Obesity and Adipose-Derived Extracellular Vesicles: Implications for Metabolic Regulation and Disease
Abstract
:1. Introduction
2. Influence of EVs in Metabolic Regulation and Obesity-Related Disease
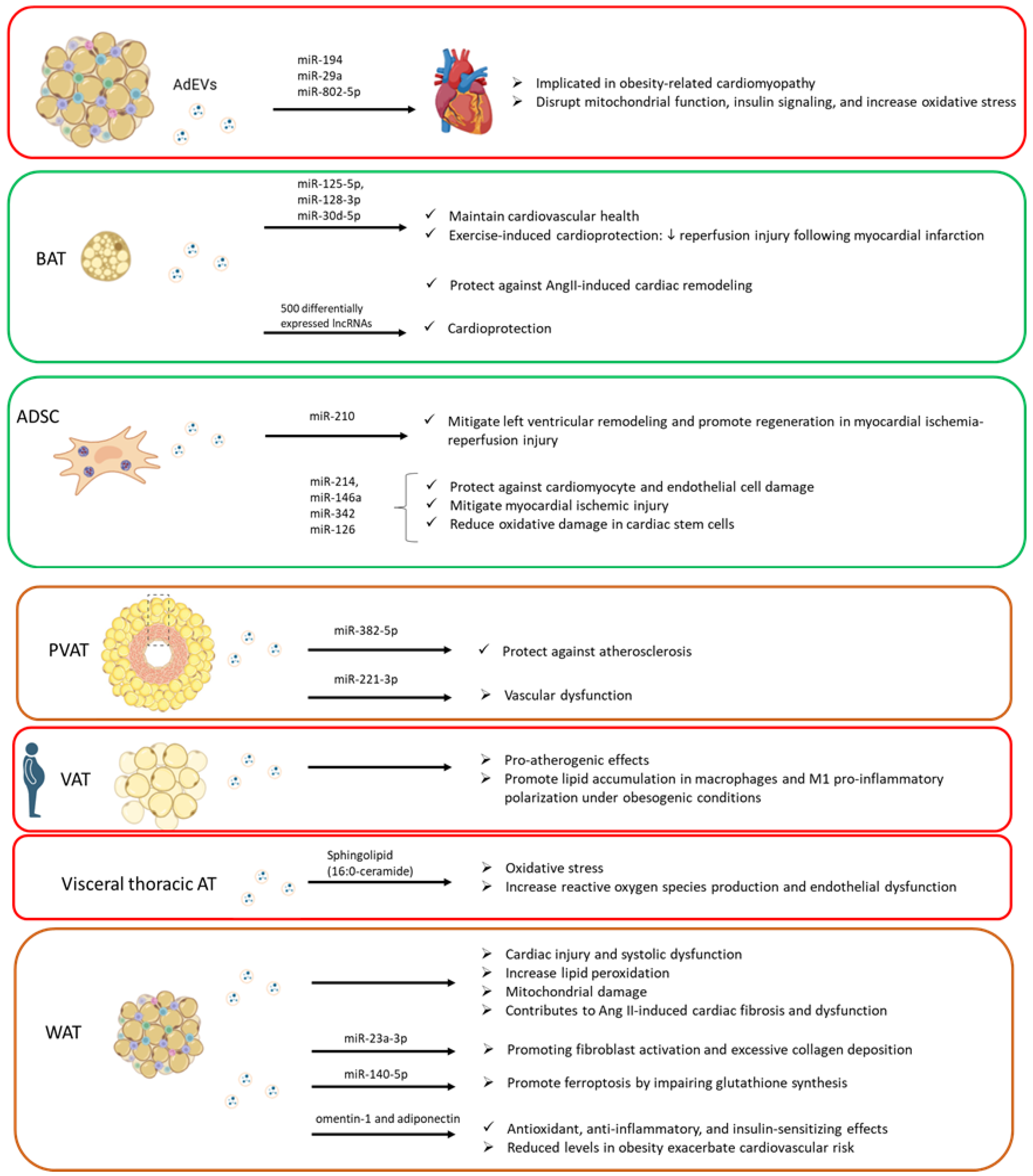
2.1. Influence of Adipose-Derived EVs on Cardiovascular System
2.2. Influence of Adipose-Derived EVs on Type 2 Diabetes
Influence of Adipose-Derived EVs on Type 2 Diabetes-Associated Comorbidities
| Identified Cargo from AdEVs | Effect on Obesity and T2D | References |
|---|---|---|
| miR-155 and miR-29a | Impairs insulin signaling by targeting insulin receptor substrates | [34,91] |
| 140 microRNAs | Angiogenic properties | [92] |
| Adiponectin and Resistin | Facilitate the transfer of their transcripts to macrophages | [92] |
| miR-500a-5p | Promotes adipocyte inflammation by suppressing Nrf2 expression | [93] |
| miR-210-3p | Delivered to neighboring adipocytes, skeletal muscle cells, and hepatocytes, it reduces insulin sensitivity and suppresses glucose transporter type 4 expression | [94] |
| miR-155 | Promotes M1 macrophage polarization by targeting the suppressor of cytokine signaling 1 gene | [95] |
| miR-34a and miR-1224 | Suppress M2 macrophage polarization by targeting the Krüppel-like factor 4 and Musashi RNA-binding protein 2 genes M1 macrophage accumulation leads to the release of pro-inflammatory cytokines in adipose tissue, contributing to insulin resistance | [127,128,129] |
| miR-4431, miR-548ab/ag, and miR-450a-5p | Exacerbate inflammation and metabolic dysfunction | [97] |
| STAT3 | Anti-inflammatory and metabolic benefits associated with obesity Activates M2 macrophages | [98] |
| Insulinotropic proteins | Glucose regulation Improve insulin secretion and glucose tolerance, highlighting their role in β-cell adaptation to insulin resistance | [92] |
| PTP1B and PP2A | Inhibition of PTP1B and PP2A activity in EVs from individuals with insulin resistance restores insulin signaling in adipocytes and hepatocytes | [99] |
| miR-122 | Promotes adipogenesis by targeting the vitamin D3 receptor gene, which serves as a negative regulator of the sterol regulatory element-binding transcription factor 1 | [101] |
| miR-27a | Induces insulin resistance by targeting the peroxisome proliferator-activated receptor gamma gene | [100] |
| miR-27a-5p | Delivered to pancreatic β-cells, impairs insulin secretion by downregulating the CaV1.2 calcium channel in β-cells. This led to glucose intolerance. | [112] |
| miR-222 | Inhibits the insulin receptor substrate 1 (IRS1) gene, impairing glucose uptake in skeletal muscle and hepatocytes | [108,109] |
| miR-99b | In hepatocytes, suppress the fibroblast growth factor 21 gene, reducing insulin sensitivity | [110] |
| miR-141-3p | Deficiency in miR-141-3p impairs insulin signaling by upregulating phosphatase and tensin homolog (PTEN), a negative regulator of the PI3K/Akt pathway, which is critical for glucose uptake | [111] |
| miR-103-3p and let-7f-5p | Disrupts insulin signaling in the liver | [113] |
| miR-130b-3p | Promotes myocardial injury | [120] |
| miR-326-3p | Suppresses Rictor expression and contributes to cardiac impairments | [114] |
| miR-802-5p | Cardiac insulin resistance Downregulates HSP60, a regulator of insulin-like growth factor-1 receptor (IGF-1R) signaling, which is inversely associated with the progression of diabetic cardiomyopathy | [80] |
| miR-9-3p | Hippocampal synaptic loss and cognitive impairment Suppresses hippocampal BDNF expression, a critical factor for synaptic function | [126] |
2.3. Influence of Adipose-Derived EVs on Liver Lipid Metabolism and Inflammation: Metabolic-Associated Fatty Liver Disease
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahase, E. Global Cost of Overweight and Obesity Will Hit $4.32tn a Year by 2035, Report Warns. BMJ 2023, 380, 523. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-Tissue Plasticity in Health and Disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Valls-Bellés, V.; Arilla-Codoñer, A.; Alonso-Iglesias, E. Oxidant Mechanisms in Childhood Obesity: The Link between Inflammation and Oxidative Stress. Transl. Res. 2011, 158, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Noor, S.; Menzel, A.; Doşa, A.; Pivina, L.; Bjørklund, G. Obesity and Insulin Resistance: Associations with Chronic Inflammation, Genetic and Epigenetic Factors. Curr. Med. Chem. 2021, 28, 800–826. [Google Scholar] [CrossRef]
- Baldelli, S.; Aiello, G.; Mansilla Di Martino, E.; Campaci, D.; Muthanna, F.M.S.; Lombardo, M. The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin. Nutrients 2024, 16, 2436. [Google Scholar] [CrossRef]
