Neuroprotective Properties of Clove (Syzygium aromaticum): State of the Art and Future Pharmaceutical Applications for Alzheimer’s Disease
Abstract
1. Introduction
2. Cloves and Mechanisms of Neuroprotection
2.1. Clove Antioxidant Effects
2.2. Clove Anti-Inflammatory Effects
2.3. Neurotrophic and Neuropharmacological Effects
3. Amino Acid and Peptide Components in Cloves with Neuroprotective Potential
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The Global Prevalence of Dementia: A Systematic Review and Meta-Analysis. Alzheimers Dement. 2013, 9, 63.e2–75.e2. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, A.M.; Winblad, B.; Jönsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zahra, W.; Rai, S.N.; Birla, H.; Singh, S.S.; Dilnashin, H.; Rathore, A.S.; Singh, S.P. The Global Economic Impact of Neurodegenerative Diseases: Opportunities and Challenges. Bioeconomy Sustain. Dev. 2019, 17, 333–345. [Google Scholar] [CrossRef]
- Roland, K.P.; Chappell, N.L. Caregiver Experiences Across Three Neurodegenerative Diseases: Alzheimer’s, Parkinson’s, and Parkinson’s With Dementia. J. Aging Health 2019, 31, 256–279. [Google Scholar] [CrossRef]
- Aza, A.; Gómez-Vela, M.; Badia, M.; Orgaz, M.B.; González-Ortega, E.; Vicario-Molina, I.; Montes-López, E. Listening to families with a person with neurodegenerative disease talk about their quality of life: Integrating quantitative and qualitative approaches. Health Qual. Life Outcomes 2022, 20, 76. [Google Scholar] [CrossRef]
- Dokholyan, N.V.; Mohs, R.C.; Bateman, R.J. Challenges and progress in research, diagnostics, and therapeutics in Alzheimer’s disease and related dementias. Alzheimers Dement. 2022, 8, e12330. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef]
- Lemche, E. Early Life Stress and Epigenetics in Late-Onset Alzheimer’s Dementia: A Systematic Review. Curr. Genomics 2018, 19, 522–602. [Google Scholar] [CrossRef]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-d.-C.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Zemek, F.; Drtinova, L.; Nepovimova, E.; Sepsova, V.; Korabecny, J.; Klimes, J.; Kuca, K. Outcomes of Alzheimer’s Disease Therapy with Acetylcholinesterase Inhibitors and Memantine. Expert Opin. Drug Saf. 2014, 13, 759–774. [Google Scholar] [CrossRef]
- Kmietowicz, Z.; Mahase, E. Lecanemab: Benefits of Alzheimer’s Drug Are “Just Too Small” to Justify Cost, Says NICE. BMJ 2024, 386, q1853. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, J.; Kaniecki, E.; Naidu, A.; Silverglate, B.D.; Grossberg, G. How Promising Are the Latest Monoclonal Antibodies Targeting Amyloid-β for the Treatment of Early Alzheimer’s Disease? Expert Opin. Emerg. Drugs 2024, 29, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2024. Alzheimers Dement. 2024, 10, e12465. [Google Scholar] [CrossRef]
- Mahase, E. Lecanemab and donanemab: NICE reconsiders controversial Alzheimer’s drugs. BMJ 2025, 388, r463. [Google Scholar] [CrossRef]
- Cummings, J. Corection to: New Approaches to Symptomatic Treatments for Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 2. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Szekely, C.A.; Mielke, M.M.; Rosenberg, P.B.; Zandi, P.P. Developing New Treatments for Alzheimer’s Disease: The Who, What, When, and How of Biomarker-Guided Therapies. Int. Psychogeriatr. 2008, 20, 871–889. [Google Scholar] [CrossRef]
- Roviello, V.; Gilhen-Baker, M.; Roviello, G.N.; Lichtfouse, E. River Therapy. Environ. Chem. Lett. 2022, 20, 2729–2734. [Google Scholar] [CrossRef]
- Costanzo, M.; De Giglio, M.A.R.; Gilhen-Baker, M.; Roviello, G.N. The Chemical Basis of Seawater Therapies: A Review. Environ. Chem. Lett. 2024. [CrossRef]
- Fik-Jaskółka, M.; Mittova, V.; Motsonelidze, C.; Vakhania, M.; Vicidomini, C.; Roviello, G.N. Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics. Antibiotics 2024, 13, 487. [Google Scholar] [CrossRef]
- Ricci, A.; Roviello, G.N. Exploring the Protective Effect of Food Drugs against Viral Diseases: Interaction of Functional Food Ingredients and SARS-CoV-2, Influenza Virus, and HSV. Life 2023, 13, 402. [Google Scholar] [CrossRef]
- Ding, H.; Reiss, A.B.; Pinkhasov, A.; Kasselman, L.J. Plants, Plants, and More Plants: Plant-Derived Nutrients and Their Protective Roles in Cognitive Function, Alzheimer’s Disease, and Other Dementias. Medicina 2022, 58, 1025. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.L.; Ma, M.J. Alzheimer Disease: Clues from Traditional and Complementary Medicine. J. Tradit. Complement Med. 2017, 7, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Fik-Jaskółka, M.A.; Mkrtchyan, A.F.; Saghyan, A.S.; Palumbo, R.; Belter, A.; Hayriyan, L.A.; Simonyan, H.; Roviello, V.; Roviello, G.N. Spectroscopic and SEM Evidence for G4-DNA Binding by a Synthetic Alkyne-Containing Amino Acid with Anticancer Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117884. [Google Scholar] [CrossRef]
- Roviello, V.; Musumeci, D.; Mokhir, A.; Roviello, G.N. Evidence of Protein Binding by a Nucleopeptide Based on a Thyminedecorated L-Diaminopropanoic Acid through CD and In Silico Studies. Curr. Med. Chem. 2021, 28, 5004–5015. [Google Scholar] [CrossRef]
- Marzano, M.; Falanga, A.P.; Marasco, D.; Borbone, N.; D’Errico, S.; Piccialli, G.; Roviello, G.N.; Oliviero, G. Evaluation of an Analogue of the Marine ε-PLL Peptide as a Ligand of G-quadruplex DNA Structures. Mar. Drugs 2020, 18, 49. [Google Scholar] [CrossRef]
