Drugs Repurposing of Molecules Modulating Human Delta Globin Gene Expression via a Model of Transgenic Foetal Liver Cells: Implications for Beta-Hemoglobinopathy Therapeutics
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Compounds for Screening
2.2. In-Silico Prediction of Chemical-Physical Properties of Drugs
2.3. Mice
2.4. Cell Lines Generation and Culture
2.5. Luciferase Assay
2.6. Real-Time Quantitative PCR (RT-qPCR)
2.7. Statistics
3. Results
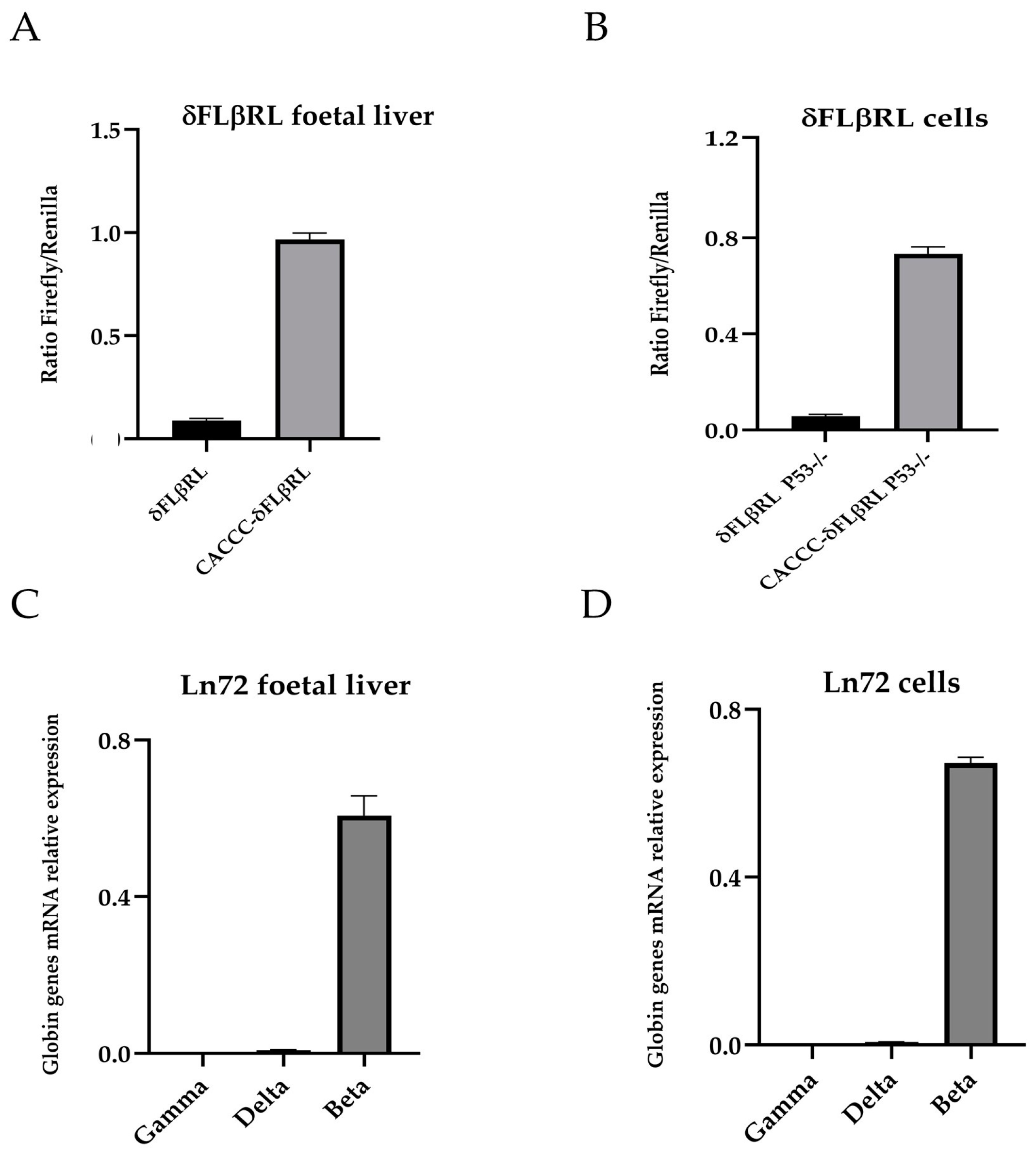
3.1. Creation of Foetal Liver Cell Lines for Drug and Small Molecules Screening
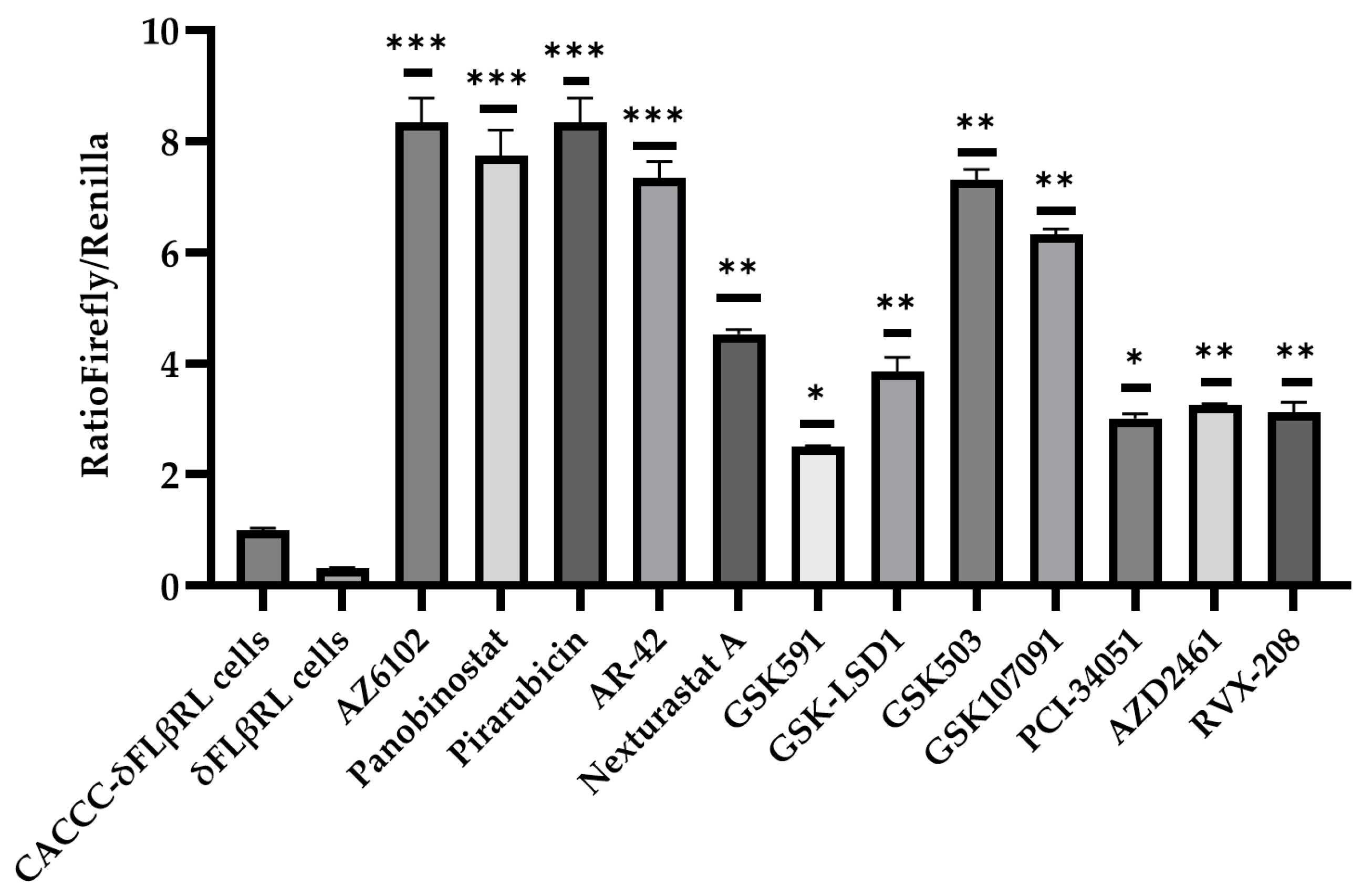
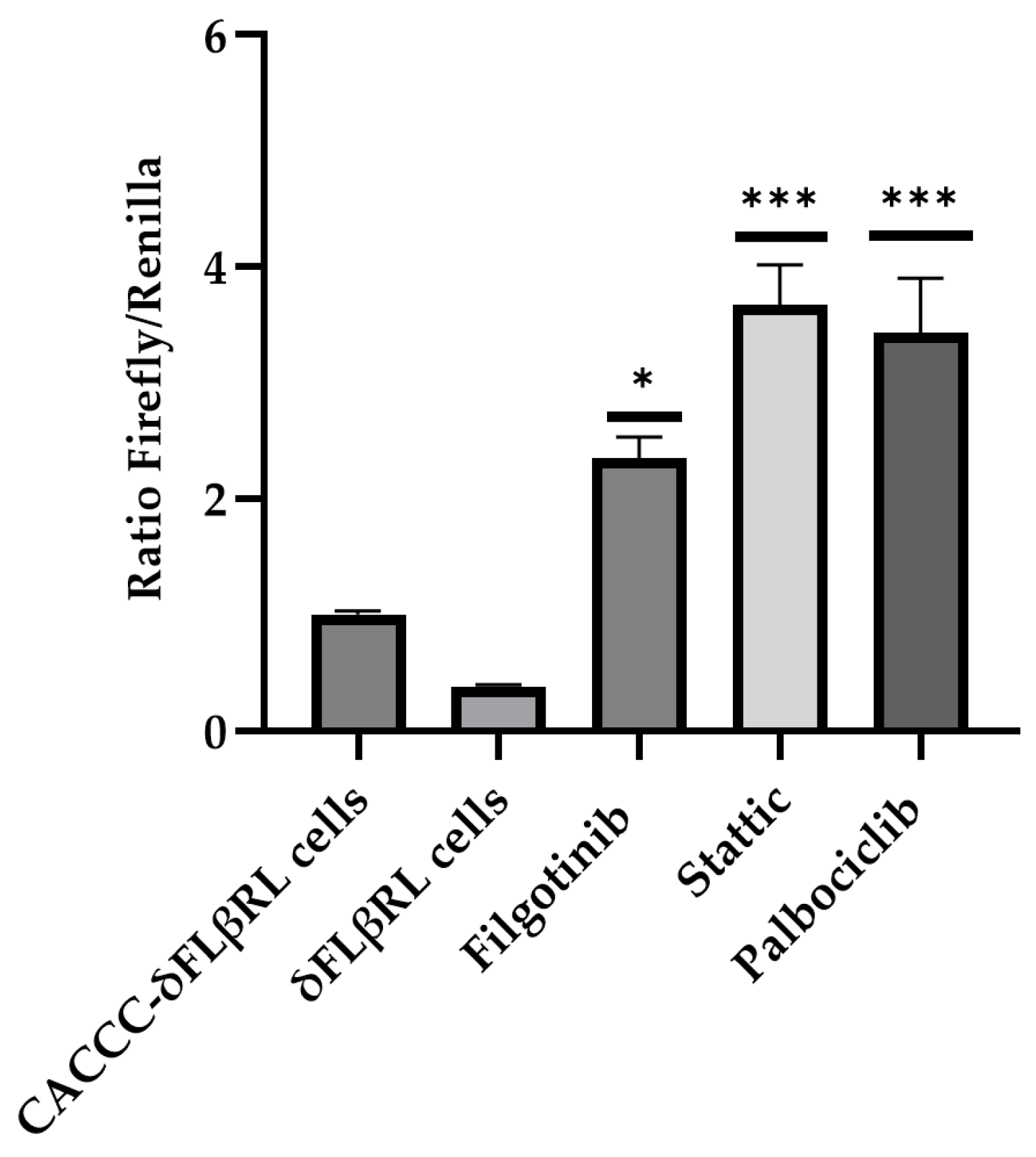
3.2. Screening of Drugs by Luciferase Assay via δFLβRL Foetal Liver Cells
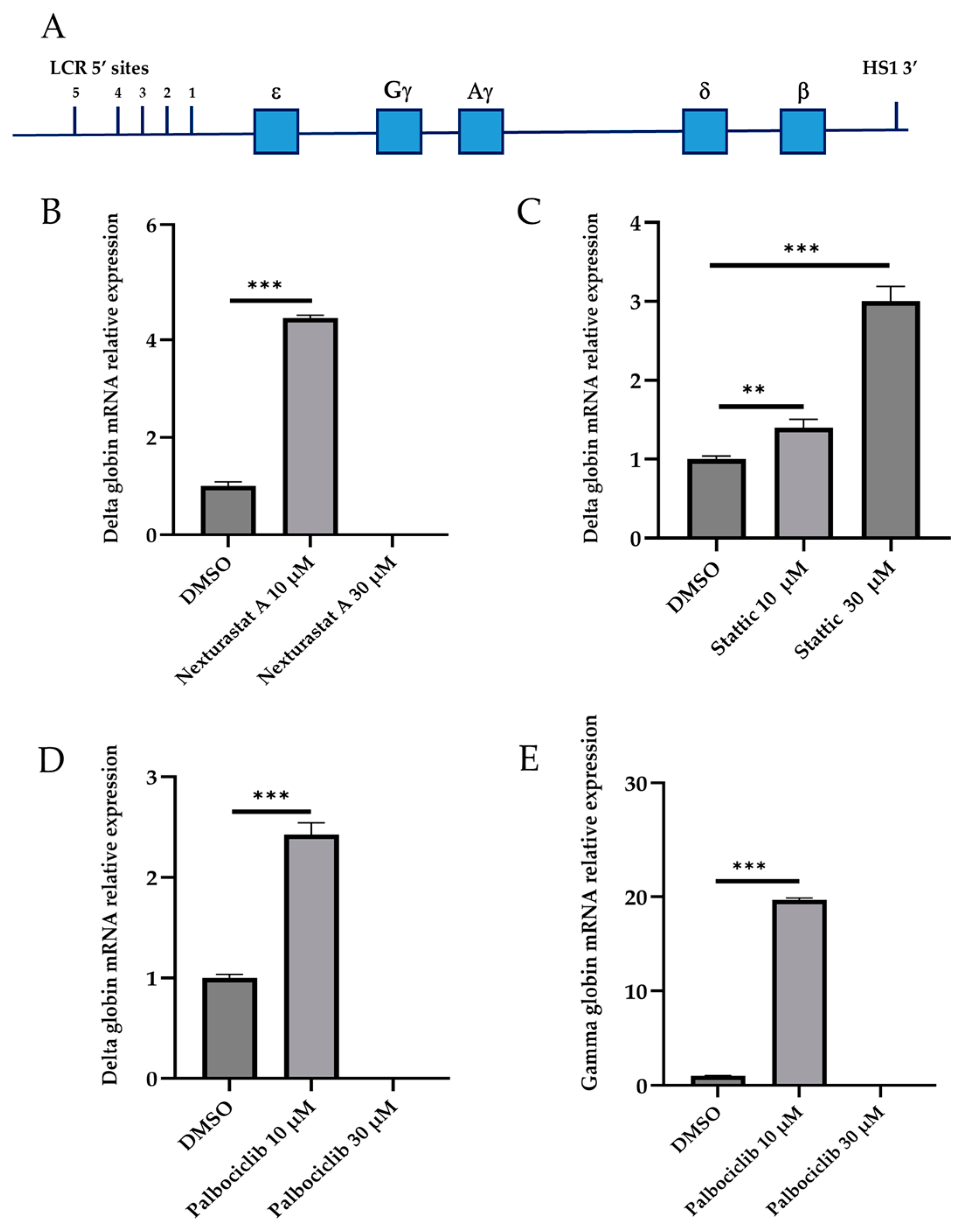
3.3. Validation of Active Drugs via Ln72 Foetal Liver Cells
3.4. In Silico Prediction of Chemical–Physical Properties of Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCD | Sickle cell disease |
| HBB | Beta globin gene |
| 3Rs | Replacement, reduction, and refinement |
| DPC | Days post-coitum |
| ADME-Tox | Absorption, distribution, metabolism, excretion and toxicity |
| MDCK | Madin-Darby canine kidney |
| CNS | Central Nervous System |
| IP | Ionisation potential |
| EV | Energy values |
References
- Weatherall, D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010, 115, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Galanello, R. Beta-thalassemia. Genet. Med. 2010, 12, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Karamperis, K.; Tsoumpeli, M.T.; Kounelis, F.; Koromina, M.; Mitropoulou, C.; Moutinho, C.; Patrinos, G.P. Genome-based therapeutic interventions for β-type hemoglobinopathies. Hum. Genom. 