Electrostatic Effects on Tau Nanocondensates
Abstract
1. Introduction
2. Materials and Methods
2.1. WT, pTau, and P301S Tau Expression and Purification
2.2. pTau Verification via Mass Spectroscopy
2.3. pTau Charge Distribution Determination
2.4. Protein Sample Preparation and Concentration Determination
2.5. Dynamic Light Scattering
2.6. Tau Microcondensate Induction and Microscopy Imaging
2.7. Electrostatic Effect on Tau Nanocondensate Formation and Dissolution
3. Results
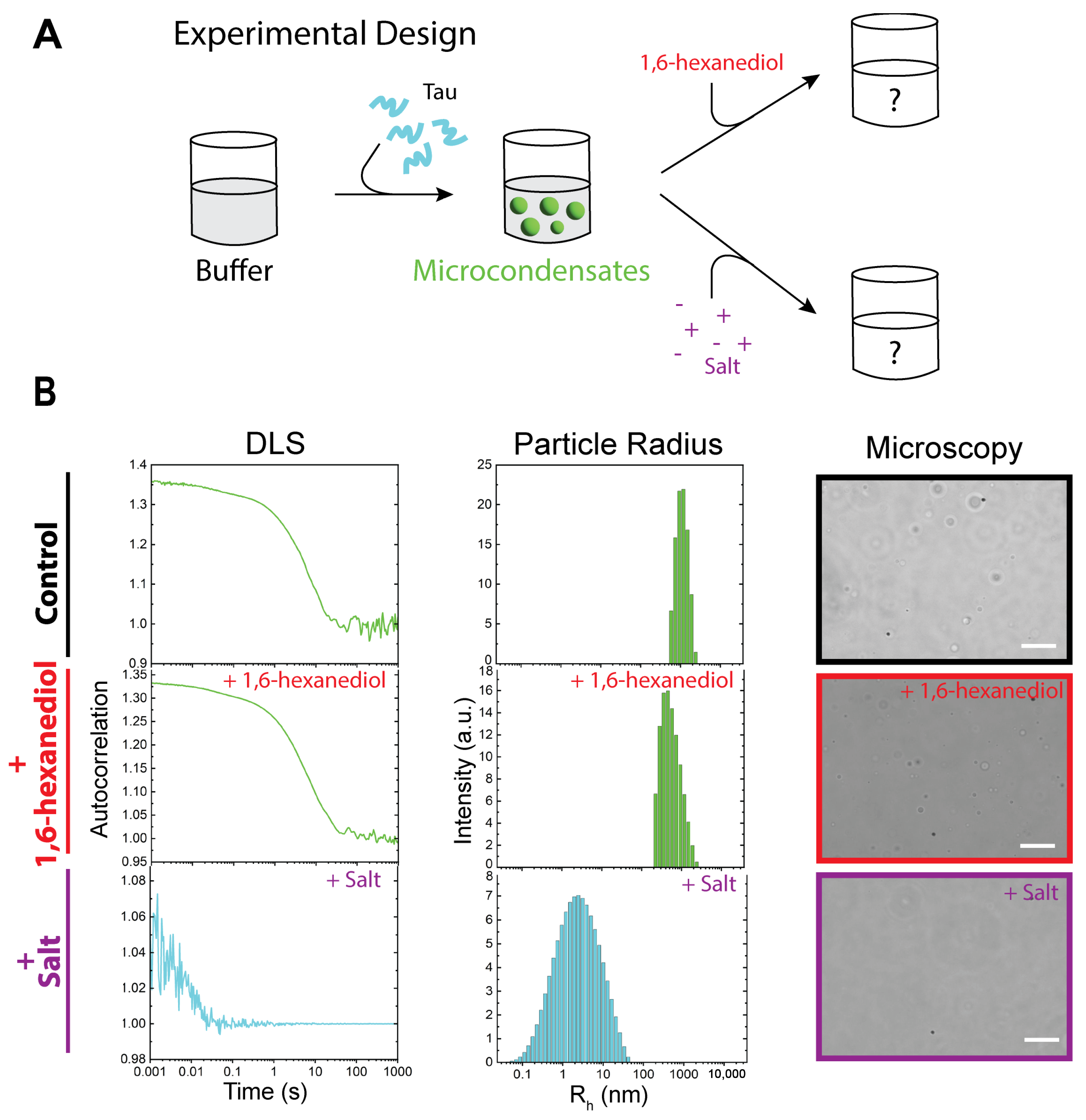
3.1. Wild-Type, Hyperphosphorylated, and P301S-Mutated Tau Can Exist as Monomers, Nanocondensates, and Microcondensates
3.2. WT Tau Condensates Are Strongly Regulated by Electrostatic Forces
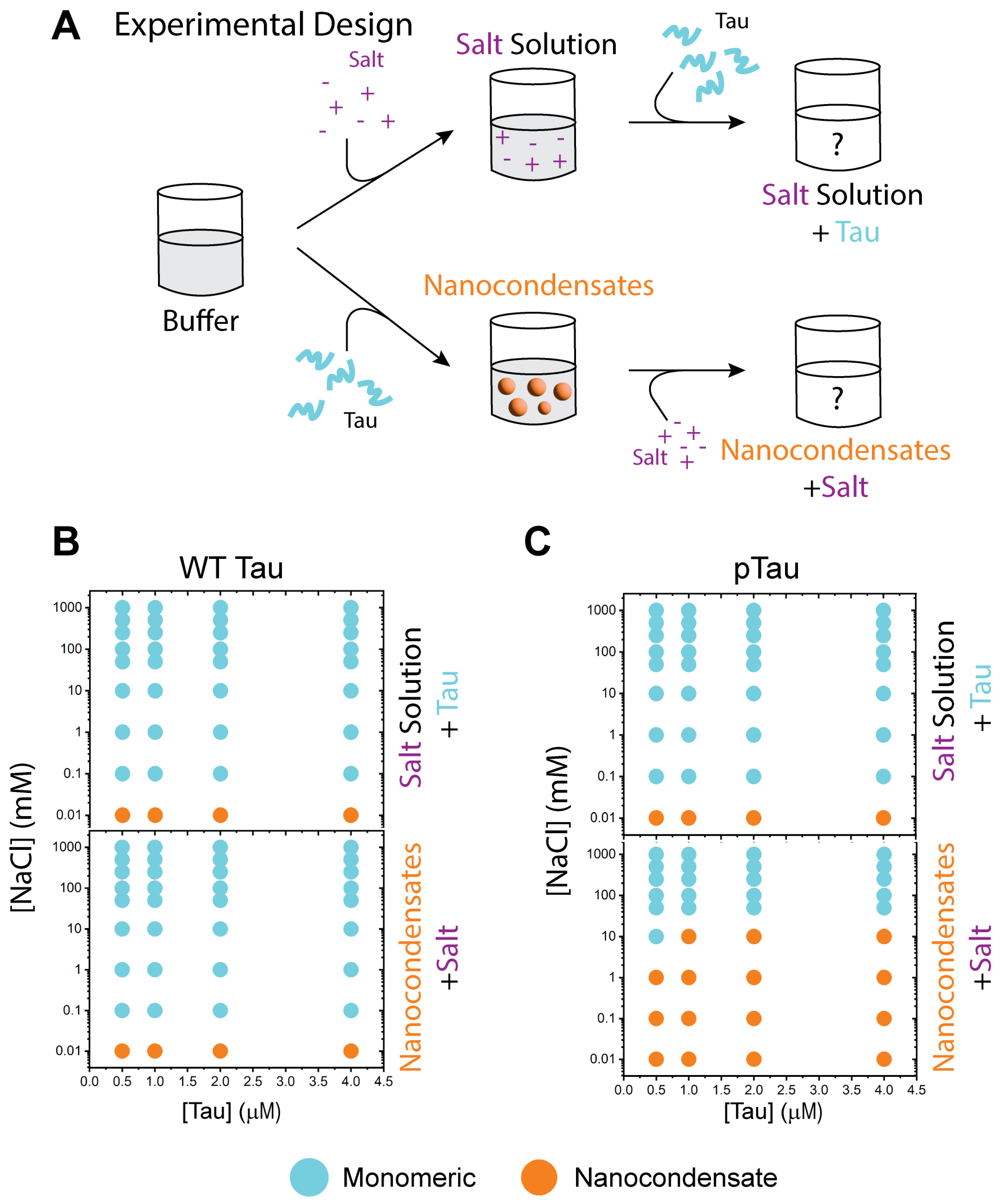
3.3. WT and pTau Share Similar Nanocondensate Formation and Dissolution Properties
3.4. P301S Tau Exhibits Distinct Dissolution Properties Relative to WT and pTau
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Gyparaki, M.T.; Arab, A.; Sorokina, E.M.; Santiago-Ruiz, A.N.; Bohrer, C.H.; Xiao, J.; Lakadamyali, M. Tau forms oligomeric complexes on microtubules that are distinct from tau aggregates. Proc. Natl. Acad. Sci. USA 2021, 118, e2021461118. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Han, S. Tau Condensates. Adv. Exp. Med. Biol. 2019, 1184, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Longfield, S.F.; Mollazade, M.; Wallis, T.P.; Gormal, R.S.; Joensuu, M.; Wark, J.R.; van Waardenberg, A.J.; Small, C.; Graham, M.E.; Meunier, F.A.; et al. Tau forms synaptic nano-biomolecular condensates controlling the dynamic clustering of recycling synaptic vesicles. Nat. Commun. 2023, 14, 7277. [Google Scholar] [CrossRef]
- Siahaan, V.; Tan, R.; Humhalova, T.; Libusova, L.; Lacey, S.E.; Tan, T.; Dacy, M.; Ori-McKenney, K.M.; McKenney, R.J.; Braun, M.; et al. Microtubule lattice spacing governs cohesive envelope formation of tau family proteins. Nat. Chem. Biol. 2022, 18, 1224–1235. [Google Scholar] [CrossRef]
- Tan, R.; Lam, A.J.; Tan, T.; Han, J.; Nowakowski, D.W.; Vershinin, M.; Simó, S.; Ori-McKenney, K.M.; McKenney, R.J. Microtubules gate tau condensation to spatially regulate microtubule functions. Nat. Cell Biol. 2019, 21, 1078–1085. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Ghetti, B.; Goedert, M. Classification of diseases with accumulation of Tau protein. Neuropathol. Appl. Neurobiol. 2022, 48, e12792. [Google Scholar] [CrossRef]
