Abstract
Serum creatinine levels are the most used clinical marker to estimate renal function as the glomerular function rate because it is simple, fast, and inexpensive. However, creatinine has limitations, as its levels can be influenced by factors such as advanced age, physical activity, protein-rich diets, male gender, medications, and ethnicity. Serum cystatin C and its combination with serum creatinine may serve as an alternative since these factors do not affect it. Most creatinine synthesis occurs in the muscles, making it a valuable marker for assessing lean body mass within body composition. This measurement is crucial for evaluating and monitoring nutritional status in patients with chronic kidney disease. This review aimed to discuss the literature on creatinine metabolism, its advantages and disadvantages in assessing renal function, and its utility in measuring lean body mass. The variability in the creatinine generation rate among individuals should be considered when assessing the glomerular function rate.
1. Introduction
Creatinine (Cr) was discovered by French chemist Michel Eugène Chevreul in 1832 while investigating the composition of the skeletal muscles. He named the component “creatinine”, derived from the Greek word kreas, meaning meat.
Cr, chemically known as α-methyl guanidinoacetic acid, is a substance that appears as white crystalline particles in its pure state. It has a molecular weight of 113.1 Daltons and behaves as a cation in aqueous solutions.
It is consistently produced as part of normal muscle cells, serving as the final product of creatine (Crn) and phosphocreatine (PCrn) metabolism, and dietary protein intake, and eliminated by extrarenal degradation and urinary excretion [1].
Serum creatinine (SCr) concentration is the most used clinical indicator for assessing the glomerular function rate (GFR). Its widespread use is based on the correlation of its concentration with the precise measurement of the GFR using the clearance of substances like inulin (the gold standard) [2]. The broad availability, technical simplicity, and low cost of Cr measurement contribute to its use in routine renal function assessment.
Despite these practical advantages, Cr has limitations as a GFR marker. These include the imprecision of conventional quantification methods, which rely on chemical reactions (Jaffé) prone to interference from other molecules. Certain medications, such as cimetidine, trimethoprim, and abemaciclib, inhibit the tubular secretion of Cr by competing for secretion pathways, thereby increasing SCr levels and leading to erroneous estimates of the GFR (decreased) [3]. Additionally, Cr levels vary with muscle mass, dietary protein intake, and creatine supplementation. Levels tend to decrease with aging, in females, and in individuals of White ethnicity [4].
In patients with chronic kidney disease (CKD), it is important to determine lean body mass (LBM) as part of nutritional status monitoring. Methods such as Bioimpedance Analysis (BIA) and Dual-Energy X-ray Absorptiometry (DEXA) are used, but these are sophisticated techniques with limited accessibility in many hospitals due to their cost and the need for specialized personnel and equipment. Consequently, the Cr index (CI) or Cr kinetic where SCr is used has been a marker of LBM [5].
The objective of this review was to describe Cr metabolism, the factors influencing its serum concentration, and the considerations required for interpreting Cr concentration as a marker of the GFR and muscle mass in healthy individuals and CKD patients.
2. Creatinine Synthesis
Cr is distributed through total body water (TBW). SCr is formed through a spontaneous non-enzymatic anhydration of Crn in muscle cells. This conversion of Crn to Cr is influenced by pH and temperature; this process is carried out at a constant rate (1% of body Crn and 2.6% of PCrn per day is changed to Cr) [6].
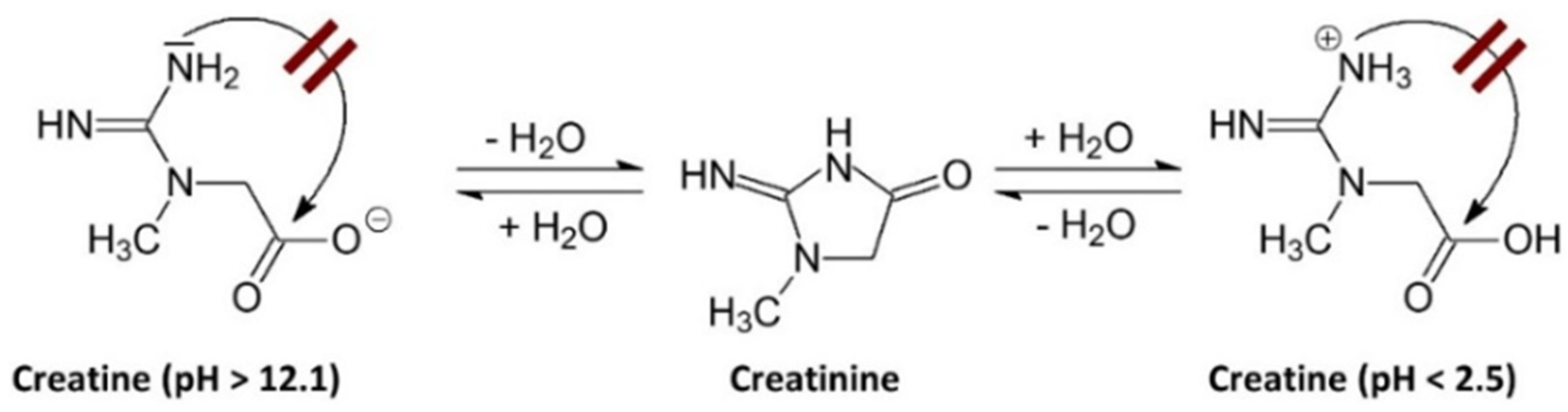
The degradation of Crn can be limited or may not occur in environments with very low or very high pH. A pH above 12.1 favors the deprotonation of the acid group, which hinders the intramolecular cyclization to form Cr. Conversely, when the pH is below 2.5, the amide functional group of the Crn molecule is protonated, also preventing intramolecular cyclization [7], as shown in Figure 1.
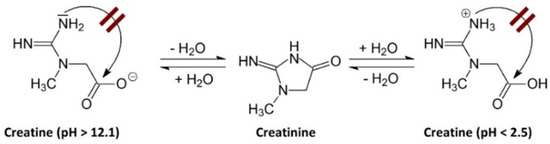
Figure 1.
Effect of pH on creatinine stability. A pH above 12.1 induces deprotonation of the acidic group, hindering intramolecular cyclization towards Cr. Conversely, when the pH is below 2.5, the amide group of the Crn molecule protonates, which also prevents intramolecular cyclization towards Cr [7].
3. Creatine Sources
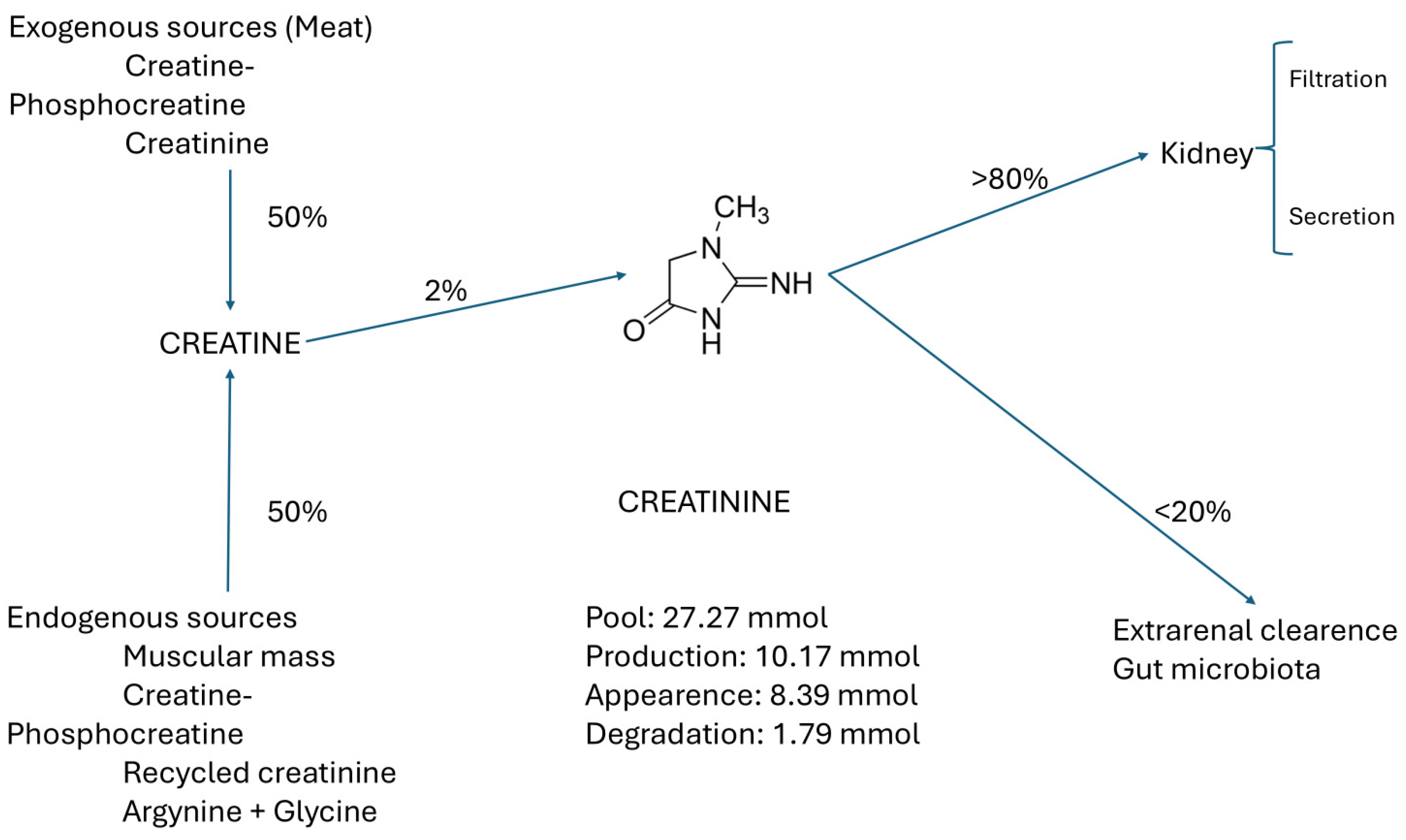
Crn comes from two main sources: 50% exogenous, from food, phosphate creatine (PCr), and commercial Crn, and 50% endogenous (from muscle, Crn, creatine phosphate (PCr), recycled Cr, and glycine + arginine), of which only 2% is converted to Cr. More than 80% of SCr is filtered and secreted in the kidney. The remaining 20% is cleared through the gut microbiota, as shown in Figure 2 [8].
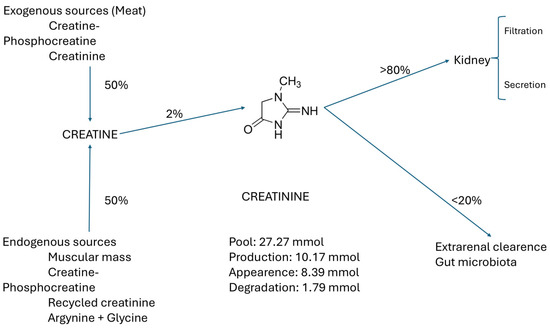
Figure 2.
Main sources and pathways of creatinine elimination.
3.1. Creatine from Exogenous Sources
3.1.1. In Foods
Exogenous Crn primarily comes from the diet and its processing. Some of the main foods where Crn can be found are shown in Table 1 [9].

Table 1.
Creatine in foods [9].
3.1.2. Cooked Meat
The cooking process is known to convert a fraction of the Crn contained in meat into Cr [9]. Around 20–30 years ago, some studies demonstrated that the intake of cooked meat (225–500 g) significantly increased the concentration of SCr [10,11].
Preiss et al. conducted a study that demonstrated that the effect of the intake of cooked meat compared to uncooked meat increases SCr by 20.5 mmol/L during the first 1 to 2 h and by 18.5 mmol/L between 3 and 4 h postprandial in healthy subjects and in stage 3 chronic CKD. Furthermore, the estimated glomerular function rate (eGFR) calculated with SCr decreased by 24.5 mL/min/1.73 m2 between the first 1 and 2 h and by 20 mL/min/1.73 m2 at 3–4 h postprandial, calculated using three methods [12]. Misclassification of CKD is possible if measurements are made after meals containing cooked meat.
Since meat is the primary source of exogenous Crn, vegans and vegetarians have a reduced intake of this essential compound, resulting in decreased muscle Crn stores, so supplementation helps to compensate for this reduction [13].
3.1.3. Creatine as a Dietary Supplement
Crn is an ergogenic supplement that has been used by athletes to increase strength gains. Various forms of commercial Crn exist; however, Crn monohydrate has been the most extensively studied, and its formulation has shown benefits in short-duration, high-intensity weightlifting, as well as in cycling [14]. In a review on the utility of Crn, it was found that its supplementation may improve post-exercise recovery, including injury prevention, thermoregulation, rehabilitation, and neuroprotection following a concussion in animal models, as well as enhancing functional capacity in individuals with spinal cord injury [15]. Additionally, its clinical applications have been investigated in neurodegenerative diseases such as muscular dystrophy, Parkinson’s disease, and Huntington’s disease, as well as in diabetes, osteoarthritis, fibromyalgia, aging, cerebral and cardiac ischemia, and depression in adolescents and pregnant women [16].
