Targeting Invasion: The Role of MMP-2 and MMP-9 Inhibition in Colorectal Cancer Therapy
Abstract
1. Introduction
2. Matrix Metalloproteinases (MMPs)
3. Gelatinase’s Role in CRC Progression
4. Natural-Based Gelatinase Inhibitors
5. Synthetic Small Molecules Inhibitors
6. MicroRNAs
7. Protein and Peptide Based Inhibitors
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedzwiedzka, E.; Arlukowicz, T.; Przybylowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef]
- Byrne, R.M.; Tsikitis, V.L. Colorectal polyposis and inherited colorectal cancer syndromes. Ann. Gastroenterol. 2018, 31, 24–34. [Google Scholar] [CrossRef]
- Skalitzky, M.K.; Zhou, P.P.; Goffredo, P.; Guyton, K.; Sherman, S.K.; Gribovskaja-Rupp, I.; Hassan, I.; Kapadia, M.R.; Hrabe, J.E. Characteristics and symptomatology of colorectal cancer in the young. Surgery 2023, 173, 1137–1143. [Google Scholar] [CrossRef]
- Holtedahl, K.; Borgquist, L.; Donker, G.A.; Buntinx, F.; Weller, D.; Campbell, C.; Mansson, J.; Hammersley, V.; Braaten, T.; Parajuli, R. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: A prospective cohort study of diagnostic accuracy in primary care. BMC Fam. Pract. 2021, 22, 148. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol 2021, 14, 101174. [Google Scholar] [CrossRef]
- Gu, M.; Thapa, S. Colorectal cancer in the United States and a review of its heterogeneity among Asian American subgroups. Asia Pac. J. Clin. Oncol. 2020, 16, 193–200. [Google Scholar] [CrossRef]
- Dutta, A.; Pratiti, R.; Kalantary, A.; Aboulian, A.; Shekherdimian, S. Colorectal Cancer: A Systematic Review of the Current Situation and Screening in North and Central Asian Countries. Cureus 2023, 15, e33424. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Amiano, P.; Fernandez de Larrea, N.; Martin, V.; Alonso, M.H.; Castano-Vinyals, G.; Perez-Gomez, B.; Olmedo-Requena, R.; Guevara, M.; Fernandez-Tardon, G.; et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur. J. Nutr. 2019, 58, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Kadla, S.A.; Rasool, V.; Ganai, B.A. A comparative overview of general risk factors associated with the incidence of colorectal cancer. Tumour Biol. 2013, 34, 2469–2476. [Google Scholar] [CrossRef]
- Cho, M.Y.; Siegel, D.A.; Demb, J.; Richardson, L.C.; Gupta, S. Increasing Colorectal Cancer Incidence Before and After Age 50: Implications for Screening Initiation and Promotion of “On-Time” Screening. Dig. Dis. Sci. 2022, 67, 4086–4091. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, A.S.; Agbaje, K.; Adesina, S.K.; Olajubutu, O. Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics 2023, 15, 2620. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16, 800. [Google Scholar] [CrossRef]
- Shoari, A.; Khalili-Tanha, G.; Coban, M.A.; Radisky, E.S. Structure and computation-guided yeast surface display for the evolution of TIMP-based matrix metalloproteinase inhibitors. Front. Mol. Biosci. 2023, 10, 1321956. [Google Scholar] [CrossRef]
- Radisky, E.S. Extracellular proteolysis in cancer: Proteases, substrates, and mechanisms in tumor progression and metastasis. J. Biol. Chem. 2024, 300, 107347. [Google Scholar] [CrossRef]
- Radisky, E.S.; Coban, M. Enzymes|Matrix Metalloproteinases. In Encyclopedia of Biological Chemistry III (Third Edition); Jez, J., Ed.; Elsevier: Oxford, UK, 2021; pp. 336–353. [Google Scholar]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Rasaee, M.J.; Kanavi, M.R.; Daraei, B. Functional mimetic peptide discovery isolated by phage display interacts selectively to fibronectin domain and inhibits gelatinase. J. Cell Biochem. 2019, 120, 19699–19711. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, G.; Gudowska-Sawczuk, M.; Mroczko, B.; Bruczko-Goralewska, M.; Romanowicz, L.; Tokarzewicz, A. Higher Content but No Specific Activity in Gelatinase B (MMP-9) Compared with Gelatinase A (MMP-2) in Human Renal Carcinoma. Cancers 2023, 15, 5475. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Singh, R.D.; Haridas, N.; Patel, J.B.; Shah, F.D.; Shukla, S.N.; Shah, P.M.; Patel, P.S. Matrix metalloproteinases and their inhibitors: Correlation with invasion and metastasis in oral cancer. Indian. J. Clin. Biochem. 2010, 25, 250–259. [Google Scholar] [CrossRef]
- Mook, O.R.; Frederiks, W.M.; Van Noorden, C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 2004, 1705, 69–89. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Buttacavoli, M.; Di Cara, G.; Roz, E.; Pucci-Minafra, I.; Feo, S.; Cancemi, P. Integrated Multi-Omics Investigations of Metalloproteinases in Colon Cancer: Focus on MMP2 and MMP9. Int. J. Mol. Sci. 2021, 22, 12389. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Gong, J.H.; Tam, E.M.; McCulloch, C.A.; Clark-Lewis, I.; Overall, C.M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 2000, 289, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.C.; Hsu, S.M.; Ho, H.N.; Lien, H.C.; Huang, S.C.; Lin, R.H. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001, 61, 237–242. [Google Scholar]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef]
- Shoari, A. Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review. Targets 2024, 2, 104–125. [Google Scholar] [CrossRef]
- Said, A.H.; Raufman, J.P.; Xie, G. The role of matrix metalloproteinases in colorectal cancer. Cancers 2014, 6, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013-2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Lei, Z.N.; Tian, Q.; Teng, Q.X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.S. Understanding and targeting resistance mechanisms in cancer. MedComm (2020) 2023, 4, e265. [Google Scholar] [CrossRef] [PubMed]
- Tune, B.X.J.; Sim, M.S.; Poh, C.L.; Guad, R.M.; Woon, C.K.; Hazarika, I.; Das, A.; Gopinath, S.C.B.; Rajan, M.; Sekar, M.; et al. Matrix Metalloproteinases in Chemoresistance: Regulatory Roles, Molecular Interactions, and Potential Inhibitors. J. Oncol. 2022, 2022, 3249766. [Google Scholar] [CrossRef] [PubMed]
