Metabolic Implications of Elevated Neutrophil Extracellular Traps in Polycystic Ovary Syndrome: A Focus on Hepatic Glycolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection from Patients
2.2. NETs Quantification
2.2.1. dsDNA
2.2.2. NE
2.3. Animals
2.3.1. Model Construction
2.3.2. Estrous Cycle Analysis
2.3.3. IPGTT
2.3.4. ITT
2.3.5. Measurement of Hormone Levels
2.4. Immunostaining
2.5. Histopathological Examination of Ovarian and Liver Tissues
2.6. Untargeted Metabolomics Analysis
2.7. Isolation and Culture of Mouse Bone Marrow Neutrophils
2.8. Cell Culture and Reagents
2.9. Western-Blot Analysis
2.10. RNA Isolation and Real-Time PCR
2.11. Enzyme Activity
2.12. Statistical Analysis
3. Results
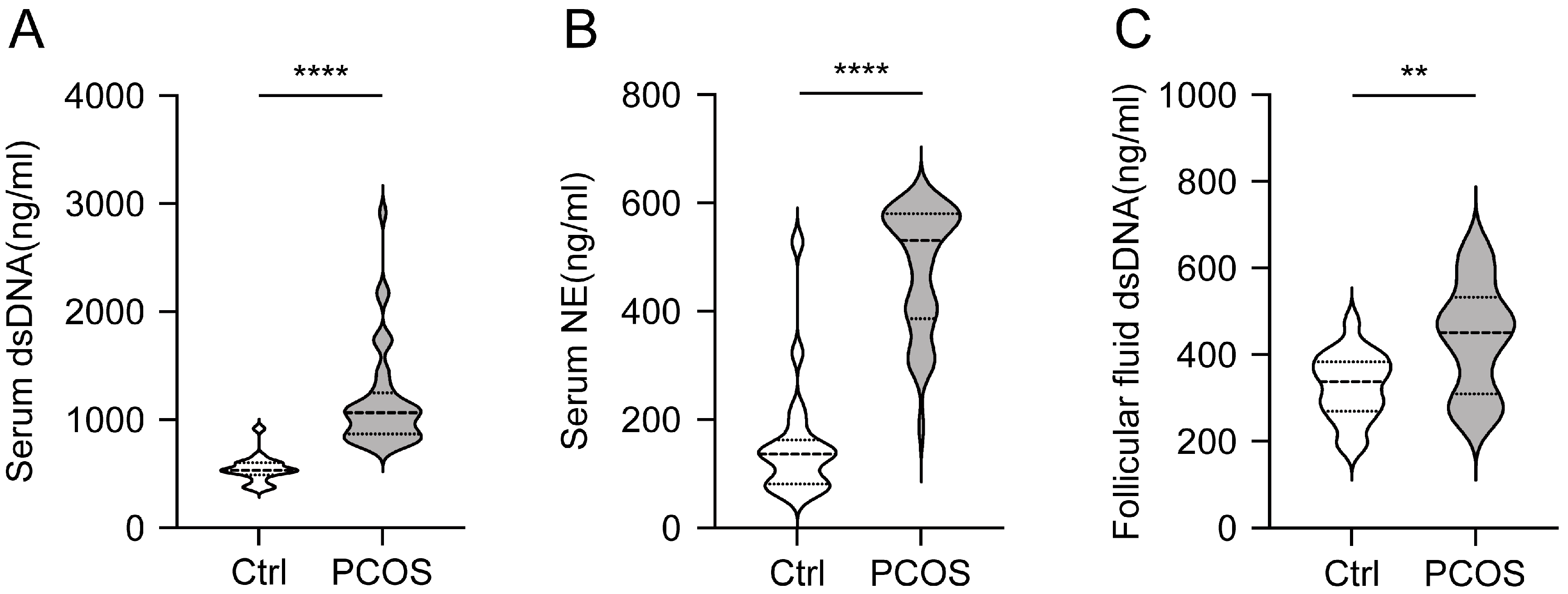
3.1. Increased Level of NETs in Serum and Follicular Fluid of PCOS Patients
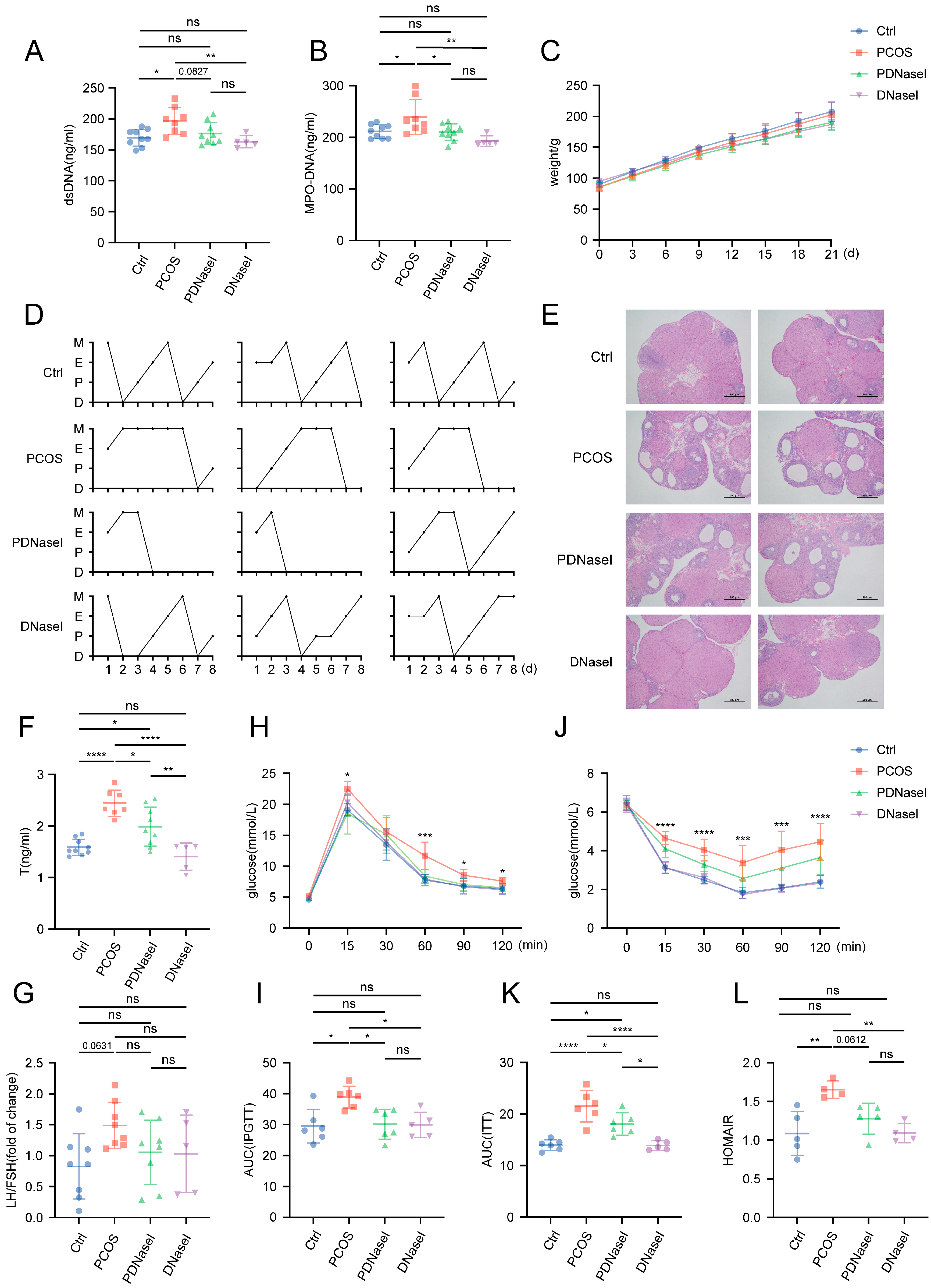
3.2. Intravenous Injection of DNase I Downregulates the Formation of NETs and Ameliorates Glucose Metabolism Disorders in PCOS Rat Models
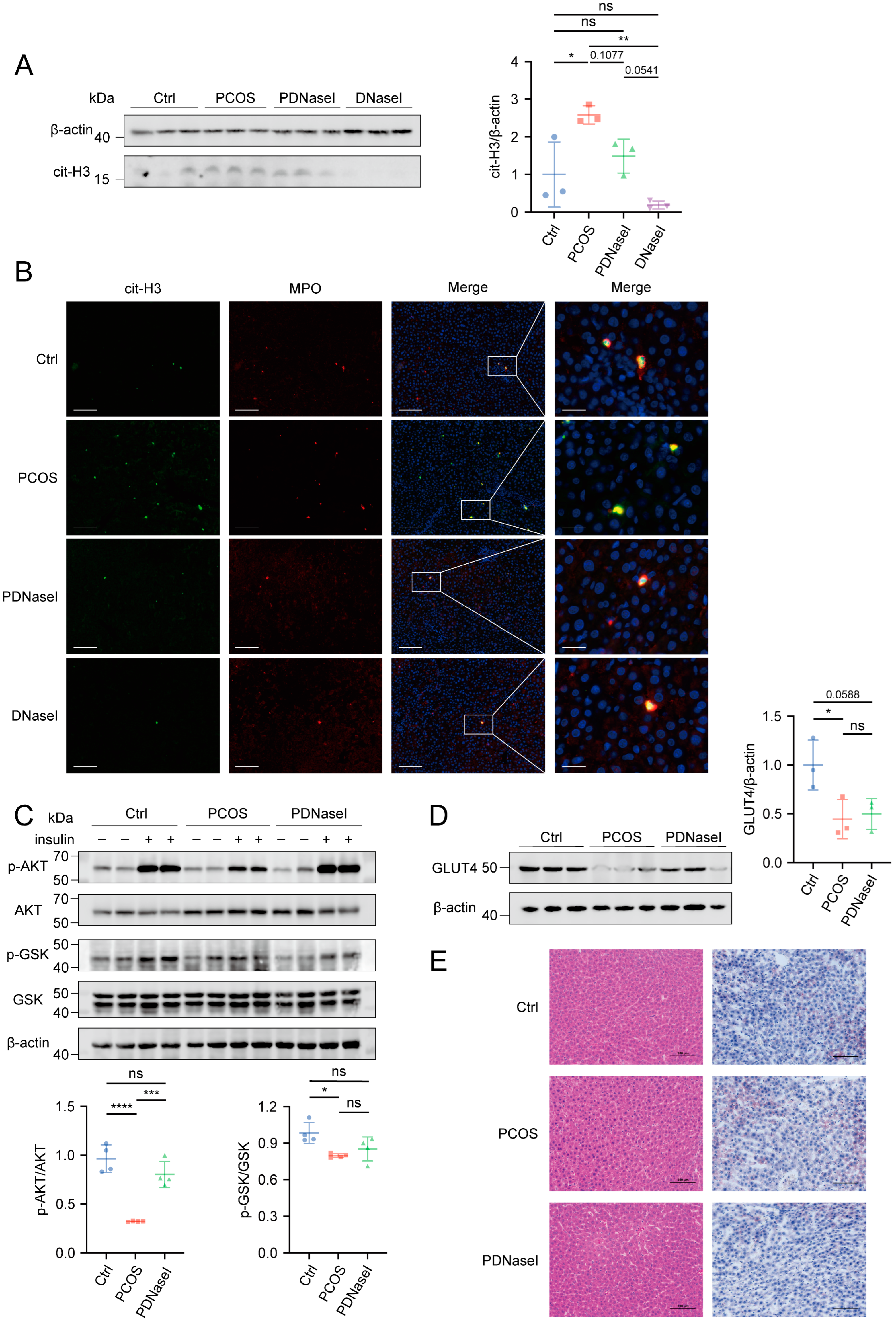
3.3. DNase I May Enhance Insulin Sensitivity by Improving Liver Function
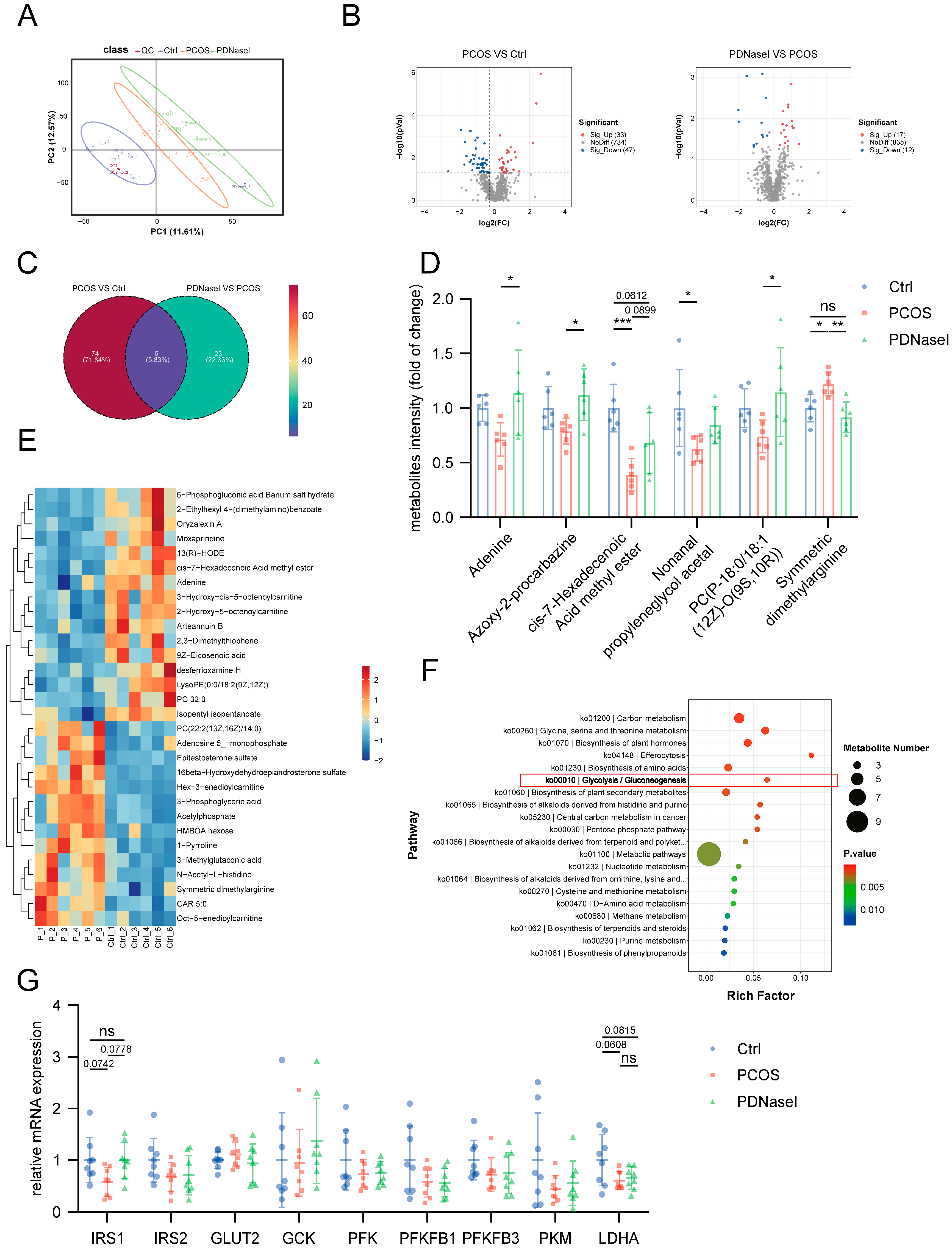
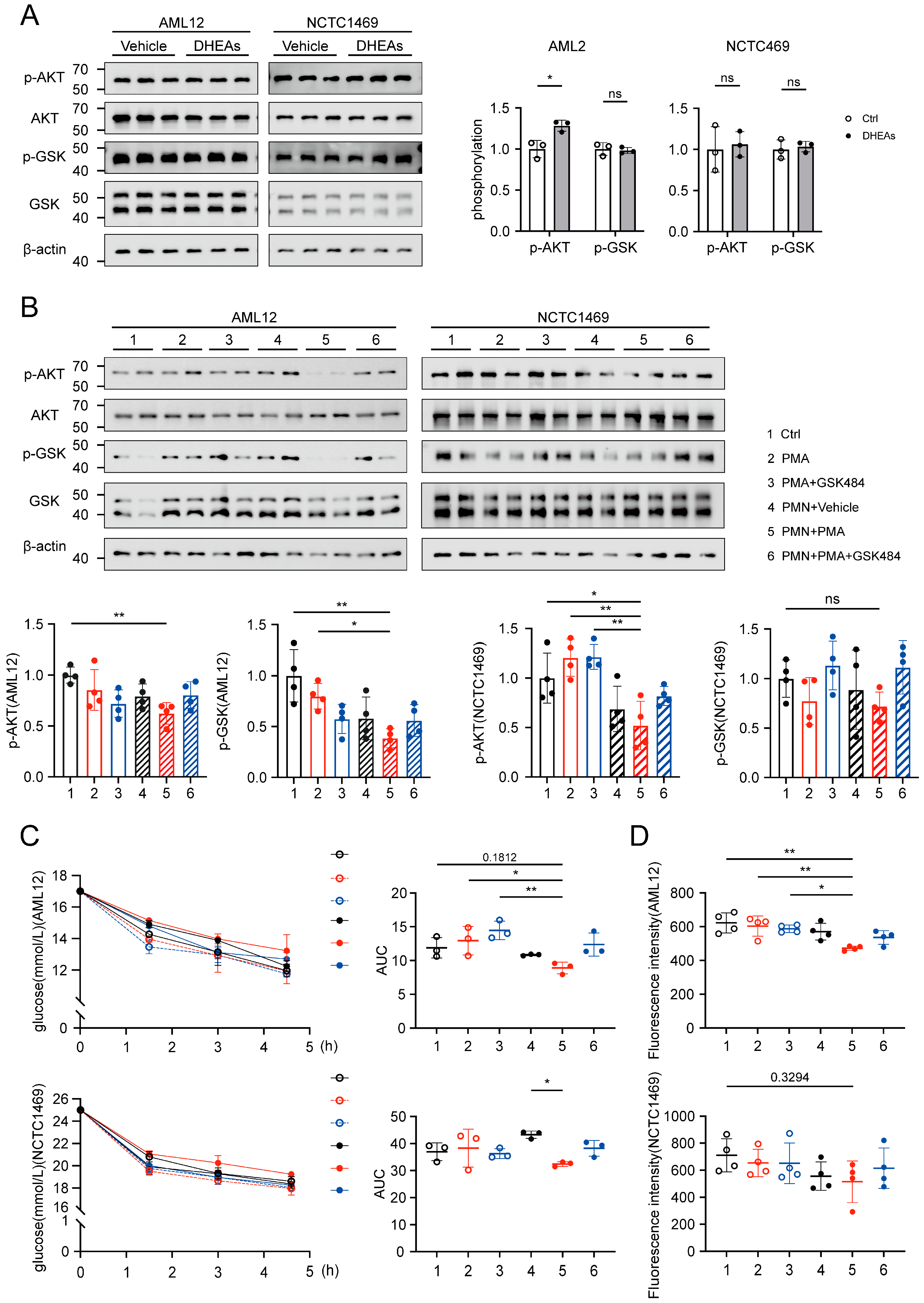
3.4. NET Infiltration in the Liver Tissue Affects the Glycolytic Pathway
3.5. NETs Induce Disruptions in Glucose Utilization Within Hepatocytes
3.6. NETs Induce a Downregulation of the Glycolytic Pathway in Hepatocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stepto, N.K.; Moreno-Asso, A.; McIlvenna, L.C.; Walters, K.A.; Rodgers, R.J. Molecular Mechanisms of Insulin Resistance in Polycystic Ovary Syndrome: Unraveling the Conundrum in Skeletal Muscle? J. Clin. Endocrinol. Metab. 