2021, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Thein, S.L. Genetic association studies in β-hemoglobinopathies. Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Bou-Fakhredin, R.; De Franceschi, L.; Motta, I.; Capellini, M.D.; Taher, A.T. Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective. Pharmaceuticals 2022, 15, 753. [Google Scholar] [CrossRef] [PubMed]
- Manchinu, M.F.; Marongiu, M.F.; Poddie, D.; Casu, C.; Latini, V.; Simbula, M.; Galanello, R.; Moi, P.; Cao, A.; Porcu, S.; et al. In vivo activation of the human δ-globin gene: The therapeutic potential in β-thalassemic mice. Haematologica 2014, 99, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Porcu, S.; Simbula, M.; Marongiu, M.F.; Perra, A.; Poddie, D.; Perseu, L.; Kowalik, M.A.; Littera, R.; Barella, S.; Caria, C.A.; et al. Delta-globin gene expression improves sickle cell disease in a humanised mouse model. Br. J. Haematol. 2021, 193, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.H.; Rodgers, G.P. HbA2: Biology, clinical relevance and a possible target for ameliorating sickle cell disease. Br. J. Haematol. 2015, 170, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, H.; Aerbajinai, W.; Kumkhaek, C.; Pirooznia, M.; Saxena, A.; Dagur, P.; Chin, K.; Rodgers, G.P. Kruppel-like factor 1-GATA1 fusion protein improves the sickle cell disease phenotype in mice both in vitro and in vivo. Blood 2022, 140, 2276–2289. [Google Scholar] [CrossRef] [PubMed]
- Manchinu, M.F.; Simbula, M.; Caria, C.A.; Musu, E.; Perseu, L.; Porcu, S.; Steri, M.; Poddie, D.; Frau, J.; Cocco, E.; et al. Delta-Globin Gene Expression Is Enhanced in vivo by Interferon Type I. Front. Med. 