Exosomal Biomarkers: A Comprehensive Overview of Diagnostic and Prognostic Applications in Malignant and Non-Malignant Disorders
Abstract
:1. Introduction
2. Physiological Functions of Exosomes
2.1. Cell-to-Cell Communication
2.2. Waste Removal
2.3. Development and Tissue Repair
2.4. Immune Response Regulation
3. Diagnostic and Prognostic Function of Exosomes
3.1. Diagnostic and Prognostic Function of Exosome in Non-Malignant Disorders
3.1.1. Diagnostic Function of Exosomes in Cardiovascular Disease
Exosomes in Coronary Artery Disease
Exosomes in Heart Failure
3.1.2. Diagnostic Function of Exosomes in Lung Disorders
Asthma
Pulmonary Fibrosis
3.1.3. Diagnostic Function of Exosomes in Liver Disorders
Acute Liver Injury
Non-Alcoholic Fatty Liver Disease and Alcohol-Associated Liver Disease
Viral Hepatitis
Liver Fibrosis
3.1.4. Diagnostic Function of Exosomes in Pancreatitis
Acute Pancreatitis
Chronic Pancreatitis
Diabetes
3.1.5. Diagnostic Function of Exosomes in Kidney Disorders
Acute Kidney Disease
Chronic Kidney Disease
Lupus Nephritis
3.1.6. Diagnostic Function of Exosomes in CNS Disorders
Exosomes in Neurodegenerative Disease
Stroke
Neuropsychiatric
3.1.7. Diagnostic Function of Exosomes in Pregnancy Disorders
Exosomes in Hypertensive Disorder of Pregnancy
Exosomes in Prenatal Screening
3.1.8. Diagnostic Function of Exosomes in Organ Transplantation
3.2. Diagnostic and Prognostic Function of Exosomes in Malignant Disorders
3.2.1. Diagnostic Function of Exosomes in Brain Tumor
3.2.2. Diagnostic Function of Exosomes in Thoracic Tumor
Lung Cancer
Breast Cancer
3.2.3. Diagnostic Function of Exosomes in Gastrointestinal Tumor
Pancreatic Cancer
Colorectal Cancer
Hepatocellular Carcinoma
3.2.4. Diagnostic Function of Exosomes in Thyroid Cancer
3.2.5. Diagnostic Function of Exosomes in Urogenital Tumor
Prostate Cancer
Bladder Cancer
Kidney Cancer
Ovarian Cancer
Cervical Cancer
3.2.6. Melanoma
3.2.7. Hematologic Malignancy
Leukemia
Lymphoma
3.2.8. The Advantage of Exosomes for Cancer Early Detection
Specificity and High Sensitivity
Noninvasive Sample and Patient Friendly
3.3. Limitations and Overcoming Limitations of Exosome Diagnostic Use in the Clinic
4. The Application of Exosomes in Clinical Trials
4.1. Trends of Trials Investigating Diagnostic and Prognostic Values of Exosomes
4.2. Trend of Exosome Application in Malignancies Has Been Higher Compared with Non-Malignant Disorders
5. Conclusions and Future Prospect
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEA | Carcinoembryonic antigen |
| EpCAM | Epithelial cell adhesion molecule |
| PD-L1 | Programmed death-ligand 1 |
| NSCLC | Non-small-cell lung cancer |
| CSF | Cerebrospinal fluid |
| BALF | Bronchoalveolar lavage fluid |
| CTCs | Circulating tumor cells |
| cfDNA | Cell-free DNA |
| SMCs | Smooth muscle cells |
| MHC | Major histocompatibility complex |
| IL | Interleukins |
| CNS | Central nervous system |
| CAD | Coronary artery disease |
| CVDs | Cardiovascular diseases |
| LDL | Low-density lipoproteins |
| MI | Myocardial infarction |
| LC-MS/MS | Liquid chromatography coupled with tandem mass spectrometry |
| ACS | Acute coronary syndrome |
| HF | Heart failure |
| AMI | Acute myocardial infarction |
| PF | Pulmonary fibrosis |
| IPF | Idiopathic pulmonary fibrosis |
| EVs | Extracellular vesicle |
| CES1 | Carboxylesterase |
| ADH1 | Alcohol dehydrogenase 1 |
| GST | Glutathione S-transferases |
| APOA1 | Apolipoprotein A1 |
| ALB | Albumin |
| HP | Haptoglobin |
| FGB | Fibrinogen |
| ALI | Acute liver injury |
| APAP | Acetaminophen |
| NAC | N acetyl-cysteine |
| NAFLD | Non-alcoholic fatty liver disease |
| FZD-7 | Frizzled’ gene family encode 7 |
| ALD | Alcohol-associated liver disease |
| hsp90 | Heat shock protein 90 |
| HBV | Hepatitis B virus |
| HBsAg | Hepatitis B surface antigens |
| LF | Liver fibrosis |
| HCV | Hepatitis C virus |
| SVR | Respiratory syncytial virus |
| HSC | Hematopoietic stem cell |
| MAFLD | Metabolically associated fatty liver disease |
| AP | Acute pancreatitis |
| PAAF | Pancreatitis-associated ascitic fluid |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| TAB2 | TAK1 binding protein2 |
