santi, L.J.; Mason, T.E.; Abbas, M.; Kittles, R.A.; Dunston, G.M.; Kanaan, Y.M. Admixture Mapping Links RACGAP1 Regulation to Prostate Cancer in African Americans. Cancer Genom. Proteom. 2018, 15, 185–191. [Google Scholar] [CrossRef]
- Hazar-Rethinam, M.; de Long, L.M.; Gannon, O.M.; Topkas, E.; Boros, S.; Vargas, A.C.; Dzienis, M.; Mukhopadhyay, P.; Simpson, F.; Endo-Munoz, L.; et al. A novel E2F/sphingosine kinase 1 axis regulates anthracycline response in squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 417–427. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Z.; Li, W.; Lu, Y.; Pu, J. HIF-1α and RACGAP1 promote the progression of hepatocellular carcinoma in a mutually regulatory way. Mol. Med. Rep. 2023, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Bao, Y.C.; Nomura, Y.; Moon, Y.; Tonozuka, Y.; Minoshima, Y.; Hatori, T.; Tsuchiya, A.; Kiyono, M.; Nosaka, T.; et al. Rac1 and a GTPase-activating protein, MgcRacGAP, are required for nuclear translocation of STAT transcription factors. J. Cell Biol. 2006, 175, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Lin, M.; Brouwer-Visser, J.; Heim, J.; Smotkin, D.; Hebert, T.; Gunter, M.J.; Goldberg, G.L.; Zheng, D.; Huang, G.S. RNA-seq Identification of RACGAP1 as a Metastatic Driver in Uterine Carcinosarcoma. Clin. Cancer Res. 2016, 22, 4676–4686. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Wang, K.; Liang, Y.; Liu, T.; Feng, L.; Yang, X. RacGAP1 promotes the malignant progression of cervical cancer by regulating AP-1 via miR-192 and p-JNK. Cell Death Dis. 2022, 13, 604. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, D.; Wang, M.; Li, E.; Hou, C.; Su, Y.; Zou, Q.; Zhou, P.; Liu, X. RACGAP1 modulates ECT2-Dependent mitochondrial quality control to drive breast cancer metastasis. Exp. Cell Res. 2021, 400, 112493. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, S.; Tu, J.; Wang, M.; Liang, W.; Cheng, J.; Guan, J.; Xu, J. RACGAP1 promotes lung cancer cell proliferation through the PI3K/AKT signaling pathway. Sci. Rep. 2024, 14, 8694. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.Y.; Zhang, H.; Zhao, B.; Zha, Z.Y.; Bai, F.; Pei, X.H.; Zhao, S.; Xiong, Y.; Guan, K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell Biol. 2008, 28, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Guan, K.L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, S.; Guo, Q.; Wei, N.; Xiao, Z.; Song, Y. Upregulation of RACGAP1 is correlated with poor prognosis and immune infiltration in hepatocellular carcinoma. Transl. Cancer Res. 2024, 13, 847–863. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, S.; Xu, S.; Sun, Z.; Liang, Y.; Ding, X. RacGAP1 ameliorates acute kidney injury by promoting proliferation and suppressing apoptosis of renal tubular cells. Biochem. Biophys. Res. Commun. 2020, 527, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, W.; Li, Y.; Ji, Z. PLAGL2 promotes bladder cancer progression via RACGAP1/RhoA GTPase/YAP1 signaling. Cell Death Dis. 2023, 14, 433. [Google Scholar] [CrossRef]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.D.; Fan, C.; Mitin, N.; Baker, N.M.; George, S.D.; Graham, D.M.; Perou, C.M.; Burridge, K.; Der, C.J.; Rossman, K.L. Rho GTPase Transcriptome Analysis Reveals Oncogenic Roles for Rho GTPase-Activating Proteins in Basal-like Breast Cancers. Cancer Res. 2016, 76, 3826–3837. [Google Scholar] [CrossRef]
- Wang, B.; Lin, H.; Li, X.; Lu, W.; Kim, J.B.; Xu, A.; Cheng, K.K.Y. The adaptor protein APPL2 controls glucose-stimulated insulin secretion via F-actin remodeling in pancreatic beta-cells. Proc. Natl. Acad. Sci. USA 2020, 117, 28307–28315. [Google Scholar] [CrossRef]
- Kobayashi, A.; Hashizume, C.; Dowaki, T.; Wong, R.W. Therapeutic potential of mitotic interaction between the nucleoporin Tpr and aurora kinase A. Cell Cycle 2015, 14, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Funasaka, T.; Hashizume, C.; Wong, R.W. Nucleoporin translocated promoter region (Tpr) associates with dynein complex, preventing chromosome lagging formation during mitosis. J. Biol. Chem. 2010, 285, 10841–10849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-m.; Fang, G. MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis. Proc. Natl. Acad. Sci. USA 2005, 102, 13158–13163. [Google Scholar] [CrossRef]
- Görlich, D.; Kutay, U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999, 15, 607–660. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Cansizoglu, A.E.; Suel, K.E.; Louis, T.H.; Zhang, Z.; Chook, Y.M. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell 2006, 126, 543–558. [Google Scholar] [CrossRef]
