Ultra-Processed Foods and Type 2 Diabetes Mellitus: What Is the Evidence So Far?
Abstract
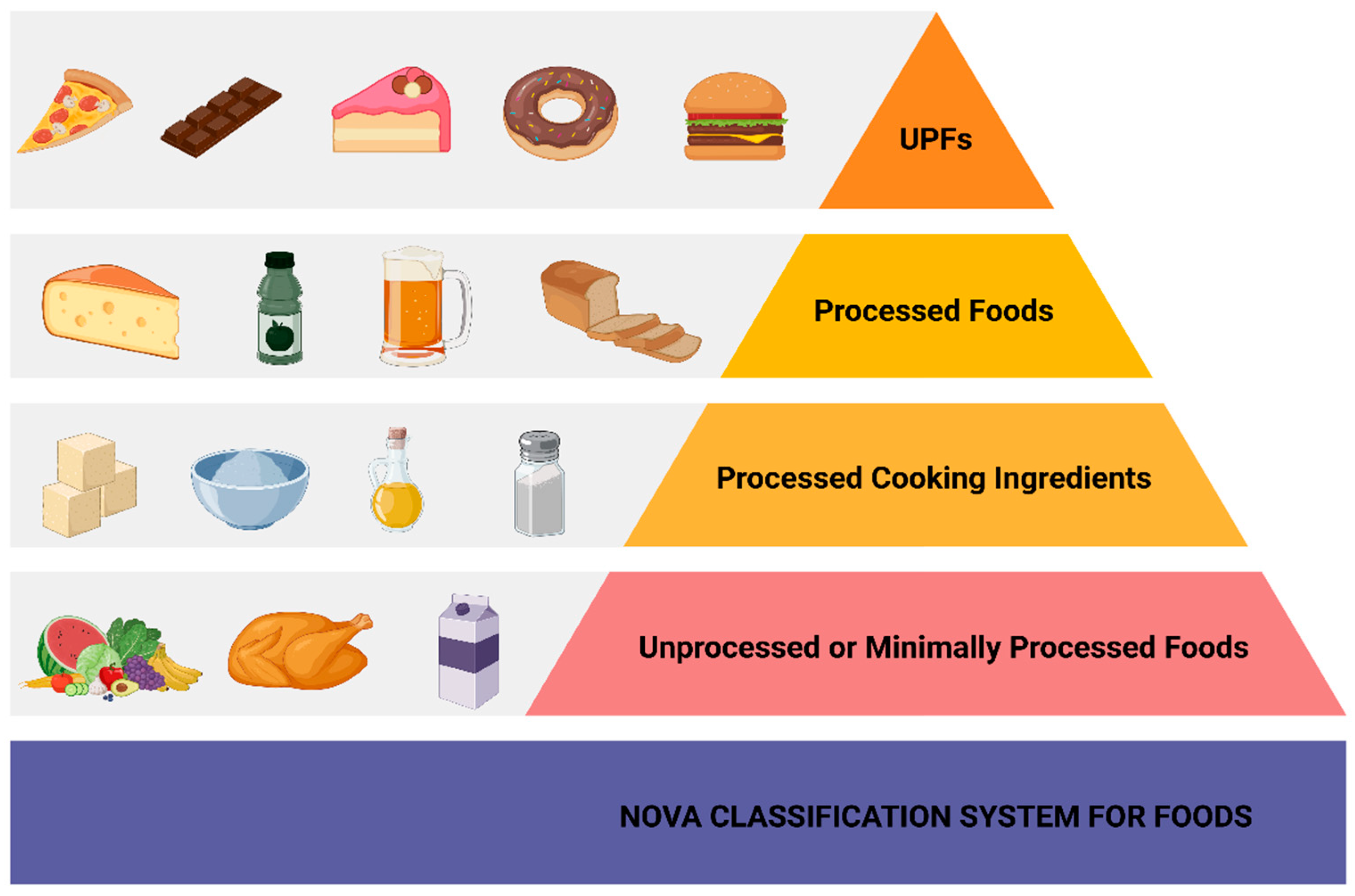
1. Introduction
2. Human Studies Interconnecting UPFs to T2DM
| Author/Year | Population/Study | Findings | Remarks |
|---|---|---|---|
| Snour et al., 2020 [12] | 104,707 participants aged > 18 y.o. (79.2% women) in the French NutriNet-Santé Study, France | A relationship between increased consumption of UPFs and T2DM risk was documented. HR: 1.15 for a 10% increment in the consumption of UPFs. | This study, after adjusting for various confounding factors, showed statistical significance only for women and not for men. |
| Levy et al., 2021 [13] | 21,730 participants aged 40–69 y.o. (52.9% women) from the UK BioBank, UK | aHR: 1.12 for a 10% increment in the consumption of UPFs. | The authors found a statistically significant association between UPF consumption and T2DM risk in men as well as in women. |
| Llavero-Valero et al., 2021 [14] | 20,060 participants, highly educated, aged > 20 y.o. (61.2% women) in the SUN Study, Spain | Higher consumption of UPFs was associated with increased T2DM risk. aHR: 1.53 in the highest tertile of UPFs consumption | This association was observed for both sexes. |
| Duan et al., 2022 [15] | 70,421 participants, aged 35–70 y.o., (58.6% women) in the LifeLines Study, The Netherlands | A diet rich in cold and hot savory snacks was associated with an increased T2DM risk. | Heterogeneity among UPFs was noted in this study. The authors concluded that further studies regarding subcategories of UPFs should be pursued. |
| Chen et al., 2023 [16] | 3 US large cohorts, including 71,871 women, from the Nurses Health Study, 87,918 women from the Nurses Health Study II, and 38,847 men from the Health Professional Follow up Study, with a meta-analysis of these 3 US cohorts | A 10% increment in the consumption of UPFs was associated with a 12% increase in T2DM risk. | The authors documented heterogeneity among UPFs, regarding T2DM risk. Sauces and sweet and artificially sweetened beverages were related to a higher T2DM risk, whereas cereals and dark-whole grain breads were associated with a reduced T2DM risk. |
| Canhada et al., 2023 [17] | 15,105 participants aged 35–74 y.o. in the ELSA-Brasil multicentered study, Brazil | A 150 g/d increment in the consumption of UPFs was associated with a RR:1.05 for T2DM risk. | The authors also documented heterogeneity among UPFs regarding T2DM risk. Sweetened beverages and processed meat were associated with a higher increase in T2DM risk than other UPFs. |
