Abstract
Colorectal cancer (CRC) is one of the deadliest neoplasia. Intrinsic or acquired resistance is the main cause of failure of therapy regimens that leads to relapse and death in CRC patients. The widely used chemotherapeutic agent 5-fluorouracil (5-FU) remains the mainstay for therapeutic combinations. Unfortunately, chemotherapeutic resistance and side effects are frequent events that compromise the success of these therapies; the dysregulation of enzymes that regulate 5-FU metabolism increases the expression and activity of efflux pumps. Additional tumor cell adaptations such as epithelial–mesenchymal transition (EMT), autophagy shaping of the tumor microenvironment, and inflammation contribute to chemoresistance. Finding new strategies and alternatives to enhance conventional chemotherapies has become necessary. Recently, the study of natural compounds has been gaining strength as an alternative to chemotherapeutics in different cancers. Curcumin, trimethylglycine, resveratrol, artemisinin, and some helminth-derived molecules, among others, are some natural compounds studied in the context of CRC. This review discusses the main benefits, mechanisms, advances, and dark side of conventional chemotherapeutics currently evaluated in CRC treatment. We also analyzed the landscape of alternative non-conventional compounds and their underlying mechanisms of action, which could, in the short term, provide fundamental knowledge to harness their anti-tumor effects and allow them to be used as alternative adjuvant therapies.
1. Introduction
Colorectal cancer (CRC) is one of the main concerns worldwide due to its increasing incidence and mortality rate in the last few years. Currently, CRC is the third most common cancer (1.9 million new cases) and the second leading cause of cancer-related death (930,000 deaths) [1]. Furthermore, the World Health Organization (WHO) estimates that by 2030 there will be 2.2 million new cases of CRC and 1.09 million deaths caused by CRC [2].
The etiological origin of CRC can be classified into two principal groups: hereditary, around 20%, and sporadic, representing 80% of total cases [3]. Sporadic CRC emerges from the accumulation of acquired somatic mutations and epigenetic alterations related to several modifiable lifestyle risk factors, such as obesity, sedentary behavior, high alcohol and tobacco intake, low fiber consumption, high-fat diets, and processed meats [4]. Three main pathways have been described for the formation of sporadic CRC tumors: (I) the adenoma–carcinoma sequence, influenced by chromosomal instability (CIN), microsatellite instability (MSI), KRAS, APC, and Tp53 mutations; (II) the serrated pathway, governed by the methylation of CpG island methylator phenotype (CIMP), MSI, BRAF, and CDKN2A mutations; and (III) the inflammatory pathway, related to the activation of inflammatory signaling pathways such as NFκB, IL-6/STAT3, COX-2/PGE2 and IL-23/Th17 [5,6].
The heterogeneity of tumor origins influences the treatment and prognosis of CRC. The classification and identification of tumor subtypes can improve disease management and predictions [7]. The WHO classifies CRC tumors according to their histological characteristics, including (I) adenocarcinoma, which accounts for about 85%; (II) mucinous carcinoma, which represents around 5–20% of the total cases; (III) medullary carcinoma (4%); and (IV) Signet-ring carcinoma, with less than 2% [8].
The staging-based classification of CRC tumors is supported by the tumor–node–metastasis (TNM) system, where T represents tumor invasion into the layers of the intestinal wall subdivided into T1: submucosa; T2: muscularis propria; T3: mesocolon or mesorectal fat; and T4: perforation of serosa or invasion into other organs. The N refers to the number of lymph nodes with metastases, where N0: no lymph nodes; N1: 1–3; N2: 4 or more. The M indicates the presence of distant metastasis, including M0, which has no distant metastasis, and M1, with metastasis beyond regional lymph nodes. In this context, colorectal tumors are classified as early stage or stage I, where T1 or T2 indicate tumors without metastases, stage II is T3 or T4 tumors without metastases, stage III is tumors with lymph node metastases, and stage IV is indicative of tumors with distant metastases [9]. Since detecting CRC in the pre-cancerous and early stages reduces mortality, periodic screening is recommended for adults aged 50–70. However, due to the increasing incidence of CRC in young patients, recent guidelines recommend screening at age 45 [10]. In this context, the treatment choice and its success depend on the tumor stage at diagnosis.
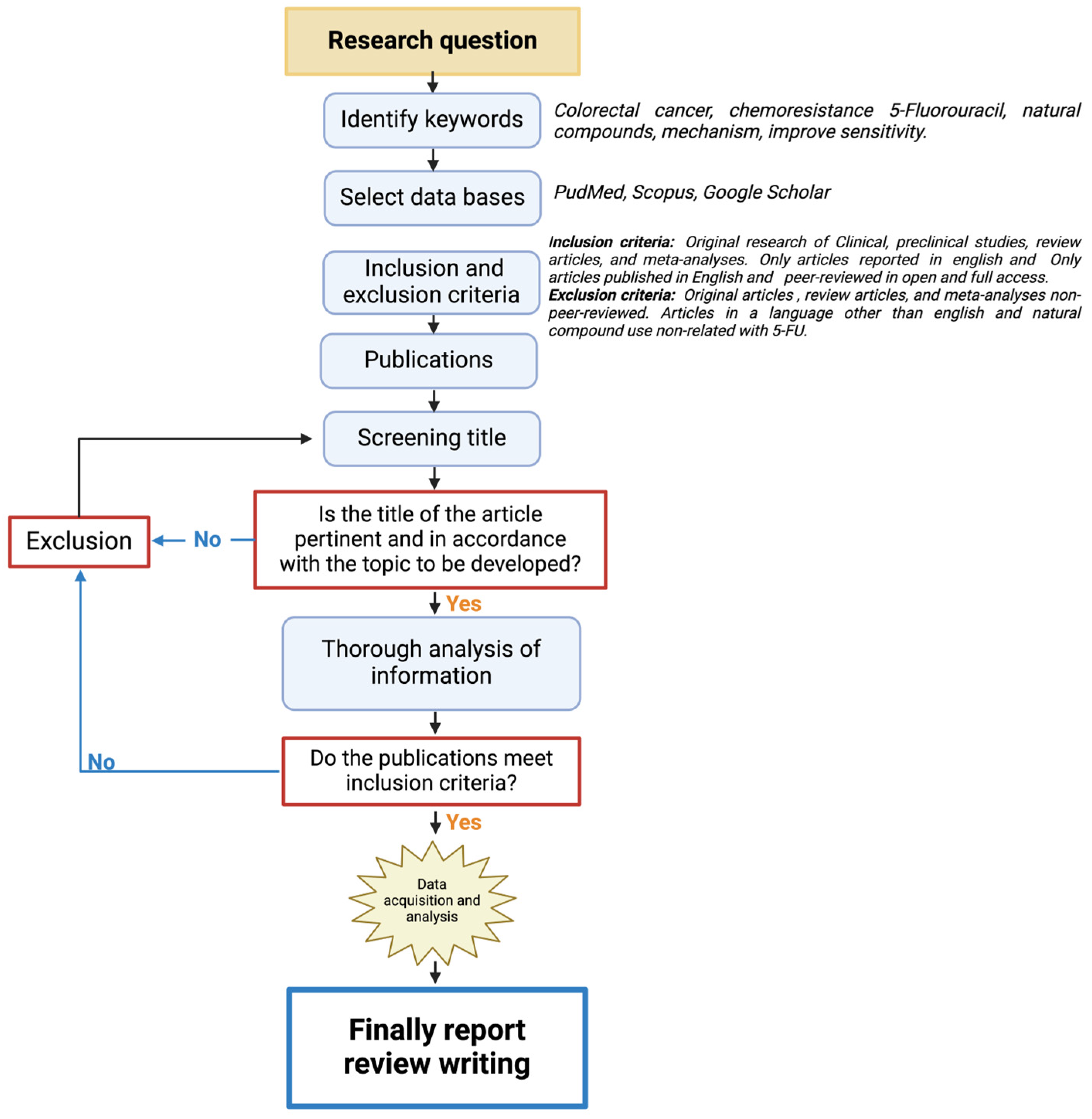
This is a narrative review structured as shown in Figure 1. First, the keywords related to the research question were identified, and recognized databases were utilized for the search (PubMed, Scopus, and Google Scholar). The inclusion criteria focused on all the works on colorectal cancer treatment and natural compounds and excluded the articles that did not have experimental validation and relation to colorectal cancer treatment with 5-Fluorouracil (5-FU). All the publications selected were thoroughly analyzed in their full-text forms to ensure alignment with the inclusion criteria. This system allowed for the development of the following topics of the review.
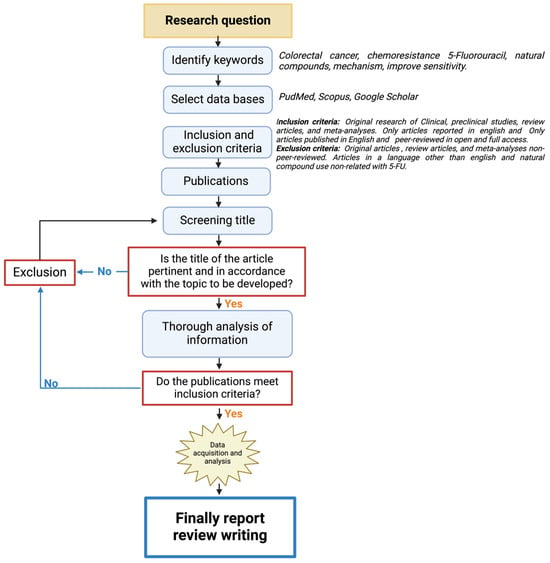
Figure 1.
A flowchart of the methodological approach that was followed for the selection/exclusion of the articles.
2. CRC Treatment
The treatment management of patients with CRC mostly depends on the stage of their diagnosis. Surgery is the primary treatment used in the early stages (I and II), and in the advanced stages, surgery accompanied by systemic therapy is the option for patients with positive lymph nodes and patients with a low-burden spread of tumors [11]. Unfortunately, it is well known that more than 50% of CRC patients will develop metastasis, mainly to the liver; in this situation, most tumors are unresectable, and systemic therapy becomes the primary treatment for metastatic CRC (mCRC) [12,13]. Systemic therapy consists of chemotherapy, targeted therapy, and immunotherapy. Chemotherapy is the primary treatment for mCRC inducing cytotoxicity against cancer cells; targeted therapy recognizes specific molecules that are involved in signaling pathways related to proliferation and survival, such as epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF); and immunotherapy blocks immune checkpoint molecules such as PD-1, PD-L1, and CTLA-4 which restore the activation of adaptative immune cells [14].
5-Fluorouracil (5-FU) is the central axis for therapeutic combinations in advanced CRC treatment. However, individual therapy’s function and success rates are limited (10–15%). Therefore, it is frequently administered together with other drugs, such as leucovorin, oxaliplatin, or irinotecan, increasing the response rates by 40–50% [15]. Nonetheless, it has been determined that 40–50% of all patients with CRC will develop metastasis either at the time of diagnosis or as a recurrent disease after therapy [13,16]. mCRC remains incurable, and the five-year relative survival rate is only about 14% [14].
It is well known that current pharmacological chemotherapeutic drugs are closely related to severe side effects in cancer patients [17]. The adverse effects of chemotherapy in CRC patients include typical symptoms such as asthenia, nausea, vomiting, hair loss, diarrhea, and immunosuppression [18]. However, other symptoms with more significant repercussions on the patient’s health may occur. For instance, the pharmacological scheme of 5-FU plus irinotecan causes intestinal mucositis in 5–15% of patients [19]. Cardiotoxicity and thrombosis have been reported in 5-FU treatment, and oxaliplatin administration can provoke peripheral neuropathy in about 89% of patients [20,21]. Usually, these side effects are intolerable for patients who are forced to reduce chemotherapeutic drug doses, therefore limiting the therapeutic response, or even requiring that they drop the treatment scheme [22], favoring the acquisition of chemoresistance, which is one of the main problems clinicians face in treating and managing CRC patients.
3. Chemoresistance in CRC
Chemoresistance is associated with a lack of response to therapy and relapse in CRC patients, representing a significant problem in medical oncology. Drug resistance occurs in about 90% of patients with mCRC, and high mortality rates can be attributed to a deficient response to chemotherapeutics [23,24].
