Heavy Chalcogen Properties of Sulfur and Selenium Enhance Nucleic Acid-Based Therapeutics
Abstract
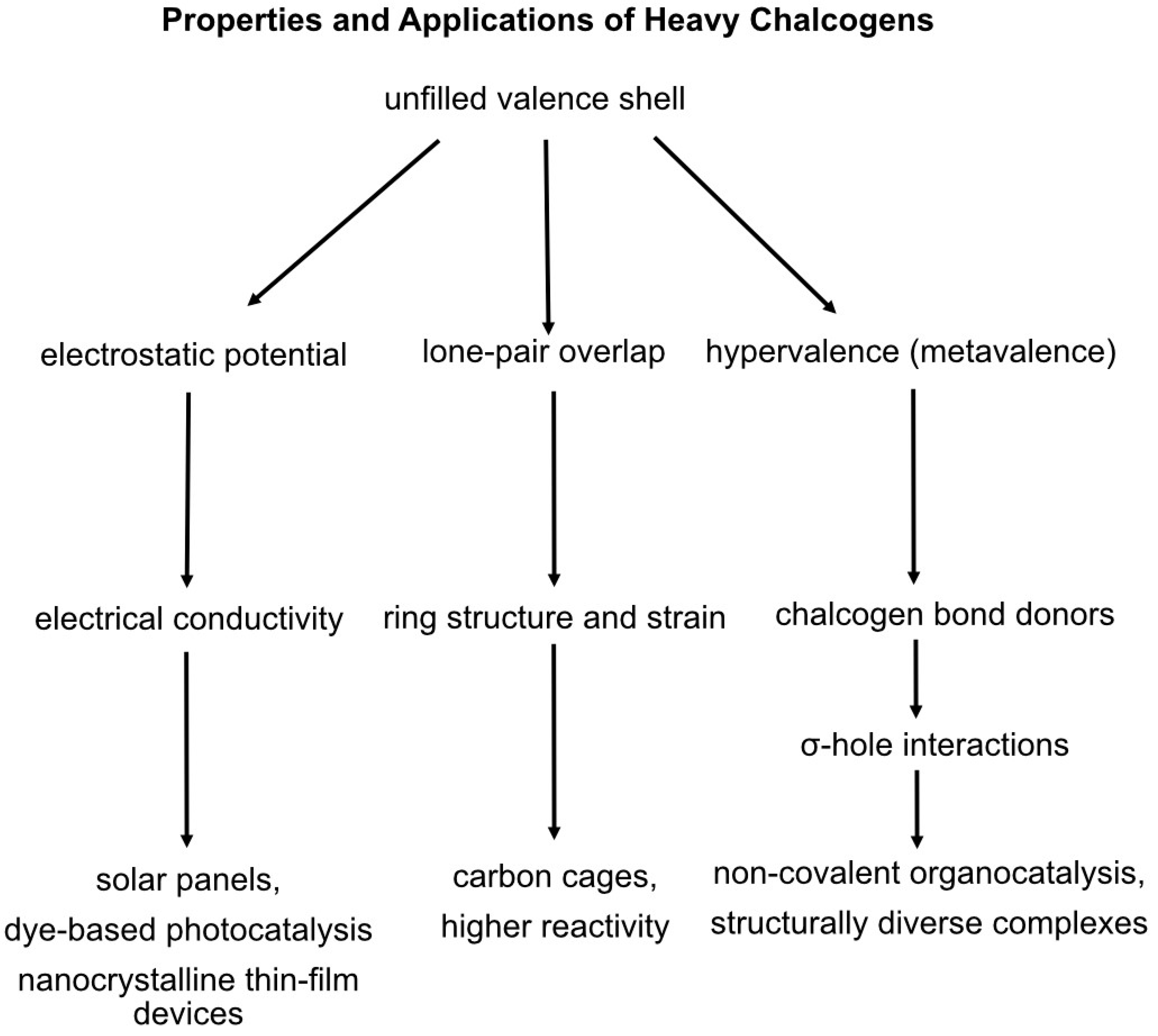
1. Chemical Properties of Chalcogens as Sources of Innovation
2. Chalcogen Electrostatics Improve Material Design
3. Metallo-Heterocyclic Rings Exhibit Greater Stability
4. Heavy Chalcogen Incorporation Primes Organic Reactivity
5. Chalcogen-Derivatized Therapeutics and Dietary Selenium Are Anticancerous
6. Geologic Source and Biologic Nature of Chalcogens in Proteins and Nucleic Acids
7. Sulfated Amino Acids Stabilize Molecular Interactions
8. Selenoproteins Facilitate Cellular Redox Reactions
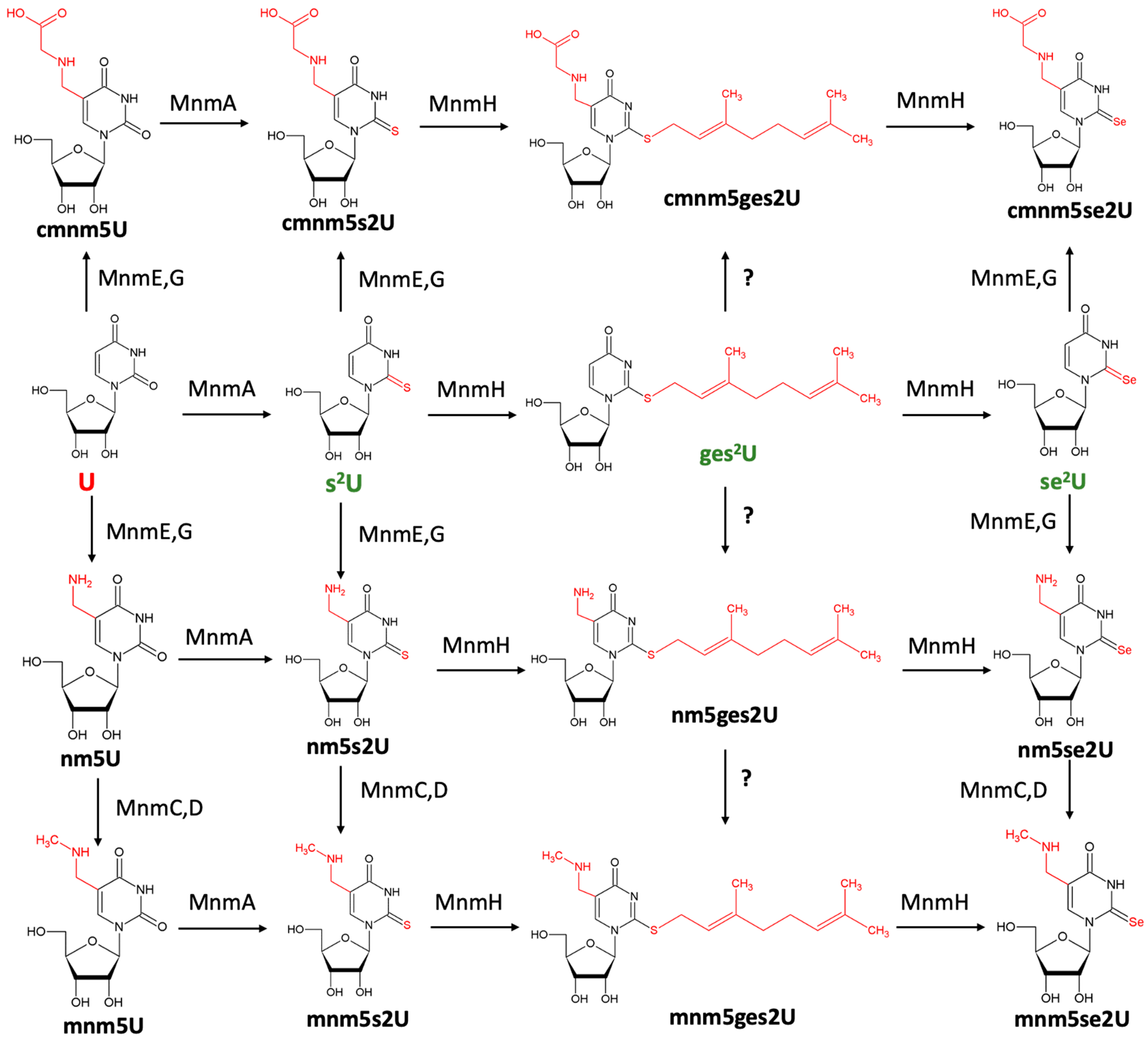
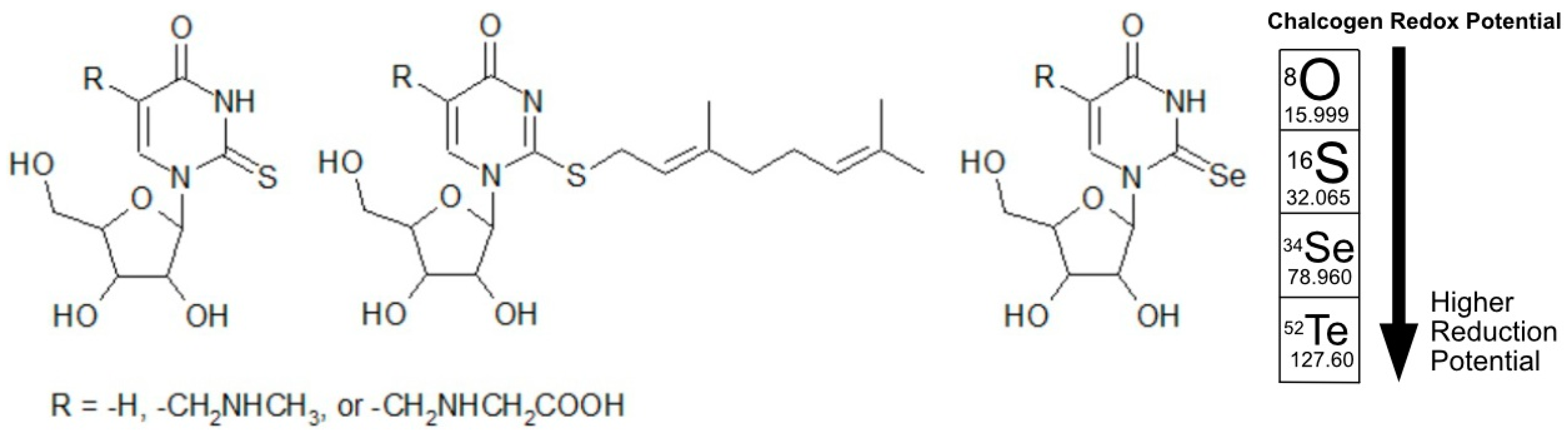
9. Mnm Enzymes Catalyze Sulfur and Selenium Substitution Within Nucleic Acids
10. Chalcogens in Nucleic Acid Mimicry and Their Detection via Analytical Methods
11. Native Uridine Modifications Customize Small Molecule Mimetics
12. Selenium-Derivatized Phosphoramidites in Synthetic Oligomers Drive Crystal Growth
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hassan, A.E.; Sheng, J.; Jiang, J.; Zhang, W.; Huang, Z. Synthesis and crystallographic analysis of 5-Se-thymidine DNAs. Org. Lett. 2009, 11, 2503–2506. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.E.; Sheng, J.; Zhang, W.; Huang, Z. High fidelity of base pairing by 2-selenothymidine in DNA. J. Am. Chem. Soc. 2010, 132, 2120–2121. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Figuereo, A.; Moreno, E.; Sanmartin, C.; Plano, D. Exploring Novel Drug Combinations: The Therapeutic Potential of Selanyl Derivatives for Leishmania Treatment. Molecules 2023, 28, 5845. [Google Scholar] [CrossRef] [PubMed]
- Mayo, R.A.; Morgan, I.S.; Soldatov, D.V.; Clerac, R.; Preuss, K.E. Heisenberg Spin Chains via Chalcogen Bonding: Noncovalent S-O Contracts Enable Long-Range Magnetic Order. Inorg. Chem. 2021, 60, 11338–11346. [Google Scholar] [CrossRef]
- Kim, J.; Almo, S.C. Structural basis for hypermodification of the wobble uridine in tRNA by bifunctional enzyme MnmC. BMC Struct. Biol. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.K.; Meirzadeh, E.; Stone, I.B.; Koay, C.S.; Nuckolls, C.; Steigerwald, M.L.; Roy, X. Superatom regiochemistry dictates the assembly and surface reactivity of a two-dimensional material. J. Am. Chem. Soc. 2022, 144, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Figuereo, A.; Morán-Serradilla, C.; Angulo-Elizari, E.; Sanmartín, C.; Plano, D. Small molecules containing chalcogen elements (S, Se, Te) as new warhead to fight neglected tropical diseases. Eur. J. Med. Chem. 2023, 246, 115002. [Google Scholar] [CrossRef] [PubMed]
- Christofferson, A.; Zhao, L.; Sun, H.; Huang, Z.; Huang, N. Theoretical studies of the base pair fidelity of selenium-modified DNA. J. Phys. Chem. B 2011, 115, 10041–10048. [Google Scholar] [CrossRef] [PubMed]
- Berseneva, A.A.; Klepov, V.V.; Pal, K.; Seeley, K.; Koury, D.; Schaeperkoetter, J.; Wright, J.T.; Kanatzidis, M.G.; Berseneva, A.A.; Gelis, A.V.; et al. Transuranium Sulfide via the Boron Chalcogen Mixture Method and Reversible Water Uptake in the NaCu T S3 Family. J. Am. Chem. Soc. 2022, 144, 13773–13786. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Data, D.; Seelam, P.P.; Bhattacharyya, D.; Mitra, A. Estimating strengths of individual hydrogen bonds in RNA base pairs: Toward a consensus between different computational approaches. ACS Omega 2019, 4, 7354–7368. [Google Scholar] [CrossRef] [PubMed]
- Chand, A.; Sahoo, D.K.; Rana, A.; Jena, S.; Biswal, H.S. The prodigious hydrogen bonds with sulfur and selenium in molecular assemblies, structural biology, and functional materials. Acc. Chem. Res. 2020, 53, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Beckett, G.J.; Arthur, J.R. Selenium and endocrine systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef]
- Wittwer, A.J.; Tsai, L.; Ching, W.M.; Stadtman, T.C. Identification and synthesis of a naturally occurring selenonucleoside in bacterial tRNAs: 5-[(methylamino) methyl]-2-selenouridine. Biochemistry 1984, 23, 4650–4655. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Sengoku, T.; Nishimoto, M.; Yokoyama, S.; Bessho, Y. Crystal structure of the bifunctional tRNA modification enzyme MnmC from Escherichia coli. Protein Sci. 2011, 20, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, B.; Sierant, M.; Szczupak, P. Sulfur-and Selenium-Modified Bacterial tRNAs. In Handbook of Chemical and Biology of Nucleic Acids; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1231–1264. [Google Scholar]