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Gutiérrez, L.; van der Linden, R.; Kong-A-San, J.; Maas, A.; Drabek, D.; Patrinos, G.P.; Philipsen, S.; Grosveld, F. A dual reporter mouse model of the human β-globin locus: Applications and limitations. PLoS ONE 2012, 5, 51272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strouboulis, J.; Dillon, N.; Grosveld, F. Developmental regulation of a complete 70-kb human beta-globin locus in transgenic mice. Genes Dev. 1992, 6, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E. The 3Rs Principle in Animal Experimentation: A Legal Review of the State of the Art in Europe and the Case in Italy. BioTech 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, J.; Turner, J.; Crossley, M. Human Krüppel-like factor 8: A CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 2000, 28, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- von Lindern, M.; Deiner, E.M.; Dolznig, H.; Parren-Van Amelsvoort, M.; Hayman, M.J.; Mullner, E.W.; Beug, H. Leukemic transformation of normal murine erythroid progenitors: V- and c-ErbB act through signaling pathways activated by the EpoR and c-Kit in stress erythropoiesis. Oncogene 2001, 20, 3651–3664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Starlard-Davenport, A.; Fitzgerald, A.; S Pace, B. Exploring epigenetic and microRNA approaches for γ-globin gene regulation. Exp. Biol. Med. 2021, 246, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Danjou, F.; Zoledziewska, M.; Sidore, C.; Steri, M.; Busonero, F.; Maschio, A.; Mulas, A.; Perseu, L.; Barella, S.; Porcu, E.; et al. Genome-wide association analyses based on whole-genome sequencing in Sardinia provide insights into regulation of hemoglobin levels. Nat. Genet. 