- Sampaio Moura, N.; Schledwitz, A.; Alizadeh, M.; Patil, S.A.; Raufman, J.P. Matrix metalloproteinases as biomarkers and therapeutic targets in colitis-associated cancer. Front. Oncol. 2023, 13, 1325095. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; Taskforce, I.N.P.S. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Guaman-Ortiz, L.M.; Orellana, M.I.; Ratovitski, E.A. Natural Compounds As Modulators of Non-apoptotic Cell Death in Cancer Cells. Curr. Genom. 2017, 18, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.M.; Yance, D.; Wong, R.K. Natural health products that inhibit angiogenesis: A potential source for investigational new agents to treat cancer-Part 1. Curr. Oncol. 2006, 13, 14–26. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.B.; Nair, B.G.; Perry, J.J.P.; Martin, D.B.C. Recent insights into natural product inhibitors of matrix metalloproteinases. Medchemcomm 2019, 10, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Shen, S.C.; Lee, T.J.; Chen, Y.C. Myricetin inhibits matrix metalloproteinase 2 protein expression and enzyme activity in colorectal carcinoma cells. Mol. Cancer Ther. 2005, 4, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Deguchi, A.; Hara, Y.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 334, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhou, H.; Wang, T.; Mu, Y.; Wu, B.; Guo, D.L.; Zhang, X.M.; Wu, Y. Epigallocatechin-3-gallate inhibits proliferation and migration of human colon cancer SW620 cells. Acta Pharmacol. Sin. 2012, 33, 120–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, C.C.; Chen, G.W.; Lin, J.G.; Wu, L.T.; Chung, J.G. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B/p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer. Res. 2006, 26, 1281–1288. [Google Scholar] [PubMed]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Harikumar, K.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Han, I.H.; Sung, M.K.; Yoo, H.; Kim, Y.G.; Kim, J.S.; Kawada, T.; Yu, R. Soybean saponin inhibits tumor cell metastasis by modulating expressions of MMP-2, MMP-9 and TIMP- 2. Cancer Lett. 2008, 261, 84–92. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chang, W.M.; Liu, Y.W.; Lee, C.Y.; Jang, Y.H.; Kuo, C.D.; Liao, H.F. A small-molecule metastasis inhibitor, norcantharidin, downregulates matrix metalloproteinase-9 expression by inhibiting Sp1 transcriptional activity in colorectal cancer cells. Chem. Biol. Interact. 2009, 181, 440–446. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lu, J.N.; Kim, G.Y.; Jung, J.M.; Kang, H.S.; Lee, W.S.; Choi, Y.H. Anti-invasive activities of anthocyanins through modulation of tight junctions and suppression of matrix metalloproteinase activities in HCT-116 human colon carcinoma cells. Oncol. Rep. 2011, 25, 567–572. [Google Scholar] [CrossRef]
- Suboj, P.; Babykutty, S.; Valiyaparambil Gopi, D.R.; Nair, R.S.; Srinivas, P.; Gopala, S. Aloe emodin inhibits colon cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB and VEGF via reduced DNA binding activity of NF-kappaB. Eur. J. Pharm. Sci. 2012, 45, 581–591. [Google Scholar] [CrossRef]
- Tseng, C.N.; Huang, C.F.; Cho, C.L.; Chang, H.W.; Huang, C.W.; Chiu, C.C.; Chang, Y.F. Brefeldin a effectively inhibits cancer stem cell-like properties and MMP-9 activity in human colorectal cancer Colo 205 cells. Molecules 2013, 18, 10242–10253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, J.; Xu, J.; Jiao, D.X.; Wang, J.; Gong, Z.Q.; Jia, J.H. Andrographolide suppresses proliferation of human colon cancer SW620 cells through the TLR4/NF-kappaB/MMP-9 signaling pathway. Oncol. Lett. 2017, 14, 4305–4310. [Google Scholar] [CrossRef]
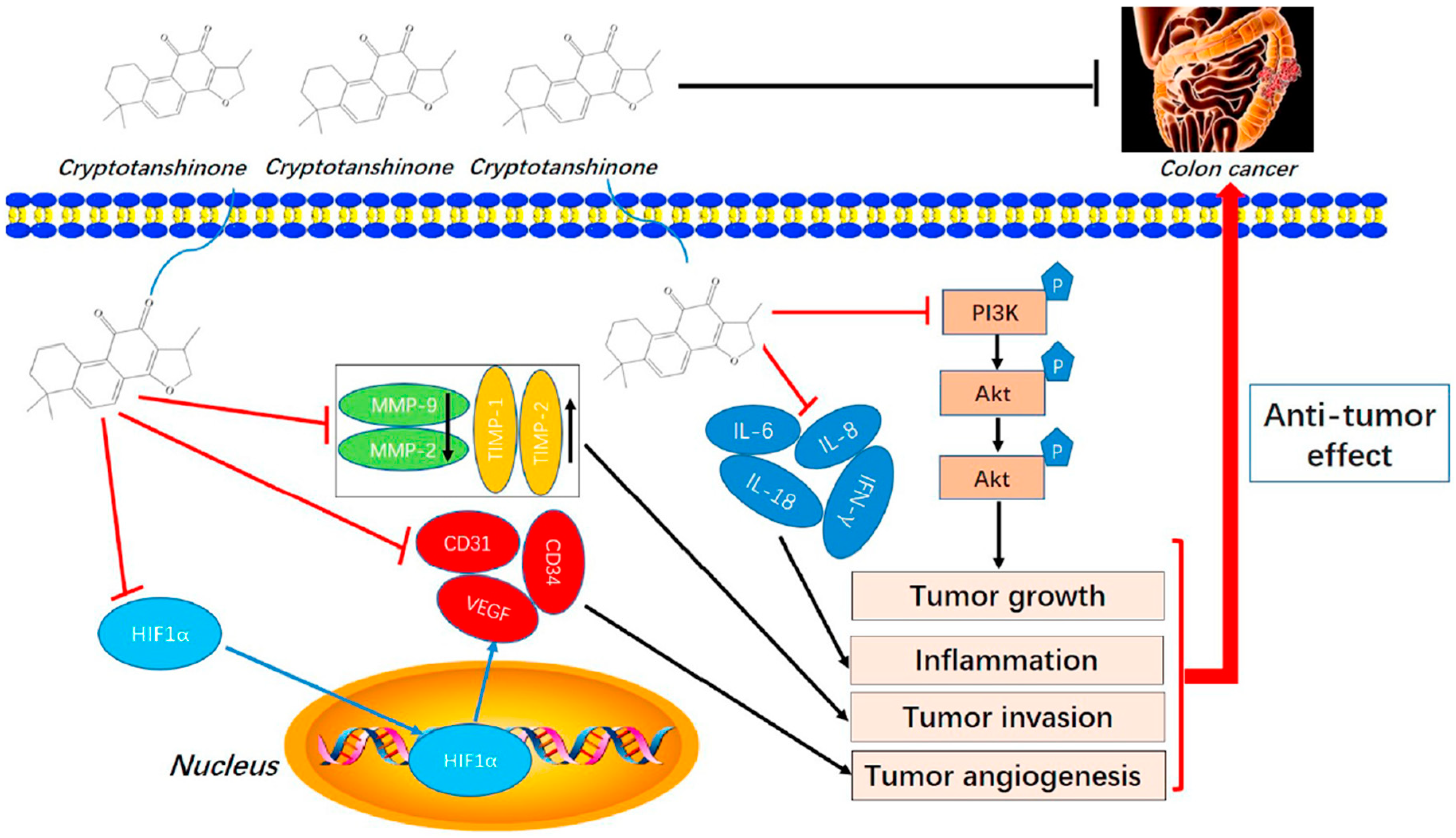
- Zhang, L.; Chen, C.; Duanmu, J.; Wu, Y.; Tao, J.; Yang, A.; Yin, X.; Xiong, B.; Gu, J.; Li, C.; et al. Cryptotanshinone inhibits the growth and invasion of colon cancer by suppressing inflammation and tumor angiogenesis through modulating MMP/TIMP system, PI3K/Akt/mTOR signaling and HIF-1alpha nuclear translocation. Int. Immunopharmacol. 2018, 65, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Kee, J.Y.; Hong, S.H. Rosmarinic Acid Activates AMPK to Inhibit Metastasis of Colorectal Cancer. Front. Pharmacol. 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Bakar-Ates, F.; Ozkan, E. The combined treatment of brassinin and imatinib synergistically downregulated the expression of MMP-9 in SW480 colon cancer cells. Phytother. Res. 2019, 33, 397–402. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-beta induces proliferation in colorectal cancer cells and resveratrol can down-modulate it. Exp. Biol. Med. 2019, 244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Manmuan, S.M.; Manmuan, P. Fucoxanthin enhances 5-FU chemotherapeutic efficacy in colorectal cancer cells by affecting MMP-9 invasive proteins. J. Appl. Pharm. Sci. 2019, 9, 007–014. [Google Scholar]
- Zare, Z.; Nayerpour Dizaj, T.; Lohrasbi, A.; Sheikhalishahi, Z.S.; Asadi, A.; Zakeri, M.; Hosseinabadi, F.; Abazari, O.; Abbasi, M.; Khanicheragh, P. Silibinin Inhibits TGF-β-induced MMP-2 and MMP-9 Through Smad Signaling Pathway in Colorectal Cancer HT-29 Cells. Basic. Clin. Cancer Res. 2021, 12, 81–90. [Google Scholar] [CrossRef]
