Toxicity Mechanisms of Microplastic and Its Effects on Ruminant Production: A Review
Abstract
1. Introduction
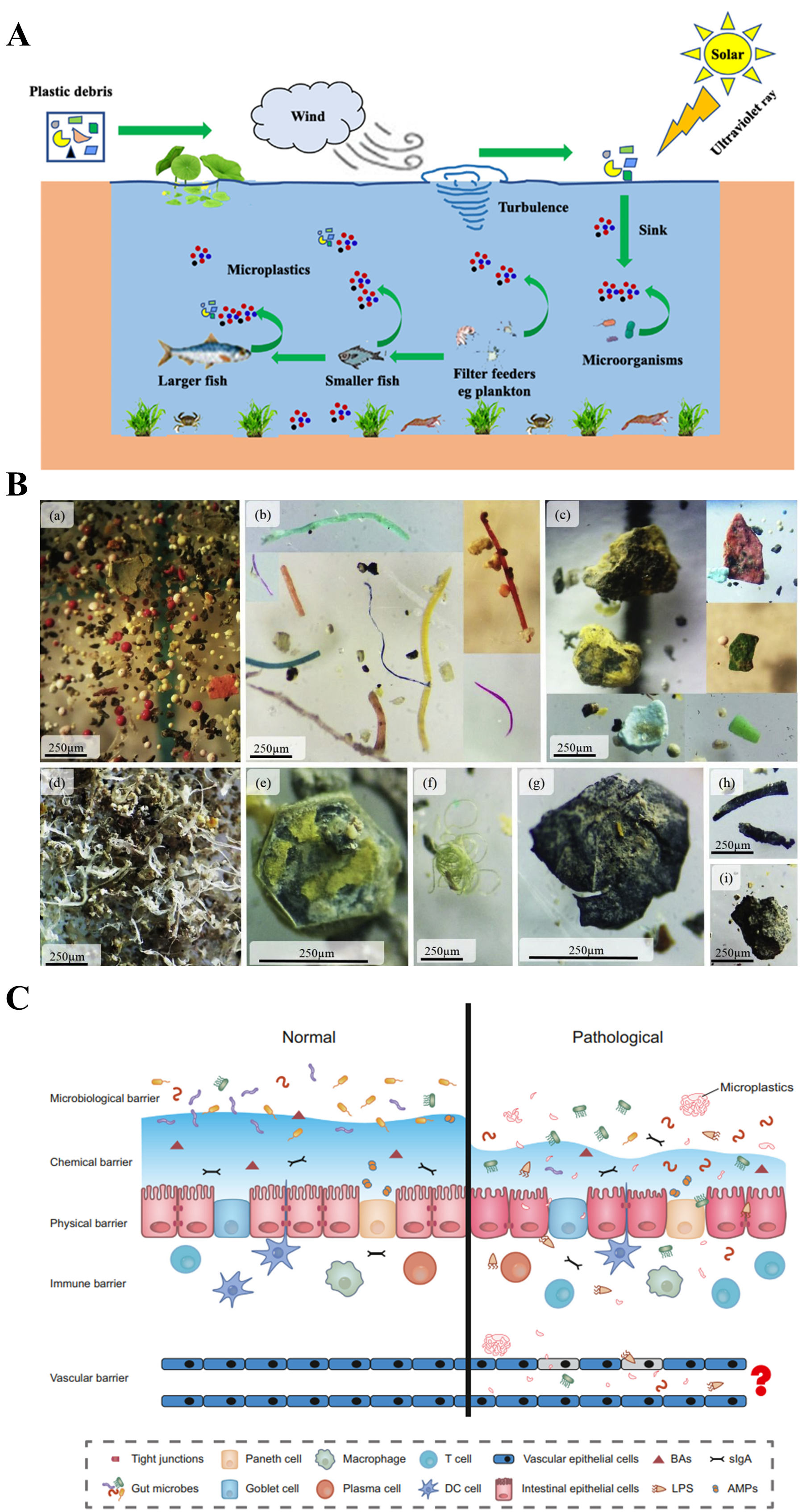
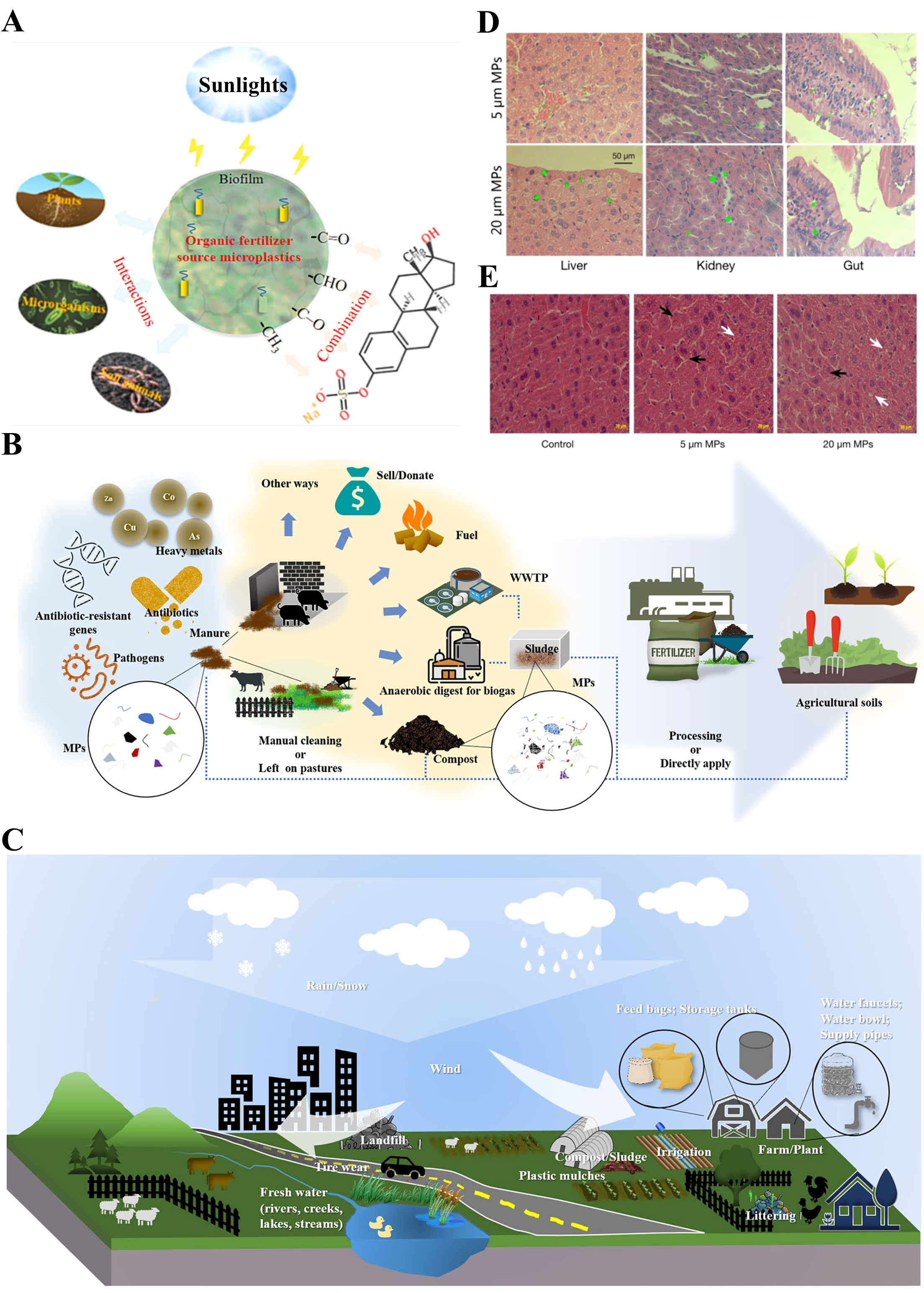
2. Methods
3. Types of Microplastics
3.1. Polyethylene Microplastics
3.2. Polypropylene Microplastics
3.3. Polystyrene Microplastics
3.4. Polyethylene Terephthalate Microplastics
3.5. Polyvinyl Chloride Microplastics
| Types | Chemical Structure of Polymer | Application | Risks | References |
|---|---|---|---|---|
| PE MPs | 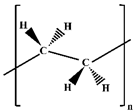 | Daily necessities (packaging bags and beverage bottles), agriculture (irrigation hoses and plastic mulch films), and construction fields (paving pads and pipes). |
| [46,47,48,52,53,57,58] |
| PP MPs |  | Fertilizer woven bags, auto parts, injector, infusion bottles, and housing for appliances (e.g., washing machines and refrigerators). |
| [60,61] |
| PS MPs |  | Packing materials, toys, disposable tableware, and bubble wrap. |
| [25,64,65,66,67,68] |
| PET MPs | 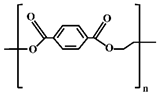 | Mineral water bottles, culture plates, plastic test tubes, insulating materials, clothing, and home textiles. |
| [71,72,74] |
| PVC MPs | 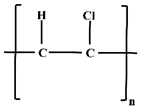 | Floors, window frames, sewer lines, and scutcheon. |
| [79,80,81,82] |
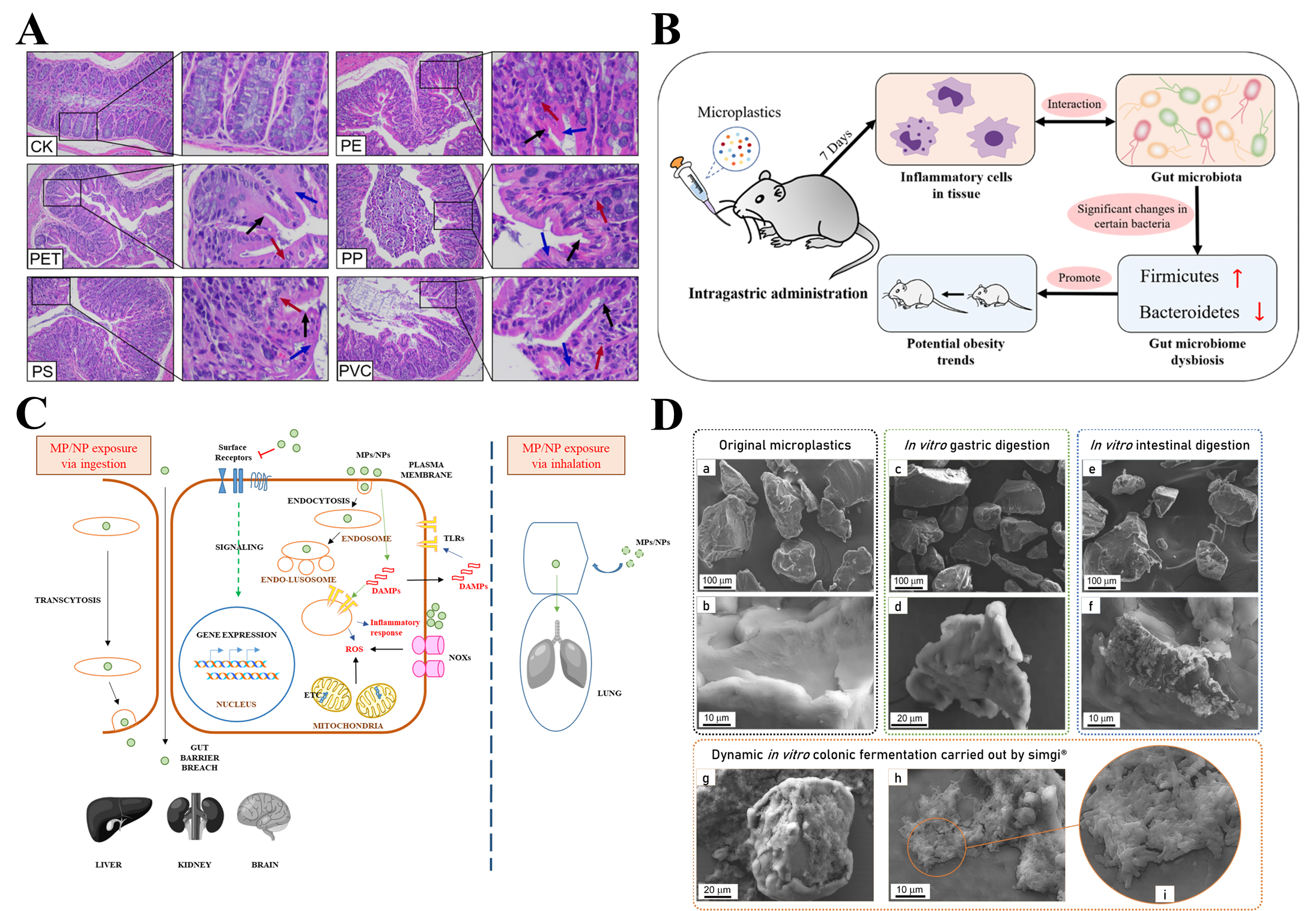
4. Toxicity Mechanism of Microplastics
4.1. Oxidative Stress
4.2. Immunotoxicity
4.3. Reproductive Toxicity
4.4. Neurotoxicity

4.5. Intestinal Injury
4.6. Inhibition of Growth and Development
| Toxic Mechanisms | Test Subjects | Type and Size | Dose and Exposure | Effects | References |
|---|---|---|---|---|---|
| Oxidative Stress | Human blood lymphocytes | PVC MPs (0.16~1.82 μm) | 24, 48, and 96 μg/mL (3 h) | ROS mass formation, lipid peroxidation, and glutathione depletion | [86] |
| Rat | PS MPs (100 nm) | 0.01 mg/kg (30 d) | The expression of Nrf-2 and antioxidant genes was decreased, and the expression of Keap-1 was increased. The activities of GSR, GSH-Px SOD, CAT, and GSH were decreased, and the levels of MDA, ROS, and inflammation were increased. | [87] | |
| Chick | PS MPs (0.5, 5, and 50 μm) | 0.5 mg/mL (35 d) | The smaller the diameter of PS MPs, the more they are deposited in skeletal muscle, and their exposure inhibited energy and lipid metabolism and induced oxidative stress and had the potential for skeletal muscle neurotoxicity. | [88] | |
| Grass carp | PS MPs (32~40 μm) | 100 and 1000 μg/L (21 d) | With the increase in MP concentration, the activity of antioxidant enzymes decreased and the mortality increased. | [89] | |
| Male mouse | MPs (0.1 and 1 μm) | 1 mg/L (24 h) | It can induce DNA damage in the nucleus and mitochondria and cause hepatotoxicity and liver fibrosis, and the content of ATP in liver tissue decreased. | [92] | |
| Mouse model | PS MPs (5~10 μm) | 100 mg/L PS MPs and 200 mg/kg DEHP (35 d) | Joint exposure can lead to ovarian damage in mice. Further research on the mechanism of ovarian granulosa cells cultured in vitro found that co-exposure had a synergistic effect, which can trigger the CNR1/CRBN/YY1/CYP2E1 signal axis, promote the excessive production of ROS, and cause oxidative stress, finally leading to oxidative DNA damage. | [94] | |
| Immunotoxicity | Mouse | PE MPs (40~48 μm) | 0.125, 0.5, and 2 mg/d (90 d) | The proportion of neutrophils in the blood increased significantly, and the persistence of PE MPs was observed in the stomach and spleen of the mice. The level of IgA in the blood of the mice was significantly increased and positively correlated with the dose of PE MPs. | [99] |
| Human and mouse cell lines | PP MPs (~20 and 25~200 μm) | 10, 50, 100, 500, and 1000 μg/mL (48 h) | Exposure of 25 μm PP MPs to 1000 μg/mL induced ROS production, which led to cytotoxicity. The secretion of TNF-α and IL-6 in human PBMCs and histamine release from mast cell lines were increased. | [100] | |
| Mouse | PS microbeads (5 μm) | 5 μg/mL (28 d) | By reducing spleen weight and the number of CD8+T cells and increasing the proportion of CD4+/CD8+T cells, the immune function of mice was significantly impaired. MPs may also induce immune and spleen damage by decreasing S100A8 levels. | [101] | |
| Mouse | PE MPs (10~150 μm) | 2, 20, and 200 μg/g (35 d) | It can increase gut microbial species, diversity of microbiota, and abundance of bacteria and decrease the percentage of Th17 and Treg cells in CD4+T cells, resulting in immunosuppression. | [25] | |
| Reproductive Toxicity | Mouse | PE MPs (0.4~5 μm) | 0.2 g/L (30 d) | Combined exposure of MPs and plastic additives had potential reproductive toxicity in male terrestrial mammals, which were reflected in spermatogenesis disorder, physiological changes in spermatozoa, and aggravation in oxidative stress. | [104] |
| Rat | PS MPs (0.2~0.5 μm) | 5 mg/kg/d (42 d) | PS MPs can lead to cystic and atretic follicles and oxidative stress in the ovaries of rats, which further confirmed that co-exposure of DEHP and PS MPs can activate the tgf-β/Smad3 signaling pathway, and the inhibition of this pathway can effectively reduce hormone imbalance, oxidative stress, and ovarian fibrosis. | [105] | |
