IgG Biomarkers in Multiple Sclerosis: Deciphering Their Puzzling Protein A Connection
Abstract
1. Introduction
2. A Brief Introduction to B Cells and Immunoglobulin Isotypes
3. The Success of B Cell Depletion Therapies Supports the Biomarker Potential of IgG Antibodies in MS
3.1. B Cell Depletion Therapies Target CD20 B Cells and May Reduce IgG Levels in the Blood
3.2. The Success of B Cell Therapies Demands Better Biomarkers for Early Treatments and Personalized Medicine
4. Antibody Gene Expressions in MS
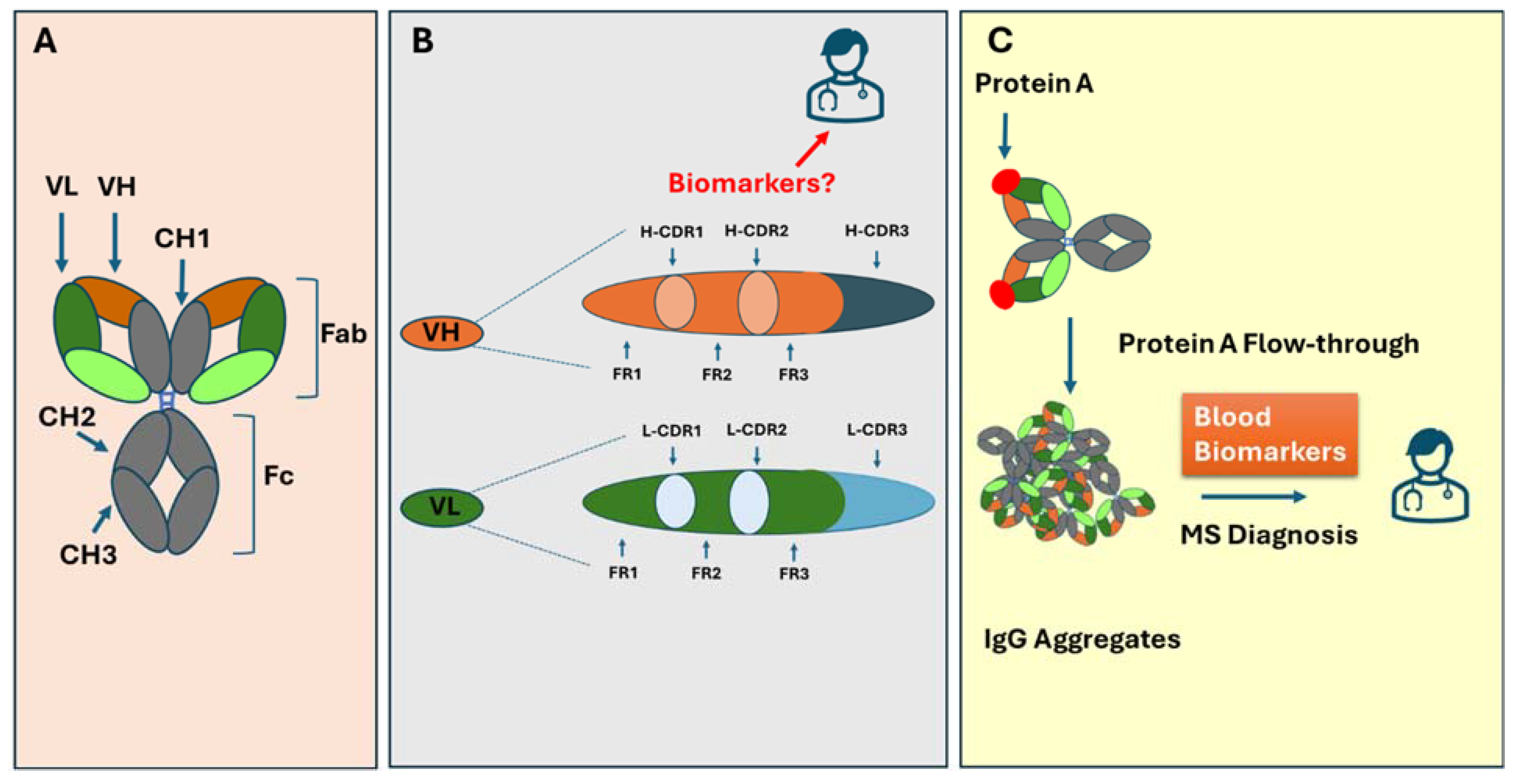
4.1. Antibody Structure Overview
4.2. Antibody Classes and Subclasses and Their Relationship to Oligoclonal Bands in MS
4.3. The Biomarker Potential of Antibody Heavy and Light Chain Gene Expression in MS
5. The Unique MS-Specific Motifs in the Antigen-Binding Variable Domain of Antibodies
5.1. The Heavy Chain CDR3 Plays a Crucial Role in Determining Antigen-Binding Characteristics
5.2. CDR-H3 as Biomarkers for MS and Other Autoimmune Disorders
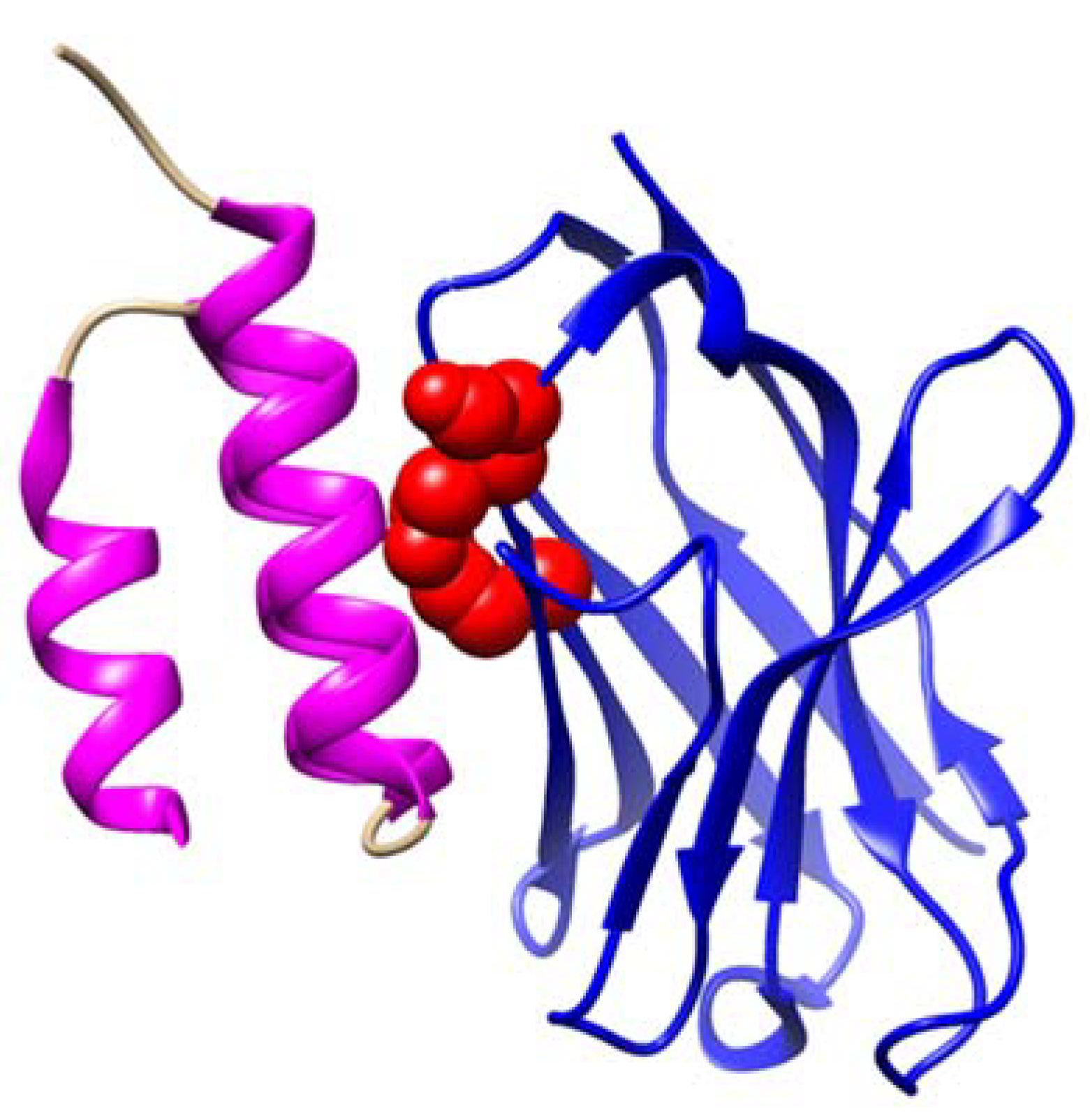
5.3. MS-Specific IGHV3 Gene Mutation in Framework 3: An Indication of the Role of Superantigen Protein A
6. Protein A Binding to IgG and Its Critical Role in Multiple Sclerosis
6.1. The Unique Feature of Protein A Binding to IgG in MS
6.2. The Enriched IgG Aggregates in Protein A Flow-Through Cause Neuron Cytotoxicity and Can Be Used as Biomarkers for MS
7. Speculative Mechanisms of IgG Aggregate Formation in MS and Neuronal Apoptosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuhlmann, T.; Antel, J. Multiple sclerosis: 2023 update. Free Neuropathol. 2023, 4, 3–4. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The Cerebrospinal Fluid in Multiple Sclerosis. Front. Immunol. 2019, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Graner, M.; Beseler, C.; Domashevich, T.; Selva, S.; Webster, G.; Ledreux, A.; Zizzo, Z.; Lundt, M.; Alvarez, E.; et al. Plasma IgG aggregates as biomarkers for multiple sclerosis. Clin. Immunol. 2023, 256, 109801. [Google Scholar] [CrossRef]
- Yam-Puc, J.C.; Zhang, L.; Zhang, Y.; Toellner, K.M. Role of B-cell receptors for B-cell development and antigen-induced differentiation. F1000Research 2018, 7, 429. [Google Scholar] [CrossRef]
- LeBien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Sabatino, J.J., Jr.; Zamvil, S.S.; Hauser, S.L. B-Cell Therapies in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a032037. [Google Scholar] [CrossRef]