- Parra Bravo, C.; Naguib, S.A.; Gan, L. Cellular and pathological functions of tau. Nat. Rev. Mol. Cell Biol. 2024, 25, 845–864. [Google Scholar] [CrossRef]
- Tsoi, P.S.; Quan, M.D.; Ferreon, J.C.; Ferreon, A.C.M. Aggregation of Disordered Proteins Associated with Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 3380. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.S.; Lipinski, W.P.; Spruijt, E. The role of biomolecular condensates in protein aggregation. Nat. Rev. Chem. 2024, 8, 686–700. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, X.; Wu, X.; Zhang, M. Formation of biological condensates via phase separation: Characteristics, analytical methods, and physiological implications. J. Biol. Chem. 2019, 294, 14823–14835. [Google Scholar] [CrossRef]
- Huggins, M.L. Thermodynamic Properties of Solutions of Long-Chain Compounds. Ann. N. Y. Acad. Sci. 1942, 43, 1–32. [Google Scholar] [CrossRef]
- Narayanan, A.; Meriin, A.; Andrews, J.O.; Spille, J.H.; Sherman, M.Y.; Cisse, I.I. A first order phase transition mechanism underlies protein aggregation in mammalian cells. eLife 2019, 8, e39695. [Google Scholar] [CrossRef]
- Posey, A.E.; Ruff, K.M.; Lalmansingh, J.M.; Kandola, T.S.; Lange, J.J.; Halfmann, R.; Pappu, R.V. Mechanistic Inferences From Analysis of Measurements of Protein Phase Transitions in Live Cells. J. Mol. Biol. 2021, 433, 166848. [Google Scholar] [CrossRef]
- Kar, M.; Dar, F.; Welsh, T.J.; Vogel, L.T.; Kühnemuth, R.; Majumdar, A.; Krainer, G.; Franzmann, T.M.; Alberti, S.; Seidel, C.A.; et al. Phase-separating RNA-binding proteins form heterogeneous distributions of clusters in subsaturated solutions. Proc. Natl. Acad. Sci. USA 2022, 119, e2202222119. [Google Scholar] [CrossRef]
- Georgalis, Y.; Umbach, P.; Saenger, W.; Ihmels, B.; Soumpasis, D.M. Ordering of Fractal Clusters in Crystallizing Lysozyme Solutions. J. Am. Chem. Soc. 1999, 121, 1627–1635. [Google Scholar] [CrossRef]
- Pan, W.; Vekilov, P.G.; Lubchenko, V. Origin of Anomalous Mesoscopic Phases in Protein Solutions. J. Phys. Chem. B 2010, 114, 7620–7630. [Google Scholar] [CrossRef]
