Varroa Volatiles Offer Chemical Cues to Honey Bees for Initial Parasitic Recognition
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee Colonies and V. destructor Mites
2.2. EAG Response to V. destructor VOCs
2.3. Identification of V. destructor VOCs
2.4. VOCs Regulated the Gene Expression Profiles of Honey Bees
2.5. Varroa Mites Regulate the Respiratory Metabolic Rate of Honey Bees
2.6. Statistical Analysis
3. Results
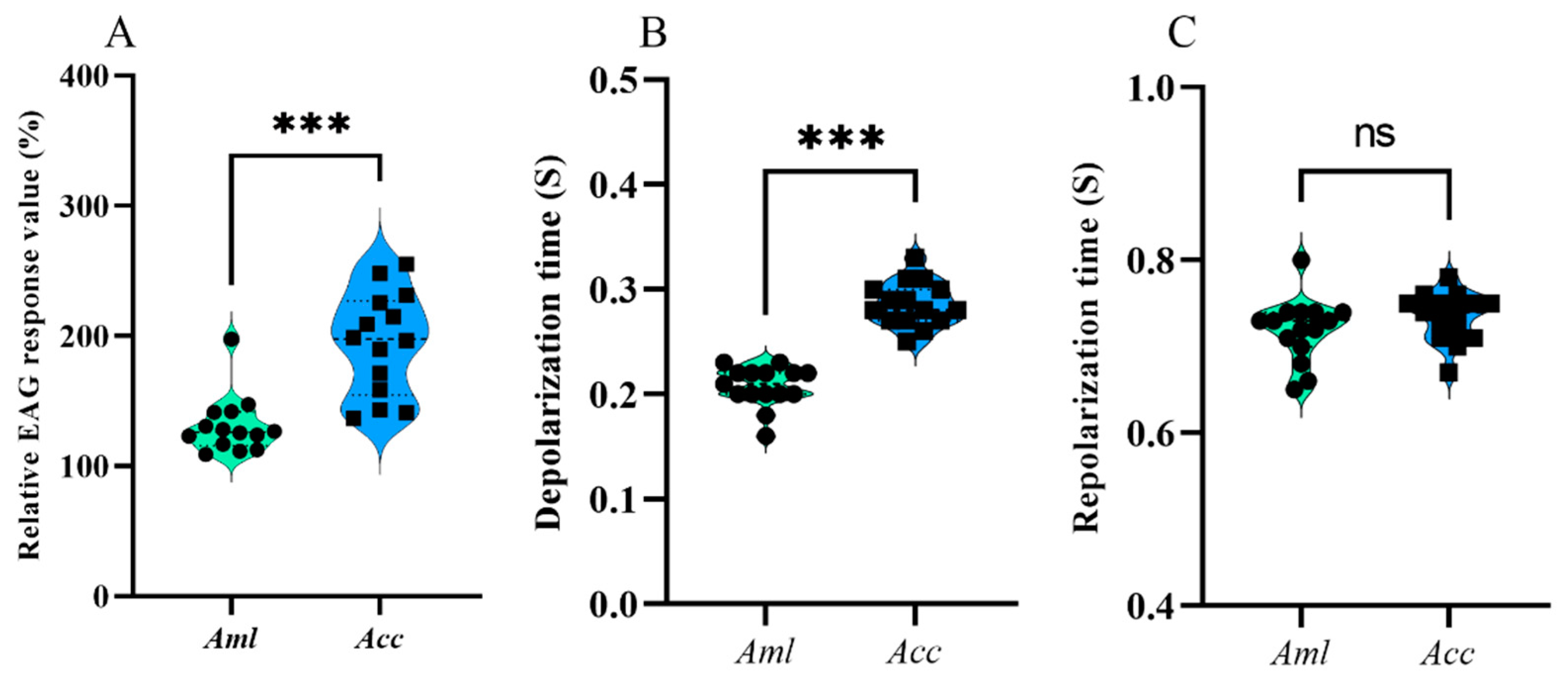
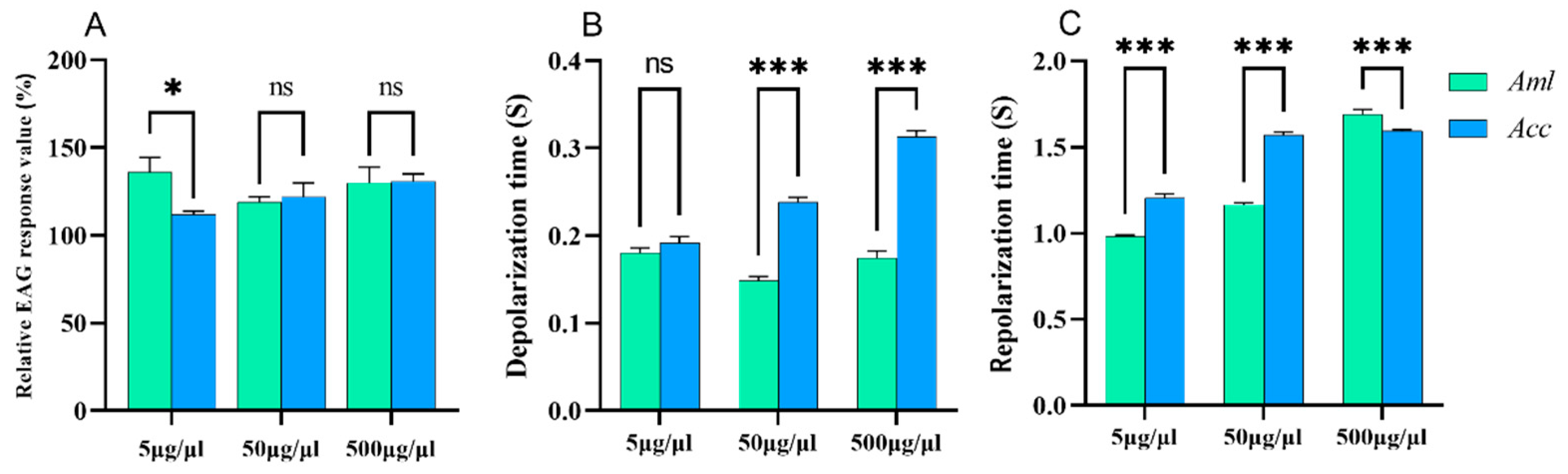
3.1. EAG Response Differences Between A. c. cerana and A. m. ligustica
3.2. Identification of V. destructor VOCs
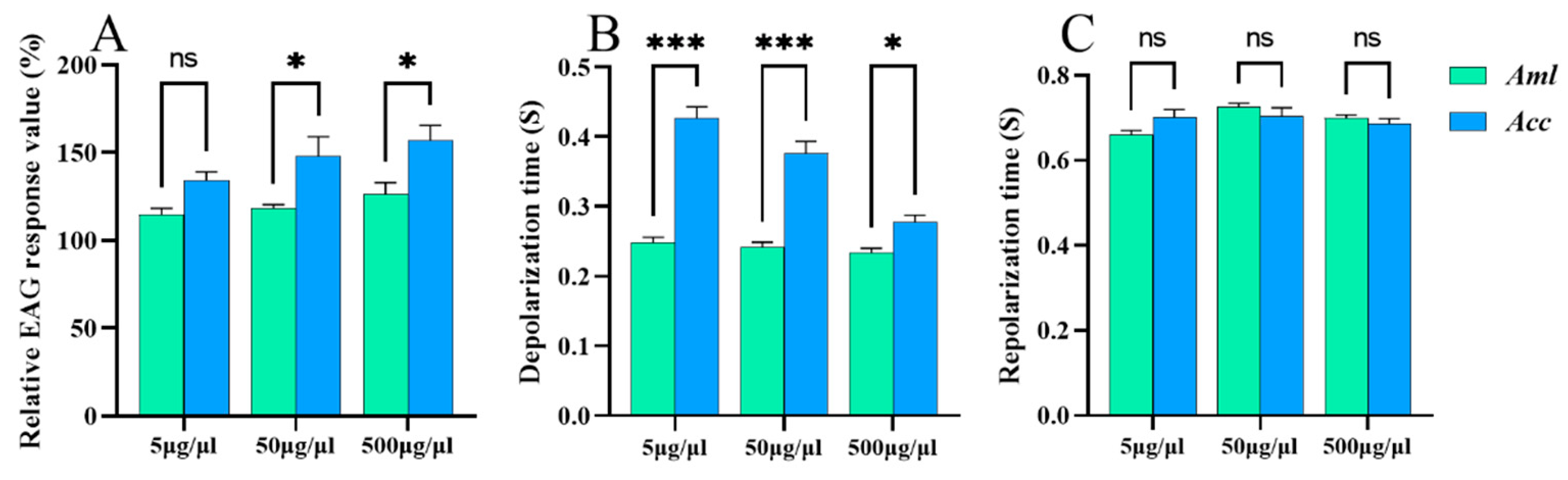
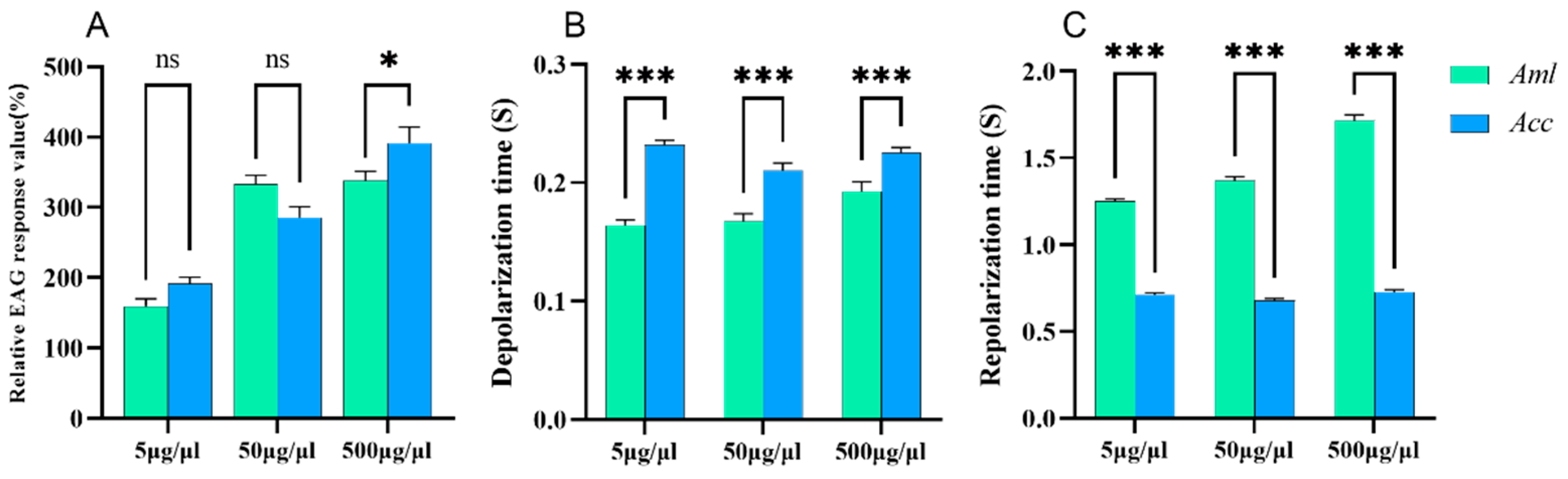
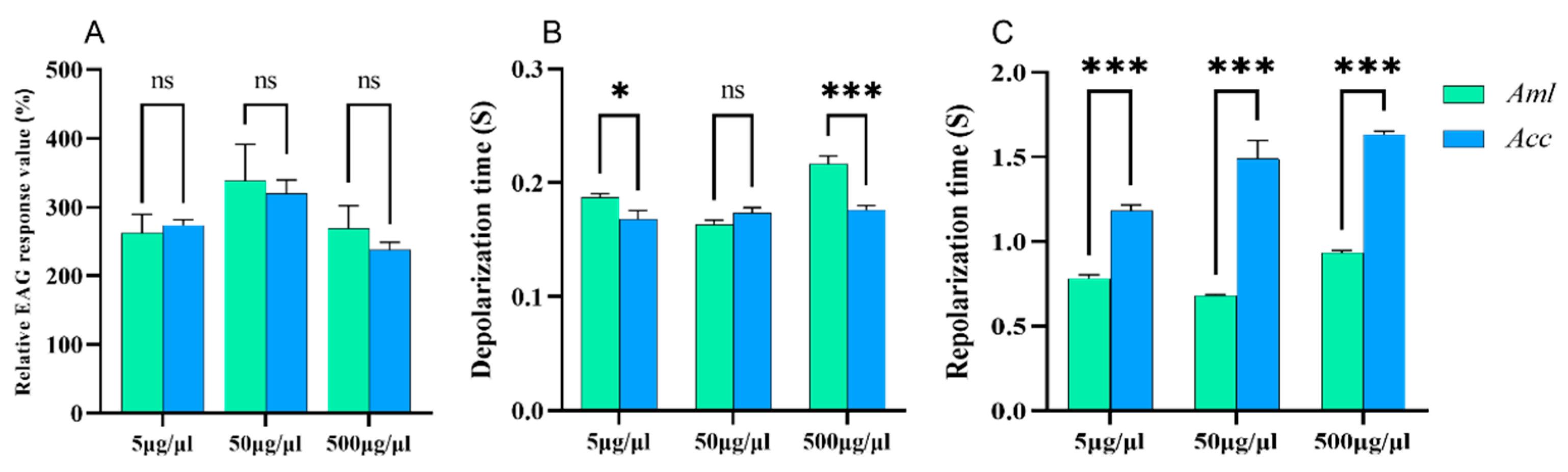
3.3. EAG Responses of Honey Bees to V. destructor VOCs
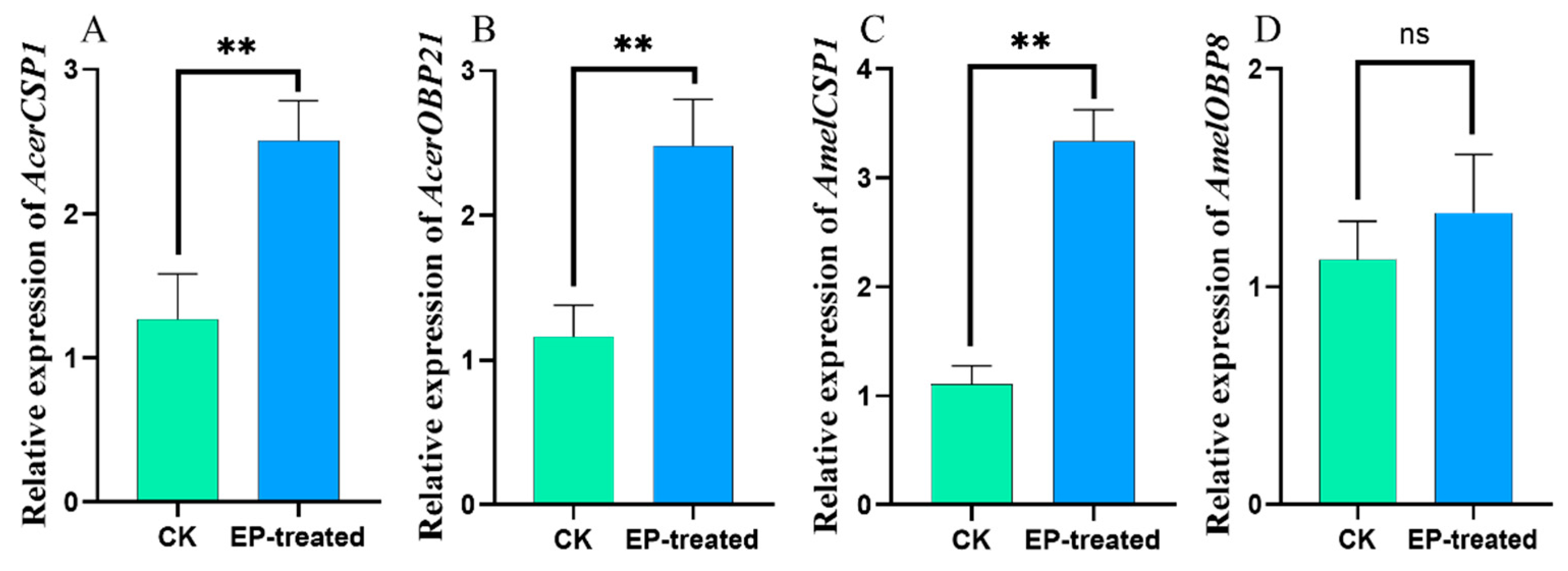
3.4. VOCs Regulate Host Gene Expression Profiles
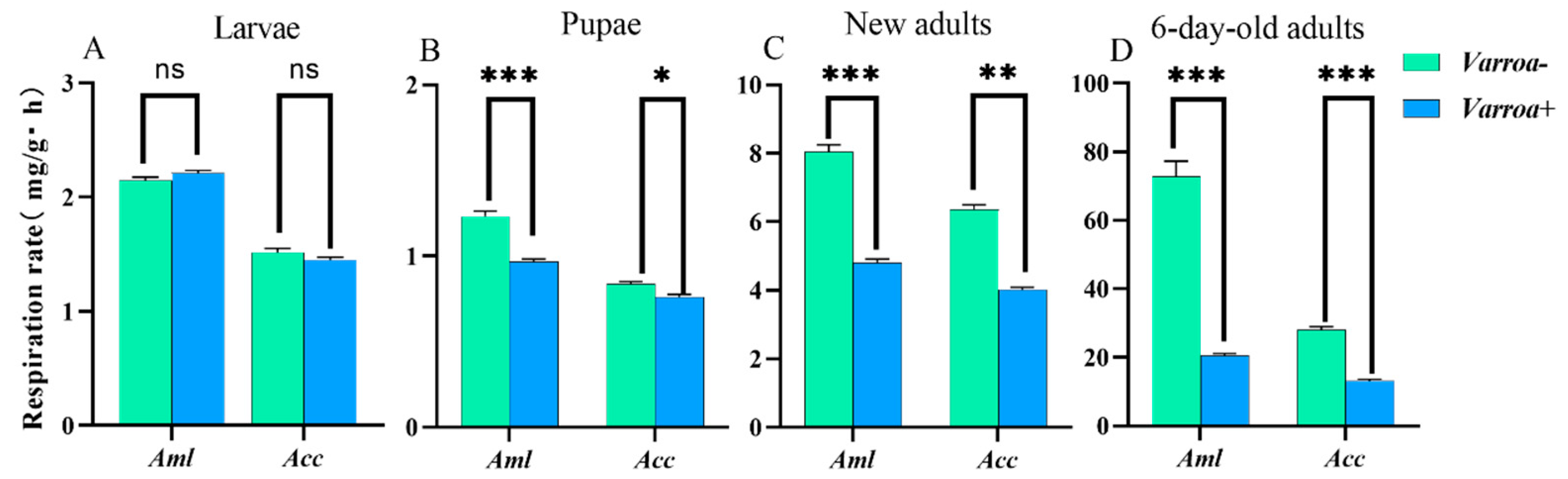
3.5. Varroa Mites Regulate the Respiratory Metabolic Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and Temporal Trends of Global Pollination Benefit. PLoS ONE 2012, 7, e35954. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Medina-Flores, C.A.; López-Carlos, M.; Carrillo-Muro, O.; Gray, A. Honey Bee Colony Losses in Mexico’s Semi-Arid High Plateau for the Winters 2016–2017 to 2021–2022. Insects 2023, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-C.; Yao, J.; Wang, Y. Varroa mite and deformed wing virus infestations interactively make honey bees (Apis mellifera) more susceptible to insecticides. Environ. Pollut. 2022, 292, 118212. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Corn, M.L. Bee Health: Background and Issues for Congress; Congressional Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Nazzi, F.; Le Conte, Y. Ecology of Varroa destructor, the Major Ectoparasite of the Western Honey Bee, Apis mellifera. Annu. Rev. Èntomol. 2016, 61, 417–432. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Ahumada, F.; Graham, H. Are Dispersal Mechanisms Changing the Host–Parasite Relationship and Increasing the Virulence of Varroa destructor (Mesostigmata: Varroidae) in Managed Honey Bee (Hymenoptera: Apidae) Colonies? Environ. Èntomol. 2017, 46, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Buawangpong, N.; de Guzman, L.I.; Khongphinitbunjong, K.; Frake, A.M.; Burgett, M.; Chantawannakul, P. Prevalence and reproduction of Tropilaelaps mercedesae and Varroa destructor in concurrently infested Apis mellifera colonies. Apidologie 2015, 46, 779–786. [Google Scholar] [CrossRef]
