Impairment of Muscle Function Causes Pupal Lethality in Flies Expressing the Mitochondrial Alternative Oxidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Diets
2.2. Developmental and Larval Mobility Assays
2.3. Measurements of Body Mass and Composition
2.4. Immunoblotting
2.5. Metabolite Extraction and Mass Spectrometry Analyses
2.6. Confocal Microscopy Imaging
2.7. Statistical Analyses
3. Results
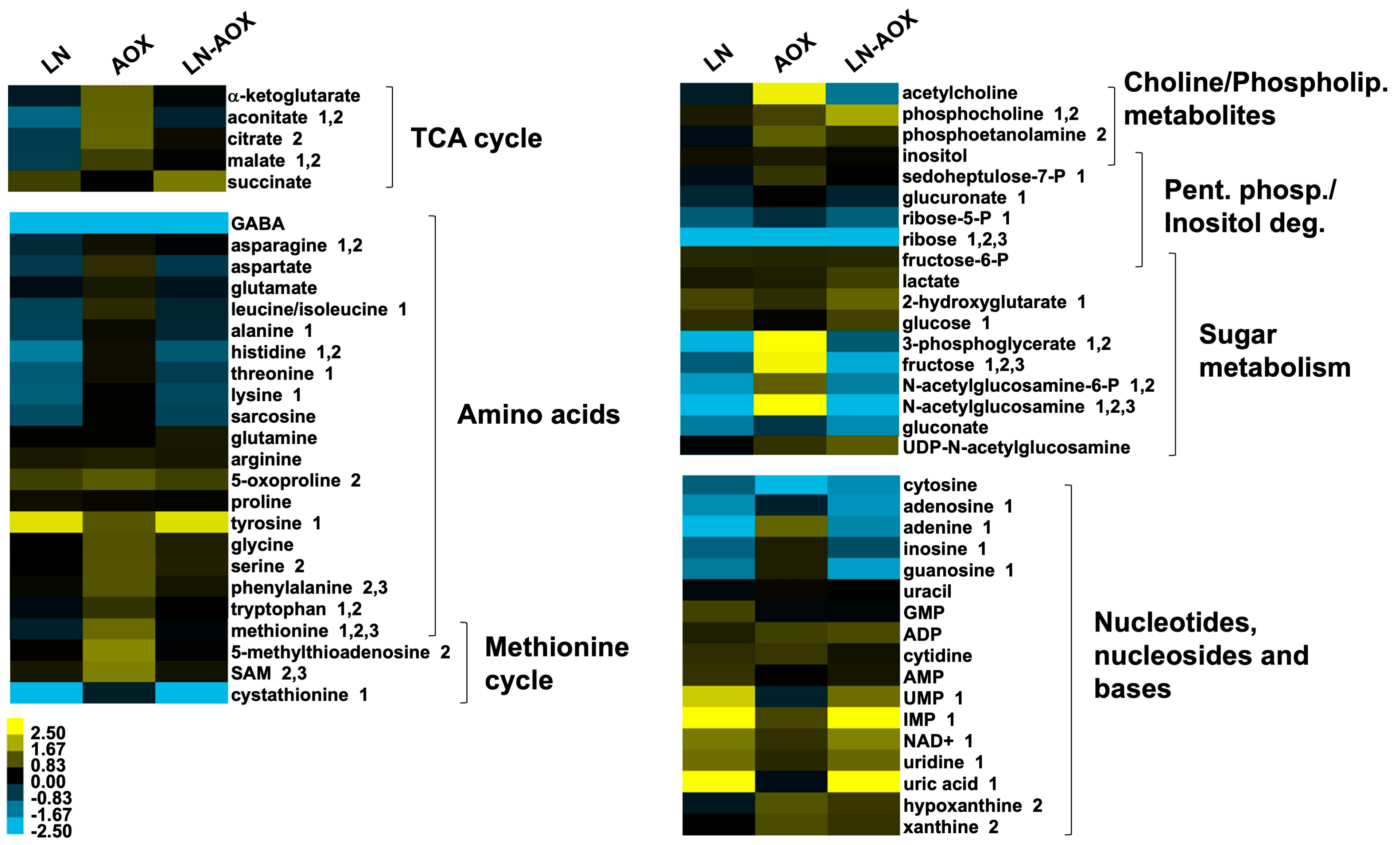
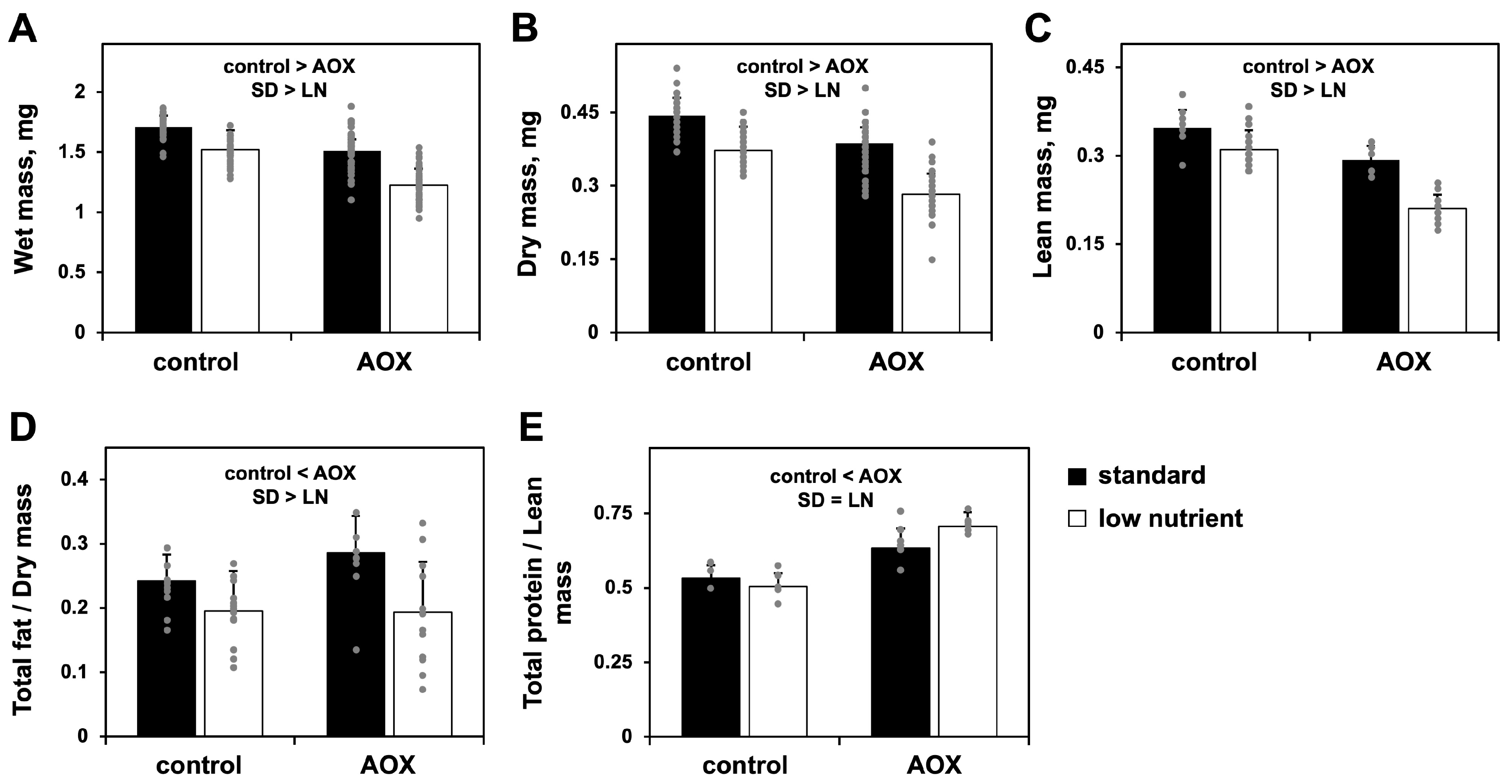
3.1. Low-Nutrient Diet and AOX Expression Cause Changes in Amino Acid Metabolism and a Severe Drop in Larval Biomass

3.2. Derangement of Myofibril Structure Is a Hallmark of AOX-Expressing Larvae Cultured on Low-Nutrient Diet
3.3. Rescue of the Phenotypes of AOX-Expressing Flies Cultured on LN Diet by Amino Acid Supplementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobs, H.T. A Century of Mitochondrial Research, 1922–2022; Kaguni, L.S., Tamanoi, F., Eds.; The Enzymes—History of the Enzymes, Current Topics and Future Perspectives; Elsevier Sciences: Amsterdam, The Netherlands, 2023; pp. 37–70. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.T.; George, J.; Kemppainen, E. Regulation of growth in Drosophila melanogaster: The roles of mitochondrial metabolism. J. Biochem. 2020, 167, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Cardona, L.R.; Kong, H.; Vasan, K.; McElroy, G.S.; Werner, M.; Kihshen, H.; Reczek, C.R.; Weinberg, S.E.; Gao, P.; et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 2020, 585, 288–292. [Google Scholar] [CrossRef]
- Kemppainen, K.K.; Rinne, J.; Sriram, A.; Lakanmaa, M.; Zeb, A.; Tuomela, T.; Popplestone, A.; Singh, S.; Sanz, A.; Rustin, P.; et al. Expression of alternative oxidase in Drosophila ameliorates diverse phenotypes due to cytochrome oxidase deficiency. Hum. Mol. Genet. 2014, 23, 2078–2093. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.E.; Vanlerberghe, G.C.; Staples, J.F. Alternative oxidase in animals: Unique characteristics and taxonomic distribution. J. Exp. Biol. 2009, 212, 2627–2634. [Google Scholar] [CrossRef]
- Sanz, A.; Fernández-Ayala, D.J.M.; Stefanatos, R.K.; Jacobs, H.T. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in drosophila. Aging 2010, 2, 200–223. [Google Scholar] [CrossRef]
- Cannino, G.; El-Khoury, R.; Pirinen, M.; Hutz, B.; Rustin, P.; Jacobs, H.T.; Dufour, E. Glucose Modulates Respiratory Complex I Activity in Response to Acute Mitochondrial Dysfunction. J. Biol. Chem. 2012, 287, 38729–38740. [Google Scholar] [CrossRef]