- Alvarez, E.; Longbrake, E.E.; Rammohan, K.W.; Stankiewicz, J.; Hersh, C.M. Secondary hypogammaglobulinemia in patients with multiple sclerosis on anti-CD20 therapy: Pathogenesis, risk of infection, and disease management. Mult. Scler. Relat. Disord. 2023, 79, 105009. [Google Scholar] [CrossRef]
- Elgenidy, A.; Abdelhalim, N.N.; Al-Kurdi, M.A.; Mohamed, L.A.; Ghoneim, M.M.; Fathy, A.W.; Hassaan, H.K.; Anan, A.; Alomari, O. Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with anti-CD20 treatments: A systematic review and meta-analysis of 19,139 multiple sclerosis patients. Front. Neurol. 2024, 15, 1380654. [Google Scholar] [CrossRef]
- Oommen, L.; Krieger, S. New Approaches to Challenge Old Assumptions—B-Cell Depletion in Multiple Sclerosis. JAMA Neurol. 2023, 80, 775–777. [Google Scholar] [CrossRef]
- Cantó, E.; Barro, C.; Zhao, C.; Caillier, S.J.; Michalak, Z.; Bove, R.; Tomic, D.; Santaniello, A.; Häring, D.A.; Hollenbach, J.; et al. Association Between Serum Neurofilament Light Chain Levels and Long-term Disease Course Among Patients With Multiple Sclerosis Followed up for 12 Years. JAMA Neurol. 2019, 76, 1359–1366. [Google Scholar] [CrossRef]
- Janeway, C. Immunobiology: The Immune System in Health and Disease, 6th ed.; Garland Science: New York, NY, USA, 2005; p. xxiii. 823p. [Google Scholar]
- Losy, J.; Mehta, P.D.; Wisniewski, H.M. Identification of IgG subclasses’ oligoclonal bands in multiple sclerosis CSF. Acta Neurol. Scand. 1990, 82, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; Fultz, M.; Phares, J.; Yu, X. Immunoglobulin G and Complement as Major Players in the Neurodegeneration of Multiple Sclerosis. Biomolecules 2024, 14, 1210. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; George, W.; Yu, X. The Possible Role of Neural Cell Apoptosis in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 7584. [Google Scholar] [CrossRef]
- Yu, X.; Burgoon, M.; Green, M.; Barmina, O.; Dennison, K.; Pointon, T.; Davis, M.; Gilden, D. Intrathecally synthesized IgG in multiple sclerosis cerebrospinal fluid recognizes identical epitopes over time. J. Neuroimmunol. 2011, 240–241, 129–136. [Google Scholar] [CrossRef]
- Villar, L.M.; Espino, M.; Cavanillas, M.L.; Roldan, E.; Urcelay, E.; de la Concha, E.G.; Sadaba, M.C.; Arroyo, R.; Gonzalez-Porque, P.; Alvarez-Cermeno, J.C. Immunological mechanisms that associate with oligoclonal IgM band synthesis in multiple sclerosis. Clin. Immunol. 2010, 137, 51–59. [Google Scholar] [CrossRef]
