Inhibitory Effect of Lactiplantibacillus plantarun HFY11 on Compound Diphenoxylate-Induced Constipation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Microorganisms
2.2. Experimental Microorganisms
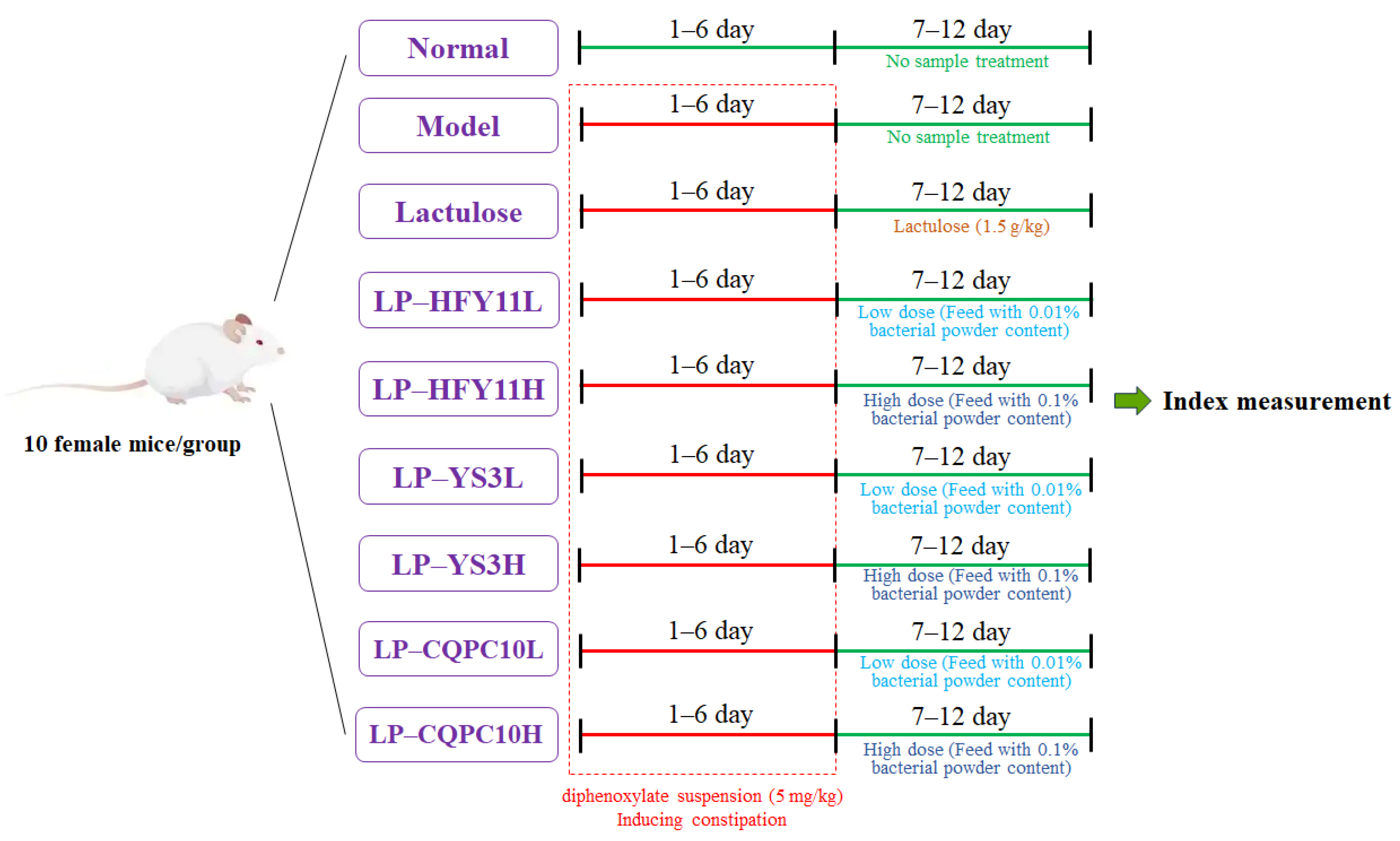
2.3. Animal Experiment
2.4. Serum Biomarker Analysis
2.5. Pathological Observation
2.6. Quantitative Polymerase Chain Reaction Analysis of Small Intestine Tissue Gene Expression
2.7. Determination of Microbial mRNA Expression in Mouse Intestinal Contents
2.8. Statistical Analysis
3. Results
3.1. Bowel Movements in Mice
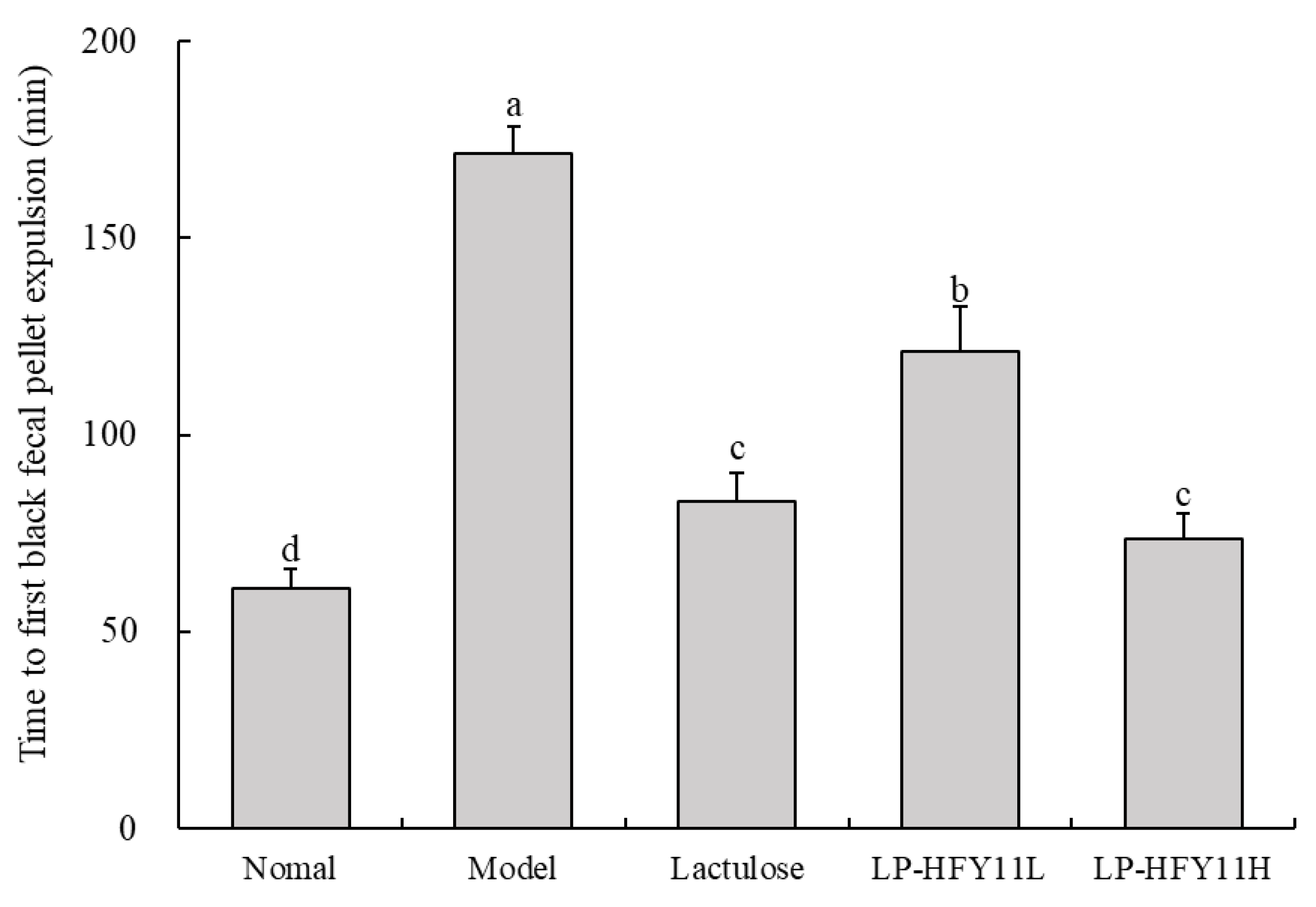
3.2. Time to First Black Fecal Pellet Expulsion in Mice
3.3. Activated Charcoal Propulsion Rate
3.4. Pathological Observation of the Mouse Colon
3.5. Mouse Serum Indicators
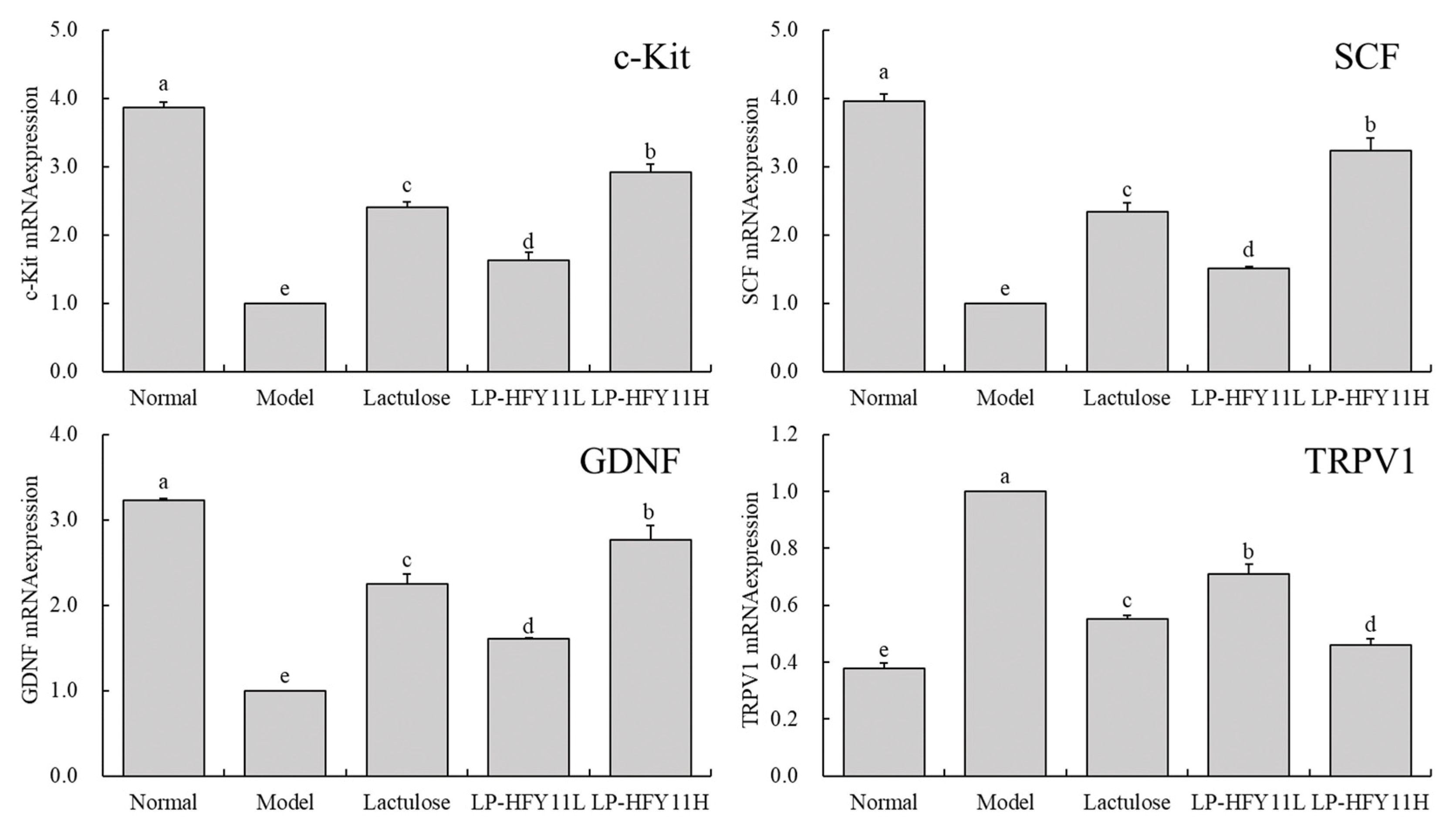
3.6. mRNA Expression of Related Genes in the Mouse Small Intestine
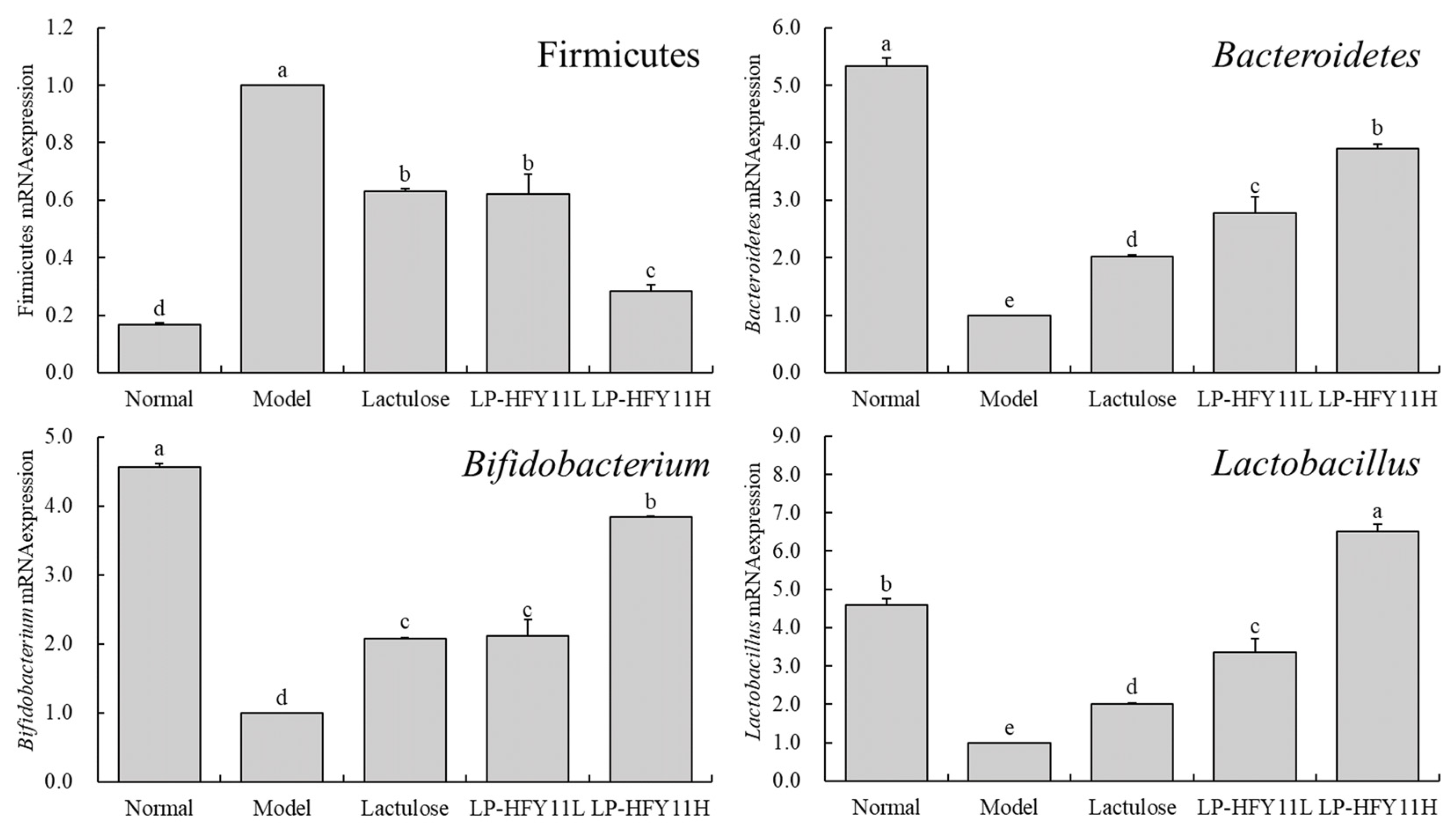
3.7. mRNA Expression in Mouse Intestinal Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Wu, M.; Zhao, W.; Kwok, L.Y.; Zhang, W. Effects of probiotics and its fermented milk on constipation: A systematic review. Food Sci. Hum. Well. 2023, 12, 2124–2134. [Google Scholar] [CrossRef]
- Yang, B.; Qin, Q.Z.; Han, L.L.; Lin, J.; Chen, Y. Spa therapy (balneotherapy) relieves mental stress, sleep disorder, and general health problems in sub-healthy people. Int. J. Biometeorol. 2018, 62, 261–272. [Google Scholar] [CrossRef]
- Tashiro, N.; Budhathoki, S.; Ohnaka, K.; Toyomura, K.; Kono, S.; Ueki, T.; Tanaka, M.; Kakeji, Y.; Maehara, Y.; Okamura, T.; et al. Constipation and colorectal cancer risk: The Fukuoka colorectal cancer study. Asian Pac. J. Cancer Prev. 2011, 12, 2025–2030. [Google Scholar]
