Stem Cell Therapies in Canine Cardiology: Comparative Efficacy, Emerging Trends, and Clinical Integration
Abstract
1. Introduction
1.1. Overview of Cardiovascular Diseases
1.2. Stem Cell Therapy: A Promising Approach
1.3. Scope and Objectives of the Review
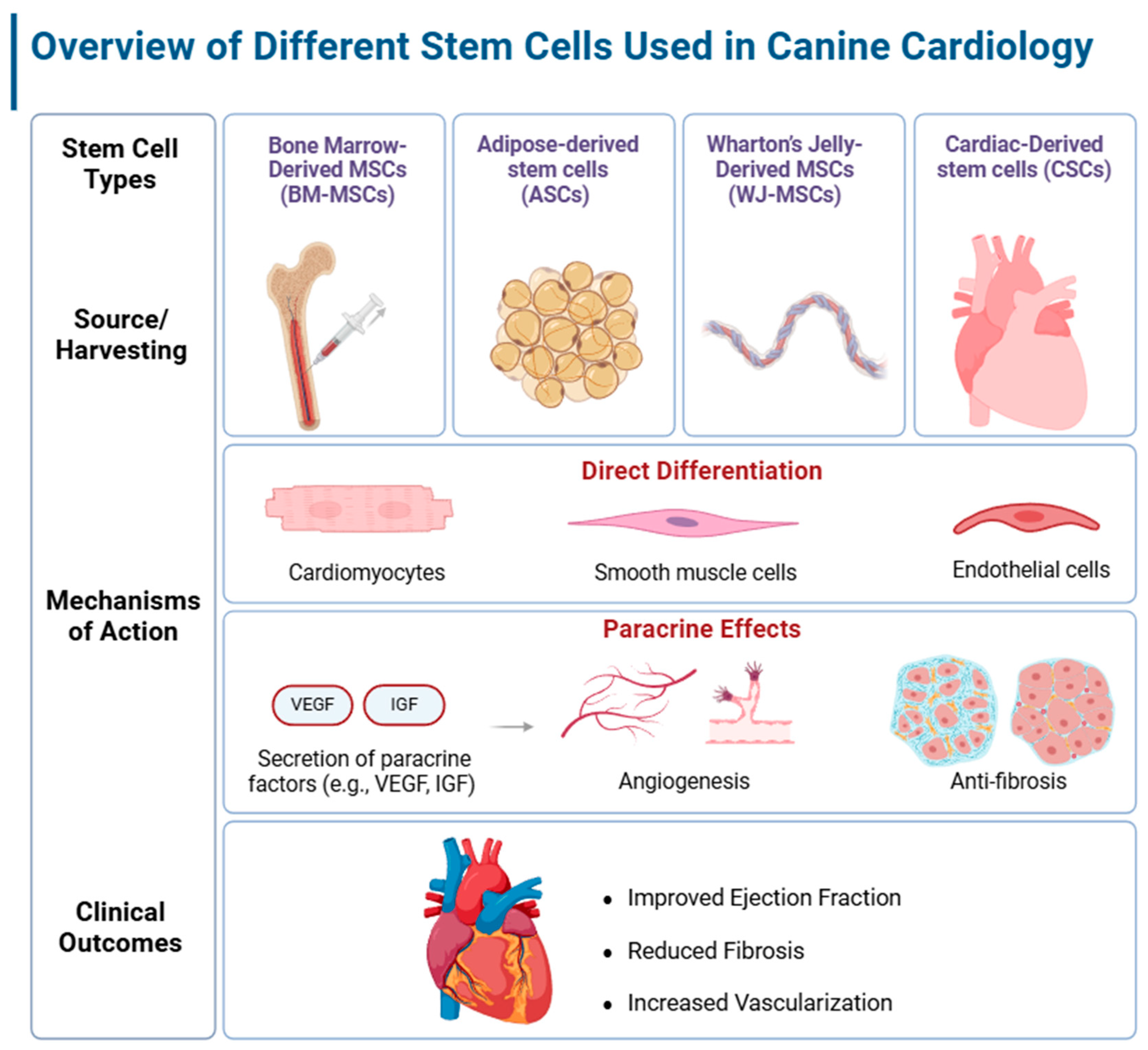
2. Efficacy of Stem Cell Therapies in Cardiology
2.1. Mesenchymal Stem Cells in Cardiac Regeneration
2.1.1. Bone Marrow-Derived MSCs
2.1.2. Adipose-Derived Stem Cells
2.1.3. Wharton’s Jelly-Derived MSCs
2.2. Cardiac Stem Cells (CSCs) and Their Potential
2.3. Effectiveness of Stem Cell Therapies in Canine Cardiac Models
3. Delivery Methods for Stem Cell Therapies
4. Integrating Stem Cell Research with Clinical Practice
4.1. Bridging the Gap
4.2. Clinical Guidelines and Protocols
4.3. Case Studies Highlighting Stem Cell Therapies in Canine Cardiology
5. Future Directions and Recommendations
5.1. Research Gaps
5.1.1. Mechanisms of Action and Differentiation
5.1.2. Cell Homing and Retention
5.1.3. Cell Differentiation and Maturation
5.1.4. Immunological Considerations
5.1.5. Comparative Effectiveness of Stem Cell Sources
5.2. Technological Advances
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsharkawy, S.H.; Torad, F.A.-S.; Abu-Aita, N.A.; Abdel-Ghany, A.K.; El-Husseiny, I.N. Acquired Cardiac Diseases in 72 Dogs: A Prospective Study 2017–2020. Int. J. Vet. Sci. 2022, 11, 427–434. [Google Scholar]
- Egenvall, A.; Bonnett, B.N.; Häggström, J. Heart Disease as a Cause of Death in Insured Swedish Dogs Younger than 10 Years of Age. J. Vet. Intern. Med. 2006, 20, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Mandour, A.S.; Hendawy, H.; Elhaieg, A.; Elfadadny, A.; Tanaka, R. A Review on Experimental Surgical Models and Anesthetic Protocols of Heart Failure in Rats. Front. Vet. Sci. 2023, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Atkins, C.; Bonagura, J.; Ettinger, S.; Fox, P.; Gordon, S.; Haggstrom, J.; Hamlin, R.; Keene, B.; Luis-Fuentes, V.; Stepien, R. Guidelines for the Diagnosis and Treatment of Canine Chronic Valvular Heart Disease. J. Vet. Intern. Med. 2009, 23, 1142–1150. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM Consensus Guidelines for the Diagnosis and Treatment of Myxomatous Mitral Valve Disease in Dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Gasparini, S.; Fonfara, S.; Kitz, S.; Hetzel, U.; Kipar, A. Canine Dilated Cardiomyopathy: Diffuse Remodeling, Focal Lesions, and the Involvement of Macrophages and New Vessel Formation. Vet. Pathol. 2020, 57, 397–408. [Google Scholar] [CrossRef]
- Tidholm, A.; Jönsson, L. Histologic Characterization of Canine Dilated Cardiomyopathy. Vet. Pathol. 2005, 42, 1–8. [Google Scholar] [CrossRef]
- Martin, M.W.S.; Stafford Johnson, M.J.; Strehlau, G.; King, J.N. Canine Dilated Cardiomyopathy: A Retrospective Study of Prognostic Findings in 367 Clinical Cases. J. Small Anim. Pract. 2010, 51, 428–436. [Google Scholar] [CrossRef]
- Lakhdhir, S.; Viall, A.; Alloway, E.; Keene, B.; Baumgartner, K.; Ward, J. Clinical Presentation, Cardiovascular Findings, Etiology, and Outcome of Myocarditis in Dogs: 64 Cases with Presumptive Antemortem Diagnosis (26 Confirmed Postmortem) and 137 Cases with Postmortem Diagnosis Only (2004–2017). J. Vet. Cardiol. 2020, 30, 44–56. [Google Scholar] [CrossRef]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM Consensus Statement: Guidelines for the Identification, Evaluation, and Management of Systemic Hypertension in Dogs and Cats. J. Vet. Intern. Med. 2018, 32, 1803–1822. [Google Scholar] [CrossRef]
- Schober, K.E.; Fox, P.R.; Abbott, J.; Côté, E.; Luis-Fuentes, V.; Matos, J.N.; Stern, J.A.; Visser, L.; Scollan, K.F.; Chetboul, V. Retrospective Evaluation of Hypertrophic Cardiomyopathy in 68 Dogs. J. Vet. Intern. Med. 2022, 36, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, E.; Pedro, B.; Dukes-McEwan, J.; Gelzer, A.R.; Blackwood, L. A Descriptive Review of Cardiac Tumours in Dogs and Cats. Vet. Comp. Oncol. 2017, 15, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A.H. Pericardial Diseases. In Veterinary Internal Medicine, 7th ed.; Ettinger, S.J., Feldman, E.C., Eds.; Saunders Elsevier: St Louis, MO, USA; Philadelphia, PA, USA, 2010; Volume 1, pp. 1342–1352. [Google Scholar]
- Grzeczka, A.; Graczyk, S.; Kordowitzki, P. Pleiotropic Effects of Resveratrol on Aging-Related Cardiovascular Diseases—What Can We Learn from Research in Dogs? Cells 2024, 13, 1732. [Google Scholar] [CrossRef]
- Kaese, S.; Frommeyer, G.; Verheule, S.; van Loon, G.; Gehrmann, J.; Breithardt, G.; Eckardt, L. The ECG in Cardiovascular-Relevant Animal Models of Electrophysiology. Herzschr. Elektrophys. 2013, 24, 84–91. [Google Scholar] [CrossRef]
- Gralinski, M.R. The Dog’s Role in the Preclinical Assessment of QT Interval Prolongation. Toxicol. Pathol. 2003, 31, 11–16. [Google Scholar]
- Macintire, D.K.; Drobatz, K.J.; Haskins, S.C.; Saxon, W.D. Manual of Small Animal Emergency and Critical Care Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118351134. [Google Scholar]
- Tilley, L.P.; Smith, F.W.K.; Sleeper, M.M.; Kraus, M. Manual of Canine and Feline Cardiology-E-BOOK: Manual of Canine and Feline Cardiology-E-BOOK; Elsevier Health Sciences: Amsterdam, The Netherlands, 2024; ISBN 0323936601. [Google Scholar]
- Varshney, J.P. Benchmarks for Normal Electrocardiogram. In Electrocardiography in Veterinary Medicine; Springer: Singapore, 2020; pp. 63–67. ISBN 978-981-15-3699-1. [Google Scholar]
- Moïse, N.S. Inherited Arrhythmias in the Dog: Potential Experimental Models of Cardiac Disease. Cardiovasc. Res. 1999, 44, 37–46. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Z.; Yan, Z.; Ji, N.; Yang, X.; Gao, D.; Hu, L.; Lv, H.; Zhang, J.; Li, M. Mesenchymal Stem Cell Immunomodulation: A Novel Intervention Mechanism in Cardiovascular Disease. Front. Cell Dev. Biol. 2022, 9, 742088. [Google Scholar] [CrossRef]
- Seow, K.S.; Ling, A.P.K. Mesenchymal Stem Cells as Future Treatment for Cardiovascular Regeneration and Its Challenges. Ann. Transl. Med. 2024, 12, 73. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Archana, S.; Verma, R.S. Emerging Trends in Mesenchymal Stem Cells Applications for Cardiac Regenerative Therapy: Current Status and Advances. Stem Cell Rev. Rep. 2022, 18, 1546–1602. [Google Scholar] [CrossRef]
- Samadi, P.; Saki, S.; Manoochehri, H.; Sheykhhasan, M. Therapeutic Applications of Mesenchymal Stem Cells: A Comprehensive Review. Curr. Stem Cell Res. Ther. 2021, 16, 323–353. [Google Scholar] [CrossRef]
- Hendawy, H.; Farag, A.; Elhaieg, A.; Metwllay, E.; Shimada, K.; Elfadadny, A.; Tanaka, R. Enhanced Bladder Regeneration with Adipose-Derived Stem Cell-Seeded Silk Fibroin Scaffolds: A Comparative Analysis. Biomimetics 2025, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Gómez-García, R.; Granados-Montiel, J.; Berebichez-Fastlicht, E.; Olivos-Meza, A.; Granados, J.; Velasquillo, C.; Ibarra, C. The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells—Their Current Uses and Potential Applications. Stem Cells Int. 2017, 2017, 2638305. [Google Scholar] [CrossRef] [PubMed]
- Ceusters, J.; Lejeune, J.-P.; Sandersen, C.; Niesten, A.; Lagneaux, L.; Serteyn, D. From Skeletal Muscle to Stem Cells: An Innovative and Minimally-Invasive Process for Multiple Species. Sci. Rep. 2017, 7, 696. [Google Scholar] [CrossRef] [PubMed]
- Radtke, C.L.; Nino-Fong, R.; Gonzalez, B.P.E.; Stryhn, H.; McDuffee, L.A. Characterization and Osteogenic Potential of Equine Muscle Tissue–and Periosteal Tissue–Derived Mesenchymal Stem Cells in Comparison with Bone Marrow–and Adipose Tissue–Derived Mesenchymal Stem Cells. Am. J. Vet. Res. 2013, 74, 790–800. [Google Scholar] [CrossRef]
- Sheng, G. The Developmental Basis of Mesenchymal Stem/Stromal Cells (MSCs). BMC Dev. Biol. 2015, 15, 44. [Google Scholar] [CrossRef]
- Farag, A.; Koung Ngeun, S.; Kaneda, M.; Aboubakr, M.; Tanaka, R. Optimizing Cardiomyocyte Differentiation: Comparative Analysis of Bone Marrow and Adipose-Derived Mesenchymal Stem Cells in Rats Using 5-Azacytidine and Low-Dose FGF and IGF Treatment. Biomedicines 2024, 12, 1923. [Google Scholar] [CrossRef]
