S-Nitrosoglutathione Is Not a Substrate of OATP1B1, but Stimulates Its Expression and Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. HepG2 Cell Line
2.2. HEK293 and HEK293-OATP1B1 Cell Line
2.3. Assessment of GSNO’s Affiliation to OATP1B1 Substrates
2.4. Assessment the Effect of GSNO on OATP1B1 and Its Mechanisms
2.5. GSNO and GSH LC-MS/MS Analysis
2.6. Determination of the Nitric Oxide Level in HepG2 Cells
2.7. Determination of Bityrosine Level in HepG2 Cells
2.8. Real-Time PCR
2.9. Western Blotting
2.10. Atorvastatin LC-MS/MS Analysis
2.11. Data Analysis
3. Results
3.1. GSNO Transport in HEK293-OATP1B1 and HEK293 Cells
3.2. Effect of GSNO on the NO Levels in HepG2 Cells
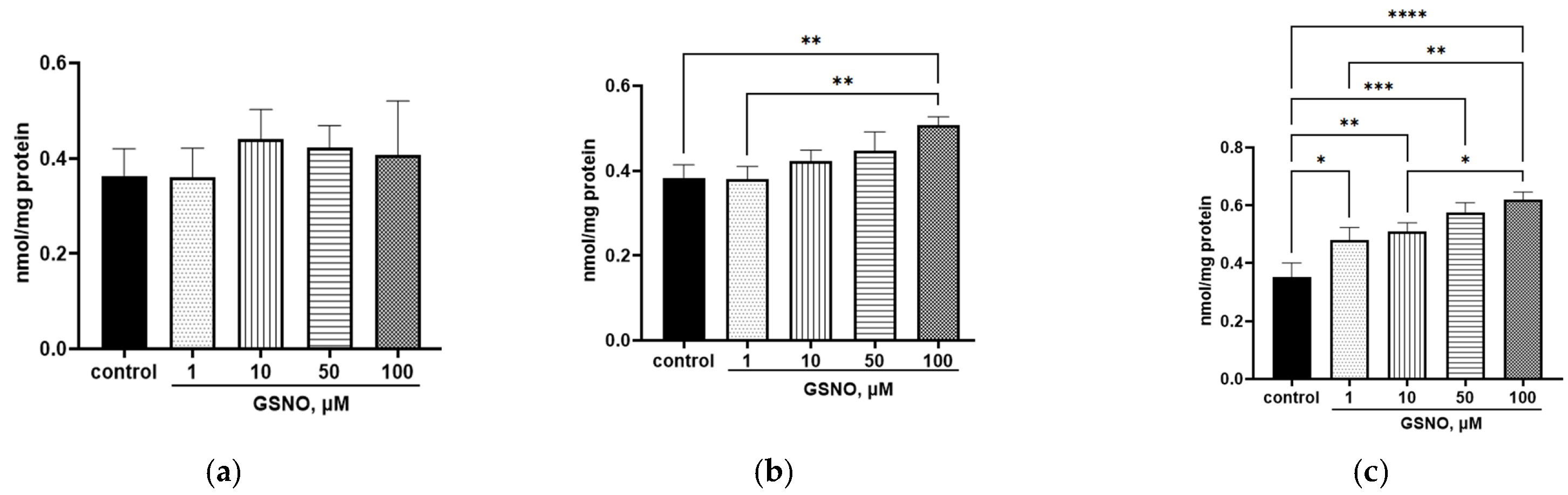
3.3. Effect of GSNO on the Expression of SLCO1B1 Gene
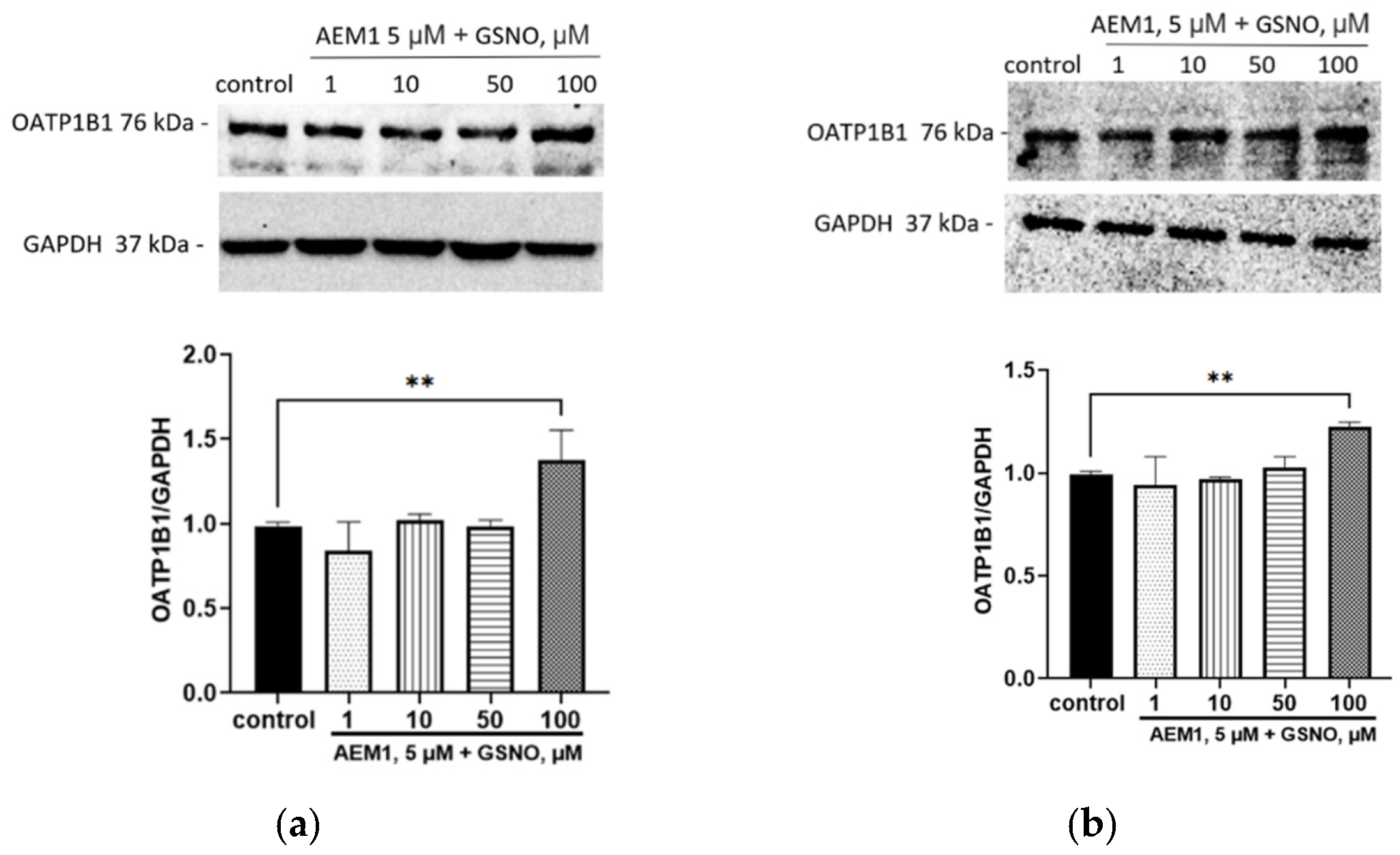
3.4. Effect of GSNO on the Expression of OATP1B1