- Chakraborty, P.; Ibáñez de Opakua, A.; Purslow, J.A.; Fromm, S.A.; Chatterjee, D.; Zachrdla, M.; Zhuang, S.; Puri, S.; Wolozin, B.; Zweckstetter, M. GSK3beta phosphorylation catalyzes the aggregation of tau into Alzheimer’s disease-like filaments. Proc. Natl. Acad. Sci. USA 2024, 121, e2414176121. [Google Scholar] [CrossRef] [PubMed]
- Savastano, A.; Flores, D.; Kadavath, H.; Biernat, J.; Mandelkow, E.; Zweckstetter, M. Disease-Associated Tau Phosphorylation Hinders Tubulin Assembly within Tau Condensates. Angew. Chem. Int. Ed. Engl. 2021, 60, 726–730. [Google Scholar] [CrossRef]
- Iqbal, K.; Alonso, A.D.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A.; et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta 2005, 1739, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Liu, F.; Gong, C.X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Johnson, G.V. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J. Biol. Chem. 2003, 278, 187–193. [Google Scholar] [CrossRef]
- Sperfeld, A.D.; Collatz, M.B.; Baier, H.; Palmbach, M.; Storch, A.; Schwarz, J.; Tatsch, K.; Reske, S.; Joosse, M.; Heutink, P.; et al. FTDP-17: An early-onset phenotype with parkinsonism and epileptic seizures caused by a novel mutation. Ann. Neurol. 1999, 46, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Mukrasch, M.D.; Bibow, S.; Korukottu, J.; Jeganathan, S.; Biernat, J.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 2009, 7, e34. [Google Scholar] [CrossRef]
- Moore, K.M.; Nicholas, J.; Grossman, M.; McMillan, C.T.; Irwin, D.J.; Massimo, L.; Van Deerlin, V.M.; Warren, J.D.; Fox, N.C.; Rossor, M.N.; et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: An international retrospective cohort study. Lancet Neurol. 2020, 19, 145–156. [Google Scholar] [CrossRef]
- Ferreon, J.C.; Jain, A.; Choi, K.J.; Tsoi, P.S.; MacKenzie, K.R.; Jung, S.Y.; Ferreon, A.C. Acetylation Disfavors Tau Phase Separation. Int. J. Mol. Sci. 2018, 19, 1360. [Google Scholar] [CrossRef]
- Edelhoch, H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 1967, 6, 1948–1954. [Google Scholar] [CrossRef]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, J.B.; Hyman, A.A.; Boke, E. Organization and Function of Non-dynamic Biomolecular Condensates. Trends Biochem. Sci. 2018, 43, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Galvanetto, N.; Ivanović, M.T.; Chowdhury, A.; Sottini, A.; Nüesch, M.F.; Nettels, D.; Best, R.B.; Schuler, B. Extreme dynamics in a biomolecular condensate. Nature 2023, 619, 876–883. [Google Scholar] [CrossRef]