- García-Barragán, N.; Villar, L.M.; Espiño, M.; Sádaba, M.C.; González-Porqué, P.; Alvarez-Cermeño, J.C. Multiple sclerosis patients with anti-lipid oligoclonal IgM show early favourable response to immunomodulatory treatment. Eur. J. Neurol. 2009, 16, 380–385. [Google Scholar] [CrossRef]
- Graner, M.; Pointon, T.; Manton, S.; Green, M.; Dennison, K.; Davis, M.; Braiotta, G.; Craft, J.; Edwards, T.; Polonsky, B.; et al. Oligoclonal IgG antibodies in multiple sclerosis target patient-specific peptides. PLoS ONE 2020, 15, e0228883. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; George, W.; Yu, X. The elusive nature of the oligoclonal bands in multiple sclerosis. J. Neurol. 2024, 271, 116–124. [Google Scholar] [CrossRef]
- Kaschka, W.P.; Theilkaes, L.; Eickhoff, K.; Skvaril, F. Disproportionate elevation of the immunoglobulin G1 concentration in cerebrospinal fluids of patients with multiple sclerosis. Infect. Immun. 1979, 26, 933–941. [Google Scholar] [CrossRef]
- Beseler, C.; Vollmer, T.; Graner, M.; Yu, X. The complex relationship between oligoclonal bands, lymphocytes in the cerebrospinal fluid, and immunoglobulin G antibodies in multiple sclerosis: Indication of serum contribution. PLoS ONE 2017, 12, e0186842. [Google Scholar] [CrossRef]
- Trend, S.; Jones, A.P.; Cha, L.; Byrne, S.N.; Geldenhuys, S.; Fabis-Pedrini, M.J.; Carroll, W.M.; Cole, J.M.; Booth, D.R.; Lucas, R.M.; et al. Higher Serum Immunoglobulin G3 Levels May Predict the Development of Multiple Sclerosis in Individuals with Clinically Isolated Syndrome. Front. Immunol. 2018, 9, 1590. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; Graner, M.W.; Fringuello, A.; Zhou, W.; Pointon, T.; Alquatli, K.; Bisel, S.; Langford, D.; Yu, X. Higher Levels of IgG3 Antibodies in Serum, But Not in CSF, Distinguish Multiple Sclerosis from Other Neurological Disorders. J. Neuroimmune Pharmacol. 2022, 17, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; Graner, M.; Pointon, T.; Li, X.; Tanimoto, K.; Dennison, K.; Im, G.; Fringuello, A.; Zhou, W.; Graner, A.; et al. Aberrant Immunoglobulin G Glycosylation in Multiple Sclerosis. J. Neuroimmune Pharmacol. 2022, 17, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, R.S.; Bryant, S.C.; McKeon, A.; Weinshenker, B.; Murray, D.L.; Pittock, S.J.; Willrich, M.A.V. CSF Kappa Free Light Chains: Cutoff Validation for Diagnosing Multiple Sclerosis. Mayo Clin. Proc. 2022, 97, 738–751. [Google Scholar] [CrossRef]
