PAX6 Downregulation Triggers HIF-1α-Mediated Ferroptosis in Glioma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Reagents
2.2. Cell Culture and Processing
2.3. Immunohistochemical Analysis of Tissue Microarrays
2.4. Establishment of Stable Cell Lines
2.5. Quantitative Real-Time PCR (qPCR)
2.6. Western Blot
2.7. CCK-8 Assay
2.8. Lipid Peroxide (LPO) Detection
2.9. Reactive Oxygen Species (ROS) Detection
2.10. Glutathione (GSH) Assay
2.11. mRNA High-Throughput Sequencing
2.12. Linear Discriminant Analysis, Identification of Differentially Expressed Genes (DEGs), and Correlation Analysis
2.13. In Vivo Tumor Formation in Nude Mice
2.14. Immunohistochemical Staining
2.15. Statistical Methods
3. Results
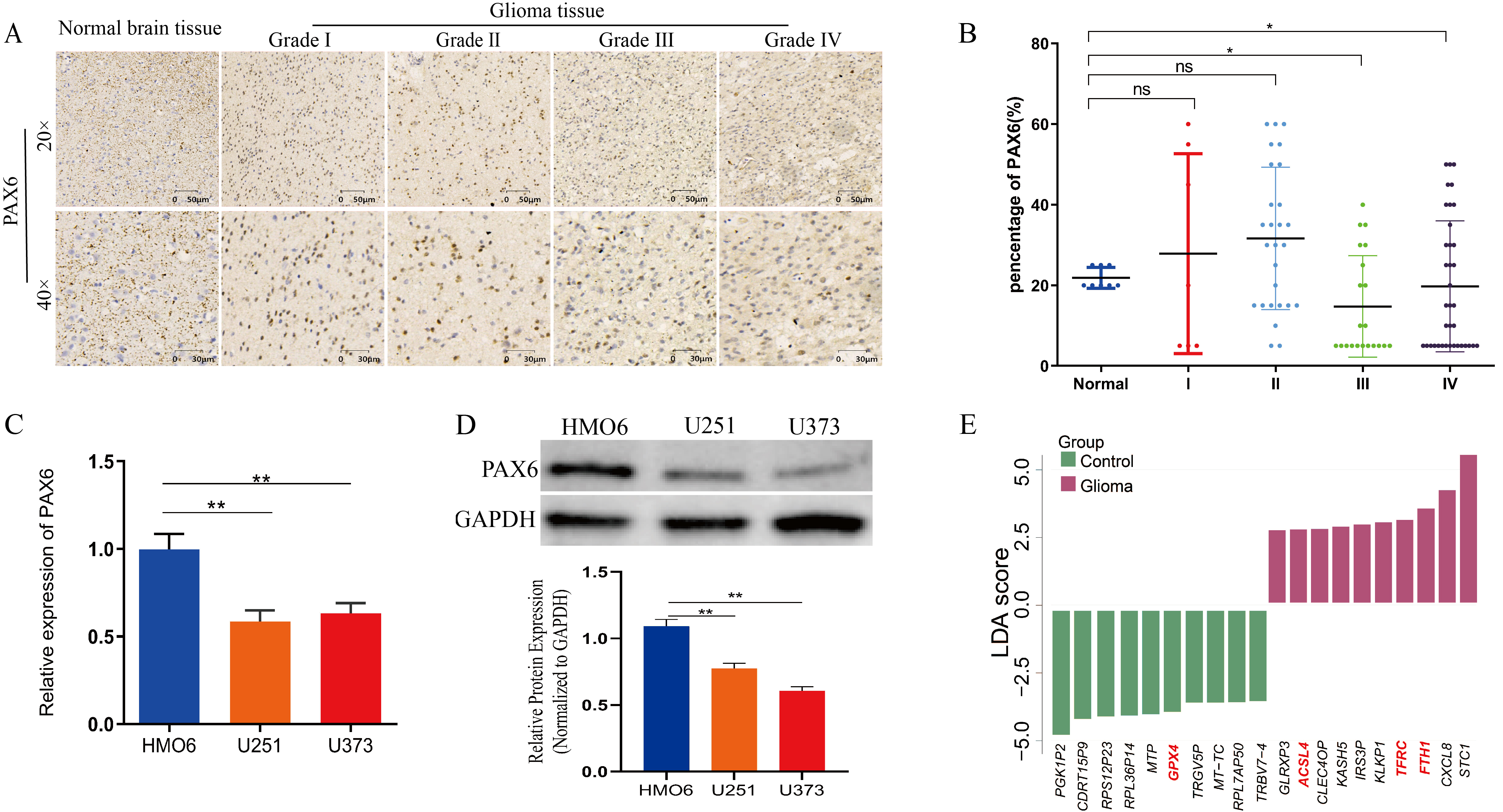
3.1. Decreased PAX6 Expression in Gliomas Exhibiting Aberrant Iron Metabolism
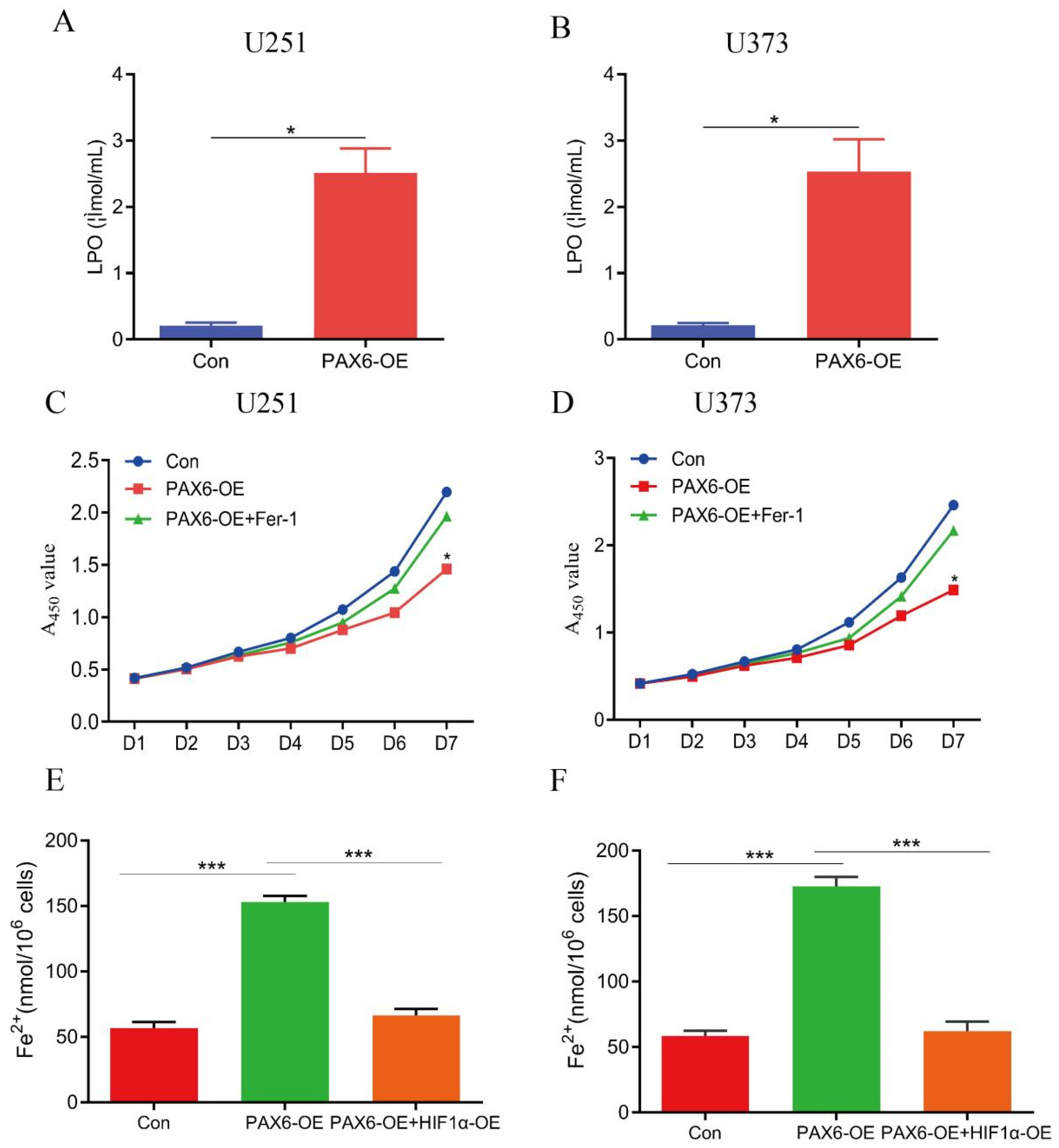
3.2. Overexpression of PAX6 Induces Ferroptosis in Glioma Cells
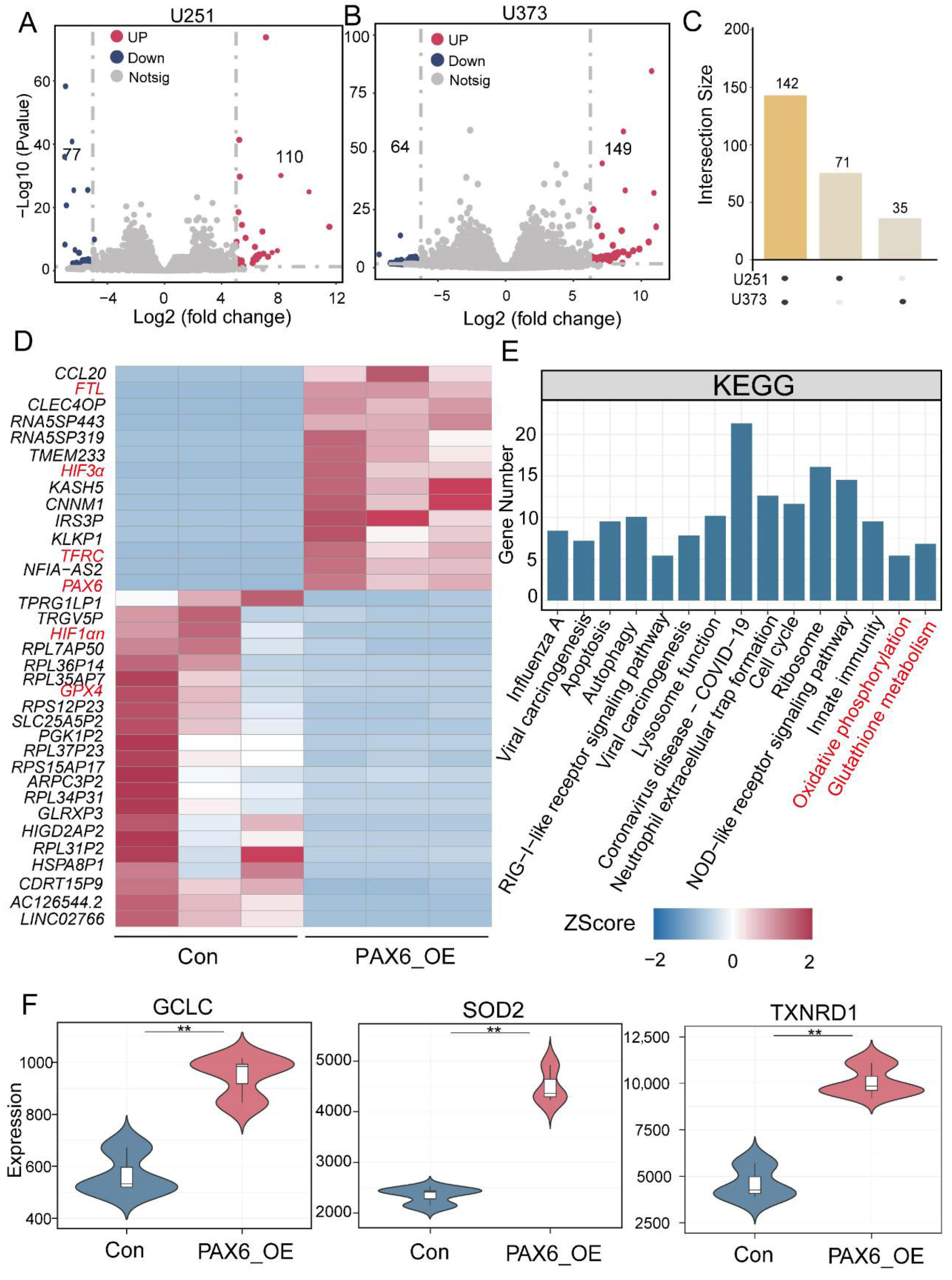
3.3. PAX6 Overexpression Alters Expression of Oxidative Stress and Iron Metabolism Genes in Glioma Cells
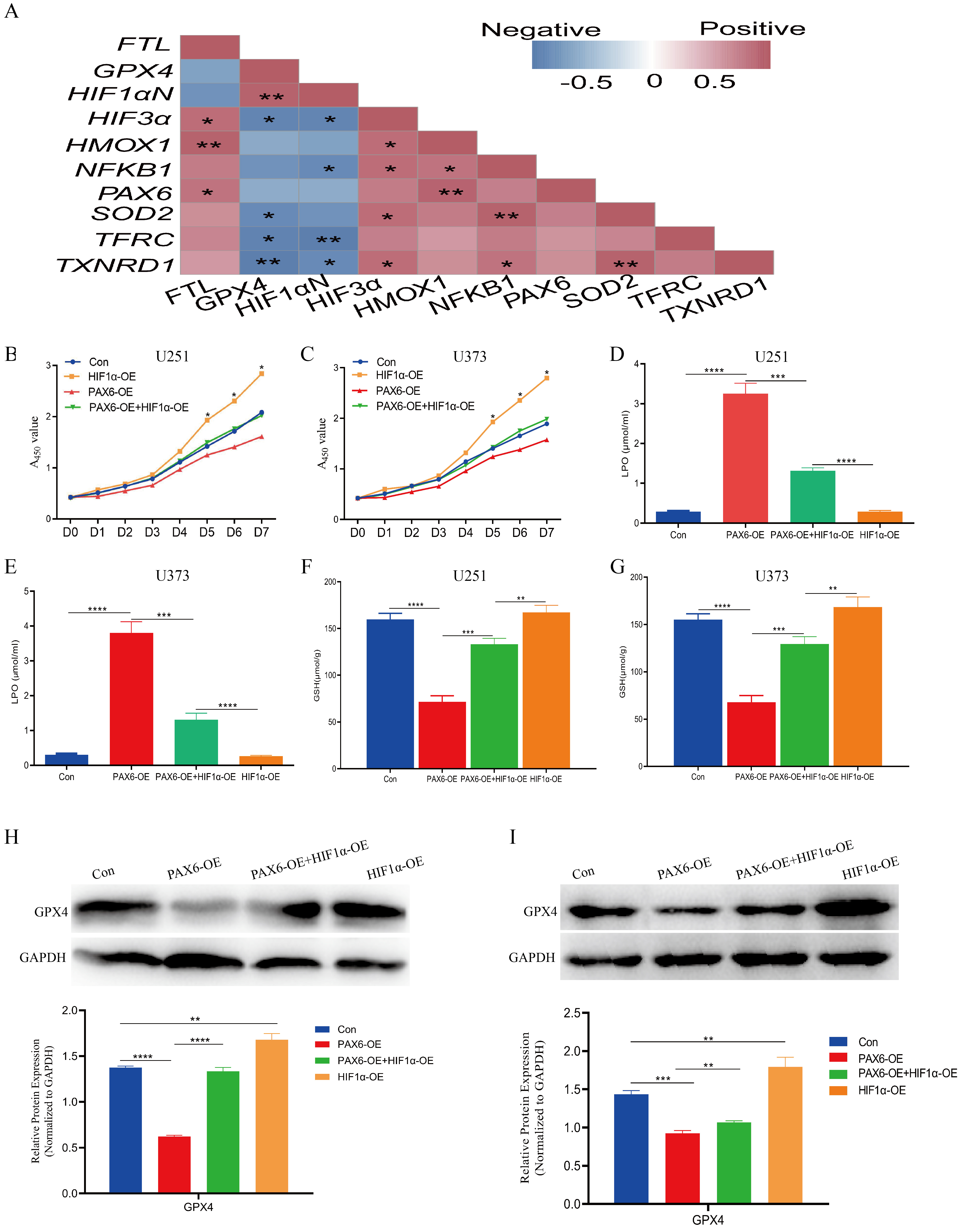
3.4. PAX6 Suppresses HIF-1α Expression via ROS Generation in Glioma Cells
3.5. PAX6 Induces Ferroptosis in Glioma Cells by Inhibiting HIF-1α
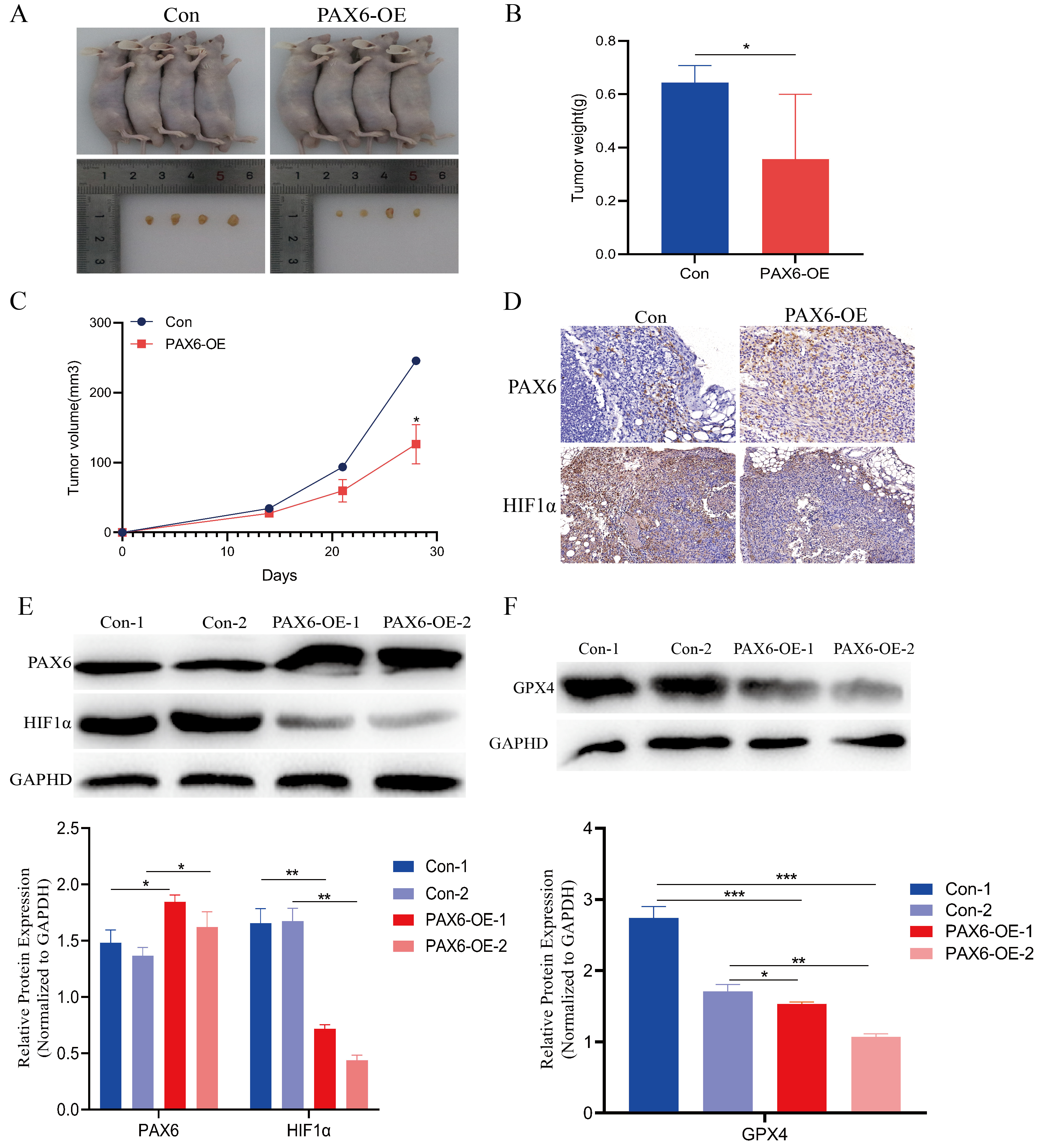
3.6. In Vivo Validation: PAX6 Overexpression Inhibits Glioma Growth by Promoting Ferroptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shergalis, A.; Bankhead, A., 3rd; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Nazir, S.; Hanif, R.; Javed, A. Glioblastoma Multiforme: Probing Solutions to Systemic Toxicity towards High-Dose Chemotherapy and Inflammatory Influence in Resistance against Temozolomide. Pharmaceutics 2023, 15, 687. [Google Scholar] [CrossRef] [PubMed]