- Salama, A.A.A.; Allam, R.M. Promising targets of chrysin and daidzein in colorectal cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharmacol. 2021, 892, 173763. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, M.; Ye, J.; Liang, Y.; Gao, J.; Ji, Z.; Wang, J. Sauchinone Inhibits the Proliferation and Immune Invasion Capacity of Colorectal Cancer Cells through the Suppression of PD-L1 and MMP2/MM9. Anticancer. Agents Med. Chem. 2023, 23, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Shahabi Nejad, F.; Karami, H.; Darvish, M. Triggering of Endoplasmic Reticulum Stress by Tannic Acid Inhibits the Proliferation and Migration of Colorectal Cancer Cells. Asian Pac. J. Cancer Prev. 2023, 24, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Zhao, Z.L. Triptolide inhibits proliferation and invasion of colorectal cancer cells by blocking Nrf2 expression. Chem. Biol. Drug Des. 2024, 103, e14410. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.P.; Huang, H.Y.; Chou, C.L.; Cheng, L.C.; Wang, W.C.; Tian, Y.F.; Fang, C.L.; Lin, K.Y. Punicalagin is cytotoxic to human colon cancer cells by modulating cell proliferation, apoptosis, and invasion. Hum. Exp. Toxicol. 2023, 42, 9603271231213979. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Wu, M.; Zhou, L.; Zheng, Y.; Ren, T.; Li, M.; Zhao, W. Hawthorn Proanthocyanidin Extract Inhibits Colorectal Carcinoma Metastasis by Targeting the Epithelial-Mesenchymal Transition Process and Wnt/beta-Catenin Signaling Pathway. Foods 2024, 13, 1171. [Google Scholar] [CrossRef]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, O.; Turkoglu, I. The investigation of some bioactive compounds and antioxidant properties of hawthorn (Crataegus monogyna subsp. monogyna Jacq). J. Intercult. Ethnopharmacol. 2014, 3, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.T.; Sun, J.; Shi, Y.J.; Zhang, X.F.; Zou, J.B.; Cheng, J.X.; Fan, Y.; Guo, D.Y.; Tian, H. Review targeted drug delivery systems for norcantharidin in cancer therapy. J. Nanobiotechnology 2022, 20, 509. [Google Scholar] [CrossRef] [PubMed]
- Intharuksa, A.; Arunotayanun, W.; Yooin, W.; Sirisa-Ard, P. A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery. Molecules 2022, 27, 4479. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Tong, W.; Wang, Q.; Sun, D.; Suo, J. Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-kappaB, uPA activator and MMP9. Oncol. Lett. 2016, 12, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Serventi, L.; Chitchumroonchokchai, C.; Riedl, K.M.; Kerem, Z.; Berhow, M.A.; Vodovotz, Y.; Schwartz, S.J.; Failla, M.L. Saponins from soy and chickpea: Stability during beadmaking and in vitro bioaccessibility. J. Agric. Food Chem. 2013, 61, 6703–6710. [Google Scholar] [CrossRef]
- Lima, A.; Oliveira, J.; Saude, F.; Mota, J.; Ferreira, R.B. Proteins in Soy Might Have a Higher Role in Cancer Prevention than Previously Expected: Soybean Protein Fractions Are More Effective MMP-9 Inhibitors Than Non-Protein Fractions, Even in Cooked Seeds. Nutrients 2017, 9, 201. [Google Scholar] [CrossRef]
- Lima, A.I.; Mota, J.; Monteiro, S.A.; Ferreira, R.M. Legume seeds and colorectal cancer revisited: Protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016, 197, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Han, B.; Li, X.; Sun, C.; Zhai, Y.; Li, M.; Jiang, M.; Zhang, W.; Liang, Y.; Kai, G. Salvia miltiorrhiza in Breast Cancer Treatment: A Review of Its Phytochemistry, Derivatives, Nanoparticles, and Potential Mechanisms. Front. Pharmacol. 2022, 13, 872085. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Kostomitsopoulos, N.G.; Tsaroucha, A.K.; Valsami, G. A Comprehensive Review of the Cardiovascular Protective Properties of Silibinin/Silymarin: A New Kid on the Block. Pharmaceuticals 2022, 15, 538. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, Z.; Wang, M.; Liu, P. Natural products in attenuating renal inflammation via inhibiting the NLRP3 inflammasome in diabetic kidney disease. Front. Immunol. 2023, 14, 1196016. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Li, X.; Lu, Q.; Zhu, Q.; Jiang, H.; Wang, T.; Huang, G.; Xu, A. Application and Mechanisms of Triptolide in the Treatment of Inflammatory Diseases-A Review. Front. Pharmacol. 2019, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.; Sibley, P.; Abdulwali, N.; Guillaume, D. Nutritional, pharmacological, and sensory properties of maple syrup: A comprehensive review. Heliyon 2023, 9, e19216. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Shiburo, R.; Moriyama, Y.; Mitamura, K.; Taga, A. Protein components of maple syrup as a potential resource for the development of novel anti-colorectal cancer drugs. Oncol. Rep. 2023, 50, 179. [Google Scholar] [CrossRef] [PubMed]
- Berdowska, I.; Matusiewicz, M.; Fecka, I. Punicalagin in Cancer Prevention-Via Signaling Pathways Targeting. Nutrients 2021, 13, 2733. [Google Scholar] [CrossRef]
- Lee, W.S.; Shin, J.S.; Jang, S.Y.; Chung, K.S.; Kim, S.D.; Cho, C.W.; Hong, H.D.; Rhee, Y.K.; Lee, K.T. Anti-Metastatic Effects of Standardized Polysaccharide Fraction from Diospyros kaki Leaves via GSK3β/β-Catenin and JNK Inactivation in Human Colon Cancer Cells. Polymers 2024, 16, 1275. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhao, J.; Fang, C.; Cao, Q.; Xing, M.; Li, X.; Hou, J.; Ji, A.; Song, S. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Mar. Drugs 2020, 18, 634. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef] [PubMed]
- Bin Sayeed, M.S.; Karim, S.M.R.; Sharmin, T.; Morshed, M.M. Critical Analysis on Characterization, Systemic Effect, and Therapeutic Potential of Beta-Sitosterol: A Plant-Derived Orphan Phytosterol. Medicines 2016, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Lee, C.F.; Chou, W.T.; Hwang, J.J.; Tyan, Y.S.; Chuang, H.Y. Liposomal beta-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System. Pharmaceutics 2022, 14, 1214. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Herbal medicines: Balancing benefits and risks. Novartis Found. Symp. 2007, 282, 154–167; discussion 167–172, 212–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef] [PubMed]
- Rombola, L.; Scuteri, D.; Marilisa, S.; Watanabe, C.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance. Life 2020, 10, 106. [Google Scholar] [CrossRef]
- Meisel, J.E.; Chang, M. Selective small-molecule inhibitors as chemical tools to define the roles of matrix metalloproteinases in disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2001–2014. [Google Scholar] [CrossRef]
- Waller, V.; Pruschy, M. Combined Radiochemotherapy: Metalloproteinases Revisited. Front. Oncol. 2021, 11, 676583. [Google Scholar] [CrossRef]
- Ogata, Y.; Matono, K.; Nakajima, M.; Sasatomi, T.; Mizobe, T.; Nagase, H.; Shirouzu, K. Efficacy of the MMP inhibitor MMI270 against lung metastasis following removal of orthotopically transplanted human colon cancer in rat. Int. J. Cancer 2006, 118, 215–221. [Google Scholar] [CrossRef]