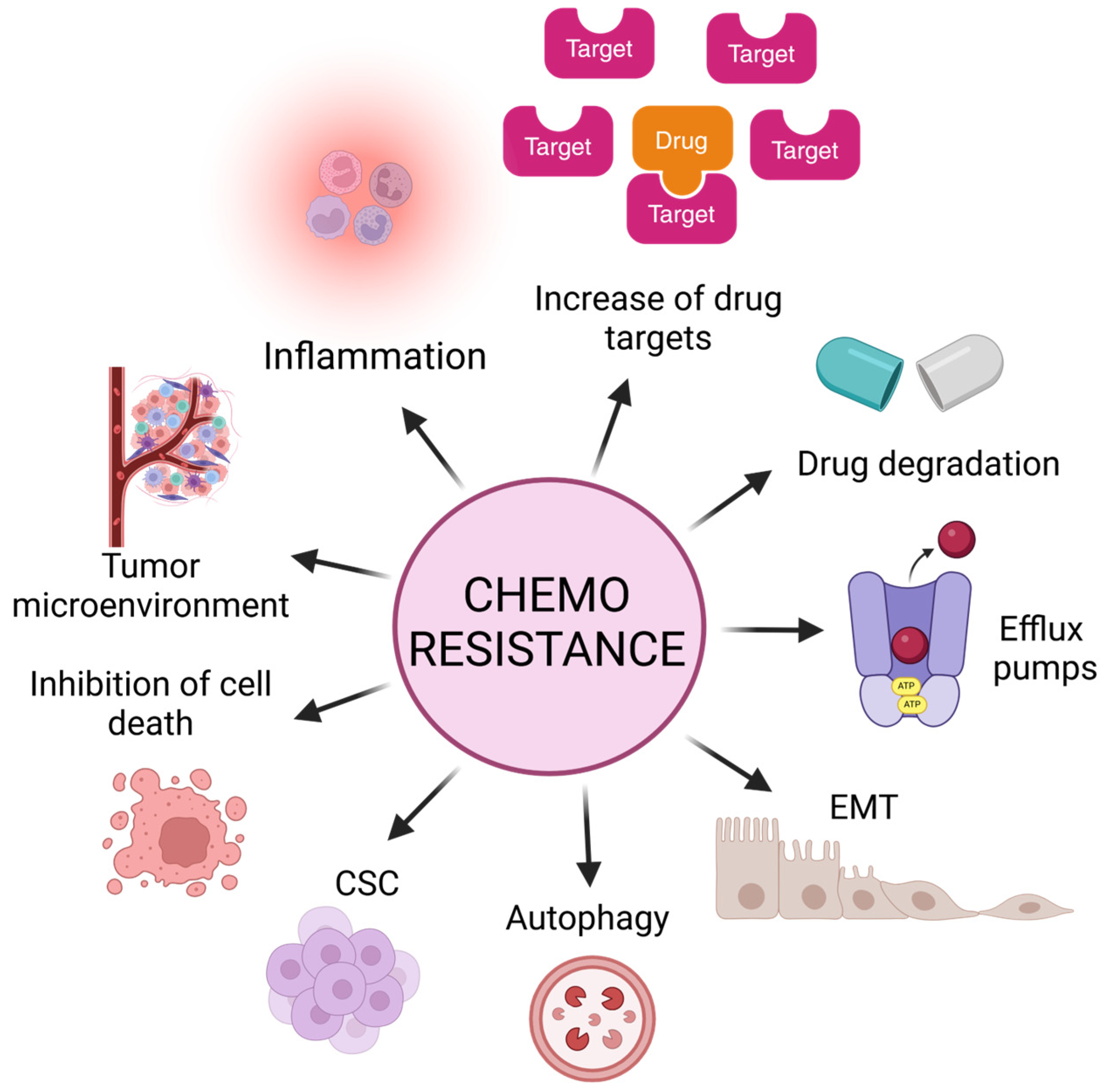
Chemoresistance can be classified into two main categories: intrinsic or acquired. Intrinsic chemoresistance exists before drug treatment, implicating pre-existing genetic and cellular alterations. These mechanisms are critical for the result of the initial response to therapies and may influence subsequent outcomes that lead to acquired resistance [25]. Thus, acquired chemoresistance is the adaptation of cancer cells after treatment exposure, in which these cells display the ability to self-renew and re-initiate the cancer-like parental tumor as well as the expression of a distinctive set of surface biomarkers; this hypothesis proposes the existence of cancer stem cells [25]. Both mechanisms lead to central hallmarks such as the evasion of tumoral cell death by increasing anti-apoptotic proteins, inflammation, increasing potential drug targets, alteration in the functions of efflux pumps, the acquisition of EMT, autophagy, the presence of cancer stem cells (CSCs), and the tumor microenvironment as the primary weapons of tumoral cells in resistance (Figure 2).
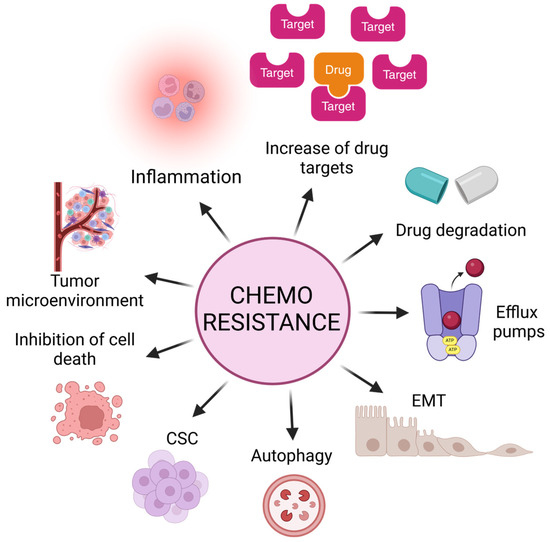
Figure 2.
Main mechanisms and alterations in tumors that lead to evasion of cell death and, consequently, chemoresistance and tumor relapse. Inflammation, increment of drug targets, alteration in functions of efflux pumps, acquisition of epithelial–mesenchymal transition (EMT) markers, autophagy, presence of cancer stem cells (CSCs), increment of anti-apoptotic proteins, and tumor microenvironment.
3.1. Enzymes Involved in the 5-FU Metabolism Processing
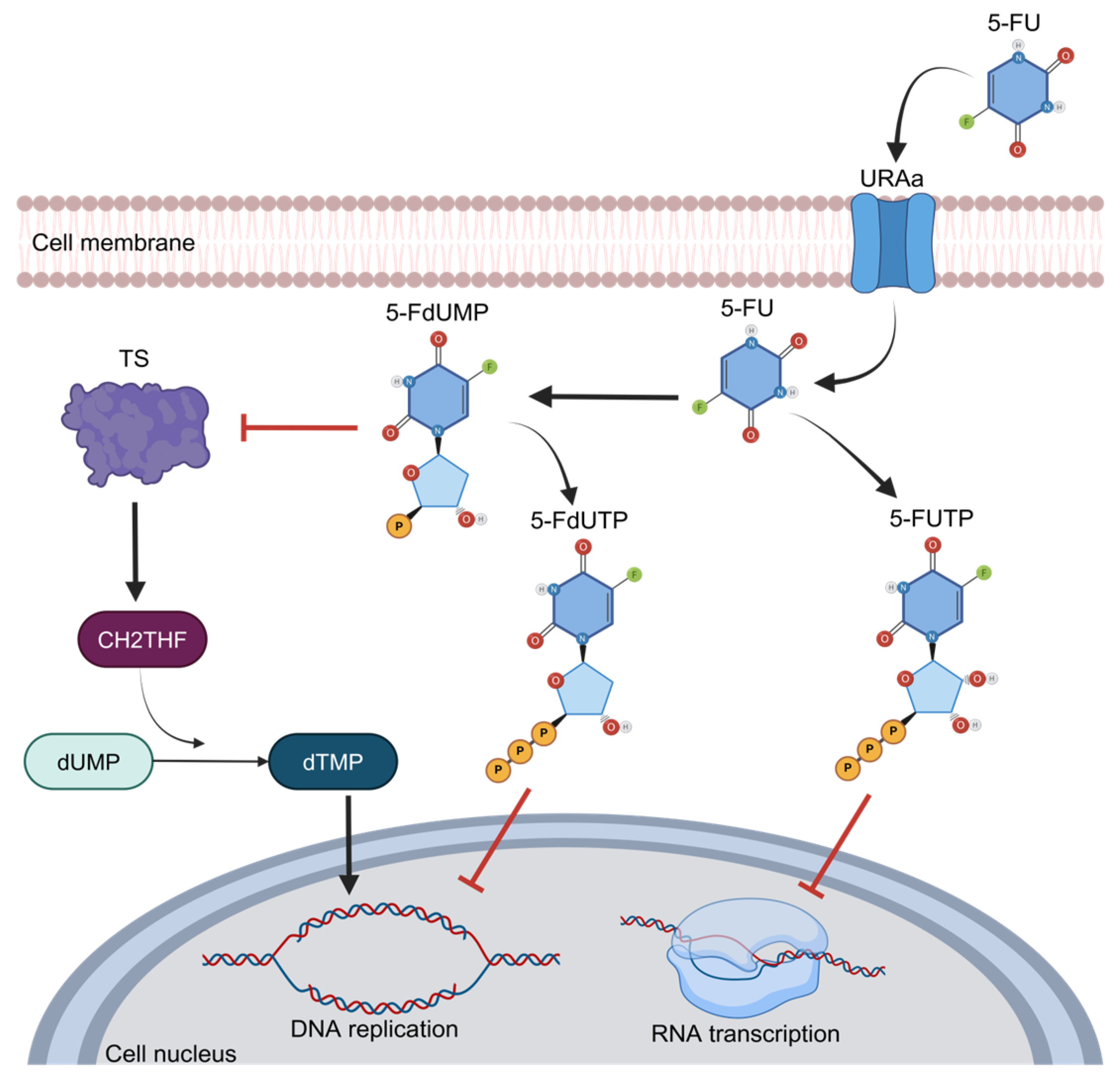
One of the main features of 5-FU is its functioning as an analog of uracil with a fluorine atom bound at the C-5 position in place of hydrogen that favors cell entry through the exact same facilitated transport mechanism as uracil [26]. Once inside the cells, this compound is converted to 5-fluorodeoxyuridine monophosphate (FdUMP), a potent thymidylate synthase (TS) inhibitor. TS is the main enzyme required for DNA replication, acting as a dimer. It contains a nucleotide-binding site for 5,10-methylenetetrahydrofolate (CH2THF) as the methyl donor necessary for the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), supplying the sole de novo source of thymidylate, which is required for DNA replication and repair [15]. This interaction of 5-FU and TS is performed when FdUMP binds to the nucleotide-binding site of TS, forming a ternary complex with the enzyme and CH2THF, thus blocking the binding site of the normal substrate dUMP and blocking dTMP synthesis and DNA replication [27] (Figure 3). Therefore, the alteration and increase in TS expression or mutation in this protein that disables the binding of FdUMP are the most well-established mechanisms of chemoresistance to 5-FU and imply poor prognosis in cancer patients [28]. As observed in breast and prostate cancer patients, the high expression of TS has been associated with a poor prognosis and survival [29,30]. In primary stomach adenocarcinoma, the expression of TS was associated with poor response and survival in patients who received 5-FU as a therapy [31,32]. In CRC, the main neoplasia treated with 5-FU, the landscape is too similar, and the high expression of TS is frequently observed in refractory patients to 5-FU treatment; in contrast, diminished TS expression correlates with a better response to chemotherapeutic treatments [32]. Indeed, when TS is found in low concentrations, CRC patients have a higher 3-year survival rate [33]. On the other hand, when TS is overexpressed, CRC patients usually do not respond to 5-FU-based schemes, such as the FOLFOX regimen (5-FU, oxaliplatin, and leucovorin) [34].
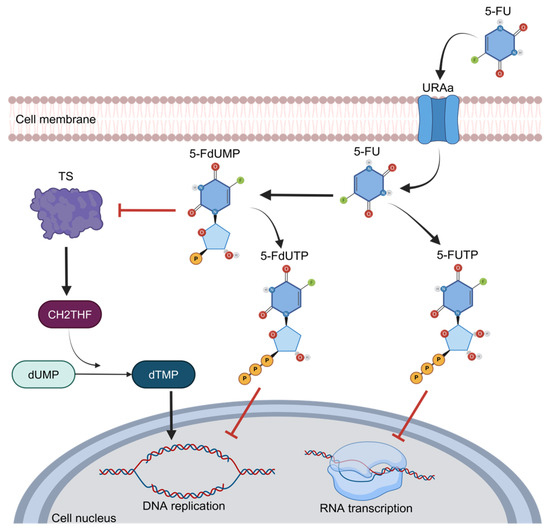
Figure 3.
The 5-FU metabolism and inhibition of thymidylate synthase activity.
Dihydropyrimidine dehydrogenase (DPD) is an enzyme involved in 5-FU catabolism. In the intravenous administration of 5-FU, a significant percentage of this drug (~80%) is metabolically degraded in the liver by the DPD enzyme, converting 5-FU to dihydro fluorouracil (DHFU), an inactive metabolite unable to stop DNA replication and synthesis due to its inability to join to TS [35]. Therefore, there is an inverse correlation between DPD expression and 5-FU-based chemotherapeutic response; in addition, DPD overexpression is associated with an unfavorable prognosis in different types of cancers, including CRC. The low expression and activity of DPD have been related to a better response to 5-FU in advanced head and neck cancer, and the same has been observed in colorectal cancer, in which the inhibition of DPD levels reverses 5-FU resistance [36].
5-FU is an analog to the nucleotide uracil; thus, its entry into cells is through facilitated transport, and it is then converted to 5-fluorouridine monophosphate (FdUMP) and may act as an inhibitor of DNA replication through the inhibition of thymidylate synthase (TS) activity, binding to the nucleotide-specific site in TS for the attachment of methylenetetrahydrofolate (CH2THF), the methyl donor necessary for the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). This is how it blocks the binding of the normal substrate dUMP as 5-FdUTP. Owing to this, the sole source of thymidylate is blocked; therefore, DNA replication is inhibited. The analogous structure of 5-FU to the nucleotide uracil allows it to disrupt the transcription of RNA.
3.2. Efflux Pumps
The activity of efflux pumps also contributes to chemoresistance, and efflux pumps are mainly members of the ATP-binding cassette (ABC) family. These drug transporters increase chemotherapeutic efflux, reducing its intracellular accumulation in cancer cells [37]. There are three main proteins involved in chemoresistance: P-glycoprotein ABCB1 (MDR1), multidrug resistance protein ABCC1 (MRP1), and breast cancer resistance protein ABCG2 [38]. Recently, compelling evidence has demonstrated that the inhibition of these transporters can improve the efficacy of chemotherapy [39]. In CRC, it has been shown that, as a secondary effect, 5-FU treatment positively regulates the expression of ABCB1, ABCC1, and ABCG2a through the activation of the IRE1α-XBP1 pathway; thus, favoring the chemoresistance and inhibition of this protein decreased the ABC transporter expression and in turn restored the efficacy of 5-FU treatment in colon cancer cells [38,40].
3.3. Epithelial–Mesenchymal Transition
One of the diverse hallmarks of malignancy in tumor cells is the acquisition of a phenotype of epithelial–mesenchymal transition (EMT). This phenotype is characterized by the loss of cell–cell adhesion and apical–basal polarity and undergoing cytoskeleton reorganization, contributing to increased migration and invasion capabilities being displayed by tumor cells [41]. These changes allow cancer cells to gain the properties of mesenchymal cells, such as a greater capacity for migration and invasion, favoring metastasis [42]. The relationship between EMT and chemoresistance to different drugs, including 5-FU in CRC and other cancers, has been broadly studied [43]. Previous reports showed that markers associated with EMT, such as Twist, Zeb1, and Zeb2, after 5-FU treatments, were overexpressed in resistant colon cancer cells (HT29) compared to non-resistant cells. Hence, they display characteristic features of loose cell–cell interaction: a spindle shape, intercellular spaces, and scattering [44]. In CRC patients receiving 5-FU, leucovorin, and oxaliplatin (FOLFOX), nuclear ZEB2 expression was a marker of a poor response to chemotherapy, favoring metastasis, early recurrence, and reduced survival [45]. Thus, the pharmacological modulation of molecules re-emerging during the EMT represents a potential target for CRC therapy [46].