- Roviello, G.N.; Ricci, A.; Buccia, E.M.; Pedone, C. Synthesis, Biological Evaluation and Supramolecular Assembly of Novel Analogues of Peptidyl Nucleosides. Mol. BioSyst. 2011, 7, 1115–1123. [Google Scholar] [CrossRef]
- Roviello, G.N.; Musumeci, D.; Buccia, E.M.; Pedone, C. Evidences for Supramolecular Organization of Nucleopeptides: Synthesis, Spectroscopic and Biological Studies of a Novel Dithymine L-Serine Tetrapeptide. Mol. BioSyst. 2011, 7, 624–633. [Google Scholar] [CrossRef]
- Roviello, G.; Musumeci, D.; Pedone, C.; Bucci, E.M. Synthesis, Characterization and Hybridization Studies of an Alternate Nucleo-Epsilon/Gamma-Peptide: Complexes Formation with Natural Nucleic Acids. Amino Acids 2010, 38, 103–111. [Google Scholar] [CrossRef]
- Musumeci, D.; Oliviero, G.; Roviello, G.N.; Bucci, E.M.; Piccialli, G. G-Quadruplex-Forming Oligonucleotide Conjugated to Magnetic Nanoparticles: Synthesis, Characterization, and Enzymatic Stability Assays. Bioconjug. Chem. 2012, 23, 382–391. [Google Scholar] [CrossRef]
- Roviello, G.N.; Di Gaetano, S.; Capasso, D.; Cesarani, A.; Bucci, E.M.; Pedone, C. Synthesis, Spectroscopic Studies and Biological Activity of a Novel Nucleopeptide with Moloney Murine Leukemia Virus Reverse Transcriptase Inhibitory Activity. Amino Acids 2010, 38, 1489–1496. [Google Scholar] [CrossRef]
- Sargsyan, T.; Stepanyan, L.; Panosyan, H.; Hakobyan, H.; Israyelyan, M.; Tsaturyan, A.; Hovhannisyan, N.; Vicidomini, C.; Mkrtchyan, A.; Saghyan, A.; et al. Synthesis and Antifungal Activity of Fmoc-Protected 1,2,4-Triazolyl-α-Amino Acids and Their Dipeptides Against Aspergillus Species. Biomolecules 2025, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, H.; Palumbo, R.; Vicidomini, C.; Scognamiglio, P.L.; Petrosyan, S.; Sahakyan, L.; Melikyan, G.; Saghyan, A.; Roviello, G.N. Exploring the Binding of c-Myc G-Quadruplex and the Structural Impact of Synthetic Non-Proteinogenic Amino Acids on Serum Albumins: Implications for Potential Intrinsic c-Myc-Associated Anticancer Activity and Drug Delivery Systems. Mol. Ther. Nucleic Acids 2025, 102478. [Google Scholar] [CrossRef]
- Falanga, A.P.; Piccialli, I.; Greco, F.; D’Errico, S.; Nolli, M.G.; Borbone, N.; Oliviero, G.; Roviello, G.N. Nanostructural Modulation of G-Quadruplex DNA in Neurodegeneration: Orotate Interaction Revealed Through Experimental and Computational Approaches. J. Neurochem. 2025, 169, e16296. [Google Scholar] [CrossRef] [PubMed]
- Yash, R.; Menghani, D.M.; Bhattad, D.M.; Chandak, K.K.; Taksande, J.R.; Umekar, M.J. A Review: Pharmacological and Herbal Remedies in the Management of Neurodegenerative Disorder (Alzheimer’s). Int. J. Pharmacogn. Life Sci. 2021, 2, 18–27. [Google Scholar] [CrossRef]
- McKeage, K.; Lyseng-Williamson, K.A. Ginkgo Biloba Extract EGb 761® in the Symptomatic Treatment of Mild-to-Moderate Dementia: A Profile of Its Use. Drugs Ther. Perspect. 2018, 34, 358–366. [Google Scholar] [CrossRef]
- Kandiah, N.; Chan, Y.F.; Chen, C.; Dasig, D.; Dominguez, J.; Han, S.H.; Jia, J.; Kim, S.; Limpawattana, P.; Ng, L.L.; et al. Treatment of Dementia and Mild Cognitive Impairment with or without Cerebrovascular Disease: Expert Consensus on the Use of Ginkgo Biloba Extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef]
- Yan, Y.-P.; Chen, J.-Y.; Lu, J.-H. Disease-Modifying Activity of Huperzine A on Alzheimer’s Disease: Evidence from Preclinical Studies on Rodent Models. Int. J. Mol. Sci. 2022, 23, 15238. [Google Scholar] [CrossRef]
- Shan, M.; Bai, Y.; Fang, X.; Lan, X.; Zhang, Y.; Cao, Y.; Zhu, D.; Luo, H. American Ginseng for the Treatment of Alzheimer’s Disease: A Review. Molecules 2023, 28, 5716. [Google Scholar] [CrossRef]
- Lee, B.C.; Choe, Y.M.; Suh, G.H.; Choi, I.G.; Kim, H.S.; Hwang, J.; Yi, D.; Jhoo, J.H.; Kim, J.W. Ginseng Intake and Alzheimer Disease-Specific Cognition in Older Adults According to Apolipoprotein ε4 Allele Status. Front. Aging Neurosci. 2023, 15, 1152626. [Google Scholar] [CrossRef]
- Mittal, S.; Prajapati, K.P.; Ansari, M.; Anand, B.G.; Kar, K. Autooxidation of Curcumin in Physiological Buffer Causes an Enhanced Synergistic Anti-Amyloid Effect. Int. J. Biol. Macromol. 2023, 235, 123629. [Google Scholar] [CrossRef]
- Sallaberry, C.A.; Voss, B.J.; Stone, W.B.; Estrada, F.; Bhatia, A.; Soto, J.D.; Griffin, C.W.; Vander Zanden, C.M. Curcumin Reduces Amyloid Beta Oligomer Interactions with Anionic Membranes. ACS Chem. Neurosci. 2023, 14, 4026–4038. [Google Scholar] [CrossRef] [PubMed]
- Goozee, K.G.; Shah, T.M.; Sohrabi, H.R.; Rainey-Smith, S.R.; Brown, B.; Verdile, G.; Martins, R.N. Examining the Potential Clinical Value of Curcumin in the Prevention and Diagnosis of Alzheimer’s Disease. Br. J. Nutr. 2016, 115, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.E.; Osman, E.E.; Saeed, A.; Ming, L.C.; Goh, K.W.; Razi, P.; Abdullah, A.D.I.; Dahab, M. Plant Extracts as Emerging Modulators of Neuroinflammation and Immune Receptors in Alzheimer’s Pathogenesis. Heliyon 2024, 10, e35943. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Jo, W.; Ji, H.W.; Pyeon, M.; Moon, M.; Yun, J.; Lee, J.H.; Sohn, S.O. Effect of a Salvia officinalis and Hypericum perforatum Mixture on Improving Memory and Cognitive Decline. Adv. Tradit. Med. 2023, 23, 633–649. [Google Scholar] [CrossRef]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative Processes and Xenobiotic Metabolism in Plants: Mechanisms of Defense and Potential Therapeutic Implications. J. Xenobiot. 2024, 14, 1541–1569. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Mittova, V.; Tsetskhladze, Z.R.; Palumbo, R.; Pastore, R.; Roviello, G.N. Georgian Medicinal Plants as Rich Natural Sources of Antioxidant Derivatives: A Review on the Current Knowledge and Future Perspectives. Curr. Med. Chem. 2024, 31, 4407–4424. [Google Scholar] [CrossRef]
