State of the Art in the Standardization of Stromal Vascular Fraction Processing
Abstract
:1. Introduction
2. Methods
2.1. Research Strategy
2.2. Selection Criteria
3. Tissue Harvesting and Transport
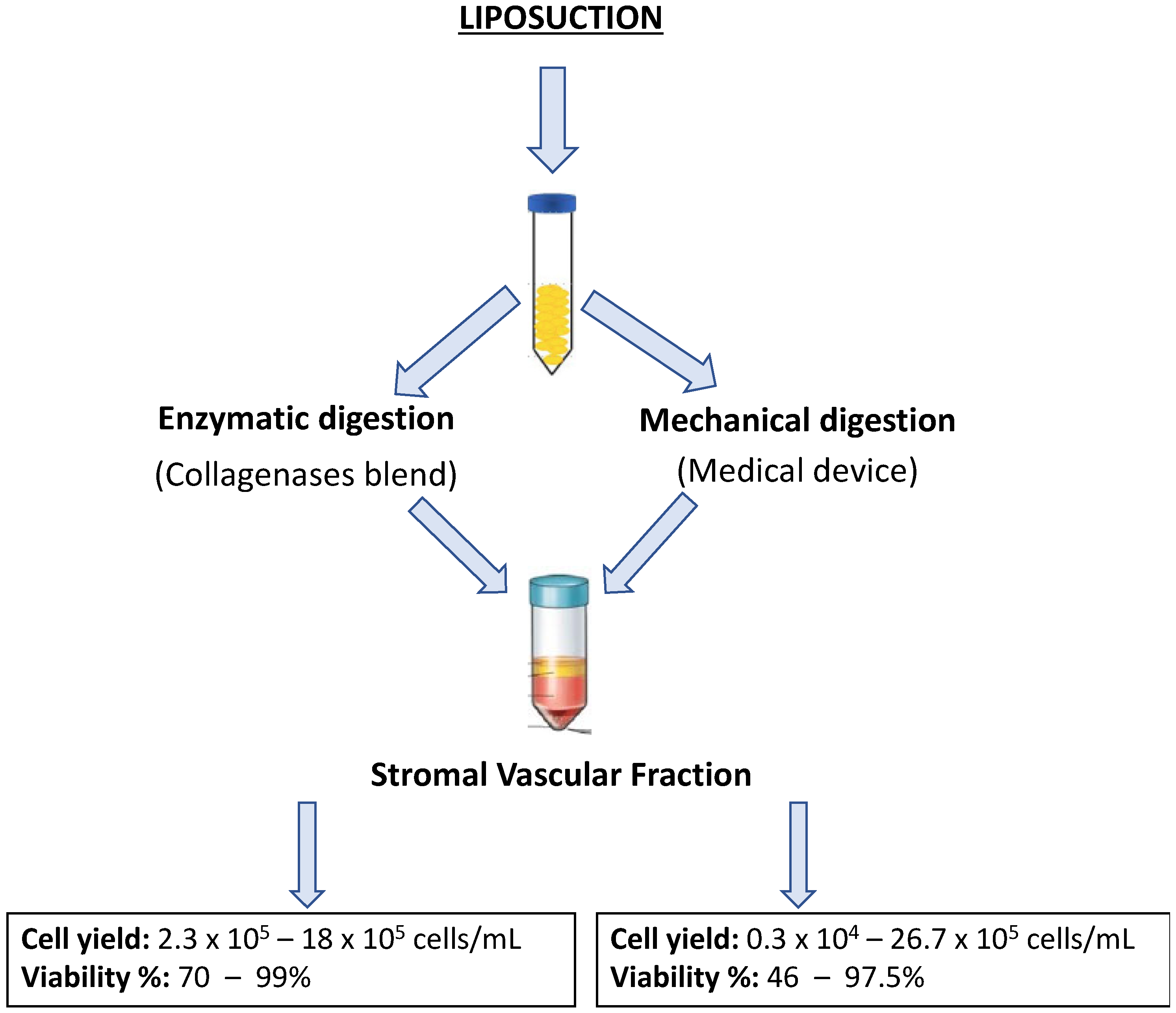
4. SVF Isolation
5. SVF Cryopreservation
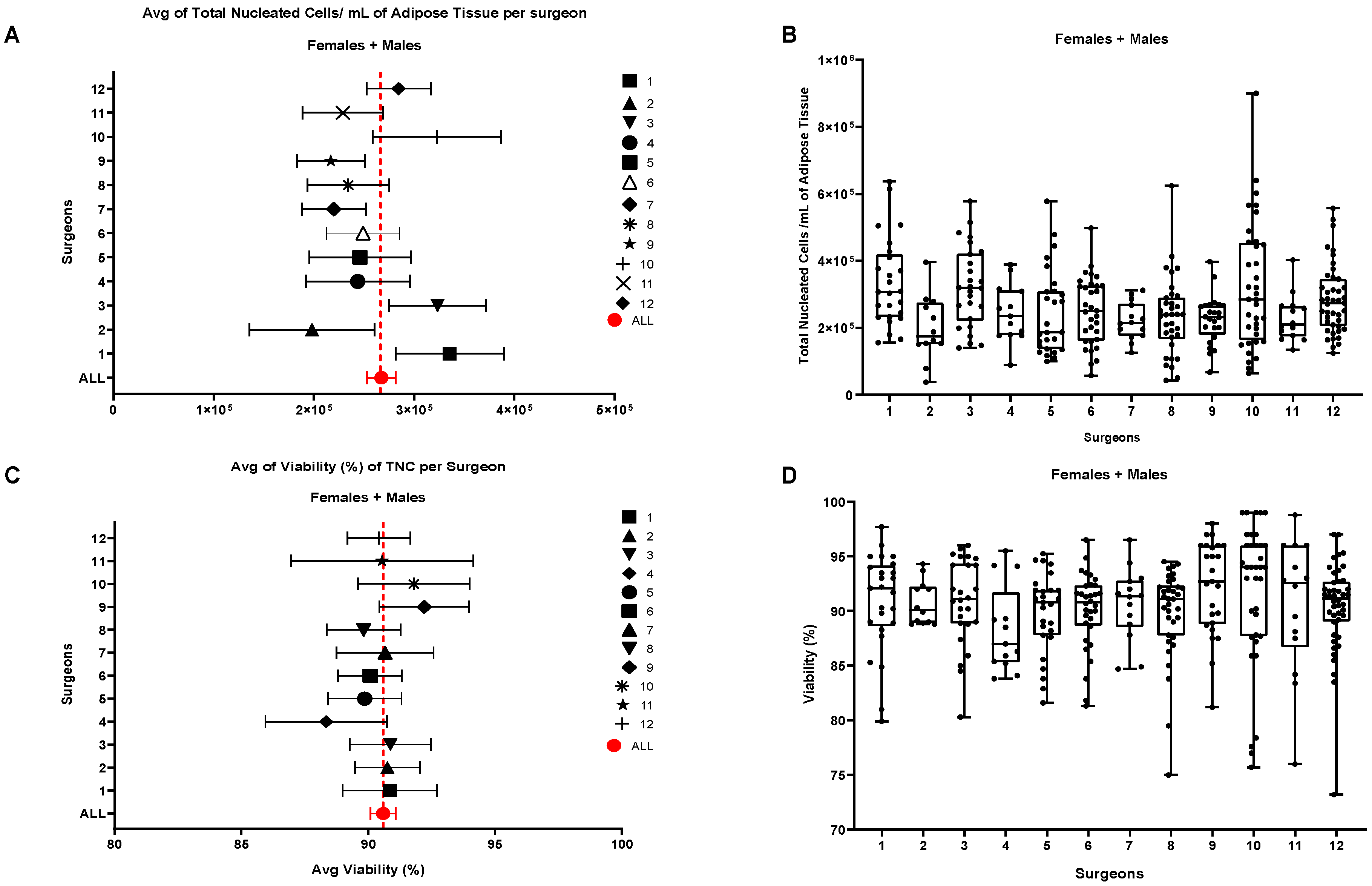
6. Quality Controls: A Brief Introduction
6.1. Microbiological Testing and Environmental Control
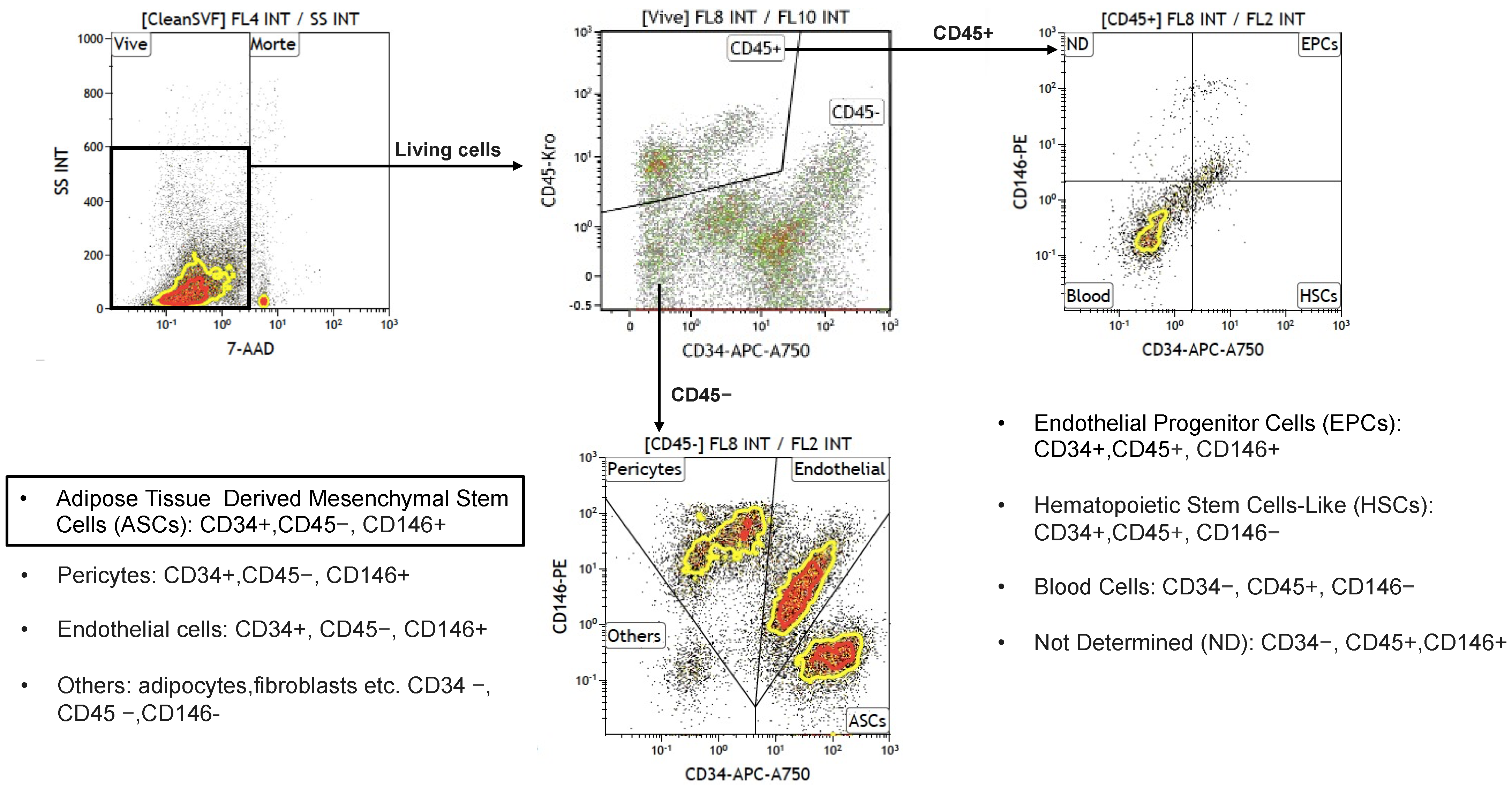
6.2. Flow Cytometry Analysis
6.3. Additional QC Tests
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCs | Adipose Stem Cells |
| AT | Adipose Tissue |
| ATMP | Advanced Therapy Medicinal Product |
| BSA | Bovine Serum Albumin |
| CD | Cluster of Differentiation |
| CFU-F | Colony Forming Unit-Fibroblast |
| CPA | Cryopreservation Agents |
| DMSO | Di-Methyl-Sulf-Oxide |
| EPCs | Endothelial Progenitor Cells |
| GMP | Good Manufacturing Practice |
| HSA | Human Serum Albumin |
| HSCs | Hematopoietic Stem Cells-Like |
| IFATS | International Federation for Adipose Therapeutics and Science |
| ISCT | International Society of Cell & Gene Therapy |
| LAL1 | Laser Assisted Liposuction |
| LAL2 | Limulus Amebocyte Lysate |
| MSC | Mesenchymal Stem Cell |
| Nano-GO | Nano Graphene Oxide |
| NC | Nucleocounter |
| ND | Not determined |
| NGS | Next Generation Sequencing |
| OOS | Out Of Specification |
| QC | Quality Control |
| PAL | Power Assisted Liposuction |
| PBS | Phosphate Buffered Saline |
| rFC | recombinant Factor C |
| RT | Room Temperature |
| SSCF | Swiss Stem Cell Foundation |
| SVF | Stromal Vascular Fraction |
| tSVF | tissue Stromal Vascular Fraction |
| TNC | Total nucleated cells |
| UAL | Ultrasound Assisted Liposuction |
| VNC | Viable Number of Cells |
| WAL | Water Assisted Liposuction |
| 7-AAD | 7-Amino-Actinomycin D |
References
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.I.N.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Rodbell, M. Metabolism of Isolated Fat Cells. J. Biol. Chem. 1964, 239, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, E.N.; Koval, O.A.; Igorevich, E.I.; Encarnacion Ramirez, M.D.J.; Nurmukhametov, R.; Valentinovich, K.K.; Montemurro, N. Analyzing the Clinical Potential of Stromal Vascular Fraction: A Comprehensive Literature Review. Medicina 2024, 60, 221. [Google Scholar] [CrossRef]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- Rodriguez, J.; Pratta, A.S.; Abbassi, N.; Fabre, H.; Rodriguez, F.; Debard, C.; Adobati, J.; Boucher, F.; Mallein-Gerin, F.; Auxenfans, C.; et al. Evaluation of Three Devices for the Isolation of the Stromal Vascular Fraction from Adipose Tissue and for ASC Culture: A Comparative Study. Stem Cells Int. 2017, 2017, 9289213. [Google Scholar] [CrossRef]
