Salivary-Gland-Mediated Nitrate Recirculation as a Modulator for Cardiovascular Diseases
Abstract
:1. Introduction
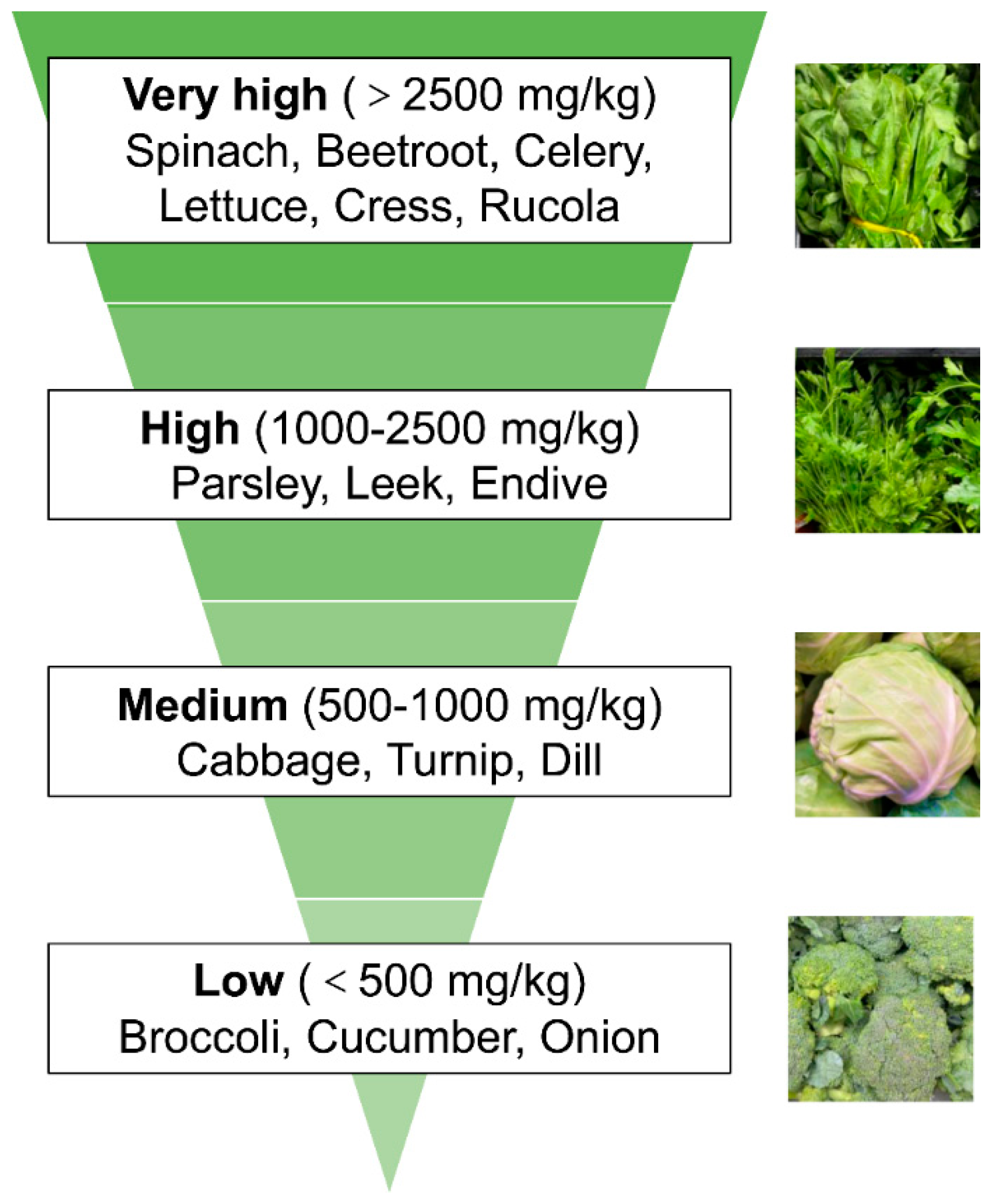
2. Vegetal Nitrate Source
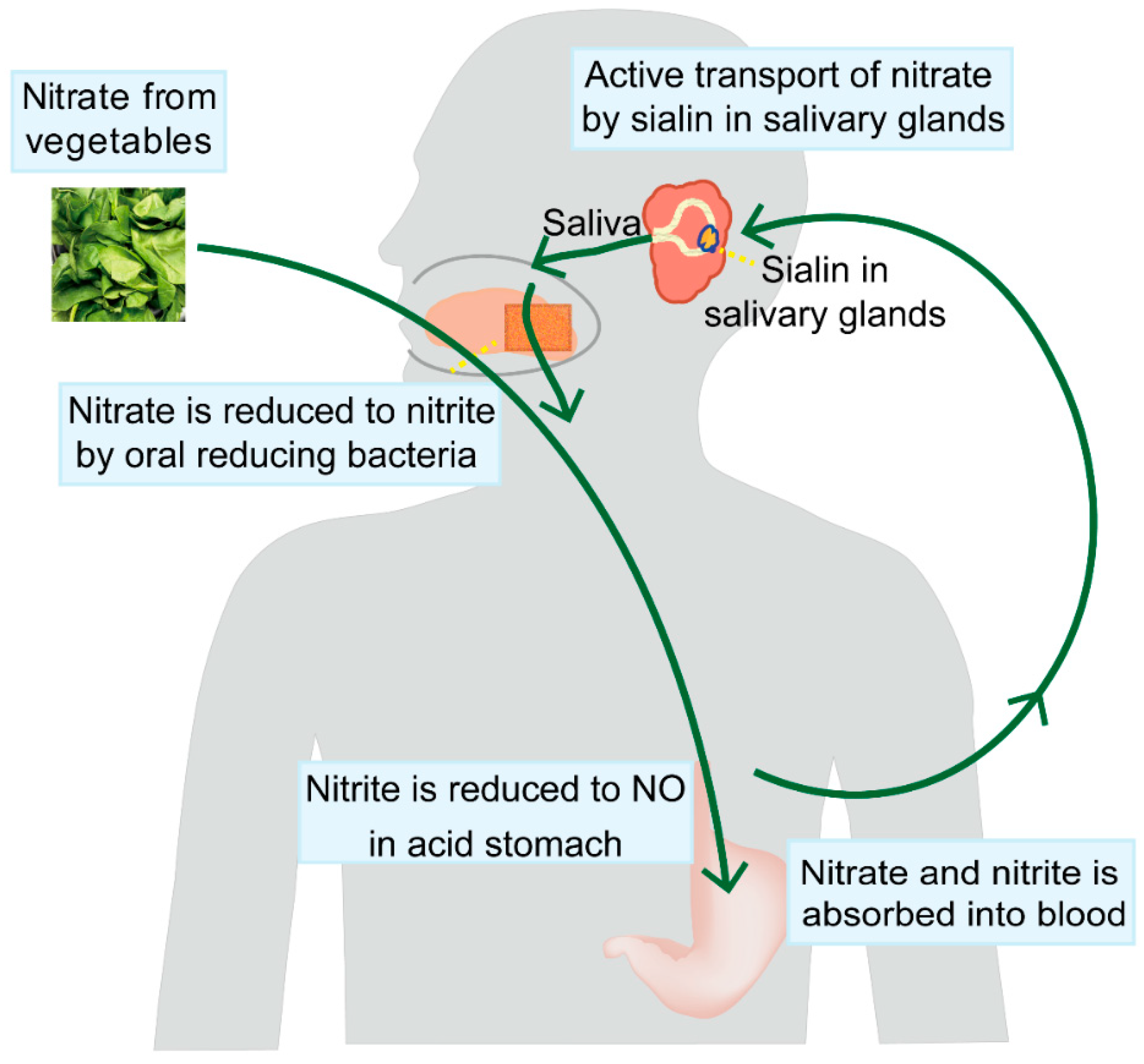
3. The Salivary-Gland-Mediated Recirculation of Nitrate
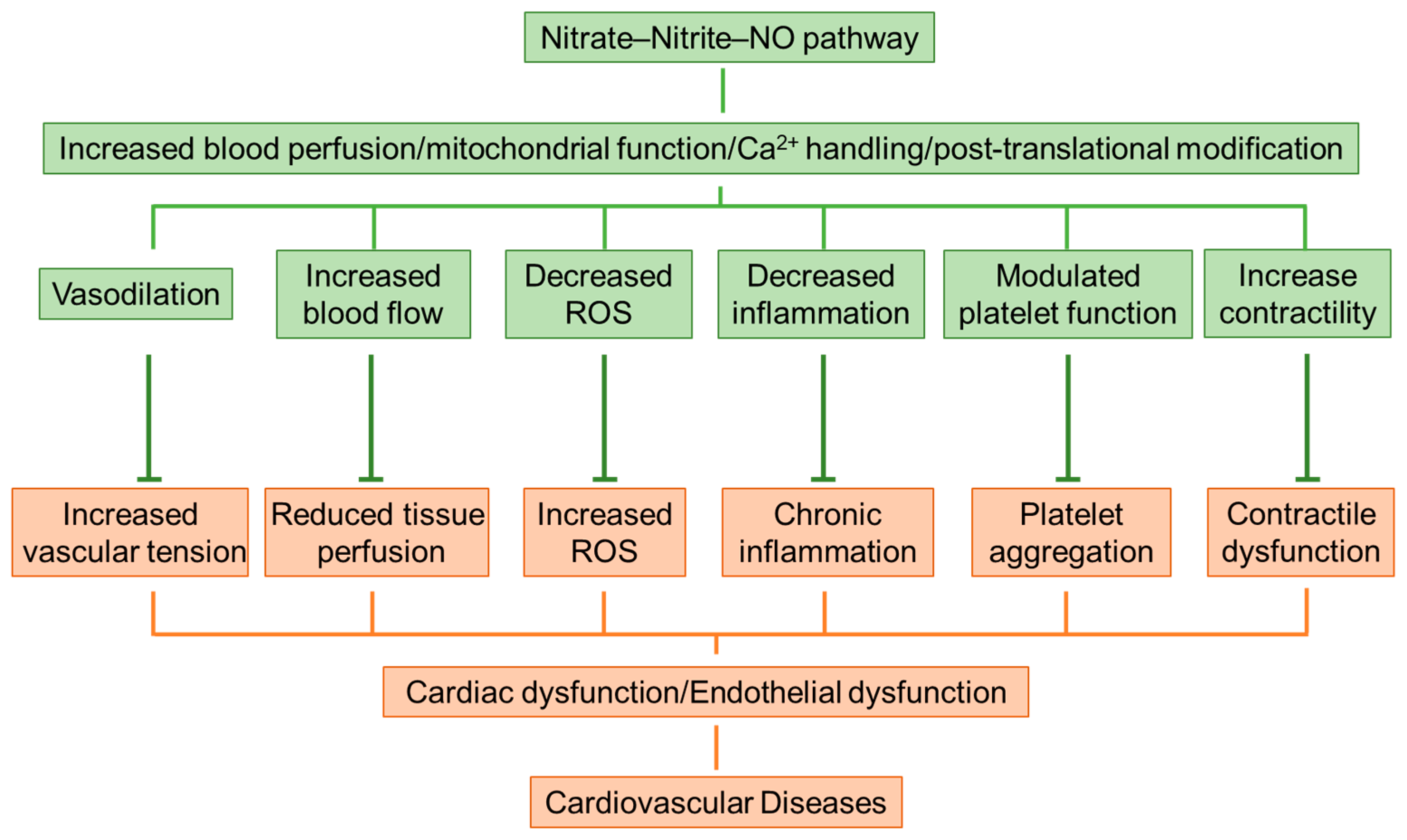
4. Protection and Mechanisms of Inorganic Nitrate on Cardiovascular Diseases
4.1. Blood Pressure Lowered by Nitrate-Rich Vegetables
4.2. Nitrate Supplementation Attenuates Heart Failure
4.3. Ischemia-Reperfusion Injury Alleviated by Nitrate–Nitrite–NO Pathway
4.4. Endothelial Dysfunction Improved by Inorganic Nitrate
4.5. Mitochondrial Reactive Oxygen Species (ROS) Inhibited by Dietary Nitrate
4.6. Reactive Nitrogen Species (RNS) and Cardiac Contractility
5. Safety of Nitrate-Rich Diet
6. Conclusions
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, J.; Addy, K.; Campbell, S.; Wilkinson, P. Primary Prevention of Cardiovascular Disease: Updated Review of Contemporary Guidance and Literature. JRSM Cardiovasc. Dis. 2020, 9, 2048004020949326. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, C.-Y.; Fang, B.; Li, B.; Li, Y.-H.; Xia, Q.-Q.; Zhao, Y.; Cheng, X.-L.; Yang, S.-M.; Zhang, M.-H.; et al. The Function and Therapeutic Potential of Transfer RNA-Derived Small RNAs in Cardiovascular Diseases: A Review. Pharmacol. Res. 2024, 206, 107279. [Google Scholar] [CrossRef]
- Bryan, N.S. Nitric Oxide Deficiency Is a Primary Driver of Hypertension. Biochem. Pharmacol. 2022, 206, 115325. [Google Scholar] [CrossRef]
- Remuzzi, G.; Perico, N.; Zoja, C.; Corna, D.; Macconi, D.; Viganò, G. Role of Endothelium-Derived Nitric Oxide in the Bleeding Tendency of Uremia. J. Clin. Investig. 1990, 86, 1768–1771. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric Oxide Signaling in Health and Disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Król, M.; Kepinska, M. Human Nitric Oxide Synthase-Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Saxena, N.; Dhammu, T.; Khan, M.; Singh, A.K.; Singh, I.; Won, J. Regulation of Endothelial Barrier Integrity by Redox-Dependent Nitric Oxide Signaling: Implication in Traumatic and Inflammatory Brain Injuries. Nitric Oxide 2019, 83, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Weitzberg, E.; Lundberg, J.O. Nitric Oxide Signaling and Regulation in the Cardiovascular System: Recent Advances. Pharmacol. Rev. 2024, 76, 1038–1062. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.-L. Nitric Oxide Signalling in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From Nitrate to NO: Potential Effects of Nitrate-Reducing Bacteria on Systemic Health and Disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef]
- Abukhodair, A.W.; Abukhudair, W.; Alqarni, M.S. The Effects of L-Arginine in Hypertensive Patients: A Literature Review. Cureus 2021, 13, e20485. [Google Scholar] [CrossRef] [PubMed]
- Massion, P.B.; Feron, O.; Dessy, C.; Balligand, J.-L. Nitric Oxide and Cardiac Function: Ten Years after, and Continuing. Circ. Res. 2003, 93, 388–398. [Google Scholar] [CrossRef]
- Danson, E.J.; Choate, J.K.; Paterson, D.J. Cardiac Nitric Oxide: Emerging Role for nNOS in Regulating Physiological Function. Pharmacol. Ther. 2005, 106, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Rastaldo, R.; Pagliaro, P.; Cappello, S.; Penna, C.; Mancardi, D.; Westerhof, N.; Losano, G. Nitric Oxide and Cardiac Function. Life Sci. 2007, 81, 779–793. [Google Scholar] [CrossRef]