- Okawa, Y.; Fukudo, S.; Sanada, H. Specific foods can reduce symptoms of irritable bowel syndrome and functional constipation: A review. Biopsychosoc. Med. 2019, 13, 10. [Google Scholar] [CrossRef]
- Araújo, M.M.; Botelho, P.B. Probiotics, prebiotics, and synbiotics in chronic constipation: Outstanding aspects to be considered for the current evidence. Front. Nutr. 2022, 9, 935830. [Google Scholar] [CrossRef]
- Hayakawa, M.; Asahara, T.; Henzan, N.; Murakami, H.; Yamamoto, H.; Mukai, N.; Minami, Y.; Sugano, M.; Kubota, N.; Uegaki, S.; et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig. Dis. Sci. 2011, 56, 2361–2365. [Google Scholar] [CrossRef]
- Higashi, B.; Mariano, T.B.; de Abreu Filho, B.A.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Effects of fructans and probiotics on the inhibition of Klebsiella oxytoca and the production of short-chain fatty acids assessed by NMR spectroscopy. Carbohydr. Polym. 2020, 248, 116832. [Google Scholar] [CrossRef]
- Gurevich, K.; Nikityuk, D.; Nikonov, E.; Zaborova, V.; Veselova, L.; Zolnikova, O. The role of probiotics and microbiota in digestion, nutrient and hormone metabolism, and hormonal background maintenance. Profil. Meditsina 2018, 21, 45. [Google Scholar] [CrossRef]
- Banaszkiewicz, A.; Szajewska, H. Ineffectiveness of Lactobacillus GG as an adjunct to lactulose for the treatment of constipation in children: A double-blind, placebo-controlled randomized trial. J. Pediatr. 2005, 146, 364–369. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Yan, Q.; Li, Y.; Jiang, Z. Effect of Konjac mannan oligosaccharides on diphenoxylate-induced constipation in mice. J. Funct. Foods 2019, 57, 399–407. [Google Scholar] [CrossRef]