- Mondet, F.; Beaurepaire, A.; McAfee, A.; Locke, B.; Alaux, C.; Blanchard, S.; Danka, B.; Le Conte, Y. Honey bee survival mechanisms against the parasite Varroa destructor: A systematic review of phenotypic and genomic research efforts. Int. J. Parasitol. 2020, 50, 433–447. [Google Scholar] [CrossRef]
- Mukogawa, B.; Nieh, J.C. The Varroa paradox: Infestation levels and hygienic behavior in feral scutellata-hybrid and managed Apis mellifera ligustica honey bees. Sci. Rep. 2024, 14, 1148. [Google Scholar] [CrossRef]
- Plettner, E.; Eliash, N.; Singh, N.K.; Pinnelli, G.R.; Soroker, V. The chemical ecology of host-parasite interaction as a target of Varroa destructor control agents. Apidologie 2017, 48, 78–92. [Google Scholar] [CrossRef]
- Fries, I.; Huazhen, W.; Wei, S.; Jin, C.S. Grooming behavior and damaged mites (Varroa jacobsoni) in Apis cerana cerana and Apis mellifera ligustica. Apidologie 1996, 27, 3–11. [Google Scholar] [CrossRef]
- Jung, J.W.; Park, K.W.; Oh, H.-W.; Kwon, H.W. Structural and functional differences in the antennal olfactory system of worker honey bees of Apis mellifera and Apis cerana. J. Asia-Pac. Èntomol. 2014, 17, 639–646. [Google Scholar] [CrossRef]
- Noël, A.; Dumas, C.; Rottier, E.; Beslay, D.; Costagliola, G.; Ginies, C.; Nicolè, F.; Rau, A.; Le Conte, Y.; Mondet, F. Detailed chemical analysis of honey bee (Apis mellifera) worker brood volatile profile from egg to emergence. PLoS ONE 2023, 18, e0282120. [Google Scholar] [CrossRef]
- Slessor, K.N.; Winston, M.L.; Le Conte, Y. Pheromone Communication in the Honeybee (Apis mellifera L.). J. Chem. Ecol. 2005, 31, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Arnold, G.; Trouiller, J.; Masson, C.; Chappe, B. Identification of a brood pheromone in honeybees. Die Naturwissenschaften 1990, 77, 334–336. [Google Scholar] [CrossRef]
- Maisonnasse, A.; Lenoir, J.-C.; Costagliola, G.; Beslay, D.; Choteau, F.; Crauser, D.; Becard, J.-M.; Plettner, E.; Le Conte, Y. A scientific note on E-β-ocimene, a new volatile primer pheromone that inhibits worker ovary development in honey bees. Apidologie 2009, 40, 562–564. [Google Scholar] [CrossRef]