| Oryzias melastigma | PS MPs (10 μm) | 2, 20, and 200 μg/L (60 d) | It can delay the gonad maturation of female fish and reduce fecundity. The HPG axis was negatively regulated. The transcription of genes associated with the estrogen production pathway was also down-regulated, resulting in lower plasma 17b-estradiol and testosterone concentrations. | [106] | |
| Mouse | PS MPs (5.0~5.9 μm) | 0.01, 0.1, and 1 mg/d (42 d) | It interferes with lipid metabolism, affects metabolism-related enzyme activity, adversely affects the reproductive system, causes oxidative stress, and activates JNK and p38 MAPK. | [109] | |
| Rat | PS MPs (0.5 μm) | 0.015, 0.15, and 1.5 mg/d (90 d) | It can induce oxidative stress, activate the p38 MAPK pathway, and reduce the nuclear Nrf2 pathway, thus affecting the quantity and quality of sperm and the integrity of the blood–testicular barrier. | [110] | |
| Neurotoxicity | Juvenile crucian carp | PA MPs | 4, 8, 16, 32, and 64 mg/L (14 d) | The AChE activity of the liver, gill, and intestine was significantly inhibited by PA MP exposure. | [115] |
| European perch | Fluorescence red polymer microspheres (1~5 μm) | 0.26 and 0.69 mg/L (96 h) | It can inhibit AChE, increase LPO in brain and muscle, and alter the activity of energy-related enzymes, including LDH and IDH, thus causing neurotoxicity. | [23] | |
| Adult zebrafish model | PS MPs (0.10~0.12 μm) | 10 and 100 μg/L (35 d) | MP exposure can induce the production of ROS and destroy the antioxidant defense system, leading to nerve damage, and it has been linked to schizophrenia, depression, attention disorders, and other mental disorders. | [119] | |
| Intestinal Injury | Mouse | PS MPs (5 μm) | 50 μg/d (17 d) | RIII was aggravated in mice with TAI, which was characterized by reduced villi height and cupped cells, as well as microbial community disturbance. | [121] |
| Inhibition of growth and development | Turbellarian | PS MPs (0.1, 1, and 10 μm) | 10, 50, and 100 mg/mL (21 d) | The growth and regeneration of planaria were delayed due to the decrease in posterior body and germ layer area. The process of stem cell proliferation and differentiation were also inhibited, and the proportion of mitotic stem cells was reduced. | [126] |
| Sebastes schlegelii | PS MPs (15 μm) | 1 × 106 microspheres/L (21 d) | It can weaken the feeding activity, affect the energy reserves, and reduce the nutritional quality of the organism. | [127] | |
| Mussel | PE MPs (1~50 μm) | 0.02, 0.04, 0.06, 0.08, and 1.0 mg/L (18 d) | It can disrupt the overall homeostasia in the mussel body, resulting in the production of stress and immune-related proteins and a decrease in the energy allocated for growth, ultimately leading to an increase in energy consumption and a decrease in growth rate. | [128] |
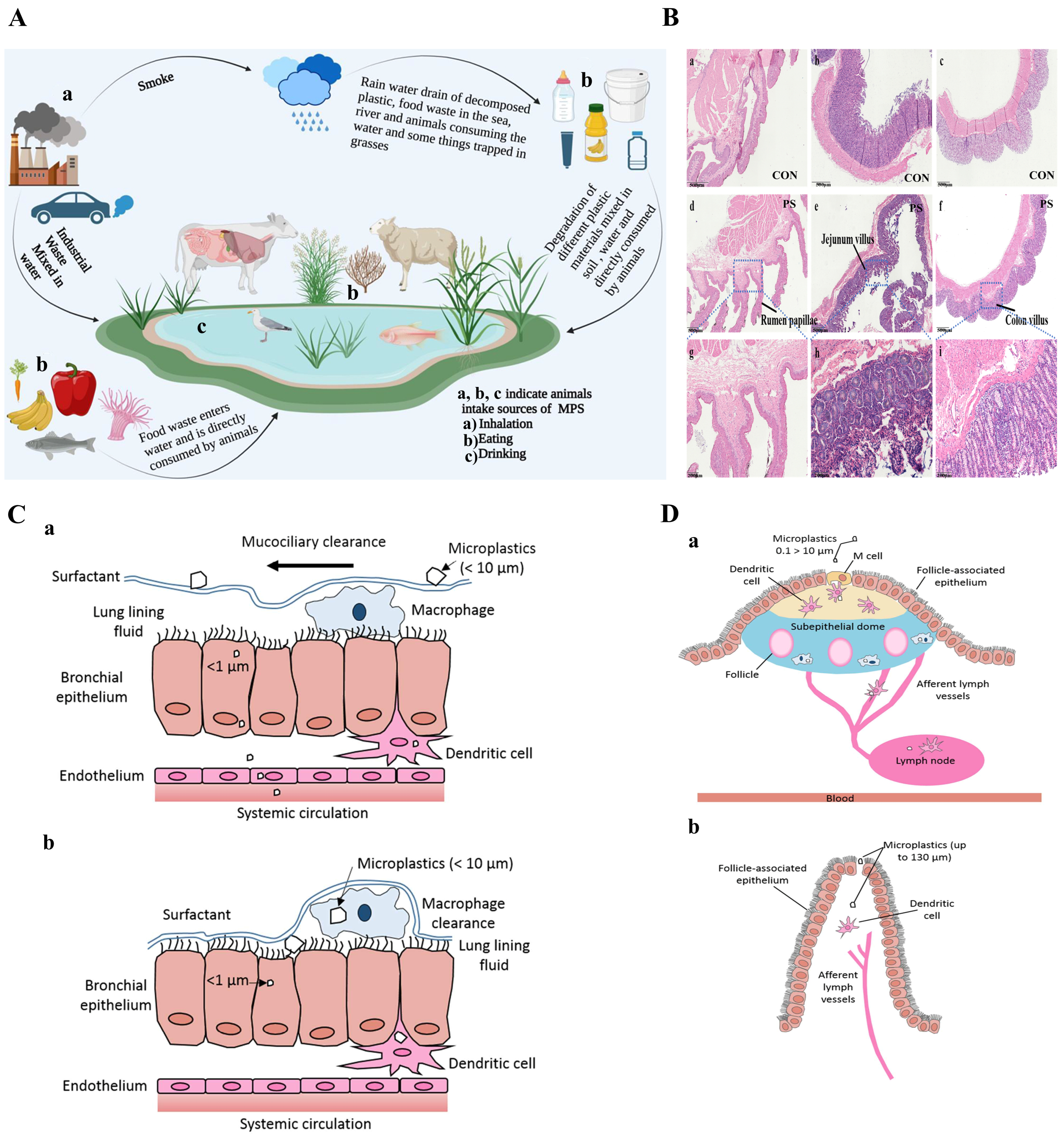
5. Primary Pathways of MPs Entering Ruminants
5.1. Ingestion
5.2. Inhalation
5.3. Dermal Contact
6. Impact of MPs on Ruminants
6.1. Production Performance
6.2. Immune Function
6.3. Rumen Microbiota
7. Conclusions and Future Viewpoints
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| NPs | Nanoplastics |
| AFM | Atomic force microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| py-GCMS | Pyrolysis-gas chromatography-mass spectrometry |
| MALDI-TOF-MS | Thermal extraction desorption-gas chromatography-mass spectrometry |
| PP MPs | Polypropylene microplastics |
| PE MPs | Polyethylene microplastics |
| PET MPs | Polyethylene terephthalate microplastics |
| PVC MPs | Polyvinyl chloride microplastics |
| PP MPs | Polypropylene microplastics |
| PY-GC/MS | Pyrolytic-gas chromatography/mass spectrometry |
| ROS/METTL3 | Reactive oxygen species/methyltransferase-like protein 3 |
| LD-IR | Laser direct infrared spectroscopy |
| DAMP | Damage-associated molecular patterns |
| Nrf-2 | Nuclear factor erythroid-2-related factor 2 |
| Keap-1 | Kelch-like ECH-associated protein 1 |
| IgA | Immunoglobulin A |
| PAEs | Phthalates |
| BPA | Bisphenol A |
| JNK | C-Jun N-terminal kinase |
| p38 MAPK | p38 mitogen-activated protein kinase |
| PB | Plastic bowls |
| miR-21 | MicroRNA-21 |
| IRAK4 | Interleukin-1 receptor-associated kinase 4 |
| NF-κB | Nuclear factor κB |
| TLR4/NF-κB | Toll-like receptor 4/nuclear factor-κB |
| HPG | Hypothalamic–pituitary–gonad |
| ROS | Reactive oxygen species |
| GSR | Glutathione reductase |
| GSH-Px | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSH | Glutathione |
| GST | Glutathione S-Transferase |
| MDA | Malondialdehyde |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| PBMCs | Peripheral blood mononuclear cells |
| DEHP | Bis(2-ethylhexyl) phthalate |
| AChE | Acetylcholinesterase |
| LPO | Lipid oxidation |
| LDH | Lactate dehydrogenase |
| IDH | Isocitrate dehydrogenase |
| TAI | Total abdominal irradiation |
| RIII | Radiation intestinal injury |
| AP-1 | Activator protein 1 |
| IRF5 | Interferon regulatory factor 5 |
| GMECs | Goat mammary epithelial cells |
| PBAT | Polybutylene adipate/terylene phthalate |
| PEF | Polyethylene 2,5-furandicarboxylate |
References
- Lim, X. Microplastics are everywhere—But are they harmful. Nature 2021, 593, 22–25. [Google Scholar] [CrossRef]