- Durant, S.T.; Zheng, L.; Wang, Y.; Chen, K.; Zhang, L.; Zhang, T.; Yang, Z.; Riches, L.; Trinidad, A.G.; Fok, J.H.L.; et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci. Adv. 2018, 4, eaat1719. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, D.G.; Hu, Y.H. The Regulation of Microglial Cell Polarization in the Tumor Microenvironment: A New Potential Strategy for Auxiliary Treatment of Glioma-A Review. Cell. Mol. Neurobiol. 2023, 43, 193–204. [Google Scholar] [CrossRef]
- Luo, F.; Liao, Y.; Cao, E.; Yang, Y.; Tang, K.; Zhou, D.; Zhou, D.; Cai, H. Hypermethylation of HIC2 is a potential prognostic biomarker and tumor suppressor of glioma based on bioinformatics analysis and experiments. CNS Neurosci. Ther. 2023, 29, 1154–1167. [Google Scholar] [CrossRef]
- Zhao, L.; Song, C.; Li, Y.; Yuan, F.; Zhao, Q.; Dong, H.; Liu, B. BZW1 as an oncogene is associated with patient prognosis and the immune microenvironment in glioma. Genomics 2023, 115, 110602. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Wei, L.; Ran, J.; Wang, K.; Zhu, S.; Liu, Q. p53 inhibits the Urea cycle and represses polyamine biosynthesis in glioma cell lines. Metab. Brain Dis. 2023, 38, 1143–1153. [Google Scholar] [CrossRef]
- Nakayama, T.; Fisher, M.; Nakajima, K.; Odeleye, A.O.; Zimmerman, K.B.; Fish, M.B.; Yaoita, Y.; Chojnowski, J.L.; Lauderdale, J.D.; Netland, P.A.; et al. Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients. Dev. Biol. 2015, 408, 328–344. [Google Scholar] [CrossRef]
- Yeh, C.H.; Chen, R.Y.; Wu, T.H.; Chang, S.Y.; Hsieh, T.Y.; Shih, Y.L.; Lin, Y.W. Promoter hypermethylation-mediated downregulation of PAX6 promotes tumor growth and metastasis during the progression of liver cancer. Clin. Epigenetics 2024, 16, 174. [Google Scholar] [CrossRef]