- McAfee, A.; Chapman, A.; Iovinella, I.; Gallagher-Kurtzke, Y.; Collins, T.F.; Higo, H.; Madilao, L.L.; Pelosi, P.; Foster, L.J. A death pheromone, oleic acid, triggers hygienic behavior in honey bees (Apis mellifera L.). Sci. Rep. 2018, 8, 5719. [Google Scholar] [CrossRef] [PubMed]
- Piou, V.; Vilarem, C.; Blanchard, S.; Armengaud, C.; Heeb, P.; Vétillard, A. Varroa destructor relies on physical cues to feed in artificial conditions. Parasite 2023, 30, 49. [Google Scholar] [CrossRef]
- Frey, E.; Odemer, R.; Blum, T.; Rosenkranz, P. Activation and interruption of the reproduction of Varroa destructor is triggered by host signals (Apis mellifera). J. Invertebr. Pathol. 2013, 113, 56–62. [Google Scholar] [CrossRef]
- Gebremedhn, H.; Claeys Bouuaert, D.; Asperges, M.; Amssalu, B.; De Smet, L.; de Graaf, D.C. Expression of Molecular Markers of Resilience against Varroa destructor and Bee Viruses in Ethiopian Honey Bees (Apis mellifera simensis) Focussing on Olfactory Sensing and the RNA Interference Machinery. Insects 2023, 14, 436. [Google Scholar] [CrossRef]
- Mondet, F.; Alaux, C.; Severac, D.; Rohmer, M.; Mercer, A.R.; Le Conte, Y. Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci. Rep. 2015, 5, 10454. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Provost, E.; Bagnères, A.G.; Roux, M.; Clément, J.L.; Le Conte, Y. Potential mechanism for detection by Apis mellifera of the parasitic mite Varroa destructor inside sealed brood cells. Physiol. Èntomol. 2002, 27, 175–188. [Google Scholar] [CrossRef]
- Gramacho, K.P.; Spivak, M. Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav. Ecol. Sociobiol. 2003, 54, 472–479. [Google Scholar] [CrossRef]
- Light, M.; Shutler, D.; Faraone, N.; Cutler, G.C.; Hillier, N.K. Locomotion behavioural responses of Varroa destructor exposed to western honey bee (Apis mellifera) semiochemicals. J. Pest Sci. 2024, 97, 757–766. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Zhao, Z.; Yang, Y.; Yan, S. Electroantennogram reveals a strong correlation between the passion of honeybee and the properties of the volatile. Brain Behav. 2020, 10, e01603. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.L.; Trueman, J.W. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Grindrod, I.; Martin, S.J. Varroa resistance in Apis cerana: A review. Apidologie 2023, 54, 14. [Google Scholar] [CrossRef]