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A.; et al. Adipose tissue derived stem cells: In vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015, 6, 2. [Google Scholar] [CrossRef]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Pizzicannella, J.; Kothari, A.; Garcovich, S. Impact of the Different Preparation Methods to Obtain Human Adipose-Derived Stromal Vascular Fraction Cells (AD-SVFs) and Human Adipose-Derived Mesenchymal Stem Cells (AD-MSCs): Enzymatic Digestion Versus Mechanical Centrifugation. Int. J. Mol. Sci. 2019, 20, 5471. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Tuin, A.J.; Spiekman, M.; Jansma, J.; van der Lei, B.; Harmsen, M.C. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: A systematic review. J. Tissue Eng. Regen. Med. 2018, 12, e261–e274. [Google Scholar] [CrossRef]
- Astori, G.; Vignati, F.; Bardelli, S.; Tubio, M.; Gola, M.; Albertini, V.; Bambi, F.; Scali, G.; Castelli, D.; Rasini, V.; et al. ‘In vitro’ and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J. Transl. Med. 2007, 5, 55. [Google Scholar] [CrossRef]
- François, P.; Rusconi, G.; Arnaud, L.; Mariotta, L.; Giraudo, L.; Minonzio, G.; Veran, J.; Bertrand, B.; Dumoulin, C.; Grimaud, F.; et al. Inter-center comparison of good manufacturing practices-compliant stromal vascular fraction and proposal for release acceptance criteria: A review of 364 productions. Stem Cell Res. Ther. 2021, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Bertozzi, N. Isolation of ready-to-use adipose-derived stem cell (ASC) pellet for clinical applications and a comparative overview of alternate methods for ASC isolation. Curr. Protoc. Stem Cell Biol. 2017, 2017, 1F-17. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Ciliberti, R.G. Clinical use of adipose-derived stem cells: European legislative issues. Ann. Med. Surg. 2017, 24, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Boada-Pladellorens, A.; Avellanet, M.; Pages-Bolibar, E.; Veiga, A. Stromal vascular fraction therapy for knee osteoarthritis: A systematic review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221117879. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel Autologous Cell Therapy in Ischemic Limb Disease Through Growth Factor Secretion by Cultured Adipose Tissue–Derived Stromal Cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Garza, J.R.; Pérez-Merino, E.M.; González-Rodríguez, A.; Gálvez, B.G.; López-Herrera, G.; González, J.A.; Rodríguez, J.M.; Salgado, M.; García-Fernández, C. The stromal vascular fraction of adipose tissue: A promising source for regenerative medicine. Front. Cell Dev. Biol. 2021, 9, 683883. [Google Scholar]