- Moawad, M.H.E.; Serag, I.; Alkhawaldeh, I.M.; Abbas, A.; Sharaf, A.; Alsalah, S.; Sadeq, M.A.; Shalaby, M.M.M.; Hefnawy, M.T.; Abouzid, M.; et al. Exploring the Mechanisms and Therapeutic Approaches of Mitochondrial Dysfunction in Alzheimer’s Disease: An Educational Literature Review. Mol. Neurobiol. 2024, 61, 1–22. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular Basis of the Therapeutical Potential of Clove (Syzygium aromaticum L.) and Clues to Its Anti-COVID-19 Utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef]
- Momo, E.J.; Nguimatsia, F.; Ateufouet Ngouango, L.; Lunga, P.K.; Pone Kamdem, B.; Jazet Dongmo, P.M. Eugenol-Rich Essential Oils from Flower Buds and Leaves of Syzygium aromaticum Show Antifungal Activity against Candida and Cryptococcus Species. Future Pharmacol. 2024, 4, 449–465. [Google Scholar] [CrossRef]
- Hickey, J.P.; Collins, A.E.; Nelson, M.L.; Chen, H.; Kalisch, B.E. Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer’s Disease. Curr. Issues Mol. Biol. 2024, 46, 4379–4402. [Google Scholar] [CrossRef]
- Saxena, B. Eugenol as Neuro-Phytomedicine: Recent Trends Pertaining to the Treatment of Neurological Disorders. In NeuroPhytomedicine, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; p. 18. [Google Scholar] [CrossRef]
- Panahzadeh, F.; Mirnasuri, R.; Rahmati, M. Exercise and Syzygium aromaticum Reverse Memory Deficits, Apoptosis, and Mitochondrial Dysfunction of the Hippocampus in Alzheimer’s Disease. J. Ethnopharmacol. 2022, 286, 114871. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, S.; Ahn, M.; Kim, J.; Moon, C.; Matsuda, H.; Tanaka, A.; Nomura, Y.; Jung, K.; Shin, T. Eugenol Alleviates the Symptoms of Experimental Autoimmune Encephalomyelitis in Mice by Suppressing Inflammatory Responses. Int. Immunopharmacol. 2024, 128, 111479. [Google Scholar] [CrossRef] [PubMed]
- Idowu, S.; Adekoya, A.E.; Igiehon, O.O.; Idowu, A.T. Clove (Syzygium aromaticum) Spices: A Review on Their Bioactivities, Current Use, and Potential Application in Dairy Products. J. Food Meas. Charact. 2021, 15, 3419–3435. [Google Scholar] [CrossRef]
- Liñán-Atero, R.; Aghababaei, F.; García, S.R.; Hasiri, Z.; Ziogkas, D.; Moreno, A.; Hadidi, M. Clove Essential Oil: Chemical Profile, Biological Activities, Encapsulation Strategies, and Food Applications. Antioxidants 2024, 13, 488. [Google Scholar] [CrossRef]
- Akopian, K.A.; Poghosyan, Y.M.; Poghosyan, S.B.; Matinyan, S.V.; Ter-Zakaryan, S.O.; Muradyan, S.A.; Movsisyan, M.R. Study of a Possible Allergic Effect of Ubivaks Ointment. New Armen. Med. J. 2015, 9, 32–35. [Google Scholar]
- Grant, W.B.; Blake, S.M. Diet’s Role in Modifying Risk of Alzheimer’s Disease: History and Present Understanding. J. Alzheimer’s Dis. 2023, 96, 1353–1382. [Google Scholar] [CrossRef]
- Muderawan, I.W.; Laksmi, P.P.D.S.; Mudianta, I.W.; Martiningsih, N.W. Chemical Constituent and Antioxidant Activity of Clove (Syzygium aromaticum) Bud and Leaf Essential Oils from Bali. Indones. J. Chem. Res. 2025, 12, mud. [Google Scholar] [CrossRef]
- Spigarelli, R.; Spisni, E.; Magalhães, M.; Cabral, C.; Gonçalves, A.C.; Saracino, I.M.; Botti, G.; Dalpiaz, A.; Beggiato, S.; Valerii, M.C. Clove Essential Oil as a Source of Antitumoral Compounds Capable of Crossing the Blood–Brain Barrier: A Focus on the Effects of β-Caryophyllene and Eugenol in a Glioblastoma Cell Line. Int. J. Mol. Sci. 2025, 26, 238. [Google Scholar] [CrossRef]
- Nagababu, E.; Lakshmaiah, N. Inhibitory Effect of Eugenol on Non-Enzymatic Lipid Peroxidation in Rat Liver Mitochondria. Biochem. Pharmacol. 1992, 43, 2393–2400. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Ashish; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive Properties of Clove (Syzygium aromaticum) Essential Oil Nanoemulsion: A Comprehensive Review. Heliyon 2024, 10, e22437. [Google Scholar] [CrossRef]
- Silva, M.V.; Lima, A.C.A.; Silva, M.G.; Caetano, V.F.; Andrade, M.F.; Silva, R.G.C.; Moraes Filho, L.E.P.T.; Silva, I.D.L.; Vinhas, G.M. Clove Essential Oil and Eugenol: A Review of Their Significance and Uses. Food Bioscience 2024, 62, 105112. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Ranđelović, P.J.; Simonović, M.; Radić, M.; Todorović, S.; Corrigan, M.; Harkin, A.; Boylan, F. Essential Oil Constituents as Anti-Inflammatory and Neuroprotective Agents: An Insight through Microglia Modulation. Int. J. Mol. Sci. 2024, 25, 5168. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, M.M.H.; Silva, M.G.; da Costa, J.G.M.; de Oliveira, A.G.; Rubio, C.; Padovani, C.R.; Rainho, C.A.; Schellini, S.A. Anti-inflammatory Effects of α-Humulene and β-Caryophyllene on Pterygium Fibroblasts. Int. J. Ophthalmol. 2022, 15, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Dalavaye, N.; Nicholas, M.; Pillai, M.; Erridge, S.; Sodergren, M.H. The Clinical Translation of α-humulene—A Scoping Review. Planta Med. 2024, 90, 664–674. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 Receptor Agonist Produces Multiple Behavioral Changes Relevant to Anxiety and Depression in Mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef]
- Cho, J.Y.; Chang, H.J.; Lee, S.K.; Kim, H.J.; Hwang, J.K.; Chun, H.S. Amelioration of Dextran Sulfate Sodium-Induced Colitis in Mice by Oral Administration of β-Caryophyllene, a Sesquiterpene. Life Sci. 2007, 80, 932–939. [Google Scholar] [CrossRef]
