Deep Thought on the HIV Cured Cases: Where Have We Been and What Lies Ahead?
Abstract
:1. Introduction
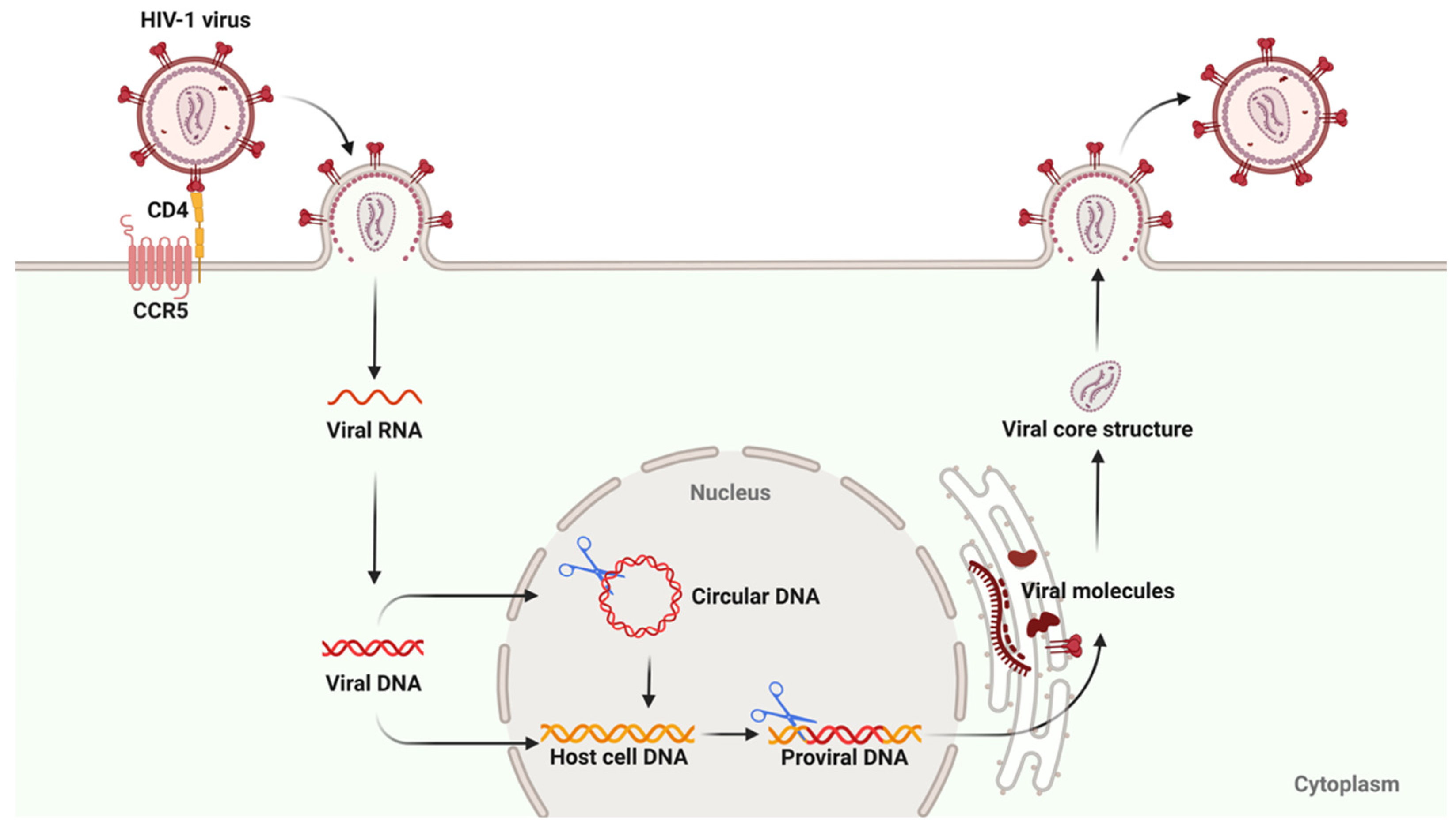
2. The HIV Reservoir
2.1. Formation Mechanism
2.2. Reservoir Duration
2.3. Immune Characteristics
3. The Definitions of HIV Cure
4. Cases of Sterilizing HIV Cured by Allo-HSCT
4.1. HIV Cure Cases
4.1.1. Berlin Patient
4.1.2. London Patient
4.1.3. Dusseldorf Patient
4.1.4. New York Patient
4.1.5. City of Hope Patient
4.1.6. Geneva Patient
4.1.7. The Second Belin Patient
4.1.8. The Eighth Cured Case
| Items | Berlin Patient | London Patient | Düsseldorf Patient | New York Patient | City of Hope Patient | Geneva Patient | The second Berlin Patient | The Eighth Cured Case |
|---|---|---|---|---|---|---|---|---|
| Basic information | ||||||||
| Gender | Male | Male | Male | Female | Male | Male | Male | Female |
| Age at transplantation | 40 | 36–37 | 43 | 59 | 63 | 47 | 51 | 50s |
| Pre-transplant HIV infection status | ||||||||
| Duration of HIV infection (Y) | >12 | >13 | >4 | >4 | >31 | >28 | >6 | >21 |
| Duration of ART (Y) | 4 | NM | 2 | >4 | ~21 | ~28 | NM | NM |
| Cancers information | ||||||||
| Type | AML | HL | AML | AML | AML | Myeloid sarcoma | AML | AML |
| Treatment | Induction CT + consolidation CT | First-line CT + salvage regimens | Induction CT + consolidation CT | Induction CT + consolidation CT | Salvage regimens | Induction CT/ hypomethylating agent | NM | NM |
| Allo-HSCT information | ||||||||
| HSC sources | PBSCs | PBSCs | PBSCs | UC/PBSCs | PBSCs | PBSCs | Bone marrow | NM |
| HLA Matching | 10/10 | 9/10 | 10/10 | 5/8 UC + 4/8 PBSCs | 11/12 | 9/10 | NM | 9/10 |
| CCR5 genotype—donor | Δ32/Δ32 | Δ32/Δ32 | Δ32/Δ32 | Δ32/Δ32 | Δ32/Δ32 | WT/WT | Δ32/WT | Δ32/Δ32 |
| CCR5 genotype—recipient | Δ32/WT | WT/WT | Δ32/WT | WT/WT | WT/WT | WT/WT | Δ32/WT | NM |
| Dose of stem cells (per kg body weight) | 2.3 × 106 (First) 2.1 × 106 (Second) | 3.6 × 106 | 8.74 × 106 | 2 × 107 CB cells + 2.2 × 105 PBSCs | NM | NM | NM | NM |
| HSC transplant times | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Post-transplant donor fusion time (D) | 13 | 30 | 34 | ~98 | 30 | <30 | NM | NM |
| Preconditioning for allo-HSCT | ||||||||
| Chemotherapy | High intensity | Reduced intensity | Reduced intensity | High intensity | Reduced intensity | High intensity | NM | Yes |
| Irradiation | Yes (200 cGy) | No | No | Yes (4 Gray) | No | Yes (8 Gray) | NM | Yes (200 cGy) |
| GVDH information | ||||||||
| Type and degree | Skin, grade I | Gastric/duodenal/ colonic biopsies, grade I | Eye, mild | No | Mild | Liver, acute/ skin, Mild/nervous system, chronic | NM | Skin, acute |
| Preventive medications | Rabbit anti-thymocyte globulin/cyclosporine/ mycophenolate mofetil | Anti-CD52/ cyclosporine/ methotrexate | Cyclosporine/ mycophenolate mofetil/ tacrolimus | Mycophenolate mofetil/ tacrolimus | Sirolimus/ tacrolimus prophylaxis | Cyclophosphamide/ Tacrolimus/ mycophenolate | NM | cyclosporin A/ mycophenolate mofetil/ cyclophosphamide |
| Treatment | Cyclosporine | No | NM | No | NM | Various drugs | NM | NM |
| ART interruption information | ||||||||
| Time of ATI | 1 day before transplantation | 16 months after transplantation | 69 months after transplantation | 37 months after transplantation | 51 months after transplantation | 40 months after transplantation | 3 years after transplantation | 39 months after transplantation |
| Length of HIV remission (ATI initiated) | 12 years (2008) | 7 years (2017) | 6 years (2018) | 4 years (2020) | 3 years (2021) | 3 years (2021) | 5 years (2019) | 1 years (2023) |
| References | [10] | [11] | [12] | [13] | [14] | [15] | [16] | [86] |
4.2. Potential Factors for Sterlizing Cure
4.2.1. Hematopoietic Stem Cells with CCR5 Δ32/Δ32 Mutation
4.2.2. Allogeneic Immunity
4.2.3. Pre-Transplant Conditioning
4.2.4. ART
5. Possible Future Strategies for Functional Cures of HIV
5.1. Transcriptional Regulation of Latent Viruses
5.1.1. Shock and Kill
5.1.2. Block and Lock
5.2. Gene Editing
5.3. Immunotherapy
5.3.1. Vaccines
5.3.2. Neutralizing Antibodies
5.3.3. Nanobodies
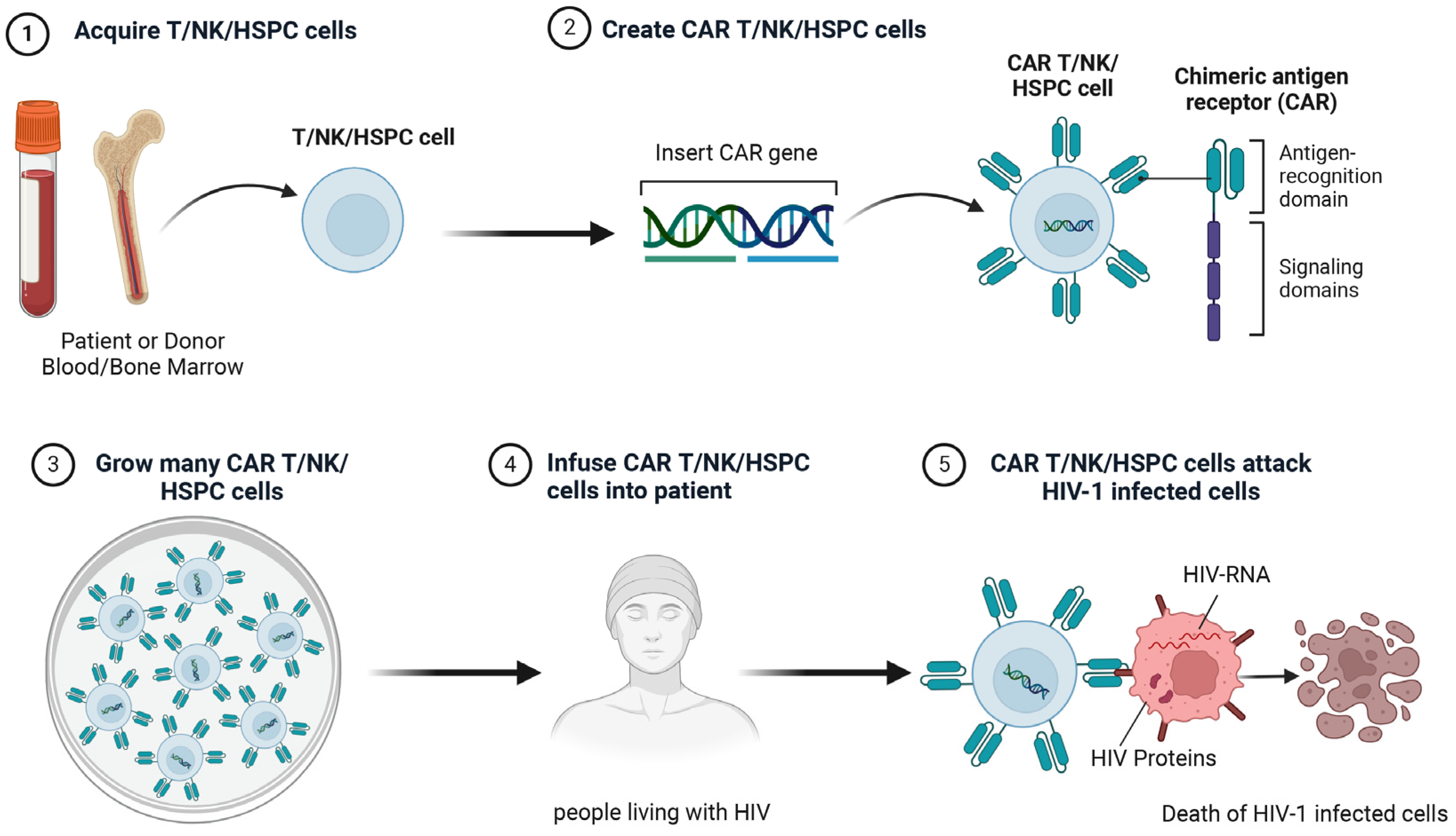
5.4. Chimeric Antigen Receptor Therapy
5.4.1. CAR-T
5.4.2. CAR-NK
5.4.3. CAR-Hematopoietic Stem and Progenitor Cells (CAR-HSPCs)
6. Clinical Research of Cell Therapy Towards HIV Cure
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, R.; Li, L.; Hu, W.; Zhuang, K.; Zhang, E.; Yan, Y.; Feng, L.; Chen, X.; Cao, Q.; Ke, H.; et al. Characteristics of refined lymphocyte subsets changes in people living with hiv/aids during antiretroviral therapy period: An observation from wuhan, china. Front. Immunol. 2023, 14, 1089379. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Zhang, M.; Tram, K.H.; Walters, M.K.; Jahagirdar, D.; Brewer, E.D.; Novotney, A.; Lasher, D.; Mpolya, E.A.; Vongpradith, A.; et al. Global, regional, and national burden of hiv/aids, 1990–2021, and forecasts to 2050, for 204 countries and territories: The global burden of disease study 2021. Lancet HIV 2024, 11, e807–e822. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.V.; Steven, G.D. Antiretroviral therapy and management of hiv infection. Lancet 2010, 376, 49–62. [Google Scholar]
- Smiley, S.T.; Singh, A.; Read, S.W.; Sharma, O.K.; Finzi, D.; Lane, C.; Rice, J.S. Progress toward curing hiv infections with hematopoietic stem cell transplantation. Clin. Infect. Dis. 2014, 60, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Nomah, D.K.; Jamarkattel, S.; Bruguera, A.; Moreno - Fornés, S.; Díaz, Y.; Alonso, L.; Aceitón, J.; Llibre, J.M.; Domingo, P.; Saumoy, M.; et al. Evolving aids- and non-aids mortality and predictors in the piscis cohort of people living with hiv in catalonia and the balearic islands (Spain), 1998–2020. Open Forum Infect. Dis. 2024, 11, ofae132. [Google Scholar] [CrossRef] [PubMed]
- Armani - Tourret, M.; Bone, B.; Tan, T.S.; Sun, W.; Bellefroid, M.; Struyve, T.; Louella, M.; Yu, X.G.; Lichterfeld, M. Immune targeting of hiv-1 reservoir cells: A path to elimination strategies and cure. Nat. Rev. Microbiol. 2024, 22, 328–344. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Bittar, C.; Silva Santos, G.S.; Oliveira, T.Y.; Huang, A.S.; Linden, N.; Ferreira, I.A.T.M.; Murdza, T.; Muecksch, F.; Jones, R.B.; et al. Transcription of hiv-1 at sites of intact latent provirus integration. J. Exp. Med. 2024, 221, e20240391. [Google Scholar] [CrossRef]
- Ikeogu, N.; Ajibola, O.; Zayats, R.; Murooka, T.T. Identifying physiological tissue niches that support the HIV reservoir in T cells. Mbio 2023, 14, e02053-23. [Google Scholar] [CrossRef]
- Kuritzkes, D.R. Hematopoietic stem cell transplantation for hiv cure. J. Clin. Investig. 2016, 126, 432–437. [Google Scholar] [CrossRef]