- Wu, D.M.; Zhang, T.; Liu, Y.B.; Deng, S.H.; Han, R.; Liu, T.; Li, J.; Xu, Y. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death Dis. 2019, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, Y.H.; Sun, J.; Deng, F.M.; Liu, Y.L. PAX6 contributes to the activation and proliferation of hepatic stellate cells via activating Hedgehog/GLI1 pathway. Biochem. Biophys. Res. Commun. 2020, 526, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, Q.; Wang, Y.A.; Hua, S.; Sauvé, C.G.; Ong, D.; Lan, Z.D.; Chang, Q.; Ho, Y.W.; Monasterio, M.M.; et al. Epigenetic Activation of WNT5A Drives Glioblastoma Stem Cell Differentiation and Invasive Growth. Cell 2016, 167, 1281–1295.e18. [Google Scholar] [CrossRef] [PubMed]
- Appolloni, I.; Calzolari, F.; Barilari, M.; Terrile, M.; Daga, A.; Malatesta, P. Antagonistic modulation of gliomagenesis by Pax6 and Olig2 in PDGF-induced oligodendroglioma. Int. J. Cancer 2012, 131, E1078–E1087. [Google Scholar] [CrossRef]
- Farheen, S.; Ahmed, S.P.; Mariyath, P.M.M.; Kausar, T.; Hoda, M.F.; Arif, S.H.; Nayeem, S.M.; Ali, A.; Chosdol, K.; Shahi, M.H. Differential role of Pax6 and its interaction with Shh-Gli1-IDH2 axis in regulation of glioma growth and chemoresistance. J. Biochem. Mol. Toxicol. 2023, 37, e23241. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Hu, Y.; Mayes, D.; Siegel, E.; Kim, J.G.; Mathews, M.S.; Hsu, N.; Eskander, D.; Yu, O.; Tromberg, B.J.; et al. PAX6 suppression of glioma angiogenesis and the expression of vascular endothelial growth factor A. J. Neuro-Oncol. 2010, 96, 191–200. [Google Scholar] [CrossRef]
- Pavlakis, E.; Tonchev, A.B.; Kaprelyan, A.; Enchev, Y.; Stoykova, A. Interaction between transcription factors PAX6/PAX6-5a and specific members of miR-183-96-182 cluster, may contribute to glioma progression in glioblastoma cell lines. Oncol. Rep. 2017, 37, 1579–1592. [Google Scholar] [CrossRef]