2019, 104, 5372–5381. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Carson-Chahhoud, K.; Sullman, M.J.M.; Collins, G.S.; Kolahi, A.A.; Avery, J. Prevalence, incidence and years lived with disability due to polycystic ovary syndrome in 204 countries and territories, 1990–2019. Hum. Reprod. 2022, 37, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, Q.; Zhou, Z.; Qian, W.; Zhang, J.; Wu, Z.; Jin, L.; Wu, X.; Zhang, C.; Zheng, B.; et al. Changes in the prevalence of polycystic ovary syndrome in China over the past decade. Lancet Reg. Health West. Pac. 2022, 25, 100494. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Deng, Q. Epigenetic inheritance of polycystic ovary syndrome—Challenges and opportunities for treatment. Nat. Rev. Endocrinol. 2021, 17, 521–533. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Benson, S.; Janssen, O.E.; Hahn, S.; Tan, S.; Dietz, T.; Mann, K.; Pleger, K.; Schedlowski, M.; Arck, P.C.; Elsenbruch, S. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav. Immun. 2008, 22, 177–184. [Google Scholar] [CrossRef]
- Wang, D.; Weng, Y.; Zhang, Y.; Wang, R.; Wang, T.; Zhou, J.; Shen, S.; Wang, H.; Wang, Y. Exposure to hyperandrogen drives ovarian dysfunction and fibrosis by activating the NLRP3 inflammasome in mice. Sci. Total Environ. 2020, 745, 141049. [Google Scholar] [CrossRef]
- Lai, Y.; Ye, Z.; Mu, L.; Zhang, Y.; Long, X.; Zhang, C.; Li, R.; Zhao, Y.; Qiao, J. Elevated Levels of Follicular Fatty Acids Induce Ovarian Inflammation via ERK1/2 and Inflammasome Activation in PCOS. J. Clin. Endocrinol. Metab. 2022, 107, 2307–2317. [Google Scholar] [CrossRef]