Research conducted in various age groups that received Crn supplements at doses of 0.3 to 0.8 g/kg/day for up to 5 years has demonstrated that supplementation with Crn does not pose negative health risks. Moreover, these studies suggest that Crn may provide several benefits for both general health and physical performance [16].
Burke et al. found that supplementation with Crn helped to improve low Crn reserves in the muscles of vegetarians, who showed more significant increases in fat-free mass (FFM), maximal strength, and type II muscle fiber area compared to those following an omnivorous diet [17].
3.2. Creatine from Endogenous Sources
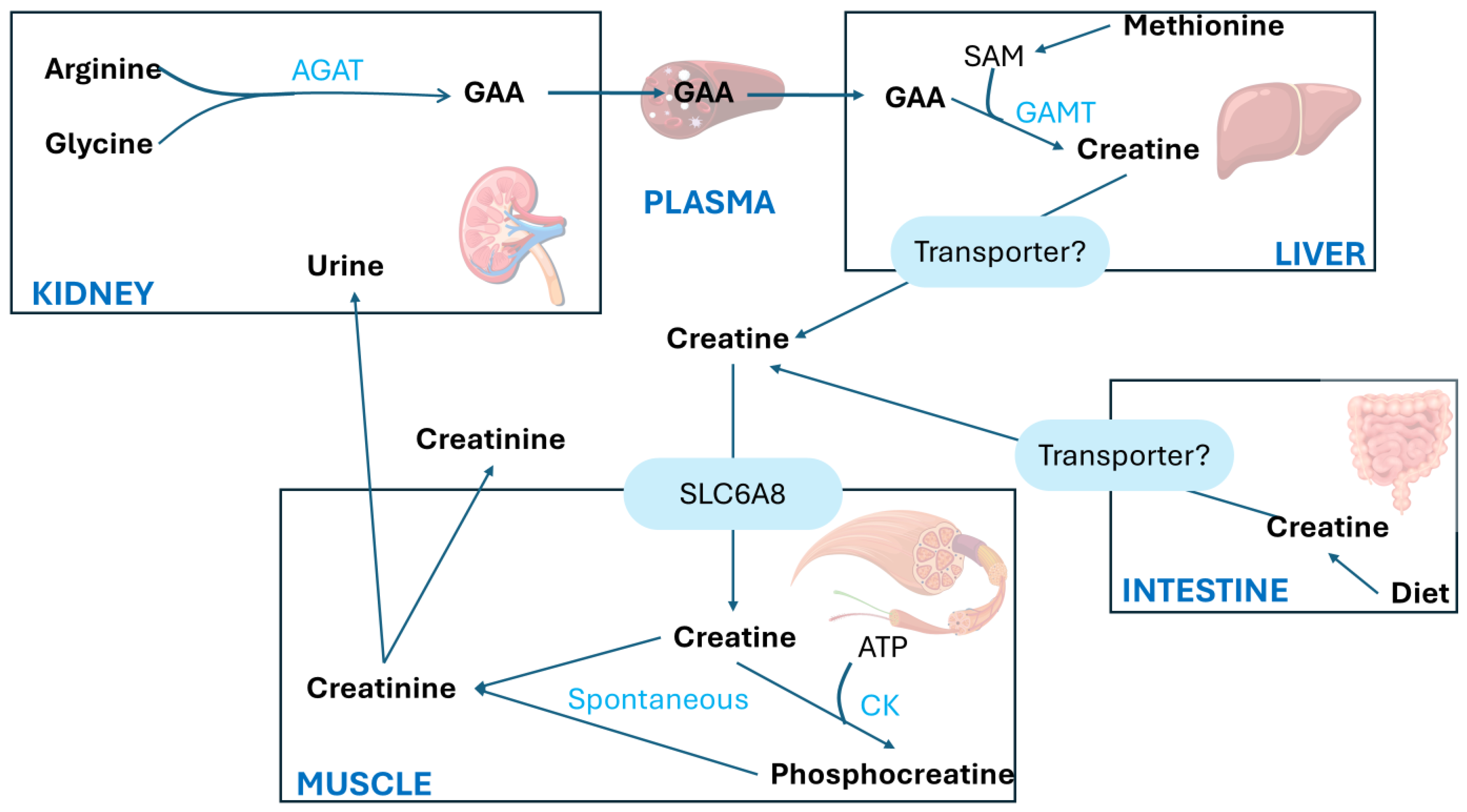
The synthesis of Crn begins in the kidneys primarily with the transfer of the amino group from arginine (Arg) to glycine (Gly), producing L-ornithine and guanidinoacetic acid (GAA). This first step is catalyzed by the enzyme L-arginine aminotransferase (AGAT). The second step involves the methylation of guanidinoacetic acid through the action of S-adenosyl-L-methionine/N-guanidoacetate methyltransferase (GAMT), which transfers a methyl group from S-adenosylmethionine (SAM), thereby forming Crn, as shown in Figure 3.
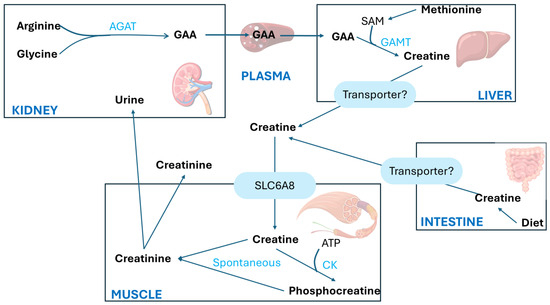
Figure 3.
Creatine metabolism adapted from Ref. [18]. AGAT, arginine/glycine amidinotransferase; CK, creatine kinase; GAA, guanidinoacetate; GAMT, guanidinoacetate methyltransferase; SAM, S-adenosylmethionine; SLC6A8, creatine transporter [18].
Crn is synthesized mainly in the liver and is transported into the bloodstream via a specific transporter to the muscles, which contains approximately 98% of the total body Crn reserves. The highest concentrations of Cr and PCr are found in the skeletal muscle, heart, sperm, and photoreceptor cells of the retina in mammals.
A large amount of PCr is available in fast-twitch skeletal muscles for the immediate regeneration of hydrolyzed adenosine triphosphate (ATP) during short periods of intense work [8]. The synthesized Crn reaches the designated tissues through the transport of blood vessels and intracellular transport mediated by a neurotransmitter called the Crn transporter, which depends on chloride and sodium ions.
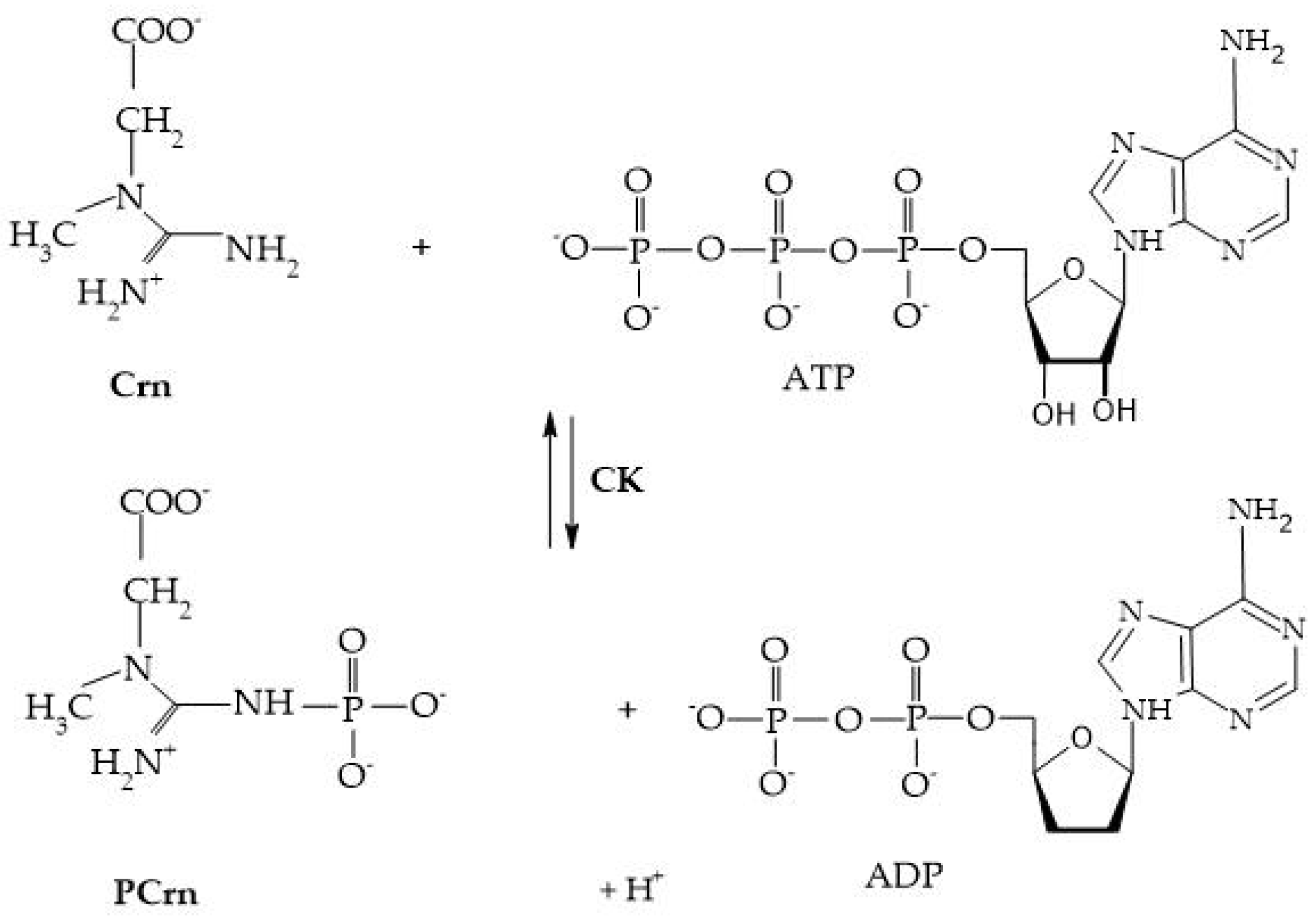
Crn and its phosphorylated form are crucial for maintaining ATP reserves in cells with high energy demands, such as myocytes, cardiomyocytes, hepatocytes, enterocytes, inner ear cells, renal cells, sperm cells, and photoreceptors. The enzyme creatine kinase (CK) initiates the reversible transfer reaction after reaching the intracellular space, leading to the creation of PCr [8], as shown in Figure 4.
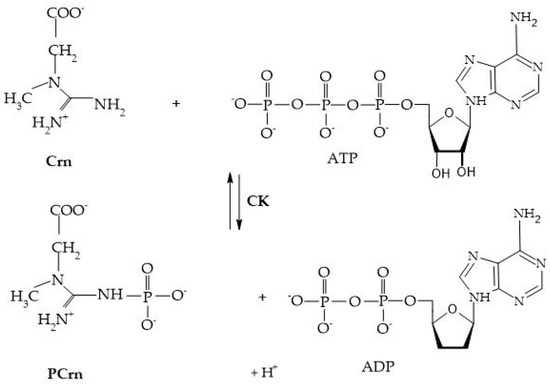
Figure 4.
Creatine kinase reaction.
4. Creatinine Metabolic Pool
Basic indicators of Cr metabolism in a patient with CKD on hemodialysis (HD). Pool: 27.27 mmol, production: 10.17 mmol, appearance: 8.39 mmol, and degradation: 1.79 mmol.
The content of Cr in the tissues and its concentration in the extracellular fluid (ECW) (and serum) remain stable unless there is acute muscular destruction, urinary tract obstruction, or acute kidney failure. The pool of Cr has two access pathways: (a) the endogenous generation in the muscles as the final product of the Crn metabolism through recycling, and (b) the intake of Cr or its precursor Crn in meat or supplements. Cr is eliminated by two pathways, primarily by the kidneys through filtration and secretion, and to a lesser extent by metabolism in the gut microbiota [4], as seen in Figure 2.
CK, creatine kinase; PCrn, phosphocreatine; Crn, creatine; ATP, adenosine triphosphate; and ADP, adenosine diphosphate [8].
4.1. Renal Clearence
Cr, being a small molecule of low molecular weight and not bound to proteins, distributes throughout the total body water and is filtered by the glomerulus. However, the estimation of its serum concentration can be significantly influenced by factors beyond glomerular filtration, such as extrarenal elimination and tubular secretion. Glomerular filtration is the initial step for its elimination from the body. Thus, SCr is predominantly excreted by glomerular ultrafiltration, so when GFR decreases, Cr accumulates.
The glomerular fenestrated endothelium allows small molecules like Cr to pass freely through its pores. The pores of this endothelium are large enough to permit the passage of water and small solutes but small enough to retain blood cells and larger proteins. The glomerular basement membrane filters molecules based on size and electrical charge, and although Cr is relatively small, its passage through the basement membrane is also facilitated by its solubility in water. Podocyte cells surround the glomerular capillaries, and their extensions (called pedicels) form filtration slits that allow Cr to pass freely into the Bowman’s capsule space [19]. Once Cr crosses the glomerular barrier, it becomes part of the glomerular filtrate and flows into the proximal tubules of the nephron.