| Cordova et al., 2023 [18] | 266,666 participants (60% women) aged 35–74 y.o. from 7 European countries in the EPIC Study, Europe | Higher consumption of UPFs was related to an increased risk of T2DM. | Heterogeneity was found by the authors. Artificially and sugar-sweetened beverages were the most highly associated UPFs with T2DM risk. |
| Cho et al., 2024 [19] | 7438 participants aged 40–69 y.o. in a Korean study from the Korean Genome and Epidemiology Study Ansan-Ansung Cohort, Korea | During a median follow up of 15 years, an association between the increased consumption of UPFs and T2DM risk was documented. | The authors also demonstrated the existence of heterogeneity among UPFs and the risk of T2DM. A higher consumption of carbonated beverages, ham/sausages, instantly made noodles, and ice-creams was related to an increased risk of T2DM. |
| Dicken et al., 2024 [6] | 311,892 participants aged 35–74 y.o. in the EPIC Study, Europe | A 10% increment in the consumption of UPFs was related to a 17% increase in T2DM risk. | The authors also reported heterogeneity among UPFs and T2DM risk. Breakfast cereals and bread were associated with a reduced risk of T2DM |
| Amirian et al., 2024 [11] | 10,047 participants aged 35–65 y.o. in the RaNCD Study in Iran, Iran | After a mean follow up time of 7.1 years, higher consumption of UPFs was associated with an increased risk of T2DM. | The authors pointed out that further studies are needed to confirm their results, as the statistical power was not significant (p = 0.665) after adjusting for multiple confounding factors. |
| Salame et al., 2024 [21] | 104,139 participants > 18 y.o. (79.2% women) in the French NutriNet-Santé Study, France | In this large cohort French study, the authors reported a correlation between food additives emulsifiers and the risk of T2DM. | The authors proposed that legislation regarding food additives should be pursued in order to reduce the burden of T2DM. |
3. Proposed Pathogenetic Mechanisms Associating Overconsumption of UPFs with T2DM Risk
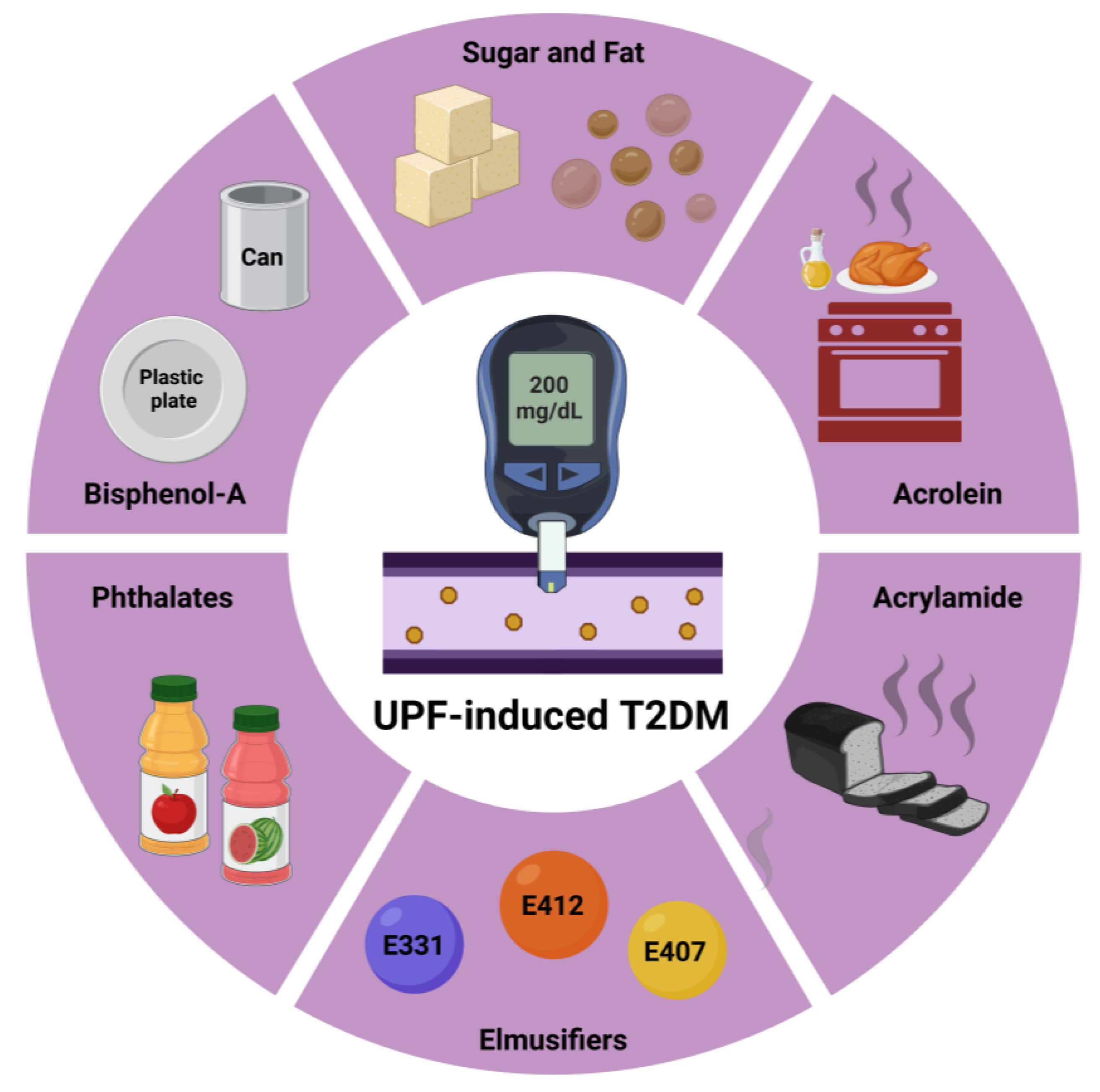
3.1. Sugar and Fat Consumption
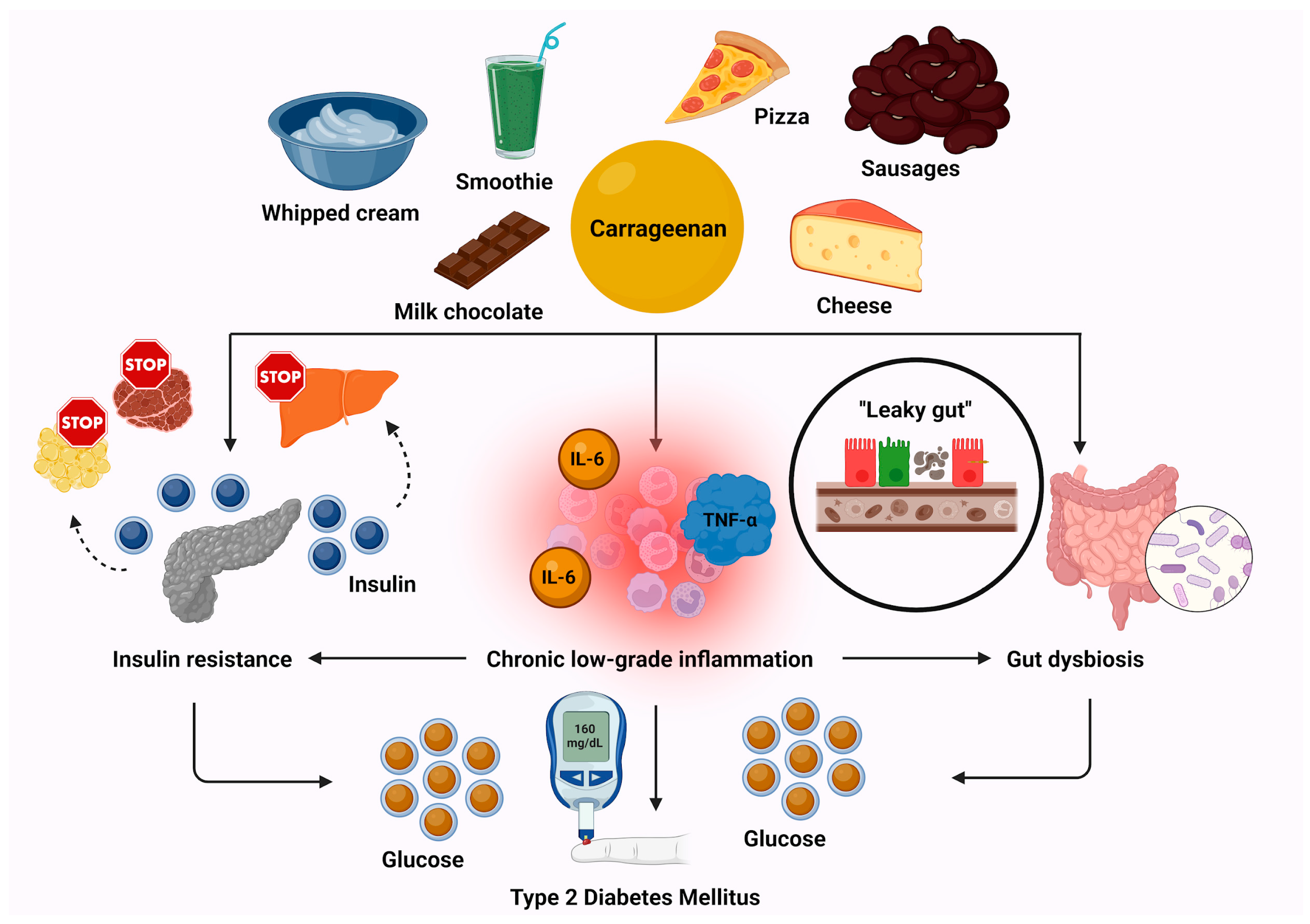
3.2. Emulsifiers
3.3. Endocrine-Disrupting Chemicals
4. Current Concepts and Future Perspectives
5. Life Expectancy in the United States of America: Is There a Connection with UPF Consumption?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Gibney, M.J. Ultra-Processed Foods: Definitions and Policy Issues. Curr. Dev. Nutr. 2019, 3, nzy077. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- WHO. Diabetes. Key Facts. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 30 November 2024).