- Tamargo, J.; Caballero, R.; Gómez, R.; Delpón, E. Cardiac Electrophysiological Effects of Nitric Oxide. Cardiovasc. Res. 2010, 87, 593–600. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Feelisch, M.; Björne, H.; Jansson, E.A.; Weitzberg, E. Cardioprotective Effects of Vegetables: Is Nitrate the Answer? Nitric Oxide 2006, 15, 359–362. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Blekkenhorst, L.C.; Liu, A.H.; Bondonno, N.P.; Ward, N.C.; Croft, K.D.; Hodgson, J.M. Vegetable-Derived Bioactive Nitrate and Cardiovascular Health. Mol. Asp. Med. 2018, 61, 83–91. [Google Scholar] [CrossRef]
- Spiegelhalder, B.; Eisenbrand, G.; Preussmann, R. Influence of Dietary Nitrate on Nitrite Content of Human Saliva: Possible Relevance to in Vivo Formation of N-Nitroso Compounds. Food Cosmet. Toxicol. 1976, 14, 545–548. [Google Scholar] [CrossRef]
- Jones, A.M.; Vanhatalo, A.; Seals, D.R.; Rossman, M.J.; Piknova, B.; Jonvik, K.L. Dietary Nitrate and Nitric Oxide Metabolism: Mouth, Circulation, Skeletal Muscle, and Exercise Performance. Med. Sci. Sports Exerc. 2021, 53, 280–294. [Google Scholar] [CrossRef]
- Rocha, B.S. The Nitrate-Nitrite-Nitric Oxide Pathway on Healthy Ageing: A Review of Pre-Clinical and Clinical Data on the Impact of Dietary Nitrate in the Elderly. Front. Aging 2021, 2, 778467. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Skibsted, L.H. Nitric Oxide and Quality and Safety of Muscle Based Foods. Nitric Oxide 2011, 24, 176–183. [Google Scholar] [CrossRef]
- Tannenbaum, S.R.; Correa, P. Nitrate and Gastric Cancer Risks. Nature 1985, 317, 675–676. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef]
- Tan, L.; Stagg, L.; Hanlon, E.; Li, T.; Fairley, A.M.; Siervo, M.; Matu, J.; Griffiths, A.; Shannon, O.M. Associations between Vegetable Nitrate Intake and Cardiovascular Disease Risk and Mortality: A Systematic Review. Nutrients 2024, 16, 1511. [Google Scholar] [CrossRef]
- Hord, N.G.; Ghannam, J.S.; Garg, H.K.; Berens, P.D.; Bryan, N.S. Nitrate and Nitrite Content of Human, Formula, Bovine, and Soy Milks: Implications for Dietary Nitrite and Nitrate Recommendations. Breastfeed. Med. 2011, 6, 393–399. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food Sources of Nitrates and Nitrites: The Physiologic Context for Potential Health Benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. The Cardioprotective Role of Nitrate-Rich Vegetables. Foods 2024, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kasama, K.; Kitabatake, M.; Okuda, M.; Imai, M. Metabolic Fate of Nitric Oxide. Int. Arch. Occup. Environ. Health 1980, 46, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Racette, S.B.; Thies, D.; Peterson, L.R.; Stratford, R.E. Simultaneous Pharmacokinetic Analysis of Nitrate and Its Reduced Metabolite, Nitrite, Following Ingestion of Inorganic Nitrate in a Mixed Patient Population. Pharm. Res. 2020, 37, 235. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, P.; de Saint Blanquat, G.; Klein, D. Excretion of Nitrates and Nitrites in Saliva and Bile in the Dog. Food Chem. Toxicol. 1985, 23, 655–659. [Google Scholar] [CrossRef]
- Chirinos, J.A. The Nitrate-Nitrite-NO Pathway as a Novel Therapeutic Target in Heart Failure with Reduced Ejection Fraction. J. Card. Fail. 2018, 24, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, N.; O’Driscoll, F.; Dougall, H.; Duncan, C.; Smith, L.; Golden, M.; McKenzie, H. Stomach NO Synthesis. Nature 1994, 368, 502. [Google Scholar] [CrossRef]