- Tan, F.; Ren, L.; Kong, C.S. Therapeutic effect of Lactiplantibacillus plantarun HFY11 isolated from naturally fermented yak yogurt on lincomycin hydrochloride–induced diarrhea in mice. Microorganisms 2024, 12, 2307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yi, R.; Qian, Y.; Park, K.Y. Lactobacillus plantarum YS-3 prevents activated carbon-induced constipation in mice. J. Med. Food 2018, 21, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Chen, B.; Long, X.; Mu, J.; Pan, Y.; Song, J.-L.; Zhao, X.; Yang, Z. Preventive effect of Lactobacillus plantarum CQPC10 on activated carbon induced constipation in institute of cancer research (ICR) mice. Appl. Sci. 2018, 8, 1498. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Suo, H.; Wang, W.; Wang, H.; Zhang, Y.; Hu, Q.; Zhao, X.; Li, J. Preventive effects of different fermentation times of Shuidouchi on diphenoxylate-induced constipation in mice. Foods 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chen, R.; Qian, Y.; Ye, K.; Long, X.; Park, K.Y.; Zhao, X. Antioxidant effect of Lactobacillus fermentum HFY02-fermented soy milk on D-galactose-induced aging mouse model. Food Sci. Hum. Well. 2022, 11, 1362–1372. [Google Scholar] [CrossRef]
- Long, X.; Pan, Y.; Zhao, X. Prophylactic effect of Kudingcha polyphenols on oxazolone induced colitis through its antioxidant capacities. Food Sci. Hum. Well. 2018, 7, 209–214. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Kan, J. Preventive effect of resistant starch on activated carbon-induced constipation in mice. Exp. Ther. Med. 2013, 6, 228–232. [Google Scholar] [CrossRef]
- Yi, R.; Qian, Y.; Wang, Q.; Mu, J.; Zhao, X. Preventive effect of Lactobacillus plantarum YS-1 on activated carbon induced constipation in mice. Food Sci. 2017, 38, 238–243. [Google Scholar]
- Suo, H.; Zhao, X.; Qian, Y.; Li, G.; Liu, Z.; Xie, J.; Li, J. Therapeutic effect of activated carbon-induced constipation mice with Lactobacillus fermentum Suo on treatment. Int. J. Mol. Sci. 2014, 15, 21875–21895. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef]