- Vallianou, N.G.; Kounatidis, D.; Tzivaki, I.; Zafeiri, G.C.M.; Rigatou, A.; Daskalopoulou, S.; Stratigou, T.; Karampela, I.; Dalamaga, M. Ultra-Processed Foods and Childhood Obesity: Current evidence and perspectives. Curr. Nutr. Rep. 2025, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Dicken, S.J.; Dahm, C.C.; Ibsen, D.B.; Olsen, A.; Tjønneland, A.; Louati-Hajji, M.; Cadeau, C.; Marques, C.; Schulze, M.B.; Jannasch, F.; et al. Food consumption by degree of food processing and risk of type 2 diabetes mellitus: A prospective cohort analysis of the European Prospective Investigation into Cancer and Nutrition (EPIC). Lancet Reg. Health Eur. 2024, 46, 101043. [Google Scholar] [CrossRef]
- Paramasivam, A.; Murugan, R.; Jeraud, M.; Dakkumadugula, A.; Periyasamy, R.; Arjunan, S. Additives in Processed Foods as a Potential Source of Endocrine-Disrupting Chemicals: A Review. J. Xenobiot. 2024, 14, 1697–1710. [Google Scholar] [CrossRef] [PubMed]
- Heidari Seyedmahalleh, M.; Nasli-Esfahani, E.; Zeinalabedini, M.; Azadbakht, L. Association of ultra-processed food consumption with cardiovascular risk factors among patients with type-2 diabetes mellitus. Nutr. Diabetes 2024, 14, 89. [Google Scholar] [CrossRef]
- Hudson, E.A.; Davis, J.N.; Haushalter, K.; Tanaka, H.; Dubois, S.K.; Steinhardt, M.A.; Burgermaster, M. Degree of Food Processing Is Associated With Glycemic Control in African American Adults With Type 2 Diabetes: Findings From Texas Strength Through Resilience in Diabetes Education Clinical Trial. J. Acad. Nutr. Diet 2024, S2212-2672(24)00877-3. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Mahdavi, M.; Mirmiran, P.; Azizi, F. Ultra-processed foods and the incidence of pre-diabetes and type 2 diabetes among Iranian adults: The Tehran lipid and glucose study. Nutr. Metab. 2024, 21, 79. [Google Scholar] [CrossRef]
- Amirian, P.; Zarpoosh, M.; Najafi, F.; Shakiba, E.; Anvari, B.; Pasdar, Y. Ultra-processed foods and type 2 diabetes mellitus incidence in RaNCD project: A prospective cohort study. Acta Diabetol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern. Med. 2020, 180, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.B.; Rauber, F.; Chang, K.; Louzada, M.L.D.C.; Monteiro, C.A.; Millett, C.; Vamos, E.P. Ultra-processed food consumption and type 2 diabetes incidence: A prospective cohort study. Clin. Nutr. 2021, 40, 3608–3614. [Google Scholar] [CrossRef] [PubMed]
- Llavero-Valero, M.; Escalada-San Martín, J.; Martínez-González, M.A.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Bes-Rastrollo, M. Ultra-processed foods and type-2 diabetes risk in the SUN project: A prospective cohort study. Clin. Nutr. 2021, 40, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.J.; Vinke, P.C.; Navis, G.; Corpeleijn, E.; Dekker, L.H. Ultra-processed food and incident type 2 diabetes: Studying the underlying consumption patterns to unravel the health effects of this heterogeneous food category in the prospective Lifelines cohort. BMC Med. 2022, 20, 7. [Google Scholar] [CrossRef]