- Harvey, I.; Boudreau, A.; Stephens, J.M. Adipose Tissue in Health and Disease. Open Biol. 2020, 10, 1281–1306. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castro, F.; Morselli, E.; Claret, M. Interplay between the Brain and Adipose Tissue: A Metabolic Conversation. EMBO Rep. 2024, 25, 5277–5293. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Chen, J.; He, Y.; Ma, W.; Liu, X.; Sun, X. Effects of Multi-Organ Crosstalk on the Physiology and Pathology of Adipose Tissue. Front. Endocrinol. 2023, 14, 1198984. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cox-Vázquez, S.J.; Cho, N.J.; Bazan, G.C.; Groves, J.T. Direct Imaging with Multidimensional Labelling and High-Content Analysis Allows Quantitative Categorization and Characterizations of Individual Small Extracellular Vesicles and Nanoparticles (SEVPs). J. Extracell. Vesicles 2024, 13, e12520. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Pastor, A. Extracellular Vesicles as Mediators of Neuroinflammation in Intercellular and Inter-Organ Crosstalk. Int. J. Mol. Sci. 2024, 25, 7041. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Cabrera-Pastor, A. Emerging Role of Extracellular Vesicles as Biomarkers in Neurodegenerative Diseases and Their Clinical and Therapeutic Potential in Central Nervous System Pathologies. Int. J. Mol. Sci. 2024, 25, 10068. [Google Scholar] [CrossRef] [PubMed]
- Bond, S.T.; Calkin, A.C.; Drew, B.G. Adipose-Derived Extracellular Vesicles: Systemic Messengers and Metabolic Regulators in Health and Disease. Front. Physiol. 2022, 13, 837001. [Google Scholar] [CrossRef] [PubMed]
- Corvera, S. Cellular Heterogeneity in Adipose Tissues. Annu. Rev. Physiol. 2021, 83, 257–278. [Google Scholar] [CrossRef]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular Characterization of the Human Beta 3-Adrenergic Receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef]
- Lowell, B.B.; S-Susulic, V.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of Obesity in Transgenic Mice after Genetic Ablation of Brown Adipose Tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. New Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Freedland, E.S. Role of a Critical Visceral Adipose Tissue Threshold (CVATT) in Metabolic Syndrome: Implications for Controlling Dietary Carbohydrates: A Review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef]
- Muzzin, P.; Revelli, J.P.; Kuhne, F.; Gocayne, J.D.; McCombie, W.R.; Venter, J.C.; Giacobino, J.P.; Fraser, C.M. An Adipose Tissue-Specific Beta-Adrenergic Receptor. Molecular Cloning and down-Regulation in Obesity. J. Biol. Chem. 1991, 266, 24053–24058. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown Adipose Tissue Is Associated with Cardiometabolic Health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of Human Brown Adipose Tissue in Lean and Obese Young Men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Chen, M.; Xu, K.; Chen, B. The Impact of Obesity on Adipocyte-Derived Extracellular Vesicles. Cell Mol. Life Sci. 2021, 78, 7275–7288. [Google Scholar] [CrossRef]
- Hartwig, S.; De Filippo, E.; Göddeke, S.; Knebel, B.; Kotzka, J.; Al-Hasani, H.; Roden, M.; Lehr, S.; Sell, H. Exosomal Proteins Constitute an Essential Part of the Human Adipose Tissue Secretome. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 140172. [Google Scholar] [CrossRef]
- Michel, L.Y.M. Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart. Int. J. Mol. Sci. 2023, 24, 7745. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gao, X.; Liu, L.; Li, Z.; Wan, Z.; Dong, Y.; Chen, X.; Niu, Y.; Zhang, J.; Yang, G. Visceral Adipose Tissue Derived Exosomes Exacerbate Colitis Severity via Pro-Inflammatory MiRNAs in High Fat Diet Fed Mice. ACS Nano 2020, 14, 5099–5110. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, H.K.; Cho, Y.; Choi, J.S.; Woo, C.H.; Lee, K.S.; Sul, J.H.; Lee, C.M.; Han, J.; Park, J.H.; et al. Cell Reprogramming Using Extracellular Vesicles from Differentiating Stem Cells into White/Beige Adipocytes. Sci. Adv. 2020, 6, eaay6721. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The Ominous Triad of Adipose Tissue Dysfunction: Inflammation, Fibrosis, and Impaired Angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Adipose Tissue Dysfunction Contributes to Obesity Related Metabolic Diseases. Best. Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Gao, H.; Dos Reis, F.C.G.; Bandyopadhyay, G.; Ofrecio, J.M.; Luo, Z.; Ji, Y.; Jin, Z.; Ly, C.; Olefsky, J.M. MiR-690, an Exosomal-Derived MiRNA from M2-Polarized Macrophages, Improves Insulin Sensitivity in Obese Mice. Cell Metab. 2021, 33, 781–790.e5. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal MiRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef] [PubMed]
- Tryggestad, J.B.; Teague, A.M.; Sparling, D.P.; Jiang, S.; Chernausek, S.D. Macrophage-Derived MicroRNA-155 Increases in Obesity and Influences Adipocyte Metabolism by Targeting Peroxisome Proliferator-Activated Receptor Gamma. Obesity 2019, 27, 1856–1864. [Google Scholar] [CrossRef]
- Tian, F.; Tang, P.; Sun, Z.; Zhang, R.; Zhu, D.; He, J.; Liao, J.; Wan, Q.; Shen, J. MiR-210 in Exosomes Derived from Macrophages under High Glucose Promotes Mouse Diabetic Obesity Pathogenesis by Suppressing NDUFA4 Expression. J. Diabetes Res. 2020, 2020, 6894684. [Google Scholar] [CrossRef]