- Toda, D.; Ota, T.; Tsukuda, K.; Watanabe, K.; Fujiyama, T.; Murakami, M.; Naito, M.; Shimizu, N. Gefitinib decreases the synthesis of matrix metalloproteinase and the adhesion to extracellular matrix proteins of colon cancer cells. Anticancer. Res. 2006, 26, 129–134. [Google Scholar]
- Ishizaki, T.; Katsumata, K.; Tsuchida, A.; Wada, T.; Mori, Y.; Hisada, M.; Kawakita, H.; Aoki, T. Etodolac, a selective cyclooxygenase-2 inhibitor, inhibits liver metastasis of colorectal cancer cells via the suppression of MMP-9 activity. Int. J. Mol. Med. 2006, 17, 357–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.K.; Yu, L.; Shen, Y.; Zhou, L.S.; Wang, Y.C.; Zhang, J.H. Inhibition of CXCR4 activity with AMD3100 decreases invasion of human colorectal cancer cells in vitro. World J. Gastroenterol. 2008, 14, 2308–2313. [Google Scholar] [CrossRef]
- Hsu, H.H.; Hu, W.S.; Lin, Y.M.; Kuo, W.W.; Chen, L.M.; Chen, W.K.; Hwang, J.M.; Tsai, F.J.; Liu, C.J.; Huang, C.Y. JNK suppression is essential for 17beta-Estradiol inhibits prostaglandin E2-Induced uPA and MMP-9 expressions and cell migration in human LoVo colon cancer cells. J. Biomed. Sci. 2011, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Müller-Edenborn, B.; Roth-Z’graggen, B.; Bartnicka, K.; Borgeat, A.; Hoos, A.; Borsig, L.; Beck-Schimmer, B. Volatile Anesthetics Reduce Invasion of Colorectal Cancer Cells through Down-regulation of Matrix Metalloproteinase-9. Anesthesiology 2012, 117, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Gungor, H.; Ilhan, N.; Eroksuz, H. The effectiveness of cyclooxygenase-2 inhibitors and evaluation of angiogenesis in the model of experimental colorectal cancer. Biomed. Pharmacother. 2018, 102, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, J.; Ding, C.; Zhou, Y.; Yang, J.; Yu, W.; Zhang, X.; Huang, H. Flavonoid GL-V9 suppresses invasion and migration of human colorectal cancer cells by inhibiting PI3K/Akt and MMP-2/9 signaling. J. Cancer 2021, 12, 4542–4551. [Google Scholar] [CrossRef] [PubMed]
- Kassassir, H.; Papiewska-Pajak, I.; Kryczka, J.; Boncela, J.; Kowalska, M.A. Platelet-derived microparticles stimulate the invasiveness of colorectal cancer cells via the p38MAPK-MMP-2/MMP-9 axis. Cell Commun. Signal. 2023, 21, 51. [Google Scholar] [CrossRef]
- Chiappetta, M.; Salvatore, L.; Congedo, M.T.; Bensi, M.; De Luca, V.; Petracca Ciavarella, L.; Camarda, F.; Evangelista, J.; Valentini, V.; Tortora, G.; et al. Management of single pulmonary metastases from colorectal cancer: State of the art. World J. Gastrointest. Oncol. 2022, 14, 820–832. [Google Scholar] [CrossRef]
- Yang, D.; Tian, X.; Ye, Y.; Liang, Y.; Zhao, J.; Wu, T.; Lu, N. Identification of GL-V9 as a novel senolytic agent against senescent breast cancer cells. Life Sci. 2021, 272, 119196. [Google Scholar] [CrossRef]
- Jones, R.A. Etodolac: An overview of a selective COX-2 inhibitor. Inflammopharmacology 1999, 7, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Nagase, H. The Road Not Taken with Pyrrole-Imidazole Polyamides: Off-Target Effects and Genomic Binding. Biomolecules 2020, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nagase, H.; Watanabe, T.; Nobusue, H.; Suzuki, T.; Asami, Y.; Shinojima, Y.; Kawashima, H.; Takagi, K.; Mishra, R.; et al. Inhibition of MMP-9 transcription and suppression of tumor metastasis by pyrrole-imidazole polyamide. Cancer Sci. 2010, 101, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Aris, A.Z.; Shamsuddin, A.S.; Praveena, S.M. Occurrence of 17alpha-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environ. Int. 2014, 69, 104–119. [Google Scholar] [CrossRef]
- White, A.; Ironmonger, L.; Steele, R.J.C.; Ormiston-Smith, N.; Crawford, C.; Seims, A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer 2018, 18, 906. [Google Scholar] [CrossRef]
- Hsu, H.H.; Liu, C.J.; Shen, C.Y.; Chen, Y.J.; Chen, L.M.; Kuo, W.H.; Lin, Y.M.; Chen, R.J.; Tsai, C.H.; Tsai, F.J.; et al. p38α MAPK mediates 17β-estradiol inhibition of MMP-2 and -9 expression and cell migration in human lovo colon cancer cells. J. Cell. Physiol. 2012, 227, 3648–3660. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Rampes, S.; Patel, S.; Hana, Z.; Ma, D. Anesthetics or anesthetic techniques and cancer surgical outcomes: A possible link. Korean J. Anesthesiol. 2021, 74, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, C.S.; Lu, Y.; Sun, S.; Huang, S. Volatile Anesthetics Regulate Anti-Cancer Relevant Signaling. Front. Oncol. 2021, 11, 610514. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.; Wu, Y.N.; Wang, J.P.; He, J.Q.; Han, X. Sevoflurane inhibits the migration and invasion of colorectal cancer cells through regulating ERK/MMP-9 pathway by up-regulating miR-203. Eur. J. Pharmacol. 2019, 850, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, B.; Huang, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R.; et al. The relationship between nonsteroidal anti-inflammatory drugs and cancer incidence: An umbrella review. Heliyon 2024, 10, e23203. [Google Scholar] [CrossRef]
- Bisht, K.S.; Bradbury, C.M.; Mattson, D.; Kaushal, A.; Sowers, A.; Markovina, S.; Ortiz, K.L.; Sieck, L.K.; Isaacs, J.S.; Brechbiel, M.W.; et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003, 63, 8984–8995. [Google Scholar]
- Zeynali-Moghaddarn, S.; Mohammadian, M.; Kheradmand, F.; Fathi-Azarbayjani, A.; Rasmi, Y.; Esna-Ashari, O.; Malekinejad, H. A molecular basis for the synergy between 17-allylamino-17-demethoxy geldanamycin with Capecitabine and Irinotecan in human colorectal cancer cells through VEFG and MMP-9 gene expression. Gene 2019, 684, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F. Chemistry, Synthesis, and Structure Activity Relationship of Anticancer Quinoxalines. Chemistry 2023, 5, 2566–2587. [Google Scholar] [CrossRef]