- Batiha, G.E.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective Effects of Oral Gallic Acid Against Oxidative Stress Induced by 6-Hydroxydopamine in Rats. Food Chem. 2013, 138, 1028–1033. [Google Scholar] [CrossRef]
- Hajipour, S.; Sarkaki, A.; Farbood, Y.; Eidi, A.; Mortazavi, P.; Valizadeh, Z. Effect of Gallic Acid on Dementia Type of Alzheimer Disease in Rats: Electrophysiological and Histological Studies. Basic Clin. Neurosci. 2016, 7, 97–106. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective Effect of Quercetin in Primary Neurons Against Aβ(1–42): Relevance to Alzheimer’s Disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The Flavonoid Quercetin Ameliorates Alzheimer’s Disease Pathology and Protects Cognitive and Emotional Function in Aged Triple Transgenic Alzheimer’s Disease Model Mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Kim, H.M.; Lee, J.S.; Lee, C.K.; Sezirahiga, J.; Woo, J.H.; Choi, J.H.; Jang, D.S. New Flavonol Glucuronides from the Flower Buds of Syzygium aromaticum (Clove). J. Agric. Food Chem. 2016, 64, 3048–3053. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective Effects of Flavone Luteolin in Neuroinflammation and Neurotrauma. Biofactors 2021, 47, 190–197. [Google Scholar] [CrossRef]
- Ahmad, S.; Jo, M.H.; Ikram, M.; Khan, A.; Kim, M.O. Deciphering the Potential Neuroprotective Effects of Luteolin against Aβ1–42-Induced Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9583. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological Effects and Mechanisms of Tannic Acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yu, Z.; Qi, J. Anti-Neuroinflammatory Effects of Tannic Acid Against Lipopolysaccharide-Induced BV2 Microglial Cells via Inhibition of NF-κB Activation. Drug Dev. Res. 2019, 80, 262–268. [Google Scholar] [CrossRef]
- Souza-Moreira, T.M.; Severi, J.A.; Lee, K.; Preechasuth, K.; Santos, E.; Gow, N.A.; Munro, C.A.; Vilegas, W.; Pietro, R.C. Anti-Candida Targets and Cytotoxicity of Casuarinin Isolated from Plinia cauliflora Leaves in a Bioactivity-Guided Study. Molecules 2013, 18, 8095–8108. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological Potential of Kaempferol, a Flavonoid in the Management of Pathogenesis via Modulation of Inflammation and Other Biological Activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojas, D.F.; de Souza, C.R.; Oliveira, W.P. Clove (Syzygium aromaticum): A Precious Spice. Asian Pac. J. Trop. Med. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Cai, L.; Wu, C.D. Compounds from Syzygium aromaticum Possessing Growth Inhibitory Activity against Oral Pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Yessirita, N.; Verawati, R.; Purnamasari, D.; Rollando, R.; Mandeli, R.S.; Albari, M.T.; Azhari, P.; Zainul, R.; Kharisma, V.D.; Jakhmola, V.; et al. In Silico Study of Rhamnocitrin Extract from Clove (Syzygium Aromaticum) in Inhibiting Adenosine A1 Adenylate Cyclase Interaction. Pharmacogn. J. 2023, 15, 512–517. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Xue, Q.; Xiang, Z.; Wang, S.; Cong, Z.; Gao, P.; Liu, X. Recent Advances in Nutritional Composition, Phytochemistry, Bioactive, and Potential Applications of Syzygium aromaticum L. (Myrtaceae). Front. Nutr. 2022, 9, 1002147. [Google Scholar] [CrossRef]
- Han, A.R. Identification and PEP Inhibitory Activity of Acetophenone Glucosides from the Clove Buds (Syzygium aromaticum). J. Korean Soc. Appl. Biol. Chem. 2010, 53, 847–851. [Google Scholar] [CrossRef]
- Kumar Pandey, V.; Shams, R.; Singh, R.; Dar, A.H.; Pandiselvam, R.; Rusu, A.V.; Trif, M. A Comprehensive Review on Clove (Caryophyllus aromaticus L.) Essential Oil and Its Significance in the Formulation of Edible Coatings for Potential Food Applications. Front. Nutr. 2022, 9, 987674. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Rizkiyah, D.N.; Qomariyah, L.; Irianto, I.; Che Yunus, M.A.; Putra, N.R. Unlocking the Full Potential of Clove (Syzygium aromaticum) Spice: An Overview of Extraction Techniques, Bioactivity, and Future Opportunities in the Food and Beverage Industry. Processes 2023, 11, 2453. [Google Scholar] [CrossRef]
- Sara, A.; Ali, S.N.; Begum, S.; Siddiqui, B.S. Chemical Constituents of Syzygium aromaticum. Chem. Nat. Comp. 2018, 54, 1192–1193. [Google Scholar] [CrossRef]
- Sen, A. Prophylactic and Therapeutic Roles of Oleanolic Acid and Its Derivatives in Several Diseases. World J. Clin. Cases 2020, 8, 1767–1792. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, J.; Perneczky, R. Anti-Dementia Medications and Anti-Alzheimer’s Disease Drugs: Side Effects, Contraindications, and Interactions. In NeuroPsychopharmacotherapy; Springer: Cham, Switzerland, 2022; pp. 1–10. [Google Scholar] [CrossRef]
- Sharma, H.; Kim, D.Y.; Shim, K.H.; Sharma, N.; An, S.S.A. Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 8148. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, M.; Ibrahim, S.S.; Said, A.M.; Elgendey, F.; Anis, A. Neuroprotective Effects of Clove Oil in Acrylamide-Induced Neurotoxicity in Rats. Pak. Vet. J. 2019, 39, 111–115. [Google Scholar] [CrossRef]
- Gandara, J.; Barreto, G.E.; Martins, N.; Sharifi-Rad, J. Oxidative Stress in Alzheimer’s Disease: Current Knowledge of Signaling Pathways and Therapeutics. Mol. Biol. Rep. 2023, 50, 1–17. [Google Scholar] [CrossRef]
- Ivanović, J.; Dimitrijević-Branković, S.; Mišić, D. Evaluation and Improvement of Antioxidant and Antibacterial Activities of Supercritical Extracts from Clove Buds. J. Funct. Foods 2013, 5, 416–423. [Google Scholar] [CrossRef]