- Yuce, K. The Application of Mesenchymal Stem Cells in Different Cardiovascular Disorders: Ways of Administration, and the Effectors. Stem Cell Rev. Rep. 2024, 20, 1671–1691. [Google Scholar] [CrossRef]
- Yang, V.K.; Meola, D.M.; Davis, A.; Barton, B.; Hoffman, A.M. Intravenous Administration of Allogeneic Wharton Jelly–Derived Mesenchymal Stem Cells for Treatment of Dogs with Congestive Heart Failure Secondary to Myxomatous Mitral Valve Disease. Am. J. Vet. Res. 2021, 82, 487–493. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Noronha, N.d.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Adelipour, M.; Lubman, D.M.; Kim, J. Potential Applications of Mesenchymal Stem Cells and Their Derived Exosomes in Regenerative Medicine. Expert Opin. Biol. Ther. 2023, 23, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mai, Z.; Cui, L.; Zhao, X. Engineering Exosomes and Biomaterial-Assisted Exosomes as Therapeutic Carriers for Bone Regeneration. Stem Cell Res. Ther. 2023, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Caplan, A.I. Cell-Based Therapies: The Nonresponder. Stem Cells Transl. Med. 2018, 7, 762–766. [Google Scholar] [CrossRef]
- Boregowda, S.V.; Krishnappa, V.; Haga, C.L.; Ortiz, L.A.; Phinney, D.G. A Clinical Indications Prediction Scale Based on TWIST1 for Human Mesenchymal Stem Cells. EBioMedicine 2016, 4, 62–73. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jung, J.; Cho, K.J.; Choi, J.-H.; Lee, H.S.; Kim, G.J.; Lee, S.G. Immunomodulatory Effects of Placenta-Derived Mesenchymal Stem Cells on T Cells by Regulation of FoxP3 Expression. Int. J. Stem Cells 2018, 11, 196–204. [Google Scholar] [CrossRef]
- Luzuriaga, J.; Polo, Y.; Pastor-Alonso, O.; Pardo-Rodríguez, B.; Larrañaga, A.; Unda, F.; Sarasua, J.-R.; Pineda, J.R.; Ibarretxe, G. Advances and Perspectives in Dental Pulp Stem Cell Based Neuroregeneration Therapies. Int. J. Mol. Sci. 2021, 22, 3546. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Bao, L.; Meng, Q.; Li, Y.; Deng, S.; Yu, Z.; Liu, Z.; Zhang, L.; Fan, H. C-Kit Positive Cardiac Stem Cells and Bone Marrow–Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function after Myocardial Infarction in a Paracrine Manner. J. Card. Fail. 2017, 23, 403–415. [Google Scholar] [CrossRef]
- Narita, T.; Suzuki, K. Bone Marrow-Derived Mesenchymal Stem Cells for the Treatment of Heart Failure. Heart Fail. Rev. 2015, 20, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Yan, J.; Jiang, Y.; Wu, L.; Li, M.; Fan, X. Exploring Cutting-Edge Approaches to Potentiate Mesenchymal Stem Cell and Exosome Therapy for Myocardial Infarction. J. Cardiovasc. Transl. Res. 2024, 17, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.E.; Ali, M.A.; Ghanem, H.G.; Shehata, A.I.; Abdelgawad, A.A.; Handal, H.R.; Talaat, K.A.; Ashaal, A.E.; El-Shal, A.S. Mesenchymal Stem Cell Therapy: A Promising Cell-based Therapy for Treatment of Myocardial Infarction. J. Gene Med. 2017, 19, e2995. [Google Scholar] [CrossRef]
- Kalou, Y.M.; Al-Khani, A.M.; Haider, K.H. The Effect of Time of Cell Delivery on Post-MI Cardiac Regeneration: A Review of Preclinical and Clinical Studies. Cardiovasc. Appl. Stem Cells 2023, 349–401. [Google Scholar]
- Wang, X.; Tang, Y.; Liu, Z.; Yin, Y.; Li, Q.; Liu, G.; Yan, B. The Application Potential and Advance of Mesenchymal Stem Cell-Derived Exosomes in Myocardial Infarction. Stem Cells Int. 2021, 2021, 5579904. [Google Scholar] [CrossRef]
- Russo, V.; Young, S.; Hamilton, A.; Amsden, B.G.; Flynn, L.E. Mesenchymal Stem Cell Delivery Strategies to Promote Cardiac Regeneration Following Ischemic Injury. Biomaterials 2014, 35, 3956–3974. [Google Scholar] [CrossRef]
- Obradović, S.; Rusović, S.; Balint, B.; Ristić-Anđelkov, A.; Romanović, R.; Baskot, B.; Vojvodić, D.; Gligić, B. Autologous Bone Marrow-Derived Progenitor Cell Transplantation for Myocardial Regeneration after Acute Infarction. Vojnosanit. Pregl. 2004, 61, 519–529. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Sotiropoulou, P.A.; Karvouni, E.; Karabinos, I.; Korovesis, S.; Perez, S.A.; Voridis, E.M.; Papamichail, M. Transcoronary Transplantation of Autologous Mesenchymal Stem Cells and Endothelial Progenitors into Infarcted Human Myocardium. Catheter. Cardiovasc. Interv. 2005, 65, 321–329. [Google Scholar] [CrossRef]
- Strauer, B.E.; Brehm, M.; Zeus, T.; Köstering, M.; Hernandez, A.; Sorg, R.V.; Kögler, G.; Wernet, P. Repair of Infarcted Myocardium by Autologous Intracoronary Mononuclear Bone Marrow Cell Transplantation in Humans. Circulation 2002, 106, 1913–1918. [Google Scholar] [CrossRef]
- Nagaya, N.; Fujii, T.; Iwase, T.; Ohgushi, H.; Itoh, T.; Uematsu, M.; Yamagishi, M.; Mori, H.; Kangawa, K.; Kitamura, S. Intravenous Administration of Mesenchymal Stem Cells Improves Cardiac Function in Rats with Acute Myocardial Infarction through Angiogenesis and Myogenesis. Am. J. Physiol. Circ. Physiol. 2004, 287, H2670–H2676. [Google Scholar] [CrossRef]
- Kawada, H.; Fujita, J.; Kinjo, K.; Matsuzaki, Y.; Tsuma, M.; Miyatake, H.; Muguruma, Y.; Tsuboi, K.; Itabashi, Y.; Ikeda, Y. Nonhematopoietic Mesenchymal Stem Cells Can Be Mobilized and Differentiate into Cardiomyocytes after Myocardial Infarction. Blood 2004, 104, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K. Monolayered Mesenchymal Stem Cells Repair Scarred Myocardium after Myocardial Infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Zhao, Q.; Zhang, Y.C.; Cheng, L.; Liu, M.; Shi, J.; Yang, Y.Z.; Pan, C.; Ge, J.; Phillips, M.I. Autologous Mesenchymal Stem Cell Transplantation Induce VEGF and Neovascularization in Ischemic Myocardium. Regul. Pept. 2004, 117, 3–10. [Google Scholar] [CrossRef]
- Iwase, T.; Nagaya, N.; Fujii, T.; Itoh, T.; Murakami, S.; Matsumoto, T.; Kangawa, K.; Kitamura, S. Comparison of Angiogenic Potency between Mesenchymal Stem Cells and Mononuclear Cells in a Rat Model of Hindlimb Ischemia. Cardiovasc. Res. 2005, 66, 543–551. [Google Scholar] [CrossRef]
- Moscoso, I.; Centeno, A.; Lopez, E.; Rodriguez-Barbosa, J.I.; Santamarina, I.; Filgueira, P.; Sanchez, M.J.; Domınguez-Perles, R.; Penuelas-Rivas, G.; Domenech, N. Differentiation “in Vitro” of Primary and Immortalized Porcine Mesenchymal Stem Cells into Cardiomyocytes for Cell Transplantation. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2005; Volume 37, pp. 481–482. [Google Scholar]
- Silva, G.V.; Litovsky, S.; Assad, J.A.R.; Sousa, A.L.S.; Martin, B.J.; Vela, D.; Coulter, S.C.; Lin, J.; Ober, J.; Vaughn, W.K. Mesenchymal Stem Cells Differentiate into an Endothelial Phenotype, Enhance Vascular Density, and Improve Heart Function in a Canine Chronic Ischemia Model. Circulation 2005, 111, 150–156. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Park, J.-S.; Tkebuchava, T.; Luedeman, C.; Losordo, D.W. Unexpected Severe Calcification after Transplantation of Bone Marrow Cells in Acute Myocardial Infarction. Circulation 2004, 109, 3154–3157. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, A.D.; Rubin, J.P.; Basse, P.; Landreneau, R.J.; Donnenberg, V.S. Regenerative Therapy and Cancer: In Vitro and in Vivo Studies of the Interaction between Adipose-Derived Stem Cells and Breast Cancer Cells from Clinical Isolates. Tissue Eng. Part A 2011, 17, 93–106. [Google Scholar] [CrossRef]
- Forslöw, U.; Blennow, O.; LeBlanc, K.; Ringdén, O.; Gustafsson, B.; Mattsson, J.; Remberger, M. Treatment with Mesenchymal Stromal Cells Is a Risk Factor for Pneumonia-related Death after Allogeneic Hematopoietic Stem Cell Transplantation. Eur. J. Haematol. 2012, 89, 220–227. [Google Scholar] [CrossRef]