The first studies on the secretion of Cr indicated that it accounted for 10% to 40% of urinary Cr excretion (UCrE) in healthy subjects [20]. More recently, studies such as the one by Imamura et al. indicated that tubular secretion contributes significantly to renal Cr elimination, accounting for 30% to 60% of the total elimination of Cr [21].
Zhang et al. analyzed data from different cohorts of the Modification of Diet in Renal Disease (MDRD) study, the African American Study of Kidney Disease and Hypertension (AASK), and the Mayo Clinic to determine the correlation between creatinine clearance (CrCl) and estimated glomerular filtration rate (eGFR) in patients with varying degrees of renal function. The results indicated that the CrCl/eGFR ratio is increased in patients with reduced eGFR due to the increased tubular secretion of Cr in this group, which is not accounted for; thus, in these cases, CrCl tends to overestimate eGFR [22].
4.2. Creatinine Transporters in Renal Proximal Tubules
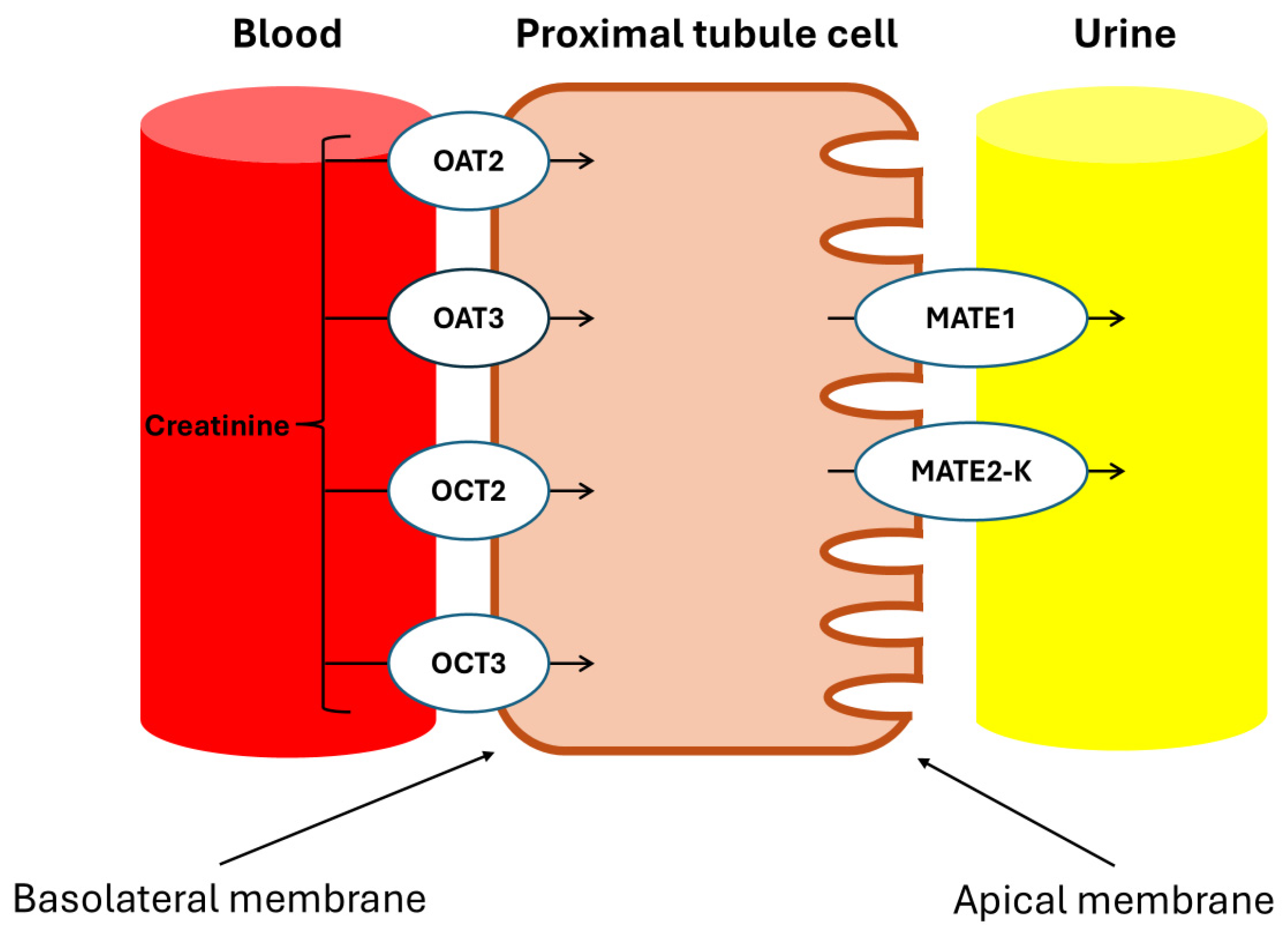
Urakami et al. demonstrated that in the renal proximal tubules, the Basolateral Organic Cation Transporters hOAT1 (SLC22A6), hOAT3 (SLC22A8), and hOCT2 (SLC22A2) are primarily expressed in the basolateral membrane and play a crucial role in the uptake of organic compounds from the blood, with Cr being an endogenous substrate of hOCT2 [23]. Additionally, Tanihara et al. revealed that the proteins MATE1 and MATE2-K, which are responsible for the exchange of protons and organic cations in the brush border membrane of the proximal tubules, also accept Cr as a substrate, facilitating efficient transcellular transport in conjunction with hOCT2 [24].
And since various drugs act as substrates for these transporters, they interfere with the tubular secretion of Cr, affecting SCr concentrations, as will be discussed later.
It is important to note that Jones and Burnett previously estimated that 16-66% of synthesized Cr has extrarenal degradation routs by intestinal microbiota in patients with decreased renal function [25].
4.3. Extrarenal Degradation of Creatinine by Gut Microbiota
Bacteria and fungi capable of degrading Crn and Cr through metabolic pathways have been identified in the droppings of chickens and pigeons, as well as in human urine, feces, and the bacterial flora of the human colon. These bacteria may be particularly relevant in kidney disease. In uremic patients with significantly elevated SCr levels, it is believed that Cr diffuses into the intestinal tract, where it induces the activity of creatininase, creatinase, and creatinine deaminase, ultimately resulting in the breakdown of part of the body’s Cr reserves and its partial recycling.
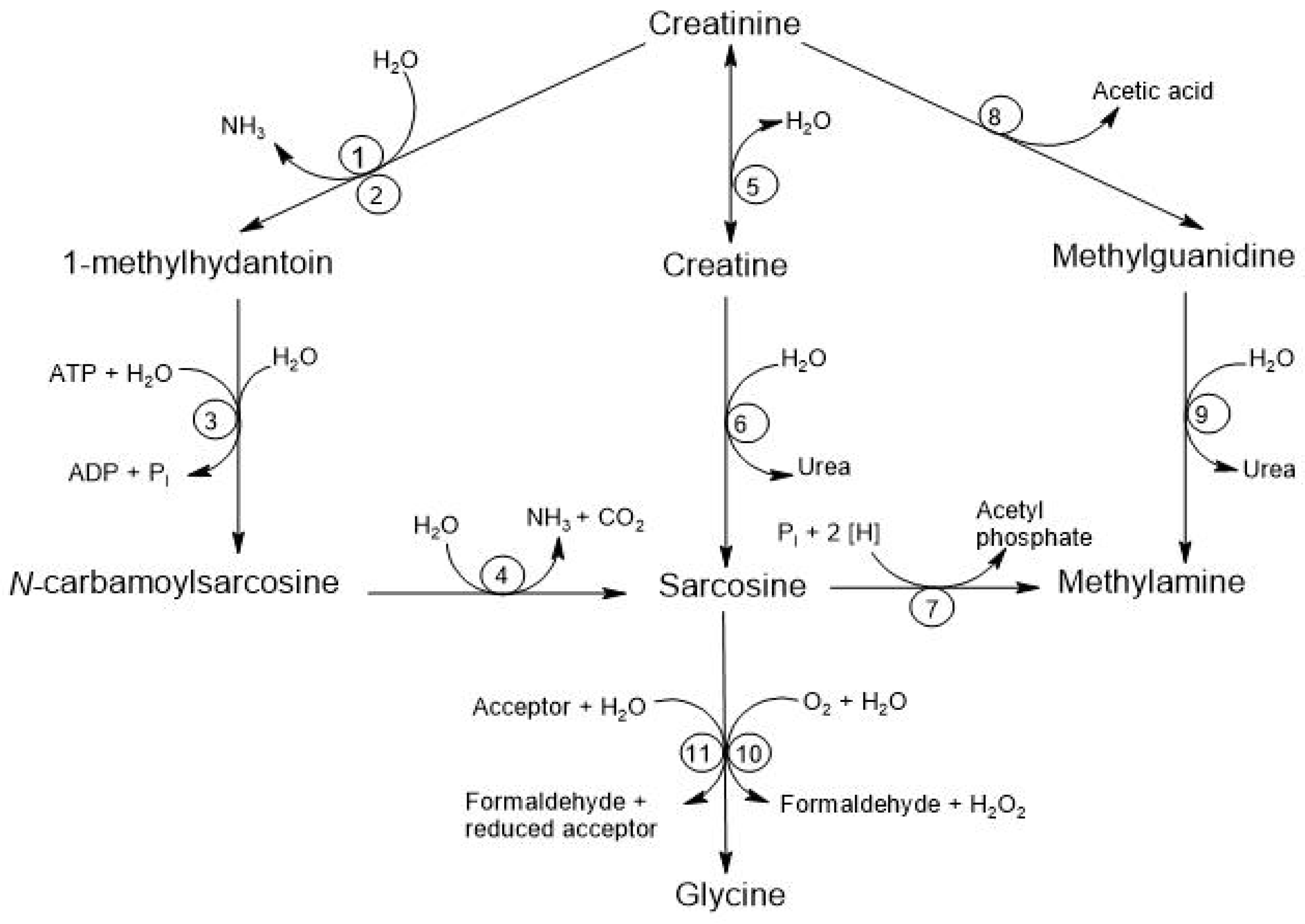
There are at least four alternative pathways for the microbial degradation of Cr. The first pathway is the degradation to 1-methylhydantoin, in which certain microorganisms, such as Bacillus, Clostridium, and Escherichia, degrade Cr into 1-methylhydantoin and ammonia, using Cr as a nitrogen source. The second pathway is the degradation to N-carbamoyl-sarcosine, where strains like Pseudomonas and Brevibacterium convert 1-methylhydantoin into N-carbamoyl-sarcosine and sarcosine. The third pathway involves degradation to Crn where different species, including Alcaligenes, Arthrobacter, and Tissierella, use creatininase to convert Cr into Crn, which is subsequently metabolized to urea and sarcosine through the action of creatinase. Finally, the fourth pathway is the degradation to methylguanidine, where Pseudomonas stutzeri has been observed to convert Cr into methylguanidine and acetic acid, and this methylguanidine can be broken down by certain Alcaligenes strains, producing methylamine and urea [8]. See Figure 5.
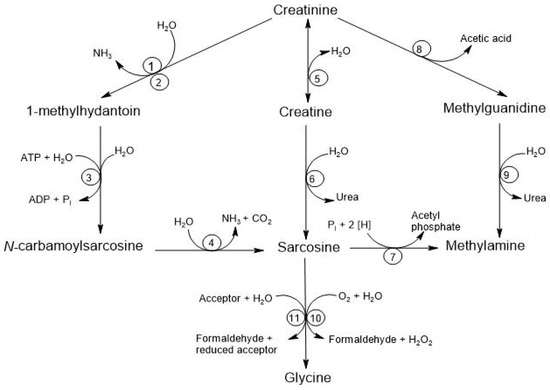
Figure 5.
Degradation of creatinine by the intestinal microbiota. The respective enzymes are denoted by numbers: (1) creatinine amidohydrolase (creatinine deaminase; EC 3.5.4.21); (2) cytosine amidohydrolase (cytosine deaminase; EC 3.5.4.1); (3) 1-methylhydantoin amidohydrolase [ATP-dependent (EC 3.5.2.14) or ATP-independent]; (4) N-carbamoyl-sarcosine amidohydrolase (EC 3.5.1.59); (5) creatinine amidohydrolase (creatinase; EC 3.5.2.10); (6) creatine amidinohydrolase (creatinase; EC 3.5.3.3); (7) sarcosine reductase (EC 1.4.4.-); (8) not yet characterized; (9) methylguanidine amidinohydrolase (EC 3.5.3.16); (10) sarcosine oxidase (EC 1.5.3.1); (11) sarcosine dehydrogenase (EC 1.5.99.1) or dimethylglycine dehydrogenase (EC 1.5.99.2) [8].