- Paneru, B.D.; Hill, D.A. The role of extracellular vesicle-derived miRNAs in adipose tissue function and metabolic health. Immunometabolism 2023, 5, e00027. [Google Scholar] [CrossRef]
- Le Lay, S.; Rome, S.; Loyer, X.; Nieto, L. Adipocyte-Derived Extracellular Vesicles in Health and Diseases: Nano-Packages with Vast Biological Properties. FASEB Bioadv. 2021, 3, 407–419. [Google Scholar] [CrossRef]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef] [PubMed]
- Camino, T.; Lago-Baameiro, N.; Bravo, S.B.; Molares-Vila, A.; Sueiro, A.; Couto, I.; Baltar, J.; Casanueva, E.F.; Pardo, M. Human Obese White Adipose Tissue Sheds Depot-Specific Extracellular Vesicles and Reveals Candidate Biomarkers for Monitoring Obesity and Its Comorbidities. Transl. Res. 2022, 239, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Lazic, M.; Armando, A.M.; Phillips, S.A.; Katebian, R.; Maraka, S.; Quehenberger, O.; Sears, D.D.; Feldstein, A.E. Circulating Adipocyte-Derived Extracellular Vesicles Are Novel Markers of Metabolic Stress. J. Mol. Med. 2016, 94, 1241–1253. [Google Scholar] [CrossRef]
- Mleczko, J.; Ortega, F.J.; Falcon-Perez, J.M.; Wabitsch, M.; Fernandez-Real, J.M.; Mora, S. Extracellular Vesicles from Hypoxic Adipocytes and Obese Subjects Reduce Insulin-Stimulated Glucose Uptake. Mol. Nutr. Food Res. 2018, 62, 1700917. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Teoh, S.L.; Das, S. MicroRNAs in Various Body Fluids and Their Importance in Forensic Medicine. Mini Rev. Med. Chem. 2022, 22, 2332–2343. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in Body Fluids--the Mix of Hormones and Biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef]
- Cui, H.; Lv, K.; Yang, N. HDL and MicroRNAs. Adv. Exp. Med. Biol. 2022, 1377, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, J.M.; Klemm, S.L.; Zheng, Y.; Sahay, A.; Blüthgen, N.; Marks, D.S.; Van Oudenaarden, A. Gene Expression. MicroRNA Control of Protein Expression Noise. Science 2015, 348, 128–131. [Google Scholar] [CrossRef]
- Fan, R.; Hilfinger, A. The Effect of MicroRNA on Protein Variability and Gene Expression Fidelity. Biophys. J. 2023, 122, 905–923. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating MiRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Gu, R.; Ke, Y.; Zhang, S.; Su, X.; Pan, X.; He, Q.; Li, G.; Zhang, Z.; et al. Development and Evaluation of Reconstructed Nanovesicles from Turmeric for Multifaceted Obesity Intervention. ACS Nano 2024, 18, 23117–23135. [Google Scholar] [CrossRef] [PubMed]
- Castaño, C.; Meza-Ramos, A.; Batlle, M.; Guasch, E.; Novials, A.; Párrizas, M. Treatment with EV-miRNAs Alleviates Obesity-Associated Metabolic Dysfunction in Mice. Int. J. Mol. Sci. 2022, 23, 14920. [Google Scholar] [CrossRef]
- Duisenbek, A.; Lopez-Armas, G.C.; Pérez, M.; Avilés Pérez, M.D.; Aguilar Benitez, J.M.; Pereira Pérez, V.R.; Gorts Ortega, J.; Yessenbekova, A.; Ablaikhanova, N.; Escames, G.; et al. Insights into the Role of Plasmatic and Exosomal microRNAs in Oxidative Stress-Related Metabolic Diseases. Antioxidants 2023, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, R.; Wang, G.; Brandão, B.B.; Zanotto, T.M.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA Sequence Codes for Small Extracellular Vesicle Release and Cellular Retention. Nature 2022, 601, 446–451. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated HnRNPA2B1 Controls the Sorting of MiRNAs into Exosomes through Binding to Specific Motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef] [PubMed]
- Temoche-Diaz, M.M.; Shurtleff, M.J.; Nottingham, R.M.; Yao, J.; Fadadu, R.P.; Lambowitz, A.M.; Schekman, R. Distinct Mechanisms of MicroRNA Sorting into Cancer Cell-Derived Extracellular Vesicle Subtypes. Elife 2019, 8, e47544. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular Vesicle-Based Interorgan Transport of Mitochondria from Energetically Stressed Adipocytes. Cell Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Ding, L.L.Q.; Xu, L.; Huang, J.; Zhang, Z.B.; Chen, X.H.; Cheng, Y.W.; Ruan, C.C.; Gao, P.J. Brown Adipocyte ADRB3 Mediates Cardioprotection via Suppressing Exosomal INOS. Circ. Res. 2022, 131, 133–147. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Hu, G.; Li, C.; Guo, L.; Zhang, L.; Sun, F.; Xia, Y.; Yan, W.; Cui, Z.; et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ. Res. 2022, 130, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Wu, Y.; Zhang, Q.; Liu, P.; Zhang, S.; Yu, M.; Tian, W. Identification of Thermogenesis-Related LncRNAs in Small Extracellular Vesicles Derived from Adipose Tissue. BMC Genomics 2022, 23, 660. [Google Scholar] [CrossRef]
- Lindgren, E.M.; Nielsen, R.; Petrovic, N.; Jacobsson, A.; Mandrup, S.; Cannon, B.; Nedergaard, J. Noradrenaline Represses PPAR (Peroxisome-Proliferator-Activated Receptor) Gamma2 Gene Expression in Brown Adipocytes: Intracellular Signalling and Effects on PPARgamma2 and PPARgamma1 Protein Levels. Biochem. J. 2004, 382, 597–606. [Google Scholar] [CrossRef]