3.5. The Effect of GSNO on the Intracellular Transport of the OATP1B1 Substrate Atorvastatin in HepG2 Cells
3.6. The Role of NO-sGC Signaling Pathway in the Up-Regulation of OATP1B1 by GSNO
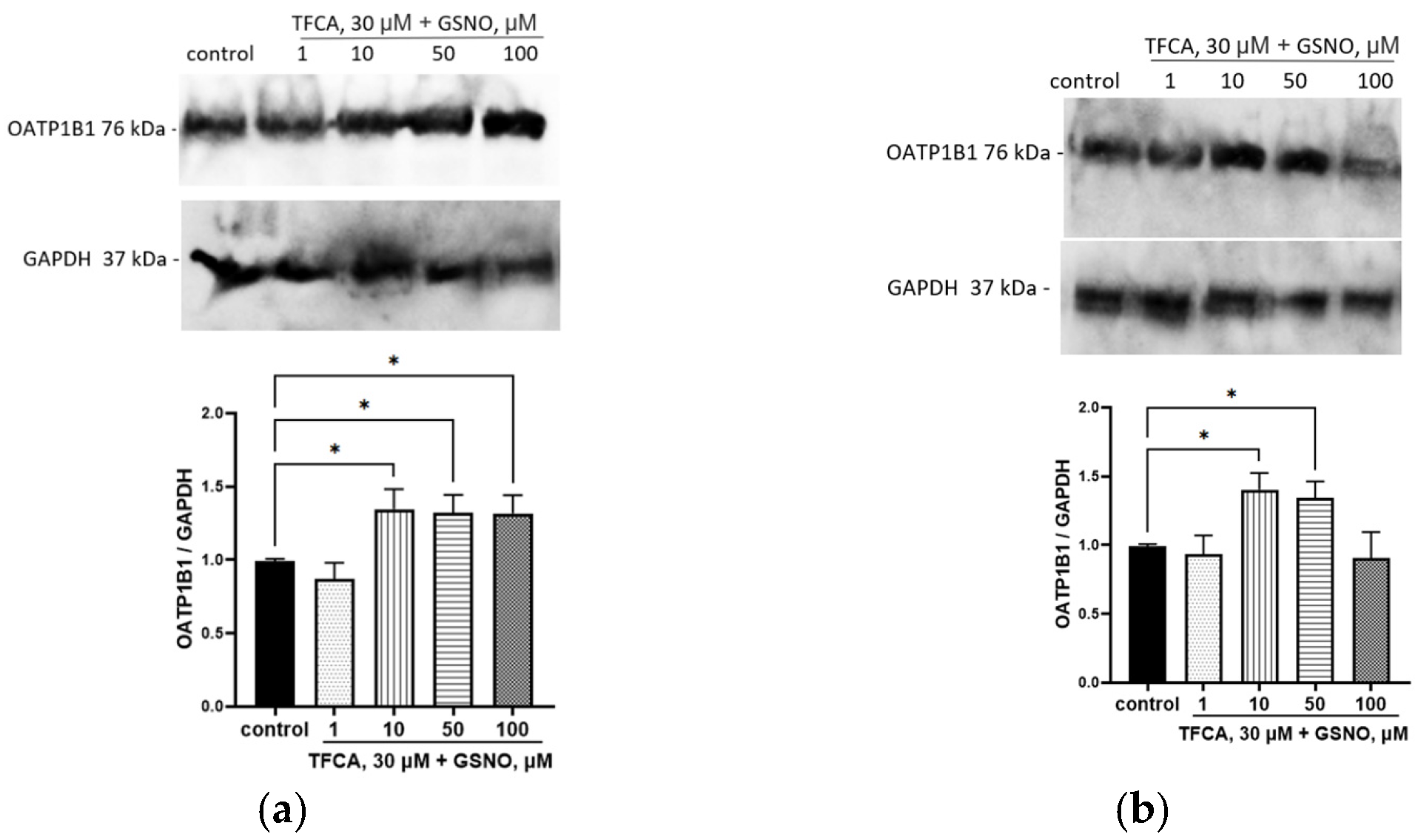
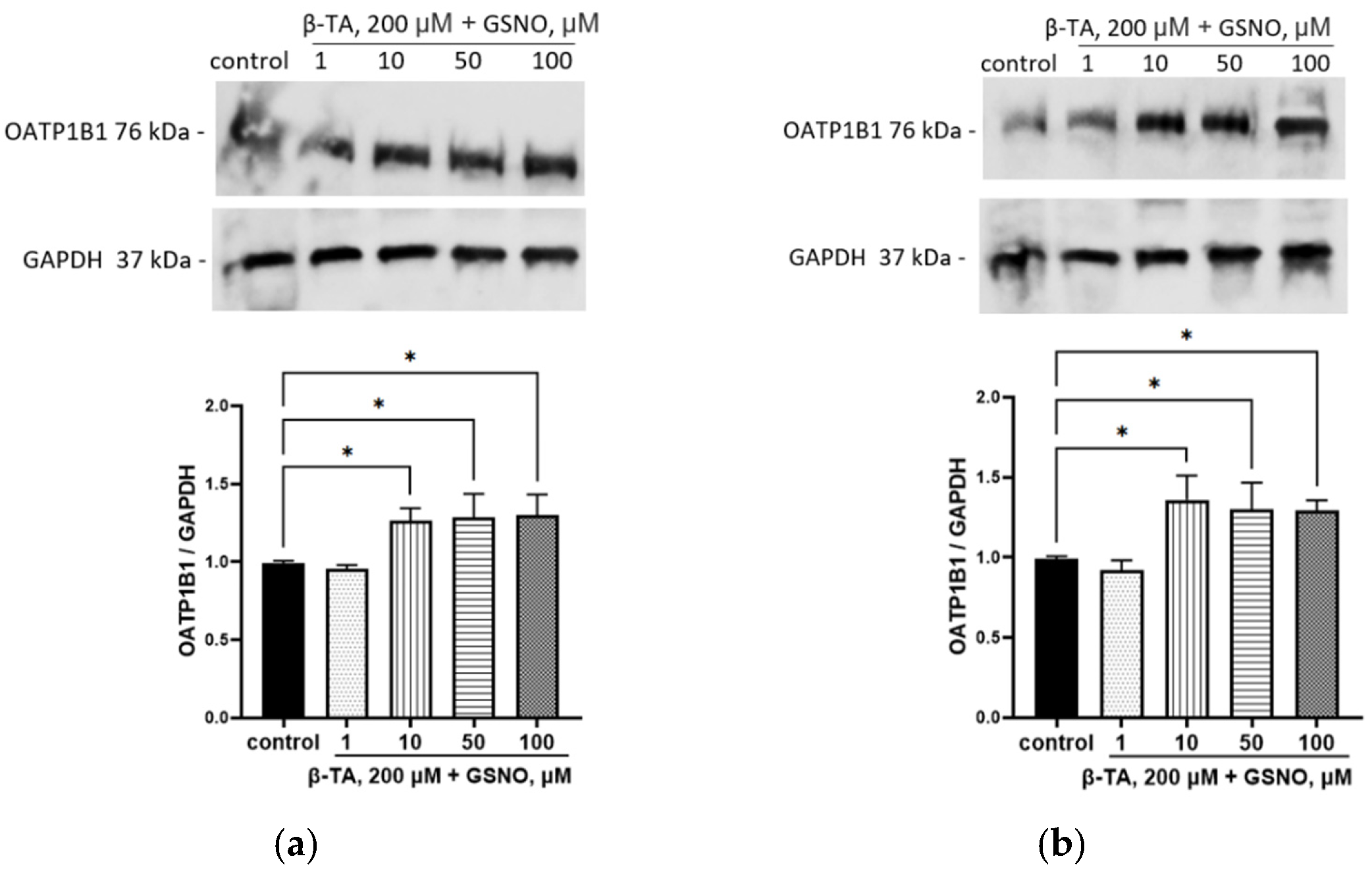
3.7. The Role of Nfr2 in the Up-Regulation of OATP1B1 by GSNO
3.8. The Role of FXR and LXRa in the Up-Regulation of OATP1B1 by GSNO
3.9. Development of Nitrosative Stress Under the Action of GSNO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-Nitrosoglutathione. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Dey, S.K.; Kundu, S. Functional implications of vascular endothelium in regulation of endothelial nitric oxide synthesis to control blood pressure and cardiac functions. Life Sci. 2020, 259, 118377. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.G.; Fischer, A.J.; Martin, S.M.; Schafer, F.Q.; Buettner, G.R. Nitric oxide as a cellular antioxidant: A little goes a long way. Free Radic. Biol. Med. 2006, 40, 501–506. [Google Scholar] [CrossRef]
- Jehle, A.; Garaschuk, O. The interplay between cGMP and calcium signaling in Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 7048. [Google Scholar] [CrossRef] [PubMed]
- Goshi, E.; Zhou, G.; He, Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas. Res. 2019, 9, 192–207. [Google Scholar] [CrossRef]
- Reis, A.; Stern, A.; Monteiro, H.P. S-nitrosothiols and H2S donors: Potential chemo-therapeutic agents in cancer. Redox Biol. 2019, 27, 101190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Z.; Li, L.L. Advanced nitric oxide donors: Chemical structure of NO drugs, NO nanomedicines and biomedical applications. Nanoscale 2021, 13, 444–459. [Google Scholar] [CrossRef]
- Miller, M.; Megson, I. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007, 151, 305–321. [Google Scholar] [CrossRef]
- Burov, O.N.; Kletskii, M.E.; Kurbatov, S.V.; Lisovin, A.V.; Fedik, N.S. Mechanisms of nitric oxide generation in living systems. Nitric Oxide 2022, 118, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, D.; Lao, K.U.; Wang, X. Buffer concentration dramatically affects the stability of S-nitrosothiols in aqueous solutions. Nitric Oxide 2022, 118, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fontana, K.; Onukwue, N.; Sun, B.L.; Lento, C.; Ventimiglia, L.; Nikoo, S.; Gauld, J.W.; Wilson, D.J.; Mutus, B. Evidence for an Allosteric S-Nitrosoglutathione Binding Site in S-Nitrosoglutathione Reductase (GSNOR). Antioxidants 2019, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S. Pathophysiological Role of S-Nitrosylation and Transnitrosylation Depending on S-Nitrosoglutathione Levels Regulated by S-Nitrosoglutathione Reductase. Biomol. Ther. 2018, 26, 533–538. [Google Scholar] [CrossRef]
- Ramachandran, N.; Root, P.; Jiang, X.-M.; Hogg, P.J.; Mutus, B. Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase. Proc. Natl. Acad. Sci. USA 2001, 98, 9539. [Google Scholar] [CrossRef] [PubMed]
- Perland, E.; Fredriksson, R. Classification systems of secondary active transporters. Trends Pharmacol. Sci. 2017, 38, 305–315. [Google Scholar] [CrossRef]
- Li, Y.; Talebi, Z.; Chen, X.; Sparreboom, A.; Hu, S. Endogenous Biomarkers for SLC Transporter-Mediated Drug-Drug Interaction Evaluation. Molecules 2021, 26, 5500. [Google Scholar] [CrossRef]
- M12 Drug Interaction Studies Guidance for Industry. Available online: https://www.fda.gov/media/161199/download (accessed on 5 March 2024).