- Kadlag, Y.; Becker, H. Highly siderophile and chalcogen element constraints on the origin of components of the Allende and Murchison meteorites. Meteorit. Planet. Sci. 2016, 51, 1136–1152. [Google Scholar] [CrossRef]
- Wang, Z.; Becker, H. Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature 2013, 499, 328–331. [Google Scholar] [CrossRef]
- Wang, Z.; Becker, H. Fractionation of highly siderophile and chalcogen elements during magma transport in the mantle: Constraints from pyroxenites of the Balmuccia peridotite massif. Geochim. Cosmochim. Acta 2015, 159, 244–263. [Google Scholar] [CrossRef]
- Benz, S.; Macchione, M.; Verolet, Q.; Mareda, J.; Sakai, N.; Matile, S. Anion Transport with Chalcogen Bonds. J. Am. Chem. Soc. 2016, 138, 9093–9096. [Google Scholar] [CrossRef] [PubMed]
- Biswal, H.S. Hydrogen bond involving sulfur: New insights from ab initio calculations and gas phase laser spectroscopy. In Noncovalent Forces; Springer: Berlin/Heidelberg, Germany, 2015; pp. 15–45. [Google Scholar]
- Bock, A. Selenium proteins containing selenocysteine. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Carlson, B.A.; Lee, B.J.; Tsuji, P.A.; Copeland, P.R.; Schweizer, U.; Gladyshev, V.N.; Hatfield, D.L. Selenocysteine tRNA[Ser]Sec, the central component of selenoprotein biosynthesis: Isolation, identification, modification, and sequencing. In Selenoproteins: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–60. [Google Scholar]
- Carlson, B.A.; Xu, X.M.; Kryukov, G.V.; Rao, M.; Berry, M.J.; Gladyshev, V.N.; Hatfield, D.L. Hatfield. Identification and characterization of phosphoseryl-tRNA[Ser]Sec kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 12848–12853. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Chakrabarti, P. Non-hydrogen bond interactions involving the methionine sulfur atom. J. Biomol. Struct. Dyn. 2001, 19, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Wilds, C.J.; Pattanayek, R.; Pan, C.; Wawrzak, Z.; Egli, M. Selenium-assisted nucleic acid crystallography: Use of phosphoroselenoates for MAD phasing of a DNA structure. J. Am. Chem. Soc. 2002, 124, 14910–14916. [Google Scholar] [CrossRef] [PubMed]
- Höbartner, C.; Rieder, R.; Kreutz, C.; Puffer, B.; Lang, K.; Polonskaia, A.; Serganov, A.; Micura, R. Syntheses of RNAs with up to 100 Nucleotides Containing Site-Specific 2 ‘-Methylseleno Labels for Use in X-ray Crystallography. J. Am. Chem. Soc. 2005, 127, 12035–12045. [Google Scholar] [CrossRef]
- Morgado, C.A.; McNamara, J.P.; Hillier, I.H.; Burton, N.A.; Vincent, M.A. Density functional and semiempirical molecular orbital methods including dispersion corrections for the accurate description of noncovalent interactions involving sulfur-containing molecules. J. Chem. Theory Comput. 2007, 3, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.C.; Geissler, A.; Button, A.; Sasuclark, A.R.; Schroll, A.L.; Ruggles, E.L.; Gladyshev, V.N.; Hondal, R.J. Comparison of the redox chemistry of sulfur- and selenium-containing analogs of uracil. Free Radic. Biol. Med. 2017, 104, 249–261. [Google Scholar] [CrossRef]
- Bethune, D.S.; Johnson, R.D.; De Vries, M.S.; Yannoni, C.S. Atoms in carbon cages: The structure and properties of endohedral fullerenes. Nature 1993, 366, 123–128. [Google Scholar] [CrossRef]
- Root, M.J.; Deutsch, E. Nucleophilicity of coordinated chalcogens as evaluated in DMF-water media. Inorg. Chem. 1981, 20, 4376–4381. [Google Scholar] [CrossRef]
- Behne, D.; Kyriakopoulos, A.; Meinhold, H.; Köhrle, J. Identification of type I iodothyronine 5’-deiodinase as a selenoenzyme. Biochem. Biophys. Res. Commun. 1990, 173, 1143–1149. [Google Scholar] [CrossRef]
- Duhovic, S.; Dinca, M. Synthesis and electrical properties of covalent organic frameworks with heavy chalcogens. Chem. Mater. 2015, 27, 5487–5490. [Google Scholar] [CrossRef]
- Xue, D.; Wu, D.; Chen, Z.; Li, Y.; Sun, W.; Liu, J.; Li, Z. On Close Parallels between the Zintl-Based Superatom Ge9Be and Chalcogen Elements. Inorg. Chem. 2021, 60, 3196–3206. [Google Scholar] [PubMed]
- Garcin, E.; Vernede, X.; Hatchikian, E.C.; Volbeda, A.; Frey, M.; Fontecilla-Camps, J.C. The crystal structure of a reduced [NiFeSe] hydrogenase provides an image of the activated catalytic center. Structure 1999, 7, 557–566. [Google Scholar] [CrossRef]
- Deacon, A.M.; Ealick, S.E. Selenium-based MAD phasing: Setting the sites on larger structures. Structure 1999, 7, R161–R166. [Google Scholar] [CrossRef]
- Ealick, S.E. Advances in multiple wavelength anomalous diffraction crystallography. Curr. Opin. Chem. Biol. 2000, 4, 495–499. [Google Scholar] [CrossRef]