3.4. Autophagy
Autophagy is a fundamental biological process where lysosomes degrade cytoplasmic materials. It is contained in double-membrane vesicles named autophagosomes. Although it is also a type of cell death, it has been shown that tumor cells exhibit increased autophagy as a metabolic adaptation to the harsh conditions present in their surrounding microenvironment. Therefore, this process can be a double-edged sword in cancer therapy because of its tumor suppressor and pro-oncogenic properties [47]. In CRC, the dual role of autophagy in 5-FU-based therapy resistance is no exception. CRC patients overexpressing the autophagy-related gene HSPB8 have shorter overall survival and disease-free survival, and higher tumor stages and lymph node invasion [48]. In some in vitro models, autophagy activation contributes to 5-FU resistance [49]. However, many studies have established that autophagy induction leads to cancer cell death [50,51]. These controversial roles require further studies to be clarified since several early and late (i.e., 3-MA and chloroquine) autophagy inhibitors exist. However, the lack of consensus impairs the pharmacological targeting of autophagy as a therapy.
3.5. Chemoresistance Induced by Components of the Tumor Microenvironment
The tumor microenvironment is a complex interaction of different cell types of the immune system, such as T cells, B cells, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs), with stromal cells like cancer-associated fibroblasts (CAFs), endothelial cells, and soluble extracellular components as growth factors, as well as cytokines, chemokines, hormones, extracellular matrix, etc. This tumor microenvironment can influence cancer patients’ therapeutic response and clinical outcomes in several ways [52,53].
In CRC, there is evidence that CAFs can promote chemoresistance to 5-FU and oxaliplatin by different mechanisms, promoting cell stemness and EMT [54,55]. Recent reports indicate an intricate regulation of CAFs and malignancy in colon cancer cells through regulating the axis molecules, STATs, involved in the EMT process and the regulation of the malignant tumor microenvironment [56,57]. CAFs secrete the pro-inflammatory cytokine IL-6 that induces STAT3 activation through its tyrosine phosphorylation, leading to translocation to the nucleus and activating pro-survival and anti-apoptotic genes [58]. Previous studies have demonstrated that the inhibition of STAT3 activity directly impacts the levels of anti-apoptotic molecules and renders colon tumor cells susceptible to 5-FU treatment [59]. Another STAT molecule involved in the pathogenesis of CRC is STAT6. Previous reports have indicated the critical role of the signaling of this molecule in tumor growth in colonic tissue in a pre-clinical model of CRC. Interestingly, the absence of STAT6 (STAT6-KO mice) and the pharmacological inhibition of STAT6 phosphorylation significantly reduced the number and size of colonic tumors while diminishing the expression of markers related to EMT, such as β-catenin nuclear expression, and increasing the response to 5-FU [60,61,62]. The regulation of these molecules is closely related to the tumor microenvironment and susceptibility to chemotherapy in CRC, making them an excellent therapeutic target that needs further study. On the other hand, the effect of TAMs can also induce oxaliplatin and 5-FU resistance by maintaining high levels of Cathepsin B, increasing the levels of efflux pump proteins and suppressing caspase-mediated apoptosis, to name a few of its effects [63,64]. The interplay between the tumor and microenvironment impacts the biological characteristics of tumoral cells frequently related to the malignancy of tumors, in which specific factors and signaling mechanisms are dysregulated.
3.6. Inflammation
It is well known that inflammation plays a crucial role in cancer initiation, progression, and malignancy [65]. Chronic intestinal inflammation is, without a doubt, a significant risk factor for CRC development and can also be involved in a patient’s prognosis and response to therapy [66]. Inflammation contributes to the drug resistance of tumoral cells by increasing the expression of efflux pumps and altering the levels of drug-metabolizing enzymes. In addition, inflammation can protect cancer cells from drug-mediated cell death by regulating DNA damage repair, activating oncogenic pathways, and modulating the influence of the tumor microenvironment [67,68]. The high expression of inflammatory molecules is associated with the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) that is considered a master regulator of the transcriptional activation of a great variety of inflammatory molecules, such as IL-1β, IL-6, COX2, and STAT3, that are closely related to malignancy and drug resistance [69]. Different studies indicate that high expression levels of NFκB are associated with perineural invasion, lymph node metastasis, and pathologic tumor node metastasis in around 62% of analyzed CRC patients [70]. Therefore, targeting inflammation is a great option to help increase therapy effectiveness. Currently, there is much interest in the use of non-steroidal anti-inflammatory drugs (NSAIDs) as possible adjuvants to conventional therapy with promising results. However, the side effects and toxicity of NSAIDs limit the establishment of their use in the clinic [71,72].
All these mechanisms involved in chemoresistance prevent therapy success. Thus, it is necessary to find new alternatives that help to increase the response to chemotherapy by counteracting chemoresistance and avoiding greater side effects and toxicity.
4. Natural Compounds as a Promising Therapeutic Option for CRC
The search for new cancer treatment strategies to improve patients’ therapeutic response has led to a broader vision of non-synthetic compounds. In this regard, natural products are a rich reservoir of bioactive compounds with therapeutic potential [73]. Moreover, around 50% of anti-cancer drugs were obtained directly or indirectly from natural products, such as alkaloids, polysaccharides, polyphenols, diterpenoids, and unsaturated fatty acids possessing various structures, among others [74]. The search for molecules of a natural origin has become relevant. It has been suggested that some of these compounds can sensitize cancer cells to chemotherapy, enhancing the effect of drugs and making them more specific to acting only on cancerous cells. Furthermore, natural compounds tend to be multi-target, so they can interfere with different signaling pathways and thus prevent the appearance of chemoresistance [75]. The origin of natural products can be diverse: microbes, plants, and other living organisms, such as protozoa and helminths, all present anti-cancer properties [73,76].
In vitro and in vivo assays have demonstrated the potential use of natural products in inhibiting cancer cell proliferation, cell cycle arrest, and tumoral cell death [77,78]. This does not mean that natural compounds (NCs) alone are the cure for all types of neoplasia. Previous reports have highlighted that the use of these compounds as a single therapy does not seem to be a viable option to cure a disease as aggressive as cancer; nonetheless, their use as adjuvants to conventional chemotherapy could be a promising therapeutic option to increase anti-cancer therapy responses [79]. Curcumin, resveratrol, artemisinin, trimethylglycine, and helminth-derived molecules are common as new NCs with potential uses as adjuvants to improve 5-FU-based therapy in CRC models. The mechanisms underlying their roles in enhancing the effect of chemotherapeutics and their abilities to revert chemoresistance are areas of intense activity.
4.1. Curcumin
Curcumin is an NC derived from the root of the turmeric plant (Curcuma longa); it has been used for many centuries as a spice and in traditional medicine [79]. This compound has anti-inflammatory, antioxidant, and anti-cancerogenic properties associated with the regulation of different signaling pathways involved in cell proliferation and survival, which make it a promising therapeutic option for being used as an adjuvant with gemcitabine, docetaxel, oxaliplatin, erlotinib, 5-FU, and celecoxib [80,81,82].
The mechanism of action of curcumin against cancer cells involves the negative regulation of NF-κB, EGFR, and PI3K/AKT signaling pathways that lead to the diminished activity and expression of proteins involved in anti-apoptotic processes, such as Bcl-2 and Bcl-xL, while inducing Bax, caspase-3, and p53 pro-apoptotic molecules. These effects have been observed in many types of cancer, mainly in breast, prostate, gastric, pancreatic, lung, and colorectal cancer [83,84]. In CRC, curcumin has been combined with 5-FU-based therapies in different models where it has been demonstrated to potentiate the chemotherapy effect and reverse chemoresistance through diverse pathways, including TS regulation in colon cancer cell lines, due to its inhibition of the nuclear translocation of NFκB and, consequently, the decrease in E2F1 transcription factor, a promoter of cell proliferation [85,86]. Table 1 summarizes the discovered mechanisms of the synergistic effects of curcumin with 5-FU-based therapies in different in vitro and in vivo models of CRC, including apoptosis inhibition, the dysregulation of survival pathways associated with inflammation such as NFκB and COX2, and the inhibition of stemness and EMT properties.

Table 1.
Effects of administration of combined curcumin and 5-FU-based therapies in different CRC models.
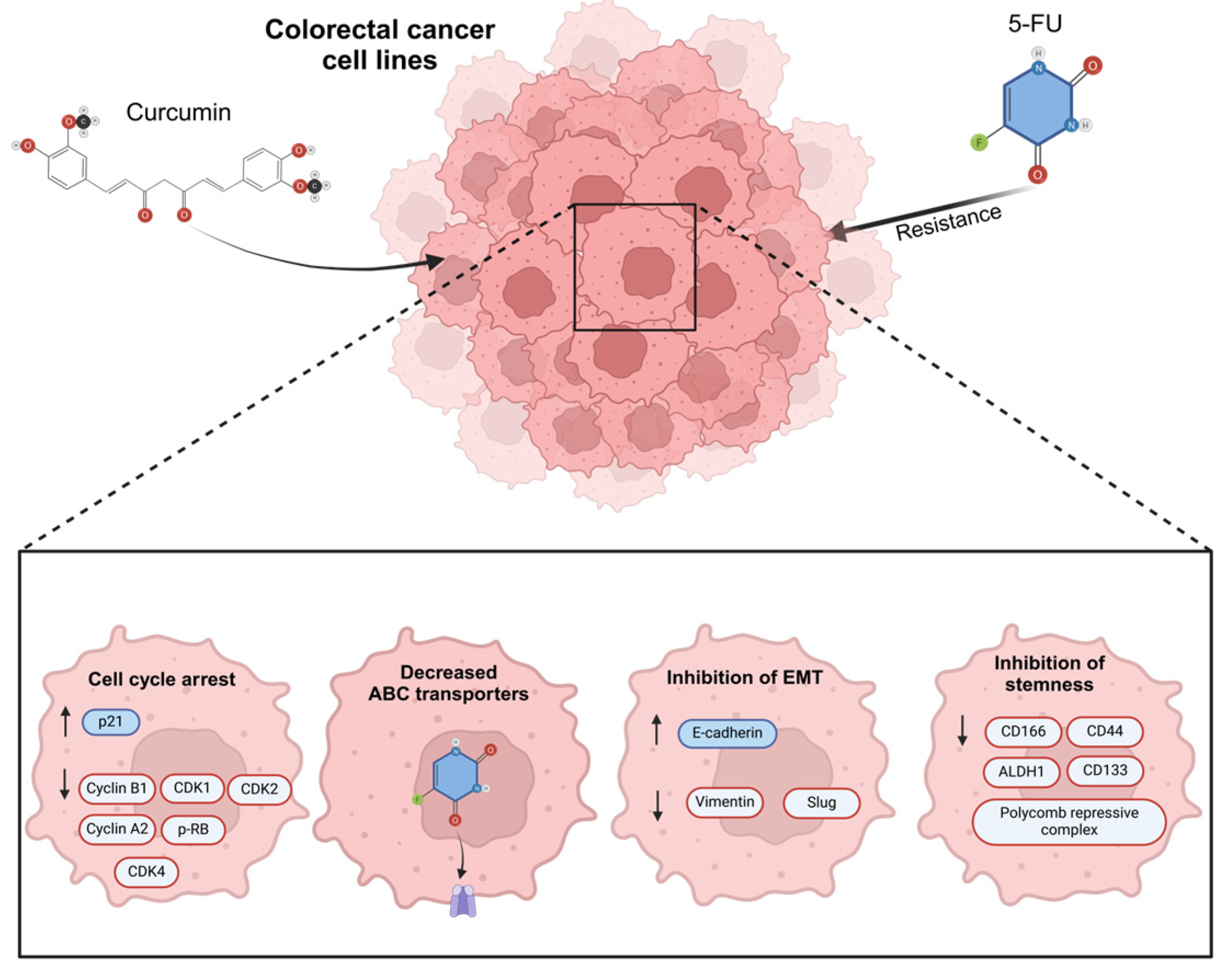
Several studies in CRC cell lines have shown that induced 5-FU resistance is reverted with exposure to curcumin through cell cycle arrest, decreased ABC-transporter protein levels, the inhibition of stemness, and a reduction in EMT; the mechanisms related to the activity of curcumin in revert 5-FU resistance (Figure 4) [93,94]. In oral cancer, using curcumin as a treatment has been demonstrated to significantly diminish EMT features via c-Met blockade and the inhibition of the ERK effector pathway [106].
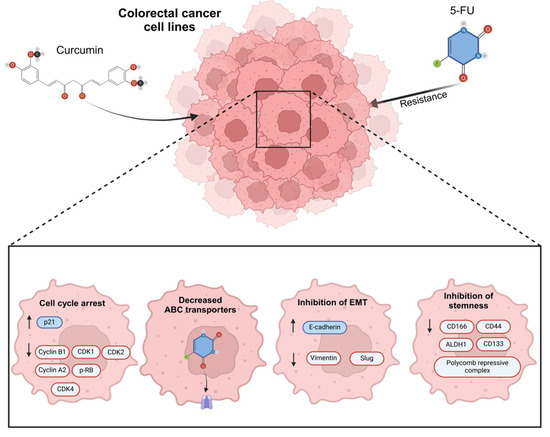
Figure 4.