- Kiki, M.J. In Vitro Antiviral Potential, Antioxidant, and Chemical Composition of Clove (Syzygium aromaticum) Essential Oil. Molecules 2023, 28, 2421. [Google Scholar] [CrossRef]
- Lionnet, L.; Beaudry, F.; Vachon, P. Intrathecal Eugenol Administration Alleviates Neuropathic Pain in Male Sprague-Dawley Rats. Phytother. Res. 2010, 24, 1645–1653. [Google Scholar] [CrossRef]
- Shekhar, S.; Yadav, Y.; Singh, A.P.; Pradhan, R.; Desai, G.R.; Dey, A.B.; Dey, S. Neuroprotection by Ethanolic Extract of Syzygium aromaticum in Alzheimer’s Disease-Like Pathology via Maintaining Oxidative Balance through SIRT1 Pathway. Exp. Gerontol. 2018, 110, 277–283. [Google Scholar] [CrossRef]
- Halder, S.; Mehta, A.K.; Kar, R.; Mustafa, M.; Mediratta, P.K.; Sharma, K.K. Clove Oil Reverses Learning and Memory Deficits in Scopolamine-Treated Mice. Planta Med. 2011, 77, 830–834. [Google Scholar] [CrossRef]
- Liu, B.B.; Luo, L.; Liu, X.L.; Geng, D.; Li, C.F.; Chen, S.M.; Chen, X.M.; Yi, L.T.; Liu, Q. Essential Oil of Syzygium aromaticum Reverses the Deficits of Stress-Induced Behaviors and Hippocampal p-ERK/p-CREB/Brain-Derived Neurotrophic Factor Expression. Planta Medica 2015, 81, 185–192. [Google Scholar] [CrossRef]
- Thapa, R.; Moglad, E.; Afzal, M.; Kumar, G.; Bhat, A.A.; Khan, M.A.; Khan, M.A.; Khan, M.I.; Khan, M.I.; Khan, M.I.; et al. The Role of Sirtuin 1 in Ageing and Neurodegenerative Disease: A Molecular Perspective. Ageing Res. Rev. 2024, 90, 102545. [Google Scholar] [CrossRef] [PubMed]
- Amir Rawa, M.S.; Mazlan, M.K.N.; Ahmad, R.; Nogawa, T.; Wahab, H.A. Roles of Syzygium in Anti-Cholinesterase, Anti-Diabetic, Anti-Inflammatory, and Antioxidant: From Alzheimer’s Perspective. Plants 2022, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y.; et al. The Neuroprotective Effects of SIRT1 in Mice Carrying the APP/PS1 Transgene. Aging Cell 2014, 13, 808–817. [Google Scholar] [CrossRef]
- Kiki, M.J. Chemical Composition, In Vivo, and In Silico Molecular Docking Studies of the Effect of Syzygium aromaticum (Clove) Essential Oil on Ochratoxin A-Induced Acute Neurotoxicity. Plants 2025, 14, 130. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.D.; Wang, Y.D. DAF-16/FOXO Transcription Factor in Aging and Longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Wang, Y.; Chen, Q.; Qiao, X. Eugenol Alleviates Lipopolysaccharide-Induced Neuroinflammation by Activating the Nrf2/HO-1 Pathway in BV2 Microglial Cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 234, 108938. [Google Scholar] [CrossRef]
- Gorgin Karaji, Z.; Fathi, M.; Mirnasori, R.; van der Zee, E.A. Swimming Exercise and Clove Oil Can Improve Memory by Molecular Responses Modification and Reduce Dark Cells in Rat Model of Alzheimer’s Disease. Exp. Gerontol. 2023, 174, 112192. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune Attack: The Role of Inflammation in Alzheimer Disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, L.; Hu, C.; Zhou, L.; Cheng, Y.; Liu, Q. Ellagic Acid (EA) Ameliorates Alzheimer’s Disease by Reducing Aβ Levels, Oxidative Stress, and Attenuating Inflammation. Eur. J. Pharmacol. 2025, 986, 177099. [Google Scholar] [CrossRef]
- Pădureanu, V.; Dop, D.; Caragea, D.C.; Rădulescu, D.; Pădureanu, R.; Forțofoiu, M.-C. Cardiovascular and Neurological Diseases and Association with Helicobacter Pylori Infection—An Overview. Diagnostics 2024, 14, 1781. [Google Scholar] [CrossRef]
- Kim, K.H.; Rateb, M.; Hassan, H.; Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A. Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Zahmatkeshan, M.; Yousefpoor, Y.; Alipanah, H.; Safari, E.; Osanloo, M. Anti-Inflammatory and Anti-Nociceptive Effects of Cinnamon and Clove Essential Oils Nanogels: An in Vivo Study. BMC Complement. Med. Ther. 2022, 22, 143. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Madhyastha, H.; Sandur, V.R.; Manikandanath, N.T.; Thiagarajan, N.; Thiagarajan, P. Anti-Inflammatory and Wound Healing Potential of a Clove Oil Emulsion. Colloids Surf. B Biointerfaces 2020, 193, 111102. [Google Scholar] [CrossRef] [PubMed]
- Akbar, L.; Juliandi, B.; Boediono, A.; Batubara, I.; Subangkit, M. Effects of Eugenol on Memory Performance, Neurogenesis, and Dendritic Complexity of Neurons in Mice Analyzed by Behavioral Tests and Golgi Staining of Brain Tissue. J. Stem Cells Regen. Med. 2021, 17, 35–41. [Google Scholar] [CrossRef]
- Revi, N.; Sankaranarayanan, S.A.; Rengan, A.K. A Study on the Role of Eugenol Encapsulated Liposomes in Facilitating Neuron-Microglia Mediated Wound Recovery. Materialia 2022, 23, 101454. [Google Scholar] [CrossRef]
- Garabadu, D.; Sharma, M. Eugenol Attenuates Scopolamine-Induced Hippocampal Cholinergic, Glutamatergic, and Mitochondrial Toxicity in Experimental Rats. Neurotoxicol. Res. 2019, 35, 848–859. [Google Scholar] [CrossRef]
- Prasad, S.N.; Bharath, M.M.; Muralidhara. Neurorestorative Effects of Eugenol, a Spice Bioactive: Evidence in Cell Model and Its Efficacy as an Intervention Molecule to Abrogate Brain Oxidative Dysfunctions in the Streptozotocin Diabetic Rat. Neurochem. Int. 2016, 95, 24–36. [Google Scholar] [CrossRef]
- Soares, G.A.B.e.; Bhattacharya, T.; Chakrabarti, T.; Tagde, P.; Cavalu, S. Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants 2022, 11, 21. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. Eugenol Enhances Memory Performance and Neurogenesis in Mice. J. Neuropharm. 2021, 9, 8372414. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8372414.