- Allen, B.W.; Stamler, J.S.; Piantadosi, C.A. Hemoglobin, Nitric Oxide and Molecular Mechanisms of Hypoxic Vasodilation. Trends Mol. Med. 2009, 15, 452–460. [Google Scholar] [CrossRef]
- Qin, L.; Liu, X.; Sun, Q.; Fan, Z.; Xia, D.; Ding, G.; Ong, H.L.; Adams, D.; Gahl, W.A.; Zheng, C.; et al. Sialin (SLC17A5) Functions as a Nitrate Transporter in the Plasma Membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 13434–13439. [Google Scholar] [CrossRef]
- Uchida, H.; Ovitt, C.E. Novel Impacts of Saliva with Regard to Oral Health. J. Prosthet. Dent. 2022, 127, 383–391. [Google Scholar] [CrossRef]
- Xia, D.S.; Deng, D.J.; Wang, S.L. Destruction of Parotid Glands Affects Nitrate and Nitrite Metabolism. J. Dent. Res. 2003, 82, 101–105. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Govoni, M. Inorganic Nitrate Is a Possible Source for Systemic Generation of Nitric Oxide. Free Radic. Biol. Med. 2004, 37, 395–400. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Hu, A.-K.; Chou, D.; Huang, L.-M.; Su, H.-Z.; Yan, F.-R.; Zhang, X.-B.; Zhang, H.-P.; Zeng, Y.-M. Analysis of Oral Microbiota in Patients with Obstructive Sleep Apnea-Associated Hypertension. Hypertens. Res. 2019, 42, 1692–1700. [Google Scholar] [CrossRef]
- Huang, L.; Borniquel, S.; Lundberg, J.O. Enhanced Xanthine Oxidoreductase Expression and Tissue Nitrate Reduction in Germ Free Mice. Nitric Oxide 2010, 22, 191–195. [Google Scholar] [CrossRef]
- Rosier, B.T.; Takahashi, N.; Zaura, E.; Krom, B.P.; MartÍnez-Espinosa, R.M.; van Breda, S.G.J.; Marsh, P.D.; Mira, A. The Importance of Nitrate Reduction for Oral Health. J. Dent. Res. 2022, 101, 887–897. [Google Scholar] [CrossRef]
- Hu, X.; Xu, H.; Bu, L.; Sun, J.; Deng, J.; Song, K.; Wang, L.; Pang, B. Exploring the Wound Healing Potential of Dietary Nitrate in Diabetic Rat Model. Front. Physiol. 2024, 15, 1475375. [Google Scholar] [CrossRef]
- Lima, L.; Gaspar, S.; Rocha, B.S.; Alves, R.; Almeida, M.G. Current Clinical Framework on Nitric Oxide Role in Periodontal Disease and Blood Pressure. Clin. Oral Investig. 2024, 28, 521. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.-L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Dey, N.; Sellak, H. Invited Review: cGMP-Dependent Protein Kinase Signaling Mechanisms in Smooth Muscle: From the Regulation of Tone to Gene Expression. J. Appl. Physiol. (1985) 2001, 91, 1421–1430. [Google Scholar] [CrossRef]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants 2019, 8, 404. [Google Scholar] [CrossRef]
- Martínez, M.C.; Andriantsitohaina, R. Reactive Nitrogen Species: Molecular Mechanisms and Potential Significance in Health and Disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Zhang, Y.; Wei, H.; Jin, S.; Huo, T.; Qin, L. Combination of Inorganic Nitrate and Vitamin C Prevents Collagen-Induced Arthritis in Rats by Inhibiting Pyroptosis. Food Funct. 2025, 16, 673–690. [Google Scholar] [CrossRef]
- Zhao, Y.; Ouyang, X.; Peng, Y.; Peng, S. Stimuli Responsive Nitric Oxide-Based Nanomedicine for Synergistic Therapy. Pharmaceutics 2021, 13, 1917. [Google Scholar] [CrossRef]
- Edosuyi, O.; Igbe, I.; Oyekan, A. Fumarate and Its Downstream Signalling Pathways in the Cardiorenal System: Recent Insights and Novel Expositions in the Etiology of Hypertension. Eur. J. Pharmacol. 2023, 961, 176186. [Google Scholar] [CrossRef]