- Hao, D.; Jun, W. Characterization and environmental impacts of microplastics. Gondwana Res. 2021, 98, 63–75. [Google Scholar]
- Brooks, A.L.; Wang, S.; Jambeck, J.R. The Chinese import ban and its impact on global plastic waste trade. Sci. Adv. 2018, 4, eaat0131. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, T.; Mayer, M.; McNally, C.; Simons, T.J.; Witte, C. How plastics waste recycling could transform the chemical industry. McKQ 2018, 12, 1. [Google Scholar]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the aquatic environment. Critical review. Mar. Anthropog. Litter 2015, 325–340. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellov, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Nene, A.; Sadeghzade, S.; Viaroli, S.; Yang, W.; Uchenna, U.P.; Kandwal, A.; Liu, X.; Somani, P.; Galluzzi, M. Recent advances and future technologies in nano-microplastics detection. Environ. Sci. Eur. 2025, 37, 7. [Google Scholar] [CrossRef]
- Jahedi, F.; Fard, N.J.H.; Turner, A. A systematic review of biomonitoring microplastics in environmental matrices: Emphasis on airborne particles, dry deposits, and comparative analysis with traditional methods. Environ. Adv. 2025, 19, 100609. [Google Scholar] [CrossRef]
- Marcuello, C. Present and future opportunities in the use of atomic force microscopy to address the physico-chemical properties of aquatic ecosystems at the nanoscale level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar]
- Driedger, A.G.J.; Dürr, H.H.; Mitchell, K.; Cappellen, P.V. Plastic debris in the Laurentian Great Lakes: A review. J. Great Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity assessment of microplastic (MPs); a threat to the ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef]
- Xu, J.L.; Lin, X.; Wang, J.J.; Gowen, A.A. A review of potential human health impacts of micro-and nanoplastics exposure. Sci. Total Environ. 2022, 851, 158111. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Mendes, L.A.; Beiras, R.; Domínguez, J. Earthworm (Eisenia andrei)-mediated degradation of commercial compostable bags and potential toxic effects. Microplastics 2024, 3, 322–338. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.S.; Wu, Y.S.; Nagandran, S.; Batumalaie, K. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef] [PubMed]
- Khaled, Z.; CorinaBianca, I.; Magdalena, M.; Marius, N.S.; Carolina, N.; Elena, M.; Doina, D.; OliviaTeodora, P. Microplastics: A real global threat for environment and food safety: A state of the art review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Jin, Y. Microplastic: A potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci. Total Environ. 2021, 785, 147365. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef] [PubMed]
- Borreani, G.; Tabacco, E. Plastics in animal production. In A guide to the Manufacture, Performance, and Potential of Plastics in Agriculture; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–185. [Google Scholar]
- Urli, S.; Corte Pause, F.; Crociati, M.; Baufeld, A.; Monaci, M.; Stradaioli, G. Impact of microplastics and nanoplastics on livestock health: An emerging risk for reproductive efficiency. Animals 2023, 13, 1132. [Google Scholar] [CrossRef]
- Galyon, H.; Vibostok, S.; Duncan, J.; Ferreira, G.; Whittington, A.; Cockrum, R. Long-term in situ ruminal degradation of biodegradable polymers in Holstein dairy cattle. JDS Commun. 2023, 4, 70–74. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf. S. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Kantono, K.; Hamid, N.; Ma, Q.; Chadha, D.; Oey, I. Consumers’ perception and purchase behaviour of meat in China. Meat Sci. 2021, 179, 108548. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Maryam, M.; Saeid, H.; Majid, M.; Enayat, B.; Morteza, Z.; Zahra, D.; Margherita, F.; Oliveri, C.G. Antibiotic residues in poultry tissues in Iran: A systematic review and meta-analysis. Environ. Res. 2021, 204, 112038. [Google Scholar]
- Atta, A.H.; Atta, S.A.; Nasr, S.M.; Mouneir, S.M. Current perspective on veterinary drug and chemical residues in food of animal origin. Environ. Sci. Pollut. R. 2022, 29, 15282–15302. [Google Scholar] [CrossRef] [PubMed]
- Sofos, J.N. Challenges to meat safety in the 21st century. Meat Sci. 2007, 78, 3–13. [Google Scholar] [CrossRef]
- Grechi, N.; Franko, R.; Rajaraman, R.; Stöckl, J.B.; Trapphoff, T.; Dieterle, S.; Fröhlich, T.; Noonan, M.J.; de AMM Ferraz, M. Microplastics are present in women’s and cows’ follicular fluid and polystyrene microplastics compromise bovine oocyte function in vitro. bioRxiv 2022. [Google Scholar] [CrossRef]
- Da Costa Filho, P.A.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; Panchaud, A. Detection and characterization of small-sized microplastics (≥ 5 µm) in milk products. Sci. Rep. 2021, 11, 24046. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Lwanga, E.H. Low density-microplastics detected in sheep faeces and soil: A case study from the intensive vegetable farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef]
