Abstract
Worldwide, nearly 40% of adults are overweight and 13% are obese. Health consequences of excess weight include cardiovascular diseases, type 2 diabetes, dyslipidemia, and increased mortality. Treating obesity is challenging and calorie restriction often leads to rebound weight gain. Treatments such as bariatric surgery create hesitancy among patients due to their invasiveness. GLP-1 medications have revolutionized weight loss and can reduce body weight in obese patients by between 15% and 25% on average after about 1 year. Their mode of action is to mimic the endogenous GLP-1, an intestinal hormone that regulates glucose metabolism and satiety. However, GLP-1 drugs carry known risks and, since their use for weight loss is recent, may carry unforeseen risks as well. They carry a boxed warning for people with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. Gastrointestinal adverse events (nausea, vomiting, diarrhea) are fairly common while pancreatitis and intestinal obstruction are rarer. There may be a loss of lean body mass as well as premature facial aging. A significant disadvantage of using these medications is the high rate of weight regain when they are discontinued. Achieving success with pharmacologic treatment and then weaning to avoid future negative effects would be ideal.
1. Introduction
Obesity as defined by The Obesity Society (TOS) is a multi-factorial chronic disease that results from excess fat accumulation that presents a risk to health [1]. Multiple organ systems are affected which leads to additional chronic diseases associated with obesity. Obesity may present itself with multiple clinical phenotypes and also varied treatment responses. These varied treatment responses likely originate from our limited understanding of the mechanisms of weight regulation. The concept that body fat storage may be regulated was first proposed by Kennedy et al. through the concept of a “set point” [2]. He suggested that adipose tissue may produce a signal that may be sensed by the brain to target a “level of body fatness”. This model of body fat regulation was widely adopted in the 1990s with the discovery of leptin [3,4].
The set point theory remains a hypothesis as the molecular mechanisms behind it continue to be ambiguous. This is also why developing pharmacologic treatments is challenging without clear targets. However, in the last 20 years, the gastrointestinal system has been identified as the largest endocrine organ and has demonstrated the key role of gut hormones in energy homeostasis [5]. The discovery of the gut hormone glucagon-like peptide-1 (GLP-1) and the synthesis of agonists for its corresponding receptor (GLP-1 receptor) has tremendously impacted treatment for weight reduction. However, significant questions remain about these drugs. Based upon the “set point” theory of weight regulation, the possibility of needing these medications over extended periods of time to avoid the inevitable weight regain may cause the clinician to consider other treatments. The lack of long-term clinical trials in weight management compels clinicians to consider potentially unforeseen long-term side effects of the GLP-1 receptor agonists. The known risks of pancreatitis, gastroparesis, and lean body mass loss are variables to be considered as well. In order to present these drugs with a balance of their pros and cons, the longer-term studies showing cardiovascular benefits are also taken into account.
In this review, we examine the health risks of obesity and the overall magnitude of the problem. A discussion of potential therapeutic strategies including dietary, physical activity, bariatric surgery, and pharmacologic therapy are described. GLP-1 receptor agonist drug therapy is a key focus with consideration of the mechanism of action, clinical trials in weight management, and the potential role of the drug category in weight maintenance. We aim to provide a balanced discussion of the benefits of GLP-1 receptor agonists, as well as the risks and unknown effects. In an ideal setting, weight loss achieved with GLP-1 agonist therapy in the short-term could be durable without pharmacotherapy for many years and perhaps over a lifetime.
2. Methodology
The search methodology employed in this narrative review was comprehensive and aimed to capture current relevant evidence pertaining to GLP-1 medication use for weight loss and discontinuation of these medications. For the purposes of gathering information and preparing this paper, PubMed, Scopus, Embase, Web of Science, and Google Scholar were searched for peer-reviewed literature in English on 8 and 9 October 2024 and again for updates on 25 January 2025. We also scrutinized details of clinical trial protocols within ClinicalTrials.gov. Key terms included “obesity”, “GLP-1 receptor agonists”, “weight regain”, “weight loss”, “liraglutide”, “semaglutide”, “tirzepatide”, “exenatide”, “dulaglutide, “ phentermine/topiramat ”, “contrave”, “bariatric surgery”, “treatment outcomes”, “adverse effects”, and “clinical care pathways”. Keywords were then refined based on the relevance of the results, and additional terms were searched to survey related areas including “cardiometabolic risk factors”, ‘sarcopenia”, “exercise”, “body mass index (BMI)”, and “appetite”. We limited our search to studies from January 1995 onwards, with further relevant studies identified from citations within papers. Articles were initially screened for relevance based on the content of their abstracts. We focused primarily on clinical trials conducted in humans and also included in vitro and animal studies for mechanistic and molecular insights.
3. Obesity: Magnitude of the Problem
3.1. A Chronic Metabolic Condition
Obesity is traditionally defined as an excess of body fat, and is classically categorized in clinical practice in terms of body mass index (BMI). A BMI (in kg/m2) in the range of 18.5–24.9 is considered normal, 25–29.9 is overweight, and ≥30 is considered obese. Severe obesity, which is defined as a BMI over 40 kg/m2, is an alarming public health issue [6]. It should be noted, however, that BMI, although easy to gauge, has limitations when utilized as a diagnostic tool because it does not account for the exact muscle mass or fat mass, especially visceral adipose tissue. Visceral adipose tissue is more metabolically active and associated with the pathophysiology seen in obesity such as insulin resistance [7]. Waist circumference is an alternative to BMI and can be used as a complement to BMI. Kim et al. found a linear association between waist circumference and all-cause mortality in a study on 23,263,878 subjects over the age of 20 years [8]. Another reason fixation on BMI is not entirely accurate is because it does not account for factors such as age, sex, and ethnic variation. BMI also does not correlate with the risk of death at a population level [9]. Therefore, although BMI is appreciated for its simplicity in categorizing subjects, it should be used with caution as a diagnostic tool and might be better used as a screening aid [10].
Obesity is a non-communicable pandemic that continues to expand and affect over 1 billion people globally [11,12,13,14]. This worldwide escalation in population BMI is associated with multiple comorbidities and is believed to have numerous causes, including dietary habits, lifestyle, environmental stimuli, genetic predisposition, endocrinological disruptions, and gut dysbiosis [15,16].
3.2. Health Complications of Obesity
Obesity-driven inflammatory processes are responsible for a large portion of the damage inflicted by excess weight (Figure 1). Obesity has harmful effects on various body systems, most notably on the cardiovascular and endocrine systems, but also on the kidneys, liver, lungs, joints, and immune system [17]. The cardiometabolic consequences of obesity such as insulin resistance, glucose intolerance, type 2 diabetes, arterial hypertension, atherosclerosis, and dyslipidemia are all stressors on the heart and vascular system [18,19]. The lipid profile in obesity is marked by an increase in triglycerides and free fatty acids [20]. Obesity leads to oxidative stress from proinflammatory cytokines and adipokines [21]. The inflammatory environment incites endothelial dysfunction further contributing to cardiovascular risk and hypertension [22,23,24].
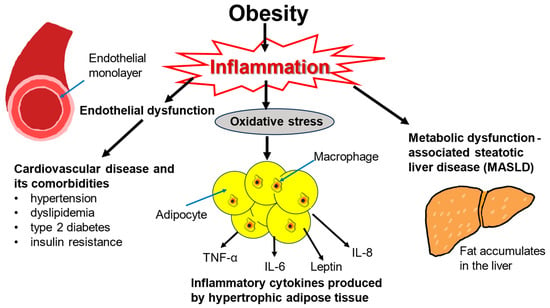
Figure 1.
Consequences of obesity-induced inflammation. Obesity leads to a physiological inflammatory response, such as oxidative stress, as illustrated by the hypertrophic adipocytes secreting pro-inflammatory cytokines, cardiometabolic conditions, and MALSD. These conditions increase the risk of mortality long-term which is generally associated with obesity.
Figure 1.
Consequences of obesity-induced inflammation. Obesity leads to a physiological inflammatory response, such as oxidative stress, as illustrated by the hypertrophic adipocytes secreting pro-inflammatory cytokines, cardiometabolic conditions, and MALSD. These conditions increase the risk of mortality long-term which is generally associated with obesity.
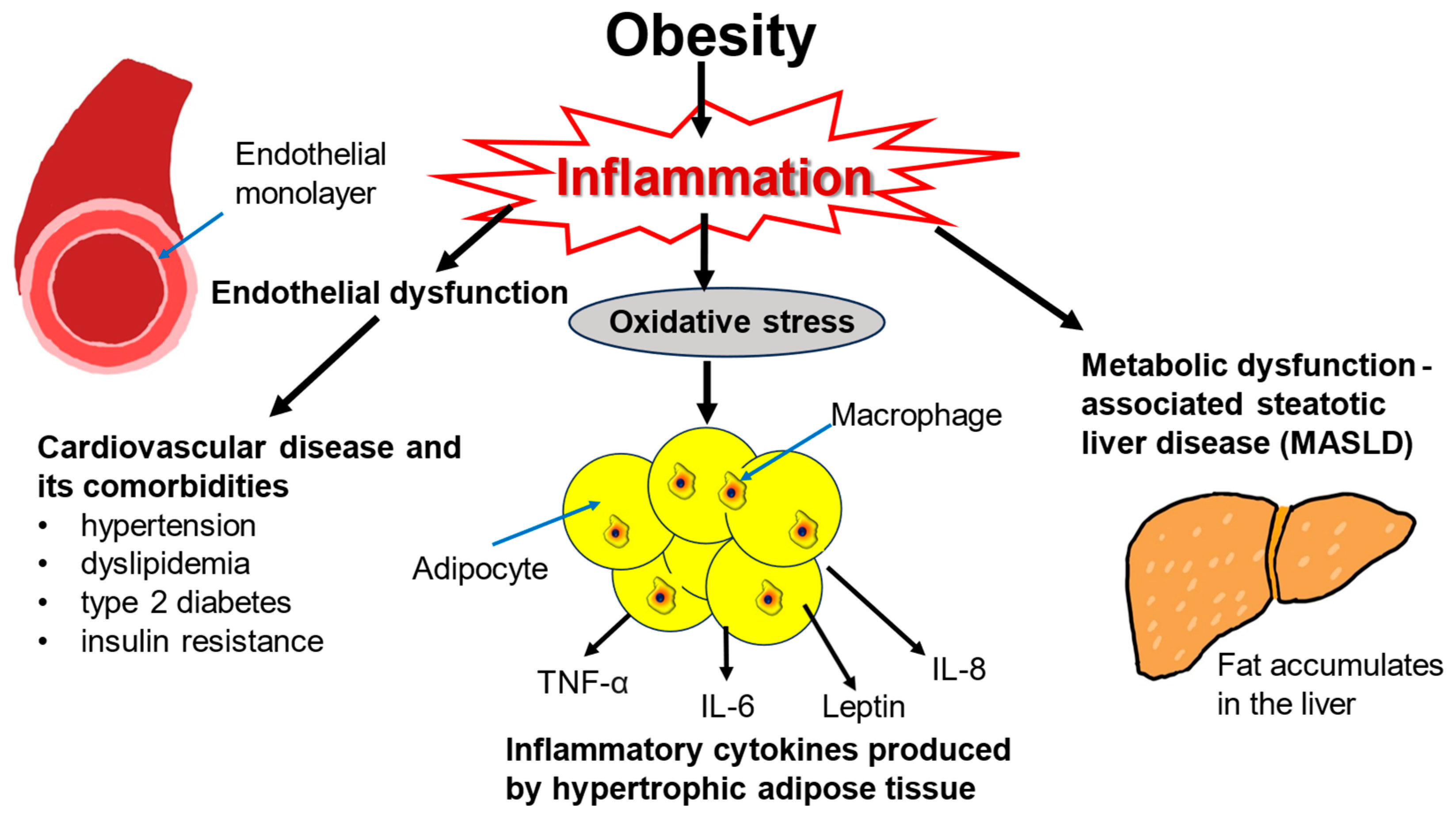
Obesity is also strongly associated with metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis, which is inflammation-driven [25,26,27]. Obesity also elevates the risk of developing some types of cancer including colorectal, esophageal, liver, and kidney malignancies [28,29,30,31,32]. Both cardiovascular disease and cancer contribute to excess mortality associated with obesity [33,34]. Obesity is the strongest risk factor for obstructive sleep apnea [35]. In this condition, excessive adiposity may restrict the lungs and impede their inflation, and adipose tissue in the diaphragm may compromise muscle strength, leading to intermittent hypoxia and hypercapnia [36,37]. Other health issues associated with obesity include depression, anxiety, and chronic kidney disease [38,39,40].
4. Treating Obesity: Current Therapies
4.1. Dietary Approaches
Treating obesity has evolved in recent years, with various shifts in dietary, pharmacological, and surgical strategies available for the management of obesity [41]. There are various dietary styles with goals of controlling macronutrient composition and calorie restriction. For instance, high protein diet, Mediterranean-style diet, low carbohydrate diet, low-calorie diet, and low-fat diet are just several options [42,43].
Dietary advice historically has seen energy restriction as the foundation for weight loss. Numerous studies have demonstrated that dietary macronutrient composition is not the most significant contributing factor for weight loss [44,45,46]. However, guidelines have started to include a more nuanced understanding that there is a synergy between dietary nutrients and their food sources [47]. When looking at macronutrient content, initially, dietary fat, carbohydrate, and protein content are scrutinized. The low-or very-low fat intake approach is recommended for inducing significant short-term weight loss, but its long-term efficacy is not superior to dietary interventions with higher fat content [48]. The low carbohydrate diet involves consuming a low content of carbohydrates and a high content of fat and protein. It is especially suitable for individuals with type 2 diabetes and/or insulin resistance [49]. Exceeding 6-12 months of use may have undesired effects by increasing LDL cholesterol and cardiovascular risk in some studies, but others have found no difference [50,51,52,53]. In a randomized study of overweight adults, following a low-fat or high-fat diet with average or high protein resulted in an average 7% weight loss at 6 months, irrespective of diet type [45]. Regular physical activity is also recommended as a component of weight loss programs not only for energy expenditure, but for cardiometabolic health as well [54]. At this time, the evolution of dietary guidelines from isolated macronutrients to broader dietary patterns is receiving major attention. One such example is the Mediterranean diet, which is rich in plant-based food with high dietary fiber and antioxidants. The Mediterranean diet has a higher composition of fatty acids, unlike the conventional Western diet. In addition to its effect on body weight, a variety of health benefits have been ascribed to it. These include favorable effects on heart and brain health and decreased diabetes risk [55,56]. Much of the recommended annual weight loss diets often seen in US News and World Report reflect a variety of these dietary patterns, highlighting that diets are more than their nutrient content [57]. Finally, the microbiome has a variety of mechanisms through which it affects obesity, and pre/probiotic therapies could be a helpful addition to a weight loss regimen [58,59].
4.2. Non-Incretin Oral Pharmacotherapies
Pharmacological treatments for weight loss have expanded and, while GLP-1 agonists are the focus of this review, other choices are available and summarized here.
Perhaps the least successful of the FDA-approved weight loss drugs in terms of achieving weight loss maintenance is Orlistat (tetrahydrolipstatin). An older drug, orlistat inhibits pancreatic lipases which break down dietary fat. By reducing absorption of fat via the intestine, it promotes weight loss [60]. Patients are advised to consume a low-fat diet to combat the side effects of oily stool [61]. This drug leads to a weight loss nadir at around 36 weeks with a weight regain that happens at around 52 weeks [62]. However, the weight regain is relatively mild and by 104 weeks there is still overall weight loss. Anti-obesity medications such as Contrave (bupropion/naltrexone) or Qsymia (phentermine/topiramate) also appear to be effective for maintaining weight loss [63]. Admittedly, the lesser potency of these drugs in the initial weight loss phase often overshadows their potential for usage for weight loss maintenance purposes. For instance, the Contrave Obesity Research studies (COR-I, COR II, COR-BMOD, and COR diabetes) were performed over a 56-week period [64,65]. Contrave appears to modulate appetite and reward centers. For Contrave, a weight loss plateau seems to occur for all the COR studies around 32 to 36 weeks with overall weight loss around 8 to 9%. Qsymia has the longest-term data of the available oral anti-obesity drugs, upwards of 108 weeks [66]. This drug also appears to induce satiety and reduce hunger. Qsymia has demonstrated overall efficacy for weight loss maintenance, achieving sustained weight loss of 9-10% at the 108-week mark compared to 1.8% for placebo [67].