- Oldroyd, B.P. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends Ecol. Evol. 1999, 14, 312–315. [Google Scholar] [CrossRef]
- Warner, S.; Pokhrel, L.R.; Akula, S.M.; Ubah, C.S.; Richards, S.L.; Jensen, H.; Kearney, G.D. A scoping review on the effects of Varroa mite (Varroa destructor) on global honey bee decline. Sci. Total Environ. 2024, 906, 167492. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wu, J.; Wei, Q.; Liu, F.; Cui, L.; Rueppell, O.; Xu, S. Life-history stage determines the diet of ectoparasitic mites on their honey bee hosts. Nat. Commun. 2024, 15, 725. [Google Scholar] [CrossRef] [PubMed]
- Light, M.; Shutler, D.; Cutler, G.C.; Hillier, N.K. Varroa destructor mite electrophysiological responses to honey bee (Apis mellifera) colony volatiles. Exp. Appl. Acarol. 2020, 81, 495–514. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Mondet, F.; Hervé, M.; Mercer, A. Honey bees performing varroa sensitive hygiene remove the most mite-compromised bees from highly infested patches of brood. Apidologie 2018, 49, 335–345. [Google Scholar] [CrossRef]
- Mondet, F.; Blanchard, S.; Barthes, N.; Beslay, D.; Bordier, C.; Costagliola, G.; Hervé, M.R.; Lapeyre, B.; Kim, S.H.; Basso, B.; et al. Chemical detection triggers honey bee defense against a destructive parasitic threat. Nat. Chem. Biol. 2021, 17, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, B.; Schmitt, T. Chemical cues in disease recognition and their immunomodulatory role in insects. Curr. Opin. Insect Sci. 2022, 50, 100884. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, K.M.; Spivak, M.; Rueppell, O. Brood Affects Hygienic Behavior in the Honey Bee (Hymenoptera: Apidae). J. Econ. Èntomol. 2018, 111, 2520–2530. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Responses to Varroa by honey bees with different levels of Varroa Sensitive Hygiene. J. Apic. Res. 2009, 48, 156–161. [Google Scholar] [CrossRef]