- Fang, X.; Stroud, M.J.; Ouyang, K.; Fang, L.; Zhang, J.; Dalton, N.D.; Gu, Y.; Wu, T.; Peterson, K.L.; Huang, H.D.; et al. Adipocyte-Specific Loss of PPAR γ Attenuates Cardiac Hypertrophy. JCI Insight 2016, 1, e89908. [Google Scholar] [CrossRef] [PubMed]
- Wallach, J.D.; Wang, K.; Zhang, A.D.; Cheng, D.; Grossetta Nardini, H.K.; Lin, H.; Bracken, M.B.; Desai, M.; Krumholz, H.M.; Ross, J.S. Updating Insights into Rosiglitazone and Cardiovascular Risk through Shared Data: Individual Patient and Summary Level Meta-Analyses. BMJ 2020, 368, l7078. [Google Scholar] [CrossRef] [PubMed]
- Song, B.W.; Lee, C.Y.; Kim, R.; Kim, W.J.; Lee, H.W.; Lee, M.Y.; Kim, J.; Jeong, J.Y.; Chang, W. Multiplexed Targeting of MiRNA-210 in Stem Cell-Derived Extracellular Vesicles Promotes Selective Regeneration in Ischemic Hearts. Exp. Mol. Med. 2021, 53, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Takefuji, M.; Sakaguchi, T.; Ishihama, S.; Mori, Y.; Tsuda, T.; Takikawa, T.; Yoshida, T.; Ohashi, K.; Shimizu, Y.; et al. Cardiomyocytes Capture Stem Cell-Derived, Anti-Apoptotic MicroRNA-214 via Clathrin-Mediated Endocytosis in Acute Myocardial Infarction. J. Biol. Chem. 2019, 294, 11665–11674. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef]
- Pan, J.; Alimujiang, M.; Chen, Q.; Shi, H.; Luo, X. Exosomes Derived from MiR-146a-Modified Adipose-Derived Stem Cells Attenuate Acute Myocardial Infarction-Induced Myocardial Damage via Downregulation of Early Growth Response Factor 1. J. Cell Biochem. 2019, 120, 4433–4443. [Google Scholar] [CrossRef]
- Xing, X.; Li, Z.; Yang, X.; Li, M.; Liu, C.; Pang, Y.; Zhang, L.; Li, X.; Liu, G.; Xiao, Y. Adipose-Derived Mesenchymal Stem Cells-Derived Exosome-Mediated MicroRNA-342-5p Protects Endothelial Cells against Atherosclerosis. Aging 2020, 12, 3880–3898. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular Adipose-Derived Exosomes Reduce Macrophage Foam Cell Formation through MiR-382-5p and the BMP4-PPARγ-ABCA1/ABCG1 Pathways. Vascul. Pharmacol. 2022, 143, 106968. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular Adipose-Derived Exosomes Reduce Foam Cell Formation by Regulating Expression of Cholesterol Transporters. Front. Cardiovasc. Med. 2021, 8, 697510. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef]
- Akawi, N.; Checa, A.; Antonopoulos, A.S.; Akoumianakis, I.; Daskalaki, E.; Kotanidis, C.P.; Kondo, H.; Lee, K.; Yesilyurt, D.; Badi, I.; et al. Fat-Secreted Ceramides Regulate Vascular Redox State and Influence Outcomes in Patients With Cardiovascular Disease. J. Am. Coll. Cardiol. 2021, 77, 2494–2513. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular Adipose Tissue-Derived Extracellular Vesicle MiR-221-3p Mediates Vascular Remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef]
- Jafari, N.; Kolla, M.; Meshulam, T.; Shafran, J.S.; Qiu, Y.; Casey, A.N.; Pompa, I.R.; Ennis, C.S.; Mazzeo, C.S.; Rabhi, N.; et al. Adipocyte-Derived Exosomes May Promote Breast Cancer Progression in Type 2 Diabetes. Sci. Signal 2021, 14, eabj2807. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Claggett, B.L.; Liu, J.; Jackson, A.M.; Jhund, P.S.; Køber, L.; Widimský, J.; Boytsov, S.A.; Chopra, V.K.; Anand, I.S.; et al. Global Differences in Heart Failure With Preserved Ejection Fraction: The PARAGON-HF Trial. Circ. Heart Fail. 2021, 14, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; Bi, Y.; Ajoolabady, A.; You, F.; Sowers, J.; Wang, Q.; Ceylan, A.F.; Zhang, Y.; Ren, J. Parkin Insufficiency Accentuates High-Fat Diet-Induced Cardiac Remodeling and Contractile Dysfunction Through VDAC1-Mediated Mitochondrial Ca2+ Overload. JACC Basic. Transl. Sci. 2022, 7, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Si, L.; Bian, J.; Pan, C.; Guo, W.; Qin, P.; Zhu, W.; Xia, Y.; Zhang, Q.; Wei, K. Adipose Tissue Macrophage-Derived Exosomes Induce Ferroptosis via Glutathione Synthesis Inhibition by Targeting SLC7A11 in Obesity-Induced Cardiac Injury. Free Radic. Biol. Med. 2022, 182, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Pan, Y.; Zhou, Y. Exosomal MicroRNA-194 Causes Cardiac Injury and Mitochondrial Dysfunction in Obese Mice. Biochem. Biophys. Res. Commun. 2018, 503, 3174–3179. [Google Scholar] [CrossRef]
- Li, F.; Zhang, K.; Xu, T.; Du, W.; Yu, B.; Liu, Y.; Nie, H. Exosomal MicroRNA-29a Mediates Cardiac Dysfunction and Mitochondrial Inactivity in Obesity-Related Cardiomyopathy. Endocrine 2019, 63, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, J.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Hypertrophic Adipocyte-Derived Exosomal MiR-802-5p Contributes to Insulin Resistance in Cardiac Myocytes Through Targeting HSP60. Obesity 2020, 28, 1932–1940. [Google Scholar] [CrossRef]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018, 175, 695–708.e13. [Google Scholar] [CrossRef] [PubMed]