The main mechanisms of action related to the effect of curcumin in 5-Fluorouracil (5-FU) resistance colorectal cancer cells. Previous studies have verified the use of curcumin in 5-FU and its effects as regulator of cell cycle arrest, increasing P21 protein expression and the negative regulation of cyclin B1, cyclin-dependent kinases 1, 2, and 4 (CDK1, CDK2, CDK4), cyclin A2, and the phosphorylation of retinoblastoma, decreasing the expression of ABC transporters, regulating EMT markers such as E-cadherin positively, and reducing Vimentin and Slung proteins. Finally, curcumin has an important effect on regulating stemness markers such as CD116, CD44, ALDH1, CD33, and the polycomb repressive complex.
Recently, different target drug delivery systems of curcumin and 5-FU have been tested to increase their specificity and cytotoxicity and reduce side effects. Moreno-Quintero et al. (2023) designed 5-FU–curcumin hybrids, tested in the malignant colorectal cancer cells SW-480 and SW-620, and showed that combinatory therapy was more effective in tumoral cells than individual treatment [103]. New alternatives for drug delivery have been proposed to increase the effects of curcumin together with 5-FU, folic acid (FA)-modified nanoparticles, or micelle-crosslinked hydrogel with 5-FU, and curcumin has been tested in HT-29 CRC cells, showing a more significant inhibitory effect than free drugs [102].
The combination of curcumin and 5-FU in animal models demonstrated that this combinatory therapy decreases the tumor volume and weight in CRC xenotransplant models [94,100]. In addition, this combination significantly reduced metastasis to different organs, such as the liver, intestine, spleen, and rectum, in an orthotopic transplant by decreasing angiogenesis and the number of NFκB, COX-2, cyclin D1, c-Myc, ICAM-1, MMP-9, CXCR4, and VEGF molecules [104].
In humans, a randomized Phase II trial evaluated the combination of curcumin with FOLFOX (folinic acid, oxaliplatin, and 5-FU) in a cohort of patients with metastatic CRC, showing a better survival in the FOLFOX plus curcumin treatment in comparison to patients receiving FOLFOX as a single therapy. This combined therapy was safe and well-tolerated, reducing neuropathic pain and neuronal functional abnormalities; however, no conclusive results were obtained regarding effectiveness; hence, more evidence is needed in clinical trials [99].
All these studies indicate that curcumin strongly enhances the effects of 5-FU-based chemotherapy. It avoids chemoresistance mainly by reducing inflammation and tumor cell stemness and enhancing apoptosis cell death, making curcumin a valuable candidate for use as a natural adjuvant in CRC therapy.
4.2. Resveratrol
Resveratrol is a polyphenol abundantly present in grape skin and seeds but also in peanuts, berries, tea, and wine [107]. Resveratrol has been reported to have anti-inflammatory and antioxidant activities that confer anti-aging and anti-cancer properties [108]. Its anti-cancer properties have been demonstrated against many types of cancers, such as breast, prostate, liver, and CRC, among others [109,110].
The administration of resveratrol as monotherapy induces apoptosis and autophagy and inhibits tumor growth in in vitro and in vivo models of CRC [111,112]. It is essential to point out that autophagy displays dual roles in cancer treatments, being both related to resistance and an inductor of death in tumoral cells. Resveratrol has demonstrated potential use in inhibiting the progression of colorectal cancer through autophagy-related apoptosis using a SIRT1/FOXQ1/ATG16L pathway axis [112]. In cisplatin-resistant human oral cancer CAR (a tongue squamous cell carcinoma cell line derived from the CAL 27 cell line), the resveratrol treatment induces cell death by an autophagic pathway, which ultimately converges with apoptotic cell death [113]. The dual role of autophagy in response to chemotherapies appears to be dependent on the origin of the neoplasia and used drugs, as observed in p53-null CRC cell lines, in which irinotecan treatment increases the autophagy favoring resistance to tumoral cell death, while data observed in hepatocellular carcinoma, colon, and pancreatic cancer showed that the restoration of autophagy favored 5-FU activity [114,115]. Therefore, the evidence suggests that resveratrol could be an inductor of autophagy and could act synergistically with 5-FU to improve the efficacy of the drug.
Several in vitro and in vivo studies reveal alternative and different mechanisms used by resveratrol to enhance 5-FU activity and cell cycle arrest and increase apoptotic proteins, mitochondrial oxidative stress, and proteins related to the integrity of cell junctions, increasing E-cadherin and claudin-2, among others [116,117,118,119]. The combination of 5-FU plus resveratrol leads to caspase-6 cleavage, increasing cancerous cell death and centrosome amplification, making CRC cells more sensitive to resveratrol and DNA damage [116,117]. Furthermore, the use of resveratrol in induced chemoresistance to the 5-FU cell line (HCT-116R) diminished the hypoxia-inducible factor (HIF) that is associated with poor prognosis in CRC patients [118]. Meanwhile, in a N-methyl nitrosourea-induced CRC model in rats, the administration of 5-FU together with resveratrol ameliorated the histopathological lesions of colon tissues and attenuated inflammation and epithelial damage, increased antioxidant molecules as superoxide dismutase (SOD) and oxidant marker malondialdehyde (MDA), and advanced oxidation protein products (AOPP) in the colon, favoring the anti-tumor response [119]. All these results and the most relevant mechanisms are presented in Table 2.

Table 2.
Effects of resveratrol and 5-FU administration in combination therapy in different colorectal cancer models.
In summary, the combinatory treatment of resveratrol and 5-FU can potentially enhance the apoptotic effect of 5-FU, regulate molecules involved in oxidative stress and autophagy, and reduce EMT markers, stemness, cell proliferation, and inflammation.
4.3. Artemisinin
Artemisinin is a sesquiterpene lactone isolated by the Chinese Professor Tu Youyou from sweet wormwood leaves (Artemisia annua L.). Initially, artemisinin and its derivates were used as antimalarial drugs. However, these compounds demonstrated immunosuppressive effects primarily associated with the suppression of T cell proliferation and the downregulation of pro-inflammatory cytokines such as IL-2 and IFN-γ [129,130]. Additionally, these NCs reduced the symptoms of autoimmune diseases in murine models of atopic dermatitis, experimental encephalomyelitis, systemic lupus erythematosus, and ulcerative colitis [131,132]. The immunosuppressive effects of artemisinin and its derivates have been tested in murine models of ulcerative colitis and colitis-associated colorectal cancer. First, in dextran sodium sulfate (DSS)-induced colitis, artemisinin and its derivates decreased the disease activity index by downregulating pro-inflammatory cytokines such as IL-12 and TNF-α, recruiting alternatively activated CD206+ macrophages, and blocking the TLR4 signaling pathway and NFκB signaling pathway, which consequently decreased the number of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. In recent reports, artemisinin and its derivates directly affected the human colorectal cancer cell lines HCT-116 and RKO by inducing apoptotic processes [133,134].
Artemisinin may also function as a chemosensitizer, as reported in esophageal cancer EC109 cells, where exposure to artemisinin reduced the activity of the Wnt/β-catenin signaling pathway, one of the main drivers associated with resistance to chemotherapies, favoring the effect of oxaliplatin [134], and, as with resveratrol, because of its additional immunosuppressive and anti-inflammatory effects, artemisinin can be considered as a potential drug repositioning candidate due to its targeting of autophagy to induce cancer cell death [135].
However, there is limited evidence for the role of artemisinin in regulating chemoresistance processes. Still, its potential beneficial activities, such as having fewer side effects than traditional cancer treatments and its beneficial use in early cancer development, need more studies to establish direct participation in these processes and their interaction with conventional therapies.
4.4. Helminth-Derived Molecules
Infections with parasites are more prevalent in developing countries than industrialized ones, correlating with the hygiene hypothesis [136]. The relationship between parasites and CRC has been controversial in the last ten years [137]. Clinical observations suggest that patients infected with Echinococcus granulossus have a lower probability of colon cancer development [138]. In concordance with this, it has been shown that early treatment using molecules excreted/secreted by Taenia crassiceps (TcES) in a colitis-associated colon cancer (CAC) mouse model leads to reduced pro-inflammatory and proliferative signaling pathways related to cancer establishment and progression, such as STAT3/NFκB, indicating that these molecules may be an excellent early adjuvant therapy in experimental CRC treatment [76]. Using other helminth antigens in vitro, such as those from Heligmosomoides po-lygyrus, increased the levels of P53 and P21 proteins in CRC cell lines from mice and humans [139]. These remarkable effects of helminth-derived molecules on CRC were reinforced when used as adjuvant therapy with 5-FU in a mouse model of CAC and the human colorectal cancer cell lines HCT-116 and RKO. Mice receiving the combination of TcES plus 5-FU developed a significantly lower colon tumor load than mice receiving single 5-FU. Moreover, this combination improved the recruitment and activity of NK cells and P53 proteins, inducing pro-apoptotic and anti-proliferative effects [140]. Previously, TcES had been demonstrated to inhibit STAT1 phosphorylation in vitro by activating protein tyrosine phosphatases, such as SHP1, in human and mouse cells [141]. Further research must determine whether this mechanism happens when TcES inhibits STAT3 phosphorylation as an adjuvant to 5-FU during CAC.
On the other hand, human CRC lines HCT-116 and RKO exposed simultaneously to TcES and 5-FU displayed lower proliferative rates and significantly reduced their migration activity [139]. Collectively, these studies show evidence of the potential use of some helminth-derived molecules as an alternative adjuvant to enhance 5-FU activity in the therapy of colorectal cancer. These molecules may negatively regulate both STAT3 and NFκB signaling, altering the interaction between tumoral cells and their microenvironment, reducing the acquisition of malignant phenotypes in tumoral cells, favoring response to therapies, and improving survival.
4.5. Trimethylglycine
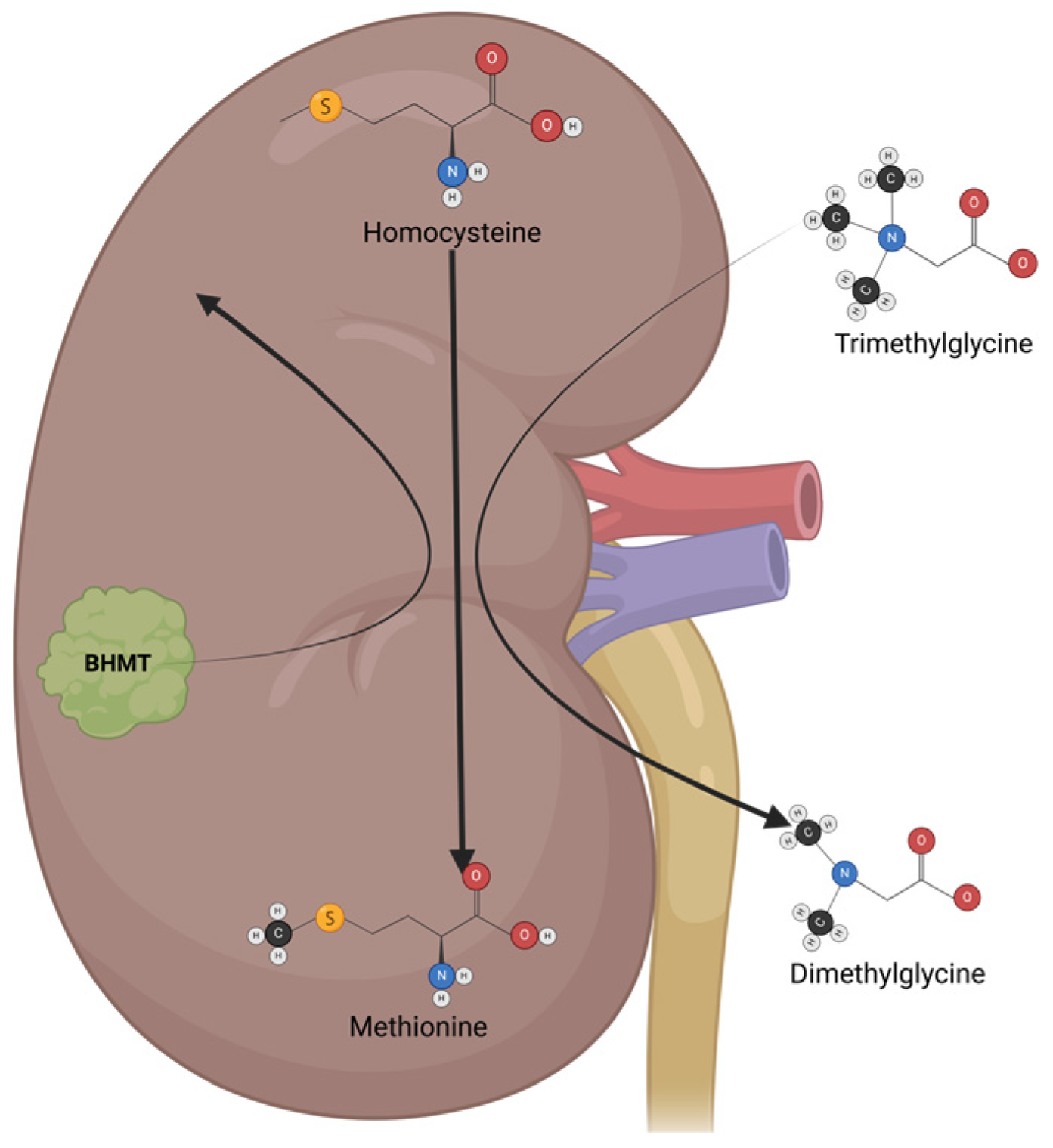
Trimethylglycine (TMG), or betaine, is a glycine-methyl derivative with a zwitterionic quaternary ammonium structure. It was first discovered in sugar beets (Beta vulgaris). It is also found in high quantities in spinach, wheat germ, wheat bran, and seafood, and is part of the metabolism of microorganisms, plants, and animals. TMG has two main physiological functions: the first is to act as an osmolyte to protect cells from stress conditions, and the second is to act as a methyl group donor [142]. In humans, TMG osmolyte function is mainly executed in the kidneys, which protects renal cells from high levels of urea and electrolytes. TMG acts as a methyl group donor mainly in the liver, where it helps to convert homocysteine to methionine via betaine–homocysteine methyl transferase (BHMT) (Figure 5) [143]. Besides its physiological functions, TMG has antioxidant and anti-inflammatory properties, making it a great option for cancer chemoprevention and cancer treatment in neoplasia with inflammatory components [144].