- Huang, B.S.; Luo, Q.Z.; Han, Y.; Li, X.B.; Cao, L.J.; Wu, L.X. microRNA-223 promotes the growth and invasion of glioblastoma cells by targeting tumor suppressor PAX6. Oncol. Rep. 2013, 30, 2263–2269. [Google Scholar] [CrossRef]
- Huang, B.S.; Luo, Q.Z.; Han, Y.; Huang, D.; Tang, Q.P.; Wu, L.X. MiR-223/PAX6 Axis Regulates Glioblastoma Stem Cell Proliferation and the Chemo Resistance to TMZ via Regulating PI3K/Akt Pathway. J. Cell. Biochem. 2017, 118, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Song, J.; Gao, Y.; Huang, S.; Dou, R.; Zhong, P.; Huang, G.; Han, L.; Zheng, J.; Zhang, X.; et al. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022, 52, 102312. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhan, R.; Zhao, G.M.; Qian, Z.H.; Gong, C.C.; Li, Y.Q. Expression of CDK13 Was Associated with Prognosis and Expression of HIF-1α and beclin1 in Breast Cancer Patients. J. Investig. Surg. 2022, 35, 442–447. [Google Scholar] [CrossRef]
- Chen, H.; Nio, K.; Tang, H.; Yamashita, T.; Okada, H.; Li, Y.; Doan, P.T.B.; Li, R.; Lv, J.; Sakai, Y.; et al. BMP9-ID1 Signaling Activates HIF-1α and VEGFA Expression to Promote Tumor Angiogenesis in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 1475. [Google Scholar] [CrossRef]
- Zhou, Z.; Mu, D.; Zhang, D.; Zhang, X.; Ding, X.; Yang, J.; Bai, X.; Hu, M. PD-L1 in Combination with CD8(+)TIL and HIF-1α are Promising Prognosis Predictors of Head and Neck Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 13233–13239. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Guo, C.; Gao, T.; Ma, D.; Su, X.; Pang, Q.; Zhang, R. Hypoxia Enhances Glioma Resistance to Sulfasalazine-Induced Ferroptosis by Upregulating SLC7A11 via PI3K/AKT/HIF-1α Axis. Oxidative Med. Cell. Longev. 2022, 2022, 7862430. [Google Scholar] [CrossRef]