- Pallan, P.S.; Egli, M. Selenium modification of nucleic acids: Preparation of phosphoroselenoate derivatives for crystallographic phasing of nucleic acid structures. Nat. Protoc. 2007, 2, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Frieben, E.E.; Amin, S.; Sharma, A.K. Development of isoselenocyanate compounds’ syntheses and biological applications. J. Med. Chem. 2019, 62, 5261–5275. [Google Scholar] [CrossRef]
- Bader, R.F.; Fang, D.C. Properties of atoms in molecules: Caged atoms and the Ehrenfest force. J. Theory Comput. 2005, 1, 403–414. [Google Scholar] [CrossRef]
- Ouhsaine, F.; Ranaivonjatovo, H.; Escudié, J.; Saffon, N.; Lazraq, M. From a Phophagermaallene -P=C=Ge< and Heavier Chalcogens (S, Se, Te): Access to 3-Phosphanylidene-1,2,Chalcogenagermiranes. Organometallics 2009, 28, 1973–1975. [Google Scholar]
- Knight, F.R.; Fuller, A.L.; Bühl, M.; Slawin, A.M.; Woollins, J.D. Hypervalent adducts of chalcogen-containing peri-substituted naphthalenes; reactions of sulfur, selenium, and tellurium with dihalogens. Inorg. Chem. 2010, 49, 7577–7596. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Olive oil phenols and their potential effects on human health. J. Agric. Food Chem. 1998, 46, 4292–4296. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, T.; Wang, Q.; Jena, P. Rational design of stable dianions and the concept of super-chalcogens. J. Phys. Chem. A 2019, 123, 5753–5761. [Google Scholar] [CrossRef]
- Rose, G.D.; Geselowitz, A.R.; Lesser, G.J.; Lee, R.H.; Zehfus, M.H. Hydrophobicity of amino acid residues in globular proteins. Sci. Adv. 1985, 229, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Werz, D.B.; Gleiter, R. [N]Chalcogena[N]pericycynes: DFT Studies on Binaric Carbon-Chalcogen Compounds. Org. Lett. 2004, 6, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Jäger, G.; Chen, P.; Björk, G.R. Transfer RNA bound to MnmH protein is enriched with geranylated tRNA-A possible intermediate in its selenation. PLoS ONE 2016, 11, e0153488. [Google Scholar] [CrossRef] [PubMed]
- Waska, H.; Kim, S.; Kim, G.; Kang, M.R.; Kim, G.B. Distribution patterns of chalcogens (S, Se, Te, and 210Po) in various tissues of a squid, Todarodes pacificus. Sci. Total Environ. 2008, 392, 218–224. [Google Scholar] [CrossRef]
- Karas, C.; Hecht, M. A strategy for combinatorial cavity design in de novo proteins. Life 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Hecht, M.H. A completely de novo ATPase from a combinatorial protein design. J. Am. Chem. Soc. 2020, 142, 15230–15234. [Google Scholar] [CrossRef]
- Hendrickson, W.A. Anomalous diffraction in crystallographic phase evaluation. Q. Rev. Biophys. 2014, 47, 49–93. [Google Scholar] [CrossRef] [PubMed]
- Moroder, H.; Kreutz, C.; Lang, K.; Serganov, A.; Micura, R. Synthesis, oxidation behavior, crystallization and structure of 2’-methylseleno guanosine containing RNAs. J. Am. Chem. Soc. 2006, 128, 9909–9918. [Google Scholar] [CrossRef] [PubMed]
- Maroney, M.J.; Hondal, R.J. Selenium versus sulfur: Reversibility of chemical reactions and resistance to permanent oxidation in proteins and nucleic acids. Free Radic. Biol. Med. 2018, 127, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Caton-Williams, J.; Huang, Z. Biochemistry of selenium-derivatized naturally occurring and unnatural nucleic acids. Chem. Biodivers. 2008, 5, 396–407. [Google Scholar] [CrossRef]
- Sheng, J.; Huang, Z. Selenium derivatization of nucleic acids for phase and structure determination in nucleic acid X-ray crystallography. Int. J. Mol. Sci. 2008, 9, 258–271. [Google Scholar] [CrossRef]
- Sheng, J.; Huang, Z. Selenium derivatization of nucleic acids for X-ray crystal-structure and function studies. Chem. Biodivers. 2010, 7, 753–785. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sheng, J.; Hassan, A.E.; Jiang, S.; Gan, J.; Huang, Z. Novel RNA base pair with higher specificity using single selenium atom. Nucleic Acids Res. 2012, 40, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, M.; Takemoto, S.; Tomoda, S. Statistical and theoretical investigations on the directionality on nonbonded S-O interactions. Implications for molecular design and protein engineering. J. Am. Chem. Soc. 2002, 124, 10613–10620. [Google Scholar] [CrossRef]