| NIK | NF-κB-inducing kinase |
| NF-κB | Nuclear factor kappa B |
| CP | Chronic pancreatitis |
| PDAC | Pancreatic ductal adenocarcinoma |
| AQP5 | Aquaporin 5 |
| DN | Diabetic nephropathy |
| AKI | Acute kidney injury |
| CKD | Chronic kidney disease |
| LN | Lupus nephritis |
| SLE | Systemic lupus erythematosus |
| PD | Parkinson’s disease |
| APS | Amyotrophic lateral sclerosis |
| VGLUT-1 | Vesicular glutamate transporter-1 |
| EAAT-2 | Excitatory amino acid transporter-2 |
| LAMP-1 | Lysosome associated membrane protein-1 |
| MS | Multiple sclerosis |
| RRMS | Relapsing-remitting multiple sclerosis |
| SPMS | Secondary progressive multiple sclerosis |
| NIHSS | National Institutes of Health Stroke Scale |
| SCZ | Schizophrenia |
| GSK-3β | Glycogen synthase kinase-3 beta |
| mTOR | Mammalian target of rapamycin |
| BD | Bipolar affective disorder |
| MDD | Major depressive disorder |
| SERPINF1 | Serpin family F member 1 |
| GRM4 | Glutamate metabotropic receptor 4 |
| PDEs | Placenta-derived exosomes |
| PE | Preeclampsia |
| PLAP | Placental alkaline phosphatase |
| RAB11FIP2 | RAB11 family interacting protein 2 |
| NB | Neuroblastoma |
| BM | Bone marrow |
| GBM | Glioblastoma multiforme |
| GDE | Glioma-derived exosomes |
| NY-ESO-1 | New York esophageal squamous cell carcinoma-1 |
| LCN2 | Lipocalin-2 |
| BC | Breast cancer |
| MS | Mass spectrometry |
| PRM | Parallel reaction monitoring |
| TJP2 | Tight junction protein 2 |
| RALGAPA2 | Ral GTPase-activating protein subunit alpha-2 |
| PKG1 | cGMP-dependent protein kinase 1 |
| HER2 | Human epidermal growth factor receptor-2 |
| EDIL3 | EGF like repeats and discoidin domains 3 |
| exo-AnxA2 | Exosomal-annexin A2 |
| TNBC | Triple-negative BC |
| PDAC | Pancreatic ductal adenocarcinoma |
| CA19-9 | Sialyl Lewis |
| GPC1 | Cell-surface proteoglycan glypican-1 |
| snoRNA | Short nucleolar RNA |
| WASF2 | WAS protein family member 2 |
| ARF6 | ADP ribosylation factor 6 |
| SNORA74A | Small nucleolar RNA, H/ACA box 74A |
| SNORA25 | Small nucleolar RNA, H/ACA box 25 |
| CRC | Colorectal cancer |
| TSPAN1 | Tetraspanin 1 |
| HCC | Hepatocellular carcinoma |
| lncRNA | Long non-coding RNA |
| TC | Thyroid cancer |
| DTC | Differentiated TC |
| SPEs | Serum-purified EVs |
| PTC | Papillary TC |
| PT | Peritumoral tissues |
| BG | Benign goiter |
| FTC | Follicular thyroid carcinoma |
| GGT1 | Gamma glutamyl transferase 1 |
| PCa | Prostate cancer |
| BPH | Benign prostatic hyperplasia |
| PSA | Prostate-specific antigen |
| CRPC | Castration-resistant prostate cancer |
| EPS8L1 | EPS8 signaling adaptor L1 |
| EGFR | Epidermal growth factor receptor |
| TPP1 | Tripeptidyl-peptidase 1 |
| TMPRSS2 | Transmembrane protease, serine 2 |
| FOLR1 | Folate receptor 1 |
| ARHGEF39 | Rho guanine nucleotide exchange factor 39 |
| FOXO3 | Forkhead box O3 |
| GALNT1 | Polypeptide N-acetylgalactosaminyltransferase 1 |
| HIF-1a | Hypoxia-inducible factor 1 subunit alpha |
| RCC | Renal cell carcinoma |
| PCBD1 | Pterin-4 alpha-carbinolamine dehydratase-1 |
| GSTA1 | Glutathione transferase alpha 1 |
| CEBPA | CCAAT enhancer binding protein alpha |
| lncRNAs | Long non-coding RNA |
| TGF-b1 | Transforming growth factor beta |
| CC | Cervical cancer |
| MAPK10 | Mitogen-activated protein kinase 10 |
| ATF1 | Activating transcription factor 1 |
| CSPG4 | Chondroitin sulfate proteoglycan 4 |
| TEXs | Tumor-derived exosomes |
| AML | Acute myeloid leukemia |
| DLBCL | Diffuse large B-cell lymphoma |
| PFS | Progression-free survival |
| IC-ELISA | Immunocapture-based ELISA |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| DEGs | Differentially expressed genes |
| cfDNA | Cell-free DNA |
| ALIX | ALG-2-interacting protein X |
| ELISA | Enzyme-linked immunosorbent assay |
| NTA | Nanoparticle tracking analysis |
| MSC | Mesenchymal stem cells |
| GI | Gastrointestinal |
| CABS | Coronary artery bypass surgery |
| DIS | Delirium in cardiovascular surgery |
| HT | Hypertension |
| SAC | Stratification of adverse cardiac |
| DR | Diabetic retinopathy |
| PDM | Post-pancreatitis diabetes mellitus |