- Jonvik, K.L.; Nyakayiru, J.; Pinckaers, P.J.; Senden, J.M.; van Loon, L.J.; Verdijk, L.B. Nitrate-Rich Vegetables Increase Plasma Nitrate and Nitrite Concentrations and Lower Blood Pressure in Healthy Adults. J. Nutr. 2016, 146, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute Blood Pressure Lowering, Vasoprotective, and Antiplatelet Properties of Dietary Nitrate via Bioconversion to Nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Benjamim, C.J.R.; da Silva, L.S.L.; Sousa, Y.B.A.; da Silva Rodrigues, G.; de Moraes Pontes, Y.M.; Rebelo, M.A.; da Silva Gonçalves, L.; Tavares, S.S.; Guimarães, C.S.; da Silva Sobrinho, A.C.; et al. Acute and Short-Term Beetroot Juice Nitrate-Rich Ingestion Enhances Cardiovascular Responses Following Aerobic Exercise in Postmenopausal Women with Arterial Hypertension: A Triple-Blinded Randomized Controlled Trial. Free Radic. Biol. Med. 2024, 211, 12–23. [Google Scholar] [CrossRef]
- Tawa, M.; Nagata, R.; Sumi, Y.; Nakagawa, K.; Sawano, T.; Ohkita, M.; Matsumura, Y. Preventive Effects of Nitrate-Rich Beetroot Juice Supplementation on Monocrotaline-Induced Pulmonary Hypertension in Rats. PLoS ONE 2021, 16, e0249816. [Google Scholar] [CrossRef]
- Bonilla Ocampo, D.A.; Paipilla, A.F.; Marín, E.; Vargas-Molina, S.; Petro, J.L.; Pérez-Idárraga, A. Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review. Biomolecules 2018, 8, 134. [Google Scholar] [CrossRef]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of Dietary Nitrate on Blood Pressure in Healthy Volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef]
- van der Avoort, C.M.T.; Jonvik, K.L.; Nyakayiru, J.; van Loon, L.J.C.; Hopman, M.T.E.; Verdijk, L.B. A Nitrate-Rich Vegetable Intervention Elevates Plasma Nitrate and Nitrite Concentrations and Reduces Blood Pressure in Healthy Young Adults. J. Acad. Nutr. Diet. 2020, 120, 1305–1317. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary Nitrate Provides Sustained Blood Pressure Lowering in Hypertensive Patients: A Randomized, Phase 2, Double-Blind, Placebo-Controlled Study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; Lopes da Silva, L.S.; Valenti, V.E.; Gonçalves, L.S.; Porto, A.A.; Tasinafo Júnior, M.F.; Walhin, J.-P.; Garner, D.M.; Gualano, B.; Bueno Júnior, C.R. Effects of Dietary Inorganic Nitrate on Blood Pressure during and Post-Exercise Recovery: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Free Radic. Biol. Med. 2024, 215, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fejes, R.; Pilat, N.; Lutnik, M.; Weisshaar, S.; Weijler, A.M.; Krüger, K.; Draxler, A.; Bragagna, L.; Peake, J.M.; Woodman, R.J.; et al. Effects of Increased Nitrate Intake from Beetroot Juice on Blood Markers of Oxidative Stress and Inflammation in Older Adults with Hypertension. Free Radic. Biol. Med. 2024, 222, 519–530. [Google Scholar] [CrossRef]
- Gee, L.C.; Massimo, G.; Lau, C.; Primus, C.; Fernandes, D.; Chen, J.; Rathod, K.S.; Hamers, A.J.P.; Filomena, F.; Nuredini, G.; et al. Inorganic Nitrate Attenuates Cardiac Dysfunction: Roles for Xanthine Oxidoreductase and Nitric Oxide. Br. J. Pharmacol. 2022, 179, 4757–4777. [Google Scholar] [CrossRef]
- Woessner, M.N.; Levinger, I.; Allen, J.D.; McIlvenna, L.C.; Neil, C. The Effect of Dietary Inorganic Nitrate Supplementation on Cardiac Function during Submaximal Exercise in Men with Heart Failure with Reduced Ejection Fraction (HFrEF): A Pilot Study. Nutrients 2020, 12, 2132. [Google Scholar] [CrossRef] [PubMed]