- Hu, C.; Pang, B.; Ma, Z.; Yi, H. Immunophenotypic Profiles in Polycystic Ovary Syndrome. Mediat. Inflamm. 2020, 2020, 5894768. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef]
- Dou, H.; Kotini, A.; Liu, W.; Fidler, T.; Endo-Umeda, K.; Sun, X.; Olszewska, M.; Xiao, T.; Abramowicz, S.; Yalcinkaya, M.; et al. Oxidized Phospholipids Promote NETosis and Arterial Thrombosis in LNK(SH2B3) Deficiency. Circulation 2021, 144, 1940–1954. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- D’Abbondanza, M.; Martorelli, E.E.; Ricci, M.A.; De Vuono, S.; Migliola, E.N.; Godino, C.; Corradetti, S.; Siepi, D.; Paganelli, M.T.; Maugeri, N.; et al. Increased plasmatic NETs by-products in patients in severe obesity. Sci. Rep. 2019, 9, 14678. [Google Scholar] [CrossRef]
- Brotfain, E.; Hadad, N.; Shapira, Y.; Avinoah, E.; Zlotnik, A.; Raichel, L.; Levy, R. Neutrophil functions in morbidly obese subjects. Clin. Exp. Immunol. 2015, 181, 156–163. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Menegazzo, L.; Ciciliot, S.; Poncina, N.; Mazzucato, M.; Persano, M.; Bonora, B.; Albiero, M.; Vigili de Kreutzenberg, S.; Avogaro, A.; Fadini, G.P. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015, 52, 497–503. [Google Scholar] [CrossRef]