- Levraut, M.; Laurent-Chabalier, S.; Ayrignac, X.; Bigaut, K.; Rival, M.; Squalli, S.; Zephir, H.; Alberto, T.; Pekar, J.D.; Ciron, J.; et al. Kappa Free Light Chain Biomarkers Are Efficient for the Diagnosis of Multiple Sclerosis: A Large Multicenter Cohort Study. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200049. [Google Scholar] [CrossRef]
- Rodriguez, O.L.; Safonova, Y.; Silver, C.A.; Shields, K.; Gibson, W.S.; Kos, J.T.; Tieri, D.; Ke, H.; Jackson, K.J.L.; Boyd, S.D.; et al. Genetic variation in the immunoglobulin heavy chain locus shapes the human antibody repertoire. Nat. Commun. 2023, 14, 4419. [Google Scholar] [CrossRef]
- Pushparaj, P.; Nicoletto, A.; Castro Dopico, X.; Sheward, D.J.; Kim, S.; Ekström, S.; Murrell, B.; Corcoran, M.; Karlsson Hedestam, G.B. Frequent use of IGHV3-30-3 in SARS-CoV-2 neutralizing antibody responses. Front. Virol. 2023, 3, 1128253. [Google Scholar] [CrossRef]
- Bashford-Rogers, R.J.M.; Smith, K.G.C.; Thomas, D.C. Antibody repertoire analysis in polygenic autoimmune diseases. Immunology 2018, 155, 3–17. [Google Scholar] [CrossRef]
- Owens, G.P.; Winges, K.M.; Ritchie, A.M.; Edwards, S.; Burgoon, M.P.; Lehnhoff, L.; Nielsen, K.; Corboy, J.; Gilden, D.H.; Bennett, J.L. VH4 gene segments dominate the intrathecal humoral immune response in multiple sclerosis. J. Immunol. 2007, 179, 6343–6351. [Google Scholar] [CrossRef]
- von Budingen, H.C.; Kuo, T.C.; Sirota, M.; van Belle, C.J.; Apeltsin, L.; Glanville, J.; Cree, B.A.; Gourraud, P.A.; Schwartzburg, A.; Huerta, G.; et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J. Clin. Investig. 2012, 122, 4533–4543. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.T.; Ramagopalan, S.V.; Morrison, K.M.; Ebers, G.C.; Breden, F. IGHV4-39 deletion polymorphism does not associate with risk or outcome of multiple sclerosis. J. Neuroimmunol. 2010, 225, 164–166. [Google Scholar] [CrossRef]
- Perez-Saldivar, M.; Nakamura, Y.; Kiyotani, K.; Imoto, S.; Katayama, K.; Yamaguchi, R.; Miyano, S.; Martinez-Barnetche, J.; Godoy-Lozano, E.E.; Ordonez, G.; et al. Comparative analysis of the B cell receptor repertoire during relapse and remission in patients with multiple sclerosis. Clin. Immunol. 2024, 269, 110398. [Google Scholar] [CrossRef]