- Petrick, H.L.; Ogilvie, L.M.; Brunetta, H.S.; Robinson, A.; Kirsh, A.J.; Barbeau, P.-A.; Handy, R.M.; Coyle-Asbil, B.; Gianetto-Hill, C.; Dennis, K.M.J.H.; et al. Dietary Nitrate and Corresponding Gut Microbiota Prevent Cardiac Dysfunction in Obese Mice. Diabetes 2023, 72, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Kondo, K.; Polhemus, D.J.; Otsuka, H.; Nicholson, C.K.; Tao, Y.-X.; Huang, H.; Georgiopoulou, V.V.; Murohara, T.; Calvert, J.W.; et al. Nitrite Therapy Improves Left Ventricular Function during Heart Failure via Restoration of Nitric Oxide-Mediated Cytoprotective Signaling. Circ. Res. 2014, 114, 1281–1291. [Google Scholar] [CrossRef]
- Yu, L.; Jin, Z.; Li, M.; Liu, H.; Tao, J.; Xu, C.; Wang, L.; Zhang, Q. Protective Potential of Hydroxysafflor Yellow A in Cerebral Ischemia and Reperfusion Injury: An Overview of Evidence from Experimental Studies. Front. Pharmacol. 2022, 13, 1063035. [Google Scholar] [CrossRef]
- Kloner, R.A.; Shi, J.; Dai, W.; Carreno, J.; Zhao, L. Remote Ischemic Conditioning in Acute Myocardial Infarction and Shock States. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 103–109. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlström, M.; Larsen, F.J.; Weitzberg, E. Roles of Dietary Inorganic Nitrate in Cardiovascular Health and Disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef]
- Eriksson, K.E.; Eidhagen, F.; Liska, J.; Franco-Cereceda, A.; Lundberg, J.O.; Weitzberg, E. Effects of Inorganic Nitrate on Ischaemia-Reperfusion Injury after Coronary Artery Bypass Surgery: A Randomised Controlled Trial. Br. J. Anaesth. 2021, 127, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Babateen, A.; Alharbi, M.; Stephan, B.; Shannon, O. Dietary Nitrate and Brain Health. Too Much Ado about Nothing or a Solution for Dementia Prevention? Br. J. Nutr. 2022, 128, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Abdel-Gaber, S.A.; Ibrahim, M.A.; Amin, E.F.; Mohammed, R.K.; Abdelrahman, A.M. Nitric Oxide Modulation as a Potential Molecular Mechanism Underlying the Protective Role of NaHS in Liver Ischemia Reperfusion Injury. Curr. Mol. Pharmacol. 2022, 15, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Branch, B.G.; Pattillo, C.B.; Hood, J.; Thoma, S.; Simpson, S.; Illum, S.; Arora, N.; Chidlow, J.H.; Langston, W.; et al. Chronic Sodium Nitrite Therapy Augments Ischemia-Induced Angiogenesis and Arteriogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 7540–7545. [Google Scholar] [CrossRef]
- Brunner, F.; Leonhard, B.; Kukovetz, W.R.; Mayer, B. Role of Endothelin, Nitric Oxide and L-Arginine Release in Ischaemia/Reperfusion Injury of Rat Heart. Cardiovasc. Res. 1997, 36, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Michel, L.; Qin, C.X.; Cao, N.; Woodman, O.L.; Ritchie, R.H. The HNO Donor Angeli’s Salt Offers Potential Haemodynamic Advantages over NO or Dobutamine in Ischaemia-Reperfusion Injury in the Rat Heart Ex Vivo. Pharmacol. Res. 2016, 104, 165–175. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.B.; Merx, M.W.; Shiva, S.; Schmitz, J.; Becher, S.; Klare, J.P.; Steinhoff, H.-J.; Goedecke, A.; Schrader, J.; Gladwin, M.T.; et al. Nitrite Reductase Activity of Myoglobin Regulates Respiration and Cellular Viability in Myocardial Ischemia-Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2008, 105, 10256–10261. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef]