- Arelaki, S.; Koletsa, T.; Sinakos, E.; Papadopoulos, V.; Arvanitakis, K.; Skendros, P.; Akriviadis, E.; Ritis, K.; Germanidis, G.; Hytiroglou, P. Neutrophil extracellular traps enriched with IL-1β and IL-17A participate in the hepatic inflammatory process of patients with non-alcoholic steatohepatitis. Virchows Arch. 2022, 481, 455–465. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, R.; Yue, H.; Zhang, X.; Pan, X.; Sun, Y.; Shi, J.; Zhu, G.; Qin, C.; Guo, Y. Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression. Signal Transduct. Target. Ther. 2023, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, S.R.; Zhou, Z.; Lam, K.S.; Xu, A. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; González, F. Circulating inflammatory markers in polycystic ovary syndrome: A systematic review and metaanalysis. Fertil. Steril. 2011, 95, 1048–1058.e2. [Google Scholar] [CrossRef]
- Zheng, F.; Ma, L.; Li, X.; Wang, Z.; Gao, R.; Peng, C.; Kang, B.; Wang, Y.; Luo, T.; Wu, J.; et al. Neutrophil Extracellular Traps Induce Glomerular Endothelial Cell Dysfunction and Pyroptosis in Diabetic Kidney Disease. Diabetes 2022, 71, 2739–2750. [Google Scholar] [CrossRef]
- Espinosa, M.E.; Melo, A.; Leon, M.; Bautista-Valarezo, E.; Zambrano, F.; Uribe, P.; Bravo, A.; Taubert, A.; Hermosilla, C.; Iturrieta, V.; et al. Vaginal Microbiota and Proinflammatory Status in Patients with Polycystic Ovary Syndrome: An Exploratory Study. J. Clin. Med. 2024, 13, 2278. [Google Scholar] [CrossRef]
- Wang, J.; Gui, R.; Li, Y.; Li, Z.; Li, Z.; Liu, S.; Zhang, M.; Qian, L.; Fan, X.; Xiong, Y. SFRP4 contributes to insulin resistance-induced polycystic ovary syndrome by triggering ovarian granulosa cell hyperandrogenism and apoptosis through the nuclear β-catenin/IL-6 signaling axis. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119822. [Google Scholar] [CrossRef]