- Yi, X.; Zhou, K.; Deng, N.; Cai, Y.; Peng, X.; Tan, Z. Simo decoction curing spleen deficiency constipation was associated with brain-bacteria-gut axis by intestinal mucosal microbiota. Front. Microbiol. 2023, 14, 1090302. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ozawa, N.; Shinozaki, T.; Wakabayashi, K.I.; Suzuki, K.; Kawano, Y.; Ohtsu, I.; Tatebayashi, Y. Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl. Psychiatry 2020, 10, 170. [Google Scholar] [CrossRef]
- Lata, J.; Novotný, I.; Príbramská, V.; Juránková, J.; Fric, P.; Kroupa, R.; Stibůrek, O. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: Results of a double-blind randomized study. Eur. J. Gastroenterol. Hepatol. 2007, 19, 1111–1113. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, B.; Liu, B.; Zhou, X.; Mu, J.; Wang, Q.; Zhao, X.; Yang, Z. Preventive effect of Lactobacillus fermentum CQPC03 on activated carbon-induced constipation in ICR mice. Medicina 2018, 54, 89. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, L.; Guo, X.; Li, G.; Guo, X. Short and long-term efficacy of combining Fuzhengliqi mixture with acupuncture in treatment of functional constipation. J. Tradit. Chin. Med. 2013, 33, 51–59. [Google Scholar] [CrossRef]
- Wang, X.; Guo, R.; Yu, Z.; Zikela, L.; Li, J.; Li, S.; Han, Q. Torreya grandis Kernel oil alleviates loperamide-induced slow transit constipation via up-regulating the colonic expressions of occludin/claudin-1/ZO-1 and 5-HT3R/5-HT4R in BALB/c mice. Mol. Nutr. Food Res. 2024, 68, 2300615. [Google Scholar] [CrossRef]
- King, S.K.; Sutcliffe, J.R.; Ong, S.Y.; Lee, M.; Koh, T.L.; Wong, S.Q.; Farmer, P.J.; Peck, C.J.; Stanton, M.P.; Keck, J.; et al. Substance P and vasoactive intestinal peptide are reduced in right transverse colon in pediatric slow-transit constipation. Neurogastroenterol. Motil. 2010, 22, 883–892. [Google Scholar] [CrossRef]