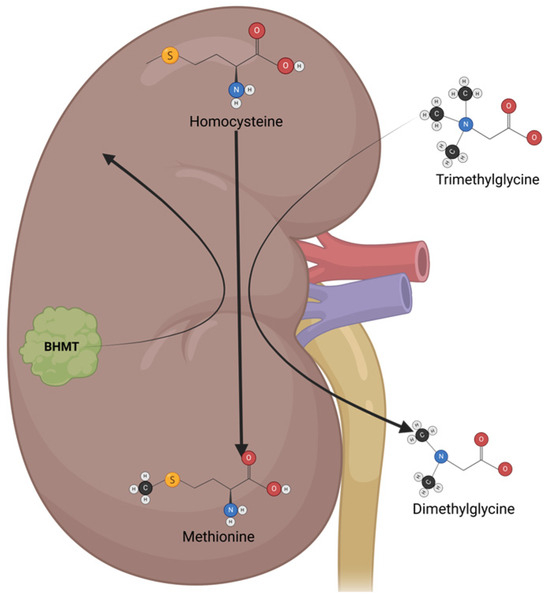
Figure 5.
The mechanisms of action and metabolism of TMG. TMG is an osmolyte that functions mainly in the kidney, protecting renal cells from high urea levels and electrolytes. TMG acts as a methyl group donor, switching from trimethylglycine to dimethylglycine, which helps to convert homocysteine to methionine via betaine–homocysteine methyl transferase (BHMT).
Some reports have indicated the potential role of TMG as a cancer chemopreventive. A meta-analysis study found a relationship between TMG levels ingested in the diet and the reduced incidence of different types of cancer, especially CRC [144]. An in vivo model of CAC indicated that the administration of TMG in the early stages of the disease significantly reduced the inflammation and development of tumors in the colon [145]. As observed in prostate and lung cancer cell lines, treatment with different doses of TMG reduces oxidative stress and inflammation, favoring the apoptosis process through the negative regulation of NFκB [146,147]. Therefore, it is possible to suggest that TMG, an NFκB activity inhibitor, could regulate mechanisms in resistance to 5-FU, such as TS expression, similarly to curcumin and resveratrol. There is evidence, shown in an in vivo CAC model, that using TMG in combination with 5-FU increases the effectiveness of 5-FU by suppressing the indirect markers of NFκB activity, such as SNAIL1 and β-catenin nuclear translocation, and recovering E-cadherin protein levels; additionally, the use of TMG in this model was shown to significantly reduce the number and size of tumors, as a potential mechanism of the downregulation of STAT6 activity [60]. Thus, TMG is an attractive molecule to be used as an adjuvant for cancer therapy; additionally, previous advantages have been reported in its apoptosis induction of tumoral cells and its anti-inflammatory effect. TMG is considered a safe compound that ameliorates the secondary effects of chemotherapy, such as nephrotoxicity and hepatic damage caused by oxaliplatin treatment [148,149]. Currently, there is limited research using TMG together with conventional chemotherapeutics. Thus, further in vitro and in vivo studies are necessary to translate these results into benefits for colon cancer patients. Nevertheless, there is strong evidence suggesting that TMG could be a great candidate as adjuvant therapy for different types of cancer.
5. Conclusions
Colorectal cancer has become a concerning disease in recent years; the high mortality rates of nearly 50% of the diagnosticated patients reflect mainly two problems: (1) a delayed time of diagnosis leading to advanced stages of CRC; (2) the failure of conventional schemes of therapeutic regimens to overcome acquired or intrinsic resistance that contributes to relapses, metastasis, and, finally, mortality in the patients. The current chemotherapeutics—5-FU, oxaliplatin, and folinic acid—are clinicians’ best weapons to fight advanced CRC. Improving these chemotherapeutic treatments and avoiding their most significant chemoresistance problems would benefit patients’ health [150].
Natural compounds have recently attracted attention, given their functions as cancer preventives and potential adjuvants in chemotherapy. NCs have displayed direct and indirect effects in regulating frequent events characteristic of intrinsic and acquired chemoresistance, such as increased drug metabolism, regulated efflux pumps, autophagy, and the acquisition of EMT markers induced by inflammation and the tumor microenvironment [74,75]. Curcumin, resveratrol, artemisinin, TMG, and some molecules derived from ancient parasites like helminths, are natural compounds that have demonstrated their safe use in combinatory therapy together with 5-FU, improving the anti-tumoral response or restoring sensitivity to the drug (Figure 5). All these data support the idea that most NCs, where safe doses for use have been proven, could be postulated as potential compounds for drug repositioning.
Interestingly, many of these natural alternatives share similar mechanisms of action, such as STAT signaling modulation and NFκB inhibition, and may have less toxicity than synthetic drugs. These characteristics make NCs an attractive field of research to support their use as adjuvant therapies against colorectal cancer in more preclinical and clinical studies (Figure 6).
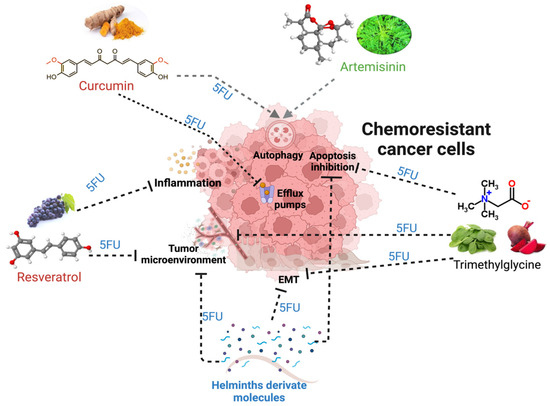
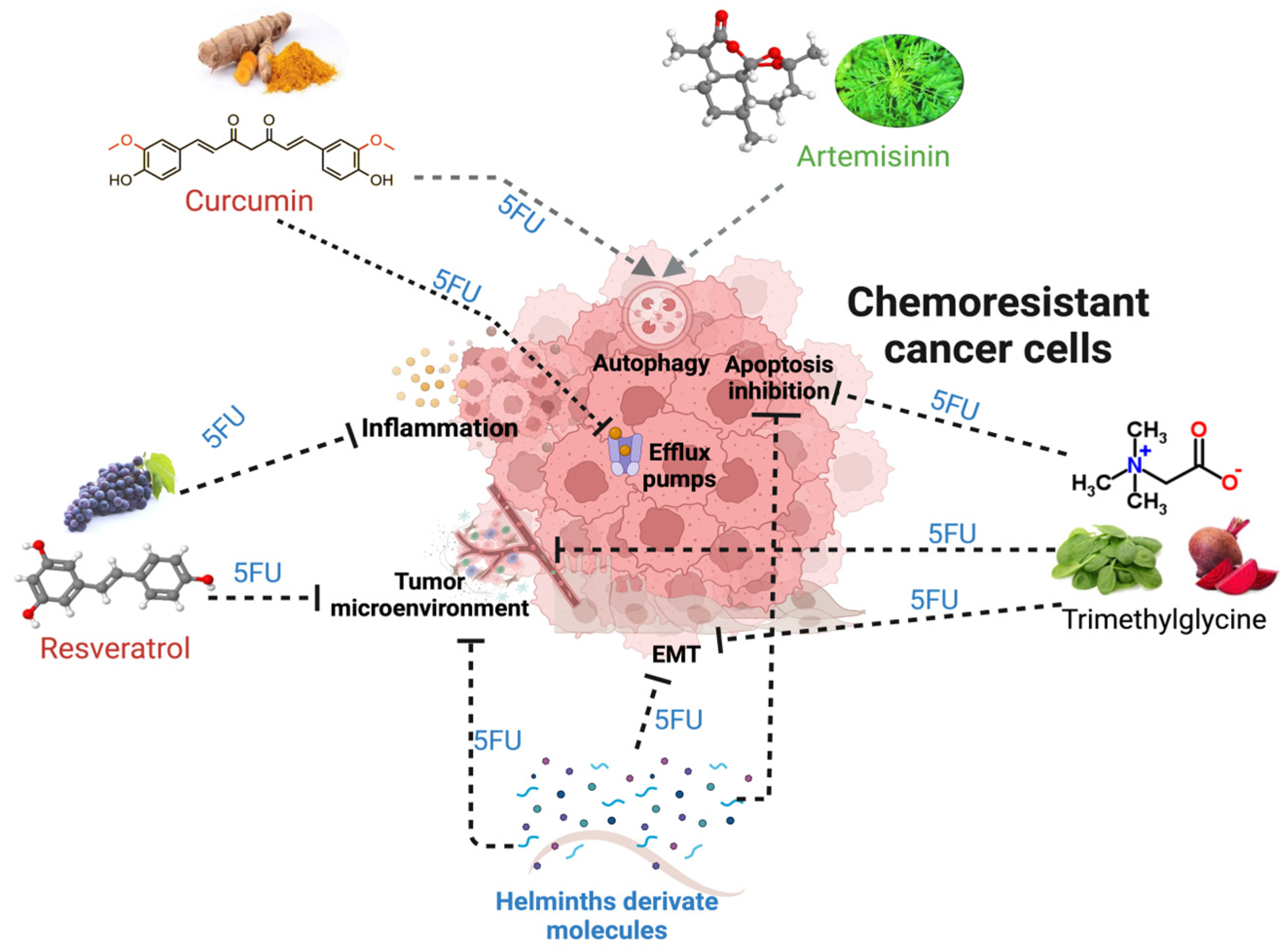
Figure 6.
The mechanisms and alterations in tumors that lead to chemoresistance and its potential regulation by natural compounds, such as trimethylglycine, curcumin, resveratrol, artemisinin, and, recently, helminth-derived molecules together with 5FU. The black dotted lines indicate the blocking effect of a natural compound, while the gray lines with arrowheads show the induction of the mechanism related to better responses to treatments.