- McDowell, S.A.C.; Luo, R.B.E.; Arabzadeh, A.; Doré, S.; Bennett, N.C.; Breton, V.; Karimi, E.; Rezanejad, M.; Yang, R.R.; Lach, K.D.; et al. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat. Cancer 2021, 2, 545–562. [Google Scholar] [CrossRef]
- Babuta, M.; Morel, C.; de Carvalho Ribeiro, M.; Calenda, C.; Ortega-Ribera, M.; Thevkar Nagesh, P.; Copeland, C.; Zhuang, Y.; Wang, Y.; Cho, Y.; et al. Neutrophil extracellular traps activate hepatic stellate cells and monocytes via NLRP3 sensing in alcohol-induced acceleration of MASH fibrosis. Gut 2024, 73, 1854–1869. [Google Scholar] [CrossRef]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018, 68, 1347–1360. [Google Scholar] [CrossRef]
- Liu, H.; Tu, M.; Yin, Z.; Zhang, D.; Ma, J.; He, F. Unraveling the complexity of polycystic ovary syndrome with animal models. J. Genet. Genom. 2024, 51, 144–158. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, H.; Fan, Y.; Chen, S.; Li, Y.; Liu, R.; Li, T.; Yin, C. Gut microbiota disorder induces liver dysfunction in polycystic ovary syndrome rats’ model by regulating metabolite rosmarinic acid. Life Sci. 2023, 330, 121912. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wu, X. A feature enhanced EEG compression model using asymmetric encoding-decoding network. J. Neural Eng. 2024, 21, 036013. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef]