- Hussain, M.; Khattak, M.N.K.; Shaheen, M.; Zaman, W.; Khan, M.S.; Saeed, M.; Rauf, A.; Anwar, F.; Shamsi, M. Clove Oil Reverses Learning and Memory Deficits in Scopolamine-Treated Mice. Brain Res. 2015, 1234, 45–52. [Google Scholar]
- Costa, J.G.; Ratti, A.L.; Filho, P.P.; Silva, L.M.; Martins, A.M.; Moreira, A.; Cavalcanti, R.P.; Araujo, S.; Rocha, A.C.; de Lima, G. Clove Extract Improves Cognitive Impairment in Septic Mice by Activating the SIRT1 Signaling Pathway. Food Sci. Technol. 2020, 40, 1234–1245. [Google Scholar]
- Liang, Z.H.; Cheng, X.H.; Ruan, Z.G.; Wang, H.; Li, S.S.; Liu, J.; Li, G.Y.; Tian, S.M. Protective Effects of Components of the Chinese Herb Grassleaf Sweetflag Rhizome on PC12 Cells Incubated with Amyloid-Beta42. Neural Regen. Res. 2015, 10, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Yaghmaei, P.; Tehrani, H.S.; Ebrahim-Habibi, A. Effects of Eugenol on Alzheimer’s Disease-like Manifestations in Insulin- and Aβ-Induced Rat Models. Neurophysiology 2019, 51, 114–119. [Google Scholar] [CrossRef]
- Jung, H.A.; Kim, S.M.; Kim, J.Y.; Yang, H.O. Eugenol Ameliorates Alzheimer’s Disease Pathologies in a 5×FAD Mouse Model. Phytomedicine 2023. [CrossRef]
- Liu, J.G.; Liu, H.P. Total Nutrient Analysis of Various Parts of Eugenia caryophyllata. Biotic Res. 2021, 43, 357–362. [Google Scholar] [CrossRef]
- Ma, S.S. Food safety standards and development of health food of clove. Ph.D. Thesis, Hainan University, Haikou, China, 2018. [Google Scholar]
- Holeček, M. Aspartic Acid in Health and Disease. Nutrients 2023, 15, 4023. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, H.; Xiong, Y.; Tong, Z.; Li, X. L-Serine, an Endogenous Amino Acid, Is a Potential Neuroprotective Agent for Neurological Disease and Injury. Front. Mol. Neurosci. 2021, 14, 726665. [Google Scholar] [CrossRef]
- Moldovan, O.L.; Sandulea, A.; Lungu, I.A.; Gâz, Ș.A.; Rusu, A. Identification of Some Glutamic Acid Derivatives with Biological Potential by Computational Methods. Molecules 2023, 28, 4123. [Google Scholar] [CrossRef]
- Petrat, F.; Boengler, K.; Schulz, R.; de Groot, H. Glycine, a Simple Physiological Compound Protecting by Yet Puzzling Mechanism(s) Against Ischaemia-Reperfusion Injury: Current Knowledge. Br. J. Pharmacol. 2012, 165, 2059–2072. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxid. Med. Cell Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A Novel Anti-Inflammatory, Immunomodulatory, and Cytoprotective Agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Canfield, C.-A.; Bradshaw, P.C. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Gupta, M.N.; Uversky, V.N. Biological Importance of Arginine: A Comprehensive Review of the Roles in Structure, Disorder, and Functionality of Peptides and Proteins. Int. J. Biol. Macromol. 2024, 257 Pt 1, 128646. [Google Scholar] [CrossRef]
- Kurhaluk, N. The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia. Int. J. Mol. Sci. 2023, 24, 8205. [Google Scholar] [CrossRef]
- Feng, L.; Peng, Y.; Wu, P.; Hu, K.; Jiang, W.D.; Liu, Y.; Jiang, J.; Li, S.H.; Zhou, X.Q. Threonine Affects Intestinal Function, Protein Synthesis and Gene Expression of TOR in Jian Carp (Cyprinus carpio var. Jian). PLoS ONE 2013, 8, e69974. [Google Scholar] [CrossRef]
- Holeček, M. Origin and Roles of Alanine and Glutamine in Gluconeogenesis in the Liver, Kidneys, and Small Intestine under Physiological and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 7037. [Google Scholar] [CrossRef]
- Jongkees, B.J.; Hommel, B.; Kühn, S.; Colzato, L.S. Effect of Tyrosine Supplementation on Clinical and Healthy Populations under Stress or Cognitive Demands—A Review. J. Psychiatr. Res. 2015, 70, 50–57. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Olaniyan, O.T.; Emeruwa, C.N.; Saha, S.; Saso, L.; Tucci, P. An Insight into the Role of Amino Acids as Antioxidants via NRF2 Activation. Amino Acids 2024, 56, 23. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Phillips, S.M. Branched-Chain Amino Acids (Leucine, Isoleucine, and Valine) and Skeletal Muscle. In Nutrition and Skeletal Muscle; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–298. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Liang, B.; Su, Y.; Sun, S.; Xia, S.; Shao, J.; Zhang, Z.; Hong, M.; Zhang, F.; et al. Methionine Metabolism in Chronic Liver Diseases: An Update on Molecular Mechanism and Therapeutic Implication. Signal Transduct. Target Ther. 2020, 5, 280. [Google Scholar] [CrossRef]
- Derouiche, F.; Djemil, R.; Sebihi, F.Z.; Douaouya, L.; Maamar, H.; Benjemana, K. High Methionine Diet Mediated Oxidative Stress and Proteasome Impairment Causes Toxicity in Liver. Sci. Rep. 2024, 14, 5555. [Google Scholar] [CrossRef]
- Xiao, C.W.; Hendry, A.; Kenney, L.; Bertinato, J. L-Lysine Supplementation Affects Dietary Protein Quality and Growth and Serum Amino Acid Concentrations in Rats. Sci. Rep. 2023, 13, 19943. [Google Scholar] [CrossRef]
- Huang, D.; Maulu, S.; Ren, M.; Liang, H.; Ge, X.; Ji, K.; Yu, H. Dietary Lysine Levels Improved Antioxidant Capacity and Immunity via the TOR and p38 MAPK Signaling Pathways in Grass Carp, Ctenopharyngodon idellus Fry. Front. Immunol. 2021, 12, 635015. [Google Scholar] [CrossRef]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Mochizuki, S.; Sugahara, K.; Yoshizawa, F. Isoleucine, a Blood Glucose-Lowering Amino Acid, Increases Glucose Uptake in Rat Skeletal Muscle in the Absence of Increases in AMP-Activated Protein Kinase Activity. J. Nutr. 2005, 135, 2103–2108. [Google Scholar] [CrossRef]