- Lin, A.Z.; Ruff, K.M.; Dar, F.; Jalihal, A.; King, M.R.; Lalmansingh, J.M.; Posey, A.E.; Erkamp, N.A.; Seim, I.; Gladfelter, A.S.; et al. Dynamical control enables the formation of demixed biomolecular condensates. Nat. Commun. 2023, 14, 7678. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Nozawa, R.S.; Jia, T.Z.; Saio, T.; Mori, E. Biological phase separation: Cell biology meets biophysics. Biophys. Rev. 2020, 12, 519–539. [Google Scholar] [CrossRef]
- Nesterov, S.V.; Ilyinsky, N.S.; Uversky, V.N. Liquid-liquid phase separation as a common organizing principle of intracellular space and biomembranes providing dynamic adaptive responses. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119102. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Mehta, S.; Zhang, J. Liquid-liquid phase separation: A principal organizer of the cell’s biochemical activity architecture. Trends Pharmacol. Sci. 2021, 42, 845–856. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Eschmann, N.A.; Zhou, H.; Rauch, J.N.; Hernandez, I.; Guzman, E.; Kosik, K.S.; Han, S. RNA stores tau reversibly in complex coacervates. PLoS Biol. 2017, 15, e2002183. [Google Scholar] [CrossRef]
- Tsoi, P.S.; Quan, M.D.; Choi, K.J.; Dao, K.M.; Ferreon, J.C.; Ferreon, A.C. Electrostatic modulation of hnRNPA1 low-complexity domain liquid-liquid phase separation and aggregation. Protein Sci. 2021, 30, 1408–1417. [Google Scholar] [CrossRef]
- Tsoi, P.S.; Ferreon, J.C.; Ferreon, A.C.M. Initiation of hnRNPA1 Low-Complexity Domain Condensation Monitored by Dynamic Light Scattering. Int. J. Mol. Sci. 2024, 25, 6825. [Google Scholar] [CrossRef]
- Boyko, S.; Qi, X.; Chen, T.H.; Surewicz, K.; Surewicz, W.K. Liquid-liquid phase separation of tau protein: The crucial role of electrostatic interactions. J. Biol. Chem. 2019, 294, 11054–11059. [Google Scholar] [CrossRef] [PubMed]
- Wegmann., S.; Eftekharzadeh, B.; Tepper, K.; Zoltowska, K.M.; Bennett, R.E.; Dujardin, S.; Laskowski, P.R.; MacKenzie, D.; Kamath, T.; Commins, C.; et al. Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 2018, 37, e98049. [Google Scholar] [CrossRef]