- Tootee, A.; Nikbin, B.; Esfahani, E.N.; Arjmand, B.; Aghayan, H.; Qorbani, M.; Ghahari, A.; Larijani, B. Clinical Outcomes of Fetal Stem Cell Transplantation in Type 1 Diabetes Are Related to Alternations to Different Lymphocyte Populations. Med. J. Islam. Repub. Iran 2022, 36, 254–261. [Google Scholar] [CrossRef]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse Events, Side Effects and Complications in Mesenchymal Stromal Cell-Based Therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat Tissue: An Underappreciated Source of Stem Cells for Biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Kornacker, M.; Mehlhorn, A.; Seckinger, A.; Vohrer, J.; Schmal, H.; Kasten, P.; Eckstein, V.; Südkamp, N.P.; Krause, U. Comparison of Immunological Properties of Bone Marrow Stromal Cells and Adipose Tissue–Derived Stem Cells before and after Osteogenic Differentiation in Vitro. Tissue Eng. 2007, 13, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Parham, A.; Mehrjerdi, H.K.; Dehghani, H. Equine Adipose-Derived Mesenchymal Stem Cells: Phenotype and Growth Characteristics, Gene Expression Profile and Differentiation Potentials. Cell J. 2015, 16, 456. [Google Scholar] [PubMed]
- Suzuki, E.; Fujita, D.; Takahashi, M.; Oba, S.; Nishimatsu, H. Adipose Tissue-Derived Stem Cells as a Therapeutic Tool for Cardiovascular Disease. World J. Cardiol. 2015, 7, 454. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, D.-W.; Yu, L.-H.; Zhang, H.-Z.; Kim, C.E.; Kim, J.-M.; Park, T.-H.; Cha, K.-S.; Seo, S.-Y.; Roh, M.-S. Mesenchymal Stem Cells Overexpressing GCP-2 Improve Heart Function through Enhanced Angiogenic Properties in a Myocardial Infarction Model. Cardiovasc. Res. 2012, 95, 495–506. [Google Scholar] [CrossRef]
- Paul, A.; Srivastava, S.; Chen, G.; Shum-Tim, D.; Prakash, S. Functional Assessment of Adipose Stem Cells for Xenotransplantation Using Myocardial Infarction Immunocompetent Models: Comparison with Bone Marrow Stem Cells. Cell Biochem. Biophys. 2013, 67, 263–273. [Google Scholar] [CrossRef]
- Shudo, Y.; Miyagawa, S.; Ohkura, H.; Fukushima, S.; Saito, A.; Shiozaki, M.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; Okano, T. Addition of Mesenchymal Stem Cells Enhances the Therapeutic Effects of Skeletal Myoblast Cell-Sheet Transplantation in a Rat Ischemic Cardiomyopathy Model. Tissue Eng. Part A 2014, 20, 728–739. [Google Scholar] [CrossRef]
- Li, T.-S.; Cheng, K.; Malliaras, K.; Smith, R.R.; Zhang, Y.; Sun, B.; Matsushita, N.; Blusztajn, A.; Terrovitis, J.; Kusuoka, H. Direct Comparison of Different Stem Cell Types and Subpopulations Reveals Superior Paracrine Potency and Myocardial Repair Efficacy with Cardiosphere-Derived Cells. J. Am. Coll. Cardiol. 2012, 59, 942–953. [Google Scholar] [CrossRef]
- Beitnes, J.O.; Øie, E.; Shahdadfar, A.; Karlsen, T.; Müller, R.M.B.; Aakhus, S.; Reinholt, F.P.; Brinchmann, J.E. Intramyocardial Injections of Human Mesenchymal Stem Cells Following Acute Myocardial Infarction Modulate Scar Formation and Improve Left Ventricular Function. Cell Transplant. 2012, 21, 1697–1709. [Google Scholar] [CrossRef]
- Henry, T.D.; Pepine, C.J.; Lambert, C.R.; Traverse, J.H.; Schatz, R.; Costa, M.; Povsic, T.J.; David Anderson, R.; Willerson, J.T.; Kesten, S. The Athena Trials: Autologous Adipose-derived Regenerative Cells for Refractory Chronic Myocardial Ischemia with Left Ventricular Dysfunction. Catheter. Cardiovasc. Interv. 2017, 89, 169–177. [Google Scholar] [CrossRef]
- Houtgraaf, J.H.; den Dekker, W.K.; van Dalen, B.M.; Springeling, T.; de Jong, R.; van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W. First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Fong, C.-Y. The Therapeutic Potential, Challenges and Future Clinical Directions of Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord. Stem Cell Rev. Rep. 2013, 9, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and Clinical Applications of Wharton’s Jelly-Derived Mesenchymal Stromal Cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef]
- Yu, H.; Lu, K.; Zhu, J.; Wang, J. Stem Cell Therapy for Ischemic Heart Diseases. Br. Med. Bull. 2017, 121, 135–154. [Google Scholar] [CrossRef]
- Fan, M.; Huang, Y.; Chen, Z.; Xia, Y.; Chen, A.; Lu, D.; Wu, Y.; Zhang, N.; Qian, J. Efficacy of Mesenchymal Stem Cell Therapy in Systolic Heart Failure: A Systematic Review and Meta-Analysis. Stem Cell Res. Ther. 2019, 10, 150. [Google Scholar] [CrossRef]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients with Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal. Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef]
- Chamuleau, S.A.J.; Vrijsen, K.R.; Rokosh, D.G.; Tang, X.L.; Piek, J.J.; Bolli, R. Cell Therapy for Ischaemic Heart Disease: Focus on the Role of Resident Cardiac Stem Cells. Neth. Hear. J. 2009, 17, 199–207. [Google Scholar] [CrossRef]
- Hosoda, T.; Kajstura, J.; Leri, A.; Anversa, P. Mechanisms of Myocardial Regeneration. Circ. J. 2010, 74, 13–17. [Google Scholar] [CrossRef]
- Leri, A.; Kajstura JA, N.; Anversa, P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol. Rev. 2005, 85, 1373–1416. [Google Scholar] [CrossRef]
- Steinhoff, G.; Nesteruk, J.; Wolfien, M.; Grosse, J.; Ruch, U.; Vasudevan, P.; Mueller, P. Stem Cells and Heart Disease-Brake or Accelerator? Adv. Drug Deliv. Rev. 2017, 120, 2–24. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Fiorini, C.; Campbell, P.M.; Goichberg, P.; Sanada, F.; Zheng, H.; Hosoda, T.; Rota, M.; Connell, J.M.; Gallegos, R.P. Functionally Competent Cardiac Stem Cells Can Be Isolated from Endomyocardial Biopsies of Patients with Advanced Cardiomyopathies. Circ. Res. 2011, 108, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.U.; Bolli, R. Cardiac Stem Cell Therapy for Cardiac Repair. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 324. [Google Scholar] [CrossRef]
- Bolli, R.; Tang, X.-L.; Sanganalmath, S.K.; Rimoldi, O.; Mosna, F.; Abdel-Latif, A.; Jneid, H.; Rota, M.; Leri, A.; Kajstura, J. Intracoronary Delivery of Autologous Cardiac Stem Cells Improves Cardiac Function in a Porcine Model of Chronic Ischemic Cardiomyopathy. Circulation 2013, 128, 122–131. [Google Scholar] [CrossRef]
- Welt, F.G.P.; Gallegos, R.; Connell, J.; Kajstura, J.; D’Amario, D.; Kwong, R.Y.; Coelho-Filho, O.; Shah, R.; Mitchell, R.; Leri, A. Effect of Cardiac Stem Cells on Left-Ventricular Remodeling in a Canine Model of Chronic Myocardial Infarction. Circ. Hear Fail. 2013, 6, 99–106. [Google Scholar] [CrossRef]
- Linke, A.; Müller, P.; Nurzynska, D.; Casarsa, C.; Torella, D.; Nascimbene, A.; Castaldo, C.; Cascapera, S.; Böhm, M.; Quaini, F. Stem Cells in the Dog Heart Are Self-Renewing, Clonogenic, and Multipotent and Regenerate Infarcted Myocardium, Improving Cardiac Function. Proc. Natl. Acad. Sci. USA 2005, 102, 8966–8971. [Google Scholar] [CrossRef]
- Bartunek, J.; Croissant, J.D.; Wijns, W.; Gofflot, S.; De Lavareille, A.; Vanderheyden, M.; Kaluzhny, Y.; Mazouz, N.; Willemsen, P.; Penicka, M. Pretreatment of Adult Bone Marrow Mesenchymal Stem Cells with Cardiomyogenic Growth Factors and Repair of the Chronically Infarcted Myocardium. Am. J. Physiol. Circ. Physiol. 2007, 292, H1095–H1104. [Google Scholar] [CrossRef]
- Hao, L.; Hao, J.; Fang, W.; Han, C.; Zhang, K.; Wang, X. Dual Isotope Simultaneous Imaging to Evaluate the Effects of Intracoronary Bone Marrow-Derived Mesenchymal Stem Cells on Perfusion and Metabolism in Canines with Acute Myocardial Infarction. Biomed. Rep. 2015, 3, 447–452. [Google Scholar] [CrossRef]
- Mathieu, M.; Bartunek, J.; El Oumeiri, B.; Touihri, K.; Hadad, I.; Thoma, P.; Metens, T.; da Costa, A.M.; Mahmoudabady, M.; Egrise, D. Cell Therapy with Autologous Bone Marrow Mononuclear Stem Cells Is Associated with Superior Cardiac Recovery Compared with Use of Nonmodified Mesenchymal Stem Cells in a Canine Model of Chronic Myocardial Infarction. J. Thorac. Cardiovasc. Surg. 2009, 138, 646–653. [Google Scholar] [CrossRef]