Understanding creatinine metabolism is important because it allows us to understand the pathophysiological mechanisms involved in its impact as a marker of renal function and prevent the misinterpretation of eGFR. Cr reflects not only renal function but also the generation, intake, and metabolism of Cr. One important aspect is its interaction with some medicaments. Cr may modify renal clearance and increase the medications’ half-life. Medication may interfere with Cr secretion and lead to a sub-estimation of renal function.
5. Factors Affecting Serum Creatinine Concentration
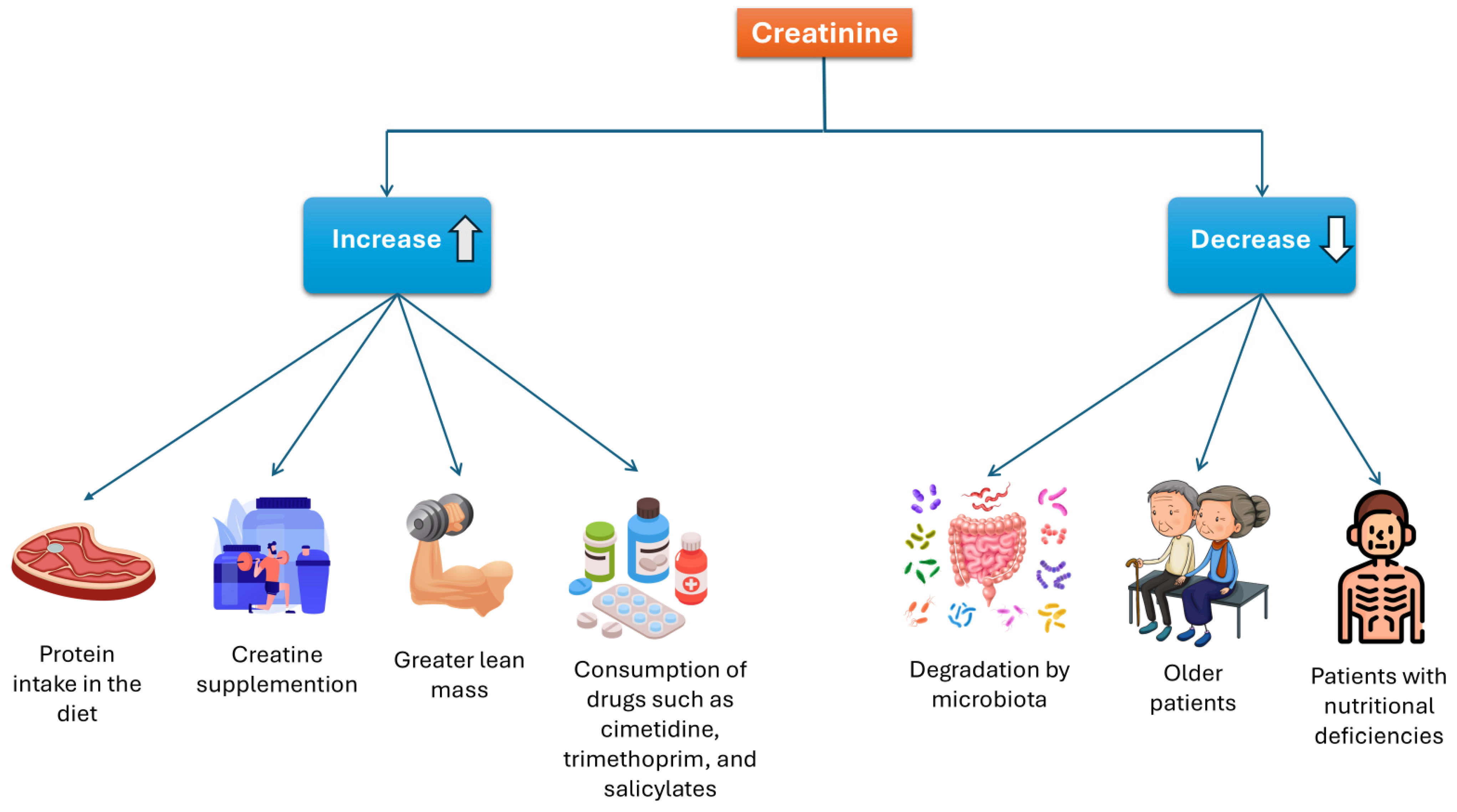
As mentioned previously, Cr levels can be affected by various factors. Some may increase Cr levels, including a high-protein diet, Crn supplementation, muscle mass, and certain medications. Conversely, factors that can decrease Cr concentrations include degradation by intestinal microbiota, advanced age, and nutritional deficiencies. See Figure 6.
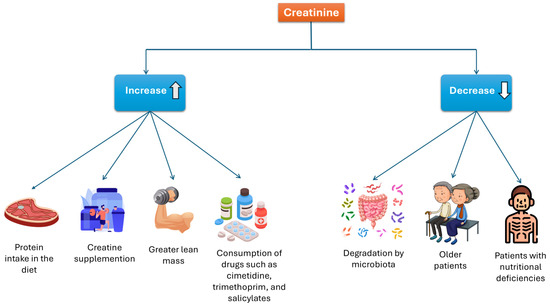
Figure 6.
Factors that influence serum creatinine concentration.
5.1. Drugs That Increase Serum Creatinine Concentrations
5.1.1. Cimetidine
Cimetidine is an H2 receptor antagonist that inhibits gastric acid secretion at a dose of 1.6 g/day. This medication ensures SCr concentrations at an average of 15% in patients with normal renal function [26,27]. High single doses of cimetidine, such as 300 mg intravenously or 800 mg orally, resulted in a reduction in both endogenous and exogenous CrCl (14C-creatinine) by 20 ± 30% in healthy volunteers. This effect is attributed to a decrease in UCrE and an accumulation of SCr until a new steady state is reached. However, there were no concomitant changes in GFR when measured with inulin [3,28].
Cimetidine has a high affinity for the organic cation transporter OCT2 and the multidrug and toxin extrusion transporters (MATE) in the luminal membrane of the proximal tubules, similar to Cr [29]. Therefore, cimetidine inhibits the tubular secretion of Cr, meaning that the absorption of cimetidine blocks the tubular secretion of Cr, resulting in a CrCl close to or identical to the clearance measured with Iohexol, which is also considered a gold standard for measuring glomerular filtration [30].
Therefore, the blockage of the tubular secretion of Cr with cimetidine has proven to be a useful tool for measuring the glomerular filtration rate in kidney transplant recipients with SCr concentrations less than 2.5 mg/L. It has been observed that the clearance calculated using the MDRD formula was similar to that obtained after the administration of cimetidine, reinforcing its viability as an alternative method in this context [31].
5.1.2. Trimethoprim
Trimethoprim is an antibacterial that inhibits the enzyme dihydrofolate reductase [32]. Clinical studies have demonstrated that at therapeutic doses, it elevates SCr concentrations by 15% to 30% and reduces CrCl by 20% to 25%, without affecting the glomerular filtration measured with iodothalamate [33,34].
This effect of trimethoprim is explained by the inhibition of Na/K ATPase present in the basal membrane of the epithelial cells of the distal tubule, as well as the OCT2 transporter and the MATE 1/2-K transporters in the proximal tubule [35,36].
5.1.3. Salicylates
Salicylates can induce an increase in SCr concentration by altering the binding of Cr to serum proteins or competitively inhibiting the tubular secretion of Cr under certain conditions (salt depletion, advanced age, liver cirrhosis, renal diseases, and renal insufficiency) [37,38,39].
5.1.4. Abemaciclib
Abemaciclib is a cyclin-dependent kinase 4 and 6 inhibitor indicated for the treatment of metastatic breast cancer. This medication has been shown to increase SCr concentrations by 10–40%, with peak Cr levels occurring 10–12 h after administration. However, this increase is reversible and demonstrates a gradual decrease over time [40,41].
The GFR calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula shows a decrease following the administration of abemaciclib, but there are no changes in GFR measured with Iohexol or in the eGFR calculated from serum concentrations of CysC. Furthermore, no significant changes were observed in urinary concentrations of neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1), which are biomarkers of renal damage, normalized to Cr.
These data, along with the changes observed in the pharmacokinetics of metformin, suggest that the elevations in SCr concentrations observed in clinical studies of abemaciclib are due to a reversible inhibition of the renal tubular secretion of Cr rather than acute kidney injury [42].
5.1.5. Integrase Inhibitors
Drugs used for the treatment of HIV, such as Dolutegravir (DTG), Raltegravir (RAL), and Elvitegravir (EVG), have been associated with an increase in SCr concentrations. In the VIKING study, which compared the efficacy of DTG versus RAL, the administration of DTG resulted in an increase in Cr levels between 0.084 and 0.105 mg/dL, with this effect being more evident on the 6th and 8th days after administration, reaching a plateau by week 4 without further progression [43].
In the SPRING-2 study, patients treated with DTG showed an increase of 14.6 μmol/L in SCr concentrations after 96 weeks of treatment. Similarly, an increase in Cr of 8.2 μmol/L was observed following the administration of RAL. Additionally, the change in CrCl calculated using the Cockcroft–Gault formula was −19.6 mL/min in the DTG group and −9.3 mL/min in the RAL group, both at week 96 [44]. Furthermore, in additional studies of patients treated with DTG, either alone or in combination with Tenofovir disoproxil fumarate (TDF), renal function estimated by Cr (eGFRCr) and by cystatin C (eGFRCys) was compared. The results indicated that treatment with DTG was associated with a significant increase in Cr and a reduction in eGFRCr, while the cystatin C concentrations and eGFR_Cys remained unchanged [45,46].
This pattern, where there is a rapid but small increase in SCr concentrations that subsequently levels off without further progression, is typical of the blockade of Cr secretion via OCT2 [47].
These transporters involved in the secretion of Cr are illustrated in Figure 7.
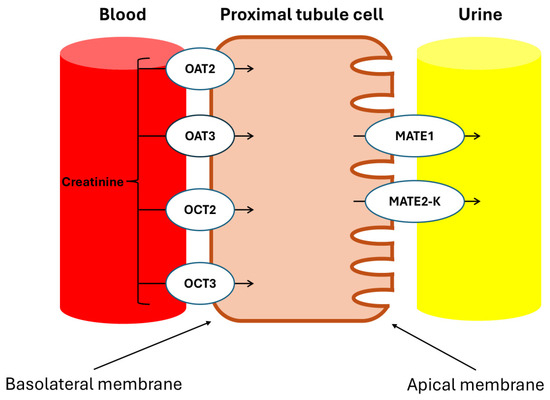
Figure 7.
Drug transporters in proximal kidney cells. OAT2: organic anion transporter 2; OAT3: organic anion transporter 3; OCT2: organic cation transporter 2; OCT3: organic cation transporter 3; MATE1: multidrug and toxin extrusion protein 1; MATE2-K: multidrug and toxin extrusion protein 2, kidney-specific. The tubular secretion of creatinine involves basolateral uptake through the OCT2 and OCT3 transporters for organic cations, and the OAT2 and OAT3 transporters for organic anions. Subsequently, the apical elimination of Cr into the tubular lumen is facilitated by the MATE1 and MATE2-K transporters, which regulate its excretion from the proximal tubular cells.
5.1.6. Glucocorticoids
Glucocorticoids are catabolic hormones from the corticosteroid family, commonly used to suppress, prevent, or reduce immune responses [48]. Due to the catabolic effect of glucocorticoids, there is a release of Crn at the muscular level, which is subsequently converted into Cr, leading to increased serum levels as well as urinary excretion [49].
5.2. Sex
A longitudinal study evaluated urinary Cr excretion and CrCl in men and women. The results showed that men excreted 33% more Cr daily than women, even after adjusting for weight. This difference was attributed to men’s greater lean body mass, contributing to higher production and, consequently, the elimination of Cr. Regarding SCr concentrations, men exhibited levels that were 21% higher compared to women [50].
The results of the Third National Health and Nutrition Examination Survey (NHANES) conducted in the United States indicated that women had Cr levels approximately 22% lower than men [51].
5.3. Race and Ethnicity
In the study mentioned above, James et al. reported that Black subjects had a Cr production rate 5% higher compared to White individuals. This difference was attributed to greater muscle mass, rather than a higher protein intake in their diet, as potassium excretion was lower in this group [50].
Hsu C-Y et al. found a 10.7% increase in SCr concentrations in comparison to non-Black subjects [52]. Similarly, this was attributed to a higher muscle mass in this population group [53]. In this same study, muscle mass and SCr were evaluated in HD patients. It was found that SCr values for Black, Asian, and Hispanic patients were higher than those for non-Hispanic White patients, with differences of more than 1.68 mg/dL, 1.61 mg/dL, and 0.83 mg/dL, respectively. However, these differences among the various groups persisted even after adjusting for intracellular water.
Black patients had SCr concentrations very similar to those of Asian patients, with a minimal difference of only 0.03 mg/dL. However, the concentrations were significantly higher compared to Hispanic and non-Hispanic White patients. Unlike other studies, this study suggests that the higher SCr concentration may not be exclusively due to a greater amount of muscle mass [54].
Since these results challenge established knowledge, further studies are essential to clarify these findings and better understand the factors involved in SCr about muscle mass.