- Veličković, N.; Mićić, B.; Teofilović, A.; Milovanovic, M.; Jovanović, M.; Djordjevic, A.; Macut, D.; Vojnović Milutinović, D. Overfeeding in the early postnatal period aggravates inflammation and hepatic insulin sensitivity in the 5α-dihydrotestosterone-induced animal model of PCOS. Front. Endocrinol. 2024, 15, 1402905. [Google Scholar] [CrossRef]
- Atsumi, T.; Nishio, T.; Niwa, H.; Takeuchi, J.; Bando, H.; Shimizu, C.; Yoshioka, N.; Bucala, R.; Koike, T. Expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase/PFKFB3 isoforms in adipocytes and their potential role in glycolytic regulation. Diabetes 2005, 54, 3349–3357. [Google Scholar] [CrossRef]
- Trefely, S.; Khoo, P.S.; Krycer, J.R.; Chaudhuri, R.; Fazakerley, D.J.; Parker, B.L.; Sultani, G.; Lee, J.; Stephan, J.P.; Torres, E.; et al. Kinome Screen Identifies PFKFB3 and Glucose Metabolism as Important Regulators of the Insulin/Insulin-like Growth Factor (IGF)-1 Signaling Pathway. J. Biol. Chem. 2015, 290, 25834–25846. [Google Scholar] [CrossRef]

| Variables N | Ctrl 19 | PCOS 40 | p-Value |
|---|---|---|---|
| Age BMI | 32.00(28.00–34.00) 21.03 ± 1.53 | 29.00(25.00–34.00) 21.80 ± 2.80 | 0.384 0.170 |
| AMH | 4.93 ± 4.08 | 5.37 ± 2.91 | 0.717 |
| LH | 5.58(4.62–7.44) | 6.00(3.48–9.58) | 0.536 |
| FSH | 6.58(5.04–7.17) | 6.00(4.60–7.17) | 0.263 |
| LH/FSH | 0.84(0.61–1.13) | 1.19(0.63–1.57) | 0.080 |
| E2 | 42.00(32.00–64.00) | 38.39(28.01–67.09) | 0.729 |
| T | 0.67 ± 0.22 | 1.94 ± 0.94 | <0.001 |
| PRL | 17.90(12.30–21.50) | 10.75(8.21–17.45) | 0.025 |
| 0’PG | 5.05(4.73–5.38) | 4.72(4.33–5.05) | 0.146 |
| 0’Ins | 7.05(5.70–9.31) | 8.27(6.26–12.70) | 0.193 |
| HOMAIR | 1.49(1.15–2.25) | 1.85 (1.28–2.99) | 0.472 |