- Zhang, P.Q.; Tan, P.C.; Gao, Y.M.; Zhang, X.J.; Xie, Y.; Zheng, D.N.; Zhou, S.B.; Li, Q.F. The effect of glycerol as a cryoprotective agent in the cryopreservation of adipose tissue. Stem Cell Res. Ther. 2022, 13, 152. [Google Scholar] [CrossRef]
- Kim, Y.S.; Oh, S.M.; Suh, D.S.; Tak, D.H.; Kwon, Y.B.; Koh, Y.G. Cartilage lesion size and number of stromal vascular fraction (SVF) cells strongly influenced the SVF implantation outcomes in patients with knee osteoarthritis. J. Exp. Orthop. 2023, 10, 28. [Google Scholar] [CrossRef]
- Moon, K.C.; Chung, H.Y.; Han, S.K.; Jeong, S.H.; Dhong, E.S. Tissue-engineered dermis grafts using stromal vascular fraction cells on the nose: A retrospective case-control study. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Choi, D.H.; Lee, J.H.; Lee, J.S.; Lee, J.; Park, H.Y.; Yang, J.D. A Prospective Study of the Efficacy of Cell-Assisted Lipotransfer with Stromal Vascular Fraction to Correct Contour Deformities of the Autologous Reconstructed Breast. Aesthetic Plast. Surg. 2021, 45, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Rowe, G.; Heng, D.S.; Beare, J.E.; Hodges, N.A.; Tracy, E.P.; Murfee, W.L.; LeBlanc, A.J. Stromal Vascular Fraction Reverses the Age-Related Impairment in Revascularization following Injury. J. Vasc. Res. 2023, 59, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yan, Y.; Song, Y.H.; Seidensticker, M.; Rabinovich, B.; Metzele, R.; Bankson, J.A.; Vykoukal, D.; Alt, E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur. Heart J. 2010, 31, 489–501. [Google Scholar] [CrossRef]
- Gareev, I.; Beylerli, O.; Ilyasova, T.; Ahmad, A.; Shi, H.; Chekhonin, V. Therapeutic application of adipose-derived stromal vascular fraction in myocardial infarction. iScience 2024, 27, 109791. [Google Scholar] [CrossRef]
- Ferreira, M.Y.; da Conceição Carvalho, J., Jr.; Ferreira, L.M. Evaluating the quality of studies reporting on clinical applications of stromal vascular fraction: A systematic review and proposed reporting guidelines (CLINIC-STRA-SVF). Regen. Ther. 2023, 24, 332–342. [Google Scholar] [CrossRef]