5.4. Physical Activity
Individuals with moderate/intense physical activity presented significantly higher SCr and albumin levels compared to those with sedentary or light physical activity, and higher urinary Cr excretion (UCrE) than sedentary individuals. There were no differences in serum CysC, urea, microalbuminuria, or measured CrCl between these groups. People with moderate/intense physical activity tended to lower CrCl and GFR (Cockcroft–Gault and MDRD equations) compared to sedentary individuals, though these differences did not reach statistical significance [55].
Individuals with higher levels of physical activity had lower body weight, BMI, waist circumference, and body fat content, as well as greater muscle mass compared to those who were sedentary or had light physical activity.
These results demonstrate that intense physical activity directly influences body composition, reducing body fat and increasing LBM. In contrast, serum CysC did not differ between groups. These findings further corroborate that CysC is not influenced by muscle mass [55].
Another study conducted by Beunders et al. showed a significant increase in SCr immediately after an exercise session, with concentrations rising from 58 ± 13 to 71 ± 11 µmol/L. The increase in SCr levels induced by exercise could imply a significant reduction in eGFR of −34 ± 33 mL/min/1.73 m2 [56].
5.5. Age
Kidney function and muscle mass decline progressively with age, the last commonly due to skeletal muscle atrophy. Serum Cr in patients with muscle mass loss might mask the loss of kidney function.
In patients over 65, a cutoff value of SCr of 1.7 mg/dL was used to identify renal insufficiency. It was found that 87.4% of patients with renal insufficiency confirmed by the Cockcroft–Gault formula to estimate GFR had SCr levels of 1.7 mg/dL or lower. These findings highlight the limitations of using SCr as a standalone marker for detecting renal insufficiency in older individuals [57].
Taking into account the aforementioned aspects, formulas that consider other parameters are recommended for a more accurate evaluation. Formulas to estimate GFR have recently eliminated the race factor to prevent race or ethnicity discrimination. Clinicians should be aware of which formula is used in their estimation.
6. Biochemical Measurement in the Laboratory
The normal range of SCr for adult men typically ranges from 0.74 to 1.35 mg/dL (65.4 to 119.3 μM), while for adult women, it ranges from 0.59 to 1.04 mg/dL (52.2 to 91.9 μM) [58]. The daily UCrE in healthy individuals ranges from 0.8 to 2.0 g/day. Elevated SCr levels, exceeding 1000 μM, can indicate renal dysfunction or muscle disorders.
Several conventional methods are available for measuring Cr levels, including colorimetric, enzymatic, and chromatographic techniques. While these methods are highly sensitive and selective, they also have drawbacks such as being time-consuming, requiring sample pre-treatment, high-cost instrumentation, and requiring skilled personnel to operate the equipment [59].
Colorimetric methods are based on the Jaffé reaction, which dates back to 1886. SCr reacts with picric acid in an alkaline medium, forming a red-colored complex at a wavelength between 510 and 520 nm. This method is simple, fast, and inexpensive.
One issue with this measurement is that, in addition to SCr, other positively charged molecules, such as proteins, glucose, acetoacetate, ascorbic acid, and uric acid, also react with picric acid as positive interferents. At the same time, there are also negative interferents, the most significant of which is bilirubin. Therefore, this technique has low specificity. In samples with elevated bilirubin levels, SCr values appear reduced because bilirubin in alkaline media oxidizes to biliverdin, forming a colorless compound that diminishes the color of the reaction. Non-creatinine chromogens can interfere with the assay, causing errors of up to 20% in normal individuals [1]. The Scr results may vary between laboratories; to validate these results and homogenize them, external quality control is necessary.
In children, particularly in the neonatal period, the Jaffé assay is more likely to be affected by non-creatinine chromogens in the sample, leading to a less accurate measurement. Additionally, the higher prevalence of jaundice and hemolyzed samples in this age group makes the results obtained less reliable [60].
Enzymatic Measurement
The Cr present in the sample is converted to creatine Crn by the action of the enzyme Cr amidohydrolase. The resulting Crn is then hydrolyzed to sarcosine and urea through the action of the enzyme Crn amidinohydrolase. Next, sarcosine oxidase promotes the oxidative demethylation of sarcosine, producing glycine, formaldehyde, and hydrogen peroxide.
In the presence of peroxidase, the hydrogen peroxide formed reacts with N-ethyl-N-sulfopropyl-m-toluidine (ESPMT) and 4-aminoantipyrine, producing a quinoneimine with a maximum absorbance at 546 nm. The intensity of the color of the reaction product is directly proportional to the Cr concentration in the sample [61].
Another enzymatic method available for measuring Cr utilizes the enzyme Cr amidohydrolase. This method involves a four-step reaction process, ultimately measuring the decrease in NADH (Nicotinamide Adenine Dinucleotide) with a reading at 340 nm. Unlike the other enzymatic method, which relies on photometric readings outside the UV range, this method uses UV readings. The error in determining Cr impacts medical decisions based on guidelines. Standardizing Cr by increasing accuracy and reducing variation between laboratories helps prevent the incorrect classification and treatment of patients [61].
The estimation of GFR from SCr is adequate for diagnosing, staging, and monitoring CKD progression in most clinical circumstances. However, like all diagnostic tests, the interpretation is influenced by the test’s variable characteristics in selected clinical circumstances and the pre-test probability of disease. In particular, an isolated reduced GFR is more likely to be a false positive in otherwise healthy individuals than in those with risk factors for kidney disease or markers of renal damage [60].
Clinicians must be aware of the characteristics of the techniques used in Cr assessment and the potential consequences of estimating GFR. The presence of known factors that interfere with the Jaffe technique warrants the use of other alternative techniques or even other biomarkers.
7. Evaluation of Glomerular Filtration Rate
The GFR is measured through clearances, CrCl = (urine vol 24 h mL/1440 min) * (UCr (mg/dL)/SCr (mg/dL), and with endogenous or exogenous metabolites of glomerular filtration.
The recommendation for GFR evaluation, according to the 2024 Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines, is to assess renal function as GFR, along with the serum concentration of endogenous filtration markers. It is suggested to express the GFR as eGFR in milliliters per minute per 1.73 m2 rather than milliliters per minute. Additionally, two-stage tests are recommended: an initial test followed by confirmation tests when necessary. Cr-based eGFR is the recommended initial test, while confirmation tests such as Cys C-based eGFR, Cr-Cys C-based eGFR, or CrCl are indicated in specific cases where Cr-based eGFR has evident limitations [60].
The equations used to estimate GFR allow it to be calculated from serum concentrations of endogenous filtration markers, eliminating the need for 24 h urine collection and direct measurements in urine. These markers include low-molecular-weight metabolites (such as Cr, 113 Da) and low-molecular-weight proteins (such as CysC, 1000–20,000 Da).
The serum concentration of these markers is inversely related to GFR. It is also influenced by other physiological factors, known as “non-GFR-related determinants,” which include their generation, renal tubular handling (reabsorption and secretion), and extrarenal elimination. For clinical practice, a specific equation is chosen for each marker or a combination of these to routinely report GFR [62]. See Table 2.

Table 2.
Formulas to determine renal function.
The combination of Cr and CysC for GFR calculation emerged because the race factor was removed to prevent discrimination.
The recalibrated MDRD equation calculates GFR adjusted for body surface area, considering SCr, age, and gender but not ethnicity or muscle mass. Therefore, in individuals with higher muscle mass, this equation often underestimates the actual GFR [63].
8. Limitations of Serum Creatinine
Due to Cr’s complex metabolism, measuring eGFR has various limitations. One of the main limitations is that changes in muscle mass, such as in patients with chronic diseases, malnutrition, or advanced age, lead to an overestimation of the GFR.
It is also important to note that all formulas for calculating the GFR do not consider other common factors, such as the intake of cooked red meat and the effect that some medications may have.
Creatinine may retain its utility in usual clinical scenarios. For instance, in individuals with stable renal function and without confounding factors such as recent changes in muscle mass or medication interference, creatinine measurement may still serve as a reliable marker of kidney function.
9. Acute Increase in Serum Creatinine
An increment in serum Cr concentration mostly means impaired kidney function. It is expected in patients affected by many chronic illnesses. The silent start and progress of CKD in many of those diseases, such as diabetes, hypertension, as well as some primary kidney diseases, is the cause of the delayed diagnosis of CKD. It is “suddenly discovered” during a routine exam or when secondary manifestations are in study, such as anemia, fatigue, and weight loss.
Actual “acute” increments of serum Cr may occur after well-identified recent events, such as accidents with hemorrhage or severe muscle damage, exercise with excessive dehydration, toxins or poisons, and pain from stones descending into the urinary tract. Under such conditions, differential diagnosis for Cr increments is necessary [64].
To distinguish if increased Cr levels mean acute kidney injury (AKI), increments ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h or an increase in SCr to ≥1.5 times over baseline values are necessary. Additional data as urine volume < 0.5 mL/kg/h for 6 h, muddy brown casts in the sediment, Uosm (mmol/kg) < 350, mild to moderate proteinuria, UNa (mmol/L) > 40, FENa (%) > 1, FEUrea (%) > 35, and novel biomarkers like KIM-1, cystatin C, and NGAL sustain the AKI diagnosis [65].
AKI syndrome encompasses various etiologies, including prerenal azotemia, which accounts for around 30–60% of cases. The reduction in the renal blood flow decreases the glomerular perfusion pressure, resulting in a lower glomerular filtration rate. Because creatinine excretion mainly depends on glomerular filtration, this reduction limits its clearance from plasma, causing elevated serum creatinine levels [66]. The ratio of serum BUN to creatinine in healthy individuals is 10 to 15:1 (when both are expressed in mg/dL or 40 to 60 when expressed in mmol/L). In prerenal AKI, the ratio may be greater than 20:1 because of a disproportionate increase in urea reabsorption resulting from elevated serum vasopressin levels. Intrinsic accounts for around 40% (acute tubular necrosis, acute interstitial nephritis, acute glomerular, and vasculitic renal diseases). In intrinsic AKI, the plasma membrane ruptures, releasing its cytoplasmic content, exacerbating inflammation and injury, reflected as a rise in SCr [67]. Acute postrenal obstructive nephropathy accounts for around 10% [68]. In postrenal AKI, hemodynamic changes combined with obstruction impede urine excretion and result in elevated blood creatinine levels [69].
Reflection Points
- Acute increase in serum creatinine (AKI) is a process in which treatment decisions must be made quickly; despite the problems of Crea interpretation, it remains the diagnostic axis for this disease. Therefore, besides SCr, assessing daily urine volume can narrow down the differential diagnosis, dividing AKI into oliguric (<500 mL) and non-oliguric causes. A careful history, physical examination, and basic laboratory tests often suffice for diagnosis.
- To improve diagnostic precision and therapeutic outcomes in AKI, a shift is needed from SCr-based staging alone to a more comprehensive approach. This includes integrating biomarkers, transcriptomic and proteomic data, and considering the pathophysiological and anatomical context of kidney injury [70,71].
- Despite its drawbacks, which have already been mentioned, SCr remains the most widely used biomarker in the diagnosis of CKD due to its low cost and rapid analysis in hospitals with limited resources. In these cases, the recommendation is to consider the clinical severity of the disease and recent changes in food consumption and medication for a better interpretation of eGFR.