- Le Conte, Y.; Huang, Z.Y.; Roux, M.; Zeng, Z.J.; Christidès, J.-P.; Bagnères, A.-G. Varroa destructor changes its cuticular hydrocarbons to mimic new hosts. Biol. Lett. 2015, 11, 20150233. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Salvy, M.; Provost, E.; Bagnères, A.-G.; Roux, M.; Crauser, D.; Clement, J.-L.; Le Conte, Y. Variations in chemical mimicry by the ectoparasitic mite Varroa jacobsoni according to the developmental stage of the host honey-bee Apis mellifera. Insect Biochem. Mol. Biol. 2001, 31, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Mohammedi, A.; Robinson, G.E. Primer effects of a brood pheromone on honeybee behavioural development. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Bécard, J.-M.; Costagliola, G.; de Vaublanc, G.; El Maâtaoui, M.; Crauser, D.; Plettner, E.; Slessor, K.N. Larval salivary glands are a source of primer and releaser pheromone in honey bee (Apis mellifera L.). Die Naturwissenschaften 2006, 93, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Trouiller, J.; Arnold, G.; Le Conte, Y.; Masson, C.; Chappe, B. Temporal pheromonal and kairomonal secretion in the brood of honeybees. Die Naturwissenschaften 1991, 78, 368–370. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Tang, R.; Zhang, Y.; Guo, R.; Xu, G.; Chen, D.; Huang, Z.Y.; Chen, Y.; Han, R.; et al. The Role of Honey Bee Derived Aliphatic Esters in the Host-Finding Behavior of Varroa destructor. Insects 2022, 14, 24. [Google Scholar] [CrossRef]
- Nazzi, F.; Milani, N.; Della Vedova, G. A semiochemical from larval food influences the entrance of Varroa destructor into brood cells. Apidologie 2004, 35, 403–410. [Google Scholar] [CrossRef]
- Nazzi, F.; Bortolomeazzi, R.; Della Vedova, G.; Del Piccolo, F.; Annoscia, D.; Milani, N. Octanoic acid confers to royal jelly varroa-repellent properties. Die Naturwissenschaften 2008, 96, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Garrido, C. Volatiles of the honey bee larva initiate oogenesis in the parasitic mite Varroa destructor. Chemoecology 2004, 14, 193–197. [Google Scholar] [CrossRef]
- Ziegelmann, B.; Tolasch, T.; Steidle, J.L.M.; Rosenkranz, P. The mating behavior of Varroa destructor is triggered by a female sex pheromone. Part 2: Identification and dose-dependent effects of components of the Varroa sex pheromone. Apidologie 2013, 44, 481–490. [Google Scholar] [CrossRef]