- Ayoup, M.S.; Abu-Serie, M.M.; Awad, L.F.; Teleb, M.; Ragab, H.M.; Amer, A. Halting colorectal cancer metastasis via novel dual nanomolar MMP-9/MAO-A quinoxaline-based inhibitors; design, synthesis, and evaluation. Eur. J. Med. Chem. 2021, 222, 113558. [Google Scholar] [CrossRef]
- Chiacchiera, F.; Matrone, A.; Ferrari, E.; Ingravallo, G.; Lo Sasso, G.; Murzilli, S.; Petruzzelli, M.; Salvatore, L.; Moschetta, A.; Simone, C. p38alpha blockade inhibits colorectal cancer growth in vivo by inducing a switch from HIF1alpha- to FoxO-dependent transcription. Cell Death Differ. 2009, 16, 1203–1214. [Google Scholar] [CrossRef]
- Serra, M.; Rubes, D.; Schinelli, S.; Paolillo, M. Small Molecules against Metastatic Tumors: Concrete Perspectives and Shattered Dreams. Cancers 2023, 15, 4173. [Google Scholar] [CrossRef]
- Liu, G.H.; Chen, T.; Zhang, X.; Ma, X.L.; Shi, H.S. Small molecule inhibitors targeting the cancers. MedComm (2020) 2022, 3, e181. [Google Scholar] [CrossRef]
- Tabana, Y.; Babu, D.; Fahlman, R.; Siraki, A.G.; Barakat, K. Target identification of small molecules: An overview of the current applications in drug discovery. BMC Biotechnol. 2023, 23, 44. [Google Scholar] [CrossRef]
- Liu, G.X.; Yang, L.N.; Chen, G.; Xu, F.H.; Yang, F.H.; Yu, H.X.; Li, L.N.; Dong, X.L.; Han, J.J.; Cao, C.; et al. A Review on Drug Delivery System for Tumor Therapy. Front. Pharmacol. 2021, 12, 735446. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Pekarek, L.; Torres-Carranza, D.; Fraile-Martinez, O.; Garcia-Montero, C.; Pekarek, T.; Saez, M.A.; Rueda-Correa, F.; Pimentel-Martinez, C.; Guijarro, L.G.; Diaz-Pedrero, R.; et al. An Overview of the Role of MicroRNAs on Carcinogenesis: A Focus on Cell Cycle, Angiogenesis and Metastasis. Int. J. Mol. Sci. 2023, 24, 7268. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.C.; Pichon, C.; Letourneur, D.; Chaubet, F. miRNA Delivery by Nanosystems: State of the Art and Perspectives. Pharmaceutics 2021, 13, 1901. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Wu, J.M.; Wu, G.; Lv, L.; Ren, Y.F.; Zhang, X.J.; Xue, Y.F.; Li, G.L.; Lu, X.C.; Sun, Z.S.; Tang, K.F. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1. Carcinogenesis 2012, 33, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Song, Y.; Liu, T.J.; Cui, Y.B.; Jiang, Y.; Xie, Z.S.; Xie, S.L. miRNA-22 suppresses colon cancer cell migration and invasion by inhibiting the expression of T-cell lymphoma invasion and metastasis 1 and matrix metalloproteinases 2 and 9. Oncol. Rep. 2013, 29, 1932–1938. [Google Scholar] [CrossRef]
- Cong, J.C.; Gong, J.; Yang, C.J.; Xia, Z.X.; Zhang, H. miR-22 Suppresses Tumor Invasion and Metastasis in Colorectal Cancer by Targeting NLRP3. Cancer Manag. Res. 2020, 12, 5419–5429. [Google Scholar] [CrossRef]
- Wang, L.; Qian, L.Y.; Li, X.R.; Yan, J. MicroRNA-195 inhibits colorectal cancer cell proliferation, colony-formation and invasion through targeting CARMA3. Mol. Med. Rep. 2014, 10, 473–478. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.B.; Mao, X.B.; Xue, L.J.; Wang, R.; Chen, L.B.; Chu, X.Y. MicroRNA-149 Suppresses Colorectal Cancer Cell Migration and Invasion by Directly Targeting Forkhead Box Transcription Factor FOXM1. Cell. Physiol. Biochem. 2015, 35, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.J.; Tao, M.L.; Zhang, W.; Han, G.D.; Zhu, Z.C.; Miao, Z.G.; Li, J.Y.; Qiao, Z.B. Up-regulation of microRNA-302a inhibited the proliferation and invasion of colorectal cancer cells by regulation of the MAPK and PI3K/Akt signaling pathways. Int. J. Clin. Exp. Pathol. 2015, 8, 4481–4491. [Google Scholar]
- Wang, X.W.; Xi, X.Q.; Wu, J.; Wan, Y.Y.; Hui, H.X.; Cao, X.F. MicroRNA-206 attenuates tumor proliferation and migration involving the downregulation of NOTCH3 in colorectal cancer. Oncol. Rep. 2015, 33, 1402–1410. [Google Scholar] [CrossRef]
- Park, Y.R.; Lee, S.T.; Kim, S.L.; Liu, Y.C.; Lee, M.R.; Shin, J.H.; Seo, S.Y.; Kim, S.H.; Kim, I.H.; Lee, S.O.; et al. MicroRNA-9 suppresses cell migration and invasion through downregulation of TM4SF1 in colorectal cancer. Int. J. Oncol. 2016, 48, 2135–2143. [Google Scholar] [CrossRef]
- Zhang, N.; Shen, Q.; Zhang, P.P. miR-497 suppresses epithelial-mesenchymal transition and metastasis in colorectal cancer cells by targeting fos-related antigen-1. Oncotargets Ther. 2016, 9, 6597–6604. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Zhan, Y.S.; Huang, J.; Chen, Y.X. MicroRNA-7 suppresses human colon cancer invasion and proliferation by targeting the expression of focal adhesion kinase. Mol. Med. Rep. 2016, 13, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.N.; Cai, X.; Li, Q.; Xue, P.; Chen, Z.X.; Dong, X.; Xue, Y. Hsa-miR-875-5p exerts tumor suppressor function through down-regulation of EGFR in colorectal carcinoma (CRC). Oncotarget 2016, 7, 42225–42240. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.B.; Qiu, H.; Chen, J.M.; Shi, W.; Chen, Y.S. MicroRNA-202-5p functions as a tumor suppressor in colorectal carcinoma by directly targeting SMARCC1. Gene 2018, 676, 329–335. [Google Scholar] [CrossRef]
- Zhou, T.C.; Wu, L.L.; Wang, Q.R.; Jiang, Z.P.; Li, Y.R.; Ma, N.; Chen, W.H.; Hou, Z.H.; Gan, W.C.; Chen, S. MicroRNA-128 targeting RPN2 inhibits cell proliferation and migration through the Akt-p53-cyclin pathway in colorectal cancer cells. Oncol. Lett. 2018, 16, 6940–6949. [Google Scholar] [CrossRef] [PubMed]
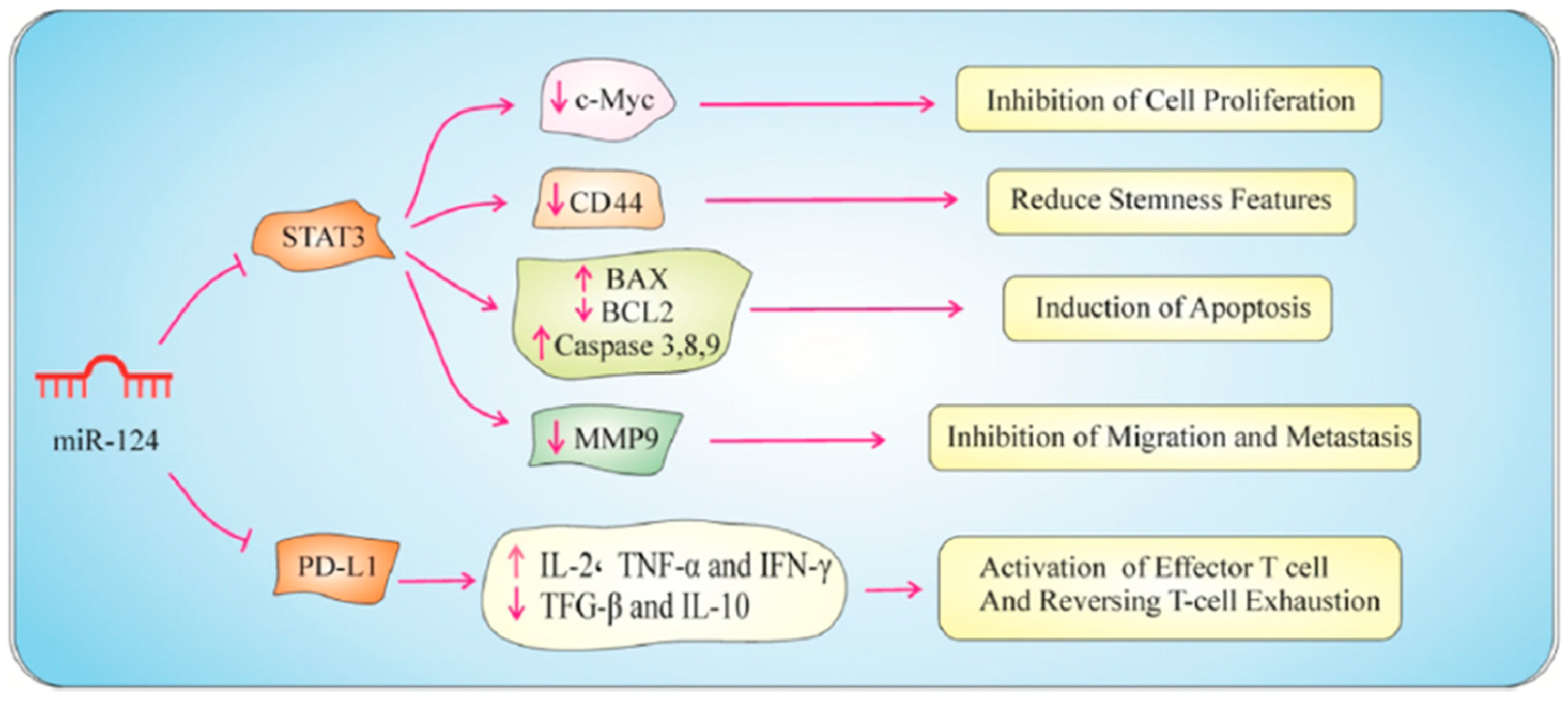
- Asl, E.R.; Rasmi, Y.; Baradaran, B. MicroRNA-124-3p suppresses PD-L1 expression and inhibits tumorigenesis of colorectal cancer cells via modulating STAT3 signaling. J. Cell. Physiol. 2021, 236, 7071–7087. [Google Scholar] [CrossRef]
- Toraih, E.A.; Ibrahiem, A.T.; Fawzy, M.S.; Hussein, M.H.; Al-Qahtani, S.A.M.; Shaalan, A.A.M. MicroRNA-34a: A Key Regulator in the Hallmarks of Renal Cell Carcinoma. Oxid. Med. Cell Longev. 2017, 2017, 3269379. [Google Scholar] [CrossRef]