- Hunter, M.S.; Yoon, C.H.; DeMirci, H.; Sierra, R.G.; Dao, E.H.; Ahmadi, R.; Aksit, F.; Aquila, A.L.; Ciftci, H.; Guillet, S.; et al. Selenium single-wavelength anomalous diffraction de novo phasing using an X-ray-free electron laser. Nat. Commun. 2016, 7, 13388. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jarhad, D.B.; Yu, J.; Lee, C.; Jeong, L.S. 2’-C-Methyl-4’-selenonucleosides as Anti-Hepatitis C Virus Agents. J. Org. Chem. 2019, 84, 14414–14426. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.E.; Schaefer, H.F., III. The tetramethyl chalcogens (Me4S, Me4Se, Me4Te): Bonding and structure. J. Am. Chem. Soc. 1994, 116, 9596–9601. [Google Scholar] [CrossRef]
- Iwaoka, M.; Isozumi, N. Hypervalent nonbonded interactions of a divalent sulfur atom. Implic. Protein Archit. Funct. 2012, 17, 7266–7283. [Google Scholar]
- Foley, J.W.; Song, X.; Demidova, T.N.; Jilal, F.; Hamblin, M.R. Synthesis ad properties of benzo [a] phenoxazinium chalcogen analogues as novel broad-spectrum antimicrobial photosensitizers. J. Med. Chem. 2006, 49, 5291–5299. [Google Scholar] [CrossRef] [PubMed]
- Andreesen, J.R.; Wagner, M.; Sonntag, D.; Kohlstock, M.; Harms, C.; Gursinsky, T.; Jäge, J.; Parther, T.; Kabisch, U.; Gräntzdöffer, A.; et al. Various functions of selenols and thiols in anaerobic Gran-positive, amino acids-utilizing bacteria. Biofactors 1999, 10, 263–270. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef]
- Bujnicki, J.M.; Oudjama, Y.; Roovers, M.; Owczarek, S.; Caillet, J.; Droogmans, L. Identification of a bifunctional enzyme MnmC involved in the biosynthesis of a hypermodified uridine in the wobble position of tRNA. RNA 2004, 10, 1236–1242. [Google Scholar] [CrossRef]
- Unrine, J.M.; Jackson, B.P.; Hopkins, W.A. Selenomethionine biotransformation and incorporation into proteins along a simulated terrestrial food chain. Environ. Sci. Technol. 2007, 41, 3601–3606. [Google Scholar] [CrossRef] [PubMed]
- Boyington, J.C.; Gladyshev, V.N.; Khangulov, S.V.; Stadtman, T.C.; Sun, P.D. Crystal structure of formate dehydrogenase H: Catalysis involving Mo, molybdopterin, selenocysteine, and an Fe4S4 cluster. Science 1997, 275, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, W.X.; Huang, X.B.; Zhou, Y.B.; Liu, M.C.; Wu, H.Y. Selective [3+2] cycloaddition of cyclopropenone derivatives and elemental chalcogens. Org. Lett. 2020, 22, 5555–5560. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Sheng, J.; Carrasco, N.; Huang, Z. Selenium derivatization of nucleic acids for crystallography. Nucleic Acids Res. 2007, 35, 477–485. [Google Scholar] [CrossRef]
- Kim, J.Y.; Carlson, B.A.; Xu, X.M.; Zeng, Y.; Chen, S.; Gladyshev, V.N.; Lee, B.J.; Hatfield, D.L. Inhibition of selenocystein tRNA [Ser] Sec aminoacylation provides evidence that aminoacylation is required for regulatory methylation of this tRNA. Biochem. Biophys. Res. Commun. 2011, 409, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Salon, J.; Jiang, J.; Sheng, J.; Gerlits, O.O.; Huang, Z. Derivatization of DNAs with selenium at 6-position of guanine for function and crystal structure studies. Nucleic Acids Res. 2008, 36, 7009–7018. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.K.; Singh, S.K.; Kumar, S.; Singh, G.; Sarkar, B.; Madhusudhan, M.S.; Das, A. Water-mediated selenium hydrogen-bonding in protein: PDB analysis and gas-phase spectroscopy of model complexes. J. Phys. Chem. A 2019, 123, 5995–6002. [Google Scholar] [CrossRef]
- Kulik, K.; Sadowska, K.; Wielgus, E.; Pacholczyk-Sienicka, B.; Sochacka, E.; Nawrot, B. 2-Selenouridine, a Modified Nucleoside of Bacterial tRNAs, Its Reactivity in the Presence of Oxidizing and Reducing Reagents. Int. J. Mol. Sci. 2022, 23, 7973. [Google Scholar] [CrossRef]
- Takahashi, K.; Avissar, N.; Whitin, J.; Cohen, H. Purification and characterization of human plasma glutathione peroxidase: A selenoglycoprotein distinct from the known cellular enzyme. Arch. Biochem. Biophys. 1987, 256, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jo, C.; Easwaramoorthi, S.; Sung, J.; Kim, D.H.; Churchill, D.G. Crytallographic, Photophysical, NMR Spectroscopic and Reactivity Manifestations of the 8-Heteroaryl Effect in 4,4-Difluoro-8-(C4H3 X)-4-bora-3 a, 4 a-diaza-s-indacene (X = 0, S, Se)(BODIPY) Systems. Inorg. Chem. 2010, 49, 4881–4894. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.L.; Kjaergaard, H.G. Hydrogen bonding to divalent sulfur. Phys. Chem. Chem. Phys. 2008, 10, 4113–4118. [Google Scholar] [CrossRef]
- Kurihara, K.; Umezawa, K.; Donnelly, A.E.; Sperling, B.; Liao, G.; Hecht, M.H.; Arai, R. Crystal structure and activity of a de novo enzyme, ferric enterobactin esterase Syn-F4. Proc. Natl. Acad. Sci. USA 2023, 120, e2218281120. [Google Scholar] [CrossRef] [PubMed]