- van der Bogt, K.E.A.; Sheikh, A.Y.; Schrepfer, S.; Hoyt, G.; Cao, F.; Ransohoff, K.J.; Swijnenburg, R.-J.; Pearl, J.; Lee, A.; Fischbein, M. Comparison of Different Adult Stem Cell Types for Treatment of Myocardial Ischemia. Circulation 2008, 118, S121–S129. [Google Scholar] [CrossRef]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases–Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.T.; Tang, J.; Woodruff, K.; Defrancesco, T.; Tou, S.; Williams, C.M.; Breen, M.; Meurs, K.; Keene, B.; Cheng, K. Intracoronary Allogeneic Cardiosphere-derived Stem Cells Are Safe for Use in Dogs with Dilated Cardiomyopathy. J. Cell. Mol. Med. 2017, 21, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.N.; Shlapakova, I.; Szabolcs, M.J.; Danilo, P., Jr.; Lorell, B.H.; Potapova, I.A.; Lu, Z.; Rosen, A.B.; Mathias, R.T.; Brink, P.R. Xenografted Adult Human Mesenchymal Stem Cells Provide a Platform for Sustained Biological Pacemaker Function in Canine Heart. Circulation 2007, 116, 706–713. [Google Scholar] [CrossRef]
- Potapova, I.A.; Doronin, S.V.; Kelly, D.J.; Rosen, A.B.; Schuldt, A.J.T.; Lu, Z.; Kochupura, P.V.; Robinson, R.B.; Rosen, M.R.; Brink, P.R. Enhanced Recovery of Mechanical Function in the Canine Heart by Seeding an Extracellular Matrix Patch with Mesenchymal Stem Cells Committed to a Cardiac Lineage. Am. J. Physiol. Circ. Physiol. 2008, 295, H2257–H2263. [Google Scholar] [CrossRef]
- Kim, U.; Shin, D.-G.; Park, J.-S.; Kim, Y.-J.; Park, S.-I.; Moon, Y.-M.; Jeong, K.-S. Homing of Adipose-Derived Stem Cells to Radiofrequency Catheter Ablated Canine Atrium and Differentiation into Cardiomyocyte-like Cells. Int. J. Cardiol. 2011, 146, 371–378. [Google Scholar] [CrossRef]
- Petchdee, S.; Sompeewong, S. Intravenous Administration of Puppy Deciduous Teeth Stem Cells in Degenerative Valve Disease. Vet. World 2016, 9, 1429. [Google Scholar] [CrossRef]
- McCall, F.C.; Telukuntla, K.S.; Karantalis, V.; Suncion, V.Y.; Heldman, A.W.; Mushtaq, M.; Williams, A.R.; Hare, J.M. Myocardial Infarction and Intramyocardial Injection Models in Swine. Nat. Protoc. 2012, 7, 1479–1496. [Google Scholar] [CrossRef]
- Wang, X.; Zhen, L.; Miao, H.; Sun, Q.; Yang, Y.; Que, B.; Lao, E.P.L.; Wu, X.; Ren, H.; Shi, S. Concomitant Retrograde Coronary Venous Infusion of Basic Fibroblast Growth Factor Enhances Engraftment and Differentiation of Bone Marrow Mesenchymal Stem Cells for Cardiac Repair after Myocardial Infarction. Theranostics 2015, 5, 995. [Google Scholar] [CrossRef]
- Chang, X.; Liu, J.; Liao, X.; Liu, G. Ultrasound-Mediated Microbubble Destruction Enhances the Therapeutic Effect of Intracoronary Transplantation of Bone Marrow Stem Cells on Myocardial Infarction. Int. J. Clin. Exp. Pathol. 2015, 8, 2221. [Google Scholar]
- Vela, D.C.; Silva, G.V.; Assad, J.A.R.; Sousa, A.L.S.; Coulter, S.; Fernandes, M.R.; Perin, E.C.; Willerson, J.T.; Buja, L.M. Histopathological Study of Healing after Allogenic Mesenchymal Stem Cell Delivery in Myocardial Infarction in Dogs. J. Histochem. Cytochem. 2009, 57, 167–176. [Google Scholar] [CrossRef]
- Sherman, W.; Martens, T.P.; Viles-Gonzalez, J.F.; Siminiak, T. Catheter-Based Delivery of Cells to the Heart. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.; Estrada, A.H.; Sosa-Samper, I.; Maisenbacher, H.W.; Lamb, K.E.; Mincey, B.D.; Erger, K.E.; Conlon, T.J. Stem-cell Therapy for Dilated Cardiomyopathy: A Pilot Study Evaluating Retrograde Coronary Venous Delivery. J. Small Anim. Pract. 2013, 54, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Brunskill, S.J.; Hyde, C.J.; Doree, C.J.; Watt, S.M.; Martin-Rendon, E. Route of Delivery and Baseline Left Ventricular Ejection Fraction, Key Factors of Bone-marrow-derived Cell Therapy for Ischaemic Heart Disease. Eur. J. Heart Fail. 2009, 11, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.G.; Paulino-Junior, D.; Pascon, J.P.E.; Pereira-Neto, G.B.; Carareto, R.; Champion, T.; Camacho, A.A. Cardiac Function in Dogs with Chronic Chagas Cardiomyopathy Undergoing Autologous Stem Cell Transplantation into the Coronary Arteries. Can. Vet. J. 2011, 52, 869. [Google Scholar]
- Sheng, C.C.; Zhou, L.; Hao, J. Current Stem Cell Delivery Methods for Myocardial Repair. Biomed. Res. Int. 2013, 2013, 547902. [Google Scholar] [CrossRef]
- Vulliet, P.R.; Greeley, M.; Halloran, S.M.; MacDonald, K.A.; Kittleson, M.D. Intra-Coronary Arterial Injection of Mesenchymal Stromal Cells and Microinfarction in Dogs. Lancet 2004, 363, 783–784. [Google Scholar] [CrossRef]
- Sun, Q.-W.; Zhen, L.; Wang, Q.; Sun, Y.; Yang, J.; Li, Y.-J.; Li, R.-J.; Ma, N.; Li, Z.-A.; Wang, L.-Y. Assessment of Retrograde Coronary Venous Infusion of Mesenchymal Stem Cells Combined with Basic Fibroblast Growth Factor in Canine Myocardial Infarction Using Strain Values Derived from Speckle-Tracking Echocardiography. Ultrasound Med. Biol. 2016, 42, 272–281. [Google Scholar] [CrossRef]
- Gathier, W.A.; van Ginkel, D.J.; van der Naald, M.; van Slochteren, F.J.; Doevendans, P.A.; Chamuleau, S.A.J. Retrograde Coronary Venous Infusion as a Delivery Strategy in Regenerative Cardiac Therapy: An Overview of Preclinical and Clinical Data. J. Cardiovasc. Transl. Res. 2018, 11, 173–181. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Pal, A.; Sharma, G.T. Dog Mesenchymal Stem Cell Basic Research and Potential Applications. In Mesenchymal Stem Cell in Veterinary Sciences; Springer: Singapore, 2020; pp. 213–282. [Google Scholar]
- Youssef, E.A.; Zhang, P.; Rogers, P.I.; Tremble, P.; Rokovich, J.; Johnstone, B.H.; March, K.L.; Hou, D. Enhancing Myocardial Plasmid Expression by Retrograde Coronary Venous Delivery. Catheter. Cardiovasc. Interv. 2005, 65, 528–534. [Google Scholar] [CrossRef]
- Kang, M.-H.; Park, H.-M. Challenges of Stem Cell Therapies in Companion Animal Practice. J. Vet. Sci. 2020, 21, e42. [Google Scholar] [CrossRef]
- Marks, P.; Gottlieb, S. Balancing Safety and Innovation for Cell-Based Regenerative Medicine. N. Engl. J. Med. 2018, 378, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Ahmad, A.; Bansal, A.; Kapoor, T.; Sipp, D.; Rasko, J.E.J. Global Distribution of Businesses Marketing Stem Cell-Based Interventions. Cell Stem Cell 2016, 19, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P. Cell Therapy Trials for Heart Regeneration—Lessons Learned and Future Directions. Nat. Rev. Cardiol. 2018, 15, 659–671. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Biondi-Zoccai, G.G.L.; Abbate, A.; Khianey, R.; Sheiban, I.; Bartunek, J.; Vanderheyden, M.; Kim, H.-S.; Kang, H.-J.; Strauer, B.E. Impact of Intracoronary Cell Therapy on Left Ventricular Function in the Setting of Acute Myocardial Infarction: A Collaborative Systematic Review and Meta-Analysis of Controlled Clinical Trials. J. Am. Coll. Cardiol. 2007, 50, 1761–1767. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Wojakowski, W.; Lemarchand, P.; Lunde, K.; Tendera, M.; Bartunek, J.; Marban, E.; Assmus, B.; Henry, T.D.; Traverse, J.H. Meta-Analysis of Cell-Based CaRdiac StUdiEs (ACCRUE) in Patients with Acute Myocardial Infarction Based on Individual Patient Data. Circ. Res. 2015, 116, 1346–1360. [Google Scholar] [CrossRef]
- Lu, W.; Yaoming, N.; Boli, R.; Jun, C.; Changhai, Z.; Yang, Z.; Zhiyuan, S. MHCN4 Genetically Modified Canine Mesenchymal Stem Cells Provide Biological Pacemaking Function in Complete Dogs with Atrioventricular Block. Pacing Clin. Electrophysiol. 2013, 36, 1138–1149. [Google Scholar] [CrossRef]
- Minguell, J.J.; Florenzano, F.M.; Ramírez, M.R.; Martínez, R.F.; Lasala, G.P. Intracoronary Infusion of a Combination of Bone Marrow-Derived Stem Cells in Dogs. Exp. Clin. Cardiol. 2010, 15, 17. [Google Scholar]
- Lee, W.S.; Suzuki, Y.; Graves, S.S.; Iwata, M.; Venkataraman, G.M.; Mielcarek, M.; Peterson, L.J.; Ikehara, S.; Torok-Storb, B.; Storb, R. Canine Bone Marrow-Derived Mesenchymal Stromal Cells Suppress Alloreactive Lymphocyte Proliferation in Vitro but Fail to Enhance Engraftment in Canine Bone Marrow Transplantation. Biol. Blood Marrow Transplant. 2011, 17, 465–475. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V. Stem Cells for the Treatment of Heart Failure. Curr. Res. Transl. Med. 2016, 64, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Liesveld, J.L.; Sharma, N.; Aljitawi, O.S. Stem Cell Homing: From Physiology to Therapeutics. Stem Cells 2020, 38, 1241–1253. [Google Scholar] [CrossRef]