- Ely, I.A.; Phillips, B.E.; Smith, K.; Wilkinson, D.J.; Piasecki, M.; Breen, L.; Larsen, M.S.; Atherton, P.J. A Focus on Leucine in the Nutritional Regulation of Human Skeletal Muscle Metabolism in Ageing, Exercise, and Unloading States. Clin. Nutr. 2023, 42, 1849–1865. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline Metabolism and Redox; Maintaining a Balance in Health and Disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research, and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef]
- Kikuchi, A.M.; Tanabe, A.; Iwahori, Y. A Systematic Review of the Effect of L-Tryptophan Supplementation on Mood and Emotional Functioning. J. Diet. Suppl. 2021, 18, 316–333. [Google Scholar] [CrossRef]
- Bon, L.I.; Maksimovich, N.Y.; Burak, I.N. Amino Acids that Play an Important Role in the Functioning of the Nervous System: A Review. Clin. Trials Clin. Res. 2023, 2, 3. [Google Scholar] [CrossRef]
- Aydin, S.; Dagli, A.F.; Ozkan, Y.; Kendir, Y.; Sahin, I.; Aksoy, A.; Ozercan, I.H. Immunohistochemical and Quantitative Analysis of Ghrelin in Syzygium aromaticum. Cell Biol. Int. 2011, 35, 437–441. [Google Scholar] [CrossRef]
- Sehnal, D.; Rose, A.S.; Koca, J.; Burley, S.K.; Velankar, S. Mol*: Towards a Common Library and Tools for Web Molecular Graphics. In Workshop on Molecular Graphics and Visual Analysis of Molecular Data; Byška, J., Krone, M., Sommer, B., Eds.; The Eurographics Association: Limassol, Cyprus, 2018. [Google Scholar] [CrossRef]
- Jeon, S.G.; Hong, S.B.; Nam, Y.; Tae, J.; Yoo, A.; Song, E.J.; Kim, K.I.; Lee, D.; Park, J.; Lee, S.M.; et al. Ghrelin in Alzheimer’s Disease: Pathologic Roles and Therapeutic Implications. Ageing Res. Rev. 2019, 55, 100945. [Google Scholar] [CrossRef]
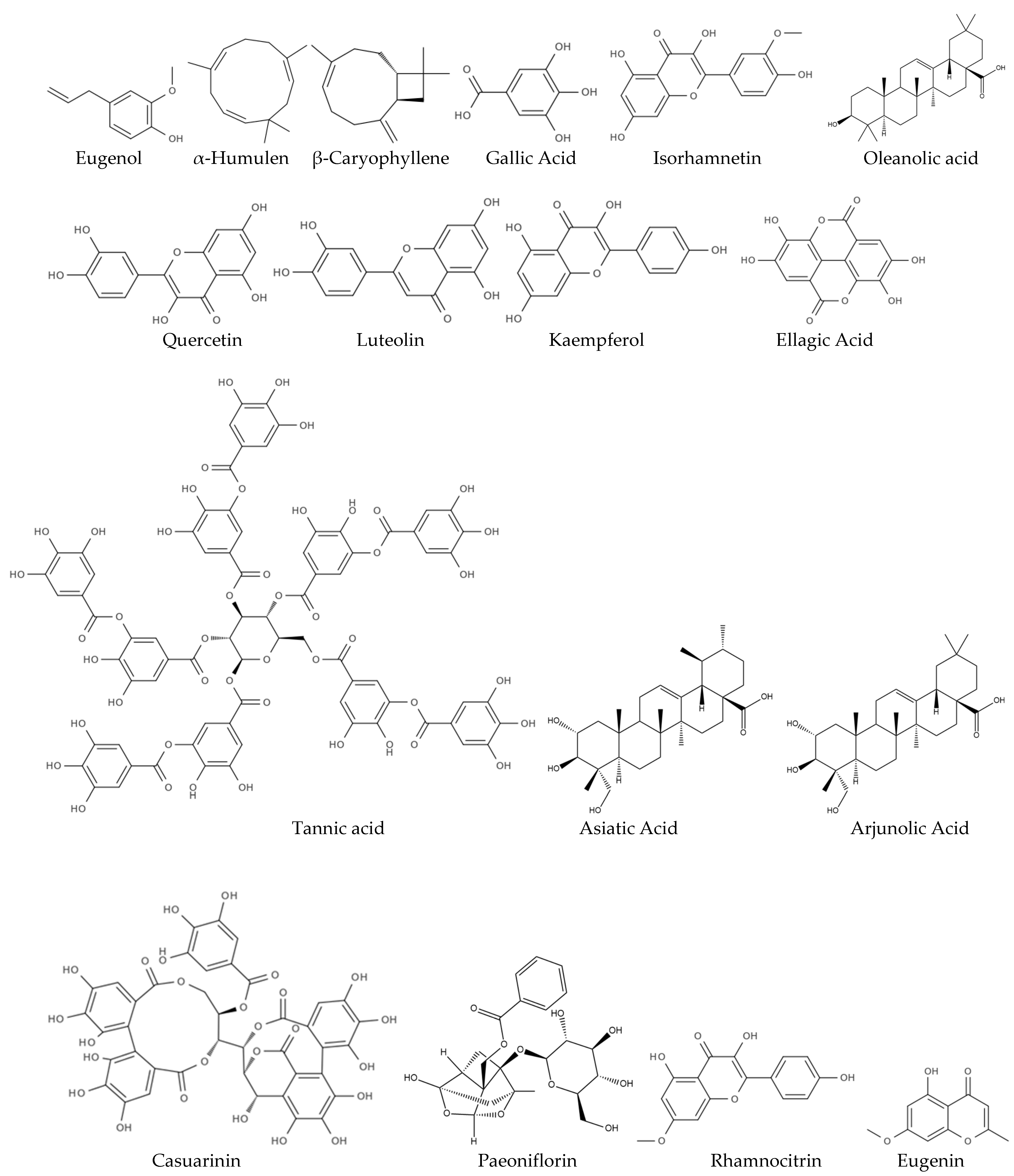

| Molecular Component | Potential Neuropharmacological Effects | Mechanism of Action | Reference |
|---|---|---|---|
| Eugenol | Neuroprotective, antioxidant, anti-inflammatory | Scavenges free radicals, inhibits neuroinflammation, modulates calcium channels | [60,61,62] |
| α-Humulen | Antioxidant, anti-inflammatory | NF-κB inhibition, ROS neutralization, COX-2 suppression, membrane disruption. | [55,63,64,65] |
| β-Caryophyllene | Neuroprotective, anti-inflammatory, anti-anxiety | CB2 receptor agonist, modulates neuroinflammation, reduces oxidative stress | [66,67,68] |
| Gallic Acid | Antioxidant, anti-apoptotic, memory enhancer | Reduces oxidative stress, prevents neuronal apoptosis | [69,70,71] |
| Quercetin | Neuroprotective, cognitive enhancer, anti-neuroinflammatory | Inhibits acetylcholinesterase, reduces pro-inflammatory mediators | [66,72,73,74] |
| Luteolin | Neuroprotective | Inhibits inflammation, promotes neuroprotection, and reduces oxidative stress | [75,76,77] |
| Tannic acid | Antioxidant, anti-inflammatory, anti-neuroinflammatory | Free radical scavenging, metal chelation, lipid protection, NF-κB inhibition, cytokine reduction, modulates cytokines, inhibits microglial activation | [61,78,79] |
| Casuarinin | Antioxidant | Scavenges free radicals, reducing oxidative stress | [80] |
| Paeoniflorin | Antioxidant, anti-inflammatory, neuroprotective | Inhibits pro-inflammatory cytokines, reduces ROS, stabilizes cell membranes | [81] |
| Kaempferol | Neuroprotective, anti-inflammatory | Suppresses pro-inflammatory pathways, protects against neuronal degeneration | [69,82] |
| Ellagic Acid | Antioxidant, neuroprotective | Scavenges free radicals, inhibits inflammation, regulates cell cycle | [69,83] |
| Rhamnocitrin | Antioxidant, neuroprotective | Free radical scavenging, reduction in neuroinflammation | [69,84,85] |
| Isorhamnetin | Antioxidant, anti-inflammatory | Free radical scavenging, inhibition of pro-inflammatory cytokines | [86,87] |
| Eugenin | Anti-inflammatory, antioxidant, neuroprotective | Neutralizes reactive oxygen species and reactive nitrogen species (RNS), inhibits the production of pro-inflammatory mediators, interferes with neuroinflammatory pathways | [87,88,89,90] |
| Oleanolic Acid | Antioxidant, anti-inflammatory | Scavenges free radicals and boosts cellular antioxidant defenses, inhibits the NF-κB pathway and reduces pro-inflammatory cytokines, modulates oxidative stress and inflammation | [87,91,92] |
| Asiatic Acid | Neuroprotective, anti-inflammatory | Protects neurons from oxidative stress and apoptosis, potentially benefiting neurodegenerative diseases like Alzheimer’s and Parkinson’s, suppresses pro-inflammatory mediators like IL-6 and TNF-α. | [87,91,92] |
| Arjunolic Acid | antioxidant, anti-inflammatory | Reduces oxidative stress, chelates metal ions and scavenges reactive oxygen species, reduces inflammation in various disease models. | [87,91,92] |
| Model | Effect | Rate | Reference |
|---|---|---|---|
| In vitro (human erythrocyte) | Inhibited human erythrocyte hemolysis | Inhibition by 53.04–63.64% | [113] |
| In vivo (rat model) | Reduced paw swelling | Reduction by 40–60% | [115] |
| In Vitro/In Vivo Model | Biological Activity | Plant Part | Extract/Oil | Reference |
|---|---|---|---|---|
| Antioxidant tests (DPPH, FRAP) (in vitro) | Antioxidant | Buds | Supercritical extract | [94] |
| Antioxidant analysis (ABTS, DPPH) (in vitro) | Antioxidant | Buds | Essential oil | [95] |
| Neuron culture, Aβ-induced damage (in vitro) | Neuroprotection (Alzheimer’s disease) | Buds | Ethanol extract | [97] |
