Congenital Zika Syndrome: Insights from Integrated Proteomic and Metabolomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Study Design
2.3. Vesicle Isolation and Purification
2.4. EV Sample Preparation for MS-Based Proteomics Analysis
2.5. EV Identification of Metabolites with GC-MS
3. Results
3.1. EV Characterization
3.2. Proteomic and Metabolomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastère, S.; Valour, F.; Baudouin, L.; Mallet, H.P.; Musso, D.; Ghawche, F. Zika Virus Infection Complicated by Guillain-Barré Syndrome—Case Report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef]
- Rozé, B.; Najioullah, F.; Fergé, J.-L.; Apetse, K.; Brouste, Y.; Cesaire, R.; Fagour, C.; Fagour, L.; Hochedez, P.; Jeannin, S.; et al. Zika Virus Detection in Urine from Patients with Guillain-Barré Syndrome on Martinique, January 2016. Eurosurveillance 2016, 21, 30154. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.N.; Marten, A.D.; Moore, G.A.; Tree, M.O.; McBrayer, S.P.; Conway, M.J. Extracellular Vesicles Restrict Dengue Virus Fusion in Aedes Aegypti Cells. Virology 2020, 541, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.O.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and Sequencing of Zika Virus from Amniotic Fluid of Fetuses with Microcephaly in Brazil: A Case Study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Lee, M.F.; Voon, G.Z.; Lim, H.X.; Chua, M.L.; Poh, C.L. Innate and Adaptive Immune Evasion by Dengue Virus. Front. Cell Infect. Microbiol. 2022, 12, 1004608. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, C.; Groß, R.; Zou, M.; Krüger, F.; Görgens, A.; Gustafsson, M.O.; El Andaloussi, S.; Münch, J.; Müller, J.A. Salivary Extracellular Vesicles Inhibit Zika Virus but Not SARS-CoV-2 Infection. J. Extracell. Vesicles 2020, 9, 1808281. [Google Scholar] [CrossRef]
- Martínez-Rojas, P.P.; Quiroz-García, E.; Monroy-Martínez, V.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Ruiz-Ordaz, B.H. Participation of Extracellular Vesicles from Zika-Virus-Infected Mosquito Cells in the Modification of Naïve Cells’ Behavior by Mediating Cell-to-Cell Transmission of Viral Elements. Cells 2020, 9, 123. [Google Scholar] [CrossRef]