- Leal, W.S.; Parra-Pedrazzoli, A.L.; Kaissling, K.-E.; Morgan, T.I.; Zalom, F.G.; Pesak, D.J.; Dundulis, E.A.; Burks, C.S.; Higbee, B.S. Unusual pheromone chemistry in the navel orangeworm: Novel sex attractants and a behavioral antagonist. Die Naturwissenschaften 2005, 92, 139–146. [Google Scholar] [CrossRef]
- Renou, M.; Nagnan, P.; Berthier, A.; Durier, C. Identification of compounds from the eggs of Ostrinia nubilalis and Mamestra brassicae having kairomone activity on Trichogramma brassicae. Èntomol. Exp. Et Appl. 1992, 63, 291–303. [Google Scholar] [CrossRef]
- Van Oystaeyen, A.; van Zweden, J.S.; Huyghe, H.; Drijfhout, F.; Bonckaert, W.; Wenseleers, T. Chemical strategies of the beetle Metoecus paradoxus, social parasite of the wasp Vespula vulgaris. J. Chem. Ecol. 2015, 41, 1137–1147. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Huttenlocher, H.; Ayasse, M. Myrmecochorous plants use chemical mimicry to cheat seed-dispersing ants. Funct. Ecol. 2010, 24, 545–555. [Google Scholar] [CrossRef]
- Kather, R.; Drijfhout, F.P.; Shemilt, S.; Martin, S.J. Evidence for Passive Chemical Camouflage in the Parasitic Mite Varroa destructor. J. Chem. Ecol. 2015, 41, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Ma, C.; Zhang, Y.; Chen, H.-S.; Guo, J.-Y.; Liu, T.-H.; Zhou, Z.-S. Characterization and Functional Analysis of OcomOBP7 in Ophraella communa Lesage. Insects 2023, 14, 190. [Google Scholar] [CrossRef]
- Weeks, E.N.I.; Logan, J.G.; Birkett, M.A.; Caulfield, J.C.; Gezan, S.A.; Welham, S.J.; Brugman, V.A.; Pickett, J.A.; Cameron, M.M. Electrophysiologically and behaviourally active semiochemicals identified from bed bug refuge substrate. Sci. Rep. 2020, 10, 4590. [Google Scholar] [CrossRef] [PubMed]
- Liendo, M.C.; Muntaabski, I.; Russo, R.M.; Lanzavecchia, S.B.; Segura, D.F.; Palacio, M.A.; Cladera, J.L.; Fernández, P.C.; Scannapieco, A.C. Temporal changes in volatile profiles of Varroa destructor-infested brood may trigger hygienic behavior in Apis mellifera. Èntomol. Exp. Et Appl. 2021, 169, 563–574. [Google Scholar] [CrossRef]
- Wagoner, K.M.; Millar, J.G.; Schal, C.; Rueppell, O. Cuticular pheromones stimulate hygienic behavior in the honey bee (Apis mellifera). Sci. Rep. 2020, 10, 7132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wen, P.; Qu, Y.; Dong, S.; Li, J.; Tan, K.; Nieh, J.C. Bees eavesdrop upon informative and persistent signal compounds in alarm pheromones. Sci. Rep. 2016, 6, 25693. [Google Scholar] [CrossRef]