- Chen, Z.; Khandpur, N.; Desjardins, C.; Wang, L.; Monteiro, C.A.; Rossato, S.L.; Fung, T.T.; Manson, J.E.; Willett, W.C.; Rimm, E.B.; et al. Ultra-Processed Food Consumption and Risk of Type 2 Diabetes: Three Large Prospective U.S. Cohort Studies. Diabetes Care 2023, 46, 1335–1344. [Google Scholar] [CrossRef]
- Canhada, S.L.; Vigo, Á.; Levy, R.; Luft, V.C.; da Fonseca, M.J.M.; Giatti, L.; Molina, M.D.C.B.; Duncan, B.B.; Schmidt, M.I. Association between ultra-processed food consumption and the incidence of type 2 diabetes: The ELSA-Brasil cohort. Diabetol. Metab. Syndr. 2023, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Cordova, R.; Viallon, V.; Fontvieille, E.; Peruchet-Noray, L.; Jansana, A.; Wagner, K.H.; Kyrø, C.; Tjønneland, A.; Katzke, V.; Bajracharya, R.; et al. Consumption of ultra-processed foods and risk of multimorbidity of cancer and cardiometabolic diseases: A multinational cohort study. Lancet Reg. Health Eur. 2023, 35, 100771. [Google Scholar] [CrossRef]
- Cho, Y.; Ryu, S.; Kim, R.; Shin, M.J.; Oh, H. Ultra-processed Food Intake and Risk of Type 2 Diabetes in Korean Adults. J. Nutr. 2024, 154, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wawro, N.; Freuer, D.; Peters, A.; Heier, M.; Meisinger, C.; Breuninger, T.A.; Linseisen, J. Differential association of dietary scores with the risk of type 2 diabetes by metabotype. Eur. J. Nutr. 2024, 63, 2137–2148. [Google Scholar] [CrossRef]
- Salame, C.; Javaux, G.; Sellem, L.; Viennois, E.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Huybrechts, I.; Pierre, F.; Coumoul, X.; et al. Food additive emulsifiers and the risk of type 2 diabetes: Analysis of data from the NutriNet-Santé prospective cohort study. Lancet Diabetes Endocrinol. 2024, 12, 339–349. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. Ultra-processed foods and type 2 diabetes: More fundamental research is needed. Lancet Reg. Health Eur. 2024, 46, 101084. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell. Metab. 2019, 30, 226, Erratum in: Cell. Metab. 2020, 32, 690. https://doi.org/10.1016/j.cmet.2020.08.014. [Google Scholar] [CrossRef]
- Taylor, R.; Barnes, A.C.; Hollingsworth, K.; Irvine, K.M.; Solovyova, A.S.; Clark, L.; Kelly, T.; Martin-Ruiz, C.; Romeres, D.; Koulman, A.; et al. Aetiology of Type 2 diabetes in people with a ’normal’ body mass index: Testing the personal fat threshold hypothesis. Clin. Sci. 2023, 137, 1333–1346. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. Overnutrition, Hyperinsulinemia and Ectopic Fat: It Is Time for A Paradigm Shift in the Management of Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 5488. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Buettner, J.; Heni, M.; Fritsche, L.; Kullmann, S.; Wagmüller, M.; Peter, A.; Preissl, H.; Machann, J.; Jumpertz von Schwartzenberg, R.; et al. Carrageenan and insulin resistance in humans: A randomised double-blind cross-over trial. BMC Med. 2024, 22, 558. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Olayinka, O.T.; Fr, J.; Nisar, M.R.; Kotha, R.; Saad-Omer, S.I.; Nath, T.S. Food Additives’ Impact on Gut Microbiota and Metabolic Syndrome: A Systematic Review. Cureus 2024, 16, e66822. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Borthakur, A.; Tobacman, J.K. Common food additive carrageenan inhibits proglucagon expression and GLP-1 secretion by human enteroendocrine L-cells. Nutr. Diabetes 2024, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Lee, S.Y.; Hong, H.C.; Choi, H.Y.; Yoo, H.; Baik, S.H.; Choi, K.M. AMPK activator-mediated inhibition of endoplasmic reticulum stress ameliorates carrageenan-induced insulin resistance through the suppression of selenoprotein P in HepG2 hepatocytes. Mol. Cell. Endocrinol. 2014, 382, 66–73. [Google Scholar] [CrossRef]
- Tuduri, E.; Marroqui, L.; Dos Santos, R.S.; Quesada, I.; Fuentes, E.; Alonso-Magdalena, P. Timing of exposure and Bisphenol-A: Implications for diabetes development. Front. Endocrinol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Kounatidis, D.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Psallida, S.; Papavassiliou, A.G. The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies. Int. J. Mol. Sci. 2024, 25, 675. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Smith, M.T.; McHale, C.M.; Heindel, J.J.; Atlas, E.; Cave, M.C.; Collier, D.; Guyton, K.Z.; Koliwad, S.; Nadal, A.; et al. Consensus on the key characteristics of metabolism disruptors. Nat. Rev. Endocrinol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wan, S.; Yu, L.; Liu, W.; Song, J.; Shi, D.; Zhang, Y.; Chen, W.; Qiu, W.; Wang, B. Phthalates exposure, biological aging, and increased risks of insulin resistance, prediabetes, and diabetes in adults with metabolic dysfunction-associated steatotic liver disease. Diabetes Metab. 2025, 51, 101602. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.A.; Wheeler, H.B.; Blumberg, B. Obesity and endocrine-disrupting chemicals. Endocr. Connect. 2021, 10, R87–R105. [Google Scholar] [CrossRef]
- Lee, H.W.; Pyo, S. Acrylamide induces adipocyte differentiation and obesity in mice. Chem. Biol. Interact. 2019, 298, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Raffan, S.; Halford, N.G. Acrylamide in food: Progress in and prospects for genetic and agronomic solutions. Ann. Appl. Biol. 2019, 175, 259–281. [Google Scholar] [CrossRef] [PubMed]