- van-der Veen, I.; Van Mourik, L.; Van-Velzen, M.; Groenewoud, Q.; Leslie, H. Plastic Particles in Livestock Feed, Milk, Meat and Blood; Department of Environment & Health: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Patrucco, S.G.; Rivoira, L.; Bruzzoniti, M.C.; Barbera, S.; Tassone, S. Development and application of a novel extraction protocol for the monitoring of microplastic contamination in widely consumed ruminant feeds. Sci. total Environ. 2024, 947, 174493. [Google Scholar] [CrossRef]
- Maganti, S.; Akkina, R. Detection and characterisation of microplastics in animal feed. Online J. Anim. Feed Res. 2023, 13, 348–356. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Jiang, L.; Chen, X.; Zhao, Y.; Shi, W.; Xing, Z. From organic fertilizer to the soils: What happens to the microplastics? A critical review. Sci. Total Environ. 2024, 919, 170217. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, Y.; Zhou, Z.; Liu, R.; Qiu, Z.; Qiu, Y.; Li, J.; Wang, W.; Li, X.; Yin, L.; et al. Are microplastics in livestock and poultry manure an emerging threat to agricultural soil safety? Environ. Sci. Pollut. Res. Int. 2024, 31, 11543–11558. [Google Scholar] [CrossRef]
- Shelver, W.L.; McGarvey, A.M.; Billey, L.O. Disposition of [14C]-polystyrene microplastics after oral administration to lactating sheep. Food Addit. Contam. A 2024, 41, 1132–1143. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Ahmad, H.; Rodrigue, D. Crosslinked polyethylene: A review on the crosslinking techniques, manufacturing methods, applications, and recycling. Polym. Eng. Sci. 2022, 62, 2376–2401. [Google Scholar] [CrossRef]
- Clere, I.K.; Ahmmed, F.; Peter III, J.; Fraser-Miller, S.J.; Gordon, K.C.; Komyakova, V.; Allan, B.J. Quantification and characterization of microplastics in commercial fish from southern New Zealand. Mar. Pollut. Bull. 2022, 184, 114121. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Nick, W. Editorial: Microbial Degradation of Plastics. Front. Microbiol. 2021, 12, 635621. [Google Scholar]
- Mat, A.M.A.; Mariatti, M. Formulation of biodegradable plastic mulch film for agriculture crop protection: A review. Polym. Rev. 2022, 62, 890–918. [Google Scholar]
- Cao, J.; Chen, P.; Gao, X.; Zou, Q.; Fang, Y.; Gu, X.; Zhao, X.; Li, Y. Effects of plastic film residue and emitter flow rate on soil water infiltration and redistribution under different initial moisture content and dry bulk density. Sci. Total Environ. 2022, 807, 151381. [Google Scholar]
- Liu, Y.; Hu, W.; Huang, Q.; Qin, J.; Zheng, Y.; Wang, J.; Li, X.; Wang, Q.; Guo, G.; Hu, S. Plastic mulch debris in rhizosphere: Interactions with soil-microbe-plant systems. Sci. Total Environ. 2022, 807, 151435. [Google Scholar] [CrossRef]
- Liu, T.; Yang, J.; Wu, J.; Sun, X.; Gao, X. Polyethylene microplastics induced inflammation via the miR-21/IRAK4/NF-κB axis resulting to endoplasmic reticulum stress and apoptosis in muscle of carp. Fish Shellfish Immun. 2024, 145, 109375. [Google Scholar] [CrossRef]
- Madjid, D.; Christophe, W.; Laurent, D.; David, L.; Cécile, V.; Mathilde, B.M. Oral exposure to polyethylene microplastics induces inflammatory and metabolic changes and promotes fibrosis in mouse liver. Ecotox. Environ. Saf. 2023, 264, 115417. [Google Scholar]
- Hailey, G.; Samuel, V.; Jane, D.; Gonzalo, F.; Abby, W.; Kirk, H.; Jason, M.; Rebecca, C. Clearance of biodegradable polymer and polyethylene films from the rumens of Holstein Bull Calves. Animals 2023, 13, 928. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. Bio-based biodegradable film to replace the standard polyethylene cover for silage conservation. J. Dairy. Sci. 2015, 98, 386–394. [Google Scholar] [CrossRef]
- Pizol, J.; Dennis, R.; Thorson, J.; Prezotto, L. Distribution and Clearance of Chopped Net Wrap in the Digestive Tract of Beef Cattle; Montana State University: Bozeman, MT, USA, 2017. [Google Scholar]
- Otsyina, H.; Mbuthia, P.; Nguhiu-Mwangi, J.; Mogoa, E.; Ogara, W. Gross and histopathologic findings in sheep with plastic bags in the rumen. Int. J. Vet. Sci. Med. 2017, 5, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Martín Martel, S.; Morales, M.; Morales, I.; Jaber, J.R.; Rodríguez-Guisado, F.; Tejedor-Junco, M.T.; Corbera, J.A. Pathological changes of the rumen in small ruminants associated with indigestible foreign objects. Ruminants 2021, 1, 118–126. [Google Scholar] [CrossRef]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Exposure to polypropylene microplastics via oral ingestion induces colonic apoptosis and intestinal barrier damage through oxidative stress and inflammation in mice. Toxics 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gong, C.; Zheng, X.; Hu, F.; Liu, J.; Wang, T.; Chen, X.; Li, M.; Zhu, Z.; Zhang, L.; et al. Early clues and molecular mechanism involved in neurodegenerative diseases induced in immature mice by combined exposure to polypropylene microplastics and DEHP. Environ. Pollut. 2023, 336, 122406. [Google Scholar] [CrossRef]
- Souza, d.N.L.; Luporini, d.O.S.; Carlino, d.C.C.; Fernanda, A.M.; Franchin, R.L.; Ives, C.; Gabriel, C.; Menegasso, M.C.F.; Kozusny, A.D.I.; Antonio, d.A.B.M. Deleterious effects of polypropylene microplastic ingestion in Nile Tilapia (Oreochromis niloticus). Bull. Environ. Contam. Toxicol. 2023, 111, 13. [Google Scholar]