- Clement, E.; Lazar, I.; Attané, C.; Carrié, L.; Dauvillier, S.; Ducoux-Petit, M.; Esteve, D.; Menneteau, T.; Moutahir, M.; Le Gonidec, S.; et al. Adipocyte Extracellular Vesicles Carry Enzymes and Fatty Acids That Stimulate Mitochondrial Metabolism and Remodeling in Tumor Cells. EMBO J. 2020, 39, e102525. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and Transfer of Mitochondrial DNA via Exosomes Regulate Escape from Dormancy in Hormonal Therapy-Resistant Breast Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef] [PubMed]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Qi, M.; Zhao, P.; Duan, Y.; Yang, G.; Yuan, L. Brown Adipose Tissue-Derived Exosomes Mitigate the Metabolic Syndrome in High Fat Diet Mice. Theranostics 2020, 10, 8197–8210. [Google Scholar] [CrossRef]
- Dracheva, K.V.; Pobozheva, I.A.; Anisimova, K.A.; Balandov, S.G.; Hamid, Z.M.; Panteleeva, A.A.; Vasilevsky, D.I.; Pchelina, S.N.; Miroshnikova, V.V. Omentin-1 and Adiponectin Secretion via Adipose Tissue Extracellular Vesicles. Atherosclerosis 2021, 331, e146. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Moraña-Fernández, S.; Anido-Varela, L.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Moscoso, I.; Gualillo, O.; González-Juanatey, J.R.; et al. Adipokines and Inflammation: Focus on Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef]
- Li, J.; Gong, L.; Liu, S.; Zhang, Y.; Zhang, C.; Tian, M.; Lu, H.; Bu, P.; Yang, J.; Ouyang, C.; et al. Adipose HuR Protects against Diet-Induced Obesity and Insulin Resistance. Nat. Commun. 2019, 10, 2375. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.R.; Anthony, S.R.; Gozdiff, A.; Green, L.C.; Fleifil, S.M.; Slone, S.; Nieman, M.L.; Alam, P.; Benoit, J.B.; Owens, A.P.; et al. Adipocyte-Specific Deletion of HuR Induces Spontaneous Cardiac Hypertrophy and Fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, 228–241. [Google Scholar] [CrossRef]
- Su, M.; Li, W.; Yuan, Y.; Liu, S.; Liang, C.; Liu, H.; Zhang, R.; Liu, Y.; Sun, L.; Wei, Y.; et al. Epididymal White Adipose Tissue Promotes Angiotensin II-Induced Cardiac Fibrosis in an Exosome-Dependent Manner. Transl. Res. 2022, 248, 51–67. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Y.C.; Cheng, P.; Shao, H.G. Adipose Tissue Macrophage-Derived Exosomal MiR-29a Regulates Obesity-Associated Insulin Resistance. Biochem. Biophys. Res. Commun. 2019, 515, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Kulaj, K.; Harger, A.; Bauer, M.; Caliskan, Ö.S.; Gupta, T.K.; Chiang, D.M.; Milbank, E.; Reber, J.; Karlas, A.; Kotzbeck, P.; et al. Adipocyte-Derived Extracellular Vesicles Increase Insulin Secretion through Transport of Insulinotropic Protein Cargo. Nat. Commun. 2023, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Tian, Y.; Yang, C.; Liu, Y.F.; Qu, S.L.; Huang, L.; Zhang, C. Adipose Tissue Macrophages-Derived Exosomal MiR-500a-5p under High Glucose Promotes Adipocytes Inflammation by Suppressing Nrf2 Expression. Int. J. Biochem. Cell Biol. 2025, 178, 106713. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Ramprasad, P.; Sharma, S.; Dey, U.; Kumar, V.; Singh, S.; Dasgupta, S.; Kumar, A.; Tikoo, K.; Pal, D. Adipose Tissue Macrophage-Derived MicroRNA-210-3p Disrupts Systemic Insulin Sensitivity by Silencing GLUT4 in Obesity. J. Biol. Chem. 2024, 300, 107328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, H.; Chang, X.; Chen, F.; Zhu, Y.; Han, X. Adipocyte-Derived Microvesicles from Obese Mice Induce M1 Macrophage Phenotype through Secreted MiR-155. J. Mol. Cell Biol. 2016, 8, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Sou, Y.L.; Chilian, W.M.; Ratnam, W.; Zain, S.M.; Syed Abdul Kadir, S.Z.; Pan, Y.; Pung, Y.F. Exosomal miRNAs and isomiRs: Potential biomarkers for type 2 diabetes mellitus. Precis. Clin. Med. 2024, 7, pbae021. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, Y.; Chen, Y.; Sun, C.; Liang, M.; Chu, X.; Wen, X.; Yuan, F.; Peng, C.; Wang, C.; et al. Palmitic Acid Promotes MiRNA Release from Adipocyte Exosomes by Activating NF-ΚB/ER Stress. Nutr. Diabetes 2024, 14, 75. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Vidal-Gómez, X.; Piquet, M.; Vergori, L.; Simard, G.; Dubois, S.; Ducluzeau, P.H.; Pomiès, P.; Kamli-Salino, S.; Delibégovic, M.; et al. Circulating Extracellular Vesicle-Carried PTP1B and PP2A Phosphatases as Regulators of Insulin Resistance. Diabetologia 2024, 68, 231–242. [Google Scholar] [CrossRef]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARγ. Theranostics 2018, 8, 2171–2188. [Google Scholar] [CrossRef]
- Huang, X.Y.; Chen, J.X.; Ren, Y.; Fan, L.C.; Xiang, W.; He, X.J. Exosomal MiR-122 Promotes Adipogenesis and Aggravates Obesity through the VDR/SREBF1 Axis. Obesity 2022, 30, 666–679. [Google Scholar] [CrossRef]
- Ojima, K.; Muroya, S.; Wada, H.; Ogawa, K.; Oe, M.; Takimoto, K.; Nishimura, T. Immature Adipocyte-Derived Exosomes Inhibit Expression of Muscle Differentiation Markers. FEBS Open Bio 2021, 11, 768–781. [Google Scholar] [CrossRef]