- Mattei, A.; Magalon, J.; Bertrand, B.; Grimaud, F.; Revis, J.; Velier, M.; Veran, J.; Dessi, P.; Sabatier, F.; Giovanni, A. Autologous adipose-derived stromal vascular fraction and scarred vocal folds: First clinical case report. Stem Cell Res. Ther. 2018, 9, 202. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N.; Burgos-Alonso, N. Stromal vascular fraction technologies and clinical applications. Expert Opin. Biol. Ther. 2019, 19, 1289–1305. [Google Scholar] [CrossRef]
- Bianchi, F.; Falanga, V.; Oliviero, U.; Miele, F.; Rocco, D.; Gallelli, L.; Scavo, M.; Moretti, G.; Manzoli, L.; Fabbri, C.; et al. Methods and Protocols for the Isolation of Stromal Vascular Fraction cells: A comparison. J. Transl. Med. 2020, 18, 1–19. [Google Scholar]
- Tallone, T.; Realini, C.; Böhmler, A.; Kornfeld, C.; Vassalli, G.; Moccetti, T.; Bardelli, S.; Soldati, G. Adult human adipose tissue contains several types of multipotent cells. J. Cardiovasc. Transl. Res. 2011, 4, 200–210. [Google Scholar] [CrossRef]
- Rodriguez, J.; Chang, S.-C.; Paduano, F.; Sykora, D.; Ayoub, N.; Bianchi, F.; De Rosa, M.; Cancedda, R.; Miele, F. Variability in SVF isolation methods: Impact on cell yield and composition. Tissue Eng. Part B Rev. 2019, 25, 435–444. [Google Scholar] [CrossRef]
- Rebelo, Â. Power-Assisted Liposuction. Clin. Plast. Surg. 2006, 33, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A. The Tumescent Technique for Lipo-Suction Surgery. Am. J. Cosmet. Surg. 1987, 4, 263–267. [Google Scholar] [CrossRef]
- Klein, J.A. Tumescent Technique for Regional Anesthesia Permits Lidocaine Doses of 35 mg/kg for Liposuction. J. Dermatol. Surg. Oncol. 1990, 16, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, M. Ultrasonic liposculpturing. Aesthetic Plast. Surg. 1992, 16, 287–298. [Google Scholar] [CrossRef]
- Goldman, A.; Gotkin, R.H. Laser-Assisted Liposuction. Perioper. Nurs. Clin. 2011, 6, 131–145. [Google Scholar] [CrossRef]
- Sasaki, G.H. Water-Assisted Liposuction for Body Contouring and Lipoharvesting: Safety and Efficacy in 41 Consecutive Patients. Aesthet. Surg. J. 2011, 31, 76–88. [Google Scholar] [CrossRef]
- Inaki, R.; Sato, Y.; Nakamura, D.; Aikawa, Y.; Takato, T.; Hoshi, K.; Hikita, A. Lipoaspirate stored at a constant low temperature by electric control suppresses intracellular metabolism and maintains high cell viability. Regen. Ther. 2023, 24, 662–669. [Google Scholar] [CrossRef]
- Rusconi, G.; Cremona, M.; Gallazzi, M.; Mariotta, L.; Gola, M.; Gandolfi, E.; Malacco, M.; Soldati, G. Good Manufacturing Practice-Compliant Cryopreserved and Thawed Native Adipose Tissue Ready for Fat Grafting. J. Clin. Med. 2024, 13, 3028. [Google Scholar] [CrossRef]