- Hütter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müssig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O.; et al. Long-term control of HIV byccr5 delta32/delta32 stem-cell transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.J.; Lee, H.; et al. HIV-1 remission following ccr5δ32/δ32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Jensen, B.O.; Knops, E.; Cords, L.; Lubke, N.; Salgado, M.; Busman-Sahay, K.; Estes, J.D.; Huyveneers, L.E.P.; Perdomo-Celis, F.; Wittner, M.; et al. In-depth virological and immunological characterization of HIV-1 cure after ccr5delta32/delta32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 2023, 29, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Van Besien, K.; Glesby, M.J.; Pahwa, S.; Coletti, A.; Warshaw, M.G.; Petz, L.; Moore, T.B.; Chen, Y.H.; Pallikkuth, S.; et al. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 2023, 186, 1115–1126. [Google Scholar] [CrossRef]
- Dickter, J.K.; Aribi, A.; Cardoso, A.A.; Gianella, S.; Gendzekhadze, K.; Li, S.; Feng, Y.; Chaillon, A.; Laird, G.M.; Browning, D.L.; et al. HIV-1 remission after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 2024, 390, 669. [Google Scholar] [CrossRef]
- Sáez-Cirión, A.; Mamez, A.-C.; Avettand-Fenoel, V.; Nabergoj, M.; Passaes, C.; Thoueille, P.; Decosterd, L.; Hentzien, M.; Perdomo-Celis, F.; Salgado, M.; et al. Sustained hiv remission after allogeneic hematopoietic stem cell transplantation with wild-type ccr5 donor cells. Nat. Med. 2024, 30, 3544–3554. [Google Scholar] [CrossRef]
- Mallapaty, S. Seventh patient ‘cured’ of HIV: Why scientists are excited. Nature 2024, 632, 235–236. [Google Scholar] [CrossRef]
- Cleary, M.; Ndhlovu, L.C.; Sacha, J.B. Stem cell transplantation and allogeneic immunity: Post treatment control or HIV cure? Curr. Opin. Hiv Aids 2025, 20, 86. [Google Scholar] [CrossRef]
- Ambinder, R.F.; Capoferri, A.A.; Durand, C.M. Haemopoietic cell transplantation in patients living with HIV. Lancet HIV 2020, 7, e652–e660. [Google Scholar] [CrossRef]
- Payra, S.; Manjhi, P.K.; Singh, S.; Kumar, R.; Singh, S.K.; Kumar, A.; Maharshi, V. HIV cure: Are we going to make history? HIV Med. 2024, 25, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Litzow, M.R.; Cummins, N.W.; Rizza, S.A.; Badley, A.D.; Navarro, W.; Hashmi, S.K. Risks and outcomes of allogeneic hematopoietic stem cell transplantation for hematologic malignancies in patients with HIV infection. Biol. Blood Marrow Transplant. 2019, 25, e260–e267. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.; Liu, Y.; Xie, L.; Su, B.; Mou, D.; Wang, L.; Liu, T.; Wang, X.; Zhang, B.; et al. Crispr-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N. Engl. J. Med. 2019, 381, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Landovitz, R.J.; Scott, H.; Deeks, S.G. Prevention, treatment and cure of HIV infection. Nat. Rev. Microbiol. 2023, 21, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, R.; Bhiman, J.N.; Burton, D.R. Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr. Opin. Immunol. 2018, 53, 143–151. [Google Scholar] [CrossRef]
- Mao, Y.; Liao, Q.; Zhu, Y.; Bi, M.; Zou, J.; Zheng, N.; Zhu, L.; Zhao, C.; Liu, Q.; Liu, L.; et al. Efficacy and safety of novel multifunctional m10 car-t cells in HIV-1-infected patients: A phase i, multicenter, single-arm, open-label study. Cell Discov. 2024, 10, 49. [Google Scholar] [CrossRef]
- Dudek, A.M.; Feist, W.N.; Sasu, E.J.; Luna, S.E.; Ben-Efraim, K.; Bak, R.O.; Cepika, A.-M.; Porteus, M.H. A simultaneous knockout knockin genome editing strategy in hspcs potently inhibits ccr5- and cxcr4-tropic HIV-1 infection. Cell Stem Cell. 2024, 31, 499–518. [Google Scholar] [CrossRef]
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.M.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an inducible hiv-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13193–13197. [Google Scholar] [CrossRef]
- Ollerton, M.T.; Berger, E.A.; Connick, E.; Burton, G.F. Hiv-1-specific chimeric antigen receptor T cells fail to recognize and eliminate the follicular dendritic cell HIV reservoir in vitro. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Vellas, C.; Martres, D.; Requena, M.; Nayrac, M.; Collercandy, N.; Latour, J.; Barange, K.; Alric, L.; Martin-Blondel, G.; Izopet, J.; et al. Compartmentalized hiv-1 reservoir in intestinal monocytes/macrophages on antiretroviral therapy. J. Infect. Dis. 2024, jiae557. [Google Scholar] [CrossRef]
- Kincer, L.P.; Joseph, S.B.; Gilleece, M.M.; Hauser, B.M.; Sizemore, S.; Zhou, S.; Di Germanio, C.; Zetterberg, H.; Fuchs, D.; Deeks, S.G.; et al. Rebound HIV-1 in cerebrospinal fluid after antiviral therapy interruption is mainly clonally amplified r5 t cell-tropic virus. Nat. Microbiol. 2023, 8, 260–271. [Google Scholar] [CrossRef]
- Avettand-Fènoël, V.; Hocqueloux, L.; Ghosn, J.; Cheret, A.; Frange, P.; Melard, A.; Viard, J.-P.; Rouzioux, C. Total HIV-1 dna, a marker of viral reservoir dynamics with clinical implications. Clin. Microbiol. Rev. 2016, 29, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Bacchus-Souffan, C.; Fitch, M.; Symons, J.; Abdel-Mohsen, M.; Reeves, D.B.; Hoh, R.; Stone, M.; Hiatt, J.; Kim, P.; Chopra, A.; et al. Relationship between cd4 t cell turnover, cellular differentiation and HIV persistence during art. PLoS Pathog. 2021, 17, e1009214. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Q.; Khadka, P.; Gowanlock, S.N.; Copertino, D.C.; Duncan, M.C.; Omondi, F.H.; Kinloch, N.N.; Kasule, J.; Kityamuweesi, T.; Buule, P.; et al. HIV-1 subtype a1, d, and recombinant proviral genome landscapes during long-term suppressive therapy. Nat. Commun. 2024, 15, 5480. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. In vivo dynamics of the latent reservoir for hiv-1: New insights and implications for cure. Annu. Rev. Pathol. 2021, 17, 271–294. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. A long-term latent reservoir for HIV-1: Discovery and clinical implications. J. Antimicrob. Chemother. 2004, 54, 6–9. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory t-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Wietgrefe, S.W.; Anderson, J.; Duan, L.; Southern, P.J.; Zuck, P.; Wu, G.; Howell, B.J.; Reilly, C.; Kroon, E.; Chottanapund, S.; et al. Initial productive and latent HIV infections originate in vivo by infection of resting T cells. J. Clin. Investig. 2023, 133, e171501. [Google Scholar] [CrossRef]
- Herrmann, C.H.; Carroll, R.G.; Wei, P.; Jones, K.A.; Rice, A.P. Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J. Virol. 1998, 72, 9881–9888. [Google Scholar] [CrossRef]
- Peterson, J.J.; Lewis, C.A.; Burgos, S.D.; Manickam, A.; Xu, Y.; Rowley, A.A.; Clutton, G.; Richardson, B.; Zou, F.; Simon, J.M.; et al. A histone deacetylase network regulates epigenetic reprogramming and viral silencing in HIV-infected cells. Cell Chem. Biol. 2023, 30, 1617–1633. [Google Scholar] [CrossRef]
- Jansen, J.; Kroeze, S.; Man, S.; Andreini, M.; Bakker, J.-W.; Zamperini, C.; Tarditi, A.; Kootstra, N.A.; Geijtenbeek, T.B.H. Noncanonical-nf-κb activation and ddx3 inhibition reduces the HIV-1 reservoir by elimination of latently infected cells ex-vivo. Microbiol. Spectr. 2023, 12, e03180-23. [Google Scholar] [CrossRef]