- Ferrero, G.; Carpi, S.; Polini, B.; Pardini, B.; Nieri, P.; Impeduglia, A.; Grioni, S.; Tarallo, S.; Naccarati, A. Intake of Natural Compounds and Circulating microRNA Expression Levels: Their Relationship Investigated in Healthy Subjects With Different Dietary Habits. Front. Pharmacol. 2021, 11, 619200. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, D.; Cui, X.; Le, P.M.; Hofseth, A.B.; Windust, A.; Nagarkatti, M.; Nagarkatti, P.S.; Schetter, A.J.; Harris, C.C.; Hofseth, L.J. A key role of microRNA-29b for the suppression of colon cancer cell migration by American ginseng. PLoS ONE 2013, 8, e75034. [Google Scholar] [CrossRef]
- Yan, C.X.; Sun, L.; Wang, G.B.; Yao, Y.Y.; Shao, J.L.; Zhou, W.H.; Zhu, Z.H.; Luo, S.C.; Han, L. Anti-tumor efficacy of emodin on colorectal cancer cells via targeting lncRNA HLA complex P5. Chem. Biol. Drug Des. 2024, 103, e14444. [Google Scholar] [CrossRef]
- Yu, W.; Liang, X.; Li, X.; Zhang, Y.; Sun, Z.; Liu, Y.; Wang, J. MicroRNA-195: A review of its role in cancers. Onco Targets Ther. 2018, 11, 7109–7123. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhu, X.; Dou, Y. The miR-302/367 cluster: A comprehensive update on its evolution and functions. Open Biol. 2015, 5, 150138. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a key modulator of skeletal muscle development and disease. Int. J. Biol. Sci. 2015, 11, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Korac, P.; Antica, M.; Matulic, M. MiR-7 in Cancer Development. Biomedicines 2021, 9, 325. [Google Scholar] [CrossRef]
- Han, D.; Dong, X.Y.; Zheng, D.M.; Nao, J.F. MiR-124 and the Underlying Therapeutic Promise of Neurodegenerative Disorders. Front. Pharmacol. 2020, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, Y.; Wang, X.; Shen, J.; An, W. Advances in Circular RNA and Its Applications. Int. J. Med. Sci. 2022, 19, 975–985. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Y.; Li, W.T.; Li, Y.; Zhu, J.F. CircFNDC3B sequestrates miR-937-5p to derepress TIMP3 and inhibit colorectal cancer progression. Mol. Oncol. 2020, 14, 2960–2984. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.Y.; Gaddam, R.R.; Kravitz, M.; Gupta, A.; Vikram, A. The Challenges and Opportunities in the Development of MicroRNA Therapeutics: A Multidisciplinary Viewpoint. Cells 2021, 10, 3097. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA delivery through nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, Z.; Lu, Z.; Xia, J.; Xie, Z.; Jiao, M.; Liu, R.; Chu, Y. MicroRNAs: Immune modulators in cancer immunotherapy. Immunother. Adv. 2021, 1, ltab006. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; De Maio, G.; Ulivi, P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018, 19, 3733. [Google Scholar] [CrossRef] [PubMed]
- Reda El Sayed, S.; Cristante, J.; Guyon, L.; Denis, J.; Chabre, O.; Cherradi, N. MicroRNA Therapeutics in Cancer: Current Advances and Challenges. Cancers 2021, 13, 2680. [Google Scholar] [CrossRef]
- Precazzini, F.; Detassis, S.; Imperatori, A.S.; Denti, M.A.; Campomenosi, P. Measurements Methods for the Development of MicroRNA-Based Tests for Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 1176. [Google Scholar] [CrossRef] [PubMed]
- Sweef, O.; Zaabout, E.; Bakheet, A.; Halawa, M.; Gad, I.; Akela, M.; Tousson, E.; Abdelghany, A.; Furuta, S. Unraveling Therapeutic Opportunities and the Diagnostic Potential of microRNAs for Human Lung Cancer. Pharmaceutics 2023, 15, 2061. [Google Scholar] [CrossRef] [PubMed]
- Nhàn, N.T.T.; Yamada, T.; Yamada, K.H. Peptide-Based Agents for Cancer Treatment: Current Applications and Future Directions. Int. J. Mol. Sci. 2023, 24, 12931. [Google Scholar] [CrossRef] [PubMed]
- Serna, N.; Sánchez-García, L.; Unzueta, U.; Díaz, R.; Vázquez, E.; Mangues, R.; Villaverde, A. Protein-Based Therapeutic Killing for Cancer Therapies. Trends Biotechnol. 2018, 36, 318–335. [Google Scholar] [CrossRef] [PubMed]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012, 19, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Wanjari, U.R.; Gopalakrishnan, A.V.; Bradu, P.; Biswas, A.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; El Allali, A.; et al. Evolving strategies and application of proteins and peptide therapeutics in cancer treatment. Biomed. Pharmacother. 2023, 163, 114832. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.S.; Gunassekaran, G.R.; Lee, S.M.; Yoon, J.W.; Lee, Y.K.; Lee, B.Y.H. Peptides as multifunctional players in cancer therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors-Novel strategies bring new prospects. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Ndinguri, M.W.; Bhowmick, M.; Tokmina-Roszyk, D.; Robichaud, T.K.; Fields, G.B. Peptide-based selective inhibitors of matrix metalloproteinase-mediated activities. Molecules 2012, 17, 14230–14248. [Google Scholar] [CrossRef] [PubMed]
- Qvit, N.; Rubin, S.J.S.; Urban, T.J.; Mochly-Rosen, D.; Gross, E.R. Peptidomimetic therapeutics: Scientific approaches and opportunities. Drug Discov. Today 2017, 22, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Kazemi, B.; Bandehpour, M.; Shoari, A.; Asgary, V.; Ardestani, M.S.; Madadkar-Sobhani, A.; Cohan, R.A. Computational and nonglycosylated systems: A simpler approach for development of nanosized PEGylated proteins. Drug Des. Dev. Ther. 2016, 10, 1193–1200. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.X.; Zhang, W.P.; Cheng, X.R.; Yan, Z.B.; Shao, G.; Wang, X.; Wang, R.; Fu, C.Y. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.; Mathew, J.K.K.; Visweswariah, S.S. Receptor Guanylyl Cyclase C and Cyclic GMP in Health and Disease: Perspectives and Therapeutic Opportunities. Front. Endocrinol. 2022, 13, 911459. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, W.J.; Zuzga, D.S.; Zhou, Z.Y.; Fu, W.L.; Pelta-Heller, J.; Muschel, R.J.; Waldman, S.A.; Pitari, G.M. Guanylyl Cyclase C Prevents Colon Cancer Metastasis by Regulating Tumor Epithelial Cell Matrix Metalloproteinase-9. Cancer Res. 2009, 69, 3529–3536. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Bian, Q.; Shao, C.H.; Li, G.; Liu, A.A.; Jing, W.; Liu, R.; Zhang, Y.J.; Zhou, Y.Q.; Hu, X.G.; et al. Ulinastatin Reduces the Resistance of Liver Cancer Cells to Epirubicin by Inhibiting Autophagy. PLoS ONE 2015, 10, e0120694. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, K.P.; Shen, F.; Xiao, H.Q.; Cai, W.S.; Li, J.L.; Liu, Q.C.; Jia, L. Ulinastatin reduces cancer recurrence after resection of hepatic metastases from colon cancer by inhibiting MMP-9 activation via the antifibrinolytic pathway. Biomed. Res. Int. 2013, 2013, 437950. [Google Scholar] [CrossRef][Green Version]