- Gavrila, D.; Liu, Y. Collection, Preservation, and Transportation of Adipose Tissue for Research Applications. Adipocyte 2015, 4, 219–225. [Google Scholar]
- Friedrich, M.; Lambert, C. Recommendations for the Collection, Storage, and Transportation of Human Adipose Tissue Samples. Clin. Chem. Lab. Med. 2016, 54, 419–427. [Google Scholar]
- Sanghvi, V.A.; Bruegger, C.M. Effect of Transport and Storage on the Quality of Adipose Tissue Samples. Sci. Rep. 2011, 11, 21418. [Google Scholar]
- Agarwal, N.; Mak, C.; Bojanic, C.; To, K.; Khan, W. Meta-analysis of adipose tissue derived cell-based therapy for the treatment of knee osteoarthritis. Cells 2021, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-J.; Fu, R.-H.; Shyu, W.-C.; Liu, S.-P.; Jong, G.-P.; Chiu, Y.-W.; Wu, H.-S.; Tsou, Y.-A.; Cheng, C.-W.; Lin, S.-Z. Adipose-Derived Stem Cells: Isolation, Characterization, and Differentiation Potential. Cell Transplant. 2013, 22, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, N.; Shrivastava, S.; Ravi, V.R.; Nallakumarasamy, A.; Pundkar, A.; Jeyaraman, M. Understanding and controlling the variables for advanced cell therapy. World J. Stem Cells 2024, 16, 784–798. [Google Scholar] [CrossRef]
- Solodeev, I.; Meilik, B.; Gur, E.; Shani, N. A Closed-system Technology for Mechanical Isolation of High Quantities of Stromal Vascular Fraction from Fat for Immediate Clinical Use. Plast. Reconstr. Surg. Glob. Open 2023, 11, E5096228. [Google Scholar] [CrossRef]
- Damia, E.; Chicharro, D.; Lopez, S.; Cuervo, B.; Rubio, M.; Sopena, J.J.; Vilar, J.M.; Carrillo, J.M. Adipose-Derived Mesenchymal Stem Cells: Are They a Good Therapeutic Strategy for Osteoarthritis? Int. J. Mol. Sci. 2018, 19, 1926. [Google Scholar] [CrossRef]
- Borić, I.; Hudetz, D.; Rod, E.; Jeleč, Ž.; Vrdoljak, T.; Skelin, A.; Polašek, O.; Plečko, M.; Trbojević-Akmačić, I.; Lauc, G.; et al. A 24-Month Follow-Up Study of the Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2019, 10, 1051. [Google Scholar] [CrossRef]
- Cremona, M.; Rusconi, G.; Ferrario, A.; Mariotta, L.; Gola, M.; Soldati, G. Processing Adipose Tissue Samples in a GMP Environment Standardizes the Use of SVF in Cell Therapy Treatments: Data on 302 Patients. Biomedicines 2023, 11, 2533. [Google Scholar] [CrossRef]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen. 2015, 4, 7. [Google Scholar] [CrossRef]