| Primary neuronal cells, scopolamine-induced memory impairment model (in vitro) | Memory enhancement, neuroprotection | Buds | Oil | [98] |
| Neuronal cell line PC12, stress-induced damage (in vitro) | Neurogenesis, memory improvement | Buds | Eugenol (oil component) | [118] |
| Neuropathic pain model, eugenol injection in cerebrospinal fluid (in vivo; rats) | Pain relief in neuropathic pain | Buds | Eugenol | [96] |
| Alzheimer’s disease model, Aβ-induced memory impairment (in vivo; mice) | Neuroprotection (SIRT1 pathway) | Buds | Extract | [101] |
| Acrylamide-induced neurotoxicity model (in vivo; rats) | Neuroprotection in toxic brain damage | Buds | Oil | [92] |
| Alzheimer’s disease model, effect of physical exercise (in vivo; rats) | Memory enhancement, reduction in damaged cells | Buds | Oil | [106] |
| Alzheimer’s disease model, mitochondrial function analysis (in vivo; rats) | Memory restoration, apoptosis reduction, improved mitochondria | Buds | Extract | [49] |
| Amino Acid | Buds (mg/kg) | Buds (mg/kg) | Fruits (mg/kg) | Branches (mg/kg) | Leaves (mg/kg) | Biological Properties | Reference |
|---|---|---|---|---|---|---|---|
| Aspartic Acid | 111.6 | 42.8 | 105.4 | - | - | Supports metabolism and neurotransmission | [129] |
| Serine | 69.8 | 80.5 | 41.5 | 57.9 | 37.9 | Supports protein synthesis and acts as a precursor for neurotransmitters | [130] |
| Glutamic Acid | 93.8 | 91.3 | 74.1 | 64.2 | 66.4 | Functions as an excitatory neurotransmitter and antioxidant | [131] |
| Glycine | 61.2 | - | 42.3 | 40.5 | 41.4 | Neurotransmitter, anti-inflammatory, cytoprotective, immunomodulatory, metabolic precursor | [132,133,134] |
| Histidine | 121.6 | - | 118.8 | 121.2 | 120.6 | Encompasses neurotransmitter synthesis, enzymatic catalysis, metal ion chelation, and plays a role in the modulation of immune responses and growth | [135,136] |
| Arginine | 133.1 | 113.7 | 96.1 | 250.1 | 89.9 | Encompasses nitric oxide production, immune enhancement, antimicrobial action, and metabolic regulation | [137,138] |
| Threonine * | 38.4 | 260.4 | 40.1 | - | 34.8 | Plays a critical role in protein synthesis, immune function, and various metabolic pathways | [139] |
| Alanine | 94.5 | - | 93.8 | 52.3 | 55.2 | Supports gluconeogenesis, insulin secretion, immune function, and longevity | [140] |
| Tyrosine | 77.5 | 40.0 | 69.3 | 64.1 | 66.7 | Precursor for hormones like dopamine and adrenaline. Affects cognition, thermoregulation, neurotransmission, and may influence lifespan at varying doses | [141] |
| Valine * | 65.9 | 106.1 | 50.2 | 45.7 | 44.9 | Contributes to muscle growth, tissue repair, has antioxidant properties, and activates NRF2 to improve cellular health and growth | [142,143] |
| Methionine * | 63.3 | 14.1 | 62.8 | - | - | Acts as an antioxidant and supports liver detoxification | [144,145] |
| Lysine * | 68.9 | - | 68.5 | 68.2 | 66.8 | Essential for protein synthesis, longevity, metabolism, and tissue repair; plays a significant role in antioxidant and anti-inflammatory activities | [146,147] |
| Isoleucine * | 59.8 | 16.8 | 53.1 | - | - | Branched-chain amino acid, affects glucose metabolism, insulin resistance, and may play a role in aging | [143,148] |
| Leucine * | 61.8 | 27.7 | 56.8 | - | - | Influences lifespan, metabolism, muscle function, and longevity regulation pathways | [143,149] |
| Phenylalanine * | 75.0 | 21.1 | 74.8 | - | 83.3 | Precursor for neurotransmitters like dopamine and norepinephrine, antioxidant. | [150] |
| Proline | 154.9 | - | 203.7 | 97.6 | 63.7 | Enhances collagen synthesis and cellular repair, supports antioxidant activity, and contributes to metabolic regulation. | [151] |
| Tryptophan * | - | 12.1 | - | - | - | Recursor to melatonin, serotonin, and vitamin B3. It influences aging, neurotransmitter synthesis, mood regulation, and sleep cycles. | [152,153] |
| Total Amino Acids (TAA) | 1351.1 | 830.2 | 1251.2 | 861.8 | 771.6 | ||
| Essential Amino Acids (EAA) | 433.1 | 461.9 | 406.9 | 113.9 | 229.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargsyan, T.; Simonyan, H.M.; Stepanyan, L.; Tsaturyan, A.; Vicidomini, C.; Pastore, R.; Guerra, G.; Roviello, G.N. Neuroprotective Properties of Clove (Syzygium aromaticum): State of the Art and Future Pharmaceutical Applications for Alzheimer’s Disease. Biomolecules 2025, 15, 452. https://doi.org/10.3390/biom15030452
Sargsyan T, Simonyan HM, Stepanyan L, Tsaturyan A, Vicidomini C, Pastore R, Guerra G, Roviello GN. Neuroprotective Properties of Clove (Syzygium aromaticum): State of the Art and Future Pharmaceutical Applications for Alzheimer’s Disease. Biomolecules. 2025; 15(3):452. https://doi.org/10.3390/biom15030452
Chicago/Turabian StyleSargsyan, Tatevik, Hayarpi M. Simonyan, Lala Stepanyan, Avetis Tsaturyan, Caterina Vicidomini, Raffaele Pastore, Germano Guerra, and Giovanni N. Roviello. 2025. "Neuroprotective Properties of Clove (Syzygium aromaticum): State of the Art and Future Pharmaceutical Applications for Alzheimer’s Disease" Biomolecules 15, no. 3: 452. https://doi.org/10.3390/biom15030452
APA StyleSargsyan, T., Simonyan, H. M., Stepanyan, L., Tsaturyan, A., Vicidomini, C., Pastore, R., Guerra, G., & Roviello, G. N. (2025). Neuroprotective Properties of Clove (Syzygium aromaticum): State of the Art and Future Pharmaceutical Applications for Alzheimer’s Disease. Biomolecules, 15(3), 452. https://doi.org/10.3390/biom15030452