- Saha, B.; Borovskii, G.; Panda, S.K. Alternative oxidase and plant stress tolerance. Plant Signal. Behav. 2016, 11, e1256530. [Google Scholar] [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Hakkaart, G.A.J.; Dassa, E.P.; Jacobs, H.T.; Rustin, P. Allotopic expression of a mitochondrial alternative oxidase confers cyanide resistance to human cell respiration. EMBO Rep. 2006, 7, 341–345. [Google Scholar] [CrossRef]
- Fernandez-Ayala, D.J.M.; Sanz, A.; Vartiainen, S.; Kemppainen, K.K.; Babusiak, M.; Mustalahti, E.; Costa, R.; Tuomela, T.; Zeviani, M.; Chung, J.; et al. Expression of the Ciona intestinalis Alternative Oxidase (AOX) in Drosophila Complements Defects in Mitochondrial Oxidative Phosphorylation. Cell Metab. 2009, 9, 449–460. [Google Scholar] [CrossRef]
- El-Khoury, R.; Dufour, E.; Rak, M.; Ramanantsoa, N.; Grandchamp, N.; Csaba, Z.; Duvillié, B.; Bénit, P.; Gallego, J.; Gressens, P.; et al. Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome c Oxidase Blockade and Limits Mitochondrial ROS Overproduction. PLoS Genet. 2013, 9, e1003182. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, R.; Kaulio, E.; Lassila, K.A.; Crowther, D.C.; Jacobs, H.T.; Rustin, P. Expression of the alternative oxidase mitigates beta-amyloid production and toxicity in model systems. Free Radic. Biol. Med. 2016, 96, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Szibor, M.; Dhandapani, P.K.; Dufour, E.; Holmström, K.M.; Zhuang, Y.; Salwig, I.; Wittig, I.; Heidler, J.; Gizatullina, Z.; Gainutdinov, T.; et al. Broad AOX expression in a genetically tractable mouse model does not disturb normal physiology. Dis. Model. Mech. 2017, 10, 163–171. [Google Scholar] [CrossRef]
- Giordano, L.; Farnham, A.; Dhandapani, P.K.; Salminen, L.; Bhaskaran, J.; Voswinckel, R.; Rauschkolb, P.; Scheibe, S.; Sommer, N.; Beisswenger, C.; et al. Alternative Oxidase Attenuates Cigarette Smoke–induced Lung Dysfunction and Tissue Damage. Am. J. Respir. Cell Mol. Biol. 2019, 60, 515–522. [Google Scholar] [CrossRef]
- Dassa, E.P.; Dufour, E.; Gonçalves, S.; Paupe, V.; Hakkaart, G.A.J.; Jacobs, H.T.; Rustin, P. Expression of the alternative oxidase complements cytochrome c oxidase deficiency in human cells. EMBO Mol. Med. 2009, 1, 30–36. [Google Scholar] [CrossRef]
- Andjelković, A.; Oliveira, M.T.; Cannino, G.; Yalgin, C.; Dhandapani, P.K.; Dufour, E.; Rustin, P.; Szibor, M.; Jacobs, H.T. Diiron centre mutations in Ciona intestinalis alternative oxidase abolish enzymatic activity and prevent rescue of cytochrome oxidase deficiency in flies. Sci. Rep. 2015, 5, 18295. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, J.; Purhonen, J.; Tegelberg, S.; Smolander, O.; Mörgelin, M.; Rozman, J.; Gailus-Durner, V.; Fuchs, H.; de Angelis, M.H.; Auvinen, P.; et al. Alternative oxidase-mediated respiration prevents lethal mitochondrial cardiomyopathy. EMBO Mol. Med. 2019, 11, e9456. [Google Scholar] [CrossRef]