- Zhao, W.; Phinney, D.G.; Bonnet, D.; Dominici, M.; Krampera, M. Mesenchymal Stem Cell Biodistribution, Migration, and Homing in Vivo. Stem Cells Int. 2014, 2014, 292109. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. Iscience 2019, 15, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Suila, H.; Hirvonen, T.; Kotovuori, A.; Ritamo, I.; Kerkelä, E.; Anderson, H.; Natunen, S.; Tuimala, J.; Laitinen, S.; Nystedt, J. Human Umbilical Cord Blood-derived Mesenchymal Stromal Cells Display a Novel Interaction between P-selectin and Galectin-1. Scand. J. Immunol. 2014, 80, 12–21. [Google Scholar] [CrossRef]
- Bailey, A.M.; Lawrence, M.B.; Shang, H.; Katz, A.J.; Peirce, S.M. Agent-Based Model of Therapeutic Adipose-Derived Stromal Cell Trafficking during Ischemia Predicts Ability to Roll on P-Selectin. PLoS Comput. Biol. 2009, 5, e1000294. [Google Scholar] [CrossRef]
- Chen, M.-S.; Lin, C.-Y.; Chiu, Y.-H.; Chen, C.-P.; Tsai, P.-J.; Wang, H.-S. IL-1β-induced Matrix Metalloprotease-1 Promotes Mesenchymal Stem Cell Migration via PAR1 and G-protein-coupled Signaling Pathway. Stem Cells Int. 2018, 2018, 3524759. [Google Scholar] [CrossRef]
- Yeo, G.C.; Weiss, A.S. Soluble Matrix Protein Is a Potent Modulator of Mesenchymal Stem Cell Performance. Proc. Natl. Acad. Sci. USA 2019, 116, 2042–2051. [Google Scholar] [CrossRef]
- Liu, H.; Xue, W.; Ge, G.; Luo, X.; Li, Y.; Xiang, H.; Ding, X.; Tian, P.; Tian, X. Hypoxic Preconditioning Advances CXCR4 and CXCR7 Expression by Activating HIF-1α in MSCs. Biochem. Biophys. Res. Commun. 2010, 401, 509–515. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Rodriguez, A.-M.; Nakhle, J.; Griessinger, E.; Vignais, M.-L. Intercellular Mitochondria Trafficking Highlighting the Dual Role of Mesenchymal Stem Cells as Both Sensors and Rescuers of Tissue Injury. Cell cycle 2018, 17, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A Brief Review: The Therapeutic Potential of Bone Marrow Mesenchymal Stem Cells in Myocardial Infarction. Stem Cell Res. Ther. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Eitoku, M.; Suganuma, N. Mesenchymal Stem/Stromal Cell Function in Modulating Cell Death. Stem Cell Res. Ther. 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Perez, J.V.D.; Bernardino, M.R.; Damasco, J.A.; Cortes, A.; Del Mundo, H.C.; San Valentin, E.M.D.; Klusman, C.; Canlas, G.M.; Heralde, F.M., III. Bioresorbable Mesenchymal Stem Cell-Loaded Electrospun Polymeric Scaffold Inhibits Neointimal Hyperplasia Following Arteriovenous Fistula Formation in a Rat Model of Chronic Kidney Disease. Adv. Healthc. Mater. 2023, 12, 2300960. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human Mesenchymal Stem Cells Differentiate to a Cardiomyocyte Phenotype in the Adult Murine Heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M. Bone Marrow Cells Regenerate Infarcted Myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef]
- Kajstura, J.; Rota, M.; Whang, B.; Cascapera, S.; Hosoda, T.; Bearzi, C.; Nurzynska, D.; Kasahara, H.; Zias, E.; Bonafé, M. Bone Marrow Cells Differentiate in Cardiac Cell Lineages after Infarction Independently of Cell Fusion. Circ. Res. 2005, 96, 127–137. [Google Scholar] [CrossRef]
- Yoon, Y.; Wecker, A.; Heyd, L.; Park, J.-S.; Tkebuchava, T.; Kusano, K.; Hanley, A.; Scadova, H.; Qin, G.; Cha, D.-H. Clonally Expanded Novel Multipotent Stem Cells from Human Bone Marrow Regenerate Myocardium after Myocardial Infarction. J. Clin. Investig. 2005, 115, 326–338. [Google Scholar] [CrossRef]
- Rota, M.; Kajstura, J.; Hosoda, T.; Bearzi, C.; Vitale, S.; Esposito, G.; Iaffaldano, G.; Padin-Iruegas, M.E.; Gonzalez, A.; Rizzi, R. Bone Marrow Cells Adopt the Cardiomyogenic Fate in Vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 17783–17788. [Google Scholar] [CrossRef]
- Balsam, L.B.; Wagers, A.J.; Christensen, J.L.; Kofidis, T.; Weissman, I.L.; Robbins, R.C. Haematopoietic Stem Cells Adopt Mature Haematopoietic Fates in Ischaemic Myocardium. Nature 2004, 428, 668–673. [Google Scholar] [CrossRef]
- Murry, C.E.; Soonpaa, M.H.; Reinecke, H.; Nakajima, H.; Nakajima, H.O.; Rubart, M.; Pasumarthi, K.B.S.; Ismail Virag, J.; Bartelmez, S.H.; Poppa, V. Haematopoietic Stem Cells Do Not Transdifferentiate into Cardiac Myocytes in Myocardial Infarcts. Nature 2004, 428, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Ageyama, N.; Shibata, H.; Yasu, T.; Misawa, Y.; Takeuchi, K.; Matsui, K.; Yamamoto, K.; Terao, K.; Shimada, K. Repair of Infarcted Myocardium Mediated by Transplanted Bone Marrow–Derived CD34+ Stem Cells in a Nonhuman Primate Model. Stem Cells 2005, 23, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, D.; Estrov, Z.; Raj, S.; Willerson, J.T.; Yeh, E.T.H. Both Cell Fusion and Transdifferentiation Account for the Transformation of Human Peripheral Blood CD34-Positive Cells into Cardiomyocytes in Vivo. Circulation 2004, 110, 3803–3807. [Google Scholar] [CrossRef]
- Bai, X.; Yan, Y.; Song, Y.-H.; Seidensticker, M.; Rabinovich, B.; Metzele, R.; Bankson, J.A.; Vykoukal, D.; Alt, E. Both Cultured and Freshly Isolated Adipose Tissue-Derived Stem Cells Enhance Cardiac Function after Acute Myocardial Infarction. Eur. Heart J. 2010, 31, 489–501. [Google Scholar] [CrossRef]
- Ellison, G.M.; Vicinanza, C.; Smith, A.J.; Aquila, I.; Leone, A.; Waring, C.D.; Henning, B.J.; Stirparo, G.G.; Papait, R.; Scarfò, M. Adult C-Kitpos Cardiac Stem Cells Are Necessary and Sufficient for Functional Cardiac Regeneration and Repair. Cell 2013, 154, 827–842. [Google Scholar] [CrossRef]
- Smits, A.M.; van Laake, L.W.; den Ouden, K.; Schreurs, C.; Szuhai, K.; van Echteld, C.J.; Mummery, C.L.; Doevendans, P.A.; Goumans, M.-J. Human Cardiomyocyte Progenitor Cell Transplantation Preserves Long-Term Function of the Infarcted Mouse Myocardium. Cardiovasc. Res. 2009, 83, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J. Cardiac Progenitor Cells from Adult Myocardium: Homing, Differentiation, and Fusion after Infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef]
- Tang, X.-L.; Li, Q.; Rokosh, G.; Sanganalmath, S.K.; Chen, N.; Ou, Q.; Stowers, H.; Hunt, G.; Bolli, R. Long-Term Outcome of Administration of c-KitPOS Cardiac Progenitor Cells after Acute Myocardial Infarction: Transplanted Cells Do Not Become Cardiomyocytes, but Structural and Functional Improvement and Proliferation of Endogenous Cells Persist for at Lea. Circ. Res. 2016, 118, 1091–1105. [Google Scholar] [CrossRef]
- Kärre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective Rejection of H-2-Deficient Lymphoma Variants Suggests Alternative Immune Defense Strategy. Nature 1986, 319, 675. [Google Scholar] [CrossRef]
- Ljunggren, H.-G.; Kärre, K. In Search of the ‘Missing Self’: MHC Molecules and NK Cell Recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Yokoyama, W.M.; Kim, S. How Do Natural Killer Cells Find Self to Achieve Tolerance? Immunity 2006, 24, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H. Hypoimmunogenic Derivatives of Induced Pluripotent Stem Cells Evade Immune Rejection in Fully Immunocompetent Allogeneic Recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Makabe, K.; Iwasaki, Y.; Fujii, S.; Takahashi, M. Natural Killer Cell Inhibition by HLA-E Molecules on Induced Pluripotent Stem Cell–Derived Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Idelson, M.; Alper, R.; Obolensky, A.; Yachimovich-Cohen, N.; Rachmilewitz, J.; Ejzenberg, A.; Beider, E.; Banin, E.; Reubinoff, B. Immunological Properties of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells. Stem Cell Rep. 2018, 11, 681–695. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The Ins and Outs of MHC Class II-Mediated Antigen Processing and Presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Marino, J.; Paster, J.; Benichou, G. Allorecognition by T Lymphocytes and Allograft Rejection. Front. Immunol. 2016, 7, 582. [Google Scholar] [CrossRef]
- Jurewicz, M.M.; Stern, L.J. Class II MHC Antigen Processing in Immune Tolerance and Inflammation. Immunogenetics 2019, 71, 171–187. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal Stem Cells Avoid Allogeneic Rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef]