- Phokasem, P.; de Guzman, L.I.; Khongphinitbunjong, K.; Frake, A.M.; Chantawannakul, P. Feeding by Tropilaelaps mercedesae on pre- and post-capped brood increases damage to Apis mellifera colonies. Sci. Rep. 2019, 9, 13044. [Google Scholar] [CrossRef]
- Wang, J.; Murphy, E.J.; Nix, J.C.; Jones, D.N.M. Aedes aegypti Odorant Binding Protein 22 selectively binds fatty acids through a conformational change in its C-terminal tail. Sci. Rep. 2020, 10, 3300. [Google Scholar] [CrossRef]
- Paula, D.P.; Togawa, R.C.; do Carmo Costa, M.M.; Grynberg, P.; Martins, N.F.; Andow, D.A. Systemic and sex-biased regulation of OBP expression under semiochemical stimuli. Sci. Rep. 2018, 8, 6035. [Google Scholar] [CrossRef]
- Ma, S.; Li, L.-L.; Yao, W.-C.; Yin, M.-Z.; Li, J.-Q.; Xu, J.-W.; Dewer, Y.; Zhu, X.-Y.; Zhang, Y.-N. Two Odorant-Binding Proteins Involved in the Recognition of Sex Pheromones in Spodoptera litura Larvae. J. Agric. Food Chem. 2022, 70, 12372–12382. [Google Scholar] [CrossRef]

| Gene Names | GenBank Accession Number | Primer Sequence (5′–3′) |
|---|---|---|
| AmelCSP1 | NM_001077820 | F: CGTGGACATATGCTGAGGAACTT R: TCTGCTAATTTGTCCAAGTTTTGT |
| AmelOBP8 | NM_001171044 | F: TTGCAGCAAGAAGAACGACACC R: CAACCTCGCATCCCTCCGTAG |
| AcerCSP1 | FJ157352 | F: ACGTGGACATATGCTGAGGAA R: GGAGTTAAACAAGGGCCTGC |
| AcerOBP21 | KP717063 | F: TGCGTTGGTGCATTGACACT R: AGACGAATGTTGGCATCAGTGA |
| Amelβ-actin | NM_001185145 | F: CCGTGATTTGACTGACTACCT R: AGTTGCCATTTCCTGTTC |
| Acerβ-actin | HM640276.1 | F: TTATATGCCAACACTGTCCTTT R: AGAATTGATCCACCAATCCA |
| # | Molecular Formula | VOC Name | CAS # | Retention Time (min) | Relative Standard Deviation (%) | Relative Content (%) | Retention Index |
|---|---|---|---|---|---|---|---|
| 1 | C6H14O2 | Acetaldehyde diethyl acetal | 105-57-7 | 3.961 | 0.710 | 4.50 ± 0.90 | 705 |
| 2 | C5H12O | Isoamyl alcohol | 123-51-3 | 4.052 | 0.711 | 14.18 ± 5.13 | 697 |
| 3 | C7H14O2 | Ethyl 2-methyl butyrate | 7452-79-1 | 8.967 | 0.242 | 1.03 ± 0.16 | 820 |
| 4 | C10H16 | DL-Limonene | 138-86-3 | 17.056 | 0.030 | 1.98 ± 1.36 | 1018 |
| 5 | C9H18O | Nonanal | 124-19-6 | 19.849 | 0.012 | 13.52 ± 2.79 | 1104 |
| 6 | C10H20O2 | Ethyl octanoate | 106-32-1 | 22.862 | 0.003 | 1.99 ± 0.10 | 1183 |