- Shiau, S.; Abrams, E.J.; Arpadi, S.M.; Kuhn, L. Early antiretroviral therapy in HIV-infected infants: Can it lead to HIV remission? Lancet HIV 2018, 5, e250–e258. [Google Scholar] [CrossRef]
- Ananworanich, J. What will it take to cure hiv? Top. Antivir. Med. 2015, 23, 80. [Google Scholar] [PubMed]
- Whitney, J.B.; Hill, A.; Sanisetty, S.; Penaloza-Macmaster, P.; Liu, J.; Shetty, M.; Parenteau, L.; Cabral, C.; Shields, J.; Blackmore, S.; et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014, 512, 74–77. [Google Scholar] [CrossRef]
- Whitney, J.B.; Lim, S.-Y.; Osuna, C.E.; Kublin, J.L.; Chen, E.; Yoon, G.; Liu, P.-T.; Abbink, P.; Borducci, E.N.; Hill, A.; et al. Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat. Commun. 2018, 9, 5429. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Cirión, A.; Bacchus, C.; Hocqueloux, L.; Avettand-Fenoel, V.; Girault, I.; Lecuroux, C.; Potard, V.; Versmisse, P.; Melard, A.; Prazuck, T.; et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy anrs visconti study. PLoS Pathog. 2013, 9, e1003211. [Google Scholar] [CrossRef] [PubMed]
- Leyre, L.; Kroon, E.; Vandergeeten, C.; Sacdalan, C.; Colby, D.J.; Buranapraditkun, S.; Schuetz, A.; Chomchey, N.; De Souza, M.; Bakeman, W.; et al. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon art initiation during acute hiv infection. Sci. Transl. Med. 2020, 12, eaav3491. [Google Scholar] [CrossRef]
- Crooks, A.M.; Bateson, R.; Cope, A.B.; Dahl, N.P.; Griggs, M.K.; Kuruc, J.D.; Gay, C.L.; Eron, J.J.; Margolis, D.M.; Bosch, R.J.; et al. Precise quantitation of the latent HIV-1 reservoir: Implications for eradication strategies. J. Infect. Dis. 2015, 212, 1361–1365. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Kajdas, J.; Finzi, D.; Quinn, T.C.; Chadwick, K.; Margolick, J.B.; Kovacs, C.; Gange, S.J.; Siliciano, R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003, 9, 727–728. [Google Scholar] [CrossRef]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef]
- Zaman, F.; Smith, M.L.; Balagopal, A.; Durand, C.M.; Redd, A.D.; Tobian, A.A.R. Spatial technologies to evaluate the HIV-1 reservoir and its microenvironment in the lymph node. Mbio 2024, 15, e01909-24. [Google Scholar] [CrossRef]
- Devanathan, A.S.; Pirone, J.R.; Akkina, R.; Remling-Mulder, L.; Luciw, P.; Adamson, L.; Garcia, J.V.; Kovarova, M.; White, N.R.; Schauer, A.P.; et al. Antiretroviral penetration across three preclinical animal models and humans in eight putative HIV viral reservoirs. Antimicrob. Agents Chemother. 2019, 64, 10–1128. [Google Scholar] [CrossRef]
- Pardons, M.; Baxter, A.E.; Massanella, M.; Pagliuzza, A.; Fromentin, R.; Dufour, C.; Leyre, L.; Routy, J.-P.; Kaufmann, D.E.; Chomont, N. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 2019, 15, e1007619. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.A.; Zerbato, J.M.; Rhodes, A.; Tumpach, C.; Dantanarayana, A.; Mcmahon, J.H.; Lau, J.S.Y.; Chang, J.J.; Gubser, C.; Brown, W.; et al. Memory CD4+ T cells that co-express pd1 and ctla4 have reduced response to activating stimuli facilitating HIV latency. Cell Rep. Med. 2022, 3, 100766. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne Naidoo, R.-B.; Steel, H.C.; Theron, A.J.; Anderson, R.; Tintinger, G.R.; Rossouw, T.M. Persistently elevated expression of systemic, soluble co-inhibitory immune checkpoint molecules in people living with HIV before and one year after antiretroviral therapy. Pathogens 2024, 13, 540. [Google Scholar] [CrossRef]
- Maldarelli, F.; Wu, X.; Su, L.; Simonetti, F.R.; Shao, W.; Hill, S.; Spindler, J.; Ferris, A.L.; Mellors, J.W.; Kearney, M.F.; et al. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014, 345, 179–183. [Google Scholar] [CrossRef]
- Mullins, J.I.; Frenkel, L.M. Clonal expansion of human immunodeficiency virus–infected cells and human immunodeficiency virus persistence during antiretroviral therapy. J. Infect. Diseases. 2017, 215 (Suppl. S3), S119–S127. [Google Scholar] [CrossRef]
- Wagner, T.A.; McLaughlin, S.; Garg, K.; Cheung, C.Y.; Larsen, B.B.; Styrchak, S.; Huang, H.C.; Edlefsen, P.T.; Mullins, J.I.; Frenkel, L.M. Hiv latency. Proliferation of cells with hiv integrated into cancer genes contributes to persistent infection. Science 2014, 345, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Dubé, M.; Tastet, O.; Dufour, C.; Sannier, G.; Brassard, N.; Delgado, G.-G.; Pagliuzza, A.; Richard, C.; Nayrac, M.; Routy, J.-P.; et al. Spontaneous HIV expression during suppressive art is associated with the magnitude and function of hiv-specific CD4+ and CD8+ T cells. Cell Host Microbe 2023, 31, 1507–1522. [Google Scholar] [CrossRef]
- Deeks, S.G.; Archin, N.; Cannon, P.; Collins, S.; Jones, R.B.; de Jong, M.A.; Lambotte, O.; Lamplough, R.; Ndung’u, T.; Sugarman, J.; et al. Research priorities for an HIV cure: International aids society global scientific strategy 2021. Nat. Med. 2021, 27, 2085–2098. [Google Scholar] [CrossRef]
- Lopez, B.; Siliciano, R.F. Analyzing the unperturbed hiv-1 t cell reservoir. Trends Immunol. 2023, 44, 147–149. [Google Scholar] [CrossRef]
- Bruner, K.M.; Murray, A.J.; Pollack, R.A.; Soliman, M.G.; Laskey, S.B.; Capoferri, A.A.; Lai, J.; Strain, M.C.; Lada, S.M.; Hoh, R.; et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016, 22, 1043–1049. [Google Scholar] [CrossRef]
- Herrera, A.; Jones, R.B. Whack-a-virus: HIV-specific T cells play an exhausting game. Cell Host Microbe 2023, 31, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, O.; Trotta, D.; Wang, K.; Wang, X.; Chu, X.; Bradley, C.; Okulicz, J.; Maves, R.C.; Kronmann, K.; Schofield, C.M.; et al. Patients with HIV-associated cancers have evidence of increased t cell dysfunction and exhaustion prior to cancer diagnosis. J. Immunother. Cancer. 2022, 10, e004564. [Google Scholar] [CrossRef]
- Martin, G.E.; Sen, D.R.; Pace, M.; Robinson, N.; Meyerowitz, J.; Adland, E.; Thornhill, J.P.; Jones, M.; Ogbe, A.; Parolini, L.; et al. Epigenetic features of HIV-induced t-cell exhaustion persist despite early antiretroviral therapy. Front. Immunol. 2021, 12, 647688. [Google Scholar] [CrossRef]
- Angamuthu, D.; Vivekanandan, S.; Hanna, L.E. Experimental models for HIV latency and molecular tools for reservoir quantification-an update. Clin. Microbiol. Rev. 2023, 36, e1323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J. HIV reservoir: How to measure it? Curr. Hiv/Aids Rep. 2023, 20, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, T.; Zhang, Y.; Luo, S.; Chen, H.; Chen, D.; Li, C.; Li, W. The reservoir of latent HIV. Front. Cell Infect. Microbiol. 2022, 12, 945956. [Google Scholar] [CrossRef]
- Rennie, S.; Siedner, M.; Tucker, J.D.; Moodley, K. The ethics of talking about ‘HIV cure’. BMC Med. Ethics 2015, 16, 18. [Google Scholar] [CrossRef]