- Petrus-Reurer, S.; Winblad, N.; Kumar, P.; Gorchs, L.; Chrobok, M.; Wagner, A.K.; Bartuma, H.; Lardner, E.; Aronsson, M.; Reyes, Á.P. Generation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells Lacking Human Leukocyte Antigen Class I and II. Stem Cell Rep. 2020, 14, 648–662. [Google Scholar] [CrossRef]
- Mattapally, S.; Pawlik, K.M.; Fast, V.G.; Zumaquero, E.; Lund, F.E.; Randall, T.D.; Townes, T.M.; Zhang, J. Human Leukocyte Antigen Class I and II Knockout Human Induced Pluripotent Stem Cell–Derived Cells: Universal Donor for Cell Therapy. J. Am. Heart Assoc. 2018, 7, e010239. [Google Scholar] [CrossRef]
- Petrus-Reurer, S.; Romano, M.; Howlett, S.; Jones, J.L.; Lombardi, G.; Saeb-Parsy, K. Immunological Considerations and Challenges for Regenerative Cellular Therapies. Commun. Biol. 2021, 4, 798. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Monetti, C.; Shutova, M.V.; Neely, E.J.; Hacibekiroglu, S.; Yang, H.; Kim, C.; Zhang, P.; Li, C.; Nagy, K. Linking a Cell-Division Gene and a Suicide Gene to Define and Improve Cell Therapy Safety. Nature 2018, 563, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Prigione, I.; Benvenuto, F.; Bocca, P.; Battistini, L.; Uccelli, A.; Pistoia, V. Reciprocal Interactions between Human Mesenchymal Stem Cells and Γδ T Cells or Invariant Natural Killer T Cells. Stem Cells 2009, 27, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noel, D. IL-6-Dependent PGE2 Secretion by Mesenchymal Stem Cells Inhibits Local Inflammation in Experimental Arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; Madala, S.; Karpati, S. Bone Marrow Stromal Cells Use TGF-β to Suppress Allergic Responses in a Mouse Model of Ragweed-Induced Asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Jitschin, R.; Johansson, C.C.; Okita, R.; Kiessling, R.; Le Blanc, K. The Impact of Inflammatory Licensing on Heme Oxygenase-1–Mediated Induction of Regulatory T Cells by Human Mesenchymal Stem Cells. Blood J. Am. Soc. Hematol. 2011, 117, 4826–4835. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent Mesenchymal Stromal Cells and the Innate Immune System. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef]
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.A.; Willemze, R.; Fibbe, W.E. Donor-Derived Mesenchymal Stem Cells Are Immunogenic in an Allogeneic Host and Stimulate Donor Graft Rejection in a Nonmyeloablative Setting. Blood 2006, 108, 2114–2120. [Google Scholar] [CrossRef]
- Gartner, S.; Kaplan, H.S. Long-Term Culture of Human Bone Marrow Cells. Proc. Natl. Acad. Sci. USA 1980, 77, 4756–4759. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.I.N.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.-J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Umbilical Cord Blood as Sources of Cell Therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef] [PubMed]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different Populations and Sources of Human Mesenchymal Stem Cells (MSC): A Comparison of Adult and Neonatal Tissue-Derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-Y.; Wang, H.-W.; Chang, S.-J.; Liao, K.-H.; Lee, I.-H.; Lin, W.-S.; Wu, C.-H.; Lin, W.-Y.; Cheng, S.-M. Mesenchymal Stem Cells from Human Umbilical Cord Express Preferentially Secreted Factors Related to Neuroprotection, Neurogenesis, and Angiogenesis. PLoS ONE 2013, 8, e72604. [Google Scholar] [CrossRef]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W. Comparative Characteristics of Mesenchymal Stem Cells from Human Bone Marrow, Adipose Tissue, and Umbilical Cord Blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Lee, R.H.; Kim, B.; Choi, I.; Kim, H.; Choi, H.S.; Suh, K.; Bae, Y.C.; Jung, J.S. Characterization and Expression Analysis of Mesenchymal Stem Cells from Human Bone Marrow and Adipose Tissue. Cell. Physiol. Biochem. 2004, 14, 311–324. [Google Scholar] [CrossRef]
- Zou, L.; Luo, Y.; Chen, M.; Wang, G.; Ding, M.; Petersen, C.C.; Kang, R.; Dagnaes-Hansen, F.; Zeng, Y.; Lv, N. A Simple Method for Deriving Functional MSCs and Applied for Osteogenesis in 3D Scaffolds. Sci. Rep. 2013, 3, 2243. [Google Scholar] [CrossRef]
- Kang, R.; Zhou, Y.; Tan, S.; Zhou, G.; Aagaard, L.; Xie, L.; Bünger, C.; Bolund, L.; Luo, Y. Mesenchymal Stem Cells Derived from Human Induced Pluripotent Stem Cells Retain Adequate Osteogenicity and Chondrogenicity but Less Adipogenicity. Stem Cell Res. Ther. 2015, 6, 144. [Google Scholar] [CrossRef]
- Farag, A.; Ngeun, S.K.; Kaneda, M.; Aboubakr, M.; Elhaieg, A.; Hendawy, H.; Tanaka, R. Exploring the Potential Effects of Cryopreservation on the Biological Characteristics and Cardiomyogenic Differentiation of Rat Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 9908. [Google Scholar] [CrossRef]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Tian, L.; Shamirzaei-Jeshvaghani, E.; Dehghani, L.; Ramakrishna, S. Structural Properties of Scaffolds: Crucial Parameters towards Stem Cells Differentiation. World J. Stem Cells 2015, 7, 728. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Mooney, D.J. Regenerative Medicine: Current Therapies and Future Directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Somasekhar, L.; Griffiths, L.G. Current Challenges and Future Promise for Use of Extracellular Matrix Scaffold to Achieve the Whole Organ Tissue Engineering Moonshot. Stem Cells Transl. Med. 2023, 12, 588–602. [Google Scholar] [CrossRef]
- Silberman, E.; Oved, H.; Namestnikov, M.; Shapira, A.; Dvir, T. Post-maturation Reinforcement of 3D-printed Vascularized Cardiac Tissues. Adv. Mater. 2023, 35, 2302229. [Google Scholar] [CrossRef]
- Xu, P.; Kankala, R.K.; Wang, S.; Chen, A. Decellularized Extracellular Matrix-Based Composite Scaffolds for Tissue Engineering and Regenerative Medicine. Regen. Biomater. 2024, 11, rbad107. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Mishra, A.; Bolinas, D.K.M.; Martin, B.M.; Melancon, M.P. Integration of Electrospun Scaffolds and Biological Polymers for Enhancing the Delivery and Efficacy of Mesenchymal Stem/Stromal Cell Therapies. Front. Biosci. 2024, 29, 228. [Google Scholar] [CrossRef]
- Hanna, J.; Markoulaki, S.; Schorderet, P.; Carey, B.W.; Beard, C.; Wernig, M.; Creyghton, M.P.; Steine, E.J.; Cassady, J.P.; Foreman, R. Direct Reprogramming of Terminally Differentiated Mature B Lymphocytes to Pluripotency. Cell 2008, 133, 250–264. [Google Scholar] [CrossRef]
- Okita, K.; Yamanaka, S. Induced Pluripotent Stem Cells: Opportunities and Challenges. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2198–2207. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Addissouky, T.A.; El Sayed, I.E.T.; Ali, M.M.A.; Wang, Y.; El Baz, A.; Elarabany, N.; Khalil, A.A. Shaping the Future of Cardiac Wellness: Exploring Revolutionary Approaches in Disease Management and Prevention. J. Clin. Cardiol. 2024, 5, 6–29. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Yin, L.; Liu, Z. The Roles of Nanoparticles in Stem Cell-Based Therapy for Cardiovascular Disease. Front. Bioeng. Biotechnol. 2020, 8, 947. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Rao, M.P.; MacDonald, N.C.; Khang, D.; Webster, T.J. Improved Endothelial Cell Adhesion and Proliferation on Patterned Titanium Surfaces with Rationally Designed, Micrometer to Nanometer Features. Acta Biomater. 2008, 4, 192–201. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Kamel, M. Advances in Nanomedical Applications: Diagnostic, Therapeutic, Immunization, and Vaccine Production. Environ. Sci. Pollut. Res. 2020, 27, 19200–19213. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, X.; You, B.; Ren, N.; Liu, H. Application of Nanomaterials in Stem Cell-Based Therapeutics for Cardiac Repair and Regeneration. Small 2023, 19, 2206487. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Lemon, G.; Hilborn, J.; Chronakis, I.S.; Fossum, M. Bladder Biomechanics and the Use of Scaffolds for Regenerative Medicine in the Urinary Bladder. Nat. Rev. Urol. 2018, 15, 155–174. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone Regenerative Medicine: Classic Options, Novel Strategies, and Future Directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N. Self-Assembling Human Heart Organoids for the Modeling of Cardiac Development and Congenital Heart Disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef]