| ALT | 14.00(11.00–23.00) | 16.00(11.00–20.25) | 0.946 |
| AST | 18.50(18.00–27.50) | 17.00(15.00–19.00) | 0.061 |
| Variables N | Ctrl 19 | PCOS 17 | p-Value |
|---|---|---|---|
| Age | 30.56 ± 3.57 | 32.36 ± 3.03 | 0.141 |
| BMI | 22.05(19.55–25.15) | 21.60(20.70–22.91) | 0.812 |
| AMH | 3.91(2.46–6.66) | 4.88(3.45–6.87) | 0.408 |
| LH | 4.68(3.46–10.25) | 5.91(4.15–8.71) | 0.945 |
| FSH | 7.40 ± 3.06 | 6.56 ± 1.68 | 0.328 |
| LH/FSH | 0.95 ± 0.57 | 0.97 ± 0.40 | 0.904 |
| E2 | 38.50(29.00–56.32) | 33.24(29.00–52.80) | 0.563 |
| T | 0.83 ± 0.34 | 1.79 ± 0.50 | <0.001 |
| PRL | 17.60(11.58–29.38) | 15.40(10.55–29.95) | 0.714 |
| 0’PG | 5.05 ± 0.42 | 5.15 ± 0.54 | 0.539 |
| ALT | 14.00(10.00–18.75) | 15.00(11.50–18.50) | 0.703 |
| AST | 16.00(14.75–21.46) | 16.00(15.00–18.75) | 0.754 |
| Target Gene | 5′-Forward Sequences-3′ | 5′-Reverse Sequence-3′ |
|---|---|---|
| IRS1 | TCTACACCCGAGACGAACACT | TGGGCCCTTTGCCCGATTATG |
| IRS2 | GACCAGTCCCACATCAGGCTT | CTGCACGGATGACCTTAGCG |
| GLUT2 | CACCAGCACATACGACACCAGAC | CCCAAGCCACCCACCAAAGAAC |
| GCK | GCCGCAGTGAGGACGTGATG | AGGTGATTTCGCAGTTGGGTGTC |
| PFKFB1 | TCAGAGCCGTACAGCCTACTACC | GCCAGAGTCACCTCCAATGCG |
| PFKFB3 | CGGCACGGCGAGAATGAGTAC | TCAGGGCATTGGCGAACTTCTTG |
| PFK | TGAATCTGCGGCTGATGCTGAAG | CTCAAGGTGCGGCGTGTGAC |
| PKM | GTGCCGCCTGGACATTGACTC | ATTCAGCCGAGCCACATTCATCC |
| LDHA | GCAATCTGGATTCGGCTCGGTTC | CGGCGACATTCACACCACTCC |
| β-actin | GCTGTGCTATGTTGCCCTAGACTTC | GGAACCGCTCATTGCCGATAGTG |
| Target Gene | 5′-Forward Sequences-3′ | 5′-Reverse Sequence-3′ |
|---|---|---|
| IRS1 | TGTCACCCAGTGGTAGTTGCTC | CTCTCAACAGGAGGTTTGGCATG |