- Han, Y.; Ye, S.; Liu, B. Roles of Extracellular Vesicles Derived from Healthy and Obese Adipose Tissue in Inter-Organ Crosstalk and Potential Clinical Implication. Front. Endocrinol. 2024, 15, 1409000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, Y.Q.; Da, M.X.; Jin, W.L.; Zhou, F.H. Adipocyte-derived extracellular vesicles: Bridging the communications between obesity and tumor microenvironment. Discov. Oncol. 2023, 14, 92. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Casu, A.; Kovacova, Z.; Petrilli, A.M.; Sideleva, O.; Tharp, W.G.; Pratley, R.E. Coordinated regulation of gene expression and microRNA changes in adipose tissue and circulating extracellular vesicles in response to pioglitazone treatment in humans with type 2 diabetes. Front Endocrinol 2022, 13, 955593. [Google Scholar] [CrossRef] [PubMed]
- Gesmundo, I.; Pardini, B.; Gargantini, E.; Gamba, G.; Birolo, G.; Fanciulli, A.; Banfi, D.; Congiusta, N.; Favaro, E.; Deregibus, M.C.; et al. Adipocyte-Derived Extracellular Vesicles Regulate Survival and Function of Pancreatic β Cells. JCI Insight 2021, 6, 141962. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, X.; Li, Y.; Jia, L.; Zhai, L.; Wei, W.; Zhang, L.; Jiang, H.; Bai, Y. Exercise-Induced Browning of White Adipose Tissue and Improving Skeletal Muscle Insulin Sensitivity in Obese/Non-Obese Growing Mice: Do Not Neglect Exosomal MiR-27a. Front. Nutr. 2022, 9, 940673. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.; Ashkezari, M.D.; Seifati, S.M.; Mehrjardi, M.Y.V.; Tezerjani, M.D.; Sadeghzadeh, S.; Ladan, S.A.B. Circulating MiR-15a and MiR-222 as Potential Biomarkers of Type 2 Diabetes. Diabetes Metab. Syndr. Obes. 2020, 13, 3461–3469. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Song, H.; Shuo, L.; Wang, L.; Xie, P.; Li, W.; Liu, J.; Tong, Y.; Zhang, C.Y.; Jiang, X.; et al. Gonadal White Adipose Tissue-Derived Exosomal MiR-222 Promotes Obesity-Associated Insulin Resistance. Aging 2020, 12, 22719–22737. [Google Scholar] [CrossRef]
- Gong, Q.; Hu, Z.; Zhang, F.; Cui, A.; Chen, X.; Jiang, H.; Gao, J.; Chen, X.; Han, Y.; Liang, Q.; et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 2016, 64, 425–438. [Google Scholar] [CrossRef]
- Dang, S.Y.; Leng, Y.; Wang, Z.X.; Xiao, X.; Zhang, X.; Wen, T.; Gong, H.Z.; Hong, A.; Ma, Y. Exosomal Transfer of Obesity Adipose Tissue for Decreased MiR-141-3p Mediate Insulin Resistance of Hepatocytes. Int. J. Biol. Sci. 2019, 15, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, B.; Yang, Y.; Niu, F.; Lin, C.; Yuan, H.; Wang, J.; Wu, T.; Shao, Y.; Shao, S.; et al. Visceral Adipocyte-Derived Extracellular Vesicle MiR-27a-5p Elicits Glucose Intolerance by Inhibiting Pancreatic β-Cell Insulin Secretion. Diabetes 2024, 73, 1832–1847. [Google Scholar] [CrossRef]
- Lino, M.; Garcia-Martin, R.; Muñoz, V.R.; Ruiz, G.P.; Nawaz, A.; Brandão, B.B.; Dreyfus, J.; Pan, H.; Kahn, C.R. Multi-Step Regulation of MicroRNA Expression and Secretion into Small Extracellular Vesicles by Insulin. Cell Rep. 2024, 43, 114491. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Pan, J.; Ke, J.; Zhang, A.; Liu, Y.; Wang, C.; Chang, A.C.Y.; Gu, J. Secretion of MiRNA-326-3p by Senescent Adipose Exacerbates Myocardial Metabolism in Diabetic Mice. J. Transl. Med. 2022, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.M.; Liu, M.L. Microvesicles and Diabetic Complications--Novel Mediators, Potential Biomarkers and Therapeutic Targets. Acta Pharmacol. Sin. 2014, 35, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, G.; Liu, M.L. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genom. Proteom. Bioinform. 2018, 16, 50–62. [Google Scholar] [CrossRef]
- Liu, M.L.; Williams, K.J. Microvesicles: Potential Markers and Mediators of Endothelial Dysfunction. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 121–127. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Liu, M.L. Pathogenic Roles of Microvesicles in Diabetic Retinopathy. Acta Pharmacol. Sin. 2018, 39, 1–11. [Google Scholar] [CrossRef]
- Le Jeune, S.; Sadoudi, S.; Charue, D.; Abid, S.; Guigner, J.M.; Helley, D.; Bihan, H.; Baudry, C.; Lelong, H.; Mirault, T.; et al. Low Grade Intravascular Hemolysis Associates with Peripheral Nerve Injury in Type 2 Diabetes. PLoS ONE 2022, 17, e0275337. [Google Scholar] [CrossRef]
- Gan, L.; Xie, D.; Liu, J.; Bond Lau, W.; Christopher, T.A.; Lopez, B.; Zhang, L.; Gao, E.; Koch, W.; Ma, X.L.; et al. Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice. Circulation 2020, 141, 968–983. [Google Scholar] [CrossRef]