Author Contributions
Conceptualization, K.V.F.-M., M.G.M.-R. and L.I.T.; formal analysis, C.Á.S.-B., M.M.-R. and E.A.P.-Y.; investigation, J.L.R. and M.T.O.-M.; writing—original draft preparation, K.V.F.-M. and C.Á.S.-B.; writing—review and editing, M.G.M.-R., L.I.T. and J.L.R.; funding acquisition, M.G.M.-R. and L.I.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Consejo Nacional de Humanidades, Ciencia y Tecnología (CF-2023-I-563), Consejo Mexiquense de Ciencia y Tecnología (FICDTEM-2023-132), Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT-UNAM IA208424 and IV200425) de la Dirección General de Asuntos de Personal Académico, and the Programa de Apoyo a Profesores de Carrera (PAPCA), (FESI-PAPCA 2021-2022-38). Karen V. Fernandez-Muñoz would like to thank the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT) for PhD scholarship number 1008708.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3-MA | 3-methyladenine |
| 5-FU | 5-fluorouracil |
| 5-FUR | 5-fluorouracil resistance |
| ABC | ATP-binding cassette |
| ABCB1 | ATP-binding cassette subfamily B member 1 |
| ABCC1 | ATP-binding cassette subfamily C member 1 |
| ABCG2 | ATP-binding cassette subfamily G member 2 |
| Alb | Albumin |
| ALDH1 | Aldehyde dehydrogenase 1 |
| ALT | Alanine aminotransferase |
| AOM | Azoxymethane |
| AOPP | Advanced oxidation protein products |
| APC | Adenomatous polyposis coli |
| AST | Aspartate transferase |
| ATG16L | Autophagy related 16-like |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra large |
| BHMT | Betaine-homocysteine methyl transferase |
| BRAF | B-Raf proto-oncogene |
| CAC | Colitis-associated colon cancer |
| CAFs | Cancer-associated fibroblasts |
| CDK1/2/4 | Cyclin-dependent kinase 1/2/4 |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| CH2THF | 5,10-methylenetetrahydrofolate |
| CIMP | CpG island methylator phenotype |
| CIN | Chromosomal instability |
| COX-2 | Cyclooxygenase 2 |
| CRC | Colorectal cancer |
| CSC | Cancer stem cells |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| Cur | Curcumin |
| CXCR2/4 | Chemokine receptor type 2/4 |
| DHFU | Dihydro fluorouracil |
| DMSO | Dimethyl sulfoxide |
| DPD | Dihydropyrimidine dehydrogenase |
| DSS | Dextran sodium sulfate |
| dTMP | Deoxythymidine monophosphate |
| dUMP | Deoxyuridine monophosphate |
| E2F1 | E2F transcription factor 1 |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| ERCC1 | Excision repair cross-complementing rodent repair deficiency, complementation group 1 |
| ERK | Extracellular signal-regulated kinase |
| FA | Folinic acid |
| FdUMP | 5-fluorodeoxyuridine monophosphate |
| FOLFOX | 5-fluorouracil, oxaliplatin, and leucovorin |
| FOXQ1 | Forkhead box Q1 |
| GO | Graphene oxide |
| GPx | Glutathione peroxidase |
| HER-2/3 | Human epidermal growth factor receptor 2/3 |
| HIF | Hypoxia-inducible factor |
| HSP27 | Heat shock protein 27 |
| HSPB8 | Heat shock protein family B member 8 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IFN-γ | Interferon gamma |
| IGF-1R | Insulin-like growth factor-1 receptor |
| IL-1β/2/6/10/12/23 | Interleukin 1β/2/6/10/12/23 |
| Ip. | Intraperitoneal |
| IRE1α | Inositol-requiring transmembrane kinase endoribonuclease-1α |
| IκBα | NFκB inhibitor alpha |
| JNK | c-Jun N-terminal kinase |
| KRAS | Kirsten rat sarcoma viral proto-oncogene |
| mCRC | Metastatic colorectal cancer |
| MDA | Malondialdehyde |
| MDR1 | Multidrug resistance protein 1 |
| MDR1 | Multidrug resistance protein 1 |
| MDSC | Myeloid-derived suppressor cells |
| MMP9/13 | Matrix metalloproteinases 9/13 |
| MRP1 | Multidrug resistance-associated protein 1 |
| MSI | Microsatellite instability |
| NC | Natural compound |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | Natural killer cell |
| NNMT | Nicotinamide N-methyltransferase |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OXA | Oxaliplatin |
| PARP | Poly (ADP-ribose) polymerase |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PGE2 | Prostaglandin E2 |
| P-gp | P-glycoprotein 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| pRb | Retinoblastoma protein |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
| SHP1 | Src homology 2 domain-containing protein tyrosine phosphatase 1 |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| STAT1/3/6 | Signal transducer and activator of transcription 1/3/6 |
| TAMs | Tumor-associated macrophages |
| TcES | Molecules excreted/secreted by Taenia crassiceps |
| TGF-β | Transforming growth factor beta |
| TGFβ-R | Transforming growth factor-β receptor |
| TLR4 | Toll-like receptor 4 |
| TMG | Trimethylglycine |
| TNF-α/β | Tumor necrosis factor alpha/beta |
| TNM | Tumor–node–metastasis |
| Tp53 | Tumor protein p53 |
| TS | Thymidylate synthase |
| VCR | Vincristine-resistant |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
| XBP1 | X-box binding protein 1 |
| Zeb1/2 | Zinc finger E-box binding homeobox ½ |
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2022, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, H.; Kuroda, H.; Imai, Y.; Hiraishi, H. Molecular Pathogenesis of Sporadic Colorectal Cancers. Chin. J. Cancer 2016, 35, 4. [Google Scholar] [CrossRef]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Góra, A.; Rudzki, S. Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control 2022, 29. [Google Scholar] [CrossRef]
- Ahmad, R.; Singh, J.K.; Wunnava, A.; Al-obeed, O.; Abdulla, M.; Srivastava, S.K. Emerging Trends in Colorectal Cancer: Dysregulated Signaling Pathways. Int. J. Mol. Med. 2021, 47, 14. [Google Scholar] [CrossRef]
- Khalyfa, A.A.; Punatar, S.; Aslam, R.; Yarbrough, A. Exploring the Inflammatory Pathogenesis of Colorectal Cancer. Diseases 2021, 9, 79. [Google Scholar] [CrossRef]
- Sánchez-Barrera, C.Á.; Olguin, J.E.; Terrazas, L. A Dual Role for Neutrophils and Myeloid-Derived Suppressor Cells in the Development of Colorectal Cancer; Morales-Montor, J., Ed.; Serie: Immunology and Immune System Disorders; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2022; pp. 145–185. [Google Scholar]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafar, A.B. General Insight into Cancer: An Overview of Colorectal Cancer. Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Cancer-Preventive Interventions. Colorectal Cancer Screening [Internet]; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789283230236. [Google Scholar]
- Wilson, L.A.M.; Browne, S.; Barnes, J.; El Hoyek, N.; Helmueller, L.; Janeiro, M.J.; Wendt, B. Opportunities and Challenges in Screening for Colorectal Cancer. Popul. Health Manag. 2023, 26, 246–253. [Google Scholar] [CrossRef]
- Buccafusca, G.; Proserpio, I.; Tralongo, A.C.; Rametta Giuliano, S.; Tralongo, P. Early Colorectal Cancer: Diagnosis, Treatment and Survivorship Care. Crit. Rev. Oncol. Hematol. 2019, 136, 20–30. [Google Scholar] [CrossRef]
- Mattar, R.E.; Al-Alem, F.; Simoneau, E.; Hassanain, M. Preoperative Selection of Patients with Colorectal Cancer Liver Metastasis for Hepatic Resection. World J. Gastroenterol. 2016, 22, 567–581. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.E.; Giancotti, F.G.; Rustgi, A.K. Metastatic Colorectal Cancer: Mechanisms and Emerging Therapeutics. Trends Pharmacol. Sci. 2023, 44, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, E.; Liu, H.; Wan, L.; Lai, M. Epithelial-Mesenchymal Transition in Colorectal Cancer Metastasis: A System Review. Pathol. Res. Pract. 2015, 211, 557–569. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Wigmore, P.M.; Mustafa, S.; El-Beltagy, M.; Lyons, L.; Umka, J.; Bennett, G. Effects of 5-FU. In Chemo Fog. Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; Volume 678. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Wanderley, C.W.S.; Wong, D.V.T.; Mota, J.M.S.C.; Leite, C.A.V.G.; Souza, M.H.L.P.; Cunha, F.Q.; Lima-Júnior, R.C.P. Irinotecan- and 5-Fluorouracil-Induced Intestinal Mucositis: Insights into Pathogenesis and Therapeutic Perspectives. Cancer Chemother. Pharmacol. 2016, 78, 881–893. [Google Scholar] [CrossRef]
- Anaka, M.; Abdel-Rahman, O. Managing 5FU Cardiotoxicity in Colorectal Cancer Treatment. Cancer Manag. Res. 2022, 14, 273–285. [Google Scholar] [CrossRef]
- Kang, L.; Tian, Y.; Xu, S.; Chen, H. Oxaliplatin-Induced Peripheral Neuropathy: Clinical Features, Mechanisms, Prevention and Treatment. J. Neurol. 2021, 268, 3269–3282. [Google Scholar] [CrossRef]
- Palmieri, L.J.; Dubreuil, O.; Bachet, J.B.; Trouilloud, I.; Locher, C.; Coriat, R.; Moryoussef, F.; Landi, B.; Perkins, G.; Hautefeuille, V.; et al. Reasons for Chemotherapy Discontinuation and End-of-Life in Patients with Gastrointestinal Cancer: A Multicenter Prospective AGEO Study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101431. [Google Scholar] [CrossRef]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic Resistance in Colorectal Cancer: A Review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, R.; Llaneza, D.C.; Landen, C.N. Emerging Therapeutics to Overcome Chemoresistance in Epithelial Ovarian Cancer: A Mini-Review. Int. J. Mol. Sci. 2017, 18, 2171. [Google Scholar] [CrossRef] [PubMed]
- Wohlhueter, R.M.; McIvor, R.S.; Plagemann, P.G. Facilitated Transport of Uracil and 5-Fluorouracil, and Permeation of Orotic Acid into Cultured Mammalian Cells. J. Cell. Physiol. 1980, 104, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.V.; McHenry, C.S.; Sommer, H. Mechanism of Interaction of Thymidylate Synthetase with 5-Fluorodeoxyuridylate. Biochemistry 1974, 13, 471–481. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Singh, H.; Thareja, S.; Kumar, P. Regulation of Thymidylate Synthase: An Approach to Overcome 5-FU Resistance in Colorectal Cancer. Med. Oncol. 2023, 40, 3. [Google Scholar] [CrossRef]
- Song, S.; Tian, B.; Zhang, M.; Gao, X.; Jie, L.; Liu, P.; Li, J. Diagnostic and Prognostic Value of Thymidylate Synthase Expression in Breast Cancer. Clin. Exp. Pharmacol. Physiol. 2021, 48, 279–287. [Google Scholar] [CrossRef]
- Li, Y.; Mizutani, Y.; Shiraishi, T.; Okihara, K.; Ukimura, O.; Kawauchi, A.; Nonomura, N.; Fukushima, M.; Sakai, T.; Miki, T. Prognostic Significance of Thymidylate Synthase Expression in Patients with Prostate Cancer Undergoing Radical Prostatectomy. Urology 2007, 69, 988–995. [Google Scholar] [CrossRef]
- Lenz, H.J.; Leichman, C.G.; Danenberg, K.D.; Danenberg, P.V.; Groshen, S.; Cohen, H.; Laine, L.; Crookes, P.; Silberman, H.; Baranda, J.; et al. Thymidylate Synthase MRNA Level in Adenocarcinoma of the Stomach: A Predictor for Primary Tumor Response and Overall Survival. J. Clin. Oncol. 1996, 14, 176–182. [Google Scholar] [CrossRef]
- Dotor, E.; Cuatrecases, M.; Martínez-Iniesta, M.; Navarro, M.; Vilardell, F.; Guinó, E.; Pareja, L.; Figueras, A.; Molleví, D.G.; Serrano, T.; et al. Tumor Thymidylate Synthase 1494del6 Genotype as a Prognostic Factor in Colorectal Cancer Patients Receiving Fluorouracil-Based Adjuvant Treatment. J. Clin. Oncol. 2006, 24, 1603–1611. [Google Scholar] [CrossRef]
- Jiang, H.; Li, B.; Wang, F.; Ma, C.; Hao, T. Expression of ERCC1 and TYMS in Colorectal Cancer Patients and the Predictive Value of Chemotherapy Efficacy. Oncol. Lett. 2019, 18, 1157–1162. [Google Scholar] [CrossRef]
- Badary, D.M.; Elkabsh, M.M.; Mady, H.H.; Gabr, A.; Kroosh, S.S. Prognostic and Predictive Role of Excision Repair Cross-Complementation Group 1 and Thymidylate Synthase in Colorectal Carcinoma Patients Received FOLFOX Chemotherapy: An Immunohistochemical Study. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, C.; Menéndez, J.C. Antimetabolites. In Medicinal Chemistry of Anticancer Drugs; Elsevier: Amsterdam, The Netherlands, 2008; pp. 9–52. [Google Scholar]
- Zhang, Y.H.; Luo, D.D.; Wan, S.B.; Qu, X.J. S1PR2 Inhibitors Potently Reverse 5-FU Resistance by Downregulating DPD Expression in Colorectal Cancer. Pharmacol. Res. 2020, 155, 104717. [Google Scholar] [CrossRef] [PubMed]
- Ughachukwu, P.; Unekwe, P. Efflux Pump-Mediated Resistance in Chemotherapy. Ann. Med. Health Sci. Res. 2012, 2, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Alketbi, L.; Al-Ali, A.; Talaat, I.M.; Hamid, Q.; Bajbouj, K. The Role of ATP-Binding Cassette Subfamily A in Colorectal Cancer Progression and Resistance. Int. J. Mol. Sci. 2023, 24, 1344. [Google Scholar] [CrossRef]
- Chen, N.; Kong, Y.; Wu, Y.; Gao, Q.; Fu, J.; Sun, X.; Geng, Q. CAC1 Knockdown Reverses Drug Resistance through the Downregulation of P-Gp and MRP-1 Expression in Colorectal Cancer. PLoS ONE 2019, 14, e0222035. [Google Scholar] [CrossRef]
- Gao, Q.; Li, X.X.; Xu, Y.M.; Zhang, J.Z.; Rong, S.D.; Qin, Y.Q.; Fang, J. IRE1α-Targeting Downregulates ABC Transporters and Overcomes Drug Resistance of Colon Cancer Cells. Cancer Lett. 2020, 476, 67–74. [Google Scholar] [CrossRef]