- Ward, A.R.; Mota, T.M.; Jones, R.B. Immunological approaches to HIV cure. Semin. Immunol. 2020, 51, 101412. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Conway, J.M.; Pagane, N.; Kreig, J.; Sambaturu, N.; Iyaniwura, S.; Li, J.Z.; Ribeiro, R.M.; Ke, R.; Perelson, A.S. Understanding early hiv-1 rebound dynamics following antiretroviral therapy interruption: The importance of effector cell expansion. PLoS Pathog. 2024, 20, e1012236. [Google Scholar] [CrossRef]
- Kwaa, A.K.; Blankson, J.N. Immune responses in controllers of HIV infection. Annu. Rev. Immunol. 2024, 42, 21–33. [Google Scholar] [CrossRef]
- Chou, T.C.; Maggirwar, N.S.; Marsden, M.D. Hiv persistence, latency, and cure approaches: Where are we now? Viruses 2024, 16, 1163. [Google Scholar] [CrossRef] [PubMed]
- Cummins, N.W.; Rizza, S.; Litzow, M.R.; Hua, S.; Lee, G.Q.; Einkauf, K.; Chun, T.-W.; Rhame, F.; Baker, J.V.; Busch, M.P.; et al. Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study. PLoS Med. 2017, 14, e1002461. [Google Scholar] [CrossRef]
- Huyveneers, L.E.; Bruns, A.; Stam, A.; Ellerbroek, P.; de Jong, D.; Nagy, N.A.; Gumbs, S.B.H.; Tesselaar, K.; Bosman, K.; Salgado, M.; et al. Autopsy study defines composition and dynamics of the HIV-1 reservoir after allogeneic hematopoietic stem cell transplantation with ccr5δ32/δ32 donor cells. Viruses 2022, 14, 2069. [Google Scholar] [CrossRef]
- Wu, H.L.; Busman-Sahay, K.; Weber, W.C.; Waytashek, C.M.; Boyle, C.D.; Bateman, K.B.; Reed, J.S.; Hwang, J.M.; Shriver-Munsch, C.; Swanson, T.; et al. Allogeneic immunity clears latent virus following allogeneic stem cell transplantation in SIV-infected art-suppressed macaques. Immunity 2023, 56, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Salgado, M.; Gálvez, C.; Nijhuis, M.; Kwon, M.; Cardozo-Ojeda, E.F.; Badiola, J.; Gorman, M.J.; Huyveneers, L.; Urrea, V.; Bandera, A.; et al. Dynamics of virological and immunological markers of HIV persistence after allogeneic haematopoietic stem-cell transplantation in the icistem cohort: A prospective observational cohort study. Lancet HIV 2024, 11, e389–e405. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Cantos, A.; Montejano, R.; Pinto-Martínez, A.; Rodríguez-Centeno, J.; Pulido, F.; Arribas, J.R. Non-suppressible viraemia during HIV-1 therapy: A challenge for clinicians. Lancet HIV 2024, 11, e333–e340. [Google Scholar] [CrossRef]
- Kalidasan, V.; Das, T.K. Lessons learned from failures and success stories of HIV breakthroughs: Are we getting closer to an hiv cure? Front. Microbiol. 2020, 11, 46. [Google Scholar] [CrossRef]
- Saez-Cirion, A.; Müller-Trutwin, M. The yellow brick road towards HIV eradication. Trends Immunol. 2019, 40, 465–467. [Google Scholar] [CrossRef]
- Karuppusamy, K.V.; Demosthenes, J.P.; Venkatesan, V.; Christopher, A.C.; Babu, P.; Azhagiri, M.K.; Jacob, A.; Ramalingam, V.V.; Rangaraj, S.; Murugesan, M.K.; et al. The ccr5 gene edited CD34CD90 hematopoietic stem cell population serves as an optimal graft source for HIV gene therapy. Front. Immunol. 2022, 13, 792684. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Bayat, A.; Rhodes, C.S.; Hoh, R.; Martin, J.N.; Fromentin, R.; Chomont, N.; Hütter, G.; Kovacs, J.A.; Deeks, S.G. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J. Infect. Dis. 2013, 209, 1613–1617. [Google Scholar] [CrossRef]
- Lee, S.A.; Bacchetti, P.; Chomont, N.; Fromentin, R.; Lewin, S.R.; O’doherty, U.; Palmer, S.; Richman, D.D.; Siliciano, J.D.; Yukl, S.A.; et al. Anti-HIV antibody responses and the hiv reservoir size during antiretroviral therapy. PLoS ONE 2016, 11, e0160192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Peppa, D.; Hill, A.L.; Gálvez, C.; Salgado, M.; Pace, M.; Mccoy, L.E.; Griffith, S.A.; Thornhill, J.; Alrubayyi, A.; et al. Evidence for HIV-1 cure after ccr5δ32/δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: A case report. Lancet HIV 2020, 7, e340–e347. [Google Scholar] [CrossRef] [PubMed]
- Gavegnano, C.; Brehm, J.H.; Dupuy, F.P.; Talla, A.; Ribeiro, S.P.; Kulpa, D.A.; Cameron, C.; Santos, S.; Hurwitz, S.J.; Marconi, V.C.; et al. Novel mechanisms to inhibit HIV reservoir seeding using jak inhibitors. PLoS Pathog. 2017, 13, e1006740. [Google Scholar] [CrossRef] [PubMed]
- Gavegnano, C.; Detorio, M.; Montero, C.; Bosque, A.; Planelles, V.; Schinazi, R.F. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob. Agents Chemother. 2014, 58, 1977–1986. [Google Scholar] [CrossRef]
- Mclaren, P.J.; Fellay, J. Hiv-1 and human genetic variation. Nat. Rev. Genet. 2021, 22, 645–657. [Google Scholar] [CrossRef]
- Zaegel-Faucher, O.; Boschi, C.; Benkouiten, S.; Laroche, H.; Dos Santos, M.C.; Motte, A.; Izadifar-Legrand, F.; Olive, D.; Colson, P.; Bregigeon-Ronot, S. Absence of viral rebound without antiretrovirals after ccr5δ32/δ32 allogeneic hematopoietic stem cell transplantation: A new case of a potential cure of hiv? In Proceedings of the HIV Drug Therapy Glasgow, Glasgow, UK, 10–13 November 2024. [Google Scholar]
- Chen, B. Molecular mechanism of hiv-1 entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef]
- Pu, J.; Wang, Q.; Xu, W.; Lu, L.; Jiang, S. Development of protein- and peptide-based HIV entry inhibitors targeting gp120 or gp41. Viruses 2019, 11, 705. [Google Scholar] [CrossRef]
- Umotoy, J.C.; De Taeye, S.W. Antibody conjugates for targeted therapy against HIV-1 as an emerging tool for hiv-1 cure. Front. Immunol. 2021, 12, 708806. [Google Scholar] [CrossRef]
- Governa, P.; Manetti, F. Recent research results have converted gp120 binders to a therapeutic option for the treatment of HIV-1 infection. A medicinal chemistry point of view. Eur. J. Med. Chem. 2022, 229, 114078. [Google Scholar] [CrossRef]
- Alkhatib, G.; Berger, E.A. HIV coreceptors: From discovery and designation to new paradigms and promise. Eur. J. Med. Res. 2007, 12, 375. [Google Scholar]
- Yandrapally, S.; Mohareer, K.; Arekuti, G.; Vadankula, G.R.; Banerjee, S. Hiv co-receptor-tropism: Cellular and molecular events behind the enigmatic co-receptor switching. Crit. Rev. Microbiol. 2021, 47, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.; Gurrola, T.; Berman, R.; Collins, M.; Sariyer, I.K.; Nonnemacher, M.R.; Wigdahl, B. Targeting ccr5 as a component of an HIV-1 therapeutic strategy. Front. Immunol. 2022, 12, 816515. [Google Scholar]
- Wilen, C.B.; Tilton, J.C.; Doms, R.W. HIV: Cell binding and entry. Cold Spring Harb. Perspect. Med. 2012, 2, a006866. [Google Scholar] [CrossRef] [PubMed]
- Panos, G.; Watson, D.C. Effect of hiv-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; Overview of viral entry inhibition. Crit. Rev. Microbiol. 2014, 41, 473–487. [Google Scholar] [CrossRef]
- Venuti, A.; Pastori, C.; Lopalco, L. The role of natural antibodies to cc chemokine receptor 5 in HIV infection. Front. Immunol. 2017, 8, 1358. [Google Scholar] [CrossRef]
- Obrien, S.J. Legacy of a magic gene-ccr5-∆32: From discovery to clinical benefit in a generation. Proc. Natl. Acad. Sci. USA 2024, 121, e2321907121. [Google Scholar] [CrossRef]