- Konidaris, K.; Daolio, A.; Pizzi, A.; Scilabra, P.; Terraneo, G.; Quici, S.; Murray, J.S.; Politzer, P.; Resnati, G. Thiazolium salts as chalcogen bond donors. Cryst. Growth Des. 2022, 22, 4987–4995. [Google Scholar] [CrossRef]
- Oliveira, V.; Kraka, E. Systematic coupled cluster study of noncovalent interactions involving halogens, chalcogens, and pnicogens. J. Phys. Chem. A 2017, 121, 9544–9556. [Google Scholar] [CrossRef]
- Leonard, K.A.; Nelen, M.I.; Anderson, L.T.; Gibson, S.L.; Hilf, R.; Detty, M.R. 2, 4, 6-Triarylchalcogenopyrylium dyes related in structure to the antitumor agent AA1 as in vitro sensitizers for the photodynamic therapy of cancer. J. Med. Chem. 1999, 41, 3942–3952. [Google Scholar] [CrossRef] [PubMed]
- Jeong, L.S.; Tosh, D.K.; Choi, W.J. Development of next generation 4’-selenonucleosides. Nucleic Acids Symp. Ser. Oxf. Univ. Press 2009, 53, 7–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lancelot, G. Hydrogen bonding between nucleic acid bases and carboxylic acids. J. Am. Chem. Soc. 1977, 99, 7037–7042. [Google Scholar] [CrossRef] [PubMed]
- Kambampati, R.; Lauhon, C.T. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry 2003, 42, 1109–1117. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, T.; Liu, R.; Tian, W.; Wu, K.; Zhu, J.; Wang, Z.; He, C.; Feng, J.; Guo, X.; et al. Chalcogen-bridged coordination polymer for the photocatalytic activation of aryl halides. Nature 2023, 14, 4002. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, G.; Du, M.; Zhao, Z.; Xiao, L.; Hu, X. Effect of selenium on increasing free radical scavenging activities of polysaccharide extracts from a Se-enriched mushroom species of the genus Ganoderma. Eur. Food Res. Technol. 2008, 226, 449–505. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Verotta, L.; Goya, L.; Ramos, S.; Martín, M.A.; Bravo, L.; Napolitano, A.; d’Ischia, M. Synthesis and bioactivity profile of 5-S-lipoylhydroxytryrosol-based multidefense antioxidants with a sizeable (poly) sulfide chain. J. Agric. Food Chem. 2013, 61, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.M.; Earnest, T.; Brunger, A.T. Single-wavelength anomalous diffraction phasing revisited. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 1413–1420. [Google Scholar] [CrossRef]
- Sierant, M.; Leszczynska, G.; Sadowska, K.; Komar, P.; Radzikowska-Cieciura, E.; Sochacka, E.; Nawrot, B. Escherichia coli tRNA 2-selenouridine synthase (SelU) converts S2U-RNA to Se2U-RNA via S-geranylated-intermediate. FEBS Lett. 2018, 592, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Rob, A.; Caton-Williams, J.; Huang, Z. Biochemistry of nucleic acids functionalized with sulfur, selenium, and tellurium: Roles of the single-atom substitution. Biochalcogen Chem. 2013, 1152, 89–126. [Google Scholar]
- Berry, M.J.; Banu, L.; Larsen, P.R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 1991, 349, 438–440. [Google Scholar] [CrossRef]
- Strub, M.P.; Hoh, F.; Sanchez, J.F.; Strub, J.M.; Böck, A.; Aumelas, A.; Dumas, C. Selenomethionine and selenocysteine double labeling strategy for crystallographic phasing. Structure 2003, 11, 1359–1367. [Google Scholar] [CrossRef]
- Roovers, M.; Oudjama, Y.; Kaminska, K.H.; Purta, E.; Caillet, J.; Droogmans, L.; Bujnicki, J.M. Sequence–structure–function analysis of the bifunctional enzyme MnmC that catalyses the last two steps in the biosynthesis of hypermodified nucleoside mnm5s2U in tRNA. Proteins: Struct. Funct. Bioinform. 2008, 71, 2076–2085. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Bingemann, R.; Klein, A. Fusion of two subunits does not impair the function of a [NiFeSe]-hydrogenase in the archaeon Methanococcus voltae. Eur. J. Biochem. 1998, 256, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Sonntag, D.; Grimm, R.; Pich, A.; Eckerskorn, C.; Söhling, B.; Andreesen, J.R. Substrate-specific selenoprotein B of glycine reductase from Eubacterium acidaminophilum: Biochemical and molecular analysis. Eur. J. Biochem. 1999, 260, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Wuttig, M.; Schön, C.F.; Kim, D.; Golub, P.; Gatti, C.; Raty, J.Y.; Kooi, B.J.; Pendás, Á.M.; Arora, R.; Waghmare, U. Metavalent or Hypervalent Bonding: Is There a Chance for Reconciliation? Adv. Sci. 2024, 11, 2308578. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Kar, M.; Pal, S.; Sarkar, P. Role of chalcogens in the exciton relaxation dynamics of chalcogenol-functionalized CdSe QD: A time-domain atomistic simulation. Chem. Mater. 2019, 31, 4042–4050. [Google Scholar] [CrossRef]