- Bagdzevicius, R.; Vaicekauskas, V.; Bagdzeviciūte, S. Experience of acetylcholinesterase histochemistry application in the diagnosis of chronic constipation in children. Medicina 2007, 43, 376–384. [Google Scholar] [CrossRef]
- Yang, M.; Hua, Y.; Wu, H.; Gao, H.; Chen, W. Clinical efficacy of danning tablets in the treatment of slow-transit constipation in elderly patients. Clin. Med. J. 2023, 21, 69–74. [Google Scholar]
- Qian, Y.; Suo, H.; Du, M.; Zhao, X.; Li, J.; Li, G.J.; Song, J.L.; Liu, Z. Preventive effect of Lactobacillus fermentum Lee onactivated carbon-induced constipation in mice. Exper. Ther. Med. 2015, 9, 272–278. [Google Scholar] [CrossRef]
- Maeda, H.; Zhu, X.; Mitsuoka, T. Effects of an exopolysaccharide (Kefiran) from Lactobacillus kefiranofaciens on blood glucose in KKAy mice and constipation in SD rats induced by a low-fiber diet. Biosci. Microflora 2004, 23, 149–153. [Google Scholar] [CrossRef]
- Rich, A.; Miller, S.M.; Gibbons, S.J.; Malysz, J.; Szurszewski, J.H.; Farrugia, G. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, 313–320. [Google Scholar] [CrossRef]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef]
- Yi, R.; Peng, P.; Zhang, J.; Du, M.; Lan, L.; Qian, Y.; Zhou, J.; Zhao, X. Lactobacillus plantarum CQPC02-fermented soybean milk improves loperamide-induced constipation in mice. J. Med. Food 2019, 22, 1208–1221. [Google Scholar] [CrossRef]
- Qin, X.; Guo, Y.; Xue, K.; Qiu, Y.; An, P.; Du, Y.; Li, X.; Liu, T.; Tang, C. GDNF’s Role in Mitigating Intestinal Reactive Gliosis and Inflammation to Improve Constipation and Depressive Behavior in Rats with Parkinson’s disease. J. Mol. Neurosci. 2024, 74, 78. [Google Scholar]