4.3. Endoscopic and Bariatric Interventions
The intragastric balloon is an anti-obesity intervention in which a silicone balloon is endoscopically deployed and filled with saline and inflated for 6 months. It is a temporary and minimally invasive therapy that reduces stomach capacity and results in decreased hunger and food intake [68,69]. It can be used as a primary treatment for obesity, as an alternative for patients who do not qualify for bariatric surgery, or as a bridge to surgery [70].
Bariatric surgery is indicated in patients with a BMI above 40 independent of coexisting comorbidities or in patients with a BMI over 35 with a history of comorbidities such as type 2 diabetes or hypertension [71]. There are two common procedures currently used: sleeve gastrectomy and gastric bypass. Emerging evidence suggests that the sleeve procedure is associated with few reoperations, but significant regain of weight while the bypass procedure can lead to more durable weight loss and glycemic control [72,73]. There is strong evidence to support that bariatric surgery results in greater long-term weight loss than even the top nonsurgical interventions and a recent retrospective study showed superior cardiovascular benefit for sleeve gastrectomy and gastric bypass compared to GLP-1 treatment over a 10-year follow-up period [74]. Variations of Roux-en-Y gastric bypass limb lengths have shown potentially increased weight loss and metabolic benefit, but also, possible early and late significant complications [75,76,77]. Possible complications of the gastric bypass include bowel obstruction and malabsorption, while possible complications of the sleeve gastrectomy include venous thromboembolism and gastroesophageal reflux disease [78,79].
The metabolic efficacy of bariatric surgery in increasing gut production of GLP-1 to supraphysiologic levels postprandially is considered a major factor in early weight loss [80]. GLP-1 agonists are being explored as an adjunctive therapy to combine with bariatric surgery to avoid the weight regain that can occur post-surgery [81,82].
This summary of current treatments for obesity highlights both its difficulty and importance, as well as obesity’s role in exacerbating other disease processes.
5. GLP-1 Drug Function and Mechanism
5.1. GLP-1—The Hormone
GLP-1 is a hormone released from the enteroendocrine cells of the small bowel in response to the arrival of a nutrient bolus [83]. GLP-1 is secreted continuously at low basal levels and rises within minutes of food ingestion [84]. The initial attention in the clinical space for GLP-1 was related to the glucose-dependent insulin secretion effect, often referred to as the incretin effect [85]. The additional properties of inhibition of glucagon secretion and inhibition of caloric intake accelerated the development of GLP-1 receptor agonists for usage in type 2 diabetes management [86]. While largely successful as an anti-diabetic drug therapy, the effects on both reducing food intake and promoting weight loss in persons with diabetes and animal models prompted further study as an anti-obesity medication [87,88].
5.2. GLP-1 and Energy Balance
Identifying the mechanism of action of GLP-1 receptor agonists for inducing weight loss naturally led to the study of the impact of this drug class on two key regulatory factors in weight regulation, namely energy intake and energy expenditure. Energy balance is dependent on nutrient intake and subsequent nutrient oxidation rates [89,90]. The energy-dense nature of fat makes it an efficient means of storing excess energy intake and thus the body favors fat for keeping energy in reserve [91,92]. Energy balance is buffered by fat stores and the adipose compartment therefore potentially producing an obesogenic state [93]. GLP-1 secretion seems to be impaired in obese subjects, which informs at least the partial role of GLP-1 in the pathophysiology of obesity [94,95,96].
There are conflicting data in animal models regarding GLP-1-related drugs stimulating energy expenditure [97]. The mechanisms behind this usually involved brown adipose tissue thermogenesis [98,99]. However, this has not translated over to human studies [100,101,102]. Therefore, most of the effect of weight loss via GLP-1-related pathways may be related to a decrease in energy intake, rather than the direct effects on energy expenditure [103]. Newer anti-obesity medications are being developed that have promise for altering energy expenditure, although these are multi-receptor agonists involving binding both GLP-1 receptors and receptors such as glucagon which has been associated with increased energy expenditure [104]. However, challenges remain in such multi-agonist receptor treatments, and the focus remains predominantly on energy intake [105]. For this reason, the role of GLP-1 within the nervous system continues to be thoroughly investigated.
5.3. GLP-1 and the Nervous System
GLP-1 receptors have been identified in numerous areas of the central nervous system (CNS) including the area postrema, hypothalamus, lateral parabrachial nucleus, nucleus accumbens, nucleus tract solitarius, and the vagal efferent neurons [106,107,108,109]. While the brain does produce GLP-1, most circulating GLP-1 is gut-derived [110,111]. There are often low levels of GLP-1 circulating systemically and the post-prandial rise in GLP-1 is of intestinal origin. However, in a murine model, knockout of glucagon genes originating from the bowel elicited no increase in food intake, and thus appetite suppression is attributed to GLP-1 produced within the CNS [112].
It is interesting to note that proglucagon and proglucagon-derived peptides (e.g., GLP-1, glucagon-like peptide-2, oxyntomodulin) are present in a small group of neurons in the nucleus tract solitarius within the brainstem and are another source of endogenous GLP-1 production [113,114]. The peripheral GLP-1 system and the central preproglucagon neurons within the brain stem have not been linked and appear to function independently of each other [106,115].
There is an existing network of neurons within the hypothalamus that is widely studied for its role in energy homeostasis. This is referred to as the melanocortin system and is composed of two differing populations of neurons involved in satiety and food intake. This includes the proopiomelanocortin and amphetamine-regulated transcript (POMC/CART) pathway, which promotes satiety and decreased hunger, often referred to as the anorectic pathway [116]. The opposing pathway is the agouti-related peptide (AgRP) neurons which stimulate hunger and increase food intake, often referred to as the orexigenic pathway [117]. In rodent models, the majority of POMC/CART neurons have GLP-1 receptors, predominantly in the arcuate nucleus of the hypothalamus [118]. However, GLP-1 activity has also been seen in the hindbrain, with infusion of GLP-1 analogs into the fourth ventricle in rodent models. Reductions in food intake and body weight have been found, implying that both the hypothalamus and brainstem are important in the control of energy intake and body weight [119,120]. If these findings are also reflected in human physiology, targeting GLP-1 within the CNS would be a constructive goal for pharmacologic strategies.
5.4. GLP-1 Benefits to Organ Systems
It is meaningful to note that GLP-1 agonists have been increasingly examined outside the effects on type 2 diabetes and obesity. A growing number of trials have studied the beneficial impact of GLP-1 agonists on metabolic dysfunction associated with steatotic liver disease (MASLD), chronic kidney disease, as well as cardiovascular disease [121,122,123,124]. The benefit of the GLP-1 agonists may be from the reduction of adiposity, or non-adiposity related. For instance, the SELECT trial demonstrated the cardiovascular benefit of the GLP-1 receptor agonist semaglutide beyond that of weight loss [125]. In this placebo-controlled trial, subjects were over the age of 45 years, had a BMI of 27 or above, and established cardiovascular disease. Cardiovascular endpoints (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) were compared in those taking semaglutide versus placebo after a mean follow-up of 39.8 months, and the drug intervention was found to be superior to placebo. Benefits to the cardiovascular system extend beyond weight loss to affect other risk factors such as triglyceride level, systolic blood pressure, risk of progression to diabetes, and the inflammatory marker C-reactive protein. SELECT also showed better cardiovascular outcomes in persons with obesity and without diabetes who had previously undergone coronary artery bypass graft surgery [126]. Weight loss was sustained over 4 years [127]. There are a number of proposed mechanisms including reduction of inflammation, improvement of endothelial and ventricular function, as well as decreasing platelet aggregation [128]. A meta-analysis from Lin et al. showed the benefit of GLP-1 drugs in peripheral artery disease and heart failure [129]. Zhang et al. found that in persons with type 2 diabetes, GLP-1 drugs offered benefits whether or not the patients were also taking metformin [130].
6. GLP-1 Receptor Agonists and Weight Loss
Liraglutide was the first GLP-1 receptor agonist that was FDA-approved for weight loss. It helped one-third of the non-diabetic study patients achieve a loss of 10% of their body weight and also helped them sustain their weight loss for upwards of 1 year [131]. The weight loss plateau occurred after about 20 weeks and continued until the drug was stopped at 56 weeks. Similarly, the subcutaneously once weekly formulation of the GLP-1 receptor agonist semaglutide showed equally promising results for weight maintenance [132]. First, there was a greater overall weight loss of 15% and therefore the weight loss plateau was delayed to around 68 weeks. Persistence of the weight loss plateau (or presumed weight loss maintenance) occurred up until 104 weeks [133].
The newest incretin-based medication is a combined GLP-1 receptor and glucose-dependent insulinotropic polypeptide (GIP) dual agonist known as tirzepatide. An even greater weight loss is seen with this novel dual agonist, achieving upwards of a 22.5% weight loss at 72 weeks [134,135,136]. At 3 years of follow-up, tirzepatide use led to a sustained mean loss of weight of 20% with less likelihood of deterioration to diabetes in persons with obesity and prediabetes when compared to placebo [137].
7. GLP-1 Usage and Adverse Effects
7.1. Usage
Since the FDA approval of liraglutide for weight loss in 2015, the use of this class of medication has exploded, particularly over the last few years with the availability of weekly GLP-1s including tirzepatide and semaglutide. In an analysis of one US health systems database a 700% increase in GLP-1 prescribing over the past four years was noted, primarily driven by prescriptions for obesity [138]. Such a rapid increase in usage led to multiple drug shortages beginning in 2022 which continued through late 2024 [139]. Under section Section 503A of the FD&C Act compounding pharmacies were permitted to produce GLP-1 compounds to increase availability [140]. The usage of compounding pharmacies does come with risks as the medications produced are not FDA-regulated [141]. Furthermore, ongoing surveillance and monitoring led to the FDA issuing a statement of counterfeit products in users’ hands [142]. It is important to note when discussing the side effects and adverse outcomes of this drug class that many adverse effects are class-related; however, the degree and severity of reported side effects in certain circumstances may have been due to drug purity or dosing issues.
7.2. GLP1 Receptor Analogs Approved for Weight Loss
The potential application of GLP-1 in diabetes was recognized as early as 1998 [143]. The emergence of GLP-1 receptor agonists has re-invigorated interest in anti-obesity medications and more effective weight management. GLP-1 receptor agonist drug development began in earnest in 2005 with the approval of exenatide, a synthetic form of a natural peptide hormone isolated from the saliva of the venomous lizard Gila monster [144]. It was found to have similar activity to GLP-1, but with a longer half-life. A synthetic version of exendin-4 was approved by the FDA in 2005 for glycemic control in type 2 diabetics [145,146]. A number of clinical trials in persons with diabetes have been subsequently performed and frequently cited for the clinical efficacy of exendin-4 [147,148]. However, the prevalence of obesity along with the need for more effective anti-obesity treatments prompted the study of the first daily GLP-1 receptor agonist, liraglutide. Liraglutide was the first injectable daily GLP-1 receptor agonist that was approved by the FDA for weight loss in 2014. The Satiety and Clinical Adiposity Liraglutide Evidence (SCALE) trial demonstrated one-third of patients lost 10% of their body weight in 1 year with sustained weight loss demonstrated at 2 years [149,150]. A 3-year extension of the SCALE trial showed that persons with overweight or obese and prediabetes taking liraglutide had a reduced risk for developing type 2 diabetes with greater weight loss compared to those taking a placebo [151]
Arguably, the arrival of semaglutide more than five years later sparked the public’s attention to GLP-1 agonist therapy. This once-weekly subcutaneous injection was FDA-approved for weight loss in June 2021. Semaglutide was studied in a comprehensive series of clinical trials known as the Semaglutide Treatment Effect in People with Obesity (STEP). The STEP-1 trial is considered the pivotal trial that demonstrated 14.9% weight loss at 68 weeks with semaglutide 2.4 mg [142]. The follow-up STEP-5 trial demonstrated that semaglutide could sustain weight loss over 104 weeks or nearly 2 years [152]. While there are newer and even more potent anti-obesity drugs on the horizon, semaglutide still garners attention for its non-diabetic and non-obesity benefits, specifically cardiovascular risk reduction, as well as improving renal outcomes [125,153].
However, tirzepatide is a novel dual agonist drug that activates GLP-1 receptors, as well as the GIP receptor. It was FDA-approved for weight loss in November 2023 [154]. Tirzepatide can uniquely induce weight loss beyond what is achieved with selective GLP-1 agonists alone. As with the STEP trials, a comprehensive series of clinical trials were performed with tirzepatide known as the SURMOUNT trials. The SURMOUNT-1 trial demonstrated a 15 mg dosage of tirzepatide in non-diabetic obese subjects leads to 20.9% weight loss at week 72 with sustained weight loss during a 3-year extension period [134,137].
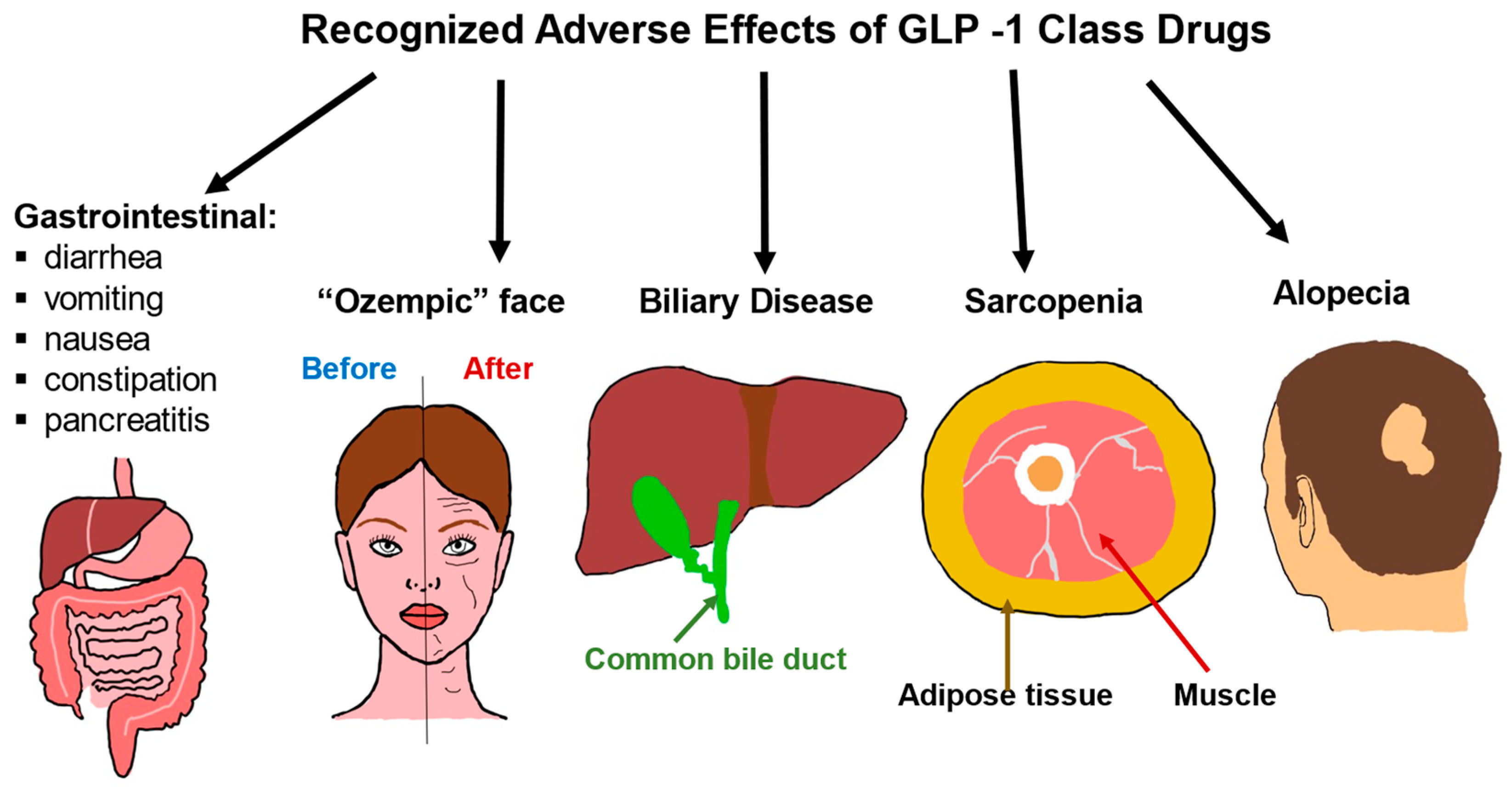
7.3. Common Adverse Effects
GLP-1 medications can cause a range of side effects related to the gastrointestinal system as well as changes in muscle mass and effects on the appearance of the face and loss of hair (Figure 2). The most common detrimental consequences of the GLP-1 class are gastrointestinal. The most often reported side effects are nausea and vomiting which are a result of activation of specific GLP-1 receptors in the hindbrain and these symptoms can be mitigated with gradual dose escalation [155,156,157]. In addition to nausea and vomiting, other GI-related side effects include diarrhea, constipation, dyspepsia, decreased appetite, and abdominal pain [158,159,160]. It is estimated that around 80–90% of patients will develop an adverse effect from the use of this class.