- Williams, C.; Palviainen, M.; Reichardt, N.-C.; Siljander, P.R.-M.; Falcón-Pérez, J.M. Metabolomics Applied to the Study of Extracellular Vesicles. Metabolites 2019, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Guo, M.; Tan, Q.; Zhou, E.; Deng, J.; Li, M.; Chen, J.; Yang, Z.; Jin, Y. Metabolomics of Extracellular Vesicles: A Future Promise of Multiple Clinical Applications. Int. J. Nanomed. 2022, 17, 6113–6129. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass Spectrometry-Based Metabolomics: A Guide for Annotation, Quantification and Best Reporting Practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Graupera, M.; Vanhaesebroeck, B. Targeting PI3K in Cancer: Impact on Tumor Cells, Their Protective Stroma, Angiogenesis, and Immunotherapy. Cancer Discov. 2016, 6, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Castel, P.; Toska, E.; Engelman, J.A.; Scaltriti, M. The Present and Future of PI3K Inhibitors for Cancer Therapy. Nat. Cancer 2021, 2, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Cantley, L.C. At a Crossroads: How to Translate the Roles of PI3K in Oncogenic and Metabolic Signalling into Improvements in Cancer Therapy. Nat. Rev. Clin. Oncol. 2022, 19, 471–485. [Google Scholar] [CrossRef]
- França, G.V.A.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.P.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika Virus Syndrome in Brazil: A Case Series of the First 1501 Livebirths with Complete Investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef]
- Moura da Silva, A.A.; Ganz, J.S.S.; Sousa, P.d.S.; Doriqui, M.J.R.; Ribeiro, M.R.C.; Branco, M.d.R.F.C.; Queiroz, R.C.d.S.; Pacheco, M.d.J.T.; Vieira da Costa, F.R.; Silva, F.d.S.; et al. Early Growth and Neurologic Outcomes of Infants with Probable Congenital Zika Virus Syndrome. Emerg. Infect. Dis. 2016, 22, 1953–1956. [Google Scholar] [CrossRef]
- Pontes, L.G.d.; Altei, W.F.; Galan, A.; Bilić, P.; Guillemin, N.; Kuleš, J.; Horvatić, A.; Ribeiro, L.N.d.M.; Paula, E.d.; Pereira, V.B.R.; et al. Extracellular Vesicles in Infectious Diseases Caused by Protozoan Parasites in Buffaloes. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190067. [Google Scholar] [CrossRef]
- Kang, S.G.; Choi, Y.Y.; Mo, S.J.; Kim, T.H.; Ha, J.H.; Hong, D.K.; Lee, H.; Park, S.D.; Shim, J.-J.; Lee, J.-L.; et al. Effect of Gut Microbiome-Derived Metabolites and Extracellular Vesicles on Hepatocyte Functions in a Gut-Liver Axis Chip. Nano Converg. 2023, 10, 5. [Google Scholar] [CrossRef]
- Chen, Z.; Zhai, J.; Ma, J.; Chen, P.; Lin, W.; Zhang, W.; Xiong, J.; Zhang, C.; Wei, H. Melatonin-Primed Mesenchymal Stem Cells-Derived Small Extracellular Vesicles Alleviated Neurogenic Erectile Dysfunction by Reversing Phenotypic Modulation. Adv. Healthc. Mater. 2023, 12, 2203087. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.E.; Jonsson, P.; Bylesjö, M.; Trygg, J.; Antti, H.; Eriksson, M.E.; Moritz, T. Changes in Diurnal Patterns within the Populus Transcriptome and Metabolome in Response to Photoperiod Variation. Plant Cell Environ. 