- Rustin, P.; Jacobs, H.T. Respiratory chain alternative enzymes as tools to better understand and counteract respiratory chain deficiencies in human cells and animals. Physiol. Plant 2009, 137, 362–370. [Google Scholar] [CrossRef]
- Giordano, L.; Aneja, M.K.; Sommer, N.; Alebrahimdehkordi, N.; Seraji, A.; Weissmann, N.; Rudolph, C.; Plank, C.; Jacobs, H.T.; Szibor, M. Alternative oxidase encoded by sequence-optimized and chemically-modified RNA transfected into mammalian cells is catalytically active. Gene Ther. 2021, 29, 655–664. [Google Scholar] [CrossRef]
- Andjelković, A.; Mordas, A.; Bruinsma, L.; Ketola, A.; Cannino, G.; Giordano, L.; Dhandapani, P.K.; Szibor, M.; Dufour, E.; Jacobs, H.T. Expression of the Alternative Oxidase Influences Jun N-Terminal Kinase Signaling and Cell Migration. Mol. Cell Biol. 2018, 38, e00110-18. [Google Scholar] [CrossRef]
- Saari, S.; Andjelković, A.; Garcia, G.S.; Jacobs, H.T.; Oliveira, M.T. Expression of Ciona intestinalis AOX causes male reproductive defects in Drosophila melanogaster. BMC Dev. Biol. 2017, 17, 9. [Google Scholar] [CrossRef]
- Saari, S.; Garcia, G.S.; Bremer, K.; Chioda, M.M.; Andjelković, A.; Debes, P.V.; Nikinmaa, M.; Szibor, M.; Dufour, E.; Rustin, P.; et al. Alternative respiratory chain enzymes: Therapeutic potential and possible pitfalls. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 854–866. [Google Scholar] [CrossRef]
- Saari, S.; Kemppainen, E.; Tuomela, T.; Oliveira, M.T.; Dufour, E.; Jacobs, H.T. Alternative oxidase confers nutritional limitation on Drosophila development. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2019, 331, 341–356. [Google Scholar] [CrossRef]
- Dogan, S.A.; Cerutti, R.; Benincá, C.; Brea-Calvo, G.; Jacobs, H.T.; Zeviani, M.; Szibor, M.; Viscomi, C. Perturbed Redox Signaling Exacerbates a Mitochondrial Myopathy. Cell Metab. 2018, 28, 764–775.e5. [Google Scholar] [CrossRef]