10. Cystatin C
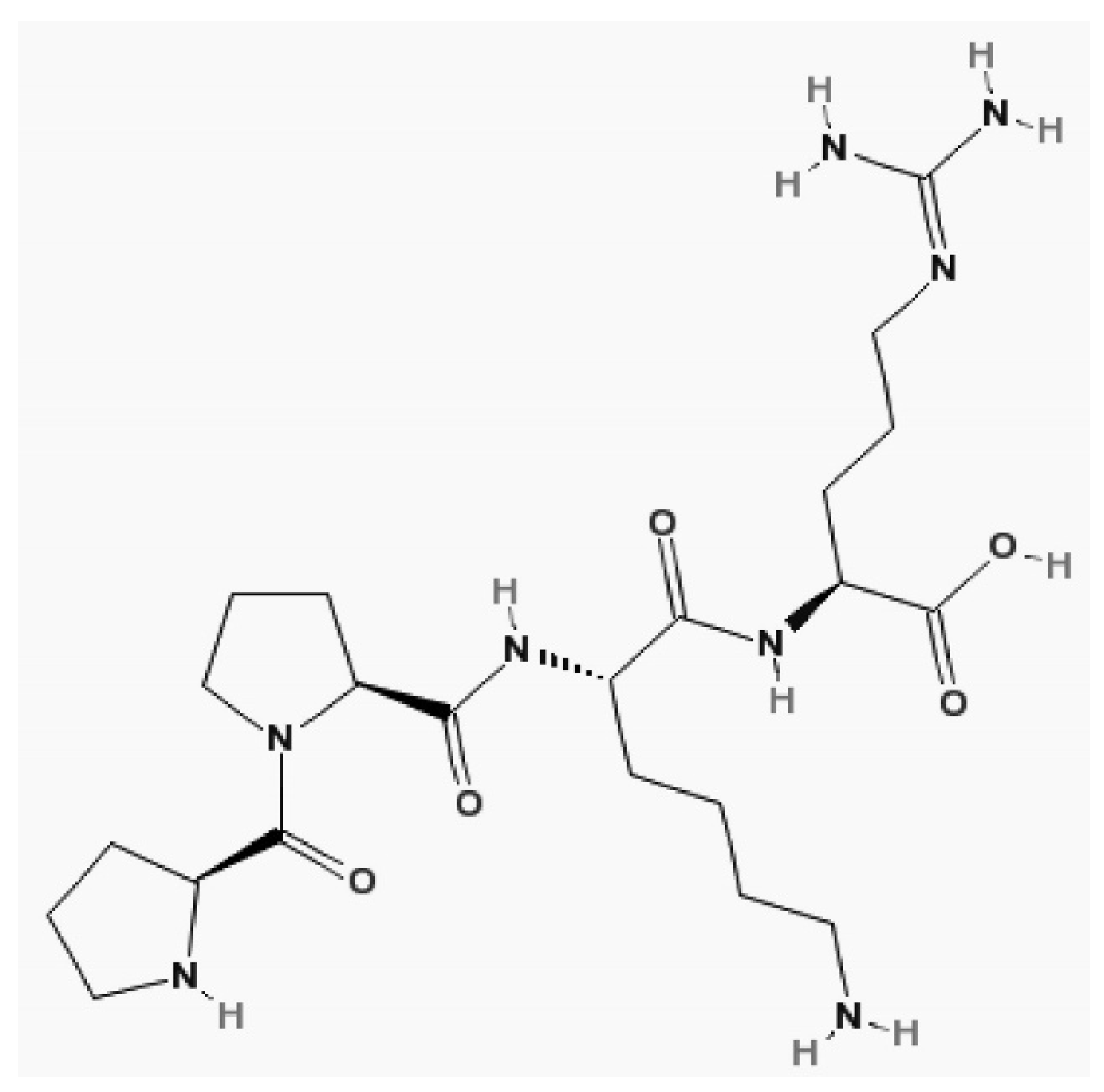
CysC is a proteinase inhibitor belonging to family 2 of the cystatin superfamily. It consists of a no glycosylated polypeptide chain of 120 amino acid residues, it has a molecular weight of 13.3 kDa, is positive charged, and has an isoelectric point of 9.3.
Grubb et al. first determined its structure in 1981 [72]. Later, it was discovered that the human CysC gene, along with its promoter, is of a constitutive type, ensuring its constant production in all nucleated cells [73]. Intracellularly, it acts as a cysteine protease inhibitor, and extracellularly, it acts as a lysosomal proteinase inhibitor [74]. CysC is distributed only in the extracellular volume and cleared by the kidney [75]. Therefore, after acute changes in GFR, the CysC serum concentration increases faster than Cr [76]. Its structure is shown in Figure 8. This molecule is freely filtered by the renal glomerulus under conditions where renal function is not impaired [77]. It is then reabsorbed and fully catabolized by the cells of the proximal tubules, with this absorption occurring through the megalin endocytic receptor. Unlike Cr, it does not undergo tubular secretion [78].
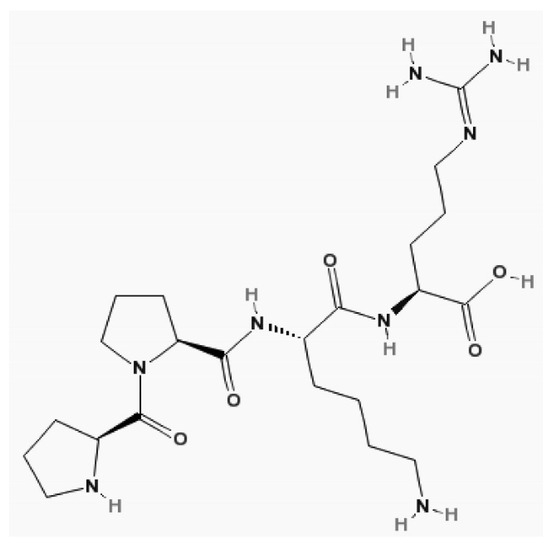
Figure 8.
The structure of cystatin C. Molecular formula of cystatin C, C22H40N8O5 [19].
However, it is essential to note that albuminuria can interfere with the process of absorption since CysC and albumin are reabsorbed in the proximal tubule via megalin-facilitated endocytosis [79]. Albuminuria, consequently, increases its excretion in urine due to the competition between the two molecules for the same transport mechanism.
A point in favor of using CysC is that its serum values are not influenced by physiological factors such as age, sex, race, diet, and muscle mass. The study conducted by Chen et al. in the UK Biobank showed that GFR based on CysC identifies five times more participants with a TFG < 60 mL/min/1.73 m2 compared to the estimate using only Cr in South Asian Individuals. Furthermore, the use of CysC allowed for the detection of a population with chronic kidney disease who were at high risk of death, heart failure, and cardiovascular atherosclerotic diseases, risks that were not identified when using Cr as a marker. Additionally, data from the UK Biobank study showed that, unlike Cr, CysC can assess kidney function independently of ethnic origin [80].
11. Factors Affecting Serum Cystatin C Levels
Pathological factors, such as hyperthyroidism and hypothyroidism, can affect serum CysC levels. It has been shown that in untreated hyperthyroid patients, serum CysC levels are higher than in euthyroid patients, and CysC levels decrease once appropriate treatment is initiated. In addition, in hypothyroid patients who were not receiving any treatment, the serum CysC levels were lower than in euthyroid patients, and the CysC levels increased after receiving the appropriate treatment [81,82,83].
One of the main advantages is that they are less susceptible to interference from factors that affect the measurement of Cr, such as hemolysis, lipemia, and jaundice, providing a more reliable assessment of renal function [84].
Although it has been shown that CysC concentrations can be influenced by pathological conditions such as obesity, inflammation, smoking, steroid treatments, and thyroid disorders this marker is not significantly influenced by physiological factors. Unlike Cr, CysC offers a more reliable and consistent measurement of renal function. CysC is a better tool for assessing renal and cardiovascular risk across different populations, ensuring greater consistency in different clinical settings.
Its measurement is typically carried out using turbidimetric (PETIA) or nephelometric (PENIA) immunoassays. This is because PETIA and PENIA provide several advantages, including fast processing times, minimal interference from other substances, and improved accuracy.
The different characteristics of Cr and CysC are summarized in Table 3.

Table 3.
Comparison of serum creatinine and cystatin C.
12. Practical Importance of Using the Difference (eGFR Cys C–eGFR Cr) or the Ratio (eGFR Cys C/eGFR Cr)
The use of Cr or CysC to estimate the GFR is not exclusive; these should be considered complementary when evident extrarenal factors may expressly limit the use of some of the biomarkers, as is the case of sarcopenia or thyroid diseases [88]. In most patients, there is concordance in eGFR based on either of the two methods. However, eGFR based on both equations is more reliable [60].
In recent years, evidence has been provided that the difference (eGFR Cys C–eGFR Cr) or the ratio (eGFR Cys C/eGFR Cr) of the two measurements has practical importance. Furthermore, they have a better predictive value for mortality than separate measurements [89]. The discrepancy between the two methods has been attributed to reduced glomerular permeability for proteins with molecular weight in the CysC range. It is known as selective glomerular hypofiltration syndrome (SGHS) [90] or known tentatively as Shrunken Pore Syndrome (SPS) [88]. Usually, these alterations are not detected in isolated evaluations of eGFR and may be present even in patients who do not meet the CKD criteria with current clinical practice guidelines. Ratios (eGFR Cys C/eGFR Cr) less than 0.70 or significant differences (eGFR Cys C–eGFR Cr) are associated with more significant kidney damage and higher mortality rates in various clinical conditions. This can affect people with pre-eclampsia/eclampsia, cardiothoracic diseases and surgery, older adults, and even healthy populations.
Serum creatinine levels should be used for the initial assessment of the GFR in detecting and staging AKI and CKD, determining CKD progression, and making treatment decisions, including those related to kidney replacement therapy [60].
Table 4 outlines the clinical indications for using eGFR calculated with creatinine, cystatin C, or a combination of both. For instance, a high-protein diet may underestimate eGFR when calculated using creatinine alone. In cases of malnutrition, reduced muscle mass can lead to an underestimation of eGFR when relying solely on SCr. Therefore, in patients with cachexia or sarcopenia, it is recommended to use a combination of creatinine and cystatin C to assess eGFR. Similarly, in patients using steroids, due to their effects on muscle mass, eGFR calculated with both markers (Cr-Cys) is suggested. In cases where medications reduce tubular secretion, it is recommended to use eGFRcys [60].

Table 4.
Clinical indications for using creatinine or cystatin C [60].
13. Utility of Creatinine as Body Composition Marker
13.1. Body Composition
Body composition evaluations include evaluations at different levels of organization, from the subcellular and cellular level to the whole body. From a clinical perspective, human body composition can be divided into two main compartments: water compartments and fat mass. Lean mass and cellular mass are calculated based on water compartments and are influenced by age, sex, and race.
Body composition measurements can be performed using different technologies, such as isotopic dilution, computed tomography, magnetic resonance imaging, X-ray densitometry (DEXA), and other more sophisticated technologies. However, indirect, low-cost, and non-invasive methods in clinical practice are commonly used to determine human body composition, such as bioelectrical impedance (BIA) and skinfold thickness. Muscle mass is highly variable between the elderly and children and can be substantially modified through physical exercise, as previously mentioned [55].
13.2. Bioimpedance
BIA is a method for estimating body composition by conducting a low-intensity multifrequency current through the human body. Higher frequencies (>50 kHz) are transmitted through the water into the cells and extracellular space, and low frequencies (<50 kHz) are transmitted through water only in the extracellular spaces. Fat mass is more resistant than non-fat cells.
Intracellular water is calculated based on total body and extracellular water differences. Lean body mass is calculated from intracellular water, assuming a constant intracellular water content, usually around 70% [91]:
Total body water = Extracellular Water + Intracellular Water
Lean body mass = ICW/0.70
In dialysis patients, the relationship between compartments is abnormal due to muscle wasting and intracellular and extracellular edema. Therefore, BIA measurements show an increased ICW and overestimate LBM because ICW is greater than 70% (used as a reference in normal subjects). This presumed edema introduces a systematic error in the calculation of LBM. An alternative method is necessary to assess how much BIA overestimates LBM. Among other alternative methods is Cr kinetics, which measures only muscle mass. However, information on this is very scarce.
13.3. Creatinine Kinetics and Creatinine Index
Cr generation is an indirect measure of muscle mass because muscle metabolism is the main source of Cr production. Mitch y col [4] conducted a study decades ago on creatine metabolism in patients with CKD, specifically the production and excretion of Cr
Despite its utility in inferring muscle mass, they observed that Cr excretion did not fully explain SCr levels. They concluded that part of this Cr is metabolized endogenously. As SCr increased, creatine was recycled rather than excreted in the urine. Therefore, the need arose to consider the complete Cr metabolism in estimating LBM. Later, Keshaviah [92] defined a formula for calculating LBM in patients on peritoneal dialysis (PD), which is still used today.
The kinetics of Cr are based on the principle that Cr generation is proportional to LBM in patients with constant protein consumption. This technique considers the sum of Cr excretion and metabolic degradation. Cr excretion includes only urinary excretion in non-dialysis patients and Cr content in drained dialysis solutions, plus urinary excretion obtained from residual renal function. At the same time, metabolic degradation is proportional to body weight and SCr concentrations. Therefore, the sum of degradation and excretion should, in a steady state, equal the Cr production rate (Table 5) [4,92].

Table 5.
Formulas used in creatinine kinetics [4,92].
Subsequently, Bhatla B et al. [93] correlated measurements of LBM using Cr kinetics, bioimpedance, and DEXA in patients with PD and found a high association. Cr kinetics have also been used to assess protein nutritional status in patients with HD [94]. Recently, the same authors found that the CI or Cr kinetics are surrogate tools for measuring muscle mass in patients with kidney damage [95] and added urea distribution to the formula in patients on HD. Since then, no studies have referred to this method in patients with PD.
Recently, Cr kinetics were recommended in the KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines to measure muscle mass in patients with CKD [96]. However, the procedure has the disadvantage that it requires a 24 h urine collection and drained PD solutions, which can sometimes be difficult for the patient. Additionally, meat consumption and creatine supplements increase UCrE, which must be accounted for when calculating Cr kinetics. In anuric HD patients, Cr kinetics are based on the SCr levels before and after HD.
The methods to calculate and use the CI according to the KDOQI guidelines are highlighted in Table 6 [5]:

Table 6.
Formulas to calculate the creatinine index.
Cr kinetics’ utility in assessing nutritional status has been questioned; it is not accepted that UCr is associated with muscle mass [97]. On the other hand, such an association is accepted as long as the subjects have no nutritional restrictions and 24 h urine was collected over three consecutive days [98]. These two studies were conducted in healthy subjects; however, in patients with kidney damage, such as those on PD, Cr excretion may be inaccurate since it does not consider recycling and Cr returns to circulation, which is why these patients have high SCr values. It is also necessary to consider the losses of Cr in the drained PD solution that the patient should collect.