- Fraser, J.K.; Hicok, K.C.; Shanahan, R.; Zhu, M.; Miller, S.; Arm, D.M. The Celution® System: Automated Processing of Adipose-Derived Regenerative Cells in a Functionally Closed System. Adv. Wound Care 2014, 3, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki, T.; Condé-Green, A.; Cohen, S.R.; Canikyan, S.; Kocak, P. A 3-step Mechanical Digestion Method to Harvest Adipose-derived Stromal Vascular Fraction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2652. [Google Scholar] [CrossRef] [PubMed]
- Cicione, C.; Vadalà, G.; Di Giacomo, G.; Tilotta, V.; Ambrosio, L.; Russo, F.; Zampogna, B.; Cannata, F.; Papalia, R.; Denaro, V. Micro-fragmented and nanofat adipose tissue derivatives: In vitro qualitative and quantitative analysis. Front. Bioeng. Biotechnol. 2023, 11, 911600. [Google Scholar] [CrossRef] [PubMed]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Raposio, E. From liposuction to adipose-derived stem cells: Indications and technique. Acta Biomed. 2019, 90, 197–208. [Google Scholar] [CrossRef]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces from Human Lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef]
- Ragni, E.; Viganò, M.; De Luca, P.; Pedrini, E.; de Girolamo, L. Adipose-Derived Stem/Stromal Cells, Stromal Vascular Fraction, and Microfragmented Adipose Tissue. In Orthobiologics: Injectable Therapies for the Musculoskeletal System; Filardo, G., Mandelbaum, B.R., Muschler, G.F., Rodeo, S.A., Nakamura, N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–61. [Google Scholar] [CrossRef]
- Schipper, J.A.M.; van Laarhoven, C.J.H.C.M.; Schepers, R.H.; Tuin, A.J.; Harmsen, M.C.; Spijkervet, F.K.L.; Jansma, J.; van Dongen, J.A. Mechanical Fractionation of Adipose Tissue—A Scoping Review of Procedures to Obtain Stromal Vascular Fraction. Bioengineering 2023, 10, 1175. [Google Scholar] [CrossRef]
- De Francesco, F.; Gravina, P.; Busato, A.; Farinelli, L.; Soranzo, C.; Vidal, L.; Zingaretti, N.; Zavan, B.; Sbarbati, A.; Riccio, M.; et al. Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease. Int. J. Mol. Sci. 2021, 22, 10197. [Google Scholar] [CrossRef]
- Jurgens, W.J.F.M.; Kroeze, R.J.; Zandieh-Doulabi, B.; van Dijk, A.; Renders, G.A.; Smit, T.H.; van Milligen, F.J.; Ritt, M.J.P.F.; Helder, M.N. One-Step Surgical Procedure for the Treatment of Osteochondral Defects with Adipose-Derived Stem Cells in a Caprine Knee Defect: A Pilot Study. BioRes. Open Access 2013, 2, 315–325. [Google Scholar] [CrossRef]