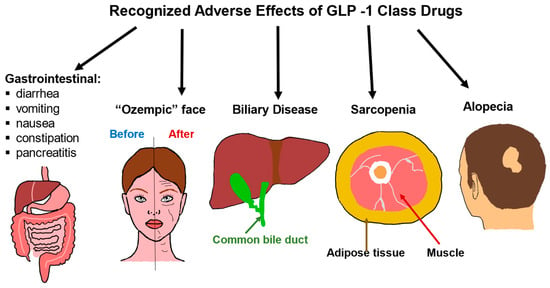
Figure 2.
Adverse effects of GLP-1 class drugs. Gastrointestinal issues are common, ranging from nausea and diarrhea to rarer and more severe consequences such as pancreatitis. Rapid weight loss due to the use of GLP-1 drugs is associated with biliary disease, sarcopenia, and alopecia. Rapid weight loss can also lead to what is known as an “Ozempic face”, where the cheeks become hollowed out, and wrinkles, as well as eye bags, become more pronounced.
A more serious gastrointestinal concern is that of pancreatitis. Early studies on patients with type 2 diabetes treated with incretin therapy including GLP-1s and dipeptidyl peptidase-4 (DPP4) inhibitors did demonstrate an association between drug usage and the development of pancreatitis [159]. GLP-1R analogs have been associated with lipase and amylase suggesting a mechanism of pancreatic inflammation [161,162]. In fact, the package insert for semaglutide suggests an increase of 13% for amylase and 22% for lipase and the dual incretin agonist tirzepatide suggests a 38% increase and lipase upwards of a 42% increase [163]. Despite this, animal studies have demonstrated decreases in pancreatic secretion in response to GLP-1 elevation, therefore the mechanism behind this potential interaction of GLP-1 receptor analogs and pancreatitis remains elusive [164]. It is important to note that these studies mostly included dipeptidyl peptidase-4 DPP4 and early GLP-1s, exenatide, and liraglutide. Furthermore, patients with type 2 diabetes are inherently at higher risk of pancreatitis [165]. The large and long-duration GLP-1 cardiovascular outcome trials did not show an increase in pancreatitis [166]. Recent trials on the newer GLP-1 agents including tirzepatide and semaglutide specific to weight loss have not demonstrated increased rates of pancreatitis, although product labeling still instructs avoidance in patients with a history of pancreatitis [167,168,169].
Rapid weight loss has long been associated with biliary disease, sarcopenia, and alopecia [170,171,172,173,174]. As the newer agents in the GLP class have become incredibly potent where users are losing an estimated 15-20% of body weight, with much of the weight loss occurring in the initial weeks of initiating the drug [175,176]. It is thus not surprising that these same side effects of rapid weight loss are seen as a class effect. The rapid weight loss can be visualized in many areas of the body and one of these manifestations known as “Ozempic face” occurs when fat pads in the face are rapidly depleted [177,178]. Patients should be aware of these potential unwanted effects and, to minimize loss of muscle mass, encouraged to participate in resistance exercises and increase protein intake [179].
While these cosmetic findings are an issue, the loss of lean body mass is another area of concern [180]. The landmark GLP-1 drug trial for semaglutide, STEP 1 (semaglutide treatment effect in people with obesity), demonstrates a significant loss of total lean body mass [132], which has been further corroborated by other investigators [181]. Tirzepatide has demonstrated total lean mass loss as well, although additional studies are needed to determine the impact of this [134,182]. The question of whether the ratio between fat mass and lean body mass is disrupted or maintained during weight loss with GLP-1 agonists is still unresolved [183,184]. Studies to clarify this issue would particularly be needed for patients who are afflicted with sarcopenic obesity, a condition of a mismatch between muscle and fat mass.
7.4. Less Recognized Adverse Effects
A common finding amongst all GLP-1 trials was that of increased heart rate attributed to a direct effect on the pacemaker cells within the sinus node of the heart [185]. This is generally a benign clinical finding with no associated arrhythmias from GLP-1 usage [186,187]. Headaches, dizziness, hypoglycemia, and injection site reactions have also been reported albeit to a much lesser degree [188,189]. Xie et al. applied a high dimensional approach to analyze associations between GLP-1 treatment and health outcomes in over 1.9 million persons over an average of 3.68 years based on data from the US Department of Veterans Affairs and found, in addition to gastrointestinal and other common symptoms, a significant increase in hypotension, joint pain, kidney stones, and nephrolithiasis [190].
7.5. Special Considerations
7.5.1. Ocular
GLP-1 therapy has two ophthalmological manifestations warranting discussion [191]. Ever since the UK Prospective Diabetes Study demonstrated modifiable retinopathy with improvements in glycemic control, clinicians and patients have aimed to improve glucose as a standard of management in type 2 diabetes [192]. A somewhat paradoxical effect has been demonstrated with GLP-1 usage and other agents for type 2 diabetes in which rapid improvement in glycemia results in worsening of retinopathy [193]. The long-term effect of GLP-1 on retinopathy in patients with type 2 diabetes may in fact be beneficial. It is important to monitor patients on therapy [194,195,196].
More recently with the increased employment of GLP-1 agents, a relatively new complication, non-arteritic anterior ischemic optic neuropathy (NAION), has been associated with their usage. Although both the association of retinopathy and NAION can be seen with GLP-1 use, it is worth noting that a majority of cases occurred in patients with type 2 diabetes. It is unclear whether those without type 2 diabetes using GLP-1 for weight loss are at the same risk [197,198].
7.5.2. Malignancy
Parks and Rosebrough first expressed concerns regarding human safety with liraglutide as early rodent trials demonstrated an increased risk of medullary thyroid carcinoma [199]. All GLP-1 agents have carried an FDA black-boxed warning of increased risk of C cell thyroid carcinoma and recommended agents used in patients with a personal or family history of multiple endocrine neoplasia type 2A or 2B. The exact risk change with other histological subtypes of thyroid carcinoma is yet to be fully established. Two trials have supported an increase in the risk of all types of thyroid carcinoma [200,201]. In contrast, a more recent Scandinavian trial did not detect a significant association between GLP-1 usage and thyroid cancer [202]. All studies are in agreement that the greatest risk if any does occur in the initial months or year of therapy [200,201,202,203,204]. Emerging data have established that GLP-1 agonist administration has not increased the risk of malignancy outside of the thyroid gland and may in fact reduce the risk of malignancy with potential for preventive applications [203,204].
7.5.3. Pregnancy
In animals exposed to GLP-1 agonists during pregnancy or lactation, studies have shown an association with reduced offspring size, delayed growth, and skeletal deformations, each of which is typically associated with reduced maternal caloric intake. Accidental human evidence has not been associated with any adverse outcomes, but overall data are scarce [205,206]. Interestingly enough, pregnancy rates may actually increase while on GLP-1 medications. Firstly, with delayed gastric emptying, nausea, vomiting, and diarrhea there is a potential for impaired absorption of oral contraceptive pills. Specific guidance on the usage of alternate methods of birth control is provided by drug manufacturers. Secondarily medically induced weight loss, particularly when totaling in excess of 5% of total body weight has demonstrated effectiveness in improving fertility [207].
7.5.4. Mental Health
Initial case reports and media reportage of an increase in suicidal ideation in persons taking GLP-1 medications were looked into further by the FDA. The FDA and studies from a cohort of Scandinavian patients concluded no association between GLP-1 use and suicidal ideation, self -harm, or new onset of depression [208,209,210]. Data are still accumulating and no definitive answer has emerged [211,212].
7.5.5. Perioperative
Early case reports called into question appropriate fasting times for pre-procedural and operative fasting due to retained gastric contents and risk of aspiration in patients taking GLP-1 medications and compounds [213,214]. A recent multi-society joint guidance statement advocated for an individualized approach based upon each patient’s unique factors rather than a one-size approach of holding this medication for all patients undergoing procedures [215]. Though more evidence is needed such guidance is useful to patients and clinicians at the present time [216,217]. As with all new medications or those whose use increases due to expanded indication, ongoing monitoring and close surveillance by both patients and clinicians continue to be necessary.
In summary, there are many side effects associated with GLP-1 drug treatment and they span a range from minor to potentially life-threatening. For many of them, measures can be taken to alleviate discomfort and risk (Table 1).

Table 1.
Common adverse effects of GLP-1 agonists and approaches to minimizing these consequences.
8. GLP-1 Receptor Agonists and Weight Regain
8.1. Forces Driving Weight Regain
The general trajectory of weight loss with initiation of GLP-1 therapy has been well-studied (Table 2) [218,219,220]. Despite the overall efficacy of the incretin-based treatments for weight loss, there is a lack of long-term controlled studies beyond about 4 years available [221,222]. While there is optimism that continuing use of GLP-1 treatments will preserve weight loss, most other anti-obesity strategies, including surgical interventions, generally have weight recidivism [127]. This can be attributed to the persistent effects of metabolic adaptation, the phenomena seen in weight regulation that may cause weight regain and potentially a weight loss plateau [223]. Definitions of weight regain may vary, namely the duration and how much is considered significant. It is worthwhile to point out that a weight loss plateau is often stated with the assumption that additional weight loss is desired, but difficult to achieve. Additionally, there are those who are attempting to prevent weight regain after already achieving a weight-reduced state. In this setting, prevention of weight regain may be better-termed weight loss maintenance.
Ultimately, understanding the existing forces that occur in a weight-reduced state may help to understand what may drive weight regain [224,225]. The conceptual framework for weight regain and weight loss maintenance is based on the theory that the human body acts to defend a particular body mass, via the hypothetical “settling point” of weight [226,227,228]. The drivers for weight regain are hypometabolism and hyperphagia in the weight-reduced state [229,230,231]. Hypometabolism, or the decrease of energy expenditure greater than what would be predicted, is known as metabolic adaptation [232]. It is therefore rational to consider these processes as targets to prevent weight regain and achieve weight loss maintenance, although some studies show that energy expenditure is not uniformly disproportionately decreased in those sustaining weight loss long-term [233]. While there are available therapies for hyperphagia and hunger and, in fact, appetite reduction is a key effect of GLP-1 agonists, there are no significant available therapies that can address the decrease in energy expenditure [234]. However, if one were to choose a mechanism to prevent weight regain, targeting hunger would seem to be the better alternative due to the greater effect of metabolic adaptation on hunger, rather than the decrease in energy expenditure [235].

Table 2.
GLP-1 agonist intervention and observed effects over time.
Table 2.
GLP-1 agonist intervention and observed effects over time.
| Duration of Intervention with GLP-1 Agonist (in Months) | Observed Beneficial Effects | References |
|---|---|---|
| BaselineStarting dose | Body adjustment, appetite reduction, decreased caloric intake | [234] |
| 1-3 monthsStep-up dose | Weight loss, improvement in insulin sensitivity, reduction in HbA1c | [121,122,158] |
| 3-6 monthsStep-up dose | Continued improvement in blood sugar levels, most effective weight loss, reduced risk for cardiovascular events | [121,158,224,225] |
| 6-12 monthsStable dose | Weight loss plateau, improvement in HbA1c, further decrease in cardiovascular events | [123,158] |
| Beyond 12 monthsStable dose | Well-controlled HbA1c, prevention of long-term diabetes complications, weight loss maintained | [123,124,132,158] |
Abbreviations: GLP-1—glucagon-like peptide-1; HbA1c—hemoglobin A1c.
8.2. Hunger
For those with untreated obesity and seeking active weight loss, decreasing hunger and achieving caloric restriction is seemingly the primary process that needs to occur. Therefore, treating hyperphagia is the strategy for both weight loss and weight loss maintenance. However, even during active weight loss, metabolic adaptation seems to be set into motion, and in fact may be triggered by achieving 11% of total body weight loss [236,237]. This suggests that decreased caloric intake can drive the initial periods of weight loss, but when weight reduction reaches 10%, decreases in energy expenditure begin with increasing strong driving forces of hunger to try to slow the weight loss process. Developing treatment paradigms for weight loss maintenance remain focused on decreasing hunger, despite the compensatory decrease in energy expenditure [94]. Incretin-based medications and other anti-obesity medicines target hunger, therefore fostering both weight loss and weight loss maintenance.
The popularity of GLP-1 receptor analogs may have to do with their profound effects on the CNS. Early studies demonstrated the anorectic actions of GLP-1 on the hypothalamus [238,239]. In situ hybridization studies in animal models demonstrated GLP-1 receptor presence in many other brain areas such as the thalamus, nucleus accumbens, and hindbrain. GLP-1 action in the hindbrain reduced food intake and body weight over time [240]. Clinical studies have supported this with GLP-1 receptor analogs such as liraglutide and semaglutide in both weight loss and weight loss maintenance trials.
8.3. GLP-1 Receptor Agonist Discontinuation
Cessation of these drugs to see if weight maintenance could be achieved was largely unsuccessful [241] (Table 3). Randomized double-blinded placebo-controlled withdrawal studies were performed in both semaglutide and tirzepatide with crossover to placebo at 20 weeks and 36 weeks, respectively [242,243]. When switched to placebo there was invariably weight regain, implying that the loss of inhibition of hyperphagia drove this process. It is worthwhile to note in both studies all participants were prescribed a reduced calorie (500 kcal/day deficit) and increased physical activity 150 min/week) regimen, which was insufficient to help preserve the initial weight loss. It is interesting to note that an extension study of semaglutide was performed to 120 weeks, but treatment was discontinued at 68 weeks. As expected, there was weight regain, but there still was an overall 5.6% net loss of weight by the end of 120 weeks [244]. The authors of this study pointed out that there appears to have been a slowing of weight regain towards the end of the study, implying a weight loss plateau below the initial pre-treatment weight. This could imply the drug’s potential altering of the settling point for weight.

Table 3.
Studies of weight regain after GLP-1 agonist or dual GLP-1/GIP agonist discontinuation.
9. Avoiding Weight Regain
9.1. Factors That Avert Weight Regain
A large public database known as the National Weight Control Registry (NWCR) was the first study to identify participants who were successful at weight loss and follow them over a 10-year period, with an attempt to identify variables that were associated with success [245]. To participate in the study, weight loss greater than 30 pounds had to have been maintained for more than 1 year at the time of enrollment. In this study population, 88% were able to keep 10% of their body weight off at year 5 and 87% at year 10. These successful subjects with weight loss maintenance reported high levels of physical activity, high levels of dietary restraint, low calorie, and fat intake, and low levels of overeating (loss of control of eating or disinhibition) [246]. Data from the NWCR database highlight the role of negative thoughts that induce eating. It is worthwhile to note these variables are all related to appetite, hunger, and caloric intake. The NWCR continues to provide information about these successful weight loss maintenance patients, but analyzing this data should be taken with the knowledge that these are select, highly motivated subjects who are attentive to their own health needs. Other studies confirm the importance of dietary restraint and physical activity in preventing weight regain [247,248].
Even more recently in 2022, a symposium was convened to discuss the state of the science of weight loss maintenance, known as the Pennington Biomedical Scientific Symposium [249]. The statement generated by the symposium broadly included nutritional strategies and physical activity recommendations. Not surprisingly, food composition is often an area of question by both scientific communities and the food industry to determine the right “mix” of macronutrients to facilitate weight loss and weight loss maintenance. However, over the years, many studies have shown no differences in achieving weight loss from a variety of macronutrient approaches [45] and calorie reduction is likely the more successful approach for weight loss maintenance [250,251]. However, as detailed in this review, calorie reduction generally leads to short-term weight loss, with poor success rates for long-term weight loss maintenance. This can be attributed to the metabolic adaptations that are seen to occur in those with obesity. Most effective weight loss from a dietary standpoint is seen over 3 to 6 months, with at least one-third of patients regaining lost weight within the first year and the majority of patients regaining the weight after five years [224,225].