- Kordelas, L.; Verheyen, J.; Beelen, D.W.; Horn, P.A.; Heinold, A.; Kaiser, R.; Trenschel, R.; Schadendorf, D.; Dittmer, U.; Esser, S. Shift of HIV tropism in stem-cell transplantation with ccr5 delta32 mutation. N. Engl. J. Med. 2014, 371, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Burwitz, B.J.; Wu, H.L.; Abdulhaqq, S.; Shriver-Munsch, C.; Swanson, T.; Legasse, A.W.; Hammond, K.B.; Junell, S.L.; Reed, J.S.; Bimber, B.N.; et al. Allogeneic stem cell transplantation in fully mhc-matched mauritian cynomolgus macaques recapitulates diverse human clinical outcomes. Nat. Commun. 2017, 8, 1418. [Google Scholar] [CrossRef]
- Hendricks, C.L.; Mellet, J.; Durandt, C.; Brittain, D.; Pepper, M.S. Haematopoietic stem-cell transplantation in an HIV endemic area: Time to consider donors exposed to or living with HIV. Lancet HIV 2023, 10, e742–e749. [Google Scholar] [CrossRef]
- Annaloro, C.; Serpenti, F.; Saporiti, G.; Galassi, G.; Cavallaro, F.; Grifoni, F.; Goldaniga, M.; Baldini, L.; Onida, F. Viral infections in hsct: Detection, monitoring, clinical management, and immunologic implications. Front. Immunol. 2021, 11, 569381. [Google Scholar] [CrossRef]
- Henrich, T.J.; Hanhauser, E.; Marty, F.M.; Sirignano, M.N.; Keating, S.; Lee, T.-H.; Robles, Y.P.; Davis, B.T.; Li, J.Z.; Heisey, A.; et al. Antiretroviral-free hiv-1 remission and viral rebound after allogeneic stem cell transplantation: Report of 2 cases. Ann. Intern. Med. 2014, 161, 319–327. [Google Scholar] [CrossRef]
- Eberhard, J.M.; Angin, M.; Passaes, C.; Salgado, M.; Monceaux, V.; Knops, E.; Kobbe, G.; Jensen, B.; Christopeit, M.; Kröger, N.; et al. Vulnerability to reservoir reseeding due to high immune activation after allogeneic hematopoietic stem cell transplantation in individuals with HIV-1. Sci. Transl. Med. 2020, 12, eaay9355. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Kröger, N. Dose intensity for conditioning in allogeneic hematopoietic cell transplantation: Can we recommend “when and for whom” in 2021? Haematologica 2021, 106, 1794–1804. [Google Scholar] [CrossRef]
- Henrich, T.J.; Hu, Z.; Li, J.Z.; Sciaranghella, G.; Busch, M.P.; Keating, S.M.; Gallien, S.; Lin, N.H.; Giguel, F.F.; Lavoie, L.; et al. Long-term reduction in peripheral blood hiv type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J. Infect. Dis. 2013, 207, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, M.; Paik, C.H.; Carrasquillo, J.A.; Maeng, J.S.; Jang, B.S.; Shin, I.S.; Srinivasula, S.; Byrum, R.; Neria, A.; Kopp, W.; et al. Noninvasive in vivo imaging of cd4 cells in simian-human immunodeficiency virus (SHIV)-infected nonhuman primates. Blood 2009, 114, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Salgado, M.; Kwon, M.; Gálvez, C.; Badiola, J.; Nijhuis, M.; Bandera, A.; Balsalobre, P.; Miralles, P.; Buño, I.; Martinez-Laperche, C.; et al. Mechanisms that contribute to a profound reduction of the HIV-1 reservoir after allogeneic stem cell transplant. Ann. Intern. Med. 2018, 169, 674–683. [Google Scholar] [CrossRef]
- Fletcher, C.V.; Staskus, K.; Wietgrefe, S.W.; Rothenberger, M.; Reilly, C.; Chipman, J.G.; Beilman, G.J.; Khoruts, A.; Thorkelson, A.; Schmidt, T.E.; et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 2307–2312. [Google Scholar] [CrossRef]
- Sigal, A.; Kim, J.T.; Balazs, A.B.; Dekel, E.; Mayo, A.; Milo, R.; Baltimore, D. Cell-to-cell spread of hiv permits ongoing replication despite antiretroviral therapy. Nature 2011, 477, 95–98. [Google Scholar] [CrossRef]
- Wu, Y.J.; Liu, H.X.; Zhu, X.L.; Fu, H.X.; He, Y.; Wang, F.R.; Zhang, Y.Y.; Mo, X.D.; Han, W.; Wang, J.Z.; et al. Predicting short-term and long-term mortalities from sepsis in patients who receive allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2023, 202, 344–355. [Google Scholar] [CrossRef]
- Board, N.L.; Moskovljevic, M.; Wu, F.; Siliciano, R.F.; Siliciano, J.D. Engaging innate immunity in HIV-1 cure strategies. Nat. Rev. Immunol. 2022, 22, 499–512. [Google Scholar] [CrossRef]
- Blanco, A.; Coronado, R.A.; Arun, N.; Ma, K.; Dar, R.D.; Kieffer, C. Monocyte to macrophage differentiation and changes in cellular redox homeostasis promote cell type-specific HIV latency reactivation. Proc. Natl. Acad. Sci. USA 2024, 121, e1981144175. [Google Scholar] [CrossRef]
- Wilhelm, E.; Poirier, M.; Da Rocha, M.; Bédard, M.; McDonald, P.P.; Lavigne, P.; Hunter, C.L.; Bell, B. Mitotic deacetylase complex (midac) recognizes the HIV-1 core promoter to control activated viral gene expression. PLoS Pathog. 2024, 20, e1011821. [Google Scholar] [CrossRef] [PubMed]
- Meeroekyai, S.; Jaimalai, T.; Suree, N.; Prangkio, P. CD4+ t cell-targeting immunoliposomes for treatment of latent hiv reservoir. Eur. J. Pharm. Biopharm. 2024, 195, 114166. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Kim, M.; Soper, A.; Kovarova, M.; Spagnuolo, R.A.; Begum, N.; Kirchherr, J.; Archin, N.; Battaglia, D.; Cleveland, D.; et al. Analysis of the effect of hdac inhibitors on the formation of the HIV reservoir. Mbio 2024, 15, e163224. [Google Scholar] [CrossRef]
- Borducchi, E.N.; Cabral, C.; Stephenson, K.E.; Liu, J.; Abbink, P.; Ng’ang’a, D.; Nkolola, J.P.; Brinkman, A.L.; Peter, L.; Lee, B.C.; et al. Ad26/MVA therapeutic vaccination with tlr7 stimulation in SIV-infected rhesus monkeys. Nature 2016, 540, 284–287. [Google Scholar] [CrossRef]
- Okoye, A.A.; Fromentin, R.; Takata, H.; Brehm, J.H.; Fukazawa, Y.; Randall, B.; Pardons, M.; Tai, V.; Tang, J.; Smedley, J.; et al. The ingenol-based protein kinase c agonist gsk445a is a potent inducer of HIV and SIV RNA transcription. PLoS Pathog. 2022, 18, e1010245. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.; Sukkestad, S.; Horner, A.M.; Neja, M.; Siddiqi, Z.; Waller, C.; Goldy, J.; Monroe, D.; Lin, A.; Schoof, N.; et al. AZD5582 plus SIV-specific antibodies reduce lymph node viral reservoirs in antiretroviral therapy-suppressed macaques. Nat. Med. 2023, 29, 2535–2546. [Google Scholar] [CrossRef]
- Vibholm, L.K.; Konrad, C.V.; Schleimann, M.H.; Frattari, G.; Winckelmann, A.; Klastrup, V.; Jensen, N.M.; Jensen, S.S.; Schmidt, M.; Wittig, B.; et al. Effects of 24-week toll-like receptor 9 agonist treatment in HIV type 1+ individuals. Aids 2019, 33, 1315–1325. [Google Scholar] [CrossRef]
- Vibholm, L.; Schleimann, M.H.; Højen, J.F.; Benfield, T.; Offersen, R.; Rasmussen, K.; Olesen, R.; Dige, A.; Agnholt, J.; Grau, J.; et al. Short-course toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin. Infect. Dis. 2017, 64, 1686–1695. [Google Scholar] [CrossRef]
- Duggan, N.N.; Dragic, T.; Chanda, S.K.; Pache, L. Breaking the silence: Regulation of HIV transcription and latency on the road to a cure. Viruses 2023, 15, 2435. [Google Scholar] [CrossRef]
- Grau-Expósito, J.; Luque-Ballesteros, L.; Navarro, J.; Curran, A.; Burgos, J.; Ribera, E.; Torrella, A.; Planas, B.; Badía, R.; Martin-Castillo, M.; et al. Latency reversal agents affect differently the latent reservoir present in distinct CD4+ t subpopulations. PLoS Pathog. 2019, 15, e1007991. [Google Scholar] [CrossRef]