- Uguten, M.; van der Sluis, N.; Vriend, L.; Coert, J.H.; Harmsen, M.C.; van der Lei, B.; van Dongen, J.A. Comparing mechanical and enzymatic isolation procedures to isolate adipose-derived stromal vascular fraction: A systematic review. Wound Repair. Regen. 2024, 32, 1008–1021. [Google Scholar] [CrossRef]
- Jaiswal, A.N.; Vagga, A. Cryopreservation: A Review Article. Cureus 2022, 14, e31564. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.; Safwani, W.K.Z.W.; Xu, F.; Abas, W.A.B.W.; Choi, J.R.; Pingguan-Murphy, B. Cryopreservation of Human Mesenchymal Stem Cells for Clinical Applications: Current Methods and Challenges. Biopreserv. Biobank. 2015, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qi, J.; Fu, S.; Luan, J.; Wang, Q. Effects of Nanographene oxide on adipose-derived stem cell cryopreservation. Cell Tissue Bank. 2024, 25, 805–830. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Tabata, Y. Hydrogen gas improves proliferation and mitochondrial activity of human adipose-derived stem cells after cryopreservation. Regen. Ther. 2024, 26, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Solodeev, I.; Orgil, M.; Bordeynik-Cohen, M.; Meilik, B.; Manheim, S.; Volovitz, I.; Sela, M.; Inbal, A.; Gur, E.; Shani, N. Cryopreservation of Stromal Vascular Fraction Cells Reduces Their Counts but Not Their Stem Cell Potency. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2321. [Google Scholar] [CrossRef]
- Shaik, S.; Wu, X.; Gimble, J.M.; Devireddy, R. Non-toxic freezing media to retain the stem cell reserves in adipose tissues. Cryobiology 2020, 96, 137–144. [Google Scholar] [CrossRef]
- Agostini, F.; Rossi, F.M.; Aldinucci, D.; Battiston, M.; Lombardi, E.; Zanolin, S.; Massarut, S.; Parodi, P.C.; Da Ponte, A.; Tessitori, G.; et al. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res. Ther. 2018, 9, 130. [Google Scholar] [CrossRef]
- Minonzio, G.; Corazza, M.; Mariotta, L.; Gola, M.; Zanzi, M.; Gandolfi, E.; De Fazio, D.; Soldati, G. Frozen adipose-derived mesenchymal stem cells maintain high capability to grow and differentiate. Cryobiology 2014, 69, 211–216. [Google Scholar] [CrossRef]
- Chaytor, J.L.; Tokarew, J.M.; Wu, L.K.; Leclère, M.; Tam, R.Y.; Capicciotti, C.J.; Guolla, L.; von Moos, E.; Findlay, C.S.; Allan, D.S.; et al. Inhibiting ice recrystallization and optimization of cell viability after cryopreservation. Glycobiology 2012, 22, 123–133. [Google Scholar] [CrossRef]
- European Pharmacopoeia, Chapter 2.6 Biological Analyses. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition (accessed on 15 November 2024).