- Zhou, W.; Graner, M.; Paucek, P.; Beseler, C.; Boisen, M.; Bubak, A.; Asturias, F.; George, W.; Graner, A.; Ormond, D.; et al. Multiple sclerosis plasma IgG aggregates induce complement-dependent neuronal apoptosis. Cell Death Dis. 2023, 14, 254. [Google Scholar] [CrossRef]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Sun, G.H.; Lee, T.P.; Huang, J.C.; Yu, C.L.; Chen, C.H.; Tang, S.J.; Sun, K.H. Arginines in the CDR of anti-dsDNA autoantibodies facilitate cell internalization via electrostatic interactions. Eur. J. Immunol. 2008, 38, 3178–3190. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.E.; Shin, W.; Seo, D.; Kim, S.; Kim, H.; Noh, J.; Lee, Y.; Kim, H.; Lim, Y.M.; et al. Distinct features of B cell receptors in neuromyelitis optica spectrum disorder among CNS inflammatory demyelinating diseases. J. Neuroinflamm. 2023, 20, 225. [Google Scholar] [CrossRef]
- Weinshenker, B.G.; Wingerchuk, D.M.; Pittock, S.J.; Lucchinetti, C.F.; Lennon, V.A. NMO-IgG: A specific biomarker for neuromyelitis optica. Dis. Markers 2006, 22, 197–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, A.D.; Moog, T.M.; Burgess, K.W.; McCreary, M.; Okuda, D.T. Factors associated with the misdiagnosis of neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 2023, 70, 104498. [Google Scholar] [CrossRef]
- Ruschil, C.; Kemmerer, C.L.; Beller, L.; Gabernet, G.; Kowarik, M.C. Next Generation Sequencing of Cerebrospinal Fluid B Cell Repertoires in Multiple Sclerosis and Other Neuro-Inflammatory Diseases-A Comprehensive Review. Diagnostics 2021, 11, 1871. [Google Scholar] [CrossRef]
- Apeltsin, L.; Wang, S.; von Budingen, H.C.; Sirota, M. A Haystack Heuristic for Autoimmune Disease Biomarker Discovery Using Next-Gen Immune Repertoire Sequencing Data. Sci. Rep. 2017, 7, 5338. [Google Scholar] [CrossRef] [PubMed]
- Deacy, A.M.; Gan, S.K.; Derrick, J.P. Superantigen Recognition and Interactions: Functions, Mechanisms and Applications. Front. Immunol. 2021, 12, 731845. [Google Scholar] [CrossRef]
- Potter, K.N.; Li, Y.; Capra, J.D. Staphylococcal protein A simultaneously interacts with framework region 1, complementarity-determining region 2, and framework region 3 on human VH3-encoded Igs. J. Immunol. 1996, 157, 2982–2988. [Google Scholar] [CrossRef] [PubMed]
- Graille, M.; Stura, E.A.; Corper, A.L.; Sutton, B.J.; Taussig, M.J.; Charbonnier, J.B.; Silverman, G.J. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: Structural basis for recognition of B-cell receptors and superantigen activity. Proc. Natl. Acad. Sci. USA 2000, 97, 5399–5404. [Google Scholar] [CrossRef]
- Langone, J.J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv. Immunol. 1982, 32, 157–252. [Google Scholar]