- JBoucau, J.; Das, J.; Joshi, N.; Gall, S. Latency reversal agents modulate HIV antigen processing and presentation to CD8 T cells. PLoS Pathog. 2020, 16, e1008442. [Google Scholar]
- Marsden, M.D.; Loy, B.A.; Wu, X.; Ramirez, C.M.; Schrier, A.J.; Murray, D.; Shimizu, A.; Ryckbosch, S.M.; Near, K.E.; Chun, T.W.; et al. In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell “kick” and “kill” in strategy for virus eradication. PLoS Pathog. 2017, 13, e1006575. [Google Scholar] [CrossRef]
- Kobayashi-Ishihara, M.; Tsunetsugu-Yokota, Y. Post-transcriptional HIV-1 latency: A promising target for therapy? Viruses 2024, 16, 666. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.C.; Deng, K. Heterogeneity of HIV-1 latent reservoirs. Chin. Med. J. 2020, 133, 2867–2873. [Google Scholar] [CrossRef]
- Janssens, J.; Kim, P.; Kim, S.J.; Wedrychowski, A.; Kadiyala, G.N.; Hunt, P.W.; Deeks, S.G.; Wong, J.K.; Yukl, S.A. Mechanisms and efficacy of small molecule latency-promoting agents to inhibit HIV reactivation ex vivo. JCI Insight 2024, 9, e183084. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; Fitzhugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar]
- Yamagishi, M.; Ishida, T.; Miyake, A.; Cooper, D.A.; Kelleher, A.D.; Suzuki, K.; Watanabe, T. Retroviral delivery of promoter-targeted shrna induces long-term silencing of hiv-1 transcription. Microbes Infect. 2009, 11, 500–508. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Ma, X.; Geng, G.; Liu, B.; Zhang, Y.; Zhang, S.; Zhong, F.; Liu, C.; Yin, Y.; et al. Long noncoding RNA NRON contributes to HIV-1 latency by specifically inducing tat protein degradation. Nat. Commun. 2016, 7, 11730. [Google Scholar] [CrossRef]
- Meredith, L.W.; Sivakumaran, H.; Major, L.; Suhrbier, A.; Harrich, D. Potent inhibition of HIV-1 replication by a tat mutant. PLoS ONE 2009, 4, e7769. [Google Scholar] [CrossRef]
- Mousseau, G.; Aneja, R.; Clementz, M.A.; Mediouni, S.; Lima, N.S.; Haregot, A.; Kessing, C.F.; Jablonski, J.A.; Thenin-Houssier, S.; Nagarsheth, N.; et al. Resistance to the tat inhibitor didehydro-cortistatin a is mediated by heightened basal hiv-1 transcription. Mbio 2019, 10, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Peng, P.; Ou, X.; Shen, K.; Wu, X. Ovarian cancer circulating extracelluar vesicles promote coagulation and have a potential in diagnosis: An itraq based proteomic analysis. BMC Cancer 2019, 19, 1095. [Google Scholar] [CrossRef]
- Ahlenstiel, C.L.; Symonds, G.; Kent, S.J.; Kelleher, A.D. Block and lock hiv cure strategies to control the latent reservoir. Front. Cell Infect. Microbiol. 2020, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Paneerselvam, N.; Lawson, B.R. Antiretrovirals to ccr5 crispr/cas9 gene editing—A paradigm shift chasing an HIV cure. Clin. Immunol. 2023, 255, 109741. [Google Scholar] [CrossRef]
- Kitawi, R.; Ledger, S.; Kelleher, A.D.; Ahlenstiel, C.L. Advances in HIV gene therapy. Int. J. Mol. Sci. 2024, 25, 2771. [Google Scholar] [CrossRef]
- Cornu, T.I.; Mussolino, C.; Bloom, K.; Cathomen, T. Editing ccr5: A novel approach to hiv gene therapy. In Gene Therapy for HIV and Chronic Infections; Ertl, H.C.J., Weinberg, M.S., Berkhout, B., Eds.; Springer: New York, NY, USA, 2015; pp. 117–130. [Google Scholar]
- Ancuta, P. Genetic haute couture to block hiv-1 at front doors. Cell Stem Cell 2024, 31, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.; Jenner, K.; Holland, M.; Khair, K. Barriers to gene therapy, understanding the concerns people with haemophilia have: An exigency sub-study. Orphanet J. Rare Dis. 2024, 19, 59. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, N.; Berkhout, B.; Das, A.T. CRISPR-cas9 can inhibit HIV-1 replication but nhej repair facilitates virus escape. Mol. Ther. 2016, 24, 522–526. [Google Scholar] [CrossRef]
- Karch, C.P.; Matyas, G.R. The current and future role of nanovaccines in HIV-1 vaccine development. Expert. Rev. Vaccines 2021, 20, 935–944. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Barton, J.P. Rational design of vaccine targets and strategies for HIV: A crossroad of statistical physics, biology, and medicine. Rep. Prog. Phys. 2017, 80, 32601. [Google Scholar] [CrossRef]
- Heger, E.; Schuetz, A.; Vasan, S. HIV vaccine efficacy trials: rv144 and beyond. In HIV Vaccines and Cure; Zhang, L., Lewin, S.R., Eds.; Springer: Singapore, 2018; pp. 3–30. [Google Scholar]
- Mdluli, T.; Jian, N.; Slike, B.; Paquin-Proulx, D.; Donofrio, G.; Alrubayyi, A.; Gift, S.; Grande, R.; Bryson, M.; Lee, A.; et al. Rv144 HIV-1 vaccination impacts post-infection antibody responses. PLoS Pathog. 2020, 16, e1009101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Julg, B. Therapeutic vaccines for the treatment of hiv. Transl. Res. J. Lab. Clin. Med. 2020, 223, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Flemming, A. Why have t cell-inducing vaccines for hiv failed so far? Nature Reviews. Immunology 2024, 24, 89. [Google Scholar]
- Havenar-Daughton, C.; Lee, J.H.; Crotty, S. Tfh cells and HIV bnAbs, an immunodominance model of the hiv neutralizing antibody generation problem. Immunol. Rev. 2017, 275, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, L.; Bettencourt, P.J. Mrna vaccines: A new opportunity for malaria, tuberculosis and HIV. Front. Immunol. 2023, 14, 1172691. [Google Scholar] [CrossRef]
- Ding, X.; Cao, K.; Wang, J.; Wan, Y.; Chen, Q.; Ren, Y.; Zheng, Y.; Zhu, M.; Tian, R.; Wang, W.; et al. Exploration of a sequential gp140-gp145 immunization regimen with heterologous envs to induce a protective cross-reactive HIV neutralizing antibody response in non-human primates. Virol. Sin. 2021, 36, 784–795. [Google Scholar] [CrossRef]
- Golding, H.; Khurana, S.; Zaitseva, M. What is the predictive value of animal models for vaccine efficacy in humans? The importance of bridging studies and species-independent correlates of protection. Cold Spring Harb. Perspect. Biol. 2017, 10, a028902. [Google Scholar]
- Zubair, A.; Bibi, B.; Habib, F.; Sujan, A.; Ali, M. Clinical trials and recent progress in HIV vaccine development. Funct. Integr. Genom. 2024, 24, 143. [Google Scholar] [CrossRef]
- Frattari, G.S.; Caskey, M.; Søgaard, O.S. Broadly neutralizing antibodies for HIV treatment and cure approaches. Curr. Opin. HIV Aids 2023, 18, 157–163. [Google Scholar] [CrossRef]
- Haynes, B.F.; Wiehe, K.; Borrow, P.; Saunders, K.O.; Korber, B.; Wagh, K.; McMichael, A.J.; Kelsoe, G.; Hahn, B.H.; Alt, F.; et al. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat. Rev. Immunol. 2023, 23, 142–158. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Asokan, M.; Gorman, J.; Zhang, B.; Liu, Q.; Louder, M.K.; Lin, B.C.; McKee, K.; Pegu, A.; Verardi, R.; et al. A matrix of structure-based designs yields improved vrc01-class antibodies for HIV-1 therapy and prevention. mAbs 2021, 13, 1946918. [Google Scholar] [CrossRef] [PubMed]
- Wu, X. Hiv broadly neutralizing antibodies: vrc01 and beyond. Adv. Exp. Med. Biol. 2018, 1075, 53–72. [Google Scholar] [PubMed]