- Buyukdere, Y.; Akyol, A. From a toxin to an obesogen: A review of potential obesogenic roles of acrylamide with a mechanistic approach. Nutr. Rev. 2023, 82, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Esfahani, F.; Beheshti, N.; Nematollahi, A.; Koochakpoor, G.; Verij-Kazemi, S.; Mirmiran, P.; Azizi, F. The association between dietary acrylamide intake and the risk of type 2 diabetes incidence in the Tehran lipid and glucose study. Sci. Rep. 2023, 13, 8235. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, W.; Yang, S.; Cao, L.; Zhu, C.; Ma, J.; Li, W.; Zhang, Z.; Xu, T.; Wang, X.; et al. Acrylamide Exposure and Oxidative DNA Damage, Lipid Peroxidation, and Fasting Plasma Glucose Alteration: Association and Mediation Analyses in Chinese Urban Adults. Diabetes Care 2020, 43, 1479–1486. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.C.; Kuo, H.K.; Hwang, J.J.; Lin, J.L.; Chen, P.C.; Lin, L.Y. Association among acrylamide, blood insulin, and insulin resistance in adults. Diabetes Care 2009, 32, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Feroe, A.G.; Attanasio, R.; Scinicariello, F. Acrolein metabolites, diabetes and insulin resistance. Environ. Res. 2016, 148, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, W.; Yu, L.; Ye, Z.; Cheng, M.; Qiu, W.; Zhou, M.; Ma, J.; Wang, X.; Yang, M.; et al. Acrolein Exposure Impaired Glucose Homeostasis and Increased Risk of Type 2 Diabetes: An Urban Adult Population-Based Cohort Study with Repeated Measures. Environ. Sci. Technol. 2023, 57, 7162–7173. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Su, G.Y.; Liu, T.Y.; Wang, H.T.; Hwu, C.M. Urinary acrolein protein conjugates-to-creatinine ratio is positively associated with diabetic peripheral neuropathy in patients with type 2 diabetes mellitus. Endocr. Connect. 2023, 12, e230253. [Google Scholar] [CrossRef] [PubMed]
- Jhuo, J.Y.; Tong, Z.J.; Ku, P.H.; Cheng, H.W.; Wang, H.T. Acrolein induces mitochondrial dysfunction and insulin resistance in muscle and adipose tissues in vitro and in vivo. Environ. Pollut. 2023, 336, 122380. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.A.; Adams, M.; Sabaté, J. Review: The Consumption of Ultra-Processed Foods and Non-communicable Diseases in Latin America. Front. Nutr. 2021, 8, 622714. [Google Scholar] [CrossRef]
- Matos, J.P.; Rodrigues, M.B.; Duarte, C.K.; Horta, P.M. A Scoping Review of Observational Studies on Food and Beverage Advertising on Social Media: A Public Health Perspective. Int. J. Environ. Res. Public. Health 2023, 20, 3615. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef]