- Yang, L.; Biswas, M.E.; Chen, P. Study of binding between protein A and immunoglobulin G using a surface tension probe. Biophys. J. 2003, 84, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Zamecnik, C.R.; Sowa, G.M.; Abdelhak, A.; Dandekar, R.; Bair, R.D.; Wade, K.J.; Bartley, C.M.; Kizer, K.; Augusto, D.G.; Tubati, A.; et al. An autoantibody signature predictive for multiple sclerosis. Nat. Med. 2024, 30, 1300–1308. [Google Scholar] [CrossRef]
- Li, W.; Prabakaran, P.; Chen, W.; Zhu, Z.; Feng, Y.; Dimitrov, D.S. Antibody Aggregation: Insights from Sequence and Structure. Antibodies 2016, 5, 19. [Google Scholar] [CrossRef]
- Petersen, B.M.; Ulmer, S.A.; Rhodes, E.R.; Gutierrez-Gonzalez, M.F.; Dekosky, B.J.; Sprenger, K.G.; Whitehead, T.A. Regulatory Approved Monoclonal Antibodies Contain Framework Mutations Predicted from Human Antibody Repertoires. Front. Immunol. 2021, 12, 728694. [Google Scholar] [CrossRef]
- Namisaki, H.; Saito, S.; Hiraishi, K.; Haba, T.; Tanaka, Y.; Yoshida, H.; Iida, S.; Takahashi, N. R409K mutation prevents acid-induced aggregation of human IgG4. PLoS ONE 2020, 15, e0229027. [Google Scholar] [CrossRef]
- Okun, E.; Mattson, M.P.; Arumugam, T.V. Involvement of Fc receptors in disorders of the central nervous system. Neuromol. Med. 2010, 12, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.R.; Boer, M.A.D.; Strasser, J.; Zwarthoff, S.A.; Beurskens, F.J.; de Haas, C.J.C.; Aerts, P.C.; Wang, G.; de Jong, R.N.; Bagnoli, F.; et al. Staphylococcal protein A inhibits complement activation by interfering with IgG hexamer formation. Proc. Natl. Acad. Sci. USA 2021, 118, e2016772118. [Google Scholar] [CrossRef]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef] [PubMed]
- Winger, R.C.; Zamvil, S.S. Antibodies in multiple sclerosis oligoclonal bands target debris. Proc. Natl. Acad. Sci. USA 2016, 113, 7696–7698. [Google Scholar] [CrossRef]
| Name (Acronym) | Description | CSF Biomarker for MS | Blood Biomarker for MS |
|---|---|---|---|
| Immunoglobulin M (IgM) | It is expressed by naïve B cells and contains four constant domains. | Found in OCBs, which are associated with disease progression. | N/A |
| Immunoglobulin G (IgG) | IgG is expressed after class-switch recombination, which contains three constant domains. | The most common and abundant Ig class in OCBs [13]. | N/A |
| Immunoglobulin G1 (IgG1) | IgG class with a short hinge region. | Primary component of OCBs. IgG1 is elevated in MS CSF [19]. | Elevated levels of IgG1 were found in the IgG aggregates in MS [4]. |