- Visentin, E.; Niero, G.; Benetti, F.; Perini, A.; Zanella, M.; Pozza, M.; Marchi, M.D. Preliminary characterization of microplastics in beef hamburgers. Meat Sci. 2024, 217, 109626. [Google Scholar] [CrossRef]
- Ullah, R.; Tsui, M.T.K.; Chow, A.; Chen, H.; Williams, C.; Ligaba-Osena, A. Micro (nano) plastic pollution in terrestrial ecosystem: Emphasis on impacts of polystyrene on soil biota, plants, animals, and humans. Environ. Monit. Assess. 2023, 195, 252. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liang, B.; Huang, Y.; Li, Z.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Deng, Y.; Xiu, J. Long-chain acyl carnitines aggravate polystyrene nanoplastics-induced atherosclerosis by upregulating MARCO. Adv. Sci. 2023, 10, 2205876. [Google Scholar] [CrossRef]
- Zou, D.; Yang, Y.; Ji, F.; Lv, R.; Wu, H.; Hou, G.; Xu, T.; Zhou, H.; Hu, C. Polystyrene Microplastics Causes Diarrhea and Impairs Intestinal Angiogenesis through the ROS/METTL3 Pathway. J. Agr. Food Chem. 2024, 72, 16638–16650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef]
- To, M.H.; Uisan, K.; Ok, Y.S.; Pleissner, D.; Lin, C.S.K. Recent trends in green and sustainable chemistry: Rethinking textile waste in a circular economy. Curr. Opin. Green Sust. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Xie, L.; Chen, T.; Liu, J.; Hou, Y.; Tan, Q.; Zhang, X.; Li, Z.; Farooq, T.H.; Yan, W.; Li, Y. Intestinal flora variation reflects the short-term damage of microplastic to the intestinal tract in mice. Ecotox. Environ. Saf. 2022, 246, 114194. [Google Scholar] [CrossRef]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveetill, S.; Tang, B.L. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Tassone, S.; Barbera, S.; Kaihara, H.; Glorio Patrucco, S.; Abid, K. First evidence of the effects of polyethylene terephthalate microplastics on ruminal degradability and gastro-intestinal digestibility of mixed hay. Animals 2024, 14, 2139. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, H.; Yang, D.; Zhang, G.; Zhang, J.; Ju, F. Polyvinyl chloride degradation by a bacterium isolated from the gut of insect larvae. Nat. Commun. 2022, 13, 5360. [Google Scholar] [CrossRef]
- Xu, Y.; Xian, Z.N.; Yue, W.; Yin, C.F.; Zhou, N.Y. Degradation of polyvinyl chloride by a bacterial consortium enriched from the gut of Tenebrio molitor larvae. Chemosphere 2023, 318, 137944. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.; Silva, L.P.; Krüger, R.H. Brazilian Cerrado soil reveals an untapped microbial potential for unpretreated polyethylene biodegradation. J. Hazard. Mater. 2017, 324, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.F.; Xu, Y.; Zhou, N.Y. Biodegradation of polyethylene mulching films by a co-culture of Acinetobacter sp. strain NyZ450 and Bacillus sp. strain NyZ451 isolated from Tenebrio molitor larvae. Int. Biodeter. Biodegr. 2020, 155, 105089. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef]
- Zhuang, J.; Chen, Q.; Xu, L.; Chen, X. Combined exposure to polyvinyl chloride and polystyrene microplastics induces liver injury and perturbs gut microbial and serum metabolic homeostasis in mice. Ecotox. Environ. Saf. 2023, 267, 115637. [Google Scholar] [CrossRef]
- Liu, X.; Liang, C.; Fan, J.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce changes in gene expression and organ histology along the HPG axis in Cyprinus carpio var. larvae. Aquat. Toxicol. 2023, 258, 106483. [Google Scholar] [CrossRef]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef]
- Liao, Y.L.; Yang, J.Y. The release process of Cd on microplastics in a ruminant digestion in-vitro method. Process Saf. Environ. Prot. 2022, 157, 266–272. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ali, A.; Maral, R.; Jalal, P. Differences in sensitivity of human lymphocytes and fish lymphocytes to polyvinyl chloride microplastic toxicity. Toxicol. Ind. Health. 2022, 38, 100–111. [Google Scholar]
- Yaqoob, S.; Hamza, A.; Batool, M.; Khatoon, A.; Hussain, S.A.; Riaz, M.N. Rhamnetin abrogates polystyrene microplastics prompted hepatic damage by regulating Nrf-2/Keap-1 pathway. J. King Saud Univ. Sci. 2024, 36, 103403. [Google Scholar] [CrossRef]
- Chen, J.; Chen, G.; Peng, H.; Qi, L.; Zhang, D.; Nie, Q.; Zhang, X.; Luo, W. Microplastic exposure induces muscle growth but reduces meat quality and muscle physiological function in chickens. Sci. Total Environ. 2023, 882, 163305. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Y.; He, Y.; Yu, H.; Liu, Y.; Shen, Y.; Xu, X.; Li, J. Exposure to microplastics induces lower survival, oxidative stress, disordered microbiota and altered metabolism in the intestines of grass carp (Ctenopharyngodon idella). Aquacult. Fish. 2024, 9, 785–794. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Kadirvelu, K.; Ramesh, M. Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in zebrafish. Chem. Biol. Interact. 2021, 345, 109550. [Google Scholar] [CrossRef]
- Paget, V.; Dekali, S.; Kortulewski, T.; Grall, R.; Gamez, C.; Blazy, K.; Aguerre-Chariol, O.; Chevillard, S.; Braun, A.; Rat, P. Specific uptake and genotoxicity induced by polystyrene nanobeads with distinct surface chemistry on human lung epithelial cells and macrophages. PLoS ONE 2015, 10, e0123297. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ. Pollut. 2022, 300, 118986. [Google Scholar] [CrossRef]