2010, 33, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Budzinski, I.G.F.; Moon, D.H.; Morosini, J.S.; Lindén, P.; Bragatto, J.; Moritz, T.; Labate, C.A. Integrated Analysis of Gene Expression from Carbon Metabolism, Proteome and Metabolome, Reveals Altered Primary Metabolism in Eucalyptus Grandis Bark, in Response to Seasonal Variation. BMC Plant Biol. 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Johansson, A.I.; Gullberg, J.; Trygg, J.; Grung, B.; Marklund, S.; Sjöström, M.; Antti, H.; Moritz, T. High-Throughput Data Analysis for Detecting and Identifying Differences between Samples in GC/MS-Based Metabolomic Analyses. Anal. Chem. 2005, 77, 5635–5642. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Jeannin, P.; Chaze, T.; Giai Gianetto, Q.; Matondo, M.; Gout, O.; Gessain, A.; Afonso, P.V. Proteomic Analysis of Plasma Extracellular Vesicles Reveals Mitochondrial Stress upon HTLV-1 Infection. Sci. Rep. 2018, 8, 5170. [Google Scholar] [CrossRef]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic Analysis of Cerebrospinal Fluid Extracellular Vesicles Reveals Synaptic Injury, Inflammation, and Stress Response Markers in HIV Patients with Cognitive Impairment. J. Neuroinflamm. 2019, 16, 254. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Porcellati, S.; Emiliani, C. The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines 2019, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, 2514. [Google Scholar] [CrossRef]
- Askeland, A.; Borup, A.; Østergaard, O.; Olsen, J.V.; Lund, S.M.; Christiansen, G.; Kristensen, S.R.; Heegaard, N.H.H.; Pedersen, S. Mass-Spectrometry Based Proteome Comparison of Extracellular Vesicle Isolation Methods: Comparison of ME-Kit, Size-Exclusion Chromatography, and High-Speed Centrifugation. Biomedicines 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.E.; Garcia, G.; Contreras, D.; Gong, D.; Sun, R.; Arumugaswami, V. Zika Virus Mucosal Infection Provides Protective Immunity. J. Virol. 2020, 94, e00067-20. [Google Scholar] [CrossRef] [PubMed]
- Hallal, S.; Tűzesi, Á.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the Extracellular Vesicle Surface for Clinical Molecular Biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration Combined with Size Exclusion Chromatography Efficiently Isolates Extracellular Vesicles from Cell Culture Media for Compositional and Functional Studies. Sci. Rep. 2017, 7, 15297. [Google Scholar] [CrossRef] [PubMed]