- Yassaghi, Y.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Myocardial Infarct Size Is Reduced by Nitrite and Nitrate Administration: A Systematic Review and Meta-Analysis of Animal Studies. EXCLI J. 2024, 23, 18–33. [Google Scholar] [CrossRef]
- Cui, H.; Feng, Y.; Shu, C.; Yuan, R.; Bu, L.; Jia, M.; Pang, B. Dietary Nitrate Protects Against Skin Flap Ischemia-Reperfusion Injury in Rats via Modulation of Antioxidative Action and Reduction of Inflammatory Responses. Front. Pharmacol. 2020, 10, 1605. [Google Scholar] [CrossRef]
- Laustiola, K.E.; Vuorinen, P.; Pörsti, I.; Metsä-Ketelä, T.; Manninen, V.; Vapaatalo, H. Exogenous GTP Enhances the Effects of Sodium Nitrite on Cyclic GMP Accumulation, Vascular Smooth Muscle Relaxation and Platelet Aggregation. Pharmacol. Toxicol. 1991, 68, 60–63. [Google Scholar] [CrossRef]
- Hu, X.; Wang, L.; Deng, J.; Xu, H.; Song, K.; Bu, L.; Pang, B. Dietary Nitrate Accelerates the Healing of Infected Skin Wounds in Mice by Increasing Microvascular Density. Biochem. Biophys. Res. Commun. 2023, 686, 149176. [Google Scholar] [CrossRef]
- Joris, P.J.; Mensink, R.P.; Adam, T.C.; Liu, T.T. Cerebral Blood Flow Measurements in Adults: A Review on the Effects of Dietary Factors and Exercise. Nutrients 2018, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Qin, L.; Xia, D.; Liu, X.; Fan, Z.; Zhang, C.; Gu, L.; He, J.; Ambudkar, I.S.; Deng, D.; et al. Active Secretion and Protective Effect of Salivary Nitrate against Stress in Human Volunteers and Rats. Free Radic. Biol. Med. 2013, 57, 61. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.-X.; Bu, L.-X.; Jia, M.-Y.; Chen, L.-Q.; Song, K.; Liu, Y.-S.; Yuan, R.-T.; Shang, W. Protective Effect of Dietary Nitrate on Stress-Induced Gastric Mucosal Injury via Enhancing Blood Perfusion in Mongolian Gerbils. Chin. Med. J. (Engl.) 2020, 133, 2141–2142. [Google Scholar] [CrossRef]
- Fuchs, D.; Nyakayiru, J.; Draijer, R.; Mulder, T.P.J.; Hopman, M.T.E.; Eijsvogels, T.M.H.; Thijssen, D.H. Impact of Flavonoid-Rich Black Tea and Beetroot Juice on Postprandial Peripheral Vascular Resistance and Glucose Homeostasis in Obese, Insulin-Resistant Men: A Randomized Controlled Trial. Nutr. Metab. 2016, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Agool, A.; van Veldhuisen, D.J.; Dierckx, R.A.; Bax, J.J. Nitrate Administration Increases Blood Flow in Dysfunctional but Viable Myocardium, Leading to Improved Assessment of Myocardial Viability: A PET Study. J. Nucl. Med. 2006, 47, 1307–1311. [Google Scholar]
- Hobbs, D.A.; George, T.W.; Lovegrove, J.A. The Effects of Dietary Nitrate on Blood Pressure and Endothelial Function: A Review of Human Intervention Studies. Nutr. Res. Rev. 2013, 26, 210–222. [Google Scholar] [CrossRef]
- Ju, J.; Liu, Y.; Liang, H.; Yang, B. The Role of Pyroptosis in Endothelial Dysfunction Induced by Diseases. Front. Immunol. 2022, 13, 1093985. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-Rich Apples and Nitrate-Rich Spinach Augment Nitric Oxide Status and Improve Endothelial Function in Healthy Men and Women: A Randomized Controlled Trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef]
- Pekas, E.J.; Wooden, T.K.; Yadav, S.K.; Park, S.-Y. Body Mass-Normalized Moderate Dose of Dietary Nitrate Intake Improves Endothelial Function and Walking Capacity in Patients with Peripheral Artery Disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R162–R173. [Google Scholar] [CrossRef]
- Lbban, E.; Ashor, A.; Shannon, O.M.; Idris, I.; Siervo, M. Is Vitamin C a Booster of the Effects of Dietary Nitrate on Endothelial Function? Physiologic Rationale and Implications for Research. Nutrition 2023, 109, 111995. [Google Scholar] [CrossRef] [PubMed]