| 7 | C10H12O | Estragole | 140-67-0 | 22.937 | 0.026 | 6.87 ± 2.13 | 1172 |
| 8 | C15H32 | 2,6,11-Trimethyldodecane | 3891-98-3 | 25.345 | 0.014 | 2.77 ± 0.96 | 1320 |
| 9 | C14H28O2 | Ethyl laurate | 106-33-2 | 38.306 | 0.030 | 2.76 ± 0.22 | 1580 |
| 10 | C15H26O | α-Cedrol | 77-53-2 | 38.430 | 0.005 | 5.83 ± 1.29 | 1543 |
| 11 | C15H26O | β-Cineole | 470-82-6 | 39.150 | 0.000 | 1.55 ± 0.10 | 1593 |
| 12 | C16H32O2 | Ethyl myristate | 124-06-1 | 40.627 | 0.030 | 4.49 ± 0.71 | 1779 |
| 13 | C18H34O2 | Ethyl 9-hexadecenoate | 54546-22-4 | 42.087 | 0.021 | 1.17 ± 0.37 | 1986 |
| 14 | C18H36O2 | Ethyl palmitate | 628-97-7 | 42.210 | 0.015 | 29.72 ± 9.89 | 1978 |
| 15 | C20H38O2 | Ethyl oleate | 111-62-6 | 43.507 | 0.013 | 7.63 ± 1.25 | 2185 |
| Protein Name | Affinity (kcal/mol) | Protein Name | Affinity (kcal/mol) |
|---|---|---|---|
| AmelCSP 1 | −7.8 | AcerCSP 1 | −8.1 |
| AmelCSP 3 | −6.2 | AcerCSP 3 | −6.5 |
| AmelOBP 1 | −5.8 | AcerOBP 1 | −5.8 |
| AmelOBP 2 | −4.7 | AcerOBP 2 | −4.7 |
| AmelOBP 4 | −5.2 | AcerOBP 4 | −6.1 |
| AmelOBP 6 | −6.7 | AcerOBP 5 | −5.2 |
| AmelOBP 8 | −7.2 | AcerOBP 14 | −4.0 |
| AmelOBP 11 | −5.7 | AcerOBP 15 | −5.8 |
| AmelOBP 16 | −6.0 | AcerOBP 19 | −4.2 |
| AmelOBP 19 | −4.8 | AcerOBP 21 | −7.4 |
| AmelOBP 21 | −4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Wang, X.; Mustafa, A.; Wang, Y.; Wang, H.; Chi, X.; Xu, B.; Liu, Z. Varroa Volatiles Offer Chemical Cues to Honey Bees for Initial Parasitic Recognition. Biomolecules 2025, 15, 66. https://doi.org/10.3390/biom15010066
Zhao Q, Wang X, Mustafa A, Wang Y, Wang H, Chi X, Xu B, Liu Z. Varroa Volatiles Offer Chemical Cues to Honey Bees for Initial Parasitic Recognition. Biomolecules. 2025; 15(1):66. https://doi.org/10.3390/biom15010066
Chicago/Turabian StyleZhao, Qinglong, Xinning Wang, Ahsan Mustafa, Ying Wang, Hongfang Wang, Xuepeng Chi, Baohua Xu, and Zhenguo Liu. 2025. "Varroa Volatiles Offer Chemical Cues to Honey Bees for Initial Parasitic Recognition" Biomolecules 15, no. 1: 66. https://doi.org/10.3390/biom15010066
APA StyleZhao, Q., Wang, X., Mustafa, A., Wang, Y., Wang, H., Chi, X., Xu, B., & Liu, Z. (2025). Varroa Volatiles Offer Chemical Cues to Honey Bees for Initial Parasitic Recognition. Biomolecules, 15(1), 66. https://doi.org/10.3390/biom15010066