The Pennington symposium highlighted potential alternative approaches for nutrition management that may be beneficial for weight loss maintenance. Specifically, a decrease in processed and ultraprocessed food consumption would be beneficial for weight loss maintenance. Processed foods are difficult to remove from the population’s food supply and public diets, due to their ubiquity as well as their low cost, convenience, and appealing taste [252,253]. However, consumption of ultraprocessed food has been shown to induce an even greater consumption of calories, and therefore leads to weight gain. Alternatively, an upcoming area in nutrition sciences involves precision medicine. Precision medicine itself is an area of medical management that tries to match personalized treatments or food content, to individual genetics, microbiome, metabolism, age, and sex. However, this treatment strategy is still in the early stages although the National Institutes for Health (NIH) recently has invested in research in the area of personalized nutrition.
9.2. Level of Physical Activity
While most of the interventions are based on targeting hunger and energy expenditure, there is little understanding of whether physical activity or exercise plays a role [254]. In simple terms, exercise is related to energy expenditure, and therefore increasing exercise increases energy expenditure and therefore weight loss. While this does seem to be true over the lower ranges of physical activity, with the upper ranges of physical activity the energy expenditure appears to plateau, consistent with a “constrained total energy expenditure model” [255,256]. Despite its somewhat attenuated impact on energy expenditure, there is evidence that exercise still helps to achieve weight loss maintenance. For instance, those with high levels of physical activity are more successful at weight loss maintenance [257,258,259]. Other studies have found little to no impact of physical activity on maintaining weight loss [260]. On the other hand, Ostendorf et al. found that high levels of physical activity including 200-300 min/week of at least moderate-intensity aerobic activity for upwards of 18 months supported weight loss maintenance [261]. Data from the NWCR also suggest successful weight maintainers can spend upwards of one hour per day in light physical activity [262]. Newer strategies for weight maintenance have focused on preserving or even increasing lean body mass to counteract the decreases in energy expenditure thereby allowing for sustained weight loss [105,263].
9.3. Targeting Mood and Providing Support
Psychological well-being is an important aspect of both eating behaviors and weight loss [264,265]. Successful weight loss management is often associated with low levels of depression [266,267]. Studies suggest that GLP-1 drugs are not a direct cause of depressive symptoms in weight loss [268]. A supportive weight management team approach considers mood changes and how they can affect quality of life [269,270]. Family encouragement and support have also been associated with successful weight loss management [271,272,273]. For health care practitioners, in-person sessions may be more successful than remote sessions, but both have value [246,274,275].
10. Present and Future of Weight Loss
It may be possible to maintain weight loss while tapering GLP-1 to a lower dosage or prolonging the time between doses [276,277]. Patients have also been reported to take “drug holidays” in which they pause the use of the drug intermittently for special occasions, but there is very little in the literature on this [278]. The effects on weight, cardiovascular health, and other parameters of decreasing the dose or pausing and resuming the use of GLP-1 agonist is an area where evidence-based studies are needed.
The approval of tirzepatide, a novel long-acting dual incretin agonist of both GLP-1 and another incretin, GIP, continues to create excitement for the development of anti-obesity medications [279]. GIP is a similar gut hormone to GLP-1 that is secreted by enteroendocrine cells. It similarly induces insulin secretion in the setting of hyperglycemia. However, earlier studies showed inconsistences of GIP as a cause of weight loss, although more recent studies have demonstrated increased weight loss efficacy [280,281,282,283]. Recent studies have now demonstrated the strongest weight loss effect with the dual agonist for GLP-1 and GIP, upwards of 22% over 1 year [134]. The effect of tirzepatide on energy expenditure appears negligible, therefore making the effect on energy intake the most impactful [284]. However, available studies are only seen in rodent studies, but the additive or synergistic effects of GLP-1 and GIP on hunger and satiety require further clinical research [285].
A multi-agonist approach is a likely road for the future of anti-obesity drug development involving novel receptors such as glucagon and amylin possibly with even more profound weight loss [286,287]. Amylin and its analogs are gaining much interest [288]. Amylin is a non-incretin hormone produced in the pancreas by β cells that is released upon nutrient intake. It delays gastric emptying and acts on areas of the brain controlling appetite and signaling fullness. Amylin analogs such as cagrilintide are being explored for obesity treatment in concert with GLP-1 drugs [289].
Drugs are in development to combat muscle loss during fat loss [290]. Monoclonal antibodies such as bimagrumab, trevogrumab, and garetosmab, prevent muscle loss by blocking the activation of receptors within the myostatin-activin-follistatin-inhibin system that cause muscle mass to decrease [291]. Clinical trial outcomes will determine whether these will be useful either on their own or in conjunction with GLP-1 agonists.
GLP-1 agonists demonstrate efficacy for weight loss maintenance, but only while the patient is continuing to use the medication. The NCWR highlights variables associated with weight loss maintenance success for upwards of 10 years, although this study is with a motivated population of individuals [243,292]. Certainly, teaching patients to be mindful of their eating and to consume adequate protein can contribute to weight loss maintenance success and overall health [293]. Physical activity also has shown significant benefits for upwards of two years. What remains to be seen is if the mixing and matching of the initial weight loss strategy, whatever this may be, with another weight loss maintenance strategy will lead to successful weight maintenance. An example would include the usage of liraglutide that helped one achieve a particular amount of weight, but continued usage of the drug led to weight regain, would switching to Contrave help to achieve weight loss maintenance?
These are questions that will need to be answered for future clinical trials. Ideally, the chosen initial intervention for weight loss would also be effective for weight loss maintenance. However, the only truly long-term strategy that has been the most successful for long-term weight loss is surgical weight loss. Upwards of 20 years has seen a sustained 22% weight loss [294]. However, even surgical weight loss reaches a peak weight nadir 1 to 2 years after surgery and weight regain tends to occur after. However, the overall weight loss is still significant, and surgical weight loss is ultimately a personal choice for patients as it does carry risks [295,296,297].
11. A Multi-Pronged Approach to Avoiding Weight Regain
The use of GLP-1 drugs as a weight loss tool is prevalent and effective, but it is preferable to find ways to keep the weight off without a lifetime of drug treatment and this is an area that needs attention [298]. Lifestyle interventions alone are not durable for most people [299,300]. Studies are clearly needed to find ways to support withdrawal of pharmacotherapy without weight rebound using a multi-disciplinary approach which would likely involve behavioral change, nutritional guidance, structured physical activity, and perhaps peer support groups [301,302,303,304,305].
12. Conclusions
Even with information on nutrition, physical activity, anti-obesity medications, and psychological support, there is no universally effective strategy in terms of weight loss maintenance. The overall mechanisms of GLP-1 agonists on weight loss are predominantly through the reduction in energy intake and not on energy expenditure. While the area of anti-obesity medication development is expanding, GLP-1 receptor agonists are already available and represent substantial progress in the growing armamentarium for use in weight loss. The effect seems principally within the CNS, but combination treatment of GLP-1 with other targets appears to further improve weight loss in early clinical trials. At this time, very little attention is being afforded to the discontinuation of GLP-1 drugs after weight loss has occurred, but this may change if serious consequences of prolonged exposure in young persons are documented.
Author Contributions
Conceptualization, A.B.R. and J.D.L.; writing—original draft preparation, A.B.R., S.G., R.L. and S.P.K.; writing—review and editing, A.B.R., A.S., H.A.R. and R.L.; visualization, S.G. and A.S.; supervision, A.B.R., J.D.L. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Robert Buescher and Edmonds Bafford. We thank Maggie Lachcik, and Rachel Schnabl from our development team. A special thank you to Amy Glass for her assistance with references. Thank you to Rick Ceo for the video abstract design.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jastreboff, A.M.; Kotz, C.M.; Kahan, S.; Kelly, A.S.; Heymsfield, S.B. Obesity as a Disease: The Obesity Society 2018 Position Statement. Obesity 2019, 27, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.C. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B Biol. Sci. 1953, 140, 578–592. [Google Scholar]
- Speakman, J.R.; Levitsky, D.A.; Allison, D.B.; Bray, M.S.; de Castro, J.M.; Clegg, D.J.; Clapham, J.C.; Dulloo, A.G.; Gruer, L.; Haw, S.; et al. Set points, settling points and some alternative models: Theoretical options to understand how genes and environments combine to regulate body adiposity. Dis. Models Mech. 2011, 4, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Bosy-Westphal, A.; Heymsfield, S.B. Is there evidence for a set point that regulates human body weight? F1000 Med. Rep. 2010, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Sun, E.W.; Keating, D.J. Mechanisms controlling hormone secretion in human gut and its relevance to metabolism. J. Endocrinol. 2019, 244, R1–R15. [Google Scholar] [CrossRef]
- Zhou, X.D.; Chen, Q.F.; Yang, W.; Zuluaga, M.; Targher, G.; Byrne, C.D.; Valenti, L.; Luo, F.; Katsouras, C.S.; Thaher, O.; et al. Burden of disease attributable to high body mass index: An analysis of data from the Global Burden of Disease Study 2021. EClinicalMedicine 2024, 76, 102848. [Google Scholar] [CrossRef]
- Choi, K.E.; Joung, C.; Pahk, K.J.; Kim, H.; Pahk, K. Metabolic activity of visceral adipose tissue is associated with age-related macular degeneration: A pilot 18F-FDG PET/CT study. Front. Endocrinol. 2024, 14, 1322326. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.M.; Han, K.D.; Jung, J.H.; Lee, S.S.; Oh, S.W.; Park, H.S.; Rhee, E.J.; Lee, W.Y.; Yoo, S.J. Waist circumference and all-cause mortality independent of body mass index in korean population from the national health insurance health checkup 2009–2015. J. Clin. Med. 2019, 8, 72. [Google Scholar] [CrossRef]
- Prillaman, M. Why BMI is flawed—And how to redefine obesity. Nature 2023, 622, 232–233. [Google Scholar] [CrossRef]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Horváth, L.; Mráz, M.; Jude, E.B.; Haluzík, M. Pharmacotherapy as an Augmentation to Bariatric Surgery for Obesity. Drugs 2024, 84, 933–952. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Long, M.W.; Gortmaker, S.L. Association of body mass index with health care expenditures in the United States by age and sex. PLoS ONE 2021, 16, e0247307. [Google Scholar] [CrossRef]
- van der Valk, E.S.; van den Akker, E.L.T.; Savas, M.; Kleinendorst, L.; Visser, J.A.; Van Haelst, M.M.; Sharma, A.M.; van Rossum, E.F.C. A comprehensive diagnostic approach to detect underlying causes of obesity in adults. Obes. Rev. 2019, 20, 795–804. [Google Scholar] [CrossRef]
- Dell’Olio, A.; Scott, W.T., Jr.; Taroncher-Ferrer, S.; San Onofre, N.; Soriano, J.M.; Rubert, J. Tailored impact of dietary fibers on gut microbiota: A multi-omics comparison on the lean and obese microbial communities. Microbiome 2024, 12, 250. [Google Scholar] [CrossRef]
- Coral, D.E.; Smit, F.; Farzaneh, A.; Gieswinkel, A.; Tajes, J.F.; Sparsø, T.; Delfin, C.; Bauvain, P.; Wang, K.; Temprosa, M.; et al. Subclassification of obesity for precision prediction of cardiometabolic diseases. Nat. Med. 2025, 31, 534–543. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-García, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef]
- Bays, H.E.; Kirkpatrick, C.F.; Maki, K.C.; Toth, P.P.; Morgan, R.T.; Tondt, J.; Christensen, S.M.; Dixon, D.L.; Jacobson, T.A. Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024. J. Clin. Lipidol. 2024, 18, e320–e350. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Varra, F.N.; Varras, M.; Varra, V.K.; Theodosis-Nobelos, P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options (Review). Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- de Lima, E.P.; Moretti, R.C., Jr.; Torres Pomini, K.; Laurindo, L.F.; Sloan, K.P.; Sloan, L.A.; Castro, M.V.M.; Baldi, E., Jr.; Ferraz, B.F.R.; de Souza Bastos Mazuqueli Pereira, E.; et al. Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways. Biology 2024, 13, 519. [Google Scholar] [CrossRef]
- Kajikawa, M.; Higashi, Y. Obesity and Endothelial Function. Biomedicines 2022, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, Y.; Cheng, L.; Huang, Y.; Rao, W.; Shi, H.; Li, J. Potential therapeutic strategies for MASH: From preclinical to clinical development. Life Metab. 2024, 3, loae029. [Google Scholar] [CrossRef]
- Alpízar Salazar, M.; Olguín Reyes, S.E.; Medina Estévez, A.; Saturno Lobos, J.A.; De Aldecoa Castillo, J.M.; Carrera Aguas, J.C.; Alaniz Monreal, S.; Navarro Rodríguez, J.A.; Alpízar Sánchez, D.M.F. Natural History of Metabolic Dysfunction-Associated Steatotic Liver Disease: From Metabolic Syndrome to Hepatocellular Carcinoma. Medicina 2025, 61, 88. [Google Scholar] [CrossRef]
- Kaya, E.; Syn, W.K.; Manka, P. Glucagon like peptide-1 receptor agonists as a promising therapeutic option of metabolic dysfunction associated steatotic liver disease and obesity: Hitting two targets with one shot. Curr. Opin. Gastroenterol. 2025. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Ghnaim, A.; Midlej, K.; Zohud, O.; Karram, S.; Schaefer, A.; Houri-Haddad, Y.; Lone, I.M.; Iraqi, F.A. Host Genetics Background Affects Intestinal Cancer Development Associated with High-Fat Diet-Induced Obesity and Type 2 Diabetes. Cells 2024, 13, 1805. [Google Scholar] [CrossRef]
- Teng, Y.; Xia, C.; Cao, M.; Yang, F.; Yan, X.; He, S.; Cao, M.; Zhang, S.; Li, Q.; Tan, N.; et al. Esophageal cancer global burden profiles, trends, and contributors. Cancer Biol. Med. 2024, 21, 656–666. [Google Scholar] [CrossRef]
- Kang, L.; Chen, X.; Qi, P.; Ma, Z.; Han, D.; Zhang, X.; Shang, P. Research progress on the correlation between obesity and the occurrence and development of kidney cancer: A narrative review. Transl. Cancer Res. 2024, 13, 5678–5690. [Google Scholar] [CrossRef]
- Mahamat-saleh, Y.; Aune, D.; Freisling, H.; Hardikar, S.; Jaafar, R.; Rinaldi, S.; Gunter, M.; Dossus, L. Association of metabolic obesity phenotypes with risk of overall and site-specific cancers: A systematic review and meta-analysis of cohort studies. Br. J. Cancer 2024, 131, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Solsona-Vilarrasa, E.; Vousden, K.H. Obesity, white adipose tissue and cancer. FEBS J. 2024. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.; Sampaio, F.; Costa, J.; Freitas, P.; Matias Dias, C.; Gaio, V.; Conde, V.; Figueira, D.; Pinheiro, B.; Silva Miguel, L. Burden of disease and cost of illness of overweight and obesity in Portugal. Obes. Facts 2024. advance online publication. [Google Scholar] [CrossRef]
- Asif, M. Obesity: A Profoundly Under Recognized Chronic Disease, And Its Impacts on Cardiovascular Disease. South Dak. Med. 2024, 77, 378–379. [Google Scholar]
- Zhang, M.; Weng, X.; Xu, J.; Xu, X. Correlation between obstructive sleep apnea and weight-adjusted-waist index: A cross-sectional study. Front. Med. 2024, 11, 1463184. [Google Scholar] [CrossRef]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. 2017, 1, 00019. [Google Scholar]
- Turnbull, C.D.; Wang, S.H.; Manuel, A.R.; Keenan, B.T.; McIntyre, A.G.; Schwab, R.J.; Stradling, J.R. Relationships between MRI fat distributions and sleep apnea and obesity hypoventilation syndrome in very obese patients. Sleep Breath. 2018, 22, 673–681. [Google Scholar] [CrossRef]
- Prasad, R.; Jha, R.K.; Keerti, A. Chronic Kidney Disease: Its Relationship With Obesity. Cureus 2022, 14, e30535. [Google Scholar] [CrossRef]
- Lam, B.C.C.; Lim, A.Y.L.; Chan, S.L.; Yum, M.P.S.; Koh, N.S.Y.; Finkelstein, E.A. The impact of obesity: A narrative review. Singap. Med. J. 2023, 64, 163–171. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Y.; Zhao, F.; Cui, R.; Xie, W.; Liu, Q.; Yang, W. Shared biological mechanisms of depression and obesity: Focus on adipokines and lipokines. Aging 2023, 15, 5917–5950. [Google Scholar] [CrossRef]
- Reid, T.J.; Korner, J. Medical and Surgical Treatment of Obesity. Med. Clin. N. Am. 2022, 106, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Moriconi, D.; Berta, R.; Baldi, S.; Quinones-Galvan, A.; Guiducci, L.; Taddei, S.; Mari, A.; Nannipieri, M. Effects of Low-Carbohydrate versus Mediterranean Diets on Weight Loss, Glucose Metabolism, Insulin Kinetics and β-Cell Function in Morbidly Obese Individuals. Nutrients 2021, 13, 1345. [Google Scholar] [CrossRef]
- Akbari, M.; Vali, M.; Rezaei, S.; Bazmi, S.; Tabrizi, R.; Lankarani, K.B. Comparison of weight loss effects among overweight/obese adults: A network meta-analysis of mediterranean, low carbohydrate, and low-fat diets. Clin. Nutr. ESPEN 2024, 64, 7–15. [Google Scholar] [CrossRef]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.M.; Springfield, S.; Van Horn, L.; Khera, A.; Lamendola, C.; Mayo, S.M.; Gardner, C.D.; Vadiveloo, M.K.; Petersen, K.S.; Joseph, J.J.; et al. Popular Dietary Patterns: Alignment With American Heart Association 2021 Dietary Guidance: A Scientific Statement From the American Heart Association. Circulation 2023, 147, 1715–1730. [Google Scholar]
- Tobias, D.K.; Chen, M.; Manson, J.E.; Ludwig, D.S.; Willett, W.; Hu, F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Brehm, B.J.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 1617–1623. [Google Scholar] [CrossRef]
- Budoff, M.; Manubolu, V.S.; Kinninger, A.; Norwitz, N.G.; Feldman, D.; Wood, T.R.; Fialkow, J.; Cury, R.; Feldman, T.; Nasir, K. Carbohydrate Restriction-Induced Elevations in LDL-Cholesterol and Atherosclerosis: The KETO Trial. JACC Adv. 2024, 3, 101109. [Google Scholar] [CrossRef]
- Kripp, A.M.; Feichter, A.; König, D. A low-carbohydrate, high-fat diet leads to unfavorable changes in blood lipid profiles compared to carbohydrate-rich diets with different glycemic indices in recreationally active men. Front. Nutr. 2024, 11, 1473747. [Google Scholar] [CrossRef] [PubMed]
- Naude, C.E.; Brand, A.; Schoonees, A.; Nguyen, K.A.; Chaplin, M.; Volmink, J. Low-carbohydrate versus balanced-carbohydrate diets for reducing weight and cardiovascular risk. Cochrane Database Syst. Rev. 2022, 1, CD013334. [Google Scholar] [CrossRef]
- Chawla, S.; Tessarolo Silva, F.; Amaral Medeiros, S.; Mekary, R.A.; Radenkovic, D. The Effect of Low-Fat and Low-Carbohydrate Diets on Weight Loss and Lipid Levels: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 3774. [Google Scholar] [CrossRef] [PubMed]
- Jakicic, J.M.; Apovian, C.M.; Barr-Anderson, D.J.; Courcoulas, A.P.; Donnelly, J.E.; Ekkekakis, P.; Hopkins, M.; Lambert, E.V.; Napolitano, M.A.; Volpe, S.L. Physical Activity and Excess Body Weight and Adiposity for Adults. American College of Sports Medicine Consensus Statement. Med. Sci. Sports Exerc. 2024, 56, 2076–2091. [Google Scholar] [CrossRef]
- D’Alessandro, A.; De Pergola, G. The Mediterranean Diet: Its definition and evaluation of a priori dietary indexes in primary cardiovascular prevention. Int. J. Food Sci. Nutr. 2018, 69, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Best Diets Overall 2025. Available online: https://health.usnews.com/best-diet/best-diets-overall (accessed on 3 January 2025).