- Gielis, J.F.; Lin, J.Y.; Wingler, K.; Van Schil, P.E.Y.; Schmidt, H.H.; Moens, A.L. Pathogenetic Role of eNOS Uncoupling in Cardiopulmonary Disorders. Free Radic. Biol. Med. 2011, 50, 765–776. [Google Scholar] [CrossRef]
- Burnley-Hall, N.; Abdul, F.; Androshchuk, V.; Morris, K.; Ossei-Gerning, N.; Anderson, R.; Rees, D.A.; James, P.E. Dietary Nitrate Supplementation Reduces Circulating Platelet-Derived Extracellular Vesicles in Coronary Artery Disease Patients on Clopidogrel Therapy: A Randomised, Double-Blind, Placebo-Controlled Study. Thromb. Haemost. 2018, 118, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.; Khambata, R.S.; Massimo, G.; Ruivo, E.; Gee, L.C.; Foster, J.; Goddard, A.; Curtis, M.; Barnes, M.R.; Wade, W.G.; et al. Local Delivery of Nitric Oxide Prevents Endothelial Dysfunction in Periodontitis. Pharmacol. Res. 2023, 188, 106616. [Google Scholar] [CrossRef]
- DesOrmeaux, G.J.; Petrick, H.L.; Brunetta, H.S.; Holloway, G.P. Independent of Mitochondrial Respiratory Function, Dietary Nitrate Attenuates HFD-Induced Lipid Accumulation and Mitochondrial ROS Emission within the Liver. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E217–E228. [Google Scholar] [CrossRef]
- Ashmore, T.; Fernandez, B.O.; Branco-Price, C.; West, J.A.; Cowburn, A.S.; Heather, L.C.; Griffin, J.L.; Johnson, R.S.; Feelisch, M.; Murray, A.J. Dietary Nitrate Increases Arginine Availability and Protects Mitochondrial Complex I and Energetics in the Hypoxic Rat Heart. J. Physiol. 2014, 592, 4715–4731. [Google Scholar] [CrossRef]
- Petrick, H.L.; Handy, R.M.; Vachon, B.; Frangos, S.M.; Holwerda, A.M.; Gijsen, A.P.; Senden, J.M.; van Loon, L.J.C.; Holloway, G.P. Dietary Nitrate Preserves Mitochondrial Bioenergetics and Mitochondrial Protein Synthesis Rates during Short-Term Immobilization in Mice. J. Physiol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Qu, Y.; Wang, X.; An, W.; Li, Z.; Han, Z.; Qin, L. Nitrate Partially Inhibits Lipopolysaccharide-Induced Inflammation by Maintaining Mitochondrial Function. J. Int. Med. Res. 2020, 48, 0300060520902605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gomez, A.M.; Wang, X.; Yan, Y.; Zheng, M.; Cheng, H. ROS Regulation of Microdomain Ca(2+) Signalling at the Dyads. Cardiovasc. Res. 2013, 98, 248–258. [Google Scholar] [CrossRef]
- Paolocci, N.; Saavedra, W.F.; Miranda, K.M.; Martignani, C.; Isoda, T.; Hare, J.M.; Espey, M.G.; Fukuto, J.M.; Feelisch, M.; Wink, D.A.; et al. Nitroxyl Anion Exerts Redox-Sensitive Positive Cardiac Inotropy in Vivo by Calcitonin Gene-Related Peptide Signaling. Proc. Natl. Acad. Sci. USA 2001, 98, 10463–10468. [Google Scholar] [CrossRef]
- Paolocci, N.; Katori, T.; Champion, H.C.; St John, M.E.; Miranda, K.M.; Fukuto, J.M.; Wink, D.A.; Kass, D.A. Positive Inotropic and Lusitropic Effects of HNO/NO- in Failing Hearts: Independence from Beta-Adrenergic Signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 5537–5542. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Wang, W.; Froehlich, J.P.; Huke, S.; Aon, M.A.; Wilson, G.M.; Di Benedetto, G.; O’Rourke, B.; Gao, W.D.; Wink, D.A.; et al. Nitroxyl Improves Cellular Heart Function by Directly Enhancing Cardiac Sarcoplasmic Reticulum Ca2+ Cycling. Circ. Res. 2007, 100, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.D.; Murray, C.I.; Tian, Y.; Zhong, X.; DuMond, J.F.; Shen, X.; Stanley, B.A.; Foster, D.B.; Wink, D.A.; King, S.B.; et al. Nitroxyl-Mediated Disulfide Bond Formation between Cardiac Myofilament Cysteines Enhances Contractile Function. Circ. Res. 2012, 111, 1002–1011. [Google Scholar] [CrossRef]