- Tong, W.D.; Liu, B.H.; Zhang, L.Y.; Zhang, S.B. Study on distribution of interstitial cells of Cajal in the sigmoid colon of patients with slow transit constipation. Chin. J. Surg. 2004, 42, 853–856. [Google Scholar]
- Tong, W.; Jia, H.; Zhang, L.; Li, C.; Ridolfi, T.J.; Liu, B. Exogenous stem cell factor improves interstitial cells of Cajal restoration after blockade of c-kit signaling pathway. Scand. J. Gastroenterol. 2010, 45, 844–851. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef]
- Zhou, B.; Jin, G.; Pang, X.; Mo, Q.; Bao, J.; Liu, T.; Wu, J.; Xie, R.; Liu, X.; Liu, J.; et al. Lactobacillus rhamnosus GG colonization in early life regulates gut-brain axis and relieves anxiety-like behavior in adulthood. Pharmacol. Res. 2022, 177, 106090. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Oscar, B.T. Therapeutic potential of the use of lactobacilli in gastroenterology and human nutrition. Rev. Chil. Nutr. 2013, 40, 290–302. [Google Scholar]
- Zhang, K.; Liu, H.; Liu, O.; Feng, Q.; Gan, L.; Yao, L.; Huang, G.; Fang, Z.; Chen, T.; Fang, N. ositive efficacy of Lactiplantibacillus plantarum MH-301 as a postoperative adjunct to endoscopic sclerotherapy for internal hemorrhoids: A randomized, double-blind, placebo-controlled trial. Food Funct. 2023, 14, 8521–8532. [Google Scholar] [CrossRef]
- Yong, C.C.; Khoo, B.Y.; Sasidharan, S.; Piyawattanametha, W.; Kim, S.H.; Khemthongcharoen, N.; Chuah, L.O.; Ang, M.Y.; Liong, M.T. Activity of crude and fractionated extracts by lactic acid bacteria (LAB) isolated from local dairy, meat, and fermented products against Staphylococcus aureus. Annl. Microbiol. 2015, 65, 1037–1047. [Google Scholar] [CrossRef]

| Gene | Primer Sequence |
|---|---|
| TRPV1 | F: 5′-CCGGCTTTTTGGGAAGGGT-3′ |
| R: 5′-GAGACAGGTAGGTCCATCCAC-3′ | |
| GDNF | F: 5′-TCAGCCATCACAGTGTTCCC-3′ |
| R: 5′-ATAGCCCGCATAGCGTATCAG-3′ | |