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Sang, Y.; Liu, M.; Wang, Q.; Yang, H.; Li, X. Gut microbiota in health and disease: Advances and future prospects. MedComm 2024, 5, e70012. [Google Scholar] [CrossRef]
- Hollywood, A.; Ogden, J. Taking Orlistat: Predicting weight loss over 6 months. J. Obes. 2011, 2011, 806896. [Google Scholar] [CrossRef]
- Jain, S.S.; Ramanand, S.J.; Ramanand, J.B.; Akat, P.B.; Patwardhan, M.H.; Joshi, S.R. Evaluation of efficacy and safety of orlistat in obese patients. Indian J. Endocrinol. Metab. 2011, 15, 99–104. [Google Scholar] [CrossRef]
- Rössner, S.; Sjöström, L.; Noack, R.; Meinders, A.E.; Noseda, G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity: European Orlistat Obesity Study Group. Obes. Res. 2000, 8, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.; Mancini, M.C. Safety assessment of combination therapies in the treatment of obesity: Focus on naltrexone/bupropion extended release and phentermine-topiramate extended release. Expert Opin. Drug Saf. 2017, 16, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L.; Fujioka, K.; Plodkowski, R.A.; Mudaliar, S.; Guttadauria, M.; Erickson, J.; Kim, D.D.; Dunayevich, E. Effect of Naltrexone plus Bupropion on Weight Loss in Overweight and Obese Adults (COR-I): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2010, 376, 595–605. [Google Scholar] [CrossRef]
- Sherman, M.M.; Ungureanu, S.; Rey, J.A. Naltrexone/Bupropion ER (Contrave): Newly Approved Treatment Option for Chronic Weight Management in Obese Adults. Pharm. Ther. 2016, 41, 164–172. [Google Scholar]
- Lonneman, D.J., Jr.; Rey, J.A.; McKee, B.D. Phentermine/Topiramate extended-release capsules (qsymia) for weight loss. Pharm. Ther. 2013, 38, 446–452. [Google Scholar]
- Garvey, W.T.; Ryan, D.H.; Look, M.; Gadde, K.M.; Allison, D.B.; Peterson, C.A.; Schwiers, M.; Day, W.W.; Bowden, C.H. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012, 95, 297–308. [Google Scholar] [CrossRef]
- Silva, L.B.; Neto, M.G. Intragastric balloon. Minim. Invasive Ther. Allied Technol. 2022, 31, 505–514. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, Y.; Qiu, X.; Lin, S.; Wen, J. Endoscopic bariatric surgery for adults with overweight and obesity: A systematic review and network meta-analysis. Int. J. Obes. 2024, 49, 237–245. [Google Scholar] [CrossRef]
- Ying, L.; Butensky, S.; Ilang-Ying, Y.; Ghiassi, S. Current State of Endoscopic Bariatric Therapies. Surg. Clin. N. Am. 2025, 105, 159–171. [Google Scholar] [CrossRef]
- Wyszomirski, K.; Walędziak, M.; Różańska-Walędziak, A. Obesity, Bariatric Surgery and Obstructive Sleep Apnea-A Narrative Literature Review. Medicina 2023, 59, 1266. [Google Scholar] [CrossRef]
- Fink, J.M.; Hetzenecker, A.; Seifert, G.; Runkel, M.; Laessle, C.; Fichtner-Feigl, S.; Marjanovic, G. Banded Versus Nonbanded Sleeve Gastrectomy: A Randomized Controlled Trial With 3 Years of Follow-up. Ann. Surg. 2020, 272, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Alaidaroos, O.; Al Jaber, A.A.; Al Jaber, A.A.; Alshehri, A.H.; Alkehaimi, M.B.; Alsannat, O.A. Long-Term Outcomes of Sleeve Gastrectomy Versus Gastric Bypass. Cureus 2024, 16, e72961. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.; Sohail, A.H.; Sulaiman, S.A.; Mansoor, L.; Cohen, E.M.; Adekolu, A.A.; Abunnaja, S.; Szoka, N.; Tabone, L.E.; Thakkar, S.; et al. Metabolic and bariatric surgery versus glucagon-like peptide-1 receptor agonist therapy: A comparison of cardiovascular outcomes in patients with obesity. Am. J. Surg. 2025, 242, 116242. [Google Scholar] [CrossRef]
- Lau, R.; Stevenson, M.; Tirumalasetty, M.B.; Lee, J.; Hall, C.; Miao, Q.; Brathwaite, C.; Ragolia, L. A Longer Biliopancreatic Limb and Shorter Common Channel Enhance Weight Loss But May Have Harmful Effects in Mouse Models of Roux-en-Y Gastric Bypass. Obes. Surg. 2025, 35, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, S.; Montrief, T.; Koyfman, A.; Long, B. High risk and low incidence diseases: Bariatric surgery complications. Am. J. Emerg. Med. 2025, 87, 113–122. [Google Scholar] [CrossRef]
- Farah, A.; Tatakis, A.; Malshy, K.; Mahajna, A.; Sayida, S. Real-Time Perfusion and Leak Assessment in Bariatric Surgery: Bridging Traditional and Advanced Techniques. Cureus 2024, 16, e71919. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Beekley, A.; Johnson, D.C.; Davis, K.A. Early and late complications of bariatric operation. Trauma Surg. Acute Care Open 2018, 3, e000219. [Google Scholar] [CrossRef]
- Çalık Başaran, N.; Dotan, I.; Dicker, D. Post metabolic bariatric surgery weight regain: The importance of GLP-1 levels. Int. J. Obes. 2025. advance online publication. [Google Scholar] [CrossRef]
- Stoll, F.; Kantowski, T.; Laaser, J.; Kloiber, U.; Plitzko, G.; Mann, O.; Aberle, J.; Lautenbach, A. Tackling suboptimal clinical response after metabolic bariatric surgery: Impact of tirzepatide on weight loss and body composition. Obes. Res. Clin. Pract. 2025, 19, 63–69. [Google Scholar] [CrossRef]
- Jensen, A.B.; Renström, F.; Aczél, S.; Folie, P.; Biraima-Steinemann, M.; Beuschlein, F.; Bilz, S. Efficacy of the Glucagon-Like Peptide-1 Receptor Agonists Liraglutide and Semaglutide for the Treatment of Weight Regain After Bariatric surgery: A Retrospective Observational Study. Obes. Surg. 2023, 33, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Gasbjerg, L.S.; Helsted, M.M.; Hartmann, B.; Jensen, M.H.; Gabe, M.B.N.; Sparre-Ulrich, A.H.; Veedfald, S.; Stensen, S.; Lanng, A.R.; Bergmann, N.C.; et al. Separate and Combined Glucometabolic Effects of Endogenous Glucose-Dependent Insulinotropic Polypeptide and Glucagon-like Peptide 1 in Healthy Individuals. Diabetes 2019, 68, 906–917. [Google Scholar] [CrossRef]
- Drucker, D.J.; Habener, J.F.; Holst, J.J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Investig. 2017, 127, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Discovery of GLP-1-Based Drugs for the Treatment of Obesity. N. Engl. J. Med. 2025, 392, 612–615. [Google Scholar] [CrossRef]
- Berning, P.; Adhikari, R.; Schroer, A.E.; Jelwan, Y.A.; Razavi, A.C.; Blaha, M.J.; Dzaye, O. Longitudinal Analysis of Obesity Drug Use and Public Awareness. JAMA Netw. Open 2025, 8, e2457232. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727.e3. [Google Scholar] [CrossRef]
- Petrovic, A.; Jovicic, S.; Dodevska, M.; Djordjevic, B.; Milinkovic, N.; Ivanovic, N.D. Effects of Specially Designed Energy-Restricted Diet on Anthropometric Parameters and Cardiometabolic Risk in Overweight and Obese Adults: Pilot Study. Nutrients 2024, 16, 3453. [Google Scholar] [CrossRef] [PubMed]
- Robbins, A.L.; Savage, D.B. The genetics of lipid storage and human lipodystrophies. Trends Mol. Med. 2015, 21, 433–438. [Google Scholar] [CrossRef]
- De Fano, M.; Malara, M.; Vermigli, C.; Murdolo, G. Adipose Tissue: A Novel Target of the Incretin Axis? A Paradigm Shift in Obesity-Linked Insulin Resistance. Int. J. Mol. Sci. 2024, 25, 8650. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Elmquist, J.K. Obesity: An evolutionary context. Life Metab. 2022, 1, 10–24. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef]
- Ranganath, L.R.; Beety, L.M.; Morgan, L.M.; Wright, J.W.; Howland, R.; Marks, V. Attenuated GLP-1 secretion in obesity: Cause or consequence? Gut 1996, 38, 916–919. [Google Scholar] [CrossRef]
- Faerch, K.; Torekov, S.S.; Vistisen, D.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; Sandbæk, A.; et al. Glucagon-like peptide-1 (GLP-1) response to oral glucose is reduced in pre-diabetes, screen-detected type 2 diabetes and obesity, and influenced by sex: The ADDITION-PRO study. Diabetes 2015, 64, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Barrera, J.G.; Sandoval, D.A.; D’Alessio, D.A.; Seeley, R.J. GLP-1 and energy balance: An integrated model of short-term and long-term control. Nat. Rev. Endocrinol. 2011, 7, 507–516. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Lockie, S.H.; Heppner, K.M.; Chaudhary, N.; Chabenne, J.R.; Morgan, D.A.; Veyrat-Durebex, C.; Ananthakrishnan, G.; Rohner-Jeanrenaud, F.; Drucker, D.J.; DiMarchi, R.; et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 2012, 61, 2753–2762. [Google Scholar] [CrossRef]
- Janssen, L.G.M.; Nahon, K.J.; Bracké, K.F.M.; van den Broek, D.; Smit, R.; Sardjoe Mishre, A.S.D.; Koorneef, L.L.; Martinez-Tellez, B.; Burakiewicz, J.; Kan, H.E.; et al. Twelve weeks of exenatide treatment increases [18F]fluorodeoxyglucose uptake by brown adipose tissue without affecting oxidative resting energy expenditure in nondiabetic males. Metabolism 2020, 106, 154167. [Google Scholar] [CrossRef]
- van Can, J.; Sloth, B.; Jensen, C.B.; Flint, A.; Blaak, E.E.; Saris, W.H.M. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 784–793. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Purnell, J.Q. The Role of Glucagon-Like Peptide-1 in Energy Homeostasis. Metab. Syndr. Relat. Disord. 2019, 17, 183–191. [Google Scholar] [CrossRef]
- Kleinert, M.; Sachs, S.; Habegger, K.M.; Hofmann, S.M.; Müller, T.D. Glucagon Regulation of Energy Expenditure. Int. J. Mol. Sci. 2019, 20, 5407. [Google Scholar] [CrossRef]
- Christoffersen, B.Ø.; Sanchez-Delgado, G.; John, L.M.; Ryan, D.H.; Raun, K.; Ravussin, E. Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obesity 2022, 30, 841–857. [Google Scholar] [CrossRef]
- Trapp, S.; Brierley, D.I. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br. J. Pharmacol. 2022, 179, 557–570. [Google Scholar] [CrossRef]
- Dossat, A.M.; Lilly, N.; Kay, K.; Williams, D.L. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J. Neurosci. 2011, 31, 14453–14457. [Google Scholar] [CrossRef]
- Richard, J.E.; Farkas, I.; Anesten, F.; Anderberg, R.H.; Dickson, S.L.; Gribble, F.M.; Reimann, F.; Jansson, J.O.; Liposits, Z.; Skibicka, K.P. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: Neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology 2014, 155, 4356–4367. [Google Scholar] [CrossRef]
- Katsurada, K.; Maejima, Y.; Nakata, M.; Kodaira, M.; Suyama, S.; Iwasaki, Y.; Kario, K.; Yada, T. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: Projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem. Biophys. Res. Commun. 2014, 451, 276–281. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Crosstalk between glucagon-like peptide 1 and gut microbiota in metabolic diseases. mBio 2024, 15, e0203223. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [PubMed]