- Kalliokoski, A.; Niemi, M. Impact of OATP transporters on pharmacokinetics. Br. J. Pharmacol. 2009, 158, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. SLC Family Transporters. In Book Drug Transporters in Drug Disposition, Effects and Toxicity, 1st ed.; Liu, X., Pan, G., Eds.; Springer: Singapore, 2019; Volume 3, pp. 101–202. [Google Scholar] [CrossRef]
- Mori, D.; Kashihara, Y.; Yoshikado, T.; Kimura, M.; Hirota, T.; Matsuki, S.; Maeda, K.; Irie, S.; Ieiri, I.; Sugiyama, Y.; et al. Effect of OATP1B1 genotypes on plasma concentrations of endogenous OATP1B1 substrates and drugs, and their association in healthy volunteers. Drug Metab. Pharmacokinet. 2019, 34, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Riha, J.; Wlcek, K.; Jaeger, W.; Thalhammer, T. Organic anion transporting polypeptides (OATPs): Regulation of expression and function. Curr. Drug Metab. 2011, 12, 139–153. [Google Scholar] [CrossRef]
- Calkin, A.; Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Meyer, Z.; Schwabedissen, H.E.; Böttcher, K.; Chaudhry, A.; Kroemer, H.K.; Schuetz, E.G.; Kim, R.B. Liver X receptor α and farnesoid X receptor are major transcriptional regulators of OATP1B1. Hepatology 2010, 52, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A Protective Role of the NRF2-Keap1 Pathway in Maintaining Intestinal Barrier Function. Oxid. Med. Cell Longev. 2019, 26, 1759149. [Google Scholar] [CrossRef]
- Li, C.Q.; Kim, M.Y.; Godoy, L.C.; Thiantanawat, A.; Trudel, L.J.; Wogan, G.N. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14547–14551. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Chen, L.; Fang, P.; Cai, H.; Tang, H.; Peng, Y.; Deng, Y.; Cao, L.; Li, H.; Zhang, B.; et al. Mechanisms of Triptolide-Induced Hepatotoxicity and Protective Effect of Combined Use of Isoliquiritigenin: Possible Roles of Nrf2 and Hepatic Transporters. Front. Pharmacol. 2018, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Kotliarova, M.S.; Shchulkin, A.V.; Erokhina, P.D.; Mylnikov, P.Y.; Yakusheva, E.N.; Nadolinskaia, N.I.; Zamakhaev, M.V.; Goncharenko, A.V. Generation of a Cell Line Selectively Producing Functionally Active OATP1B1 Transporter. Biochemistry 2023, 88, 1267–1273. [Google Scholar] [CrossRef]
- Hwang, T.L.; Wu, C.C.; Teng, C.M. Comparison of two soluble guanylyl cyclase inhibitors, methylene blue and ODQ, on sodium nitroprusside-induced relaxation in guinea-pig trachea. Br. J. Pharmacol. 1998, 125, 1158–1163. [Google Scholar] [CrossRef]
- Bollong, M.J.; Yun, H.; Sherwood, L.; Woods, A.K.; Lairson, L.L.; Schultz, P.G. A small molecule inhibits deregulated NRF2 transcriptional activity in cancer. ACS Chem. Biol. 2015, 10, 2193–2198. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Sim, W.C.; Kim, D.G.; Lee, K.J.; Choi, Y.J.; Choi, Y.J.; Shin, K.J.; Jun, D.W.; Park, S.J.; Park, H.J.; Kim, J.; et al. Cinnamamides, novel liver X receptor antagonists that inhibit ligand-induced lipogenesis and fatty liver. J. Pharmacol. Exp. Ther. 2015, 355, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Balcerczyk, A.; Soszynski, M.; Bartosz, G. On the specificity of 4-amino-5-methylamino-2′,7′-difluorofluorescein as a probe for nitric oxide. Free Radic. Biol. Med. 2005, 39, 327–335. [Google Scholar] [CrossRef]
- Amadò, R.; Aeschbach, H.; Neukom, R. Dityrosine: In vitro production and characterization. Methods Enzymol. 1984, 107, 377–388. [Google Scholar] [PubMed]