- Rodrigues, A.P.C.; Camargo, A.F.; Andjelković, A.; Jacobs, H.T.; Oliveira, M.T. Developmental arrest in Drosophila melanogaster caused by mitochondrial DNA replication defects cannot be rescued by the alternative oxidase. Sci. Rep. 2018, 8, 10882. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.F.; Chioda, M.M.; Rodrigues, A.P.C.; Garcia, G.S.; McKinney, E.A.; Jacobs, H.T.; Oliveira, M.T. Xenotopic expression of alternative electron transport enzymes in animal mitochondria and their impact in health and disease. Cell Biol. Int. 2018, 42, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, K.K.; Kemppainen, E.; Jacobs, H.T. The Alternative Oxidase AOX Does Not Rescue the Phenotype of tko25t Mutant Flies. G3 Genes|Genomes|Genet. 2014, 4, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Wodarz, A.; Hinz, U.; Engelbert, M.; Knust, E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of drosophila. Cell 1995, 82, 67–76. [Google Scholar] [CrossRef]
- Lin, D.M.; Goodman, C.S. Ectopic and increased expression of fasciclin II alters motoneuron growth cone guidance. Neuron 1994, 13, 507–523. [Google Scholar] [CrossRef]
- Schuster, C.M.; Davis, G.W.; Fetter, R.D.; Goodman, C.S. Genetic Dissection of Structural and Functional Components of Synaptic Plasticity. I. Fasciclin II Controls Synaptic Stabilization and Growth. Neuron 1996, 17, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Park, J.H. Hemolymph Sugar Homeostasis and Starvation-Induced Hyperactivity Affected by Genetic Manipulations of the Adipokinetic Hormone-Encoding Gene in Drosophila melanogaster. Genetics 2004, 167, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Barolo, S.; Carver, L.A.; Posakony, J.W. GFP and β-Galactosidase Transformation Vectors for Promoter/Enhancer Analysis in Drosophila. Biotechniques 2000, 29, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.S.; Othonicar, M.F.; Oliveira, M.T.; Couto-Lima, C.A. An Affordable and Efficient “Homemade” Platform for Drosophila Behavioral Studies, and an Accompanying Protocol for Larval Mitochondrial Respirometry. J. Vis. Exp. 2021, 175, e62669. [Google Scholar] [CrossRef]
- Salminen, T.S.; Oliveira, M.T.; Cannino, G.; Lillsunde, P.; Jacobs, H.T.; Kaguni, L.S. Mitochondrial genotype modulates mtDNA copy number and organismal phenotype in Drosophila. Mitochondrion 2017, 34, 75–83. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Kaiser, P. Methionine Dependence of Cancer. Biomolecules 2020, 10, 568. [Google Scholar] [CrossRef]
- Paudel, S.; Wu, G.; Wang, X. Amino Acids in Cell Signaling: Regulation and Function. In Amino Acids in Nutrition and Health, 1st ed.; Wu, G., Ed.; Springer: Cham, Switzerland, 2021; pp. 17–33. [Google Scholar] [CrossRef]
- Haunerland, N.H. Insect storage proteins: Gene families and receptors. Insect Biochem. Mol. Biol. 1996, 26, 755–765. [Google Scholar] [CrossRef]
- Beckett, K.; Baylies, M.K. The Development of The Drosophila Larval Body Wall Muscles. Int. Rev. Neurobiol. 2006, 75, 55–70. [Google Scholar] [CrossRef]