- Lv, J.L.; Wei, Y.F.; Sun, J.N.; Shi, Y.C.; Liu, F.H.; Sun, M.H.; Chang, Q.; Wu, Q.J.; Zhao, Y.H. Ultra-processed food consumption and metabolic disease risk: An umbrella review of systematic reviews with meta-analyses of observational studies. Front. Nutr. 2024, 11, 1306310. [Google Scholar] [CrossRef]
- Barbaresko, J.; Bröder, J.; Conrad, J.; Szczerba, E.; Lang, A.; Schlesinger, S. Ultra-processed food consumption and human health: An umbrella review of systematic reviews with meta-analyses. Crit. Rev. Food Sci. Nutr. 2024, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Grinshpan, L.S.; Eilat-Adar, S.; Ivancovsky-Wajcman, D.; Kariv, R.; Gillon-Keren, M.; Zelber-Sagi, S. Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: A systematic review. JHEP Rep. 2023, 6, 100964. [Google Scholar] [CrossRef]
- Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Boyland, E.; Cuthbertson, D.J. Ultra-processed food and non-communicable diseases in the United Kingdom: A narrative review and thematic synthesis of literature. Obes. Rev. 2024, 25, e13682. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, O.M.; Alhomaid, T.A.; Alshuaibi, A.M.; Ahmad Alahmad, R.M.; Al Mardhamah, N.H.; Alamri, T. The Influence of Eating Habits on Type 2 Diabetes in Saudi Arabia: A Systematic Review. Cureus 2023, 15, e42638. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Deierlein, A.L.; Vaidean, G.; Quatromoni, P.A.; Parekh, N. Ultra-processed Foods and Cardiometabolic Health Outcomes: From Evidence to Practice. Curr. Atheroscler. Rep. 2022, 24, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Almarshad, M.I.; Algonaiman, R.; Alharbi, H.F.; Almujaydil, M.S.; Barakat, H. Relationship between Ultra-Processed Food Consumption and Risk of Diabetes Mellitus: A Mini-Review. Nutrients 2022, 14, 2366. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Bielemann, R.M.; da Silva, B.G.C.; Dos Santos, F.S.; Mintem, G.C.; Flores, T.R.; Arcêncio, R.A.; Nunes, B.P. Ultra-processed food and risk of type 2 diabetes: A systematic review and meta-analysis of longitudinal studies. Int. J. Epidemiol. 2022, 51, 1120–1141. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, M.; Lara-Castor, L.; Cudhea, F.; Miller, V.; Reedy, J.; Shi, P.; Zhang, J.; Wong, J.B.; Economos, C.D.; Micha, R.; et al. Incident type 2 diabetes attributable to suboptimal diet in 184 countries. Nat. Med. 2023, 29, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Food Marketing Exposure and Power and Their Associations with Food-Related Attitudes, Beliefs and Behaviours: A Narrative Review. 2022. Available online: https://www.who.int/publications/i/item/9789240041783 (accessed on 7 February 2022).