| Immunoglobulin G3 (IgG3) | IgG3 has a longer, more flexible hinge region compared to IgG1. | IgG3 OCBs are observed in approximately 66% of MS patients [13]. | Elevated levels of IgG3 were found in the IgG aggregates in MS [4,23]. |
| Fragment Antigen-Binding Region (Fab) | Region of the antibody that binds to antigens, composed of a heavy chain and a light chain. | N/A | N/A |
| Immunoglobulin Kappa Light Chain (IGK) | Light chain originating from the Ig Kappa locus on chromosome 2, which is composed of one variable domain and one constant domain. | Abnormally elevated free kappa chains are observed in the CSF of most MS patients [26,27]. | N/A |
| Immunoglobulin Heavy Chain (IGH) | The heavy chain is composed of one variable domain and three to four constant domains. The variable domain is formed through the recombination of three distinct gene segments: V, D, and J. | N/A | N/A |
| Immunoglobulin Heavy Variable Gene (IGHV) | Seven unique V families are located on chromosome 14. | N/A | N/A |
| Immunoglobulin Heavy Variable 4 Gene (IGHV4) | One of the seven IGHV families. | IGHV4-34 and IGHV4-39 are overrepresented within the CSF of MS patients [32,36]. | N/A |
| Immunoglobulin Heavy Variable 3 Gene (IGHV3) | One of the seven IGHV families. | N/A | The IGHV3-23 and IGHV3-74 subfamilies are overrepresented in MS [29]. |
| Heavy Chain Complementarity Determining Region 3 (CDR3-H) | The most diverse CDR region is produced through VDJ gene recombination [5]. | N/A | Disease-specific patterns in CDR3-H have been identified in other autoimmune diseases but not in MS [37,38]. |
| IGHV3 Framework Region 3 (IGHV3 FR3) | The region between CDR2-H and CDR3-H uniquely interacts with Protein A [44,45]. | N/A | A distinct indel mutation in the IGHV3 FR3 region was observed in MS blood [42]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apeltsin, L.; Yu, X. IgG Biomarkers in Multiple Sclerosis: Deciphering Their Puzzling Protein A Connection. Biomolecules 2025, 15, 369. https://doi.org/10.3390/biom15030369
Apeltsin L, Yu X. IgG Biomarkers in Multiple Sclerosis: Deciphering Their Puzzling Protein A Connection. Biomolecules. 2025; 15(3):369. https://doi.org/10.3390/biom15030369
Chicago/Turabian StyleApeltsin, Leonard, and Xiaoli Yu. 2025. "IgG Biomarkers in Multiple Sclerosis: Deciphering Their Puzzling Protein A Connection" Biomolecules 15, no. 3: 369. https://doi.org/10.3390/biom15030369
APA StyleApeltsin, L., & Yu, X. (2025). IgG Biomarkers in Multiple Sclerosis: Deciphering Their Puzzling Protein A Connection. Biomolecules, 15(3), 369. https://doi.org/10.3390/biom15030369