- Tan, Y.; Coyle, R.C.; Barrs, R.W.; Silver, S.E.; Li, M.; Richards, D.J.; Lin, Y.; Jiang, Y.; Wang, H.; Menick, D.R. Nanowired Human Cardiac Organoid Transplantation Enables Highly Efficient and Effective Recovery of Infarcted Hearts. Sci. Adv. 2023, 9, eadf2898. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Wang, J.; Yin, J.; Wang, Z.; Pei, R. Cardiac Organoid: Multiple Construction Approaches and Potential Applications. J. Mater. Chem. B 2023, 32, 7567–7581. [Google Scholar] [CrossRef] [PubMed]
- Drakhlis, L.; Biswanath, S.; Farr, C.-M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H. Human Heart-Forming Organoids Recapitulate Early Heart and Foregut Development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Pahlavan, S.; Ansari, H.; Halvaei, M.; Kostin, S.; Feiz, M.-S.; Latifi, H.; Aghdami, N.; Braun, T.; Baharvand, H. Human Cardiomyocytes Undergo Enhanced Maturation in Embryonic Stem Cell-Derived Organoid Transplants. Biomaterials 2019, 192, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Filippo Buono, M.; von Boehmer, L.; Strang, J.; Hoerstrup, S.P.; Emmert, M.Y.; Nugraha, B. Human Cardiac Organoids for Modeling Genetic Cardiomyopathy. Cells 2020, 9, 1733. [Google Scholar] [CrossRef]
- Altyar, A.E.; El-Sayed, A.; Abdeen, A.; Piscopo, M.; Mousa, S.A.; Najda, A.; Abdel-Daim, M.M. Future Regenerative Medicine Developments and Their Therapeutic Applications. Biomed. Pharmacother. 2023, 158, 114131. [Google Scholar] [CrossRef]
- Park, B.-W.; Jung, S.-H.; Das, S.; Lee, S.M.; Park, J.-H.; Kim, H.; Hwang, J.-W.; Lee, S.; Kim, H.-J.; Kim, H.-Y. In Vivo Priming of Human Mesenchymal Stem Cells with Hepatocyte Growth Factor–Engineered Mesenchymal Stem Cells Promotes Therapeutic Potential for Cardiac Repair. Sci. Adv. 2020, 6, eaay6994. [Google Scholar] [CrossRef]
- Guo, X.; Bai, Y.; Zhang, L.; Zhang, B.; Zagidullin, N.; Carvalho, K.; Du, Z.; Cai, B. Cardiomyocyte Differentiation of Mesenchymal Stem Cells from Bone Marrow: New Regulators and Its Implications. Stem Cell Res. Ther. 2018, 9, 44. [Google Scholar] [CrossRef]
- Marotta, P.; Cianflone, E.; Aquila, I.; Vicinanza, C.; Scalise, M.; Marino, F.; Mancuso, T.; Torella, M.; Indolfi, C.; Torella, D. Combining Cell and Gene Therapy to Advance Cardiac Regeneration. Expert Opin. Biol. Ther. 2018, 18, 409–423. [Google Scholar] [CrossRef]
- Williams, A.R.; Hatzistergos, K.E.; Addicott, B.; McCall, F.; Carvalho, D.; Suncion, V.; Morales, A.R.; Da Silva, J.; Sussman, M.A.; Heldman, A.W. Enhanced Effect of Combining Human Cardiac Stem Cells and Bone Marrow Mesenchymal Stem Cells to Reduce Infarct Size and to Restore Cardiac Function after Myocardial Infarction. Circulation 2013, 127, 213–223. [Google Scholar] [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]

| Type of Stem Cells | Accessibility | Clinical and Preclinical Efficacy | Safety | Mechanism of Action |
|---|---|---|---|---|
| Bone Marrow-Derived Stem Cells (BM-MSCs) | Harvested from bone marrow, invasive procedure. | Demonstrated improvement in cardiac function, reduced infarct size, and increased vascularization in ischemic heart disease [60,94]. | No major safety concerns were reported. | Differentiation into cardiomyocytes, smooth muscle, endothelial cells; paracrine signaling [44,96]. |
| Adipose-Derived Stem Cells (ASCs) | Easily harvested from subcutaneous adipose tissue. | Improved cardiac function, reduced fibrosis, and enhanced angiogenesis in preclinical models [70,71,72,74]. | No evidence of tumorigenicity or arrhythmogenic risk in preclinical/clinical testing [73,75]. | Differentiation into endothelial and smooth muscle cells, paracrine effects promote vascularization and tissue survival [73,75]. |
| Cardiosphere-Derived Cells (CDCs) | Requires access to adult cardiac tissue; autologous use possible. | Improved cardiac function in dilated cardiomyopathy (DCM); mixed results in long-term survival [97]. | Generally safe, no signs of immune rejection, though more studies are needed [97]. | Stimulate endogenous repair through paracrine signaling and promote neovascularization [97]. |
| Wharton Jelly-Derived MSCs (WJ-MSCs) | Easily harvested from umbilical cord tissue, non-invasive. | Effective in immunomodulation but limited efficacy in improving heart function in CHF models [32]. | Safe administration with no significant side effects, though therapeutic benefits are modest [32]. | Paracrine signaling, angiogenesis, homing to damaged tissues; low differentiation into cardiomyocytes [79,80,81]. |
| Human MSCs (hMSCs) | Generally harvested from bone marrow or adipose tissue. | Effective in biological pacemaking and improving cardiac function; beneficial in chronic ischemia models [98,99]. | No cellular or humoral rejection was observed, and catecholamine responsiveness was confirmed in pacemaking studies [98]. | Differentiation into cardiac-like cells, and paracrine effects; also effective in gene-modified therapies [98,100]. |
| Deciduous Teeth Stem Cells (pDSCs) | Harvested from puppy deciduous teeth, limited availability. | Significant improvement in cardiac function and quality of life in dogs with chronic valvular heart disease [101]. | No significant adverse effects were observed in clinical studies [101]. | Paracrine signaling promotes cardiac function; and minimal direct cardiac differentiation [101]. |
| Cardiac Stem Cells (CSCs) | Obtained from cardiac tissue; requires a cardiac biopsy, making it a more invasive procedure. | Shows potential in enhancing myocardial regeneration and improving cardiac function through differentiation into cardiomyocytes and promoting angiogenesis; results vary across studies [87,91]. | Generally safe with low immunogenicity, though long-term effects and potential for arrhythmias require further investigation [87]. | Differentiates into cardiomyocytes and vascular cells; releases paracrine factors that stimulate resident cardiac cells, angiogenesis, and tissue repair [87,90,91]. |
| Condition | Type of Cells | Number of Animals | Dose of Cells | Route of Administration | Frequency of Treatments | Evaluations | Results |
|---|---|---|---|---|---|---|---|
| Ischemic heart disease [60] | Bone marrow-derived stem cells (BM-MSCs) | 12 | 100 × 106 MSCs/10 mL saline | Intramyocardial injections | Single dose | Resting and stress 2D echocardiography | Increased vascularity, and improved cardiac function through smooth muscle and endothelial cell differentiation. |
| Chronic myocardial infarction [94] | Bone marrow mononuclear cells (BMNCs) | 24 | 227 ± 32 × 106 MSCs and 232 ± 40 × 106 BMNCs | Intramyocardial injections | Single dose | Echocardiographic analysis | Improved cardiac contractility, regional systolic function, reduced infarct size, and increased angiogenesis. |
| Chronic Chagas cardiomyopathy [109] | Autologous BM-MSCs | 5 | 100 × 106 MSCs | Intracoronary injection | Single dose | Electrocardiography, echocardiography | Significant improvement in peak velocity of aortic flow. |
| Chronic valvular heart disease [101] | Deciduous teeth stem cells (pDSCs) | 20 client-owned | 1 × 106 cells of pDSC/kg B.W. | Intravenous injections | Two injections on day 0 and day 14 following the initial pDSCs administration. | ECG, echocardiography, radiography, blood pressure | Improved LVEF and quality of life scores; the control group showed no significant improvement. |
| Non-ischemic dilated cardiomyopathy (DCM) [97] | Cardiosphere-derived cells (CDCs) | 8 with spontaneous DCM | 30 million allogeneic CDCs | Via coronary vessels | Single dose | Echocardiography, histology (12-month follow-up) | No adverse events, no immune rejection, no significant survival advantage. |