- Alnajar, N.; Jha, A.N.; Turner, A. Impacts of microplastic fibres on the marine mussel, Mytilus galloprovinciallis. Chemosphere 2021, 262, 128290. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Yang, N.; Xu, S. Polystyrene-microplastics and DEHP co-exposure induced DNA damage, cell cycle arrest and necroptosis of ovarian granulosa cells in mice by promoting ROS production. Sci. Total Environ. 2023, 871, 161962. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Hollman, P.C.; Peters, R.J. Potential health impact of environmentally released micro-and nanoplastics in the human food production chain: Experiences from nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef]
- Yoo, J.W.; Doshi, N.; Mitragotri, S. Adaptive micro and nanoparticles: Temporal control over carrier properties to facilitate drug delivery. Adv. Drug Deliver. Rev. 2011, 63, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Li, Z.; Su, Z.; Wang, J. Immunotoxicity of microplastics: Carrying pathogens and destroying the immune system. TrAC Trends Anal. Chem. 2024, 177, 117817. [Google Scholar] [CrossRef]
- Park, E.J.; Han, J.S.; Park, E.J.; Seong, E.; Lee, G.H.; Kim, D.W.; Son, H.Y.; Han, H.Y.; Lee, B.S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhang, C.; Zhou, X. Microplastics induce immune suppression via S100A8 downregulation. Ecotox. Environ. Saf. 2022, 242, 113905. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid. Biochem. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Darnerud, P. Brominated flame retardants as possible endocrine disrupters. Int. J. Androl. 2008, 31, 152–160. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Huang, Y.; Ren, H.; Zhang, Y. Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus). J. Hazard. Mater. 2021, 406, 124644. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Gao, X.; Wei, Z.; Han, Q.; Wang, S.; Du, W.; Wan, J.; Wan, C.; Chen, M. Polystyrene microplastics and di-2-ethylhexyl phthalate co-exposure: Implications for female reproductive health. Environ. Sci. Ecotech. 2024, 22, 100471. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Liu, Y.; Zhao, H.; Peng, R. Toxic effects of microplastic and nanoplastic on the reproduction of teleost fish in aquatic environments. Environ. Sci. Pollut. Res. 2024, 31, 62530–62548. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Buriro, R.S.; Gandahi, J.A.; Chen, Y.; Aabdin, Z.U.; Bhutto, S.; Sun, J.; Zhu, J.; Liu, Z.; Zou, H. A critical review on male-female reproductive and developmental toxicity induced by micro-plastics and nano-plastics through different signaling pathways. Chem-Biol Interact. 2024, 394, 110976. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotox. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Li, S.; Wang, Q.; Yu, H.; Yang, L.; Sun, Y.; Xu, N.; Wang, N.; Lei, Z.; Hou, J.; Jin, Y. Polystyrene microplastics induce blood–testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ. Sci. Pollut. R. 2021, 28, 47921–47931. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, A.; Gupta, V.K.; Mishra, Y.K. Nano/Micro-Plastic, an invisible threat getting into the Brain. Chemosphere 2024, 361, 142380. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Yin, J.; Zhu, Q.; Liao, C.; Jiang, G. Neurotoxicities induced by micro/nanoplastics: A review focusing on the risks of neurological diseases. J. Hazard. Mater. 2024, 469, 134054. [Google Scholar] [CrossRef]
- Hasan, A.K.M.M.; Hamed, M.; Hasan, J.; Martyniuk, C.J.; Niyogi, S.; Chivers, D.P. A review of the neurobehavioural, physiological, and reproductive toxicity of microplastics in fishes. Ecotox. Environ. Saf. 2024, 282, 116712. [Google Scholar] [CrossRef]
- Li, L.; Ma, R.; Yuan, Y.; Yao, Q.; Han, Y.; Cao, H.; Qi, J. Neurotoxicity induced by aged microplastics from plastic bowls: Abnormal neurotransmission in Caenorhabditis elegans. Sci. Total Environ. 2024, 952, 175939. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, J.H.; Jo, A.H.; Choi, Y.J.; Choi, C.Y.; Kang, J.C.; Kim, J.H. Microplastic polyamide toxicity: Neurotoxicity, stress indicators and immune responses in crucian carp, Carassius carassius. Ecotox. Environ. Saf. 2023, 265, 115469. [Google Scholar] [CrossRef]
- Fang, C.; Gu, L.; Smerin, D.; Mao, S.; Xiong, X. The Interrelation between Reactive Oxygen Species and Autophagy in Neurological Disorders. Oxid. Med. Cell. Longev. 2017, 2017, 8495160. [Google Scholar] [CrossRef]