- Shan, Y.X.; Yang, T.L.; Mestril, R.; Wang, P.H. Hsp10 and Hsp60 Suppress Ubiquitination of Insulin-like Growth Factor-1 Receptor and Augment Insulin-like Growth Factor-1 Receptor Signaling in Cardiac Muscle: Implications on Decreased Myocardial Protection in Diabetic Cardiomyopathy. J. Biol. Chem. 2003, 278, 45492–45498. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Wang, Y.; Cao, Y.; Yao, D.; Sun, L.; Qin, L.; Qiu, H.; Zhan, X. Adipocyte-Derived Extracellular Vesicles Modulate Appetite and Weight through MTOR Signalling in the Hypothalamus. Acta Physiol. 2020, 228, e13339. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S. ichiro Extracellular Vesicle-Contained ENAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019, 30, 329–342.e5. [Google Scholar] [CrossRef] [PubMed]
- Tokizane, K.; Brace, C.S.; Imai, S. ichiro DMHPpp1r17 Neurons Regulate Aging and Lifespan in Mice through Hypothalamic-Adipose Inter-Tissue Communication. Cell Metab. 2024, 36, 377–392.e11. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Despa, F. Cognitive Decline and Dementia in Diabetes Mellitus: Mechanisms and Clinical Implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular Vesicles Mediate the Communication of Adipose Tissue with Brain and Promote Cognitive Impairment Associated with Insulin Resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Singh, P.; Dohare, R.; Jha, R.; Rahmani, A.H.; Almatroodi, S.A.; Ali, S.; Syed, M.A. Inhibition of miRNA-34a Promotes M2 Macrophage Polarization and Improves LPS-Induced Lung Injury by Targeting Klf4. Genes 2020, 11, 966. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-Secreted Exosomal MicroRNA-34a Inhibits M2 Macrophage Polarization to Promote Obesity-Induced Adipose Inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yao, X.; Teng, Y.; Zhao, T.; Lin, L.; Li, Y.; Shang, H.; Jin, Y.; Jin, Q. Adipocytes-Derived Exosomal MicroRNA-1224 Inhibits M2 Macrophage Polarization in Obesity-Induced Adipose Tissue Inflammation via MSI2-Mediated Wnt/β-Catenin Axis. Mol. Nutr. Food Res. 2022, 66, e2100889. [Google Scholar] [CrossRef]
- Kumar, V.; Kiran, S.; Kumar, S.; Singh, U.P. Extracellular Vesicles in Obesity and Its Associated Inflammation. Int. Rev. Immunol. 2022, 41, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Q. Macrophage Recruitment in Obese Adipose Tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef]
- Zhou, Z.; Tao, Y.; Zhao, H.; Wang, Q. Adipose Extracellular Vesicles: Messengers From and to Macrophages in Regulating Immunometabolic Homeostasis or Disorders. Front. Immunol. 2021, 12, 666344. [Google Scholar] [CrossRef]
- Ortega, F.J.; Moreno, M.; Mercader, J.M.; Moreno-Navarrete, J.M.; Fuentes-Batllevell, N.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Inflammation Triggers Specific MicroRNA Profiles in Human Adipocytes and Macrophages and in Their Supernatants. Clin. Epigenetics 2015, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, M.C.; Lambooij, J.M.; Pu, X.; Fagundes, R.R.; Enciso-Martinez, A.; Kats, K.; Giepmans, B.N.G.; Guigas, B.; Zaldumbide, A. Extracellular Vesicles Derived from Stressed Beta Cells Mediate Monocyte Activation and Contribute to Islet Inflammation. Front. Immunol. 2024, 15, 1393248. [Google Scholar] [CrossRef] [PubMed]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How Is It Different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Yus, M.; Hörndler, C.; Borlan, S.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Unraveling Adipose Tissue Dysfunction: Molecular Mechanisms, Novel Biomarkers, and Therapeutic Targets for Liver Fat Deposition. Cells 2024, 13, 380. [Google Scholar] [CrossRef]
- Basil, B.; Myke-Mbata, B.K.; Eze, O.E.; Akubue, A.U. From Adiposity to Steatosis: Metabolic Dysfunction-Associated Steatotic Liver Disease, a Hepatic Expression of Metabolic Syndrome—Current Insights and Future Directions. Clin. Diabetes Endocrinol. 2024, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, D.; Vesković, M.; Šutulović, N.; Hrnčić, D.; Stanojlović, O.; Radić, L.; Macut, J.B.; Macut, D. Adipose-Derived Extracellular Vesicles—A Novel Cross-Talk Mechanism in Insulin Resistance, Non-Alcoholic Fatty Liver Disease, and Polycystic Ovary Syndrome. Endocrine 2024, 85, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, M.F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.W.; Shen, D.; Zhang, J.Z.; Zhou, N.; He, J.; et al. Liver Governs Adipose Remodelling via Extracellular Vesicles in Response to Lipid Overload. Nat. Commun. 2020, 11, 719. [Google Scholar] [CrossRef]
- Payet, T.; Gabinaud, E.; Landrier, J.F.; Mounien, L. Role of Micro-RNAs Associated with Adipose-Derived Extracellular Vesicles in Metabolic Disorders. Obes. Rev. 2024, 25, e13755. [Google Scholar] [CrossRef]
- Kranendonk, M.E.G.; Visseren, F.L.J.; Van Herwaarden, J.A.; Nolte-’t Hoen, E.N.M.; De Jager, W.; Wauben, M.H.M.; Kalkhoven, E. Effect of Extracellular Vesicles of Human Adipose Tissue on Insulin Signaling in Liver and Muscle Cells. Obesity 2014, 22, 2216–2223. [Google Scholar] [CrossRef]