- Kaiser, S.J.; Mutters, N.T.; Backhaus, J.; Frank, U.; Günther, F. Sterility testing of injectable products: Evaluation of the growth based BacT/ALERT® 3DTM Dual T culture system. PDA J. Pharm. Sci. Technol. 2016, 70, 568–576. [Google Scholar] [CrossRef]
- Jimenez, L.; Rana, N.; Amalraj, J.; Walker, K.; Travers, K. Validation of the BacT/ALERT® 3D System for Rapid Sterility Testing of Biopharmaceutical Samples. PDA J. Pharm. Sci. Technol. 2012, 66, 38. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.L.; Ho, B. Endotoxin Detection—From Limulus Amebocyte Lysate to Recombinant Factor C. In Endotoxins: Structure, Function and Recognition; Wang, X., Quinn, P.J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 187–208. [Google Scholar] [CrossRef]
- Dubczak, J.; Reid, N.; Tsuchiya, M. Evaluation of limulus amebocyte lysate and recombinant endotoxin alternative assays for an assessment of endotoxin detection specificity. Eur. J. Pharm. Sci. 2021, 159, 105716. [Google Scholar] [CrossRef] [PubMed]
- Eudralex, Annex 1. Available online: https://www.gmp-compliance.org/files/guidemgr/20220825_gmp-an1_en_0.pdf (accessed on 15 November 2024).
- PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEXES. Available online: https://picscheme.org/docview/6606 (accessed on 15 November 2024).
- Verma, S.K.; Sharma, P.C. NGS-based characterization of microbial diversity and functional profiling of solid tannery waste metagenomes. Genomics 2020, 112, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Zenic, L.; Polancec, D.; Hudetz, D.; Jelec, Z.; Rod, E.; Vidovic, D.; Staresinic, M.; Sabalic, S.; Vrdoljak, T.; Petrovic, T.; et al. Polychromatic Flow Cytometric Analysis of Stromal Vascular Fraction from Lipoaspirate and Microfragmented Counterparts Reveals Sex-Related Immunophenotype Differences. Genes 2021, 12, 1999. [Google Scholar] [CrossRef]
- Gunetti, M.; Castiglia, S.; Rustichelli, D.; Mareschi, K.; Sanavio, F.; Muraro, M.; Signorino, E.; Castello, L.; Ferrero, I.; Fagioli, F. Validation of analytical methods in GMP: The disposable Fast Read 102® device, an alternative practical approach for cell counting. J. Transl. Med. 2012, 10, 112. [Google Scholar] [CrossRef]
- Radrizzani, M.; Soncin, S.; Cicero, V.L.; Andriolo, G.; Bolis, S.; Turchetto, L. Quality control assays for clinical-grade human mesenchymal stromal cells: Methods for ATMP release. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1416, pp. 313–337. [Google Scholar] [CrossRef]
- Robb, K.P.; Fitzgerald, J.C.; Barry, F.; Viswanathan, S. Mesenchymal stromal cell therapy: Progress in manufacturing and assessments of potency. Cytotherapy 2019, 21, 289–306. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cremona, M.; Gallazzi, M.; Rusconi, G.; Mariotta, L.; Gola, M.; Soldati, G. State of the Art in the Standardization of Stromal Vascular Fraction Processing. Biomolecules 2025, 15, 199. https://doi.org/10.3390/biom15020199
Cremona M, Gallazzi M, Rusconi G, Mariotta L, Gola M, Soldati G. State of the Art in the Standardization of Stromal Vascular Fraction Processing. Biomolecules. 2025; 15(2):199. https://doi.org/10.3390/biom15020199
Chicago/Turabian StyleCremona, Martina, Matteo Gallazzi, Giulio Rusconi, Luca Mariotta, Mauro Gola, and Gianni Soldati. 2025. "State of the Art in the Standardization of Stromal Vascular Fraction Processing" Biomolecules 15, no. 2: 199. https://doi.org/10.3390/biom15020199
APA StyleCremona, M., Gallazzi, M., Rusconi, G., Mariotta, L., Gola, M., & Soldati, G. (2025). State of the Art in the Standardization of Stromal Vascular Fraction Processing. Biomolecules, 15(2), 199. https://doi.org/10.3390/biom15020199