- Erokhina, P.D.; Myl’nikov, P.Y.; Ganina, S.O.; Konyakhin, E.A.; Shulkin, A.V.; Slepnev, A.A.; Yakusheva, E.N. Development and Validation of the Quantitative Determination of Atorvastatin in HepG2 Cell Line Using High-Performance Liquid Chromatography with Mass-Spectrometric Detection. IP Pavlov Russ. Med. Biol. Her. 2022, 30, 149–158. [Google Scholar] [CrossRef]
- Oestreicher, J.; Morgan, B. Glutathione: Subcellular distribution and membrane transport. Biochem. Cell Biol. 2019, 97, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Stessel, H.; Pirker, T.; Gorren, A.C.F.; Mayer, B.; Schrammel, A. S-nitrosoglutathione inhibits adipogenesis in 3T3-L1 preadipocytes by S-nitrosation of CCAAT/enhancer-binding protein β. Sci. Rep. 2019, 9, 15403. [Google Scholar] [CrossRef]
- Kim, J.; Islam, S.M.T.; Qiao, F.; Singh, A.K.; Khan, M.; Won, J.; Singh, I. Regulation of B cell functions by S-nitrosoglutathione in the EAE model. Redox Biol. 2021, 45, 102053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.; Xiao, G.; Shan, H.; Tang, L.; Yi, Y.; Yu, W.; Gu, Y. S-nitrosylation of the Peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway. Cell Death Dis. 2019, 10, 329. [Google Scholar] [CrossRef]
- Vildhede, A.; Karlgren, M.; Svedberg, E.K.; Wisniewski, J.R.; Lai, Y.; Norén, A.; Artursson, P. Hepatic uptake of atorvastatin: Influence of variability in transporter expression on uptake clearance and drug-drug interactions. Drug Metab. Dispos. 2014, 42, 1210–1218. [Google Scholar] [CrossRef]
- Torstensson, M.; Hansen, A.H.; Leth-Møller, K.; Jørgensen, T.S.; Sahlberg, M.; Andersson, C.; Kristensen, K.E.; Ryg, J.; Weeke, P.; Torp-Pedersen, C.; et al. Danish register-based study on the association between specific cardiovascular drugs and fragility fractures. BMJ Open 2015, 5, e009522. [Google Scholar] [CrossRef]








| Rifampicin/GSNO Concentrations | Rifampicin | Duration of Incubation with GSNO | ||
|---|---|---|---|---|
| 3 h | 24 h | 72 h | ||
| Control | 4.78 ± 0.49 | 4.95 ± 0.52 | 4.85 ± 0.29 | 4.65 ± 0.41 |
| 1 μM | 4.52 ± 0.13 | 5.07 ± 1.67 | 4.62 ± 0.36 | 4.79 ± 0.52 |
| 10 μM | 3.63 ± 0.32 ** | 4.69 ± 0.69 | 6.11 ± 0.13 * | 6.95 ± 0.99 # |
| 50 μM | 3.39 ± 0.35 ** | 5.12 ± 1.17 | 6.76 ± 0.77 ** | 7.20 ± 1.55 * |
| 100 μM | 2.93 ± 0.34 *** | 3.95 ± 0.39 | 5.82 ± 0.41 # | 5.88 ± 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abalenikhina, Y.V.; Shchulkin, A.V.; Suchkova, O.N.; Ananyeva, P.D.; Mylnikov, P.Y.; Yakusheva, E.N.; Suchkov, I.A.; Kalinin, R.E. S-Nitrosoglutathione Is Not a Substrate of OATP1B1, but Stimulates Its Expression and Activity. Biomolecules 2025, 15, 428. https://doi.org/10.3390/biom15030428
Abalenikhina YV, Shchulkin AV, Suchkova ON, Ananyeva PD, Mylnikov PY, Yakusheva EN, Suchkov IA, Kalinin RE. S-Nitrosoglutathione Is Not a Substrate of OATP1B1, but Stimulates Its Expression and Activity. Biomolecules. 2025; 15(3):428. https://doi.org/10.3390/biom15030428
Chicago/Turabian StyleAbalenikhina, Yulia V., Aleksey V. Shchulkin, Olga N. Suchkova, Pelageya D. Ananyeva, Pavel Yu. Mylnikov, Elena N. Yakusheva, Igor A. Suchkov, and Roman E. Kalinin. 2025. "S-Nitrosoglutathione Is Not a Substrate of OATP1B1, but Stimulates Its Expression and Activity" Biomolecules 15, no. 3: 428. https://doi.org/10.3390/biom15030428
APA StyleAbalenikhina, Y. V., Shchulkin, A. V., Suchkova, O. N., Ananyeva, P. D., Mylnikov, P. Y., Yakusheva, E. N., Suchkov, I. A., & Kalinin, R. E. (2025). S-Nitrosoglutathione Is Not a Substrate of OATP1B1, but Stimulates Its Expression and Activity. Biomolecules, 15(3), 428. https://doi.org/10.3390/biom15030428