- Saleem, S.M.; Bhattacharya, S.; Deshpande, N. Non-communicable diseases, type 2 diabetes, and influence of front of package nutrition labels on consumer’s behaviour: Reformulations and future scope. Diabetes Metab. Syndr. 2022, 16, 102422. [Google Scholar] [CrossRef]
- Moradi, S.; Hojjati Kermani, M.A.; Bagheri, R.; Mohammadi, H.; Jayedi, A.; Lane, M.M.; Asbaghi, O.; Mehrabani, S.; Suzuki, K. Ultra-Processed Food Consumption and Adult Diabetes Risk: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2021, 13, 4410. [Google Scholar] [CrossRef]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2022, 23, e13366. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. Making Sense of the Relationship Between Ultra-Processed Foods, Obesity, and Other Chronic Diseases. Nutrients 2024, 16, 4039. [Google Scholar] [CrossRef]
- Juul, F.; Bere, E. Ultra-processed foods—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2024, 68. [Google Scholar] [CrossRef]
- Vepsäläinen, H.; Lindström, J. Dietary patterns—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2024, 68. [Google Scholar] [CrossRef] [PubMed]
- Sherling, D.H.; Hennekens, C.H.; Ferris, A.H. Newest Updates to Health Providers on the Hazards of Ultra-Processed Foods and Proposed Solutions. Am. J. Med. 2024, 137, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Vadiveloo, M.K.; Gardner, C.D. Not All Ultra-Processed Foods Are Created Equal: A Case for Advancing Research and Policy That Balances Health and Nutrition Security. Diabetes Care 2023, 46, 1327–1329. [Google Scholar] [CrossRef]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef]
- Wang, L.; Martínez Steele, E.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in Consumption of Ultra processed Foods Among US Youths Aged 2-19 Years, 1999-2018. JAMA 2021, 326, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, E.; Esposito, S.; Costanzo, S.; Di Castelnuovo, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M.; INHES Study Investigators. Ultra-processed food consumption and its correlates among Italian children, adolescents and adults from the Italian Nutrition & Health Survey (INHES) cohort study. Public Health Nutr. 2021, 24, 6258–6271. [Google Scholar] [CrossRef]
- Fedde, S.; Stolte, A.; Plachta-Danielzik, S.; Müller, M.J.; Bosy-Westphal, A. Ultra-processed food consumption and overweight in children, adolescents and young adults: Long-term data from the Kiel Obesity Prevention Study (KOPS). Pediatr. Obes. 2024, e13192. [Google Scholar] [CrossRef]
- Dittmann, A.; Werner, L.; Hörz, L.; Luft, T.; Finkbeiner, F.; Storcksdieck Genannt Bonsmann, S. Sociodemographic and behavioural differences between frequent and non-frequent users of convenience food in Germany. Front. Nutr. 2024, 11, 1369137. [Google Scholar] [CrossRef]
- Reddy, K.R.; Aggarwal, M.; Freeman, A.M. Food Is Medicine: The Time Is Now. Am. J. Med. 2024, 137, 1180–1183. [Google Scholar] [CrossRef]
- O’ Leary, K. The harms of ultra processed foods. Nat. Med. 2024, 30, 3392. [Google Scholar] [CrossRef] [PubMed]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in the food industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.; Poelman, M.P.; Aaspõllu, A.; Bica, M.; Bouzas, C.; Carrano, E.; De Miguel-Etayo, P.; Djojosoeparto, S.; Blenkuš, M.G.; Graca, P.; et al. Policy implementation and priorities to create healthy food environments using the Healthy Food Environment Policy Index (Food-EPI): A pooled level analysis across eleven European countries. Lancet Reg. Health Eur. 2022, 23, 100522. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.D.E.; Anghinoni, I.C.A.B.; Gomes, M.B. Plant-Based Food for the Prevention of Type 2 Diabetes: Scoping Review. Nutrients 2024, 16, 1671. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallianou, N.G.; Evangelopoulos, A.; Tzivaki, I.; Daskalopoulou, S.; Adamou, A.; Michalaki Zafeiri, G.C.; Karampela, I.; Dalamaga, M.; Kounatidis, D. Ultra-Processed Foods and Type 2 Diabetes Mellitus: What Is the Evidence So Far? Biomolecules 2025, 15, 307. https://doi.org/10.3390/biom15020307
Vallianou NG, Evangelopoulos A, Tzivaki I, Daskalopoulou S, Adamou A, Michalaki Zafeiri GC, Karampela I, Dalamaga M, Kounatidis D. Ultra-Processed Foods and Type 2 Diabetes Mellitus: What Is the Evidence So Far? Biomolecules. 2025; 15(2):307. https://doi.org/10.3390/biom15020307
Chicago/Turabian StyleVallianou, Natalia G., Angelos Evangelopoulos, Ilektra Tzivaki, Stavroula Daskalopoulou, Andreas Adamou, Georgia Chrysi Michalaki Zafeiri, Irene Karampela, Maria Dalamaga, and Dimitris Kounatidis. 2025. "Ultra-Processed Foods and Type 2 Diabetes Mellitus: What Is the Evidence So Far?" Biomolecules 15, no. 2: 307. https://doi.org/10.3390/biom15020307
APA StyleVallianou, N. G., Evangelopoulos, A., Tzivaki, I., Daskalopoulou, S., Adamou, A., Michalaki Zafeiri, G. C., Karampela, I., Dalamaga, M., & Kounatidis, D. (2025). Ultra-Processed Foods and Type 2 Diabetes Mellitus: What Is the Evidence So Far? Biomolecules, 15(2), 307. https://doi.org/10.3390/biom15020307