- Sneller, M.C.; Blazkova, J.; Justement, J.S.; Shi, V.; Kennedy, B.D.; Gittens, K.; Tolstenko, J.; McCormack, G.; Whitehead, E.J.; Schneck, R.F.; et al. Combination anti-HIV antibodies provide sustained virological suppression. Nature 2022, 606, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Lynn, K.; Azzoni, L.; Cocchella, G.; Papasavvas, E.; Fair, M.; Karanam, B.; Sharma, P.; Reeves, J.D.; Petropoulos, C.J.; et al. Susceptibility to 3bnc117 and 10-1074 in art suppressed chronically infected persons. Aids 2023, 37, 1203–1207. [Google Scholar] [CrossRef]
- van den Berg, F.T.; Makoah, N.A.; Ali, S.A.; Scott, T.A.; Mapengo, R.E.; Mutsvunguma, L.Z.; Mkhize, N.N.; Lambson, B.E.; Kgagudi, P.D.; Crowther, C.; et al. Aav-mediated expression of broadly neutralizing and vaccine-like antibodies targeting the HIV-1 envelope v2 region. Mol. Ther. Methods Clin. Dev. 2019, 14, 100–112. [Google Scholar] [CrossRef]
- Fuchs, S.P.; Desrosiers, R.C. Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol. Ther. Methods Clin. Dev. 2016, 3, 16068. [Google Scholar] [CrossRef]
- Paneerselvam, N.; Khan, A.; Lawson, B.R. Broadly neutralizing antibodies targeting HIV: Progress and challenges. Clin. Immunol. 2023, 257, 109809. [Google Scholar] [CrossRef]
- Thavarajah, J.J.; Hønge, B.L.; Wejse, C.M. The use of broadly neutralizing antibodies (bnAbs) in HIV-1 treatment and prevention. Viruses 2024, 16, 911. [Google Scholar] [CrossRef]
- Wan, S.F.; Happe, M.; Hofstetter, A.R.; Gama, L. Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr. Opin. HIV Aids 2022, 17, 247–257. [Google Scholar]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Weiss, R.A.; Verrips, C.T. Nanobodies that neutralize HIV. Vaccines 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Jähnichen, S.; Blanchetot, C.; Maussang, D.; Gonzalez-Pajuelo, M.; Chow, K.Y.; Bosch, L.; De Vrieze, S.; Serruys, B.; Ulrichts, H.; Vandevelde, W.; et al. Cxcr4 nanobodies (vhh-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 20565–20570. [Google Scholar] [CrossRef]
- Vercruysse, T.; Pardon, E.; Vanstreels, E.; Steyaert, J.; Daelemans, D. An intrabody based on a llama single-domain antibody targeting the n-terminal alpha-helical multimerization domain of HIV-1 rev prevents viral production. J. Biol. Chem. 2010, 285, 21768–21780. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.R.; Brookes, J.C.; Caillat, C.; Turbé, V.; Webb, B.L.; Granger, L.A.; Miller, B.S.; McCoy, L.E.; El Khattabi, M.; Verrips, C.T.; et al. Unravelling the molecular basis of high affinity nanobodies against HIV p24: In vitro functional, structural, and in silico insights. ACS Infect. Dis. 2017, 3, 479–491. [Google Scholar] [CrossRef]
- Turbé, V.; Gray, E.R.; Lawson, V.E.; Nastouli, E.; Brookes, J.C.; Weiss, R.A.; Pillay, D.; Emery, V.C.; Verrips, C.T.; Yatsuda, H.; et al. Towards an ultra-rapid smartphone- connected test for infectious diseases. Sci. Rep. 2017, 7, 11971. [Google Scholar] [CrossRef]
- Bouchet, J.; Basmaciogullari, S.E.; Chrobak, P.; Stolp, B.; Bouchard, N.; Fackler, O.T.; Chames, P.; Jolicoeur, P.; Benichou, S.; Baty, D. Inhibition of the nef regulatory protein of HIV-1 by a single-domain antibody. Blood 2011, 117, 3559–3568. [Google Scholar] [CrossRef]
- Matz, J.; Hérate, C.; Bouchet, J.; Dusetti, N.; Gayet, O.; Baty, D.; Benichou, S.; Chames, P. Selection of intracellular single-domain antibodies targeting the HIV-1 vpr protein by cytoplasmic yeast two-hybrid system. PLoS ONE 2014, 9, e113729. [Google Scholar] [CrossRef] [PubMed]
- Diorio, C.; Teachey, D.T.; Grupp, S.A. Allogeneic chimeric antigen receptor cell therapies for cancer: Progress made and remaining roadblocks. Nat. Rev. Clin. Oncol. 2025, 22, 10–27. [Google Scholar] [CrossRef]
- Gong, Y.; Klein Wolterink, R.G.; Wang, J.; Bos, G.M.; Germeraad, W.T. Chimeric antigen receptor natural killer (car-nk) cell design and engineering for cancer therapy. J. Hematol. Oncol. 2021, 14, 73. [Google Scholar] [CrossRef]
- Chung, J.B.; Brudno, J.N.; Borie, D.; Kochenderfer, J.N. Chimeric antigen receptor t cell therapy for autoimmune disease. Nat. Rev. Immunol. 2024, 24, 830–845. [Google Scholar] [CrossRef]
- Pitman, M.C.; Lau, J.S.Y.; Mcmahon, J.H.; Lewin, S.R. Barriers and strategies to achieve a cure for HIV. Lancet HIV 2018, 5, e317–e328. [Google Scholar] [CrossRef]
- Collins, K.L.; Chen, B.K.; Kalams, S.A.; Walker, B.D.; Baltimore, D. HIV-1 nef protein protects infected primary cells against killing by cytotoxic t lymphocytes. Nature 1998, 391, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Maldini, C.R.; Gayout, K.; Leibman, R.S.; Dopkin, D.L.; Mills, J.P.; Shan, X.; Glover, J.A.; Riley, J.L. HIV-resistant and hiv-specific car-modified CD4+ T cells mitigate hiv disease progression and confer CD4+ T cell help in vivo. Mol. Ther. 2020, 28, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Ayala, V.I.; Deleage, C.; Trivett, M.T.; Jain, S.; Coren, L.V.; Breed, M.W.; Kramer, J.A.; Thomas, J.A.; Estes, J.D.; Lifson, J.D.; et al. Cxcr5-dependent entry of CD8 T cells into rhesus macaque b-cell follicles achieved through t-cell engineering. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyasu, R.T.; Anton, P.A.; Deeks, S.G.; Scadden, D.T.; Connick, E.; Downs, M.T.; Bakker, A.; Roberts, M.R.; June, C.H.; Jalali, S.; et al. Prolonged survival and tissue trafficking following adoptive transfer of cd4zeta gene-modified autologous CD4+ and CD8+ T cells in human immunodeficiency virus-infected subjects. Blood 2000, 96, 785–793. [Google Scholar] [CrossRef]
- Walker, R.E.; Bechtel, C.M.; Natarajan, V.; Baseler, M.; Hege, K.M.; Metcalf, J.A.; Stevens, R.; Hazen, A.; Blaese, R.M.; Chen, C.C.; et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood 2000, 96, 467–474. [Google Scholar]
- Schroeder, T.; Martens, T.; Fransecky, L.; Valerius, T.; Schub, N.; Pott, C.; Baldus, C.; Stölzel, F. Management of chimeric antigen receptor t (car-t) cell-associated toxicities. Intensive Care Med. 2024, 50, 1459–1469. [Google Scholar] [CrossRef]
- Liu, D.; Tian, S.; Zhang, K.; Xiong, W.; Lubaki, N.M.; Chen, Z.; Han, W. Chimeric antigen receptor (car)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and hiv. Protein Cell 2017, 8, 861–877. [Google Scholar] [CrossRef]
- Mikulak, J.; Oriolo, F.; Zaghi, E.; Di Vito, C.; Mavilio, D. Natural killer cells in HIV-1 infection and therapy. Aids 2017, 31, 2317–2330. [Google Scholar] [CrossRef]
- Strauss-Albee, D.M.; Fukuyama, J.; Liang, E.C.; Yao, Y.; Jarrell, J.A.; Drake, A.L.; Kinuthia, J.; Montgomery, R.R.; John-Stewart, G.; Holmes, S.; et al. Human nk cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci. Transl. Med. 2015, 7, 297ra115. [Google Scholar] [CrossRef]
- Mavilio, D.; Lombardo, G.; Benjamin, J.; Kim, D.; Follman, D.; Marcenaro, E.; O’Shea, M.A.; Kinter, A.; Kovacs, C.; Moretta, A.; et al. Characterization of CD56-/CD16+ natural killer (nk) cells: A highly dysfunctional nk subset expanded in hiv-infected viremic individuals. Proc. Natl. Acad. Sci. USA 2005, 102, 2886–2891. [Google Scholar] [CrossRef]