- Huang, W.; Ding, X.; Ye, H.; Wang, J.; Shao, J.; Huang, T. Hypoxia enhances the migration and invasion of human glioblastoma U87 cells through PI3K/Akt/mTOR/HIF-1α pathway. Neuroreport 2018, 29, 1578–1585. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Wang, M.; Luo, W.; Xue, Y. GDNF regulates lipid metabolism and glioma growth through RET/ERK/HIF—1/SREBP—1. Int. J. Oncol. 2022, 61, 109. [Google Scholar] [CrossRef]
- Nguyen, A.; Moussallieh, F.M.; Mackay, A.; Cicek, A.E.; Coca, A.; Chenard, M.P.; Weingertner, N.; Lhermitte, B.; Letouzé, E.; Guérin, E.; et al. Characterization of the transcriptional and metabolic responses of pediatric high grade gliomas to mTOR-HIF-1α axis inhibition. Oncotarget 2017, 8, 71597–71617. [Google Scholar] [CrossRef]
- Almiron Bonnin, D.A.; Havrda, M.C.; Lee, M.C.; Liu, H.; Zhang, Z.; Nguyen, L.N.; Harrington, L.X.; Hassanpour, S.; Cheng, C.; Israel, M.A. Secretion-mediated STAT3 activation promotes self-renewal of glioma stem-like cells during hypoxia. Oncogene 2018, 37, 1107–1118. [Google Scholar] [CrossRef]
- Li, J.; Shen, J.; Wang, Z.; Xu, H.; Wang, Q.; Chai, S.; Fu, P.; Huang, T.; Anas, O.; Zhao, H.; et al. ELTD1 facilitates glioma proliferation, migration and invasion by activating JAK/STAT3/HIF-1α signaling axis. Sci. Rep. 2019, 9, 13904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Doughman, Y.Q.; Gu, S.; Jarrell, A.; Aota, S.; Cvekl, A.; Watanabe, M.; Dunwoodie, S.L.; Johnson, R.S.; van Heyningen, V.; et al. Cited2 is required for the proper formation of the hyaloid vasculature and for lens morphogenesis. Development 2008, 135, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, M.; Simko, V.; Takacova, M.; Barathova, M.; Bartosova, M.; Hunakova, L.; Sedlakova, O.; Hudecova, S.; Krizanova, O.; Dequiedt, F.; et al. Sulforaphane reduces molecular response to hypoxia in ovarian tumor cells independently of their resistance to chemotherapy. Int. J. Oncol. 2015, 47, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Mo, J.; Zhang, J.; Chen, Y. HIF—1α inhibits ferroptosis and promotes malignant progression in non—Small cell lung cancer by activating the Hippo—YAP signalling pathway. Oncol. Lett. 2023, 25, 90. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, H.; Zheng, J.; Yang, K. The PER1/HIF-1alpha negative feedback loop promotes ferroptosis and inhibits tumor progression in oral squamous cell carcinoma. Transl. Oncol. 2022, 18, 101360. [Google Scholar] [CrossRef] [PubMed]
- Mayes, D.A.; Hu, Y.; Teng, Y.; Siegel, E.; Wu, X.; Panda, K.; Tan, F.; Yung, W.K.; Zhou, Y.H. PAX6 suppresses the invasiveness of glioblastoma cells and the expression of the matrix metalloproteinase-2 gene. Cancer Res. 2006, 66, 9809–9817. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Wu, X.; Tan, F.; Shi, Y.X.; Glass, T.; Liu, T.J.; Wathen, K.; Hess, K.R.; Gumin, J.; Lang, F.; et al. PAX6 suppresses growth of human glioblastoma cells. J. Neuro-Oncol. 2005, 71, 223–229. [Google Scholar] [CrossRef]
- Jiang, Q.; Xing, W.; Cheng, J.; Yu, Y. Knockdown of lncRNA XIST Suppresses Cell Tumorigenicity in Human Non-Small Cell Lung Cancer by Regulating miR-142-5p/PAX6 Axis. OncoTargets Ther. 2020, 13, 4919–4929. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, Q.; Hu, Y.; Zhang, T.; Li, J.; Liu, Z.; Zheng, H.; Gao, Y.; Jia, W.; Hu, A.; et al. Investigating the mechanism by which SMAD3 induces PAX6 transcription to promote the development of non-small cell lung cancer. Respir. Res. 2018, 19, 262. [Google Scholar] [CrossRef] [PubMed]