| IRS2 | CCAGTAAACGGAGGTGGCTACA | CCATAGACAGCTTGGAGCCACA |
| GLUT2 | GTTGGAAGAGGAAGTCAGGGCA | ATCACGGAGACCTTCTGCTCAG |
| GCK | GCATCTCTGACTTCCTGGACAAG | CTTGGTCCAGTTGAGCAGGATG |
| PFKFB1 | AGAGGCAGTGAGCTACAGGAAC | TGACCTTCCTCACGGCTGAGAT |
| PFKFB3 | TCATCGAGTCGGTCTGTGACGA | CATGGCTTCTGCTGAGTTGCAG |
| PFK | CCATCAGCAACAATGTGCCTGG | TGAGGCTGACTGCTTGATGCGA |
| PKM | CAGAGAAGGTCTTCCTGGCTCA | GCCACATCACTGCCTTCAGCAC |
| LDHA | ACGCAGACAAGGAGCAGTGGAA | ATGCTCTCAGCCAAGTCTGCCA |
| β-actin | CATTGCTGACAGGATGCAGAAGG | TGCTGGAAGGTGGACAGTGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Li, Y.; Liu, W.; Du, Y.; Tao, T. Metabolic Implications of Elevated Neutrophil Extracellular Traps in Polycystic Ovary Syndrome: A Focus on Hepatic Glycolysis. Biomolecules 2025, 15, 572. https://doi.org/10.3390/biom15040572
Lin S, Li Y, Liu W, Du Y, Tao T. Metabolic Implications of Elevated Neutrophil Extracellular Traps in Polycystic Ovary Syndrome: A Focus on Hepatic Glycolysis. Biomolecules. 2025; 15(4):572. https://doi.org/10.3390/biom15040572
Chicago/Turabian StyleLin, Siyu, Yushan Li, Wei Liu, Yanzhi Du, and Tao Tao. 2025. "Metabolic Implications of Elevated Neutrophil Extracellular Traps in Polycystic Ovary Syndrome: A Focus on Hepatic Glycolysis" Biomolecules 15, no. 4: 572. https://doi.org/10.3390/biom15040572
APA StyleLin, S., Li, Y., Liu, W., Du, Y., & Tao, T. (2025). Metabolic Implications of Elevated Neutrophil Extracellular Traps in Polycystic Ovary Syndrome: A Focus on Hepatic Glycolysis. Biomolecules, 15(4), 572. https://doi.org/10.3390/biom15040572