| c-Kit | F: 5′-AGACCGAACGCAACTT-3′ |
| R: 5′-GGTGCCATCCACTTCA-3′ | |
| SCF | F: 5′-AAACTGGTGGCGAATC-3′ |
| R: 5′-CACGGGTAGCAAGAAC-3′ | |
| R: 5′-GTGCTCCGGTTGTATAAGATGAC-3′ | |
| GAPDH | F: 5′-AATGGATTTGGACGCATTGGT-3′ |
| R: 5′-TTTGCACTGGTACGTGTTGAT-3′ | |
| Firmicutes | F: 5′-GCGTGAGTGAAGAAGT-3′ |
| R: 5′-CTACGCTCCCTTTACAC-3′ | |
| Bacteroidetes | F: 5′-ACGCTAGCTACAGGCTTAACA-3′ |
| R: 5′-ACGCTACTTGGCTGGTTCA-3′ | |
| Lactobacillus | F: 5′-CACCGCTACACATGGAG-3′ |
| R: 5′-AGCAGTAGGGAATCTTCCA-3′ | |
| Bifidobacterium | F: 5′-TCGCGTCYGGTGTGAAAG-3′ |
| R: 5′-CCACATCCAGCRTCCAC-3′ | |
| Total bacteria | F: 5′-ACTCCTACGGGAGGCAGCAGT-3′ |
| R: 5′-ATTACCGCGGCTGCTGGC-3′ |
| Group | Normal | Model | Lactulose | LP-HFY11L | LP-HFY11H | LP-YS3L | LP-YS3H | LP-CQPC10L | LP-CQPC10H |
|---|---|---|---|---|---|---|---|---|---|
| 1–6 day (diphenoxylate suspension treated, samples untreated) | |||||||||
| Fecal weight (g) | 0.90 ± 0.06 a | 0.48 ± 0.06 b | 0.50 ± 0.07 b | 0.47 ± 0.05 b | 0.46 ± 0.06 b | 0.46 ± 0.03 b | 0.47 ± 0.05 b | 0.48 ± 0.04 b | 0.47 ± 0.07 b |
| Fecal particle number | 36 ± 3 a | 21 ± 4 b | 23 ± 5 b | 21 ± 5 b | 23 ± 2 b | 22 ± 2 b | 23 ± 4 b | 23 ± 3 b | 22 ± 3 b |
| Fecal water content (%) | 49.7 ± 3.5 a | 14.7 ± 3.8 b | 14.9 ± 4.1 b | 14.6 ± 4.4 b | 14.9 ± 4.3 b | 14.6 ± 2.9 b | 14.8 ± 3.2 b | 14.7 ± 4.1 b | 14.8 ± 3.9 b |
| 7–12 day (samples treated) | |||||||||
| Fecal weight (g) | 0.95 ± 0.06 a | 0.46 ± 0.07 d | 0.75 ± 0.06 b | 0.71 ± 0.06 b | 0.86 ± 0.05 ab | 0.59 ± 0.05 c | 0.72 ± 0.05 b | 0.70 ± 0.04 b | 0.85 ± 0.07 ab |
| Fecal particle number | 39 ± 4 a | 20 ± 4 c | 30 ± 3 a | 29 ± 4 ab | 35 ± 4 a | 24 ± 3 bc | 28 ± 2 b | 28 ± 3 b | 33 ± 3 a |
| Fecal water content (%) | 50.1 ± 3.6 a | 14.4 ± 3.1 d | 34.6 ± 4.5 c | 32.7 ± 3.7 c | 42.6 ± 3.9 b | 27.6 ± 3.8 c | 33.1 ± 4.0 c | 31.9 ± 3.3 c | 40.5 ± 3.9 b |