- Petroff, M.G.; Kim, S.H.; Pepe, S.; Dessy, C.; Marbán, E.; Balligand, J.L.; Sollott, S.J. Endogenous Nitric Oxide Mechanisms Mediate the Stretch Dependence of Ca2+ Release in Cardiomyocytes. Nat. Cell Biol. 2001, 3, 867–873. [Google Scholar] [CrossRef]
- Swann, P.F. Carcinogenic Risk from Nitrite, Nitrate and N-Nitrosamines in Food. Proc. R. Soc. Med. 1977, 70, 113–115. [Google Scholar] [PubMed]
- Hezel, M.P.; Liu, M.; Schiffer, T.A.; Larsen, F.J.; Checa, A.; Wheelock, C.E.; Carlström, M.; Lundberg, J.O.; Weitzberg, E. Effects of Long-Term Dietary Nitrate Supplementation in Mice. Redox Biol. 2015, 5, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Qu, X.; Tran, S.D.; Schmidt, L.L.; Qin, L.; Zhang, C.; Cui, X.; Deng, D.; Wang, S. Histological Characteristics Following a Long-Term Nitrate-Rich Diet in Miniature Pigs with Parotid Atrophy. Int. J. Clin. Exp. Pathol. 2015, 8, 6225–6234. [Google Scholar]
- Sindelar, J.J.; Milkowski, A.L. Human Safety Controversies Surrounding Nitrate and Nitrite in the Diet. Nitric Oxide 2012, 26, 259–266. [Google Scholar] [CrossRef]
- Clements, W.T.; Lee, S.-R.; Bloomer, R.J. Nitrate Ingestion: A Review of the Health and Physical Performance Effects. Nutrients 2014, 6, 5224–5264. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J. Processed Meat: The Real Villain? Proc. Nutr. Soc. 2016, 75, 233–241. [Google Scholar] [CrossRef]
- Moretti, C.H.; Schiffer, T.A.; Montenegro, M.F.; Larsen, F.J.; Tsarouhas, V.; Carlström, M.; Samakovlis, C.; Weitzberg, E.; Lundberg, J.O. Dietary Nitrite Extends Lifespan and Prevents Age-Related Locomotor Decline in the Fruit Fly. Free Radic. Biol. Med. 2020, 160, 860–870. [Google Scholar] [CrossRef]
- Carvalho, L.R.R.A.; Guimarães, D.D.; Flôr, A.F.L.; Leite, E.G.; Ruiz, C.R.; de Andrade, J.T.; Monteiro, M.M.O.; Balarini, C.M.; de Lucena, R.B.; Sandrim, V.C.; et al. Effects of Chronic Dietary Nitrate Supplementation on Longevity, Vascular Function and Cancer Incidence in Rats. Redox Biol. 2021, 48, 102209. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; de Almeida, J.R.; Watson, E.; Glogauer, M.; Xu, W.; Keshavarzi, S.; O’Sullivan, B.; Ringash, J.; Hope, A.; Bayley, A.; et al. Short-Term and Long-Term Unstimulated Saliva Flow Following Unilateral vs Bilateral Radiotherapy for Oropharyngeal Carcinoma. Head Neck 2021, 43, 456–466. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, B.; Qi, X.; Zhang, H. Salivary-Gland-Mediated Nitrate Recirculation as a Modulator for Cardiovascular Diseases. Biomolecules 2025, 15, 439. https://doi.org/10.3390/biom15030439
Pang B, Qi X, Zhang H. Salivary-Gland-Mediated Nitrate Recirculation as a Modulator for Cardiovascular Diseases. Biomolecules. 2025; 15(3):439. https://doi.org/10.3390/biom15030439
Chicago/Turabian StylePang, Baoxing, Xingyun Qi, and Huiliang Zhang. 2025. "Salivary-Gland-Mediated Nitrate Recirculation as a Modulator for Cardiovascular Diseases" Biomolecules 15, no. 3: 439. https://doi.org/10.3390/biom15030439
APA StylePang, B., Qi, X., & Zhang, H. (2025). Salivary-Gland-Mediated Nitrate Recirculation as a Modulator for Cardiovascular Diseases. Biomolecules, 15(3), 439. https://doi.org/10.3390/biom15030439