2015, 47, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Caria, C.A.; Faà, V.; Porcu, S.; Marongiu, M.F.; Poddie, D.; Perseu, L.; Meloni, A.; Vaccargiu, S.; Ristaldi, M.S. Post-GWAS Validation of Target Genes Associated with HbF and HbA2 Levels. Cells 2024, 13, 1185. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.R.; Rotili, D.; Nebbioso, A.; Atweh, G.; Mai, A. Histone deacetylase inhibitors and hemoglobin F induction in beta-thalassemia. Int. J. Biochem. Cell Biol. 2008, 40, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Dellavalle, S.; Pisano, C.; Zunino, F. Development and therapeutic impact of HDAC6-selective inhibitors. Biochem. Pharmacol. 2012, 84, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Selenica, M.L.; Benner, L.; B Housley, S.; Manchec, B.; C Lee, D.; R Nash, K.; Kalin, J.; A Bergman, J.; Kozikowski, A.; N Gordon, M.; et al. Histone deacetylase 6 inhibition improves memory and reduces total tau levels in a mouse model of tau deposition. Alzheimers Res. Ther. 2014, 27, 12. [Google Scholar] [CrossRef] [PubMed]
- Foley, H.A.; Ofori-Acquah, S.F.; Yoshimura, A.; Critz, S.; Surendra Baliga, B.; Pace, B.S. Stat3 beta inhibits gamma-globin gene expression in erythroid cells. J. Biol. Chem. 2002, 277, 16211–16219. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Kodeboyina, S.; Liu, L.; Dzandu, J.; Sangerman, J.; Ofori-Acquah, S.F.; Pace, B.S. Role of STAT3 and GATA-1 interactions in gamma-globin gene expression. Exp. Hematol. 2009, 37, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, A.A.; Eekels, J.J.M.; Woytschak, J.; van Drogen, A.; Bosch, A.; Prader, S.; Felber, M.; Heeg, M.; Opitz, L.; Trück, J.; et al. Erythropoiesis defect observed in STAT3 GOF patients with severe anemia. J. Allergy Clin. Immunol. 2020, 145, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.; Bonetta, L.; MacAllan, D.; Parry, D.; Holder, A.; Dickson, C.; Peters, G. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene 1994, 9, 71–79. [Google Scholar] [PubMed]