- Rong, B.; Feng, R.; Liu, C.; Wu, Q.; Sun, C. Reduced Delivery of Epididymal Adipocyte-Derived Exosomal Resistin Is Essential for Melatonin Ameliorating Hepatic Steatosis in Mice. J. Pineal Res. 2019, 66, e12561. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yang, K.; Shen, Z.; Jia, K.; Liu, P.; Pan, M.; Sun, C. ER Stress-Induced Adipocytes Secrete-Aldo-Keto Reductase 1B7-Containing Exosomes That Cause Nonalcoholic Steatohepatitis in Mice. Free Radic. Biol. Med. 2021, 163, 220–233. [Google Scholar] [CrossRef]
- Song, H.; Canup, B.S.B.; Ngo, V.L.; Denning, T.L.; Garg, P.; Laroui, H. Internalization of Garlic-Derived Nanovesicles on Liver Cells Is Triggered by Interaction with CD98. ACS Omega 2020, 5, 23118–23128. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Fang, Q.H.; Liu, M.L.; Lin, J.N. Current Understanding of the Role of Adipose-Derived Extracellular Vesicles in Metabolic Homeostasis and Diseases: Communication from the Distance between Cells/Tissues. Theranostics 2020, 10, 7422–7435. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; Cannataro, R.; Gallelli, L.; De Sarro, G.; Caroleo, M.C. Exosome MicroRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options. Pharmaceuticals 2021, 14, 1257. [Google Scholar] [CrossRef]
- Koeck, E.S.; Iordanskaia, T.; Sevilla, S.; Ferrante, S.C.; Hubal, M.J.; Freishtat, R.J.; Nadler, E.P. Adipocyte Exosomes Induce Transforming Growth Factor Beta Pathway Dysregulation in Hepatocytes: A Novel Paradigm for Obesity-Related Liver Disease. J. Surg. Res. 2014, 192, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Kaufman, R.J. Endoplasmic Reticulum Stress in Liver Disease. J. Hepatol. 2011, 54, 795–809. [Google Scholar] [CrossRef]
- Lu, M.M.; Ren, Y.; Zhou, Y.W.; Xu, L.L.; Zhang, M.M.; Ding, L.P.; Cheng, W.X.; Jin, X. Antagonizing Adipose Tissue-Derived Exosome MiR-103-Hepatocyte Phosphatase and Tensin Homolog Pathway Alleviates Autophagy in Non-Alcoholic Steatohepatitis: A Trans-Cellular Crosstalk. World J. Gastroenterol. 2023, 29, 4528–4541. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Chavey, C.; Mari, B.; Monthouel, M.N.; Bonnafous, S.; Anglard, P.; Van Obberghen, E.; Tartare-Deckert, S. Matrix Metalloproteinases Are Differentially Expressed in Adipose Tissue during Obesity and Modulate Adipocyte Differentiation. J. Biol. Chem. 2003, 278, 11888–11896. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Khokha, R. Metalloproteinases in Extracellular Vesicles. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R.; Maquoi, E.; Demeulemeester, D.; Van Hoef, B.; Collen, D. Modulation of Fibrinolytic and Gelatinolytic Activity during Adipose Tissue Development in a Mouse Model of Nutritionally Induced Obesity. Thromb. Haemost. 2002, 88, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Liu, Y.; Chen, Y.; Yu, D.; Williams, K.J.; Liu, M.L. Novel Proteolytic Microvesicles Released from Human Macrophages after Exposure to Tobacco Smoke. Am. J. Pathol. 2013, 182, 1552–1562. [Google Scholar] [CrossRef]
- Marino, L.; Ni, B.; Farrar, J.S.; Lownik, J.C.; Pearce, J.V.; Martin, R.K.; Celi, F.S. Adipose Tissue-Selective Ablation of ADAM10 Results in Divergent Metabolic Phenotypes Following Long-Term Dietary Manipulation. Adipocyte 2024, 13, 2339418. [Google Scholar] [CrossRef]
- Matthews, J.; Villescas, S.; Herat, L.; Schlaich, M.; Matthews, V. Implications of ADAM17 Activation for Hyperglycaemia, Obesity and Type 2 Diabetes. Biosci. Rep. 2021, 41, BSR20210029. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, M.; Li, C.; Frebelius, S.; Swedenborg, J.; Wågsäter, D.; Williams, K.J.; Eriksson, P.; Roy, J.; Liu, M.L. Proteolytically Active ADAM10 and ADAM17 Carried on Membrane Microvesicles in Human Abdominal Aortic Aneurysms. Thromb. Haemost. 2015, 114, 1165–1174. [Google Scholar] [CrossRef]
- DiStefano, J.K.; Piras, I.S.; Wu, X.; Sharma, R.; Garcia-Mansfield, K.; Willey, M.; Lovell, B.; Pirrotte, P.; Olson, M.L.; Shaibi, G.Q. Changes in Proteomic Cargo of Circulating Extracellular Vesicles in Response to Lifestyle Intervention in Adolescents with Hepatic Steatosis. Clin. Nutr. ESPEN 2024, 60, 333–342. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaguarnera, M.; Cauli, O.; Cabrera-Pastor, A. Obesity and Adipose-Derived Extracellular Vesicles: Implications for Metabolic Regulation and Disease. Biomolecules 2025, 15, 231. https://doi.org/10.3390/biom15020231
Malaguarnera M, Cauli O, Cabrera-Pastor A. Obesity and Adipose-Derived Extracellular Vesicles: Implications for Metabolic Regulation and Disease. Biomolecules. 2025; 15(2):231. https://doi.org/10.3390/biom15020231
Chicago/Turabian StyleMalaguarnera, Michele, Omar Cauli, and Andrea Cabrera-Pastor. 2025. "Obesity and Adipose-Derived Extracellular Vesicles: Implications for Metabolic Regulation and Disease" Biomolecules 15, no. 2: 231. https://doi.org/10.3390/biom15020231
APA StyleMalaguarnera, M., Cauli, O., & Cabrera-Pastor, A. (2025). Obesity and Adipose-Derived Extracellular Vesicles: Implications for Metabolic Regulation and Disease. Biomolecules, 15(2), 231. https://doi.org/10.3390/biom15020231