- Fialho, E.M.S.; Veras, E.M.; Jesus, C.M.d.; Gomes, L.N.; Khouri, R.; Sousa, P.S.; Ribeiro, M.R.C.; Batista, R.F.L.; Costa, L.C.; Nascimento, F.R.F.; et al. Maternal Th17 Profile after Zika Virus Infection Is Involved in Congenital Zika Syndrome Development in Children. Viruses 2023, 15, 1320. [Google Scholar] [CrossRef]
- Oosthuizen, D.; Sturrock, E.D. Exploring the Impact of ACE Inhibition in Immunity and Disease. J. Renin-Angiotensin-Aldosterone Syst. 2022, 2022, 9028969. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/MTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/MTOR Interactive Pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, D.; Kim, E.-Y.; Rahman, A.H.; Raghunathan, R.; Kim-Schulze, S.; Che, Y.; Kalayci, S.; Gümüş, Z.H.; Kuan, G.; Balmaseda, A.; et al. Comprehensive Immunoprofiling of Pediatric Zika Reveals Key Role for Monocytes in the Acute Phase and No Effect of Prior Dengue Virus Infection. Cell Rep. 2020, 31, 107569. [Google Scholar] [CrossRef]
- Srivastava, M.; Zhang, Y.; Chen, J.; Sirohi, D.; Miller, A.; Zhang, Y.; Chen, Z.; Lu, H.; Xu, J.; Kuhn, R.J.; et al. Chemical Proteomics Tracks Virus Entry and Uncovers NCAM1 as Zika Virus Receptor. Nat. Commun. 2020, 11, 3896. [Google Scholar] [CrossRef]
- Tatara, J.M.; Santi, L.; Beys-da-Silva, W.O. Proteome Alterations Promoted by Zika Virus Infection. In Zika Virus Biology, Transmission, and Pathology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 307–317. [Google Scholar]
- Sun, X.; Hua, S.; Gao, C.; Blackmer, J.E.; Ouyang, Z.; Ard, K.; Ciaranello, A.; Yawetz, S.; Sax, P.E.; Rosenberg, E.S.; et al. Immune-Profiling of ZIKV-Infected Patients Identifies a Distinct Function of Plasmacytoid Dendritic Cells for Immune Cross-Regulation. Nat. Commun. 2020, 11, 2421. [Google Scholar] [CrossRef]
- Giacobino, C.; Canta, M.; Fornaguera, C.; Borrós, S.; Cauda, V. Extracellular Vesicles and Their Current Role in Cancer Immunotherapy. Cancers 2021, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. In Cancer Cell Signaling: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2021; pp. 143–170. [Google Scholar]
- Akhmerov, A.; Parimon, T. Extracellular Vesicles, Inflammation, and Cardiovascular Disease. Cells 2022, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wu, L.; Wang, L.; Reiter, R.J.; Lip, G.Y.H.; Ren, J. Extracellular Vesicles in Cardiovascular Diseases: From Pathophysiology to Diagnosis and Therapy. Cytokine Growth Factor. Rev. 2023, 74, 40–55. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas Aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Peregrino, E.S.; Castañeda-Casimiro, J.; Vázquez-Flores, L.; Estrada-Parra, S.; Wong-Baeza, C.; Serafín-López, J.; Wong-Baeza, I. The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 6210. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The Roles of Extracellular Vesicles in the Immune System. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Flores, V.; Romero, R.; Xu, Y.; Theis, K.R.; Arenas-Hernandez, M.; Miller, D.; Peyvandipour, A.; Bhatti, G.; Galaz, J.; Gershater, M.; et al. Maternal-Fetal Immune Responses in Pregnant Women Infected with SARS-CoV-2. Nat. Commun. 2022, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, D.M.; Schuepbach, R.A. Protease-Activated Receptors (PARs): Mechanisms of Action and Potential Therapeutic Modulators in PAR-Driven Inflammatory Diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tato, C.M.; Davis, M.M. How the Immune System Talks to Itself: The Varied Role of Synapses. Immunol. Rev. 2013, 251, 65–79. [Google Scholar] [CrossRef]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-Specific Regulation of Extracellular Vesicle Biogenesis and Cargo Selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Wright, S.S.; Rathinam, V.A. Role of Extracellular Vesicles in Immunity and Host Defense. Immunol. Investig. 2024, 53, 10–25. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Sun, X.; Wu, C.; Yao, L.; Zhang, Y.; Liu, S.; Jiang, Y.; Li, Y.; Wang, M.; Xu, Y. PROS1, a Clinical Prognostic Biomarker and Tumor Suppressor, Is Associated with Immune Cell Infiltration in Breast Cancer: A Bioinformatics Analysis Combined with Experimental Verification. Cell Signal 2023, 112, 110918. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The Role of Extracellular Vesicles in the Physiological and Pathological Regulation of the Blood–Brain Barrier. FASEB Bioadv. 2021, 3, 665–675. [Google Scholar] [CrossRef]
- Gamage, T.K.J.B.; Fraser, M. The Role of Extracellular Vesicles in the Developing Brain: Current Perspective and Promising Source of Biomarkers and Therapy for Perinatal Brain Injury. Front. Neurosci. 2021, 15, 744840. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Cai, Y.; Lin, J.; Ma, L.; Han, H.; Li, F. Advancement in Modulation of Brain Extracellular Space and Unlocking Its Potential for Intervention of Neurological Diseases. Med-X 2024, 2, 6. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Voltarelli, V.A.; Alves de Souza, R.W.; Miyauchi, K.; Hauser, C.J.; Otterbein, L.E. Heme: The Lord of the Iron Ring. Antioxidants 2023, 12, 1074. [Google Scholar] [CrossRef]