- Song, Y.; Koehler, J.A.; Baggio, L.L.; Powers, A.C.; Sandoval, D.A.; Drucker, D.J. Gut-Proglucagon-Derived Peptides Are Essential for Regulating Glucose Homeostasis in Mice. Cell Metab. 2019, 30, 976–986.e3. [Google Scholar] [CrossRef] [PubMed]
- Vrang, N.; Larsen, P.J. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: Role of peripherally secreted and centrally produced peptides. Prog. Neurobiol. 2010, 92, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.J.; Vrang, N.; Tang-Christensen, M. Central pre-proglucagon derived peptides: Opportunities for treatment of obesity. Curr. Pharm. Des. 2003, 9, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Brierley, D.I.; Holt, M.K.; Singh, A.; de Araujo, A.; McDougle, M.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.; Maske, C.; et al. Central and peripheral GLP-1 systems independently suppress eating. Nat. Metab. 2021, 3, 258–273. [Google Scholar] [CrossRef]
- Niu, Y.; Yu, W.; Kou, X.; Wu, S.; Liu, M.; Chen, C.; Ji, J.; Shao, Y.; Xue, Z. Bioactive compounds regulate appetite through the melanocortin system: A review. Food Funct. 2024, 15, 11811–11833. [Google Scholar] [CrossRef]
- Baldini, G.; Phelan, K.D. The melanocortin pathway and control of appetite-progress and therapeutic implications. J. Endocrinol. 2019, 241, R1–R33. [Google Scholar] [CrossRef]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Hayes, M.R.; Leichner, T.M.; Zhao, S.; Lee, G.S.; Chowansky, A.; Zimmer, D.; De Jonghe, B.C.; Kanoski, S.E.; Grill, H.J.; Bence, K.K. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011, 13, 320–330, Correction in Cell Metab. 2016, 23, 745. [Google Scholar] [CrossRef]
- Reiner, D.J.; Mietlicki-Baase, E.G.; McGrath, L.E.; Zimmer, D.J.; Bence, K.K.; Sousa, G.L.; Konanur, V.R.; Krawczyk, J.; Burk, D.H.; Kanoski, S.E.; et al. Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance. J. Neurosci. 2016, 36, 3531–3540. [Google Scholar] [CrossRef]
- Masaki, T.; Ozeki, Y.; Yoshida, Y.; Okamoto, M.; Miyamoto, S.; Gotoh, K.; Shibata, H. Glucagon-Like Peptide-1 Receptor Agonist Semaglutide Improves Eating Behavior and Glycemic Control in Japanese Obese Type 2 Diabetic Patients. Metabolites 2022, 12, 147. [Google Scholar] [CrossRef]
- Mellbin, L.G.; Bhatt, D.L.; David, J.P.; Ekström, K.; Petrie, M.C.; Rasmussen, S.; Vilsbøll, T. Semaglutide and cardiovascular outcomes by baseline HbA1c in diabetes: The SUSTAIN 6 and PIONEER 6 trials. Eur. Heart J. 2024, 45, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo-Garcia, M.I.; Merino-Torres, J.F. GLP-1 Receptor Agonists and Cardiovascular Disease in Patients with Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 4020492. [Google Scholar] [CrossRef]
- Badve, S.V.; Bilal, A.; Lee, M.M.Y.; Sattar, N.; Gerstein, H.C.; Ruff, C.T.; McMurray, J.J.V.; Rossing, P.; Bakris, G.; Mahaffey, K.W.; et al. Effects of GLP-1 receptor agonists on kidney and cardiovascular disease outcomes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2025, 13, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Emerson, S.; Plutzky, J.; Kahn, S.E.; Stensen, S.; Weeke, P.E.; Musinga, D.; Poirier, P.; Lingvay, I.; Lincoff, A.M. Semaglutide Improves Cardiovascular Outcomes in Patients With History of Coronary Artery Bypass Graft and Obesity. J. Am. Coll. Cardiol. 2025, 85, 541–545. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat. Med. 2024, 30, 2049–2057. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Qi, X.; Fan, G.; Zhou, L.; Peng, Z.; Yang, J. Anti-atherosclerotic effect of incretin receptor agonists. Front. Endocrinol. 2024, 15, 1463547. [Google Scholar] [CrossRef]
- Lin, Y.M.; Wu, J.Y.; Lee, M.C.; Su, C.L.; Toh, H.S.; Chang, W.T.; Chen, S.Y.; Kuo, F.H.; Tang, H.J.; Liao, C.T. Comparative cardiovascular effectiveness of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors in atherosclerotic cardiovascular disease phenotypes: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2025. advance online publication. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Hao, Y. Comparative efficacy of GLP-1 RAs/SGLT-2 inhibitors in reducing cardiovascular events in type 2 diabetes according to baseline use of metformin: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Med. Res. 2025, 30, 13. [Google Scholar] [CrossRef]
- Wadden, T.A.; Hollander, P.; Klein, S.; Niswender, K.; Woo, V.; Hale, P.M.; Aronne, L.; NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int. J. Obes. 2013, 37, 1443–1451. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Gao, L.; Lee, B.W.; Chawla, M.; Kim, J.; Huo, L.; Du, L.; Huang, Y.; Ji, L. Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the asia-pacific region: The SURPASS-AP-combo trial. Nat. Med. 2023, 29, 1500–1510. [Google Scholar] [CrossRef]
- de Mesquita, Y.L.L.; Pera Calvi, I.; Reis Marques, I.; Almeida Cruz, S.; Padrao, E.M.H.; Carvalho, P.E.P.; da Silva, C.H.A.; Cardoso, R.; Moura, F.A.; Rafalskiy, V.V. Efficacy and safety of the dual GIP and GLP-1 receptor agonist tirzepatide for weight loss: A meta-analysis of randomized controlled trials. Int. J. Obes. 2023, 47, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; le Roux, C.W.; Stefanski, A.; Aronne, L.J.; Halpern, B.; Wharton, S.; Wilding, J.P.H.; Perreault, L.; Zhang, S.; Battula, R.; et al. Tirzepatide for Obesity Treatment and Diabetes Prevention. N. Engl. J. Med. 2024, 392, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.H.; Rezaie, A.; Hsieh, T.Y.; Hu, X.; Gaddam, S.; Ma, K.S.; Gastrointestinal Motility and Metabolic Pharmacoepidemiology Group; Mohamed, G.; Lee, G.Y.; Huang, P.C.; et al. Shifting Trends in the Indication of Glucagon-like Peptide-1 Receptor Agonist Prescriptions: A Nationwide Analysis. Ann. Intern. Med. 2024, 177, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Fitch, A.; Francavilla Brown, C.; Younglove, C.; Christensen, S.M.; Alexander, L.C. Frequently asked questions to the 2023 obesity medicine association position statement on compounded peptides: A call for action. Obes. Pillars 2024, 11, 100122. [Google Scholar] [CrossRef]
- Hung, J.C.; Augustine, S.C.; Cheng, K.T.; Green, R.L.; Hopkins, W.M.; Laven, D.L.; Nelson, B.R.; Petry, N.A.; Ponto, J.A.; Quinton, T.M.; et al. Explanations and unresolved issues pertaining to the development of the Nuclear Pharmacy Compounding Guidelines. J. Am. Pharm. Assoc. 2002, 42, 789–798. [Google Scholar] [CrossRef]
- Watson, C.J.; Whitledge, J.D.; Siani, A.M.; Burns, M.M. Pharmaceutical Compounding: A History, Regulatory Overview, and Systematic Review of Compounding Errors. J. Med. Toxicol. 2021, 17, 197–217. [Google Scholar] [CrossRef]
- DA Warns Consumers Not to Use Counterfeit Ozempic (Semaglutide) Found in U.S. Drug Supply Chain. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-use-counterfeit-ozempic-semaglutide-found-us-drug-supply-chain (accessed on 27 November 2024).
- Ahrén, B. Glucagon-like peptide-1 (GLP-1): A gut hormone of potential interest in the treatment of diabetes. Bioessays 1998, 20, 642–651. [Google Scholar] [CrossRef]
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.V.; McLaughlin, C.M.; Flatt, P.R. Role of exendin-4 in the Gila monster: Further lessons regarding human oral glucagon-like peptide-1 therapy? Diabetes Obes. Metab. 2020, 22, 2509–2511. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E.; Egan, J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007, 113, 546–593. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ratner, R.E.; Han, J.; Kim, D.D.; Fineman, M.S.; Baron, A.D. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005, 28, 1092–1100. [Google Scholar] [CrossRef]
- Yap, M.K.K.; Misuan, N. Exendin-4 from Heloderma suspectum venom: From discovery to its latest application as type II diabetes combatant. Basic Clin. Pharmacol. Toxicol. 2019, 124, 513–527. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Astrup, A.; Carraro, R.; Finer, N.; Harper, A.; Kunesova, M.; Lean, M.E.; Niskanen, L.; Rasmussen, M.F.; Rissanen, A.; Rössner, S.; et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 2012, 36, 843–854. [Google Scholar] [CrossRef]
- le Roux, C.W.; Astrup, A.; Fujioka, K.; Greenway, F.; Lau, D.C.W.; Van Gaal, L.; Ortiz, R.V.; Wilding, J.P.H.; Skjøth, T.V.; Manning, L.S.; et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017, 389, 1399–1409. [Google Scholar] [CrossRef]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jódar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. Two-year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, J.; Han, Y.; Zhang, W.; Wang, Y.; Jiang, Z. A real-world disproportionality analysis of tirzepatide-related adverse events based on the FDA Adverse Event Reporting System (FAERS) database. Endocr. J. 2024, 72, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Basheer, F.T.; Poojari, P.G.; Thunga, G.; Chandran, V.P.; Acharya, L.D. Adverse drug reactions of GLP-1 agonists: A systematic review of case reports. Diabetes Metab. Syndr. 2022, 16, 102427. [Google Scholar] [CrossRef]
- Gorgojo-Martínez, J.J.; Mezquita-Raya, P.; Carretero-Gómez, J.; Castro, A.; Cebrián-Cuenca, A.; de Torres-Sánchez, A.; García-de-Lucas, M.D.; Núñez, J.; Obaya, J.C.; Soler, M.J.; et al. Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus. J. Clin. Med. 2022, 12, 145. [Google Scholar] [CrossRef]
- Huang, K.P.; Acosta, A.A.; Ghidewon, M.Y.; McKnight, A.D.; Almeida, M.S.; Nyema, N.T.; Hanchak, N.D.; Patel, N.; Gbenou, Y.S.K.; Adriaenssens, A.E.; et al. Dissociable hindbrain GLP1R circuits for satiety and aversion. Nature 2024, 632, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ghusn, W.; Hurtado, M.D. Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks. Obes. Pillars 2024, 12, 100127. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Incretin-based therapies: Facing the realities of benefits versus side effects. Diabetes Technol. Ther. 2013, 15, 909–913. [Google Scholar] [CrossRef]
- Guo, H.; Yang, J.; Huang, J.; Xu, L.; Lv, Y.; Wang, Y.; Ren, J.; Feng, Y.; Zheng, Q.; Li, L. Comparative efficacy and safety of GLP-1 receptor agonists for weight reduction: A model-based meta-analysis of placebo-controlled trials. Obes. Pillars 2025, 13, 100162. [Google Scholar] [CrossRef]
- Lando, H.M.; Alattar, M.; Dua, A.P. Elevated amylase and lipase levels in patients using glucagonlike peptide-1 receptor agonists or dipeptidyl-peptidase-4 inhibitors in the outpatient setting. Endocr. Pract. 2012, 18, 472–477. [Google Scholar] [CrossRef]
- Seo, Y.G. Side Effects Associated with Liraglutide Treatment for Obesity as Well as Diabetes. J. Obes. Metab. Syndr. 2021, 30, 12–19. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf (accessed on 5 December 2024).