- Marshall, D.C.; Lyman, S.K.; McCauley, S.; Kovalenko, M.; Spangler, R.; Liu, C.; Lee, M.; O’Sullivan, C.; Barry-Hamilton, V.; Ghermazien, H.; et al. Selective Allosteric Inhibition of MMP9 Is Efficacious in Preclinical Models of Ulcerative Colitis and Colorectal Cancer. PLoS ONE 2015, 10, e0127063. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Chang, H.M.; Zhao, H.C.; Yu, Y.; Li, R.; Qiao, J. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum. Reprod. Update 2019, 25, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Chen, W.; Zhan, X.; Lin, S.Y.; Chen, Z.H. Overexpression of KiSS-1 reduces colorectal cancer cell invasion by downregulating MMP-9 via blocking PI3K/Akt/NF-κB signal pathway. Int. J. Oncol. 2016, 48, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, L.; Soto, B.; Meier, R.; Geraghty, P. The Biology and Function of Tissue Inhibitor of Metalloproteinase 2 in the Lungs. Pulm. Med. 2022, 2022, 3632764. [Google Scholar] [CrossRef]
- Wang, W.M.; Li, D.; Xiang, L.L.; Lv, M.Y.; Tao, L.; Ni, T.Y.; Deng, J.L.; Gu, X.C.; Masatara, S.; Liu, Y.Q.; et al. TIMP-2 inhibits metastasis and predicts prognosis of colorectal cancer via regulating MMP-9. Cell Adhes. Migr. 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Park, J.; Moon, S.K.; Lee, C. N-methylsansalvamide elicits antitumor effects in colon cancer cells in vitro and in vivo by regulating proliferation, apoptosis, and metastatic capacity. Front. Pharmacol. 2023, 14, 1146966. [Google Scholar] [CrossRef] [PubMed]
- Kremsmayr, T.; Aljnabi, A.; Blanco-Canosa, J.B.; Tran, H.N.T.; Emidio, N.B.; Muttenthaler, M. On the Utility of Chemical Strategies to Improve Peptide Gut Stability. J. Med. Chem. 2022, 65, 6191–6206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.G.; Chen, Z.J.; Paul, P.K.; Lu, Y.; Wu, W.; Qi, J.P. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm. Sin. B 2021, 11, 2416–2448. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef]
- Shoari, A.; Tooyserkani, R.; Tahmasebi, M.; Löwik, D.W.P.M. Delivery of Various Cargos into Cancer Cells and Tissues via Cell-Penetrating Peptides: A Review of the Last Decade. Pharmaceutics 2021, 13, 1391. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.J.; Xu, S.; Wang, H.M.; Ling, Y.; Dong, J.; Xia, R.D.; Sun, X.H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv. Drug Deliv. Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef]
- Moore, T.J.; Mouslim, M.C.; Blunt, J.L.; Alexander, G.C.; Shermock, K.M. Assessment of Availability, Clinical Testing, and US Food and Drug Administration Review of Biosimilar Biologic Products. JAMA Intern. Med. 2021, 181, 52–60. [Google Scholar] [CrossRef] [PubMed]





| Name | Type and Source | Structure | Cell Type | Function | Refs. |
|---|---|---|---|---|---|
| Myricetin | Plants | 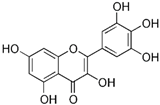 | HT-29, COLO 205 | Inhibitory effects on MMP-2 enzyme activity, Blocked TPA-stimulated invasion | [52] |
| EGCG | Phenol/green tea | 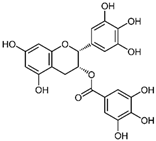 | SW837, SW620 | Significant reduction in the cellular levels of MMP-9 mRNAs, Suppressed the proliferation and migration | [53,54] |
| Curcumin | Turmeric |  | COLO 205, HCT 116, HT- 29, SW 620 | Inhibiting MMP-2 and MMP-9 enzymes and blocking the invasion, Blocked MMP-9 activation and gene production by inhibiting the NF-κB, Reduced tumor volume in nude mice | [55,56] |
| Saponin | Soybean |  | CT-26 | Reduced mRNA expression and the secretion of MMP-2 and MMP-9, Moderate reduction in the occurrence of metastatic lung tumors in the mice | [57] |
| Norcantharidin | Mylabris phalerata Pall. | 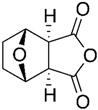 | CT-26 | Reduced MMP-9 mRNA and protein levels and its gelatinolytic activity | [58] |
| Anthocyanins | Plants |  | HCT 116 | Anti-invasive properties on cancer cells, Suppression of MMP-2 and MMP-9 protein expression and activity | [59] |
| Aloe emodin | Aloe vera | 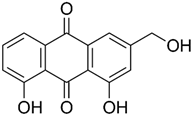 | WiDr | Inhibited mRNA expression and gelatinolytic activity of MMP-2 and MMP-9, Blocking the PMA induced migration and invasion | [60] |
| Brefeldin A | Eupenicillium brefeldianum |  | COLO 205 | Significantly decreased the viability of cells by promoting apoptosis, Diminished the activity of MMP-9 | [61] |
| Andrographolide | Andrographis paniculata |  | SW620 | Inhibit cell proliferation, Enhance cytotoxicity, Induce apoptosis, Suppression of MMP-9 signaling pathways | [62] |
| Cryptotanshinone | Salvia miltiorrhiza | 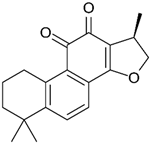 | CT-26 | Effectively inhibited cell invasion in vitro, Reduced the protein levels of MMP-2 and MMP-9, Increased TIMP-1 and TIMP-2 | [63] |
| Rosmarinic Acid | Rosmarinus officinalis | 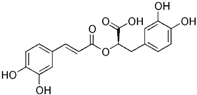 | CT-26, HCT 116 | Cell cycle arrest and apoptosis. Hindered the invasion and migration of cells, Decreased the levels of MMP-2 and MMP-9 | [64] |
| Brassinin | Cruciferous vegetables | 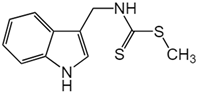 | SW-480 | Substantial reduction in MMP-9 activity, Induced apoptosis | [65] |
| Resveratrol | Plants | 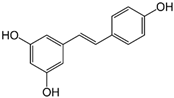 | HCT 116 | The anti-proliferative and anti-cancer properties related to MMP-9 reduction | [66] |
| Fucoxanthin | Brown seaweeds |  | SW620 | Significantly inhibited the proliferation of cells, Downregulation of MMP-9 mRNA and protein expression | [67] |
| Silibinin | Silybum marianum | 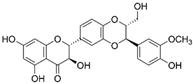 | HT-29 | Reduced the viability of cell, Significantly inhibited MMP-2 and MMP-9 mRNA and protein expression | [68] |