- P, M.M.; Farheen, S.; Sharma, R.M.; Shahi, M.H. Differential regulation of Shh-Gli1 cell signalling pathway on homeodomain transcription factors Nkx2.2 and Pax6 during the medulloblastoma genesis. Mol. Biol. Rep. 2024, 51, 1096. [Google Scholar] [CrossRef]
- Shyr, C.R.; Tsai, M.Y.; Yeh, S.; Kang, H.Y.; Chang, Y.C.; Wong, P.L.; Huang, C.C.; Huang, K.E.; Chang, C. Tumor suppressor PAX6 functions as androgen receptor co-repressor to inhibit prostate cancer growth. Prostate 2010, 70, 190–199. [Google Scholar] [CrossRef]
- Chang, J.Y.; Hu, Y.; Siegel, E.; Stanley, L.; Zhou, Y.H. PAX6 increases glioma cell susceptibility to detachment and oxidative stress. J. Neuro-Oncol. 2007, 84, 9–19. [Google Scholar] [CrossRef]
- Hegge, B.; Sjøttem, E.; Mikkola, I. Generation of a PAX6 knockout glioblastoma cell line with changes in cell cycle distribution and sensitivity to oxidative stress. BMC Cancer 2018, 18, 496. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Luo, B.; Song, L.; Chen, L.; Cai, Y.; Zhang, M.; Wang, S. Ganoderic acid D attenuates gemcitabine resistance of triple-negative breast cancer cells by inhibiting glycolysis via HIF-1α destabilization. Phytomedicine 2024, 129, 155675. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, X.; Han, J. KIF20A Promotes CRC Progression and the Warburg Effect through the C-Myc/HIF-1α Axis. Protein Pept. Lett. 2024, 31, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, B.; Liu, Y.; Deng, W.; Zhang, Y. Angiogenesis in residual cancer and roles of HIF-1α, VEGF, and MMP-9 in the development of residual cancer after radiofrequency ablation and surgical resection in rabbits with liver cancer. Folia Morphol. 