- Wettergren, A.; Wøjdemann, M.; Holst, J.J. Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am. J. Physiol. 1998, 275, G984–G992. [Google Scholar] [CrossRef] [PubMed]
- Noel, R.A.; Braun, D.K.; Patterson, R.E.; Bloomgren, G.L. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: A retrospective cohort study. Diabetes Care 2009, 32, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yang, S.; Zhou, Z. GLP-1 receptor agonists and pancreatic safety concerns in type 2 diabetic patients: Data from cardiovascular outcome trials. Endocrine 2020, 68, 518–525. [Google Scholar] [CrossRef]
- Baker, D.E.; Walley, K.; Levien, T.L. Tirzepatide. Hosp. Pharm. 2023, 58, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Alenzi, K.A.; Alsuhaibani, D.; Batarfi, B.; Alshammari, T.M. Pancreatitis with use of new diabetic medications: A real-world data study using the post-marketing FDA adverse event reporting system (FAERS) database. Front. Pharmacol. 2024, 15, 1364110. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, M.; Wang, Z.; Hu, M.; Xie, D.; Wang, X.; Guo, Z.; Zhu, J.; Zhang, W.; Luo, Z.; et al. A real-world disproportionality analysis of semaglutide: Post-marketing pharmacovigilance data. J. Diabetes Investig. 2024, 15, 1422–1433. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. Short-term medical benefits and adverse effects of weight loss. Ann. Intern. Med. 1993, 119, 722–726. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Butsch, W.S.; Christensen, S.M.; Hamdy, O.; Li, Z.; Prado, C.M.; Heymsfield, S.B. Strategies for minimizing muscle loss during use of incretin-mimetic drugs for treatment of obesity. Obes. Rev. 2025, 26, e13841. [Google Scholar]
- Kim, H.J.; Kang, T.U.; Kim, M.J.; Swan, H.; Park, S.M. Long-term weight patterns and physical activity in gallstones. Sci. Rep. 2024, 14, 25817. [Google Scholar] [CrossRef]
- Alessandrini, A.; Bruni, F.; Piraccini, B.M.; Starace, M. Common causes of hair loss—Clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 629–640. [Google Scholar] [CrossRef]
- Desai, D.D.; Sikora, M.; Nohria, A.; Bordone, L.; Caplan, A.S.; Shapiro, J.; Lo Sicco, K.I. GLP-1 agonists and hair loss: A call for further investigation. Int. J. Dermatol. 2024, 63, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.L.; Brønden, A.; Karstoft, K.; Sonne, D.P.; Christensen, M.B. The Body weight Reducing Effects of Tirzepatide in People with and without Type 2 Diabetes: A Review on Efficacy and Adverse Effects. Patient Prefer. Adherence 2024, 18, 373–382. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mahle, C.D.; Bell, T.; Kushner, R.F. Healthcare professionals’ perceptions and management of obesity & knowledge of glucagon, GLP-1, GIP receptor agonists, and dual agonists. Obes. Sci. Pract. 2024, 10, e756. [Google Scholar] [PubMed]
- Humphrey, C.D.; Lawrence, A.C. Implications of Ozempic and Other Semaglutide Medications for Facial Plastic Surgeons. Facial Plast. Surg. 2023, 39, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.R.; Hannawa, O.M.; Yaldo, M.M.; Nageeb, E.M.; Chaiyasate, K. The rise of “Ozempic Face”: Analyzing trends and treatment challenges associated with rapid facial weight loss induced by GLP-1 agonists. J. Plast. Reconstr. Aesthet. Surg. 2024, 96, 225–227. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Heymsfield, S.B. Fundamental Body Composition Principles Provide Context for Fat-Free and Skeletal Muscle Loss With GLP-1 RA Treatments. J. Endocr. Soc. 2024, 8, bvae164. [Google Scholar] [CrossRef]
- Neeland, I.J.; Linge, J.; Birkenfeld, A.L. Changes in lean body mass with glucagon-like peptide-1-based therapies and mitigation strategies. Diabetes Obes. Metab. 2024, 26, 16–27. [Google Scholar] [CrossRef]
- Bikou, A.; Dermiki-Gkana, F.; Penteris, M.; Constantinides, T.K.; Kontogiorgis, C. A systematic review of the effect of semaglutide on lean mass: Insights from clinical trials. Expert Opin. Pharmacother. 2024, 25, 611–619. [Google Scholar] [CrossRef]
- Rochira, V.; Greco, C.; Boni, S.; Costantino, F.; Dalla Valentina, L.; Zanni, E.; Itani, L.; El Ghoch, M. The Effect of Tirzepatide on Body Composition in People with Overweight and Obesity: A Systematic Review of Randomized, Controlled Studies. Diseases 2024, 12, 204. [Google Scholar] [CrossRef]
- Schmidt, P.H.S.; Pasqualotto, E.; Dos Santos, H.V.; de Souza, L.S.N.; Dos Santos, B.E.; Chavez, M.P.; Ferreira, R.O.M.; Hohl, A.; Ronsoni, M.F.; van de Sande-Lee, S. Effects of liraglutide on body composition in people living with obesity or overweight: A systematic review. Obes. Res. Clin. Pract. 2025, 19, 11–18. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Mantzoros, C.S. Effect of glucagon-like peptide-1 receptor agonists and co-agonists on body composition: Systematic review and network meta-analysis. Metabolism 2025, 164, 156113. [Google Scholar] [CrossRef] [PubMed]
- Lubberding, A.F.; Veedfald, S.; Achter, J.S.; Nissen, S.D.; Soattin, L.; Sorrentino, A.; Vega, E.T.; Linz, B.; Eggertsen, C.H.E.; Mulvey, J.; et al. Glucagon-like peptide-1 increases heart rate by a direct action on the sinus node. Cardiovasc. Res. 2024, 120, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, R.; Ye, H.; Wang, Y.; Wang, L.; Zhang, X. Effects of GLP-1 receptor agonists on arrhythmias and its subtypes in patients with type 2 diabetes: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 910256. [Google Scholar] [CrossRef] [PubMed]
- Boulmpou, A.; Patoulias, D.; Papadopoulos, C.E.; Teperikidis, E.; Doumas, M.; Vassilikos, V. Meta-analysis of cardiovascular outcome trials assessing the impact of glucagon-like peptide-1 receptor agonists on major cardiac arrhythmias. Acta Cardiol. 2023, 78, 519–524. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, Y.; Hu, Y.; Zhu, H.; Chen, X.; Zhao, B. Hypoglycemia following the use of glucagon-like peptide-1 receptor agonists: A real-world analysis of post-marketing surveillance data. Ann. Transl. Med. 2021, 9, 1482. [Google Scholar] [CrossRef]
- Gao, L.; Yu, S.; Cipriani, A.; Wu, S.; Huang, Y.; Zhang, Z.; Yang, J.; Sun, Y.; Yang, Z.; Chai, S.; et al. Neurological Manifestation of Incretin-Based Therapies in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Aging Dis. 2019, 10, 1311–1319. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat. Med. 2025. advance online publication. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, W.; Xie, Y.; Shen, H.; Liu, M.; Wu, X. Risk of ophthalmic adverse drug reactions in patients prescribed glucagon-like peptide 1 receptor agonists: A pharmacovigilance study based on the FDA adverse event reporting system database. Endocrine 2024. advance online publication. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Bain, S.C.; Klufas, M.A.; Ho, A.; Matthews, D.R. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes. Metab. 2019, 21, 454–466. [Google Scholar] [CrossRef]
- Bethel, M.A.; Diaz, R.; Castellana, N.; Bhattacharya, I.; Gerstein, H.C.; Lakshmanan, M.C. HbA1c Change and Diabetic Retinopathy During GLP-1 Receptor Agonist Cardiovascular Outcome Trials: A Meta-analysis and Meta-regression. Diabetes Care 2021, 44, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Joshi, P.; Barri, S.; Wang, J.; Corder, A.L.; O’Connell, S.S.; Fonseca, V.A. Progression of retinopathy with glucagon-like peptide-1 receptor agonists with cardiovascular benefits in type 2 diabetes—A systematic review and meta-analysis. J. Diabetes Complicat. 2022, 36, 108255. [Google Scholar] [CrossRef]
- Wai, K.M.; Mishra, K.; Koo, E.; Ludwig, C.A.; Parikh, R.; Mruthyunjaya, P.; Rahimy, E. Impact of GLP-1 Agonists and SGLT-2 Inhibitors on Diabetic Retinopathy Progression: An Aggregated Electronic Health Record Data Study. Am. J. Ophthalmol. 2024, 265, 39–47. [Google Scholar] [CrossRef]
- Hathaway, J.T.; Shah, M.P.; Hathaway, D.B.; Zekavat, S.M.; Krasniqi, D.; Gittinger, J.W., Jr.; Cestari, D.; Mallery, R.; Abbasi, B.; Bouffard, M.; et al. Risk of Nonarteritic Anterior Ischemic Optic Neuropathy in Patients Prescribed Semaglutide. JAMA Ophthalmol. 2024, 142, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kesavadev, J.; Tiwaskar, M. Nonarteritic Anterior Ischemic Optic Neuropathy and Semaglutide: What is This All About? J. Assoc. Physicians India 2024, 72, 11–12. [Google Scholar] [PubMed]
- Parks, M.; Rosebraugh, C. Weighing risks and benefits of liraglutide—The FDA’s review of a new antidiabetic therapy. N. Engl. J. Med. 2010, 362, 774–777. [Google Scholar] [CrossRef]
- Bezin, J.; Gouverneur, A.; Pénichon, M.; Mathieu, C.; Garrel, R.; Hillaire-Buys, D.; Pariente, A.; Faillie, J.L. GLP-1 Receptor Agonists and the Risk of Thyroid Cancer. Diabetes Care 2023, 46, 384–390. [Google Scholar] [CrossRef]
- Silverii, G.A.; Monami, M.; Gallo, M.; Ragni, A.; Prattichizzo, F.; Renzelli, V.; Ceriello, A.; Mannucci, E. Glucagon-like peptide-1 receptor agonists and risk of thyroid cancer: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2024, 26, 891–900. [Google Scholar] [CrossRef]
- Pasternak, B.; Wintzell, V.; Hviid, A.; Eliasson, B.; Gudbjörnsdottir, S.; Jonasson, C.; Hveem, K.; Svanström, H.; Melbye, M.; Ueda, P. Glucagon-like peptide 1 receptor agonist use and risk of thyroid cancer: Scandinavian cohort study. BMJ 2024, 385, e078225. [Google Scholar] [CrossRef]
- Wang, J.; Kim, C.H. Differential Risk of Cancer Associated with Glucagon-like Peptide-1 Receptor Agonists: Analysis of Real-world Databases. Endocr. Res. 2022, 47, 18–25. [Google Scholar] [CrossRef]
- Wang, L.; Xu, R.; Kaelber, D.C.; Berger, N.A. Glucagon-Like Peptide 1 Receptor Agonists and 13 Obesity-Associated Cancers in Patients With Type 2 Diabetes. JAMA Netw. Open 2024, 7, e2421305. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.F.; Seif, K.E.; Reece, E.A. Glucagon-like peptide-1 receptor agonist use in pregnancy: A review. Am. J. Obstet. Gynecol. 2024, 232, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Maslin, K.; Alkutbe, R.; Gilbert, J.; Pinkney, J.; Shawe, J. What is known about the use of weight loss medication in women with overweight/obesity on fertility and reproductive health outcomes? A scoping review. Clin. Obes. 2024, 14, e12690. [Google Scholar] [CrossRef]
- Pavli, P.; Triantafyllidou, O.; Kapantais, E.; Vlahos, N.F.; Valsamakis, G. Infertility Improvement after Medical Weight Loss in Women and Men: A Review of the Literature. Int. J. Mol. Sci. 2024, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, D.S. Preliminary Review Finds No Link Between GLP-1 Receptor Agonists and Suicidality. Am. J. Nurs. 2024, 124, 20. [Google Scholar] [CrossRef]
- Ueda, P.; Söderling, J.; Wintzell, V.; Svanström, H.; Pazzagli, L.; Eliasson, B.; Melbye, M.; Hviid, A.; Pasternak, B. GLP-1 Receptor Agonist Use and Risk of Suicide Death. JAMA Intern. Med. 2024, 184, 1301–1312. [Google Scholar] [CrossRef]
- Tian, C.; Yang, Z.; Zhao, S.; Zhang, P.; Li, R. Adverse event reporting of combining SGLT2 inhibitor and GLP1 receptor agonist: A real-world study from FAERS. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103758. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Mansur, R.B.; Rosenblat, J.D.; Rhee, T.G.; Cao, B.; Teopiz, K.M.; Wong, S.; Le, G.H.; Ho, R.; Kwan, A.T.H. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: A replication study using reports to the World Health Organization pharmacovigilance database (VigiBase®). J. Affect. Disord. 2025, 369, 922–927. [Google Scholar] [CrossRef]
- Kerem, L.; Stokar, J. Risk of Suicidal Ideation or Attempts in Adolescents With Obesity Treated With GLP1 Receptor Agonists. JAMA Pediatr. 2024, 178, 1307–1315. [Google Scholar] [CrossRef]
- Kittner, S.L.; Talbott, A.L.; Vishneski, S.R.; Narbaiza, J.; Shields, J.S. Retained Gastric Contents After Adequate Fasting Associated with GLP-1 Receptor Agonist Use: A Report of 3 Cases. JBJS Case Connect. 2023, 13, e23.00506. [Google Scholar] [CrossRef]
- Goron, A.R.; Connolly, C.; Valdez-Sinon, A.N.; Hesson, A.; Helou, C.; Kirschen, G.W. Anti-Hyperglycemic Medication Management in the Perioperative Setting: A Review and Illustrative Case of an Adverse Effect of GLP-1 Receptor Agonist. J. Clin. Med. 2024, 13, 6259. [Google Scholar] [CrossRef]
- Kindel, T.L.; Wang, A.Y.; Wadhwa, A.; Schulman, A.R.; Sharaiha, R.Z.; Kroh, M.; Ghanem, O.M.; Levy, S.; Joshi, G.P.; LaMasters, T.L.; et al. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Surg. Obes. Relat. Dis. 2024, 20, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.B.; Mizubuti, G.B.; da Silva, L.M.; Silveira, S.Q.; Nersessian, R.S.F.; Abib, A.C.V.; Bellicieri, F.N.; Lima, H.O.; Ho, A.M.; Dos Anjos, G.S.; et al. Effect of various perioperative semaglutide interruption intervals on residual gastric content assessed by esophagogastroduodenoscopy: A retrospective single center observational study. J. Clin. Anesth. 2024, 99, 111668. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, P. Glucagon-Like Peptide-1 Receptor Agonists in the Peri-Operative Period. Br. J. Hosp. Med. 2025, 86, 1–4. [Google Scholar] [CrossRef]
- Fornes, A.; Huff, J.; Pritchard, R.I.; Godfrey, M. Once-Weekly Semaglutide for Weight Management: A Clinical Review. J. Pharm. Technol. 2022, 38, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.R.; Franco, D.R.; Gieremek, H.W.; Vidal, C.M.; Bronzeri, F.; de Cassia Rocha, A.; de Carvalho Cara, L.G.; Fogo, S.L.; Eliaschewitz, F.G. GLP-1 Agonist to Treat Obesity and Prevent Cardiovascular Disease: What Have We Achieved so Far? Curr. Atheroscler. Rep. 2022, 24, 867–884. [Google Scholar] [CrossRef]
- Jones, L.A.; Brierley, D.I. GLP-1 and the Neurobiology of Eating Control: Recent Advances. Endocrinology 2025, 166, bqae167. [Google Scholar] [CrossRef]
- Chetty, A.K.; Rafi, E.; Bellini, N.J.; Buchholz, N.; Isaacs, D. A Review of Incretin Therapies Approved and in Late-Stage Development for Overweight and Obesity Management. Endocr. Pract. 2024, 30, 292–303. [Google Scholar] [CrossRef]
- Jensen, S.B.K.; Blond, M.B.; Sandsdal, R.M.; Olsen, L.M.; Juhl, C.R.; Lundgren, J.R.; Janus, C.; Stallknecht, B.M.; Holst, J.J.; Madsbad, S.; et al. Healthy weight loss maintenance with exercise, GLP-1 receptor agonist, or both combined followed by one year without treatment: A post-treatment analysis of a randomised placebo-controlled trial. EClinicalMedicine 2024, 69, 102475. [Google Scholar] [CrossRef]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 2016, 24, 1612–1619. [Google Scholar] [CrossRef]
- Maclean, P.S.; Bergouignan, A.; Cornier, M.A.; Jackman, M.R. Biology’s response to dieting: The impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R581–R600. [Google Scholar] [CrossRef] [PubMed]
- Falkenhain, K.; Martin, C.K.; Ravussin, E.; Redman, L.M. Energy expenditure, metabolic adaptation, physical activity and energy intake following weight loss: Comparison between bariatric surgery and low-calorie diet. Eur. J. Clin. Nutr. 2024. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Geisler, C.; Heymsfield, S.B.; Bosy-Westphal, A. Recent advances in understanding body weight homeostasis in humans. F1000Research 2018, 7, F1000. [Google Scholar]
- Hall, K.D.; Heymsfield, S.B. Models use leptin and calculus to count calories. Cell Metab. 2009, 9, 3–4. [Google Scholar] [CrossRef]
- Garvey, W.T. Is Obesity or Adiposity-Based Chronic Disease Curable: The Set Point Theory, the Environment, and Second-Generation Medications. Endocr. Pract. 2022, 28, 214–222. [Google Scholar] [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Foster, G. Differential mechanisms affecting weight loss and weight loss maintenance. Nat. Metab. 2023, 5, 1266–1274. [Google Scholar] [CrossRef]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’Rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef]
- Martínez-Gómez, M.G.; Roberts, B.M. Metabolic Adaptations to Weight Loss: A Brief Review. J. Strength Cond. Res. 2022, 36, 2970–2981. [Google Scholar] [CrossRef]
- Ostendorf, D.M.; Melanson, E.L.; Caldwell, A.E.; Creasy, S.A.; Pan, Z.; MacLean, P.S.; Wyatt, H.R.; Hill, J.O.; Catenacci, V.A. No consistent evidence of a disproportionately low resting energy expenditure in long-term successful weight-loss maintainers. Am. J. Clin. Nutr. 2018, 108, 658–666. [Google Scholar] [CrossRef]