| Chrysin | Plants | 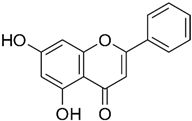 | SW620 | At dosages of 125 and 150 mg/kg, significantly reduced the MMP-9 levels2, Anti-invasion activity versus | [69] |
| Sauchinone | Saururus chinensis | 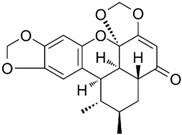 | SW840, HCT 116 | Decreased the expression of MMP2 and MMP9, Enhanced the cytotoxicity of cells co-cultured with CD8+ T cells | [70] |
| Tannic Acid | Plants |  | SW48 | Suppressed the survival, colony formation, and migration, significantly reduced the levels of MMP-9 expression | [71] |
| Triptolide | Tripterygium wilfordii |  | HT-29 | Significantly decreased the proliferation and invasion of cells, Enhanced apoptosis, Reduced levels of MMP-2 and MMP-9 | [72] |
| Punicalagin | Punica granatum | 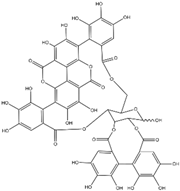 | HCT 116, HT-29, LoVo | Cytotoxic effects on colon cancer cells, Suppression of MMP-2 and MMP-9 expression | [73] |
| Hawthorn Proanthocyanidin | Hawthorn | 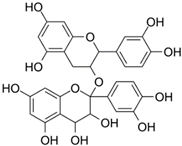 | HCT 116 | The levels of N-cadherin and MMP-9 were significantly reduced and led to inhibitory effect on cells migration | [74] |
| Name | Structure | Cell Type | Function | Ref. |
|---|---|---|---|---|
| MMI270 | 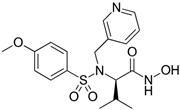 | KM12SM | Significantly inhibited lung metastasis, Higher survival rates, Suppressed MMP-9 activities, Reduced the relative MMP-2 activity | [102] |
| Gefitinib | 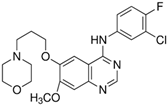 | HT-29 | Inhibited the secretion and mRNA expression of MMP-9 and MMP-2, Reduced the cells’ ability to adhere to laminin and type IV collagen | [103] |
| Etodolac | 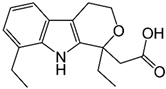 | Colon 26 | Reduced expression of MMP-9 mRNA, Decreased metastatic nodules on the liver | [104] |
| AMD3100 | 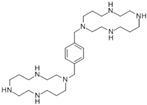 | SW480 | Cell viability blocked, Hindered the invasion ability, Significantly decreased the expression of MMP-9 but not MMP-2 | [105] |
| 17β-estradiol |  | LoVo | Decreased MMP-2 and MMP-9 expression, Reduced cell mobility by suppressing activation of JNK1/2 and p38α MAPK signaling pathway | [106] |
| Volatile Anesthetics | 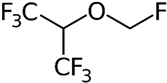 | MC-38 | Reduced MMP-9 release by IL-8-stimulated human neutrophils in vitro, Reduced mouse colon carcinoma cell migration across simulated extracellular matrix in vitro | [107] |
| Celecoxib | 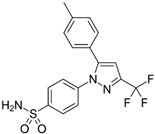 | - | Decrease in gelatinases levels, TIMP-2 levels were significantly higher in the celecoxib-treated group | [108] |
| GL-V9 |  | HCT116, SW480, SW620, LS174T | Reduces cell viability, migration, and invasion in a concentration-dependent fashion, Significantly decreased both the protein expression levels and activities of MMP-2 and MMP-9 | [109] |
| β-Sitosterol |  | CT-26/luc cells | Effectively inhibited metastases, Reduced MMP-9 expression | [95] |
| SB202190 |  | HT-29, SW480, SW620 | Reduced levels of MMP-2 and MMP-9 and related invasiveness in cells | [110] |
| Name | Target Gene | Cell Type | Function | Refs. |
|---|---|---|---|---|
| miRNA-34a | Fra-1 | HCT 116 | Significantly suppressed cell migration and invasion, The levels of MMP-9 were reduced | [136] |
| miRNA-22 | TIAM1/NLRP3 | HCT 116 | Significantly reduced the viability and migration and invasion capabilities. Decrease in the levels of the pro-invasive MMP-2 and MMP-9 expression | [137,138] |
| miRNA-195 | CARMA3 | SW480, HT-29 | Modulated MMP-9 via targeting CARMA3 | [139] |
| miRNA-149 | FOXM1 | HCT 116, LoVo, SW480 | mRNA expression levels of MMP-2 and MMP-9 were downregulated | [140] |
| miRNA-302a | MAPK, PI3K/Akt | SW620, SW480, HT-29, HCT 116 | Inhibited the proliferation and invasion of cells, The expression and secretion of MMP-9 and MMP-2 were notably reduced | [141] |
| miRNA-206 | NOTCH3 | SW480, SW620 | Prevented cancer cell proliferation and migration, Arrested the cell cycle, Triggered apoptosis, Inhibition of MMP-9 expression | [142] |
| miRNA 9 | TM4SF1 | SW480 | Impaired transwell migration and invasion, Led to a decrease in MMP-2 and MMP-9 levels | [143] |
| miRNA-497 | Fra-1 | HCT 116, SW480 | The levels of MMP-2 and MMP-9 proteins and invasion were significantly reduced | [144] |
| miRNA-7 | FAK | HCT-8, Caco-2 | Inhibited proliferation and migration of cells, impacted the expression of MMP-2 and MMP-9 | [145] |
| miRNA-875-5p | EGFR | HCT 116, SW480 | Reduced cell proliferation, Induced apoptosis, Impaired cellular migration and invasion by inhibiting MMP-9 | [146] |
| miRNA-202-5p | SMARCC1 | HCT 116, SW480 | Suppressed cell growth and metastasis, Inhibited MMP-9 | [147] |
| miRNA-128 | RPN2 | HT-29 | Significantly suppressed cell proliferation, migration, and invasion, Led to lower levels of MMP-2 and MMP-9, while increasing levels of TIMP-2 | [148] |
| miRNA-124-3p | PD-L1 | HT-29, SW480 | Proliferation of cells was reduced, Cell cycle was halted at the G1 phase, Reduced MMP-9 expression leading to the inhibition of cell motility and invasion | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoari, A.; Ashja Ardalan, A.; Dimesa, A.M.; Coban, M.A. Targeting Invasion: The Role of MMP-2 and MMP-9 Inhibition in Colorectal Cancer Therapy. Biomolecules 2025, 15, 35. https://doi.org/10.3390/biom15010035
Shoari A, Ashja Ardalan A, Dimesa AM, Coban MA. Targeting Invasion: The Role of MMP-2 and MMP-9 Inhibition in Colorectal Cancer Therapy. Biomolecules. 2025; 15(1):35. https://doi.org/10.3390/biom15010035
Chicago/Turabian StyleShoari, Alireza, Arghavan Ashja Ardalan, Alexandra M. Dimesa, and Mathew A. Coban. 2025. "Targeting Invasion: The Role of MMP-2 and MMP-9 Inhibition in Colorectal Cancer Therapy" Biomolecules 15, no. 1: 35. https://doi.org/10.3390/biom15010035
APA StyleShoari, A., Ashja Ardalan, A., Dimesa, A. M., & Coban, M. A. (2025). Targeting Invasion: The Role of MMP-2 and MMP-9 Inhibition in Colorectal Cancer Therapy. Biomolecules, 15(1), 35. https://doi.org/10.3390/biom15010035