- Mattaj, I.W.; Englmeier, L. Nucleocytoplasmic transport: The soluble phase. Annu. Rev. Biochem. 1998, 67, 265–306. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, Y.; Kawashima, T.; Hirose, K.; Tonozuka, Y.; Kawajiri, A.; Bao, Y.C.; Deng, X.; Tatsuka, M.; Narumiya, S.; May, W.S.; et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev. Cell 2003, 4, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, C.; Wei, Z.; He, W.; Xu, N.; Xu, Y.; Li, T.; Ren, K.; Kuang, Y.; Zhu, X.; et al. EGF-induced nuclear translocation of SHCBP1 promotes bladder cancer progression through inhibiting RACGAP1-mediated RAC1 inactivation. Cell Death Dis. 2022, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, J.; Wang, X.; Geng, Y.; Sun, L.; Zhang, H. The centralspindlin complex regulates cytokinesis and morphogenesis in the C. elegans spermatheca. Development 2023, 150, 200840. [Google Scholar] [CrossRef]
- Sander, E.E.; ten Klooster, J.P.; van Delft, S.; van der Kammen, R.A.; Collard, J.G. Rac downregulates Rho activity: Reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 1999, 147, 1009–1022. [Google Scholar] [CrossRef]
- Rottner, K.; Hall, A.; Small, J.V. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 1999, 9, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Vigil, D.; Cherfils, J.; Rossman, K.L.; Der, C.J. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat. Rev. Cancer 2010, 10, 842–857. [Google Scholar] [CrossRef]
- Aguilar-Aragon, M.; Bonello, T.T.; Bell, G.P.; Fletcher, G.C.; Thompson, B.J. Adherens junction remodelling during mitotic rounding of pseudostratified epithelial cells. EMBO Rep. 2020, 21, e49700. [Google Scholar] [CrossRef]
- Yang, A.; Liu, J.; Li, M.; Zhang, H.; Zhang, X.; Wu, L. Integrating bioinformatics and machine learning methods to analyze diagnostic biomarkers for HBV-induced hepatocellular carcinoma. Diagn. Pathol. 2024, 19, 105. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Jia, X.; Song, W.; Wu, H.; Zhu, H.; Xuan, Z.; Du, Y.; Zhu, X.; Song, G.; et al. Targeting anillin inhibits tumorigenesis and tumor growth in hepatocellular carcinoma via impairing cytokinesis fidelity. Oncogene 2022, 41, 3118–3130. [Google Scholar] [CrossRef]
- Kim, H.; Guo, F.; Brahma, S.; Xing, Y.; Burkard, M.E. Centralspindlin assembly and 2 phosphorylations on MgcRacGAP by Polo-like kinase 1 initiate Ect2 binding in early cytokinesis. Cell Cycle 2014, 13, 2952–2961. [Google Scholar] [CrossRef][Green Version]
- Matthews, H.K.; Delabre, U.; Rohn, J.L.; Guck, J.; Kunda, P.; Baum, B. Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev. Cell 2012, 23, 371–383. [Google Scholar] [CrossRef]
- Wu, C.G.; Chen, H.; Guo, F.; Yadav, V.K.; McIlwain, S.J.; Rowse, M.; Choudhary, A.; Lin, Z.; Li, Y.; Gu, T.; et al. PP2A-B’ holoenzyme substrate recognition, regulation and role in cytokinesis. Cell Discov. 2017, 3, 17027. [Google Scholar] [CrossRef]
- Sana, S.; Rajeevan, A.; Kotak, S. Membrane compartmentalization of Ect2/Cyk4/Mklp1 and NuMA/dynein regulates cleavage furrow formation. J. Cell Biol. 2022, 221, e202203127. [Google Scholar] [CrossRef]
- Chen, J.; Xia, H.; Zhang, X.; Karthik, S.; Pratap, S.V.; Ooi, L.L.; Hong, W.; Hui, K.M. ECT2 regulates the Rho/ERK signalling axis to promote early recurrence in human hepatocellular carcinoma. J. Hepatol. 2015, 62, 1287–1295. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Zhu, Y.; Lin, Z.; Yu, J.; Lin, X.; Lai, W.; Tong, B.; Xu, L.; Li, E.; Long, L. The Expression Regulation and Cancer-Promoting Roles of RACGAP1. Biomolecules 2025, 15, 3. https://doi.org/10.3390/biom15010003
Lin J, Zhu Y, Lin Z, Yu J, Lin X, Lai W, Tong B, Xu L, Li E, Long L. The Expression Regulation and Cancer-Promoting Roles of RACGAP1. Biomolecules. 2025; 15(1):3. https://doi.org/10.3390/biom15010003
Chicago/Turabian StyleLin, Jiacheng, Yuhao Zhu, Zhaoping Lin, Jindong Yu, Xiaobing Lin, Weiyuan Lai, Beibei Tong, Liyan Xu, Enmin Li, and Lin Long. 2025. "The Expression Regulation and Cancer-Promoting Roles of RACGAP1" Biomolecules 15, no. 1: 3. https://doi.org/10.3390/biom15010003
APA StyleLin, J., Zhu, Y., Lin, Z., Yu, J., Lin, X., Lai, W., Tong, B., Xu, L., Li, E., & Long, L. (2025). The Expression Regulation and Cancer-Promoting Roles of RACGAP1. Biomolecules, 15(1), 3. https://doi.org/10.3390/biom15010003