- Detty, M.R.; Merkel, P.B.; Hilf, R.; Gibson, S.L.; Powers, S.K. Chalcogenapyrylium dyes as photochemotherapeutic agens. 2. Tumor uptake, mitochondrial targeting, and singlet-oxygen-induced inhibition of cytochrome c oxidase. J. Med. Chem. 1990, 33, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, M.; Takemoto, S.; Okada, M.; Tomoda, S. Statistical characterization of nonbonded S-O interactions in proteins. Chem. Lett. 2001, 30, 132–133. [Google Scholar] [CrossRef]
- Höbartner, C.; Micura, R. Chemical synthesis of selenium-modified oligoribonucleotides and their enzymatic ligation leading to an U6 SnRNA stem−loop segment. J. Am. Chem. Soc. 2004, 126, 1141–1149. [Google Scholar] [CrossRef]
- Moustafa, M.E.; El-Saadani, M.A.; Kandeel, K.M.; Mansur, D.B.; Lee, B.J.; Hatfield, D.L.; Diamond, A.M. Overproduction of selenocysteine tRNA in Chinese hamster ovary cells following transfection of the mouse tRNA [Ser] Sec gene. RNA 1998, 4, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Fickl, M.; Bauer, I.; Brunner, M.; Rázková, A.; Rieder, D.; Delazer, I.; Micura, R.; Lusser, A. 6-Thioguanosine monophosphate prodrugs display enhanced performance against thiopurine-resistant leukemia and breast cancer cells. J. Med. Chem. 2022, 65, 15165–15173. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Rinklebe, J.; Tsang, D.C.W.; Tack, F.M.G.; Abbasi, G.H.; Hussain, A.; Igalavithana, A.D.; Lee, B.C.; et al. Effects of Selenium on the Uptake of Toxic Trace Elements by Crop Plants: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2531–2566. [Google Scholar] [CrossRef]
- Shigi, N.; Horitani, M.; Miyauchi, K.; Suzuki, T.; Kuroki, M. An ancient type of MnmA protein is an iron–sulfur cluster-dependent sulfurtransferase for tRNA anticodons. RNA 2020, 26, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, N.; Ginsburg, D.; Du, Q.; Huang, Z. Synthesis of selenium-derivatized nucleosides and oligonucleotides for X-ray crystallography. Nucleosides Nucleotides Nucleic Acids 2001, 20, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, N.; Buzin, Y.; Tyson, E.; Halpert, E.; Huang, Z. Selenium derivatization and crystallization of DNA and RNA oligonucleotides for X-ray crystallography using multiple anomalous dispersion. Nucleic Acids Res. 2004, 32, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Korotkikh, N.I.; Rayenko, G.F.; Shvaika, O.P.; Pekhtereva, T.M.; Cowley, A.H.; Jones, J.N.; Macdonald, C.L. Synthesis of 1,2,4-triazol-5-ylidenes and their interaction with acetonitrile and chalcogens. J. Org. Chem. 2003, 68, 5762–5765. [Google Scholar] [CrossRef]
- Norberg, J.; Nilsson, L. Solvent influence on base stacking. Biophys. J. 1998, 74, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, N.; Satoh, K.; Kurosawa, R.; Yaoita, N.; Elias-Al-Mamun, M.; Siddique, M.A.H.; Omura, J.; Satoh, T.; Nogi, M.; Sunamura, S.; et al. Selenoprotein P promotes the development of pulmonary arterial hypertension: Possible therapeutic target. Circulation 2018, 138, 600–623. [Google Scholar] [CrossRef] [PubMed]
- Carugo, O.; Resnati, G.; Metrangolo, P. Chalcogen bonds involving selenium in protein structures. ACS Chem. Biol. 2021, 16, 1622–1627. [Google Scholar] [CrossRef]
- Begines, P.; Oliete, A.; Lopez, O.; Maya, I.; Plata, G.B.; Padron, J.M.; Fernandez-Bolanos, J.G. Chalcogen-containing phenolics as antiproliferative agens. Future Medininal Chem. 2018, 10, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Scilabra, P.; Terraneo, G.; Resnati, G. The chalcogen bond in crystalline solids: A world parallel to halogen bond. Acc. Chem. Res. 2019, 52, 1313–1324. [Google Scholar] [CrossRef]
- Haruehanroengra, P.; Vangaveti, S.; Ranganathan, S.V.; Mao, S.; Su, M.D.; Chen, A.A.; Sheng, J. Terpene Chain Length Affects the Base Pairing Discrimination of S-geranyl-2-thiouridine in RNA Duplex. Iscience 2020, 23, 101866. [Google Scholar] [CrossRef]
- Haruehanroengra, P.; Zheng, Y.Y.; Ma, G.; Lan, T.H.; Hassan, A.E.; Zhou, Y.; Sheng, J. Probing the Substrate Requirements of the In Vitro Geranylation Activity of Selenouridine Synthase (SelU). ChemBioChem 2022, 23, e202200089. [Google Scholar] [CrossRef] [PubMed]
- Durant, P.C.; Bajji, A.C.; Sundaram, M.; Kumar, R.K.; Davis, D.R. Structural effects of hypermodified nucleosides in the Escherichia coli and human tRNALys anticodon loop: The effect of nucleosides s2U, mcm5U, mcm5s2U, t6A, and ms2t6A. Biochemistry 2005, 44, 8078–8089. [Google Scholar] [CrossRef]
- Bommisetti, P.; Young, A.; Bandarian, V. Elucidation of the substrate of tRNA-modifying enzymes MnmEG leads to in vitro reconstitution of an evolutionarily conserved uridine hypermodification. J. Biol. Chem. 2022, 298, 102548. [Google Scholar] [CrossRef]