2020, 79, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chan, Y.C.; Chang, W.M.; Lin, Y.F.; Yang, C.J.; Su, C.Y.; Huang, M.S.; Wu, A.T.H.; Hsiao, M. Feedback regulation of ALDOA activates the HIF-1α/MMP9 axis to promote lung cancer progression. Cancer Lett. 2017, 403, 28–36. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, D.; Yang, J.; Li, M.; Ling, C.; Lv, D.; Cao, Y.; Chen, Z.; Shi, C.; Shen, H.; et al. Iron deficiency in hepatocellular carcinoma cells induced sorafenib resistance by upregulating HIF-1α to inhibit apoptosis. Biomed. Pharmacother. 2023, 163, 114750. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequences (5′-3′) |
|---|---|
| PAX6-F | CCGTGTGCCTCAACCGTA |
| PAX6-R | CACGGTTTACTGGGTCTGG |
| HIF-1α-F | TCGGCGAAGTAAAGAATC |
| HIF-1α-R | TTCCTCACACGCAAATAG |
| GAPDH-F | CACCAGGGCTGCTTTTAACTC |
| GAPDH-R | GAAGATGGTGATGGGATTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Fu, L.; Zhang, J.; Zhang, S.; Wu, L.; Zhu, Q.; Huang, B. PAX6 Downregulation Triggers HIF-1α-Mediated Ferroptosis in Glioma Cells. Biomolecules 2025, 15, 1462. https://doi.org/10.3390/biom15101462
Luo Q, Fu L, Zhang J, Zhang S, Wu L, Zhu Q, Huang B. PAX6 Downregulation Triggers HIF-1α-Mediated Ferroptosis in Glioma Cells. Biomolecules. 2025; 15(10):1462. https://doi.org/10.3390/biom15101462
Chicago/Turabian StyleLuo, Qizhi, Li Fu, Jie Zhang, Shashuang Zhang, Lixiang Wu, Quan Zhu, and Baisheng Huang. 2025. "PAX6 Downregulation Triggers HIF-1α-Mediated Ferroptosis in Glioma Cells" Biomolecules 15, no. 10: 1462. https://doi.org/10.3390/biom15101462
APA StyleLuo, Q., Fu, L., Zhang, J., Zhang, S., Wu, L., Zhu, Q., & Huang, B. (2025). PAX6 Downregulation Triggers HIF-1α-Mediated Ferroptosis in Glioma Cells. Biomolecules, 15(10), 1462. https://doi.org/10.3390/biom15101462