- Grandison, R.C.; Piper, M.D.W.; Partridge, L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 2009, 462, 1061–1064. [Google Scholar] [CrossRef]
- Sanz, A.; Soikkeli, M.; Portero-Otín, M.; Wilson, A.; Kemppainen, E.; McIlroy, G.; Ellilä, S.; Kemppainen, K.K.; Tuomela, T.; Lakanmaa, M.; et al. Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc. Natl. Acad. Sci. USA 2010, 107, 9105–9110. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Calleja, M.; Peralta, S.; Matsushima, Y.; Hernandez-Sierra, R.; Whitworth, A.J.; Kaguni, L.S.; Garesse, R. Modeling pathogenic mutations of human Twinkle in Drosophila suggests an apoptosis role in response to mitochondrial defects. PLoS ONE 2012, 7, e43954. [Google Scholar] [CrossRef] [PubMed]
- Adán, C.; Matsushima, Y.; Hernández-Sierra, R.; Marco-Ferreres, R.; Fernández-Moreno, M.Á.; González-Vioque, E.; Calleja, M.; Aragón, J.J.; Kaguni, L.S.; Garesse, R. Mitochondrial transcription factor B2 is essential for metabolic function in Drosophila melanogaster development. J. Biol. Chem. 2008, 283, 12333–12342. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, S.; Erol, H.; Özsoy, B.; Onbaşılar, I.; Yalçın, S. Effects of the usage of dried brewing yeast in the diets on the performance, egg traits and blood parameters in quails. Animal 2008, 2, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H. Amino acid metabolism, redox balance and epigenetic regulation in cancer. FEBS J. 2024, 291, 412–429. [Google Scholar] [CrossRef]
- Dai, Z.; Mentch, S.J.; Gao, X.; Nichenametla, S.N.; Locasale, J.W. Methionine metabolism influences genomic architecture and gene expression through H3K4me3 peak width. Nat. Commun. 2018, 9, 1955. [Google Scholar] [CrossRef]
- Yang, M.; Vousden, K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Sanderson, S.M.; Gao, X.; Dai, Z.; Locasale, J.W. Methionine metabolism in health and cancer: A nexus of diet and precision medicine. Nat. Rev. Cancer 2019, 19, 625–637. [Google Scholar] [CrossRef]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Qi, C.; Bajaj, J.; Lee, D. Serotonin receptor 5-HT7 in Drosophila mushroom body neurons mediates larval appetitive olfactory learning. Sci. Rep. 2020, 10, 21267. [Google Scholar] [CrossRef] [PubMed]
- Gabrawy, M.M.; Westbrook, R.; King, A.; Khosravian, N.; Ochaney, N.; DeCarvalho, T.; Wang, Q.; Yu, Y.; Huang, Q.; Said, A.; et al. Dual treatment with kynurenine pathway inhibitors and NAD+ precursors synergistically extends life span in Drosophila. Aging Cell 2024, 23, e14102. [Google Scholar] [CrossRef]