- Zhang, N.; Ng, A.S.; Cai, S.; Li, Q.; Yang, L.; Kerr, D. Novel Therapeutic Strategies: Targeting Epithelial–Mesenchymal Transition in Colorectal Cancer. Lancet Oncol. 2021, 22, e358–e368. [Google Scholar] [CrossRef]
- Du, F.; Liu, H.; Lu, Y.; Zhao, X.; Fan, D. Epithelial-to-Mesenchymal Transition: Liaison between Cancer Metastasis and Drug Resistance. Crit. Rev. Oncog. 2017, 22, 275–282. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Manavi, M.S.; Faghihkhorasani, F.; Fakhr, S.S.; Baei, F.J.; Khorasani, F.F.; Zare, M.M.; Far, N.P.; Rezaei-Tazangi, F.; Ren, J.; et al. Harnessing Function of EMT in Cancer Drug Resistance: A Metastasis Regulator Determines Chemotherapy Response. Cancer Metastasis Rev. 2024, 43, 457–479. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kwak, J.H.; Je, N.K.; Lee, Y.H.; Jung, Y.S. Epithelial-Mesenchymal Transition Is Associated with Acquired Resistance to 5-Fluorocuracil in HT-29 Colon Cancer Cells. Toxicol. Res. 2015, 31, 151–156. [Google Scholar] [CrossRef]
- Sreekumar, R.; Al-Saihati, H.; Emaduddin, M.; Moutasim, K.; Mellone, M.; Patel, A.; Kilic, S.; Cetin, M.; Erdemir, S.; Navio, M.S.; et al. The ZEB2-Dependent EMT Transcriptional Programme Drives Therapy Resistance by Activating Nucleotide Excision Repair Genes ERCC1 and ERCC4 in Colorectal Cancer. Mol. Oncol. 2021, 15, 2065–2083. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Z.; Miow, Q.H.; Miki, Y.; Noda, T.; Mori, S.; Huang, R.Y.; Thiery, J.P. Epithelial-mesenchymal Transition Spectrum Quantification and Its Efficacy in Deciphering Survival and Drug Responses of Cancer Patients. EMBO Mol. Med. 2014, 6, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a Molecular Target for Cancer Treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [CrossRef]
- Gao, T.; Yuan, D.; He, B.; Gao, Y.; Liu, C.; Sun, H.; Nie, J.; Wang, S.; Nie, Z. Identification of Autophagy Related Genes in Predicting the Prognosis and Aiding 5- Fluorouracil Therapy of Colorectal Cancer. Heliyon 2022, 8, e09033. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, R.; Liu, Y.; Mei, H.; Liu, X.; Peng, Z. USP11 Induce Resistance to 5-Fluorouracil in Colorectal Cancer through Activating Autophagy by Stabilizing VCP. J. Cancer 2021, 12, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, J.; Chen, C.; Jiang, Y.; Feng, X.; Liao, Y.; Yang, Z. NGFR Increases the Chemosensitivity of Colorectal Cancer Cells by Enhancing the Apoptotic and Autophagic Effects of 5-Fluorouracil via the Activation of S100A9. Front. Oncol. 2021, 11, 652081. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, Z.; Zhou, L.; Zhang, H.Y.; Chen, Y.; Tang, Y. Repurposing Ziyuglycoside II Against Colorectal Cancer via Orchestrating Apoptosis and Autophagy. Front. Pharmacol. 2020, 11, 576547. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor Microenvironment and Therapeutic Response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil Resistance Mechanisms in Colorectal Cancer: From Classical Pathways to Promising Processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Hu, J.L.; Wang, W.; Lan, X.L.; Zeng, Z.C.; Liang, Y.S.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.J.; et al. CAFs Secreted Exosomes Promote Metastasis and Chemotherapy Resistance by Enhancing Cell Stemness and Epithelial-Mesenchymal Transition in Colorectal Cancer. Mol. Cancer 2019, 18, 91. [Google Scholar] [CrossRef]
- Linares, J.; Sallent-Aragay, A.; Badia-Ramentol, J.; Recort-Bascuas, A.; Méndez, A.; Manero-Rupérez, N.; Re, D.L.; Rivas, E.I.; Guiu, M.; Zwick, M.; et al. Long-Term Platinum-Based Drug Accumulation in Cancer-Associated Fibroblasts Promotes Colorectal Cancer Progression and Resistance to Therapy. Nat. Commun. 2023, 14, 746. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial– Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramírez, D.; Mendoza-Rodríguez, M.G.; Alemán, O.R.; Candanedo-González, F.A.; Rodríguez-Sosa, M.; Montesinos-Montesinos, J.J.; Salcedo, M.; Brito-Toledo, I.; Vaca-Paniagua, F.; Terrazas, L.I. Impact of STAT-Signaling Pathway on Cancer-Associated Fibroblasts in Colorectal Cancer and Its Role in Immunosuppression. World J. Gastrointest. Oncol. 2024, 16, 1705–1724. [Google Scholar] [CrossRef] [PubMed]
- Tošić, I.; Frank, D.A. STAT3 as a Mediator of Oncogenic Cellular Metabolism: Pathogenic and Therapeutic Implications. Neoplasia 2021, 23, 1167–1178. [Google Scholar] [CrossRef]
- Qin, A.; Yu, Q.; Gao, Y.; Tan, J.; Huang, H.; Qiao, Z.; Qian, W. Inhibition of STAT3/CyclinD1 Pathway Promotes Chemotherapeutic Sensitivity of Colorectal Caner. Biochem. Biophys. Res. Commun. 2015, 457, 681–687. [Google Scholar] [CrossRef]
- Mendoza-Rodríguez, M.G.; Sánchez-Barrera, C.Á.; Callejas, B.E.; García-Castillo, V.; Beristain-Terrazas, D.L.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; León-Cabrera, S.A.; Rodríguez-Sosa, M.; Gutierrez-Cirlos, E.B.; et al. Use of STAT6 Phosphorylation Inhibitor and Trimethylglycine as New Adjuvant Therapies for 5-Fluorouracil in Colitis-Associated Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 2130. [Google Scholar] [CrossRef]
- Leon-Cabrera, S.A.; Molina-Guzman, E.; Delgado-Ramirez, Y.G.; Vázquez-Sandoval, A.; Ledesma-Soto, Y.; Pérez-Plasencia, C.G.; Chirino, Y.I.; Delgado-Buenrostro, N.L.; Rodríguez-Sosa, M.; Vaca-Paniagua, F.; et al. Lack of STAT6 Attenuates Inflammation and Drives Protection against Early Steps of Colitis-Associated Colon Cancer. Cancer Immunol. Res. 2017, 5, 385–396. [Google Scholar] [CrossRef]
- Jayakumar, A.; Bothwell, A.L.M. Stat6 Promotes Intestinal Tumorigenesis in a Mouse Model of Adenomatous Polyposis by Expansion of MDSCs and Inhibition of Cytotoxic CD8 Response. Neoplasia 2017, 19, 595–605. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Xu, Y.; La, X.; Tian, J.; Li, A.; Li, H.; Wu, C.; Xi, Y.; Song, G.; et al. Tumor-Associated Macrophages Confer Colorectal Cancer 5-Fluorouracil Resistance by Promoting MRP1 Membrane Translocation via an Intercellular CXCL17/CXCL22–CCR4–ATF6–GRP78 Axis. Cell Death Dis. 2023, 14, 582. [Google Scholar] [CrossRef]
- Shi, Q.; Shen, Q.; Liu, Y.; Shi, Y.; Huang, W.; Wang, X.; Li, Z.; Chai, Y.; Wang, H.; Hu, X.; et al. Increased Glucose Metabolism in TAMs Fuels O-GlcNAcylation of Lysosomal Cathepsin B to Promote Cancer Metastasis and Chemoresistance. Cancer Cell 2022, 40, 1207–1222.e10. [Google Scholar] [CrossRef]
- Niu, T.; Zhou, F. Inflammation and Tumor Microenvironment. J. Cent. South Univ. (Med. Sci.) 2023, 48, 1899–1913. [Google Scholar] [CrossRef]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic Inflammation in Colorectal Cancer: Underlying Factors, Effects, and Prognostic Significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, Y.; Zhu, C.; Wang, W.; Zhou, Y. Managing Cancer Drug Resistance from the Perspective of Inflammation. J. Oncol. 2022, 2022, 3426407. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.A.; Dinmohammadi, F.; Alizadeh, A.; Elahian, F. Inflammatory Pathway Interactions and Cancer Multidrug Resistance Regulation. Life Sci. 2019, 235, 116825. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, B.; Peng, J.; Tang, H.; Wang, S.; Peng, S.; Ye, F.; Wang, J.; Ouyang, K.; Li, J.; et al. Inhibition of NF-ΚB Signaling Unveils Novel Strategies to Overcome Drug Resistance in Cancers. Drug Resist. Updat. 2024, 73, 101042. [Google Scholar] [CrossRef]
- Pyo, J.S.; Kim, E.K. Clinicopathological Significance and Prognostic Implication of Nuclear Factor-ΚB Activation in Colorectal Cancer. Pathol. Res. Pract. 2019, 215, 152469. [Google Scholar] [CrossRef]
- Thiruchenthooran, V.; Sánchez-López, E.; Gliszczyńska, A. Perspectives of the Application of Non-Steroidal Anti-Inflammatory Drugs in Cancer Therapy: Attempts to Overcome Their Unfavorable Side Effects. Cancers 2023, 15, 475. [Google Scholar] [CrossRef]
- Hua, X.; Phipps, A.I.; Burnett-Hartman, A.N.; Adams, S.V.; Hardikar, S.; Cohen, S.A.; Kocarnik, J.M.; Ahnen, D.J.; Lindor, N.M.; Baron, J.A.; et al. Timing of Aspirin and Other Nonsteroidal Anti-Inflammatory Drug Use among Patients with Colorectal Cancer in Relation to Tumor Markers and Survival. J. Clin. Oncol. 2017, 35, 2806–2813. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural Compounds and Combination Therapy in Colorectal Cancer Treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Callejas, B.E.; Mendoza-Rodríguez, M.G.; Villamar-Cruz, O.; Reyes-Martínez, S.; Sánchez-Barrera, C.A.; Rodríguez-Sosa, M.; Delgado-Buenrostro, N.L.; Martínez-Saucedo, D.; Chirino, Y.I.; León-Cabrera, S.A.; et al. Helminth-Derived Molecules Inhibit Colitis-Associated Colon Cancer Development through NF-ΚB and STAT3 Regulation. Int. J. Cancer 2019, 145, 3126–3139. [Google Scholar] [CrossRef] [PubMed]
- Calibasi-Kocal, G.; Pakdemirli, A.; Bayrak, S.; Ozupek, N.M.; Sever, T.; Basbinar, Y.; Ellidokuz, H.; Yigitbasi, T. Curcumin Effects on Cell Proliferation, Angiogenesis and Metastasis in Colorectal Cancer. J. BUON 2019, 24, 1482–1487. [Google Scholar]
- Liang, Z.; Xie, H.; Shen, W.; Shao, L.; Zeng, L.; Huang, X.; Zhu, Q.; Zhai, X.; Li, K.; Qiu, Z.; et al. The Synergism of Natural Compounds and Conventional Therapeutics against Colorectal Cancer Progression and Metastasis. Front. Biosci. 2022, 27, 263. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by Targeting the Nuclear Factor-ΚB Pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef]
- Yoysungnoen, P.; Wirachwong, P.; Changtam, C.; Suksamram, A.; Patumraj, S. Anti-Cancer and Anti-Angiogenic Effects of Curcumin and Tetrahydrocurcumin on Implanted Hepatocellular Carcinoma in Nude Mice. World J. Gastroenterol. 2008, 14, 2003–2009. [Google Scholar] [CrossRef]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal Curcumin with and without Oxaliplatin: Effects on Cell Growth, Apoptosis, and Angiogenesis in Colorectal Cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Rajitha, B.; Belalcazar, A.; Nagaraju, G.P.; Shaib, W.L.; Snyder, J.P.; Shoji, M.; Pattnaik, S.; Alam, A.; El-Rayes, B.F. Inhibition of NF-κB Translocation by Curcumin Analogs Induces G0/G1 Arrest and Downregulates Thymidylate Synthase in Colorectal Cancer. Cancer Lett. 2016, 373, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, S.; Wei, Y.; Cao, S.; Pi, C.; Feng, T.; Liang, J.; Zhao, L.; Ren, G. Curcumin Enhances the Anticancer Effect of 5-Fluorouracil against Gastric Cancer through down-Regulation of COX-2 and NF-ΚB Signaling Pathways. J. Cancer 2017, 8, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jiang, L.; Xia, Q.; Zhong, L. Synergistic Inhibitory Effects of Curcumin and 5-Fluorouracil on the Growth of the Human Colon Cancer Cell Line HT-29. Chemotherapy 2005, 52, 23–28. [Google Scholar] [CrossRef]
- Patel, B.B.; Sengupta, R.; Qazi, S.; Vachhani, H.; Yu, Y.; Rishi, A.K.; Majumdar, A.P.N. Curcumin Enhances the Effects of 5-Fluorouracil and Oxaliplatin in Mediating Growth Inhibition of Colon Cancer Cells by Modulating EGFR and IGF-1R. Int. J. Cancer 2008, 122, 267–273. [Google Scholar] [CrossRef]
- Yu, Y.; Kanwar, S.S.; Patel, B.B.; Nautiyal, J.; Sarkar, F.H.; Majumdar, A.P.N. Elimination of Colon Cancer Stem-like Cells by the Combination of Curcumin and FOLFOX. Transl. Oncol. 2009, 2, 321–328. [Google Scholar] [CrossRef]
- Patel, B.B.; Gupta, D.; Elliott, A.A.; Sengupta, V.; Yu, Y.; Majumdar, A.P.N. Curcumin Targets FOLFOX-Surviving Colon Cancer Cells via Inhibition of EGFRs and IGF-1R. Anticancer Res. 2010, 30, 319–325. [Google Scholar]
- Shakibaei, M.; Mobasheri, A.; Lueders, C.; Busch, F.; Shayan, P.; Goel, A. Curcumin Enhances the Effect of Chemotherapy against Colorectal Cancer Cells by Inhibition of NF-ΚB and Src Protein Kinase Signaling Pathways. PLoS ONE 2013, 8, e57218. [Google Scholar] [CrossRef]
- Lu, W.D.; Qin, Y.; Yang, C.; Li, L.; Fu, Z.X. Effect of Curcumin on Human Colon Cancer Multidrug Resistance in Vitro and in Vivo. Clinics 2013, 68, 694–701. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin Chemosensitizes 5-Fluorouracil Resistant MMR-Deficient Human Colon Cancer Cells in High Density Cultures. PLoS ONE 2014, 9, e85397. [Google Scholar] [CrossRef]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Richard Boland, C.; Goel, A. Curcumin Mediates Chemosensitization to 5-Fluorouracil through MiRNA-Induced Suppression of Epithelialto-Mesenchymal Transition in Chemoresistant Colorectal Cancer. Carcinogenesis 2014, 36, 355–367. [Google Scholar] [CrossRef]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin Suppresses Crosstalk between Colon Cancer Stem Cells and Stromal Fibroblasts in the Tumor Microenvironment: Potential Role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin Potentiates Antitumor Activity of 5-Fluorouracil in a 3D Alginate Tumor Microenvironment of Colorectal Cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, L.-J.; Ye, H.-Z.; Liu, D.-F.; Zhu, Y.-B.; Miao, D.-D.; Zhang, S.-P.; Chen, Y.-Y.; Jua, Y.-W.; Shen, J.; et al. Nrf2 Is a Key Factor in the Reversal Effect of Curcumin on Multidrug Resistance in the HCT-8/5-Fu Human Colorectal Cancer Cell Line. Mol. Med. Rep. 2018, 18, 5409–5416. [Google Scholar] [CrossRef] [PubMed]