- Gabe, M.B.N.; Breitschaft, A.; Knop, F.K.; Hansen, M.R.; Kirkeby, K.; Rathor, N.; Adrian, C.L. Effect of oral semaglutide on energy intake, appetite, control of eating and gastric emptying in adults living with obesity: A randomized controlled trial. Diabetes Obes. Metab. 2024, 26, 4480–4489. [Google Scholar] [CrossRef]
- Polidori, D.; Sanghvi, A.; Seeley, R.J.; Hall, K.D. How Strongly Does Appetite Counter Weight Loss? Quantification of the Feedback Control of Human Energy Intake. Obesity 2016, 24, 2289–2295. [Google Scholar] [CrossRef]
- Knuth, N.D.; Johannsen, D.L.; Tamboli, R.A.; Marks-Shulman, P.A.; Huizenga, R.; Chen, K.Y.; Abumrad, N.N.; Ravussin, E.; Hall, K.D. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity 2014, 22, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Lecoultre, V.; Ravussin, E.; Redman, L.M. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J. Clin. Endocrinol. Metab. 2011, 96, E1512–E1516. [Google Scholar] [CrossRef] [PubMed]
- Marinho, T.S.; Fabiano, M.M.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Principal components analysis on genes related to inflammasome complex and microglial activation in the hypothalamus of obese mice treated with semaglutide (GLP-1 analog). Brain Res. 2024, 1846, 149225. [Google Scholar] [CrossRef] [PubMed]
- Imbernon, M.; Saponaro, C.; Helms, H.C.C.; Duquenne, M.; Fernandois, D.; Deligia, E.; Denis, R.G.P.; Chao, D.H.M.; Rasika, S.; Staels, B.; et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab. 2022, 34, 1054–1063. [Google Scholar] [CrossRef]
- Drucker, D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef]
- Taylor, R. Hunger and the Obesity Epidemic: Old Insights Reaffirmed by New Medicines? Ann. Intern. Med. 2023, 176, 995–996. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G.; Bond, D.S.; Phelan, S.; Hill, J.O.; Wing, R.R. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am. J. Prev. Med. 2014, 46, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lillis, J.; Thomas, J.G.; Niemeier, H.; Wing, R.R. Internal disinhibition predicts 5-year weight regain in the National Weight Control Registry (NWCR). Obes. Sci. Pract. 2016, 2, 83–87. [Google Scholar] [CrossRef]
- Paixão, C.; Dias, C.M.; Jorge, R.; Carraça, E.V.; Yannakoulia, M.; de Zwaan, M.; Soini, S.; Hill, J.O.; Teixeira, P.J.; Santos, I. Successful weight loss maintenance: A systematic review of weight control registries. Obes. Rev. 2020, 21, e13003. [Google Scholar] [CrossRef]
- Mauldin, K.; May, M.; Clifford, D. The consequences of a weight-centric approach to healthcare: A case for a paradigm shift in how clinicians address body weight. Nutr. Clin. Pract. 2022, 37, 1291–1306. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Spann, R.; Berry, S.E.; Berthoud, H.R.; Broyles, S.; Foster, G.D.; Krakoff, J.; Loos, R.J.F.; Lowe, M.R.; Ostendorf, D.M.; et al. New insights in the mechanisms of weight-loss maintenance: Summary from a Pennington symposium. Obesity 2023, 31, 2895–2908. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Eng. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Minderis, P.; Fokin, A.; Povilonis, T.; Kvedaras, M.; Ratkevicius, A. Effects of Diet Macronutrient Composition on Weight Loss during Caloric Restriction and Subsequent Weight Regain during Refeeding in Aging Mice. Nutrients 2023, 15, 4836. [Google Scholar] [CrossRef]
- Neve, K.L.; Isaacs, A. How does the food environment influence people engaged in weight management? A systematic review and thematic synthesis of the qualitative literature. Obes. Rev. 2022, 23, e13398. [Google Scholar] [CrossRef]
- Cordova, R.; Kliemann, N.; Huybrechts, I.; Rauber, F.; Vamos, E.; Levy, R.B.; Wagner, K.H.; Viallon, V.; Casagrande, C.; Nicolas, G.; et al. Consumption of ultra-processed foods associated with weight gain and obesity in adults: A multi-national cohort study. Clin. Nutr. 2021, 40, 5079–5088. [Google Scholar] [CrossRef]
- Foright, R.M.; Presby, D.M.; Sherk, V.D.; Kahn, D.; Checkley, L.A.; Giles, E.D.; Bergouignan, A.; Higgins, J.A.; Jackman, M.R.; Hill, J.O.; et al. Is regular exercise an effective strategy for weight loss maintenance? Phys. Behav. 2018, 188, 86–93. [Google Scholar] [CrossRef]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr. Biol. 2016, 26, 410–417. [Google Scholar] [CrossRef]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Marcus, B.H.; Lang, W.; Janney, C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch. Intern. Med. 2008, 168, 1550–1560. [Google Scholar] [CrossRef]
- Jakicic, J.; Marcus, B.; Gallagher, K.; Napolitano, M.; Lang, W. Effect of exercise duration and intensity on weight loss in overweight sedentary women. JAMA 2003, 290, 1323–1330. [Google Scholar] [CrossRef]
- Cox, C.E. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017, 30, 157–160. [Google Scholar] [CrossRef]
- Ghoreishy, S.M.; Noormohammadi, M.; Zeraattalab-Motlagh, S.; Shoaibinobarian, N.; Hasan Rashedi, M.; Movahed, S.; Hemmati, A.; Nazarian, A.; Fernandez, M.L.; Shidfar, F. The Effectiveness of Nonsurgical Interventions for Weight Loss Maintenance in Adults: An Updated, GRADE-Assessed Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutr. Rev. 2024, nuae128, advance online publication. [Google Scholar] [CrossRef]
- Ostendorf, D.M.; Blankenship, J.M.; Grau, L.; Arbet, J.; Mitchell, N.S.; Creasy, S.A.; Caldwell, A.E.; Melanson, E.L.; Phelan, S.; Bessesen, D.H.; et al. Predictors of long-term weight loss trajectories during a behavioral weight loss intervention: An exploratory analysis. Obes. Sci. Pract. 2021, 7, 569–582. [Google Scholar] [CrossRef]
- Ostendorf, D.M.; Lyden, K.; Pan, Z.; Wyatt, H.R.; Hill, J.O.; Melanson, E.L.; Catenacci, V.A. Objectively Measured Physical Activity and Sedentary Behavior in Successful Weight Loss Maintainers. Obesity 2018, 26, 53–60. [Google Scholar] [CrossRef]
- McCarthy, D.; Berg, A. Weight Loss Strategies and the Risk of Skeletal Muscle Mass Loss. Nutrients 2021, 13, 2473. [Google Scholar] [CrossRef]
- Hooker, A.R.; Sagui-Henson, S.J.; Daubenmier, J.; Moran, P.J.; Hartogensis, W.; Acree, M.; Kristeller, J.; Epel, E.S.; Mason, A.E.; Hecht, F.M. Effects of a Mindfulness-Based Weight Loss Intervention on Long-Term Psychological Well-Being Among Adults with Obesity: Secondary Analyses from the Supporting Health by Integrating Nutrition and Exercise (SHINE) Trial. Mindfulness 2022, 13, 2227–2242. [Google Scholar] [CrossRef]
- Preiss, K.; Brennan, L.; Clarke, D. A systematic review of variables associated with the relationship between obesity and depression. Obes. Rev. 2013, 14, 906–918. [Google Scholar] [CrossRef]
- Jackson, S.E.; Steptoe, A.; Beeken, R.J.; Kivimaki, M.; Wardle, J. Psychological changes following weight loss in overweight and obese adults: A prospective cohort study. PLoS ONE 2014, 9, e104552. [Google Scholar] [CrossRef]
- Wing, R.R.; Papandonatos, G.; Fava, J.L.; Gorin, A.A.; Phelan, S.; McCaffery, J.; Tate, D.F. Maintaining large weight losses: The role of behavioral and psychological factors. J. Consult. Clin. Psychol. 2008, 76, 1015–1021. [Google Scholar] [CrossRef]
- Wadden, T.A.; Brown, G.K.; Egebjerg, C.; Frenkel, O.; Goldman, B.; Kushner, R.F.; McGowan, B.; Overvad, M.; Fink-Jensen, A. Psychiatric Safety of Semaglutide for Weight Management in People Without Known Major Psychopathology: Post Hoc Analysis of the STEP 1, 2, 3, and 5 Trials. JAMA Intern. Med. 2024, 184, 1290–1300. [Google Scholar] [CrossRef]
- Marwood, J.; Brown, T.; Kaiseler, M.; Clare, K.; Feeley, A.; Blackshaw, J.; Ells, L.J. Psychological support within tier 2 adult weight management services, are we doing enough for people with mental health needs? A mixed-methods survey. Clin. Obes. 2023, 13, e12580. [Google Scholar] [CrossRef]
- Cambi, M.P.C.; Baretta, G.A.P.; Magro, D.O.; Boguszewski, C.L.; Ribeiro, I.B.; Jirapinyo, P.; de Moura, D.T.H. Multidisciplinary Approach for Weight Regain-how to Manage this Challenging Condition: An Expert Review. Obes. Surg. 2021, 31, 1290–1303. [Google Scholar] [CrossRef]
- Karfopoulou, E.; Anastasiou, C.A.; Avgeraki, E.; Kosmidis, M.H.; Yannakoulia, M. The role of social support in weight loss maintenance: Results from the MedWeight study. J. Behav. Med. 2016, 39, 511–518. [Google Scholar] [CrossRef]
- Reyes, N.R.; Oliver, T.L.; Klotz, A.A.; Lagrotte, C.A.; Vander Veur, S.S.; Virus, A.; Bailer, B.A.; Foster, G.D. Similarities and differences between weight loss maintainers and regainers: A qualitative analysis. J. Acad. Nutr. Diet. 2012, 112, 499–505. [Google Scholar] [CrossRef]
- Soini, S.; Mustajoki, P.; Eriksson, J.G. Long-term Weight Maintenance after Successful Weight Loss: Motivational Factors, Support, Difficulties, and Success Factors. Am. J. Health Behav. 2018, 42, 77–84. [Google Scholar] [CrossRef]
- Fischer, M.; Weimann, T.; Oberänder, N.; Schupitza, L.; Hösel, J.; Weimann, A. Remote Treatment Successfully Delivers a Usual Care Weight Loss and Lifestyle Intervention in Adults with Morbid Obesity. Ann. Nutr. Metab. 2022, 78, 328–335. [Google Scholar] [CrossRef]
- Patel, M.L.; Cleare, A.E.; Smith, C.M.; Rosas, L.G.; King, A.C. Detailed Versus Simplified Dietary Self-monitoring in a Digital Weight Loss Intervention Among Racial and Ethnic Minority Adults: Fully Remote, Randomized Pilot Study. JMIR Form. Res. 2022, 6, e42191. [Google Scholar] [CrossRef]
- Cengiz, A.; Wu, C.C.; Lawley, S.D. Alternative dosing regimens of GLP-1 receptor agonists may reduce costs and maintain weight loss efficacy. Diabetes Obes. Metab. 2025, 27, 2251–2258. [Google Scholar] [CrossRef]
- Manne-Goehler, J.; Teufel, F.; Venter, W.D.F. GLP-1 Receptor Agonists and the Path to Sustainable Obesity Care. JAMA Intern. Med. 2025, 185, 8–10. [Google Scholar] [CrossRef]
- Marroquin-Harris, M.; Olesnicky, B. Aspiration risk with glucagon-like peptide 1 (GLP-1) agonists. Anaesthesia 2023, 78, 1524. [Google Scholar] [CrossRef]
- De Block, C.; Peleshok, J.; Wilding, J.P.H.; Kwan, A.Y.M.; Rasouli, N.; Maldonado, J.M.; Wysham, C.; Liu, M.; Aleppo, G.; Benneyworth, B.D. Post Hoc Analysis of SURPASS-1 to -5: Efficacy and Safety of Tirzepatide in Adults with Type 2 Diabetes are Independent of Baseline Characteristics. Diabetes Ther. 2024, 16, 43–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Delessa, C.T.; Augustin, R.; Bakhti, M.; Colldén, G.; Drucker, D.J.; Feuchtinger, A.; Caceres, C.G.; Grandl, G.; Harger, A.; et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 2021, 33, 833–844.e5. [Google Scholar] [CrossRef]
- Mroz, P.A.; Finan, B.; Gelfanov, V.; Yang, B.; Tschöp, M.H.; DiMarchi, R.D.; Perez-Tilve, D. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol. Metab. 2019, 20, 51–62. [Google Scholar] [CrossRef]
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015, 21, 27–36. [Google Scholar] [CrossRef]
- Rodriguez, P.J.; Goodwin Cartwright, B.M.; Gratzl, S.; Brar, R.; Baker, C.; Gluckman, T.J.; Stucky, N.L. Semaglutide vs Tirzepatide for Weight Loss in Adults With Overweight or Obesity. JAMA Intern. Med. 2024, 184, 1056–1064. [Google Scholar] [CrossRef]
- Heise, T.; DeVries, J.H.; Urva, S.; Li, J.; Pratt, E.J.; Thomas, M.K.; Mather, K.J.; Karanikas, C.A.; Dunn, J.; Haupt, A.; et al. Tirzepatide Reduces Appetite, Energy Intake, and Fat Mass in People With Type 2 Diabetes. Diabetes Care 2023, 46, 998–1004. [Google Scholar] [CrossRef]
- Nicze, M.; Dec, A.; Borówka, M.; Krzyżak, D.; Bołdys, A.; Bułdak, Ł.; Okopień, B. Molecular Mechanisms behind Obesity and Their Potential Exploitation in Current and Future Therapy. Int. J. Mol. Sci. 2024, 25, 8202. [Google Scholar] [CrossRef] [PubMed]
- Janket, S.J.; Chatanaka, M.K.; Sohaei, D.; Tamimi, F.; Meurman, J.H.; Diamandis, E.P. Does Incretin Agonism Have Sustainable Efficacy? Cells 2024, 13, 1842. [Google Scholar] [CrossRef] [PubMed]
- Enyew Belay, K.; Jemal, R.H.; Tuyizere, A. Innovative Glucagon-based Therapies for Obesity. J. Endocr. Soc. 2024, 8, bvae197. [Google Scholar] [CrossRef]
- Panou, T.; Gouveri, E.; Popovic, D.S.; Papanas, N. Amylin analogs for the treatment of obesity without diabetes: Present and future. Expert Rev. Clin. Pharmacol. 2024, 17, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, D.; Li, Y.; Shi, J.; Zhang, X.; Yi, T. Identification and utility exploration of a highly potent and long-acting bullfrog GLP-1 analogue in GLP-1 and amylin combination therapy. Peptides 2024, 177, 171203. [Google Scholar] [CrossRef]
- Stefanakis, K.; Kokkorakis, M.; Mantzoros, C.S. The impact of weight loss on fat-free mass, muscle, bone and hematopoiesis health: Implications for emerging pharmacotherapies aiming at fat reduction and lean mass preservation. Metabolism 2024, 161, 156057. [Google Scholar] [CrossRef]
- Nunn, E.; Jaiswal, N.; Gavin, M.; Uehara, K.; Stefkovich, M.; Drareni, K.; Calhoun, R.; Lee, M.; Holman, C.D.; Baur, J.A.; et al. Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism. Mol. Metab. 2024, 80, 101880. [Google Scholar] [CrossRef]
- Dalle Grave, R. The Benefit of Healthy Lifestyle in the Era of New Medications to Treat Obesity. Diabetes Metab. Syndr. Obes. 2024, 17, 227–230. [Google Scholar] [CrossRef]
- Papathanasiou, T.; Strathe, A.; Agersø, H.; Lund, T.M.; Overgaard, R.V. Impact of dose-escalation schemes and drug discontinuation on weight loss outcomes with liraglutide 3.0 mg: A model-based approach. Diabetes Obes. Metab. 2020, 22, 969–977. [Google Scholar] [CrossRef]
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef]
- Duarte-Medrano, G.; Nuño-Lámbarri, N.; Minutti-Palacios, M.; Dominguez-Cherit, G.; Dominguez-Franco, A.; La Via, L.; Paternò, D.S.; Sorbello, M. Perioperative Rhabdomyolysis in Obese Individuals Undergoing Bariatric Surgery: Current Status. Healthcare 2024, 12, 2029. [Google Scholar] [CrossRef]
- Pories, W.J. Bariatric Surgery: Risks and Rewards. J. Clin. Endocrinol. Metab. 2008, 93, S89–S96. [Google Scholar] [CrossRef]
- Inaba, C.S.; Koh, C.Y.; Sujatha-Bhaskar, S.; Silva, J.P.; Chen, Y.; Nguyen, D.V.; Nguyen, N.T. One-Year Mortality after Contemporary Laparoscopic Bariatric Surgery: An Analysis of the Bariatric Outcomes Longitudinal Database. J. Am. Coll. Surg. 2018, 226, 1166–1174. [Google Scholar] [CrossRef]
- Kim, D.D.; Hwang, J.H.; Fendrick, A.M. Balancing innovation and affordability in anti-obesity medications: The role of an alternative weight-maintenance program. Health Aff. Sch. 2024, 2, qxae055. [Google Scholar] [CrossRef]
- Davidson, M.B. Should Prediabetes be Treated Pharmacologically? Diabetes Ther. 2023, 14, 1585–1593. [Google Scholar] [CrossRef]
- Khattab, R. Weight Loss Programs: Why Do They Fail? A Multidimensional Approach for Obesity Management. Curr. Nutr. Rep. 2024, 13, 478–499. [Google Scholar] [CrossRef]
- Zhou, B.; Roberts, S.B.; Das, S.K.; Naumova, E.N. Weight Loss Trajectories and Short-Term Prediction in an Online Weight Management Program. Nutrients 2024, 16, 1224. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Mire, E.F.; Horswell, R.; Chu, S.T.; Zhang, D.; Martin, C.K.; Newton, R.L.; Apolzan, J.W.; Price-Haywood, E.G.; Fort, D.; et al. Four-year follow-up of weight loss maintenance using electronic medical record data: The PROPEL trial. Obes. Sci. Pract. 2024, 10, e70017. [Google Scholar] [CrossRef]
- Borer, K.T. Why We Eat Too Much, Have an Easier Time Gaining Than Losing Weight, and Expend Too Little Energy: Suggestions for Counteracting or Mitigating These Problems. Nutrients 2021, 13, 3812. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Odgen, L.; Phelan, S.; Thomas, J.G.; Hill, J.; Wing, R.R.; Wyatt, H. Dietary habits and weight maintenance success in high versus low exercisers in the National Weight Control Registry. J. Phys. Act. Health 2014, 11, 1540–1548. [Google Scholar] [CrossRef]
- Sforzo, G.A.; Gordon, N.F.; Peeke, P.M.; Moore, M. Health and Well-Being Coaching Adjuvant to GLP-1 Induced Weight Loss. Am. J. Lifestyle Med. 2024. advance online publication. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).