- Hu, G.; Ling, C.; Chi, L.; Thind, M.K.; Furse, S.; Koulman, A.; Swann, J.R.; Lee, D.; Calon, M.M.; Bourdon, C.; et al. The role of the tryptophan-NAD + pathway in a mouse model of severe malnutrition induced liver dysfunction. Nat. Commun. 2022, 13, 7576. [Google Scholar] [CrossRef]
- Kim, H.; Kim, K.; Yim, J. Biosynthesis of drosopterins, the red eye pigments of Drosophila melanogaster. IUBMB Life 2013, 65, 334–340. [Google Scholar] [CrossRef]
- Ryall, R.L.; Howells, A.J. Ommochrome biosynthetic pathway of Drosophila melanogaster: Variations in levels of enzyme activities and intermediates during adult development. Insect Biochem. 1974, 4, 47–61. [Google Scholar] [CrossRef]
- Maddison, D.C.; Alfonso-Núñez, M.; Swaih, A.M.; Breda, C.; Campesan, S.; Allcock, N.; Straatman-Iwanowska, A.; Kyriacou, C.P.; Giorgini, F. A novel role for kynurenine 3-monooxygenase in mitochondrial dynamics. PLoS Genet. 2020, 16, e1009129. [Google Scholar] [CrossRef]
- Sullivan, D.T.; Sullivan, M.C. Transport defects as the physiological basis for eye color mutants of Drosophila melanogaster. Biochem. Genet. 1975, 13, 603–613. [Google Scholar] [CrossRef]
- Carvalho, M.; Sampaio, J.L.; Palm, W.; Brankatschk, M.; Eaton, S.; Shevchenko, A. Effects of diet and development on the Drosophila lipidome. Mol. Syst. Biol. 2012, 8, 600. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto-Lima, C.A.; Saari, S.; Garcia, G.S.; Rocha, G.H.; ten Hoeve, J.; Dufour, E.; Oliveira, M.T. Impairment of Muscle Function Causes Pupal Lethality in Flies Expressing the Mitochondrial Alternative Oxidase. Biomolecules 2025, 15, 570. https://doi.org/10.3390/biom15040570
Couto-Lima CA, Saari S, Garcia GS, Rocha GH, ten Hoeve J, Dufour E, Oliveira MT. Impairment of Muscle Function Causes Pupal Lethality in Flies Expressing the Mitochondrial Alternative Oxidase. Biomolecules. 2025; 15(4):570. https://doi.org/10.3390/biom15040570
Chicago/Turabian StyleCouto-Lima, Carlos A., Sina Saari, Geovana S. Garcia, Gabriel H. Rocha, Johanna ten Hoeve, Eric Dufour, and Marcos T. Oliveira. 2025. "Impairment of Muscle Function Causes Pupal Lethality in Flies Expressing the Mitochondrial Alternative Oxidase" Biomolecules 15, no. 4: 570. https://doi.org/10.3390/biom15040570
APA StyleCouto-Lima, C. A., Saari, S., Garcia, G. S., Rocha, G. H., ten Hoeve, J., Dufour, E., & Oliveira, M. T. (2025). Impairment of Muscle Function Causes Pupal Lethality in Flies Expressing the Mitochondrial Alternative Oxidase. Biomolecules, 15(4), 570. https://doi.org/10.3390/biom15040570