- Pawluk, H.; Kaczmarek, A.T.; Sopońska, M.; Porzych, M.; Modrzejewska, M.; Pawluk, M.; Kurhaluk, N.; Tkaczenko, H.; Kołodziejska, R. The influence of oxidative stress markers in patients with ischemic stroke. Biomolecules 2024, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, S.; Priyadarshinee, S.; Bhattacharjee, M.; Kadirvelu, K.; Ramesh, M. Exposure to polystyrene microplastics induced gene modulated biological responses in zebrafish (Danio rerio). Chemosphere 2021, 281, 128592. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, N.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.; Liu, H.P.; Xu, Z. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat. Toxicol. 2018, 195, 67–76. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, Q.; Luo, Y.; Song, M.; He, X.; Sheng, H.; Gao, X.; Zhu, Z.; Sun, J.; Cao, C. Polystyrene microplastics aggravate radiation-induced intestinal injury in mice. Ecotox. Environ. Saf. 2024, 283, 116834. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Domenech, J.; Hernández, A.; Demir, E.; Marcos, R.; Cortés, C. Interactions of graphene oxide and graphene nanoplatelets with the in vitro Caco-2/HT29 model of intestinal barrier. Sci. Rep. 2020, 10, 2793. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wegner, A.; Foekema, E.M.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Sun, B.; Xu, Z.; Chen, Q.; Yang, M.; Wan, Q.; Song, L.; Chen, G.; Jing, C.; Zeng, E.Y. Exposure to polystyrene microplastics reduces regeneration and growth in planarians. J. Hazard. Mater. 2022, 432, 128673. [Google Scholar] [CrossRef]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef]
- Détrée, C.; Gallardo-Escárate, C. Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immun. 2018, 83, 52–60. [Google Scholar] [CrossRef]
- Khan, A.; Qadeer, A.; Wajid, A.; Ullah, Q.; Rahman, S.U.; Ullah, K.; Safi, S.Z.; Ticha, L.; Skalickova, S.; Chilala, P. Microplastics in Animal Nutrition: Occurrence; spread; and hazard in animals. J. Agr. Food. Res. 2024, 17, 101258. [Google Scholar] [CrossRef]
- Chang, X.; Li, Y.; Han, Y.; Fang, Y.; Xiang, H.; Zhao, Z.; Zhao, B.; Zhong, R. Polystyrene exposure induces lamb gastrointestinal injury, digestive disorders and inflammation, decreasing daily gain, and meat quality. Ecotox. Environ. Saf. 2024, 277, 116389. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Yang, J.; Li, R.; Zhou, Q.; Li, L.; Li, Y.; Tu, C.; Zhao, X.; Xiong, K.; Christie, P.; Luo, Y. Abundance and morphology of microplastics in an agricultural soil following long-term repeated application of pig manure. Environ. Pollut. 2020, 272, 116028. [Google Scholar] [CrossRef]
- Ashbell, G.; Kipnis, T.; Titterton, M.; Hen, Y.; Azrieli, A.; Weinberg, Z.G. Examination of a technology for silage making in plastic bags. Anim. Feed. Sci. Technol. 2001, 91, 213–222. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Ameer, K.; Jiang, G.; Hong, G.P. Micro- and nanoplastic contamination in livestock production: Entry pathways; potential effects and analytical challenges. Sci. Total Environ. 2022, 844, 157234. [Google Scholar] [CrossRef]
- Wang, R.; Huang, Y.; Dong, S.; Wang, P.; Su, X. The occurrence of bisphenol compounds in animal feed plastic packaging and migration into feed. Chemosphere 2021, 265, 129022. [Google Scholar] [CrossRef]
- Giacomo, R.; Francesco, B.; Eleonora, C.; Margherita, F.; Stefania, A.; Lucia, G. Bisphenol A and Bisphenol S release in milk under household conditions from baby bottles marketed in Italy. J. Environ. Sci. Health 2018, 53, 116–120. [Google Scholar]
- Wang, F.; Wang, B.; Duan, L.; Zhang, Y.; Zhou, Y.; Sui, Q.; Xu, D.; Qu, H.; Yu, G. Occurrence and distribution of microplastics in domestic; industrial; agricultural and aquacultural wastewater sources: A case study in Changzhou; China. Water Res. 2020, 182, 115956. [Google Scholar] [CrossRef]
- Dong, X.; Liu, X.; Hou, Q.; Wang, Z. From natural environment to animal tissues: A review of microplastics (nanoplastics) translocation and hazards studies. Sci. Total Environ. 2023, 855, 158686. [Google Scholar] [CrossRef]
- Li, H.; Chang, X.; Zhang, J.; Wang, Y.; Zhong, R.; Wang, L.; Wei, J.; Wang, Y. Uptake and distribution of microplastics of different particle sizes in maize (Zea mays) seedling roots. Chemosphere 2023, 313, 137491. [Google Scholar] [CrossRef]
- Jin, T.; Tang, J.; Lyu, H.; Wang, L.; Gillmore, A.B.; Schaeffer, S.M. Activities of Microplastics (MPs) in Agricultural Soil: A Review of MPs Pollution from the Perspective of Agricultural Ecosystems. J. Agr. Food Chem. 2022, 70, 4182–4201. [Google Scholar] [CrossRef]
- Meyer, G.; Puig-Lozano, R.; Fernández, A. Anthropogenic litter in terrestrial flora and fauna: Is the situation as bad as in the ocean? A field study in Southern Germany on five meadows and 150 ruminants in comparison with marine debris. Environ. Pollut. 2023, 323, 121304. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Werbowski, L.M.; Gilbreath, A.; Munno, K.; Zhu, X.; Grbi, J.; Wu, T.; Sutton, R.; Sedlak, M.D.; Deshpande, A.; Rochman, C. Urban stormwater runoff: A major pathway for anthropogenic particles; black rubbery fragments; and other types of microplastics to urban receiving waters. Acs Est Water 2021, 1, 1420–1428. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Lee, S.; Kang, K.K.; Sung, S.E.; Choi, J.H.; Sung, M.; Seong, K.Y.; Lee, S.; Yang, S.Y.; Seo, M.S.; Kim, K. Toxicity study and quantitative evaluation of polyethylene microplastics in ICR mice. Polymers 2022, 14, 402. [Google Scholar] [CrossRef]
- Smit, L.A.; Heederik, D. Impacts of intensive livestock production on human health in densely populated regions. GeoHealth 2017, 1, 272–277. [Google Scholar] [CrossRef]
- Anna, C.; Beata, T.; Leszek, T.; Hanna, B.; Łukasz, M. Microbial contamination of the air in livestock buildings as a threat to human and animal health–a review. Ann. Anim. Sci. 2021, 21, 417–431. [Google Scholar]