- Meyerson, M.; Harlow, E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell Biol. 1994, 14, 2077–2086. [Google Scholar] [PubMed]
- Sankaran, V.G.; Ludwing, L.S.; Sicinska, E.; Xu, J.; Bauer, D.E.; Eng, J.C.; Patterson, H.C.; Metcalf, R.A.; Natkunam, Y.; Orkin, S.H.; et al. Cyclin D3 coordinates the cell cycle during differentiation to regulate erythrocyte size and number. Genes Dev. 2012, 26, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Wojda, U.; Noel, P.; Miller, J.L. Fetal and adult hemoglobin production during adult erythropoiesis: Coordinate expression correlates with cell proliferation. Blood 2002, 99, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Menzel, S.; Garner, C.; Rooks, H.; D Spector, T.; Lay Thein, S. HbA2 levels in normal adults are influenced by two distinct genetic mechanisms. Br. J. Haematol. 2013, 160, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.J.; Shani, M. Are animal models as good as we think? Theriogenology 2008, 69, 2–9. [Google Scholar] [CrossRef] [PubMed]
- McColl, B.; Vadolas, J. Animal models of β-hemoglobinopathies: Utility and limitations. J. Blood Med. 2016, 7, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.M.; Pham, C.T.; Ley, T.J. Transgenic analysis of a 100-kb human beta-globin cluster-containing DNA fragment propagated as a bacterial artificial chromosome. Blood 1999, 94, 3178–3184. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.R.; Li, Q.L.; Clegg, C.H.; Furukawa, T.; Navas, P.A.; Norton, E.J.; Kimbrough, T.G.; Stamatoyannopoulos, G. Use of yeast artificial chromosomes (YACs) in studies of mammalian development: Production of beta-globin locus YAC mice carrying human globin developmental mutants. Proc. Natl. Acad. Sci. USA 1995, 92, 5655–5659. [Google Scholar] [CrossRef] [PubMed]