| Dilated cardiomyopathy [107] | Adipose-derived MSCs (ASCs) transduced with stromal-derived factor-1 | 15 | 1 × 107 cells suspended in 20 mL PBS | Retrograde coronary sinus infusion | Single dose | ECG, echocardiography, Holter monitoring (2-year follow-up) | The procedure was safe; no survival advantage over existing treatments; one dog developed ventricular fibrillation and died. |
| CHF secondary to MMVD [32] | Wharton jelly-derived MSCs (WJ-MSCs) | 10 | 2 × 106 cells/kg IV | Intravenous injections | Three injections administered three weeks apart. | Echocardiography, ECG, cardiac biomarkers | Lymphocyte and eosinophil counts decreased, with no significant improvement in echocardiography or survival. |
| Myocardial infarction [105] | Allogeneic MSCs | 7 | 100 × 106 DAPI-labeled MSCs | Transendocardial (TE) or intracoronary (IC). | Single dose | Histopathology after 21 days | MSCs reduced necrosis, increased extracellular matrix deposition; no cardiac differentiation. |
| AV block [123] | mHCN4-modified canine MSCs | - | 3.0 to 3.5 × 106 viable cells | Subepicardial injection into left ventricular anterior wall | Single dose | Heart rate variability (HRV), cardiac parameters (6 weeks), histology, Western blot | Improved maximum heart rate and impulse generation at the injection site, stable heart rate by Week 4, increased HRV during exercise, survival of modified cMSCs, HCN4 expression confirmed in heart tissues. |
| Coronary ischemia [124] | Endothelial progenitor cells (EPCs), BM-MSCs | 9 | 35 × 106 cells/kg heart weight | Intracoronary infusion | Single dose | Electrocardiogram, cardiac enzyme tests, echocardiography, histopathology | Safe and feasible procedure with no significant changes in vital signs, ECG, cardiac function, or heart histology |
| Radiofrequency ablation [100] | Human adipose-derived stem cells (h-ASCs) | 14 | 1 × 107 cells | Intravenous injections | Single dose | Prussian blue staining, immunohistochemistry, flow cytometry | h-ASCs homed to injured atrial tissue, expressed cardiomyocyte-like markers (α-actinin, troponin-I, connexin 43, VEGFR-2); no immunorejection or neoplasm-like cells; h-ASCs also detected in lungs and spleen. |
| Complete heart block [98] | Human MSCs (hMSCs) | - | ≥700,000 hMSCs | subepicardial at 3 closely apposed sites in the left ventricular anterior wall | Single dose | Pacemaker function, catecholamine responsiveness, histology, rejection studies | Stable pacemaker function for 6 weeks, catecholamine-responsive rhythms, no cellular or humoral rejection, doses >700,000 hMSCs sufficient for stable impulse generation. |
| Right ventricular defect [99] | Spheroid-derived human mesenchymal stem cells (hMSCs) | - | 250,000 hMSCs were used to form each spheroid. 2 million hMSCs were used for comparison in the experiment | The cells were placed on a scaffold (ECM) and implanted into the heart tissue | Cells were cultured for 3 days to form spheroids and then for 7–10 days before implantation | Calcium channel analysis, cardiac protein expression, mechanical function evaluation, histology | hMSC spheroids expressed cardiac-specific proteins (α-actinin, cardiotin, ANP), showed functional calcium currents, and improved regional mechanical function compared to unmanipulated hMSCs. |
| Chronic myocardial infarction [90] | Autologous c-kit–positive cardiac stem cells (CSCs) | 19 | 1 × 106 cells/mL concentration. 0.8 mL of the cell suspension | Intramyocardial injection | Single dose | Cardiac MRI at 6 and 30 weeks post-infarct, LV volumes, ejection fraction | Attenuation of adverse left ventricular remodeling, reduced increase in end-systolic volume, and preservation of left ventricular ejection fraction over 30 weeks. |
| Category | Current Trends | Challenges | Future Directions |
|---|---|---|---|
| Cell Sources | Increasing use of MSCs from bone marrow, adipose tissue, and Wharton’s jelly [175,176,177]. | Limited differentiation into cardiac cells; ethical concerns with some cell types [176]. | Explore genetically modified stem cells, induced pluripotent stem cells (iPSCs), and engineered cells for enhanced regeneration [166,186]. |
| Delivery Methods | Retrograde coronary venous delivery, intracoronary injections, and intramyocardial injections are common [112,113] | Risks of cell embolization, inefficient homing, and invasiveness of procedures [110,124]. | Development of less invasive delivery systems such as targeted nanoparticles or scaffold-based cell delivery [202]. |
| Mechanism of Action | Emphasis on paracrine signaling for angiogenesis, anti-fibrosis, and immune modulation [96,142]. | Limited direct differentiation into functional cardiomyocytes; poor long-term engraftment [154]. | Enhance cell survival and efficacy by preconditioning, and genetic engineering to increase paracrine factor secretion (e.g., exosomes, growth factors) [197,199]. |
| Preclinical/Clinical Efficacy | Positive results in improving cardiac function in myocardial infarction and heart failure models [128]. | Heterogeneous responses across species, lack of long-term data, and modest improvements in survival [125]. | Conduct large-scale, standardized trials across species and explore combination therapies (stem cells + drugs/growth factors) [121]. |
| Stem Cell Tracking and Retention | Imaging techniques like MRI and SPECT are used to track cells post-delivery [137,183]. | Poor retention of stem cells at the target site; rapid clearance or apoptosis post-injection [139]. | Investigate scaffolds, hydrogels, and biomaterials to enhance cell retention and integration with host tissue [189,191]. |
| Combination Therapies | Combining MSCs with growth factors, gene therapy, or cardiac stem cells [212,213,214,215]. | Lack of optimized combinations, and unclear mechanisms of synergistic effects [117]. | Further explore synergies between stem cells and adjunctive therapies (e.g., CRISPR gene editing, biomaterials) [126,198]. |
| Ethical and Regulatory Concerns | Autologous stem cell therapies reduce ethical concerns, and allogeneic therapies gaining traction [119]. | Regulatory hurdles for new therapies, long and expensive approval processes, and lack of standardization [120]. | Develop global regulatory frameworks and ethical guidelines for stem cell therapies in veterinary medicine [122]. |
| Technology Integration | Advances in tissue engineering (3D scaffolds, bio-printed tissues) and nanotechnology [203,204]. | The complexity of integrating technologies into routine clinical practice; high costs [204]. | Leverage bioengineering advances to create “off-the-shelf” stem cell products for cardiac repair [209]. |
| Patient Selection and Stratification | Identifying specific subgroups (e.g., age, disease stage) that benefit most from stem cell therapies [216]. | Variability in disease progression, and patient-specific responses to treatment [99]. | Personalize stem cell therapies based on genetic, molecular, and disease profiles for optimized outcomes [180]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, A.; Hendawy, H.; Emam, M.H.; Hasegawa, M.; Mandour, A.S.; Tanaka, R. Stem Cell Therapies in Canine Cardiology: Comparative Efficacy, Emerging Trends, and Clinical Integration. Biomolecules 2025, 15, 371. https://doi.org/10.3390/biom15030371
Farag A, Hendawy H, Emam MH, Hasegawa M, Mandour AS, Tanaka R. Stem Cell Therapies in Canine Cardiology: Comparative Efficacy, Emerging Trends, and Clinical Integration. Biomolecules. 2025; 15(3):371. https://doi.org/10.3390/biom15030371
Chicago/Turabian StyleFarag, Ahmed, Hanan Hendawy, Mahmoud H. Emam, Mizuki Hasegawa, Ahmed S. Mandour, and Ryou Tanaka. 2025. "Stem Cell Therapies in Canine Cardiology: Comparative Efficacy, Emerging Trends, and Clinical Integration" Biomolecules 15, no. 3: 371. https://doi.org/10.3390/biom15030371
APA StyleFarag, A., Hendawy, H., Emam, M. H., Hasegawa, M., Mandour, A. S., & Tanaka, R. (2025). Stem Cell Therapies in Canine Cardiology: Comparative Efficacy, Emerging Trends, and Clinical Integration. Biomolecules, 15(3), 371. https://doi.org/10.3390/biom15030371