- Mense, S.M.; Zhang, L. Heme: A Versatile Signaling Molecule Controlling the Activities of Diverse Regulators Ranging from Transcription Factors to MAP Kinases. Cell Res. 2006, 16, 681–692. [Google Scholar] [CrossRef]
- Ellis-Guardiola, K.; Soule, J.; Clubb, R. Methods for the Extraction of Heme Prosthetic Groups from Hemoproteins. Bio Protoc. 2021, 11, e4156. [Google Scholar] [CrossRef]
- Bandu, R.; Oh, J.W.; Kim, K.P. Mass Spectrometry-Based Proteome Profiling of Extracellular Vesicles and Their Roles in Cancer Biology. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Oh, I.; Lee, N.; Lee, K.; Yoon, Y.J.; Lee, E.Y.; Kim, B.; Kim, D. Integrating Cell-free Biosyntheses of Heme Prosthetic Group and Apoenzyme for the Synthesis of Functional P450 Monooxygenase. Biotechnol. Bioeng. 2013, 110, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.C.; Sarkar, S.; Elliott, E.C.; Henry, H.R.; Powell, S.M.; Diaz Ludovico, I.; You, Y.; Huang, F.; Payne, S.H.; Ramanadham, S.; et al. A Proteomic Meta-Analysis Refinement of Plasma Extracellular Vesicles. Sci. Data 2023, 10, 837. [Google Scholar] [CrossRef]
- Chang, C.-J.; Huang, Y.-N.; Lu, Y.-B.; Zhang, Y.; Wu, P.-H.; Huang, J.-S.; Yang, W.; Chiang, T.-Y.; Hsieh, H.-S.; Chung, W.-H.; et al. Proteomic Analysis of Serum Extracellular Vesicles from Biliary Tract Infection Patients to Identify Novel Biomarkers. Sci. Rep. 2024, 14, 5707. [Google Scholar] [CrossRef] [PubMed]
- Badosa, C.; Roldán, M.; Fernández-Irigoyen, J.; Santamaria, E.; Jimenez-Mallebrera, C. Proteomic and Functional Characterisation of Extracellular Vesicles from Collagen VI Deficient Human Fibroblasts Reveals a Role in Cell Motility. Sci. Rep. 2023, 13, 14622. [Google Scholar] [CrossRef] [PubMed]
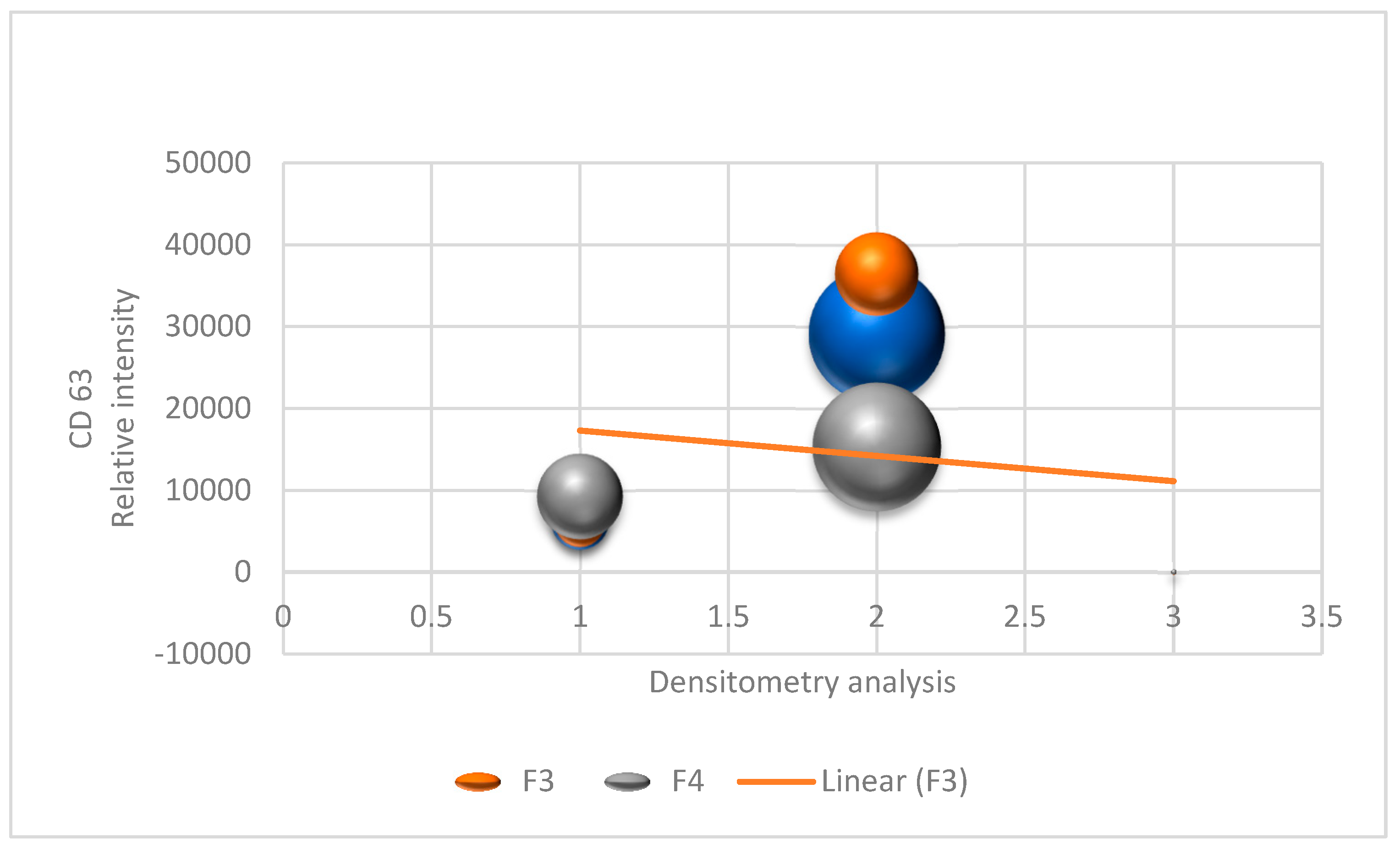

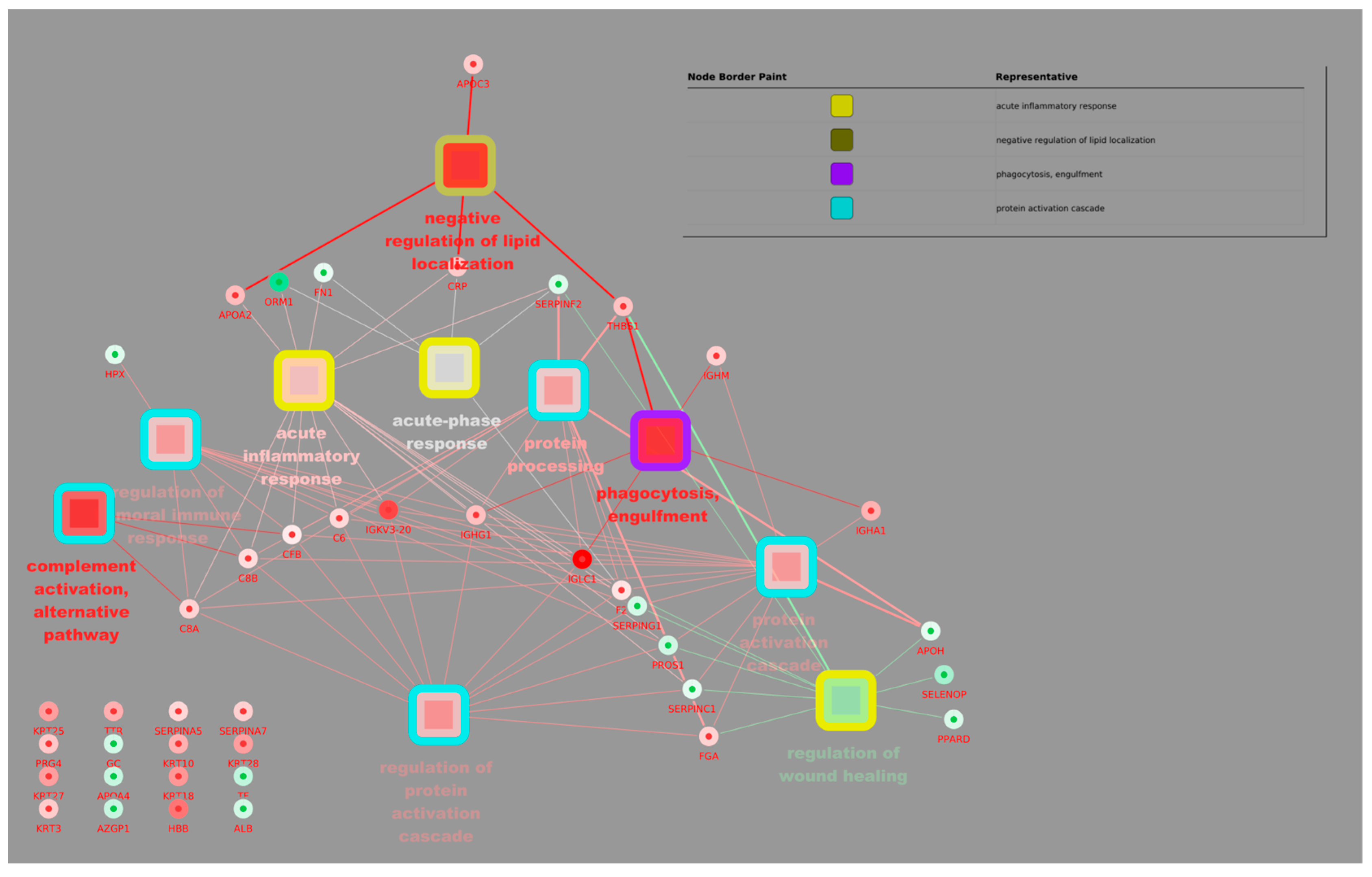
| Sample | Group | Average Size (nm) | Minimum Size (nm) | Maximum Size (nm) | Dispersion (PDI) | Concentration (Particles/mL) | Quantification (ng/ µL) |
|---|---|---|---|---|---|---|---|
| CZS +1 | CZS+ | 175.0 | 140 | 220 | 0.22 | 3.8 × 10 8 | 52.3 |
| CZS +2 | CZS+ | 190.2 | 135 | 225 | 0.20 | 4.1 × 10 8 | 60.1 |
| CZS +3 | CZS+ | 180.0 | 150 | 230 | 0.23 | 4.0 × 10 8 | 45.0 |
| CZS +4 | CZS+ | 195.3 | 140 | 210 | 0.19 | 3.9 × 10 8 | 70.5 |
| CZS +5 | CZS+ | 178.5 | 145 | 220 | 0.21 | 3.7 × 10 8 | 50.7 |
| CZS +6 | CZS+ | 192.6 | 160 | 235 | 0.24 | 4.2 × 10 8 | 80.4 |
| CZS +7 | CZS+ | 170.0 | 150 | 225 | 0.21 | 4.0 × 10 8 | 55.3 |
| CZS +8 | CZS+ | 196.4 | 130 | 220 | 0.22 | 3.9 × 10 8 | 65.2 |
| CZS +9 | CZS+ | 188.9 | 145 | 225 | 0.20 | 4.1 × 10 8 | 61.7 |
| CZS +10 | CZS+ | 179.7 | 135 | 215 | 0.22 | 4.0 × 10 8 | 48.6 |