- Akhbarizadeh, R.; Dobaradaran, S.; Torkmahalleh, M.A.; Saeedi, R.; Aibaghi, R.; Ghasemi, F.F. Suspended fine particulate matter (PM2. 5); microplastics (MPs); and polycyclic aromatic hydrocarbons (PAHs) in air: Their possible relationships and health implications. Environ. Res. 2021, 192, 110339. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Schmidt, T.C.; Nabipour, I.; Spitz, J. Worldwide bottled water occurrence of emerging contaminants: A review of the recent scientific literature. J. Hazard. Mater. 2020, 392, 122271. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: A threat to human health? Food. Addit. Contam. A 2019, 36, 1696–1708. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County; Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Bahar, M.M.; Megharaj, M.; Fang, C.; Rahman, M.M. The unseen threat of the synergistic effects of microplastics and heavy metals in aquatic environments: A critical review. Curr. Pollut. Rep. 2024, 10, 478–497. [Google Scholar] [CrossRef]
- Liang, J.; Ji, F.; Abdullah, A.L.B.; Qin, W.; Zhu, T.; Tay, Y.J.; Li, Y.; Han, M. Micro/nano-plastics impacts in cardiovascular systems across species. Sci. Total Environ. 2024, 942, 173770. [Google Scholar] [CrossRef]
- Lackner, M.; Branka, M. Microplastics in farmed animals—A review. Microplastics 2024, 3, 559–588. [Google Scholar] [CrossRef]
- Corte Pause, F.; Urli, S.; Crociati, M.; Stradaioli, G.; Baufeld, A. Connecting the dots: Livestock animals as missing links in the chain of microplastic contamination and human health. Animals 2024, 14, 350. [Google Scholar] [CrossRef]
- Glorio Patrucco, S.; Rivoira, L.; Bruzzoniti, M.C.; Fortina, R.; Tassone, S. Microplastics contamination in dairy cows’ total mixed ration. Ital. J. Anim. Sci. 2023, 22, 242–243. [Google Scholar]
- Ramaswamy, V.; Sharma, H.R. Plastic bags–Threat to environment and cattle health: A retrospective study from Gondar City of Ethiopia. IIOAB J. 2011, 2, 6–11. [Google Scholar]
- Anteneh, B.; Cheru, H.; Shumet, A.; Tadesse, A. Study on rumen and reticulum foreign body in slaughtered cattle at Gondar Elfora abattoir. Researcher 2017, 9. [Google Scholar] [CrossRef]
- Priyanka, M.; Dey, S. Ruminal impaction due to plastic materials-An increasing threat to ruminants and its impact on human health in developing countries. Vet. World 2018, 11, 1307–1315. [Google Scholar] [CrossRef]
- Hailat, N.; Al-Darraji, A.; Lafi, S.; Barakat, S.; Al-Ani, F.; El-Magrhaby, H.; Al-Qudah, K.; Gharaibeh, S.; Rousan, M.; Al-Smadi, M. Pathology of the rumen in goats caused by plastic foreign bodies with reference to its prevalence in Jordan. Small Ruminant Res. 1998, 30, 77–83. [Google Scholar] [CrossRef]
- Bwatota, S.; Makungu, M.; Nonga, H. Occurrences of indigestible foreign bodies in cattle slaughtered at Morogoro Municipal Slaughterhouse, Tanzania. J. Vet. Med. 2018, 2018, 4818203. [Google Scholar] [CrossRef] [PubMed]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The influence of gut microbiota on oxidative stress and the immune system. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Cheng, Z.; Xu, G.; Li, F.; Bu, Q.; Zhang, L.; Song, Y.; An, X. Endoplasmic reticulum stress exacerbates microplastics-induced toxicity in animal cells. Food Res. Int. 2024, 175, 113818. [Google Scholar] [CrossRef]
- Steele, M.A.; Penner, G.B.; Chaucheyras-Durand, F.; Guan, L.L. Development and physiology of the rumen and the lower gut: Targets for improving gut health 1. J. Dairy Sci. 2016, 99, 4955–4966. [Google Scholar] [CrossRef]
- Newbold, C.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal. 2020, 14, s78–s86. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Xiang, K.; Zhao, C.; He, Z.; Qiu, M.; Hu, X.; Zhang, N. The role of rumen microbiota and its metabolites in subacute ruminal acidosis (SARA)-induced inflammatory diseases of ruminants. Microorganisms 2022, 10, 1495. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, R.; Wang, D.; Zhu, W. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar] [CrossRef]
- Quartinello, F.; Kremser, K.; Schoen, H.; Tesei, D.; Ploszczanski, L.; Nagler, M.; Podmirseg, S.M.; Insam, H.; Piñar, G.; Sterflingler, K. Together is better: The rumen microbial community as biological toolbox for degradation of synthetic polyesters. Front. Bioeng. Biotech. 2021, 9, 684459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Gan, S.; Gao, R.; Du, C.; Wei, C.; Shah, A.M.; Ma, J. Toxicity Mechanisms of Microplastic and Its Effects on Ruminant Production: A Review. Biomolecules 2025, 15, 462. https://doi.org/10.3390/biom15040462
Su M, Gan S, Gao R, Du C, Wei C, Shah AM, Ma J. Toxicity Mechanisms of Microplastic and Its Effects on Ruminant Production: A Review. Biomolecules. 2025; 15(4):462. https://doi.org/10.3390/biom15040462
Chicago/Turabian StyleSu, Mengrong, Shangquan Gan, Rui Gao, Chunmei Du, Chen Wei, Ali Mujtaba Shah, and Jian Ma. 2025. "Toxicity Mechanisms of Microplastic and Its Effects on Ruminant Production: A Review" Biomolecules 15, no. 4: 462. https://doi.org/10.3390/biom15040462
APA StyleSu, M., Gan, S., Gao, R., Du, C., Wei, C., Shah, A. M., & Ma, J. (2025). Toxicity Mechanisms of Microplastic and Its Effects on Ruminant Production: A Review. Biomolecules, 15(4), 462. https://doi.org/10.3390/biom15040462