BIA and creatinine kinetics are useful tools for assessing body composition, though each has limitations. Fluid imbalances can influence BIA, while creatinine kinetics require careful urine collection. Despite these challenges, creatinine kinetics provide a more reliable estimate of lean mass, particularly in patients with kidney issues, making it a valuable clinical tool.
Measurement of LBM is important in the nutritional management of patients to quantify sarcopenia, energy waste, and mortality outcomes.
13.4. Conclusions and Future Directions
Creatinine measurements are an easy and widely available technique for routinely estimating the glomerular filtration rate and muscle mass. Despite its utility in daily clinical work, the medical team should know its limitations and be aware of misinterpretation. Serum creatinine should be used for the initial assessment of GFR in detecting and staging acute kidney disease and CKD. Alternative or complementary measurements of cystatin C must be considered when evaluating kidney function, including dosing medications and making treatment decisions. However, the utility of Cr is good when using Cr kinetics as a direct measurement of lean body mass compared to electrical bioimpedance, which underestimates muscle mass in patients with edema, such as those with CKD.
Author Contributions
Conceptualization, R.P. and M.Á.; investigation R.P., M.Á., and M.G.M.S.; writing—original draft preparation, R.P. and A.S.B.A.; writing—review and editing, M.Á., M.G.M.S.; conduction, M.Á. All authors have read and agreed to the published version of this manuscript.
Funding
APC of this Review was funding by Baxter, México, and administrated by ÍMSS Fundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AASK | African American Study of Kidney Disease and Hypertension |
| ADP | Adenosine diphosphate |
| AGAT | L-arginine amino transferase |
| Arg | Arginine |
| ATP | Adenosine triphosphate |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body mass index |
| CI | Creatinine index |
| CK | Creatine kinase |
| CKD | Chronic kidney disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| Cr | Creatinine |
| CrCl | Creatinine clearance |
| Crn | Creatine |
| CysC | Cystatin C |
| Da | Dalton |
| DTG | Dolutegravir |
| DEXA | Dual-Energy X-ray Absorptiometry |
| eGFR | Estimated glomerular filtration rate |
| ECW | Extracellular fluid |
| ESPMT | N-ethyl-N-sulfopropyl-m-toluidine |
| FFM | Fat-free mass |
| GFR | Estimated glomerular filtration rate |
| GAA | Guanidinoacetic acid |
| GAMT | S-adenosyl-L-methionine methyltransferase |
| Gly | Glycine |
| GFRcys | Estimated glomerular filtration rate from cystatin C |
| GFRcr | Estimated glomerular filtration rate from creatinine |
| GFRcr-cys | Estimated glomerular filtration rate from creatinine and cystatin C |
| HD | Hemodialysis |
| ICW | Intracellular fluid |
| kDa | Kilodalton |
| KDIGO | Kidney Disease Improving Global Outcomes |
| KDOQI | Kidney Disease Outcomes Quality Initiative |
| LBM | Lean body mass |
| MATE1 | Multidrug and toxin extrusion protein 1 |
| MATE2-K | Kidney-specific multidrug and toxin extrusion protein 2 |
| MDRD | Modification of diet in renal disease |
| NADH | Nicotinamide adenine dinucleotide |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| NHANES | National Health and Nutrition Examination Survey |
| OAT2 | Organic anion transporter 2 |
| OAT3 | Organic anion transporter 3 |
| OCT2 | Organic cation transporter 2 |
| OCT3 | Organic cation transporter 3 |
| PD | Peritoneal dialysis |
| SAM | S-adenosylmethionine |
| SCr | Serum creatinine |
| TBW | Total body water |
| TDF | Tenofovir disoproxil fumarate |
| UCr | Urinary creatinine concentration |
| UCrE | Urinary creatinine excretion |
| UK Biobank | UK Biobank Health Database |
| UV | Ultraviolet |
| μM | Micromolar |
References
- Levey, A.; Inker, L. Assessment of Glomerular Filtration Rate in Health and Disease: A State of the Art Review. Clin. Pharmacol. Ther. 2017, 102, 405–419. [Google Scholar] [CrossRef]
- Dodge, W.F. Comparison of Endogenous Creatinine Clearance With Inulin Clearance. Arch. Pediatr. Adolesc. Med. 1967, 113, 683. [Google Scholar] [CrossRef] [PubMed]
- Andreev, E.; Koopman, M.; Arisz, L. A rise in plasma creatinine that is not a sign of renal failure: Which drugs can be responsible? J. Intern. Med. 1999, 246, 247–252. [Google Scholar] [CrossRef]
- Mitch, W.E.; Collier, V.U.; Walser, M. Creatinine Metabolism in Chronic Renal Failure. Clin. Sci. 1980, 58, 327–335. [Google Scholar] [CrossRef]
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am. J. Kidney Dis. 2000, 35, S17–S104. [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef]
- Kreider, R.; Jäger, R.; Purpura, M. Bioavailability, Efficacy, Safety, and Regulatory Status of Creatine and Related Compounds: A Critical Review. Nutrients 2022, 14, 1035. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Balsom, P.D.; Söderlund, K.; Ekblom, B. Creatine in humans with special reference to creatine supplementation. Sports Med. 1994, 18, 268–280. [Google Scholar]
- Jacobsen, F.K.; Christensen, C.K.; Mogensen, C.E.; Andreasen, F.; Heilskov, N.S. Pronounced increase in serum creatinine concentration after eating cooked meat. BMJ 1979, 1, 1049–1050. [Google Scholar] [CrossRef]
- Mayersohn, M.; Conrad, K.; Achari, R. The influence of a cooked meat meal on creatinine plasma concentration and creatinine clearance. Br. J. Clin. Pharmacol. 1983, 15, 227–230. [Google Scholar] [CrossRef]
- Preiss, D.J.; Godber, I.M.; Lamb, E.J.; Dalton, R.N.; Gunn, I.R. The influence of a cooked-meat meal on estimated glomerular filtration rate. Ann. Clin. Biochem. Int. J. Lab. Med. 2007, 44, 35–42. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan diets: Practical advice for athletes and exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Trojian, T.H. Creatine Supplementation. Curr. Sports Med. Rep. 2013, 12, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.L.; Mahoney, E.T.; Cohn, K.A.; Sheradsky, L.F.; Green, B.A. Oral creatine supplementation enhances upper extremity work capacity in persons with cervical-level spinal cord injury. Arch. Phys. Med. Rehabil. 2002, 83, 19–23. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Candow, D.G.; Mahoney, D.; Tarnopolsky, M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med. Sci. Sports Exerc. 2003, 35, 1946–1955. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- McMurray, M.D.; Trivax, J.E.; McCullough, P.A. Serum Cystatin C, Renal Filtration Function, and Left Ventricular Remodeling. Circ. Heart Fail. 2009, 2, 86–89. [Google Scholar] [CrossRef]
- Levey, A.S.; Perrone, R.D.; Madias, N.E. Serum Creatinine and Renal Function. Annu. Rev. Med. 1988, 39, 465–490. [Google Scholar] [CrossRef]
- Imamura, Y.; Murayama, N.; Okudaira, N.; Kurihara, A.; Okazaki, O.; Izumi, T.; Inoue, K.; Yuasa, H.; Kusuhara, H.; Sugiyama, Y. Prediction of Fluoroquinolone-Induced Elevation in Serum Creatinine Levels: A Case of Drug–Endogenous Substance Interaction Involving the Inhibition of Renal Secretion. Clin. Pharmacol. Ther. 2011, 89, 81–88. [Google Scholar] [CrossRef]
- Zhang, X.; Rule, A.D.; McCulloch, C.E.; Lieske, J.C.; Ku, E.; Hsu, C.Y. Tubular secretion of creatinine and kidney function: An observational study. BMC Nephrol. 2020, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Urakami, Y.; Kimura, N.; Okuda, M.; Inui, K.-I. Creatinine Transport by Basolateral Organic Cation Transporter hOCT2 in the Human Kidney. Pharm. Res. 2004, 21, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, Y.; Masuda, S.; Sato, T.; Katsura, T.; Ogawa, O.; Inui, K.-I. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem. Pharmacol. 2007, 74, 359–371. [Google Scholar] [CrossRef]
- Jones, J.D.; Burnett, P.C. Creatinine Metabolism in Humans with Decreased Renal Function: Creatinine Deficit. Clin. Chem. 1974, 20, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, W.S.; Pickard, R.G.; Maudgal, D.P.; Lawrence, D.; Northfield, T.C. CIMETIDINE IN DUODENAL ULCER Controlled Trial. Lancet 1976, 308, 174–176. [Google Scholar] [CrossRef]
- Haggie, S.J.; Fermont, D.C.; Wyllie, J.H. Treatment of duodenal ulcer with cimetidine. Lancet 1976, 307, 983–984. [Google Scholar] [CrossRef]
- Dubb, J.W.; Stote, R.M.; Familiar, R.G.; Lee, K.; Alexander, F. Effect of cimetidine on renal function in normal man. Clin. Pharmacol. Ther. 1978, 24, 76–83. [Google Scholar] [CrossRef]
- Burt, H.J.; Neuhoff, S.; Almond, L.; Gaohua, L.; Harwood, M.D.; Jamei, M.; Rostami-Hodjegan, A.; Tucker, G.T.; Rowland-Yeo, K. Metformin and cimetidine: Physiologically based pharmacokinetic modelling to investigate transporter mediated drug–drug interactions. Eur. J. Pharm. Sci. 2016, 88, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Stehlé, T.; El Karoui, K.; Sakka, M.; Ismail, A.; Matignon, M.; Grimbert, P.; Canoui-Poitrine, F.; Prié, D.; Audard, V. Creatinine clearance after cimetidine administration in a new short procedure: Comparison with plasma and renal clearances of iohexol. Clin. Kidney J. 2020, 13, 587–596. [Google Scholar] [CrossRef]
- Kabat-Koperska, J.; Safranow, K.; Gołembiewska, E.; Domański, L.; Ciechanowski, K. Creatinine Clearance after Cimetidine Administration—Is It Useful in the Monitoring of the Function of Transplanted Kidney? Ren. Fail. 2007, 29, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.A.; O’Bryan, T.A.; Zurlo, J.; Miller, D.Q.; Joshi, N. Trimethoprim-Sulfamethoxazole Revisited. Arch. Intern. Med. 2003, 163, 402. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.T.; First, M.R.; Myre, S.A.; Cacini, W. Effect of Co-trimoxazole and Sulfamethoxazole on Serum Creatinine in Normal Subjects. Ther. Drug Monit. 1982, 4, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Petersen, P.; Bartram, R.; Hansen, J.M. The Effect of Trimethoprim on Serum Creatinine. Br. J. Urol. 1985, 57, 265–268. [Google Scholar] [CrossRef]
- Yokoyama, S.; Nakagawa, J.; Aiuchi, N.; Seito, T.; Niioka, T. Impact of trimethoprim on serum creatinine, sodium, and potassium concentrations in patients taking trimethoprim-sulfamethoxazole without changes in glomerular filtration rate. J. Clin. Pharm. Ther. 2022, 47, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Kudo, T.; Ito, K. Quantitative Consideration of Clinical Increases in Serum Creatinine Caused by Renal Transporter Inhibition. Drug Metab. Dispos. 2023, 51, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V. Does aspirin cause acute or chronic renal failure in experimental animals and in humans? Am. J. Kidney Dis. 1996, 28, S24–S29. [Google Scholar] [CrossRef]
- Muther, R.S. Aspirin-Induced Depression of Glomerular Filtration Rate in Normal Humans: Role of Sodium Balance. Ann. Intern. Med. 1981, 94, 317. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.J. Acute effects of acetylsalicylic acid in patients with chronic renal insufficiency. Eur. J. Clin. Pharmacol. 1977, 11, 111–116. [Google Scholar] [CrossRef]
- Patnaik, A.; Rosen, L.S.; Tolaney, S.M.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Hilton, J.F.; et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non–Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016, 6, 740–753. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Chappell, J.C.; Turner, P.K.; Pak, Y.A.; Bacon, J.; Chiang, A.Y.; Royalty, J.; Hall, S.D.; Kulanthaivel, P.; Bonventre, J.V. Abemaciclib Inhibits Renal Tubular Secretion Without Changing Glomerular Filtration Rate. Clin. Pharmacol. Ther. 2019, 105, 1187–1195. [Google Scholar] [CrossRef]
- Eron, J.J.; Clotet, B.; Durant, J.; Katlama, C.; Kumar, P.; Lazzarin, A.; Poizot-Martin, I.; Richmond, G.; Soriano, V.; Ait-Khaled, M.; et al. Safety and Efficacy of Dolutegravir in Treatment-Experienced Subjects With Raltegravir-Resistant HIV Type 1 Infection: 24-Week Results of the VIKING Study. J. Infect. Dis. 2013, 207, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Raffi, F.; Jaeger, H.; Quiros-Roldan, E.; Albrecht, H.; Belonosova, E.; Gatell, J.M.; Baril, J.-G.; Domingo, P.; Brennan, C.; Almond, S.; et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2013, 13, 927–935. [Google Scholar] [CrossRef]
- Lu, L.; Li, X.; Liu, X.; Han, Y.; Qiu, Z.; Song, X.; Li, Y.; Li, X.; Cao, W.; Li, T. Comparison of Renal Function Biomarkers of Serum Creatinine and Cystatin C in HIV-Infected People on Dolutegravir-Containing Therapy. Infect. Drug Resist. 2022, 15, 1695–1706. [Google Scholar] [CrossRef]
- Palich, R.; Tubiana, R.; Abdi, B.; Mestari, F.; Guiguet, M.; Imbert-Bismut, F.; Katlama, C.; Bonnefont–Rousselot, D.; Isnard-Bagnis, C. Plasma cystatin C as a marker for estimated glomerular filtration rate assessment in HIV-1-infected patients treated with dolutegravir-based ART. J. Antimicrob. Chemother. 2018, 73, 1935–1939. [Google Scholar] [CrossRef]
- Koteff, J.; Borland, J.; Chen, S.; Song, I.; Peppercorn, A.; Koshiba, T.; Cannon, C.; Muster, H.; Piscitelli, S.C. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br. J. Clin. Pharmacol. 2013, 75, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M. Clinical Pharmacology of Corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, B.A.C.; Prummel, M.F.; Weberc, J.A.; Wiersinga, W.M.; Arisz, L. Effect of Prednisone on Renal Function in Man. Nephron 1993, 65, 254–259. [Google Scholar] [CrossRef]
- James, G.D.; Sealey, J.E.; Alderman, M.; Ljungman, S.; Mueller, F.B.; Pecker, M.S.; Laragh, J.H. A Longitudinal Study of Urinary Creatinine and Creatinine Clearance in Normal Subjects: Race, Sex, and Age Differences. Am. J. Hypertens. 1988, 1, 124–131. [Google Scholar] [CrossRef]
- Köttgen, A.; Selvin, E.; Stevens, L.A.; Levey, A.S.; Van Lente, F.; Coresh, J. Serum cystatin C in the United States: The Third National Health and Nutrition Examination Survey (NHANES III). Am. J. Kidney Dis. 2008, 51, 385–394. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Yang, W.; Parikh, R.V.; Anderson, A.H.; Chen, T.K.; Cohen, D.L.; He, J.; Mohanty, M.J.; Lash, J.P.; Mills, K.T.; et al. Race, Genetic Ancestry, and Estimating Kidney Function in CKD. N. Engl. J. Med. 2021, 385, 1750–1760. [Google Scholar] [CrossRef]
- Goldwasser, P.; Aboul-Magd, A.; Maru, M. Race and creatinine excretion in chronic renal insufficiency. Am. J. Kidney Dis. 1997, 30, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Powe, N.R.; Chertow, G.M.; Grimes, B.; Johansen, K.L. Muscle Mass and Serum Creatinine Concentration by Race and Ethnicity among Hemodialysis Patients. J. Am. Soc. Nephrol. 2024, 35, 66–73. [Google Scholar] [CrossRef]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of Muscle Mass and Physical Activity on Serum and Urinary Creatinine and Serum Cystatin C. Clin. J. Am. Soc. Nephrol. 2008, 3, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Beunders, R.; Bongers, C.C.W.G.; Pickkers, P. The effects of physical exercise on the assessment of kidney function. J. Appl. Physiol. 2020, 128, 1459–1460. [Google Scholar] [CrossRef]
- Swedko, P.J.; Clark, H.D.; Paramsothy, K.; Akbari, A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch. Intern. Med. 2003, 163, 356–360. [Google Scholar] [CrossRef] [PubMed]
- American Board of Internal Medicine. ABIM Laboratory Test Reference Ranges—January 2024. Available online: https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf (accessed on 18 July 2024).