| CZS +11 | CZS+ | 193.8 | 140 | 220 | 0.19 | 3.8 × 10 8 | 72.4 |
| CZS +12 | CZS+ | 180.5 | 150 | 230 | 0.21 | 4.1 × 10 8 | 58.9 |
| CZS +13 | CZS+ | 194.2 | 140 | 220 | 0.22 | 3.9 × 10 8 | 69.5 |
| CZS +14 | CZS+ | 185.4 | 130 | 220 | 0.21 | 4.0 × 10 8 | 57.2 |
| CZS -1 | CZS- | 150.0 | 120 | 200 | 0.19 | 3.5 × 10 8 | 44.1 |
| CZS -2 | CZS- | 160.1 | 115 | 195 | 0.18 | 3.4 × 10 8 | 36.5 |
| CZS -3 | CZS- | 152.0 | 110 | 200 | 0.18 | 3.5 × 10 8 | 38.9 |
| CZS -4 | CZS- | 159.5 | 125 | 205 | 0.19 | 3.6 × 10 8 | 42.6 |
| CZS -5 | CZS- | 149.0 | 130 | 210 | 0.20 | 3.7 × 10 8 | 49.2 |
| CZS -6 | CZS- | 160.30 | 120 | 200 | 0.18 | 3.5 × 10 8 | 46.7 |
| CZS -7 | CZS- | 155.5 | 125 | 205 | 0.19 | 3.6 × 10 8 | 43.8 |
| CZS -8 | CZS- | 153.8 | 110 | 195 | 0.17 | 3.4 × 10 8 | 37.6 |
| CZS -9 | CZS- | 148.9 | 120 | 200 | 0.18 | 3.5 × 10 8g | 41.4 |
| CZS -10 | CZS- | 161.0 | 130 | 210 | 0.20 | 3.6 × 10 8 | 49.8 |
| CZS -11 | CZS- | 156.7 | 115 | 195 | 0.18 | 3.5 × 10 8 | 44.9 |
| CZS -12 | CZS- | 151.2 | 120 | 200 | 0.17 | 3.4 × 10 8 | 39.2 |
| CZS -13 | CZS- | 157.0 | 125 | 205 | 0.19 | 3.6 × 10 8 | 45.3 |
| CZS -14 | CZS- | 153.4 | 120 | 200 | 0.18 | 3.5 × 10 8 | 42.0 |
| CZS -15 | CZS- | 154.9 | 120 | 198 | 0.20 | 3.6 × 10 8 | 40.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes-de-Pontes, L.; Barreiros, L.A.; Gomes, L.N.; Salgado, R.C.; da Silva Napoleão, S.M.; Soeiro-Pereira, P.V.; Passos, S.D.; Condino-Neto, A. Congenital Zika Syndrome: Insights from Integrated Proteomic and Metabolomic Analysis. Biomolecules 2025, 15, 32. https://doi.org/10.3390/biom15010032
Gomes-de-Pontes L, Barreiros LA, Gomes LN, Salgado RC, da Silva Napoleão SM, Soeiro-Pereira PV, Passos SD, Condino-Neto A. Congenital Zika Syndrome: Insights from Integrated Proteomic and Metabolomic Analysis. Biomolecules. 2025; 15(1):32. https://doi.org/10.3390/biom15010032
Chicago/Turabian StyleGomes-de-Pontes, Leticia, Lucila Akune Barreiros, Lillian Nunes Gomes, Ranieri Coelho Salgado, Sarah Maria da Silva Napoleão, Paulo V. Soeiro-Pereira, Saulo Duarte Passos, and Antonio Condino-Neto. 2025. "Congenital Zika Syndrome: Insights from Integrated Proteomic and Metabolomic Analysis" Biomolecules 15, no. 1: 32. https://doi.org/10.3390/biom15010032
APA StyleGomes-de-Pontes, L., Barreiros, L. A., Gomes, L. N., Salgado, R. C., da Silva Napoleão, S. M., Soeiro-Pereira, P. V., Passos, S. D., & Condino-Neto, A. (2025). Congenital Zika Syndrome: Insights from Integrated Proteomic and Metabolomic Analysis. Biomolecules, 15(1), 32. https://doi.org/10.3390/biom15010032