- Walker-Sperling, V.E.; Pohlmeyer, C.W.; Veenhuis, R.T.; May, M.; Luna, K.A.; Kirkpatrick, A.R.; Laeyendecker, O.; Cox, A.L.; Carrington, M.; Bailey, J.R.; et al. Factors associated with the control of viral replication and virologic breakthrough in a recently infected hiv-1 controller. Ebiomedicine 2017, 16, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mazarzaei, A.; Vafaei, M.; Ghasemian, A.; Mirforughi, S.A.; Rajabi Vardanjani, H.; Alwan, N.A. Memory and car-nk cell-based novel approaches for HIV vaccination and eradication. J. Cell Physiol. 2019, 234, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Nolting, A.; Dugast, A.S.; Rihn, S.; Luteijn, R.; Carrington, M.F.; Kane, K.; Jost, S.; Toth, I.; Nagami, E.; Faetkenheuer, G.; et al. Mhc class i chain-related protein a shedding in chronic hiv-1 infection is associated with profound nk cell dysfunction. Virology 2010, 406, 12–20. [Google Scholar] [CrossRef]
- Perera Molligoda Arachchige, A.S. Nk cell-based therapies for HIV infection: Investigating current advances and future possibilities. J. Leukoc. Biol. 2022, 111, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Noy, A. Optimizing treatment of HIV-associated lymphoma. Blood 2019, 134, 1385–1394. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, J.; Parikh, K.; Brinkley, A.; Lin, S.; Zakarian, C.; Pernet, O.; Shimizu, S.; Khamaikawin, W.; Hacke, K.; et al. In vivo selection of anti-HIV-1 gene-modified human hematopoietic stem/progenitor cells to enhance engraftment and HIV-1 inhibition. Mol. Ther. 2024, 32, 384–394. [Google Scholar] [CrossRef]
- Suryawanshi, G.W.; Khamaikawin, W.; Wen, J.; Shimizu, S.; Arokium, H.; Xie, Y.; Wang, E.; Kim, S.; Choi, H.; Zhang, C.; et al. The clonal repopulation of HSPC gene modified with anti-HIV-1 RNAi is not affected by preexisting HIV-1 infection. Sci. Adv. 2020, 6, eaay9206. [Google Scholar] [CrossRef]
- Zhen, A.; Kamata, M.; Rezek, V.; Rick, J.; Levin, B.; Kasparian, S.; Chen, I.S.Y.; Yang, O.O.; Zack, J.A.; Kitchen, S.G. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol. Ther. 2015, 23, 1358–1367. [Google Scholar] [CrossRef]
- Rust, B.J.; Kiem, H.P.; Uldrick, T.S. Car t-cell therapy for cancer and hiv through novel approaches to HIV-associated haematological malignancies. Lancet Haematol. 2020, 7, e690–e696. [Google Scholar] [CrossRef]
- Veeraraghavan, V.P.; Needamangalam Balaji, J.; Prakash, S.; Prashar, L.; Mony, U.; Surapaneni, K.M. HIV and immunotherapy: Will car—t cell therapy cure hiv? Int. J. Surg. 2023, 109, 3224–3225. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Carrillo, E.; Berkhout, B. Potential mechanisms for cell-based gene therapy to treat HIV/AIDS. Expert. Opin. Ther. Targets 2015, 19, 245–263. [Google Scholar] [CrossRef] [PubMed]

| Cell Therapy Clinical Trials | Trail Registry Identifiers | Sponsors | Phase | Estimated End Date |
|---|---|---|---|---|
| CAR-T Cells for HIV Infection | NCT04648046 | Steven Deeks | Phase I/II | 2028-12-31 |
| CMV-specific HIV-CAR T Cells as Immunotherapy for HIV/AIDS | NCT06252402 | City of Hope Medical Center | Early Phase I | 2028-03-30 |
| Immune Cell Therapy (CAR-T) for the Treatment of Patients With HIV and B-Cell Non-Hodgkin Lymphoma | NCT05077527 | AIDS Malignancy Consortium | Phase I | 2027-01-31 |
| Safety and Survival of Genetically Modified White Blood Cells in HIV-infected Twins: The Gemini Study | NCT04799483 | National Institute of Allergy and Infectious Diseases (NIAID) | Not listed | 2030-01-30 |
| CD4+ CAR ZFN-modified T Cells in HIV Therapy | NCT03617198 | University of Pennsylvania | Phase I | 2027-12 |
| HIV-1-Specific T Cells (HST-NEETs) for HIV-Infected Individuals | NCT03485963 | Catherine Bollard | Phase I | 2024-12 |
| Study of Anti-HIV Cellular Therapy Based on Dendritic Cells Pulsed With Chemically Inactivated Virus | NCT02766049 | University of Sao Paulo General Hospital | Phase I/II | 2014-05 |
| Lymphocyte Infusions for the Treatment of HIV-Infected Patients Failing Anti-HIV Therapy | NCT00559416 | National Institutes of Health Clinical Center (CC) | Phase I | 2016-09-30 |
| Redirected High-Affinity Gag-Specific Autologous T Cells for HIV Gene Therapy | NCT00991224 | University of Pennsylvania | Phase I | 2014-01 |
| Adoptive Transfer of Haploidentical NK Cells and N-803 | NCT03899480 | University of Minnesota | Phase I | 2021-04-01 |
| Phase I Study of HIV-1 Antigen Expanded Specific T Cell Therapy | NCT02208167 | University of North Carolina, Chapel Hill | Phase I | 2017-11-27 |
| Safety and Effectiveness of Immunotherapy With Autologous HIV-Specific CD8 Cells in HIV-Infected Adults | NCT00110578 | National Institute of Allergy and Infectious Diseases (NIAID) | Phase I | 2005-04 |
| Effect of Chidamide Combined With CAT-T or TCR-T Cell Therapy on HIV-1 Latent Reservoir | NCT03980691 | Guangzhou 8th People’s Hospital | Phase I | 2020-05-31 |
| Evaluate the Tolerability and Therapeutic Effects of Repeated Doses of Autologous T Cells With VRX496 in HIV | NCT00295477 | University of Pennsylvania | Phase I | 2013-12 |
| An Efficacy and Safety Study of shRNA-modified CD34+ Cells in HIV-infected Patients | NCT03517631 | Shanghai Public Health Clinical Center | Phase I | 2020-12-31 |
| Redirected MazF-CD4 Autologous T Cells for HIV Gene Therapy | NCT01787994 | University of Pennsylvania | Phase I | 2017-07 |
| Safety and Survival of Genetically Modified White Blood Cells in HIV-Infected Persons—A Study in Identical Twin Pairs | NCT00001353 | National Institute of Allergy and Infectious Diseases (NIAID) | Phase I/II | 2002-03 |
| A Study of Cytotoxic T Lymphocyte (CTL) Therapy in HIV-Infected Patients | NCT00000824 | National Institute of Allergy and Infectious Diseases (NIAID) | Not listed | 2005-06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Q.; He, S.; Wang, C.; Zhou, Y.; Zeng, C.; Liu, J.; Liu, T.; Li, T.; Quan, X.; Wang, L.; et al. Deep Thought on the HIV Cured Cases: Where Have We Been and What Lies Ahead? Biomolecules 2025, 15, 378. https://doi.org/10.3390/biom15030378
Xiao Q, He S, Wang C, Zhou Y, Zeng C, Liu J, Liu T, Li T, Quan X, Wang L, et al. Deep Thought on the HIV Cured Cases: Where Have We Been and What Lies Ahead? Biomolecules. 2025; 15(3):378. https://doi.org/10.3390/biom15030378
Chicago/Turabian StyleXiao, Qing, Sanxiu He, Chaoyu Wang, Yixing Zhou, Chensi Zeng, Jun Liu, Tingting Liu, Tingting Li, Xi Quan, Linyue Wang, and et al. 2025. "Deep Thought on the HIV Cured Cases: Where Have We Been and What Lies Ahead?" Biomolecules 15, no. 3: 378. https://doi.org/10.3390/biom15030378
APA StyleXiao, Q., He, S., Wang, C., Zhou, Y., Zeng, C., Liu, J., Liu, T., Li, T., Quan, X., Wang, L., Zhai, L., Liu, Y., Li, J., Zhang, X., & Liu, Y. (2025). Deep Thought on the HIV Cured Cases: Where Have We Been and What Lies Ahead? Biomolecules, 15(3), 378. https://doi.org/10.3390/biom15030378