| Group | Small Intestine Length (cm) | Propulsion Distance (cm) | Propulsion Rate (%) |
|---|---|---|---|
| Normal | 46.5 ± 3.7 a | 46.5 ± 3.7 a | 100.00 ± 0.00 a |
| Model | 44.9 ± 4.1 a | 10.8 ± 2.9 e | 24.99 ± 3.70 e |
| Lactulose | 45.6 ± 3.3 a | 28.7 ± 3.5 c | 64.31 ± 11.36 c |
| LP-HFY11L | 45.1 ± 3.8 a | 19.5 ± 3.1 d | 45.07 ± 8.47 d |
| LP-HFY11H | 45.9 ± 3.5 a | 35.1 ± 2.6 b | 75.83 ± 4.69 b |
| Group | MTL (pg/mL) | ET-1 (pg/mL) | VIP (pg/mL) | AchE (nmol/L) |
|---|---|---|---|---|
| Normal | 60.44 ± 4.32 a | 10.81 ± 1.57 e | 16.23 ± 1.44 e | 19.68 ± 1.67 a |
| Model | 23.25 ± 3.04 e | 28.12 ± 2.18 a | 40.15 ± 2.78 a | 2.42 ± 0.41 e |
| Lactulose | 46.56 ± 4.83 c | 17.72 ± 1.66 c | 27.21 ± 2.24 c | 12.04 ± 1.54 c |
| LP-HFY11L | 36.72 ± 3.81 d | 22.56 ± 1.79 b | 33.84 ± 2.22 b | 6.17 ± 1.36 d |
| LP-HFY11H | 53.18 ± 2.98 b | 15.25 ± 1.57 d | 23.63 ± 2.04 d | 15.59 ± 1.40 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, F.; Kong, C.-S. Inhibitory Effect of Lactiplantibacillus plantarun HFY11 on Compound Diphenoxylate-Induced Constipation in Mice. Biomolecules 2025, 15, 358. https://doi.org/10.3390/biom15030358
Tan F, Kong C-S. Inhibitory Effect of Lactiplantibacillus plantarun HFY11 on Compound Diphenoxylate-Induced Constipation in Mice. Biomolecules. 2025; 15(3):358. https://doi.org/10.3390/biom15030358
Chicago/Turabian StyleTan, Fang, and Chang-Suk Kong. 2025. "Inhibitory Effect of Lactiplantibacillus plantarun HFY11 on Compound Diphenoxylate-Induced Constipation in Mice" Biomolecules 15, no. 3: 358. https://doi.org/10.3390/biom15030358
APA StyleTan, F., & Kong, C.-S. (2025). Inhibitory Effect of Lactiplantibacillus plantarun HFY11 on Compound Diphenoxylate-Induced Constipation in Mice. Biomolecules, 15(3), 358. https://doi.org/10.3390/biom15030358