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Kunz, J.B.; Tagliaferri, L. Sickle Cell Disease. Transfus. Med. Hemother 2024, 51, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Beachler, D.C.; de Luise, C.; Jamal-Allial, A.; Yin, R.; Taylor, D.H.; Suzuki, A.; Lewis, J.H.; Freston, J.W.; Lanes, S. Real-world safety of palbociclib in breast cancer patients in the United States: A new user cohort study. BMC Cancer 2021, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Haque, T.; Murakhovskaya, I.; Wang, Y.; Bachiashvili, K.; Papazoglu, C.; Pradhan, K.; Steidl, U.G.; Sparano, J.A.; Verma, A. Macrocytosis and dysplastic anemia is associated with the cyclin-dependent kinase 4/6 inhibitor palbociclib in metastatic breast cancer. Haematologica 2018, 103, e98–e102. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Leone, G.; Cappellini, M.D. Therapeutic Gene Editing for Hemoglobinopathies. Mediterr. J. Hematol. Infect. Dis. 2024, 16, e2024068. [Google Scholar] [CrossRef] [PubMed]

| Principal Descriptor | Range 95% of Drugs | Stattic | Palbociclib | Nexturastat |
|---|---|---|---|---|
| #starts | 0 to 5 | 2 | 0 | 0 |
| #rtvFG | 0 to 2 | 0 | 0 | 1 |
| Predicted CNS Activity | a −2 (inactive) to +2 (active) | −2 | 0 | −2 |
| Apparent MDCK Permeability (nm/s) | (<25 po or, >500 great) | 67.78 | 62.49 | 145 |
| Apparent Caco-2 Permeability (nm/s) | (<25 po or, >500 great) | 156.88 | 134.31 | 216.72 |
| QP logP for octanol/water | (−2.0/6.5) | −0.19 | 2.13 | 2.285 |
| No. of Primary Metabolites | (1.0/8.0) | 1 | 3 | 2 |
| Human Oral Absorption | 1 low, 2 medium, 3 high | 2 | 3 | 3 |