- Boyko, S.; Surewicz, K.; Surewicz, W.K. Regulatory mechanisms of tau protein fibrillation under the conditions of liquid-liquid phase separation. Proc. Natl. Acad. Sci. USA 2020, 117, 31882–31890. [Google Scholar] [CrossRef] [PubMed]
- Boyko, S.; Surewicz, W.K. Tau liquid-liquid phase separation in neurodegenerative diseases. Trends Cell Biol. 2022, 32, 611–623. [Google Scholar] [CrossRef]
- Ambadipudi, S.; Biernat, J.; Riedel, D.; Mandelkow, E.; Zweckstetter, M. Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat. Commun. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Abasi, L.S.; Elathram, N.; Movva, M.; Deep, A.; Corbett, K.D.; Debelouchina, G.T. Phosphorylation regulates tau’s phase separation behavior and interactions with chromatin. Commun. Biol. 2024, 7, 251. [Google Scholar] [CrossRef]
- Li, S.C.; Goto, N.K.; Williams, K.A.; Deber, C.M. Alpha-helical, but not beta-sheet, propensity of proline is determined by peptide environment. Proc. Natl. Acad. Sci. USA 1996, 93, 6676–6681. [Google Scholar] [CrossRef]
- Williamson, M.P. The structure and function of proline-rich regions in proteins. Biochem. J. 1994, 297 Pt 2, 249–260. [Google Scholar] [CrossRef]
- Morimoto, A.; Irie, K.; Murakami, K.; Masuda, Y.; Ohigashi, H.; Nagao, M.; Fukuda, H.; Shimizu, T.; Shirasawa, T. Analysis of the secondary structure of beta-amyloid (Abeta42) fibrils by systematic proline replacement. J. Biol. Chem. 2004, 279, 52781–52788. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoi, P.S.; Lucas, L.; Rhoades, D.; Ferreon, J.C.; Ferreon, A.C.M. Electrostatic Effects on Tau Nanocondensates. Biomolecules 2025, 15, 406. https://doi.org/10.3390/biom15030406
Tsoi PS, Lucas L, Rhoades D, Ferreon JC, Ferreon ACM. Electrostatic Effects on Tau Nanocondensates. Biomolecules. 2025; 15(3):406. https://doi.org/10.3390/biom15030406
Chicago/Turabian StyleTsoi, Phoebe S., Lathan Lucas, Derek Rhoades, Josephine C. Ferreon, and Allan Chris M. Ferreon. 2025. "Electrostatic Effects on Tau Nanocondensates" Biomolecules 15, no. 3: 406. https://doi.org/10.3390/biom15030406
APA StyleTsoi, P. S., Lucas, L., Rhoades, D., Ferreon, J. C., & Ferreon, A. C. M. (2025). Electrostatic Effects on Tau Nanocondensates. Biomolecules, 15(3), 406. https://doi.org/10.3390/biom15030406