- Pundir, C.S.; Kumar, P.; Jaiwal, R. Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Peake, M.; Whiting, M. Measurement of serum creatinine--current status and future goals. Clin. Biochem. Rev. 2006, 27, 173–184. [Google Scholar] [PubMed]
- Levey, A.S.; Titan, S.M.; Powe, N.R.; Coresh, J.; Inker, L.A. Kidney Disease, Race, and GFR Estimation. Clin. J. Am. Soc. Nephrol. 2020, 15, 1203–1212. [Google Scholar] [CrossRef]
- Levey, A.; Coresh, J.; Greene, T.; Marsh, J.; Stevens, L.; Kusek, J.; Van Lente, F. Expressing the MDRD study equation for estimating GFR with IDMS traceable (gold standard) serum creatinine values. J. Am. Soc. Nephrol. 2005, 16, 69A. [Google Scholar]
- Johnson, R.J.; Feehally, J.; Floege, J.r. Comprehensive Clinical Nephrology, 6th ed.; Elsevier: Edinburgh, Scotland, 2019. [Google Scholar]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Blantz, R.C. Pathophysiology of pre-renal azotemia. Kidney Int. 1998, 53, 512–523. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of Acute Kidney Injury. Compr. Physiol. 2012, 2, 1303. [Google Scholar] [CrossRef]
- Molitoris, B.A. Low-Flow Acute Kidney Injury: The Pathophysiology of Prerenal Azotemia, Abdominal Compartment Syndrome, and Obstructive Uropathy. Clin. J. Am. Soc. Nephrol. 2022, 17, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Nørregaard, R.; Mutsaers, H.A.M.; Frøkiær, J.; Kwon, T.H. Obstructive nephropathy and molecular pathophysiology of renal interstitial fibrosis. Physiol. Rev. 2023, 103, 2827–2872. [Google Scholar] [CrossRef] [PubMed]
- Barasch, J.; Zager, R.; Bonventre, J.V. Acute kidney injury: A problem of definition. Lancet 2017, 389, 779–781. [Google Scholar] [CrossRef]
- Moore, P.K.; Hsu, R.K.; Liu, K.D. Management of Acute Kidney Injury: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 72, 136–148. [Google Scholar] [CrossRef]
- Grubb, A.; Löfberg, H. Human gamma-trace, a basic microprotein: Amino acid sequence and presence in the adenohypophysis. Proc. Natl. Acad. Sci. USA 1982, 79, 3024–3027. [Google Scholar] [CrossRef]
- Abrahamson, M.; Olafsson, I.; Palsdottir, A.; Ulvsbäck, M.; Lundwall, Å.; Jensson, O.; Grubb, A. Structure and expression of the human cystatin C gene. Biochem. J. 1990, 268, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, M.; Barrett, A.J.; Salvesen, G.; Grubb, A. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J. Biol. Chem. 1986, 261, 11282–11289. [Google Scholar] [CrossRef]
- Jacobsson, B.; Lignelid, H.; Bergerheim, U.S. Transthyretin and cystatin C are catabolized in proximal tubular epithelial cells and the proteins are not useful as markers for renal cell carcinomas. Histopathology 1995, 26, 559–564. [Google Scholar] [CrossRef]
- Nejat, M.; Pickering, J.W.; Walker, R.J.; Endre, Z.H. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol. Dial. Transpl. 2010, 25, 3283–3289. [Google Scholar] [CrossRef]
- Grubb, A.O. Cystatin C-Properties and Use as Diagnostic Marker; Elsevier: Amsterdam, The Netherlands, 2001; pp. 63–99. [Google Scholar] [CrossRef]
- Kaseda, R.; Iino, N.; Hosojima, M.; Takeda, T.; Hosaka, K.; Kobayashi, A.; Yamamoto, K.; Suzuki, A.; Kasai, A.; Suzuki, Y.; et al. Megalin-mediated endocytosis of cystatin C in proximal tubule cells. Biochem. Biophys. Res. Commun. 2007, 357, 1130–1134. [Google Scholar] [CrossRef]
- Nejat, M.; Hill, J.V.; Pickering, J.W.; Edelstein, C.L.; Devarajan, P.; Endre, Z.H. Albuminuria increases cystatin C excretion: Implications for urinary biomarkers. Nephrol. Dial. Transplant. 2012, 27, iii96–iii103. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.C.; Lu, K.; Scherzer, R.; Lees, J.S.; Rutherford, E.; Mark, P.B.; Potok, O.A.; Rifkin, D.E.; Ix, J.H.; Shlipak, M.G.; et al. Cystatin C- and Creatinine-based Estimated GFR Differences: Prevalence and Predictors in the UK Biobank. Kidney Med. 2024, 6, 100796. [Google Scholar] [CrossRef]
- Jayagopal, V.; Keevil, B.G.; Atkin, S.L.; Jennings, P.E.; Kilpatrick, E.S. Paradoxical Changes in Cystatin C and Serum Creatinine in Patients with Hypo- and Hyperthyroidism. Clin. Chem. 2003, 49, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Wiesli, P.; Schwegler, B.; Spinas, G.A.; Schmid, C. Serum cystatin C is sensitive to small changes in thyroid function. Clin. Chim. Acta 2003, 338, 87–90. [Google Scholar] [CrossRef]
- Fricker, M.; Wiesli, P.; Brändle, M.; Schwegler, B.; Schmid, C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003, 63, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Emara, M.; El Moselhi, H.; Shoker, A. Comparing Measures of Cystatin C in Human Sera by Three Methods. Am. J. Nephrol. 2009, 29, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Welle, S.; Thornton, C.; Totterman, S.; Forbes, G. Utility of creatinine excretion in body-composition studies of healthy men and women older than 60 y. Am. J. Clin. Nutr. 1996, 63, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Schmid, C.H.; Greene, T.; Li, L.; Beck, G.J.; Joffe, M.M.; Froissart, M.; Kusek, J.W.; Zhang, Y.; Coresh, J.; et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009, 75, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A. Cystatin C as a Biomarker in Kidney Disease; Elsevier: Amsterdam, The Netherlands, 2011; pp. 291–312. [Google Scholar] [CrossRef]
- Grubb, A.; Lindström, V.; Jonsson, M.; Bäck, S.E.; Åhlund, T.; Rippe, B.; Christensson, A. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: ‘Shrunken pore syndrome’. Scand. J. Clin. Lab. Invest. 2015, 75, 333–340. [Google Scholar] [CrossRef]
- Åkesson, A.; Malmgren, L.; Leion, F.; Nyman, U.; Christensson, A.; Björk, J.; Grubb, A. Different ways of diagnosing selective glomerular hypofiltration syndromes such as shrunken pore syndrome and the associated increase in mortality. J. Intern. Med. 2024, 297, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Malmgren, L.; Öberg, C.; den Bakker, E.; Leion, F.; Siódmiak, J.; Åkesson, A.; Lindström, V.; Herou, E.; Dardashti, A.; Xhakollari, L.; et al. The complexity of kidney disease and diagnosing it—Cystatin C, selective glomerular hypofiltration syndromes and proteome regulation. J. Intern. Med. 2023, 293, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deurenberg, P.; Wang, W.; Pietrobelli, A.; Baumgartner, R.N.; Heymsfield, S.B. Hydration of fat-free body mass: New physiological modeling approach. Am. J. Physiol.-Endocrinol. Metab. 1999, 276, E995–E1003. [Google Scholar] [CrossRef]
- Keshaviah, P.R.; Nolph, K.D.; Moore, H.L.; Prowant, B.; Emerson, P.F.; Meyer, M.; Twardowski, Z.J.; Khanna, R.; Ponferrada, L.; Collins, A. Lean body mass estimation by creatinine kinetics. J. Am. Soc. Nephrol. 1994, 4, 1475–1485. [Google Scholar] [CrossRef]
- Bhatla, B.; Moore, H.; Emerson, P.; Keshaviah, P.; Prowant, B.; Nolph, K.D.; Singh, A. Lean Body Mass Estimation by Creatinine Kinetics, Bioimpedance, and Dual Energy X-Ray Absorptiometry in Patients on Continuous Ambulatory Peritoneal Dialysis. ASAIO J. 1995, 41, M442–M446. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Garred, L.J.; Argiles, A.; Flavier, J.L.; Bouloux, C.; Mion, C. Creatinine kinetic modelling: A simple and reliable tool for the assessment of protein nutritional status in haemodialysis patients. Nephrol. Dial. Transpl. 1995, 10, 1405–1410. [Google Scholar]
- Canaud, B.; Ye, X.; Usvyat, L.; Kooman, J.; Van Der Sande, F.; Raimann, J.; Wang, Y.; Kotanko, P. Clinical and predictive value of simplified creatinine index used as muscle mass surrogate in end-stage kidney disease haemodialysis patients—Results from the international MONitoring Dialysis Outcome initiative. Nephrol. Dial. Transplant. 2020, 35, 2161–2171. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Iacone, R.; D’Elia, L.; Guida, B.; Barbato, A.; Scanzano, C.; Strazzullo, P. Validation of daily urinary creatinine excretion measurement by muscle-creatinine equivalence. J. Clin. Lab. Anal. 2018, 32, e22407. [Google Scholar] [CrossRef]
- Forbes, G.; Bruining, G. Urinary creatinine excretion and lean body mass. Am. J. Clin. Nutr. 1976, 29, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).