- Du, Q.; Carrasco, N.; Teplova, M.; Wilds, C.J.; Egli, M.; Huang, Z. Internal derivatization of oligonucleotides with selenium for X-ray crystallography using MAD. J. Am. Chem. Soc. 2002, 124, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Mundlapati, V.R.; Sahoo, D.K.; Ghosh, S.; Purame, U.K.; Pandey, S.; Acharya, R.; Pal, N.; Tiwari, P.; Biswal, H.S. Spectroscopic evidences for strong hydrogen bonds with selenomethionine in proteins. J. Phys. Chem. Lett. 2017, 8, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Arguello-Garcia, R.; Medina-Campos, O.N.; Perez-Hernandez, N.; Pedraza-Chaverri, J.; Ortega-Pierres, G. Hypochlorous acid scavening and activities of thioallyl compounds from garlic. J. Agric. Food Chem. 2010, 58, 11226–11233. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.E., Jr.; Parthasarathy, R.; Dunitz, J.D. Directional preferences of nonbonded atomic contacts with divalent sulfur. 1. Electrophiles and nucleophiles. J. Am. Chem. Soc. 1977, 99, 4860–4862. [Google Scholar] [CrossRef]
- Reid, R.C.; Yau, M.K.; Singh, R.; Lim, J.; Fairlie, D.P. Stereoelectronic effects dictate molecular conformation and biological function of heterocyclic amides. J. Am. Chem. Soc. 2014, 136, 11914–11917. [Google Scholar] [CrossRef] [PubMed]
- Fick, R.J.; Kroner, G.M.; Nepal, B.; Magnani, R.; Horowitz, S.; Houtz, R.L.; Scheiner, S.; Trievel, R.C. Sulfur-oxygen chalcogen bonding mediates adomet recognition in the lysine methyltransferase SET7/9. ACS Chem. Biol. 2016, 11, 748–754. [Google Scholar]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Vangaveti, S.; Ranganathan, S.V.; Basanta-Sanchez, M.; Haruehanroengra, P.; Chen, A.; Sheng, J. Synthesis, base pairing and structural studies of geranylated RNA. Nucleic Acids Res. 2016, 44, 6036–6045. [Google Scholar] [CrossRef] [PubMed]
- Masuda, R.; Kimura, R.; Karasaki, T.; Sase, S.; Goto, K. Modeling the catalytic cycle of glutathione peroxidase by nuclear magnetic resonance spectroscopic analysis of selenocysteine selenenic acids. J. Am. Chem. Soc. 2021, 143, 6345–6350. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: Implications for insulin resistance, inflammation, and atherosclerosis. J. Clin. Endocrinol. Metab. 2011, 96, E1325–E1329. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, R.; Saito, Y. Selenoprotein P; P for plasma, prognosis, prophylaxis, and more. Biol. Pharm. Bull. 2020, 43, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Benz, S.; López-Andarias, J.; Mareda, J.; Sakai, N.; Matile, S. Catalysis with chalcogen bonds. Angew. Chem. Int. Ed. 2017, 56, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kim, J.R.; Kwon, K.S.; Yoon, H.W.; Levine, R.L.; Ginsburg, A.; Rhee, S.G. Molecular cloning and characterization of a mitochondrial selenocysteine-containing thioredoxin reductase from rat liver. J. Biol. Chem. 1999, 274, 4722–4734. [Google Scholar] [CrossRef]
- Boggon, T.J.; Shapiro, L. Screening for phasing atoms in protein crystallography. Structure 2000, 8, R143–R149. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J. Synthesis, Structure and Function Studies of Selenium and Tellurium Derivatized Nucleic Acids. Ph.D. Thesis, Georgia State University, Atlanta, GA, USA, 2009. [Google Scholar]
- Watabe, S.; Makino, Y.; Ogawa, K.; Hiroi, T.; Yamamoto, Y.; Takahashi, S.Y. Mitochondrial thioredoxin reductase in bovine adrenal cortex: Its purification, properties, nucleotide/amino acid sequences, and identification of selenocysteine. Eur. J. Biochem. 1999, 264, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Singh, A.; Morihiro, K.; Moai, Y.; Kodama, T.; Obika, S.; Maiti, S. Systematic evaluation of biophysical and functional characteristics of selenomethylene-locked nucleic acid-mediated inhibition of miR-21. Biochemistry 2016, 55, 7023–7032. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, T. New biologic functions—Selenium-dependent nucleic acids and proteins. Fundam. Appl. Toxicol. 1983, 3, 420–423. [Google Scholar] [CrossRef]
- Tamura, T.; Stadtman, T.C. A new selenoprotein from human lung adenocarcinoma cells: Purification, properties, and thioredoxin reductase activity. Proc. Natl. Acad. Sci. USA 1996, 93, 1006–1011. [Google Scholar] [CrossRef]
- Sasamori, T.; Sasaki, T.; Takeda, N.; Tokitoh, N. Reactions of a Germacyclopropabenzene with Elemental Chalcogens: Synthesis and Structures of a Series of Stable 2 H-Benzo [c][1,2] chalcogenagermetes. Organometallics 2005, 24, 612–618. [Google Scholar] [CrossRef]