| % Human Oral Absorption in GI | (<25% is poor) | 65% | 78% | 82% |
| Lipinski Rule of 5 Violations | (maximum is 4) | 0 | 0 | 0 |
| Jorgensen Rule of 3 Violations | (maximum is 3) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simbula, M.; Manchinu, M.F.; Olla, S.; Congiu, M.; Vaccargiu, S.; Caria, C.A.; Poddie, D.; Ristaldi, M.S. Drugs Repurposing of Molecules Modulating Human Delta Globin Gene Expression via a Model of Transgenic Foetal Liver Cells: Implications for Beta-Hemoglobinopathy Therapeutics. Biomolecules 2025, 15, 565. https://doi.org/10.3390/biom15040565
Simbula M, Manchinu MF, Olla S, Congiu M, Vaccargiu S, Caria CA, Poddie D, Ristaldi MS. Drugs Repurposing of Molecules Modulating Human Delta Globin Gene Expression via a Model of Transgenic Foetal Liver Cells: Implications for Beta-Hemoglobinopathy Therapeutics. Biomolecules. 2025; 15(4):565. https://doi.org/10.3390/biom15040565
Chicago/Turabian StyleSimbula, Michela, Maria Francesca Manchinu, Stefania Olla, Michela Congiu, Simona Vaccargiu, Cristian Antonio Caria, Daniela Poddie, and Maria Serafina Ristaldi. 2025. "Drugs Repurposing of Molecules Modulating Human Delta Globin Gene Expression via a Model of Transgenic Foetal Liver Cells: Implications for Beta-Hemoglobinopathy Therapeutics" Biomolecules 15, no. 4: 565. https://doi.org/10.3390/biom15040565
APA StyleSimbula, M., Manchinu, M. F., Olla, S., Congiu, M., Vaccargiu, S., Caria, C. A., Poddie, D., & Ristaldi, M. S. (2025). Drugs Repurposing of Molecules Modulating Human Delta Globin Gene Expression via a Model of Transgenic Foetal Liver Cells: Implications for Beta-Hemoglobinopathy Therapeutics. Biomolecules, 15(4), 565. https://doi.org/10.3390/biom15040565