- He, W.-T.; Zhu, Y.-H.; Zhang, T.; Abulimiti, P.; Zeng, F.-Y.; Zhang, L.-P.; Luo, L.-J.; Xie, X.-M.; Zhang, H.-L. Curcumin Reverses 5-Fluorouracil Resistance by Promoting Human Colon Cancer HCT-8/5-FU Cell Apoptosis and Down-Regulating Heat Shock Protein 27 and P-Glycoprotein. Chin. J. Integr. Med. 2019, 25, 416–424. [Google Scholar] [CrossRef]
- Li, G.; Fang, S.; Shao, X.; Li, Y.; Tong, Q.; Kong, B.; Chen, L.; Wang, Y.; Yang, J.; Yu, H.; et al. Curcumin Reverses Nnmt-Induced 5-Fluorouracil Resistance via Increasing Ros and Cell Cycle Arrest in Colorectal Cancer Cells. Biomolecules 2021, 11, 1295. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low Curcumin Concentration Enhances the Anticancer Effect of 5-Fluorouracil against Colorectal Cancer. Phytomedicine 2021, 85, 153547. [Google Scholar] [CrossRef]
- Sadeghi-Abandansari, H.; Pakian, S.; Nabid, M.R.; Ebrahimi, M.; Rezalotfi, A. Local Co-Delivery of 5-Fluorouracil and Curcumin Using Schiff’s Base Cross-Linked Injectable Hydrogels for Colorectal Cancer Combination Therapy. Eur. Polym. J. 2021, 157, 110646. [Google Scholar] [CrossRef]
- Bardania, H.; Jafari, F.; Baneshi, M.; Mahmoudi, R.; Ardakani, M.T.; Safari, F.; Barmak, M.J. Folic Acid-Functionalized Albumin/Graphene Oxide Nanocomposite to Simultaneously Deliver Curcumin and 5-Fluorouracil into Human Colorectal Cancer Cells: An in Vitro Study. BioMed Res. Int. 2023, 2023, 8334102. [Google Scholar] [CrossRef]
- Moreno-Quintero, G.; Betancur-Zapata, E.; Herrera-Ramírez, A.; Cardona-Galeano, W. New Hybrid Scaffolds Based on 5-FU/Curcumin: Synthesis, Cytotoxic, Antiproliferative and Pro-Apoptotic Effect. Pharmaceutics 2023, 15, 1221. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Kuzhuvelil, H.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin Sensitizes Human Colorectal Cancer to Capecitabine by Modulation of Cyclin D1, COX-2, MMP-9, VEGF and CXCR4 Expression in an Orthotopic Mouse Model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R.; Sajini, D.V.; Ashraf, G.M.; Rahman, M.H. 5-Fluorouracil and Curcumin with Pectin Coating as a Treatment Regimen for Titanium Dioxide with Dimethylhydrazine-Induced Colon Cancer Model. Environ. Sci. Pollut. Res. 2022, 29, 63202–63215. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Sakamoto, T.; Li, Z.; Yasui, H.; Hamada, H.; Kubo, H.; Nakajima, M. Curcumin Inhibits Epithelial-Mesenchymal Transition in Oral Cancer Cells via c-Met Blockade. Oncol. Lett. 2020, 19, 4177–4182. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health Benefits of Resveratrol Administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and Cancer: Focus on in Vivo Evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Honari, M.; Shafabakhsh, R.; Reiter, R.J.; Mirzaei, H.; Asemi, Z. Resveratrol Is a Promising Agent for Colorectal Cancer Prevention and Treatment: Focus on Molecular Mechanisms. Cancer Cell Int. 2019, 19, 180. [Google Scholar] [CrossRef]
- Zhou, M.; Niu, H.; Cui, D.; Huang, G.; Li, J.; Tian, H.; Xu, X.; Liang, F.; Chen, R. Resveratrol Triggers Autophagy-related Apoptosis to Inhibit the Progression of Colorectal Cancer via Inhibition of FOXQ1. Phytother. Res. 2024, 38, 3218–3239. [Google Scholar] [CrossRef]
- Chang, C.H.; Lee, C.Y.; Lu, C.C.; Tsai, F.J.; Hsu, Y.M.; Tsao, J.W.; Juan, Y.N.; Chiu, H.Y.; Yang, J.S.; Wang, C.C. Resveratrol-Induced Autophagy and Apoptosis in Cisplatin-Resistant Human Oral Cancer CAR Cells: A Key Role of AMPK and Akt/MTOR Signaling. Int. J. Oncol. 2017, 50, 873–882. [Google Scholar] [CrossRef]
- Wu, J.; Hu, D.; Zhang, R. Depletion of Bmi-1 Enhances 5-Fluorouracil-Induced Apoptosisand Autophagy in Hepatocellular Carcinoma Cells. Oncol. Lett. 2012, 4, 723–726. [Google Scholar] [CrossRef]
- He, X.X.; Huang, C.K.; Xie, B.S. Autophagy Inhibition Enhanced 5-FU-Induced Cell Death in Human Gastric Carcinoma BGC-823 Cells. Mol. Med. Rep. 2018, 17, 6768–6776. [Google Scholar] [CrossRef]
- Chan, J.Y.; Meng, S.P.; Clement, M.V.; Pervaiz, S.; Shao, C.L. Resveratrol Displays Converse Dose-Related Effects on 5-Fluorouracilevoked Colon Cancer Cell Apoptosis: The Roles of Caspase-6 and P53. Cancer Biol. Ther. 2008, 7, 1305–1312. [Google Scholar] [CrossRef]
- Lee, S.C.; Chan, J.Y.; Pervaiz, S. Spontaneous and 5-Fluorouracil-Induced Centrosome Amplification Lowers the Threshold to Resveratrol-Evoked Apoptosis in Colon Cancer Cells. Cancer Lett. 2010, 288, 36–41. [Google Scholar] [CrossRef]
- Baba, Y.; Nosho, K.; Shima, K.; Irahara, N.; Chan, A.T.; Meyerhardt, J.A.; Chung, D.C.; Giovannucci, E.L.; Fuchs, C.S.; Ogino, S. HIF1A Overexpression Is Associated with Poor Prognosis in a Cohort of 731 Colorectal Cancers. Am. J. Pathol. 2010, 176, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latif, Y.; El-Bana, M.; Hussein, J.; El-Khayat, Z.; Farrag, A.R. Effects of Resveratrol in Combination with 5-Fluorouracil on N-Methylnitrosourea-Induced Colon Cancer in Rats. Comp. Clin. Pathol. 2019, 28, 1351–1362. [Google Scholar] [CrossRef]
- Mohapatra, P.; Preet, R.; Choudhuri, M.; Choudhuri, T.; Kundu, C.N. 5-Fluorouracil Increases the Chemopreventive Potentials of Resveratrol through DNA Damage and MAPK Signaling Pathway in Human Colorectal Cancer Cells. Oncol. Res. 2011, 19, 311–321. [Google Scholar] [CrossRef]
- Santandreu, F.M.; Valle, A.; Oliver, J.; Roca, P. Resveratrol Potentiates the Cytotoxic Oxidative Stress Induced by Chemotherapy in Human Colon Cancer Cells. Cell. Physiol. Biochem. 2011, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Kraehe, P.; Popper, B.; Goel, A.; Shakibaei, M. Resveratrol Induces Chemosensitization to 5-Fluorouracil through up-Regulation of Intercellular Junctions, Epithelial-to-Mesenchymal Transition and Apoptosis in Colorectal Cancer. Biochem. Pharmacol. 2015, 98, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Blanquer-Rosselló, M. del M.; Hernández-López, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol Induces Mitochondrial Respiration and Apoptosis in SW620 Colon Cancer Cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 431–440. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells. Nutrients 2018, 10, 888. [Google Scholar] [CrossRef]
- Chung, S.S.; Dutta, P.; Austin, D.; Wang, P.; Awad, A.; Vadgama, J.V. Combination of Resveratrol and 5-Flurouracil Enhanced Antitelomerase Activity and Apoptosis by Inhibiting STAT3 and Akt Signaling Pathways in Human Colorectal Cancer Cells. Oncotarget 2018, 9, 32943–32957. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, S.; Zhou, J.; Li, X. Effect of Resveratrol on Drug Resistance in Colon Cancer Chemotherapy. RSC Adv. 2019, 9, 2572–2580. [Google Scholar] [CrossRef]
- Brockmueller, A.; Girisa, S.; Kunnumakkara, A.B.; Shakibaei, M. Resveratrol Modulates Chemosensitisation to 5-FU via Β1-Integrin/HIF-1α Axis in CRC Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4988. [Google Scholar] [CrossRef]
- Soliman, B.A.; Farrag, A.R.H.; Khaled, H.A.S. Combinational Effect of 5-Flourouracil and Resveratrol against N-Nitroso-N-Methyl Urea Induced Colorectal Cancer. Egypt. J. Hosp. Med. 2018, 70, 994–1006. [Google Scholar] [CrossRef]
- Shi, C.; Li, H.; Yang, Y.; Hou, L. Anti-Inflammatory and Immunoregulatory Functions of Artemisinin and Its Derivatives. Mediat. Inflamm. 2015, 2015, 435713. [Google Scholar] [CrossRef] [PubMed]
- Pras, N.; Visser, J.F.; Batterman, S.; Woerdenbag, H.J.; Malingré, T.M.; Lugt, C.B. Laboratory Selection of Artemisia annua L. for High Artemisinin Yielding Types. Phytochem. Anal. 1991, 2, 80–83. [Google Scholar] [CrossRef]
- Xie, K.; Li, Z.; Zhang, Y.; Wu, H.; Zhang, T.; Wang, W. Artemisinin and Its Derivatives as Promising Therapies for Autoimmune Diseases. Heliyon 2024, 10, e27972. [Google Scholar] [CrossRef]
- Khakzad, M.R.; Ganji, A.; Ariabod, V.; Farahani, I. Artemisinin Therapeutic Efficacy in the Experimental Model of Multiple Sclerosis. Immunopharmacol. Immunotoxicol. 2017, 39, 348–353. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Ren, W.; Liu, Z.L.; Cheng, Y.F.; Zhang, X.M. Combination Treatment with Artemisinin and Oxaliplatin Inhibits Tumorigenesis in Esophageal Cancer EC109 Cell through Wnt/β-Catenin Signaling Pathway. Thorac. Cancer 2020, 11, 2316–2324. [Google Scholar] [CrossRef]
- Jung, E.J.; Paramanantham, A.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.M.; Ryu, C.H.; Hong, S.C.; Chung, K.H.; Kim, C.W.; et al. Artemisia annua L. Polyphenol-Induced Cell Death Is Ros-Independently Enhanced by Inhibition of Jnk in Hct116 Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1366. [Google Scholar] [CrossRef]
- Ma, Z.; Woon, C.Y.N.; Liu, C.G.; Cheng, J.T.; You, M.; Sethi, G.; Wong, A.L.A.; Ho, P.C.L.; Zhang, D.; Ong, P.; et al. Repurposing Artemisinin and Its Derivatives as Anticancer Drugs: A Chance or Challenge? Front. Pharmacol. 2021, 12, 828856. [Google Scholar] [CrossRef]
- Rastogi, T.; Devesa, S.; Mangtani, P.; Mathew, A.; Cooper, N.; Kao, R.; Sinha, R. Cancer Incidence Rates among South Asians in Four Geographic Regions: India, Singapore, UK and US. Int. J. Epidemiol. 2008, 37, 147–160. [Google Scholar] [CrossRef]
- Callejas, B.E.; Martínez-Saucedo, D.; Terrazas, L.I. Parasites as Negative Regulators of Cancer. Biosci. Rep. 2018, 38, BSR20180935. [Google Scholar] [CrossRef]
- Rostamirad, S.; Daneshpour, S.; Mofid, M.R.; Andalib, A.; Eskandariyan, A.; Mousavi, S.; Yousofi Darani, H. Inhibition of Mouse Colon Cancer Growth Following Immunotherapy with a Fraction of Hydatid Cyst Fluid. Exp. Parasitol. 2023, 249, 108501. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.A.; Prince, S.; Smith, K.A. Gastrointestinal Nematode-Derived Antigens Alter Colorectal Cancer Cell Proliferation and Migration through Regulation of Cell Cycle and Epithelial-Mesenchymal Transition Proteins. Int. J. Mol. Sci. 2020, 21, 7845. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Rodríguez, M.G.; Medina-Reyes, D.; Sánchez-Barrera, C.A.; Fernández-Muñoz, K.V.; García-Castillo, V.; Ledesma-Torres, J.L.; González-González, M.I.; Reyes, J.L.; Pérez-Plascencia, C.; Rodríguez-Sosa, M.; et al. Helminth-Derived Molecules Improve 5-Fluorouracil Treatment on Experimental Colon Tumorigenesis. Biomed. Pharmacother. 2024, 175, 116628. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Díaz, M.; Terrazas, L.I. Taenia Crassiceps Infection and Its Excreted/Secreted Products Inhibit STAT1 Activation in Response to IFN-γ. Int. J. Parasitol. 2014, 44, 613–623. [Google Scholar] [CrossRef]
- Dobrijević, D.; Pastor, K.; Nastić, N.; Özogul, F.; Krulj, J.; Kokić, B.; Bartkiene, E.; Rocha, J.M.; Kojić, J. Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules 2023, 28, 4824. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Youn, J.; Cho, E.; Lee, J.E. Association of Choline and Betaine Levels with Cancer Incidence and Survival: A Meta-Analysis. Clin. Nutr. 2019, 38, 100–109. [Google Scholar] [CrossRef]
- Kim, D.H.; Sung, B.; Kang, Y.J.; Jang, J.Y.; Hwang, S.Y.; Lee, Y.; Kim, M.; Im, E.; Yoon, J.-H.; Kim, C.M.; et al. Anti-Inflammatory Effects of Betaine on AOM/DSS-induced Colon Tumorigenesis in ICR Male Mice. Int. J. Oncol. 2014, 45, 1250–1256. [Google Scholar] [CrossRef]
- Bingula, R.; Dupuis, C.; Pichon, C.; Berthon, J.Y.; Filaire, M.; Pigeon, L.; Filaire, E. Study of the Effects of Betaine and/or C-Phycocyanin on the Growth of Lung Cancer A549 Cells In Vitro and In Vivo. J. Oncol. 2016, 2016, 8162952. [Google Scholar] [CrossRef]
- Kar, F.; Hacioglu, C.; Kacar, S.; Sahinturk, V.; Kanbak, G. Betaine Suppresses Cell Proliferation by Increasing Oxidative Stress–Mediated Apoptosis and Inflammation in DU-145 Human Prostate Cancer Cell Line. Cell Stress Chaperones 2019, 24, 871–881. [Google Scholar] [CrossRef]
- Hagar, H.; Husain, S.; Fadda, L.M.; Attia, N.M.; Attia, M.M.A.; Ali, H.M. Inhibition of NF-ΚB and the Oxidative Stress -Dependent Caspase-3 Apoptotic Pathway by Betaine Supplementation Attenuates Hepatic Injury Mediated by Cisplatin in Rats. Pharmacol. Rep. 2019, 71, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Al Za’abi, M.; Ali, H.; Al Sabahi, M.; Ali, B.H. The Salutary Action of Melatonin and Betaine, given Singly or Concomitantly, on Cisplatin-Induced Nephrotoxicity in Mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).