- Nauser, T.; Steinmann, D.; Grassi, G.; Koppenol, W.H. Why selenocysteine replaces cysteine in thioredoxin reductase: A radical hypothesis. Biochemistry 2014, 53, 5017–5022. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Ikeuchi, Y.; Fukai, S.; Adachi, H.; Matsumura, H.; Takano, K.; Murakami, S.; Inoue, T.; Mori, Y.; Sasaki, T.; et al. Crystallization and preliminary X-ray analysis of the tRNA thiolation enzyme MnmA from Escherichia coli complexed with tRNAGlu. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Nobori, H.; Sato, A.; Toba, S.; Kusakabe, S.; Sasaki, M.; Tabata, K.; Matsuno, K.; Maeda, N.; Ito, S.; et al. 2-Thiouridine is a broad-spectrum antiviral nucleoside analogue against positive-strand RNA viruses. Proc. Natl. Acad. Sci. USA 2023, 120, e2304139120. [Google Scholar] [CrossRef] [PubMed]
- Olieric, V.; Rieder, U.; Lang, K.; Serganov, A.; Schulze-Briese, C.; Micura, R.; Dumas, P.; Ennifar, E. A fast selenium derivatization strategy for crystallization and phasing of RNA structures. RNA 2009, 15, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Vishal, A.; Adhav, B.P.; Saikrishnan, K. Probing the Directionality of S O/N Chalcogen Bond and Its Interplay with Weak C-H O/N/S Hydrogen Bond Using Molecular Electrostatic Potential. J. Phys. Chem. B 2022, 126, 7818–7832. [Google Scholar]
- Waters, M.L. Aromatic interactions in peptides: Impact on structure and function. Pept. Sci. Orig. Res. Biomol. 2004, 76, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, W.A.; Horton, J.R.; LeMaster, D.M. Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): A vehicle for direct determination of three-dimensional structure. EMBO J. 1990, 9, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Szostak, J.W.; Huang, Z. Nucleic acid crystallization and X-ray crystallography facilitated by single selenium atom. Front. Chem. Sci. Eng. 2016, 10, 196–202. [Google Scholar] [CrossRef]
- Wang, W.; Walter, M.J.; Brodholt, J.P.; Huang, S.; Petaev, M.I. Chacogen isotopes reveal limited volatile contribution from late veneer to Earth. Sci. Adv. 2023, 9, eadh0670. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.F.; Werz, D.B. Caged Chalcogens: Theoretical Studies on a Tetracoordinated Oxonium Dication and Its Higher Homologues. Org. Lett. 2010, 12, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; An, X.; Li, Q. Se-N Chalcogen Bond and Se-X Halogen Bond Involving F2C=Se: Influence of Hybridization, Substitution, and Cooperativity. J. Phys. Chem. A 2015, 119, 3518–3527. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Luo, L.Y.; Fu, P.N.; Wang, Q.; Li, H.F. Selenium uptake and biotransformation in Brassica rapa supplied with selenite and selenate: A hydroponic work with HPLC speciation and RNA-sequencing. J. Agric. Food Chem. 2019, 2019, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, L.; Sun, C.; Zeng, Y. Organocatalysis by halogen, chalcogen, and pnictogen bond donors in halide abstraction reactions: An alternative to hydrogen bond-based catalysis. J. Phys. Chem. A 2020, 124, 3815–3824. [Google Scholar] [CrossRef]
- Kyogoku, Y.; Lord, R.C.; Rich, A. Hydrogen bonding specificity of nucleic acid purines and pyrimidines in solution. Science 1966, 154, 518–520. [Google Scholar] [CrossRef]
- Mita, Y.; Nakayama, K.; Inari, S.; Nishito, Y.; Yoshioka, Y.; Sakai, N.; Sotani, K.; Nagamura, T.; Kuzuhara, Y.; Inagaki, K.; et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat. Commun. 2017, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Buzin, Y.; Carrasco, N.; Huang, Z. Synthesis of selenium-derivatized cytidine and oligonucleotides for X-ray crystallography using MAD. Org. Lett. 2004, 6, 1099–1102. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dansereau, S.J.; Sheng, J. Heavy Chalcogen Properties of Sulfur and Selenium Enhance Nucleic Acid-Based Therapeutics. Biomolecules 2025, 15, 218. https://doi.org/10.3390/biom15020218
Dansereau SJ, Sheng J. Heavy Chalcogen Properties of Sulfur and Selenium Enhance Nucleic Acid-Based Therapeutics. Biomolecules. 2025; 15(2):218. https://doi.org/10.3390/biom15020218
Chicago/Turabian StyleDansereau, Stephen J., and Jia Sheng. 2025. "Heavy Chalcogen Properties of Sulfur and Selenium Enhance Nucleic Acid-Based Therapeutics" Biomolecules 15, no. 2: 218. https://doi.org/10.3390/biom15020218
APA StyleDansereau, S. J., & Sheng, J. (2025). Heavy Chalcogen Properties of Sulfur and Selenium Enhance Nucleic Acid-Based Therapeutics. Biomolecules, 15(2), 218. https://doi.org/10.3390/biom15020218