| OSAS | Obstructive sleep apnea syndrome |
| PN | Pulmonary nodules |
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| GI | Gastrointestinal |
| MMB | Metastatic meningitis from breast |
References
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O'Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Statello, L.; Maugeri, M.; Garre, E.; Nawaz, M.; Wahlgren, J.; Papadimitriou, A.; Lundqvist, C.; Lindfors, L.; Collen, A.; Sunnerhagen, P. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS ONE 2018, 13, e0195969. [Google Scholar] [CrossRef]
- Kwantwi, L.B. Exosome-mediated crosstalk between tumor cells and innate immune cells: Implications for cancer progression and therapeutic strategies. J. Cancer Res. Clin. Oncol. 2023, 149, 9487–9503. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, K. Stem cell-derived exosome versus stem cell therapy. Nat. Rev. Bioeng. 2023, 1, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Anees, A.; Harsiddharay, R.K.; Kumar, P.; Tripathi, P.K. A Comprehensive Review on Exosome: Recent Progress and Outlook. Pharm. Nanotechnol. 2024, 12, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; Van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef]
- Jakobsen, K.R.; Paulsen, B.S.; Bæk, R.; Varming, K.; Sorensen, B.S.; Jørgensen, M.M. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles 2015, 4, 26659. [Google Scholar] [CrossRef]
- Rodríguez Zorrilla, S.; Pérez-Sayans, M.; Fais, S.; Logozzi, M.; Gallas Torreira, M.; García García, A. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers 2019, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, Q.; Zeng, H.; Zhao, Q.; Guo, Z.; Zhong, R.; Xie, M.; Cai, X.; Su, J.; He, Z. Exosomal CA125 as a promising biomarker for ovarian cancer diagnosis. J. Cancer 2020, 11, 6445. [Google Scholar] [CrossRef]
- Sun, N.; Lee, Y.-T.; Zhang, R.Y.; Kao, R.; Teng, P.-C.; Yang, Y.; Yang, P.; Wang, J.J.; Smalley, M.; Chen, P.-J. Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat. Commun. 2020, 11, 4489. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Egan, T.K. Monitoring patients undergoing cancer therapy. Lab. Med. 2000, 31, 666–671. [Google Scholar] [CrossRef]
- Car, L.T.; Papachristou, N.; Urch, C.; Majeed, A.; El–Khatib, M.; Aylin, P.; Atun, R.; Car, J.; Vincent, C. Preventing delayed diagnosis of cancer: Clinicians’ views on main problems and solutions. J. Glob. Health 2016, 6, 020901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-c.; Lu, S.; Zhang, L.; Wang, C.-l.; Cheng, Y.; Mok, T.; Huang, C.; Liu, X.-q.; Wang, J.; Wang, M.-z. Consensus on dignosis for ALK positive non-small cell lung cancer in China, the 2013 version. Chin. J. Pathol. 2013, 42, 402–406. [Google Scholar]
- Bassani, S.; Lee, Y.K.; Campagnari, V.; Eccher, A.; Monzani, D.; Nocini, R.; Sacchetto, L.; Molteni, G. From hype to reality: A narrative review on the promising role of artificial intelligence in larynx cancer detection and transoral microsurgery. Crit. Rev. Oncog. 2023, 28, 21–24. [Google Scholar] [CrossRef]
- Kawada, T.; Shim, S.R.; Quhal, F.; Rajwa, P.; Pradere, B.; Yanagisawa, T.; Bekku, K.; Laukhtina, E.; von Deimling, M.; Teoh, J.Y.-C. Diagnostic accuracy of liquid biomarkers for clinically significant prostate cancer detection: A systematic review and diagnostic meta-analysis of multiple thresholds. Eur. Urol. Oncol. 2023, 7, 649–662. [Google Scholar] [CrossRef]
- Young, E.; Edwards, L.; Singh, R. The role of artificial intelligence in colorectal cancer screening: Lesion detection and lesion characterization. Cancers 2023, 15, 5126. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.-A.; Gwak, H.; Lee, J.; Kwak, B.; Jung, H.-I. Salivary exosome and cell-free DNA for cancer detection. Micromachines 2018, 9, 340. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef]
- Street, J.M.; Barran, P.E.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, R.T.; Webb, D.J.; Dear, J.W. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 2012, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.-A.A.; van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Runz, S.; Keller, S.; Rupp, C.; Stoeck, A.; Issa, Y.; Koensgen, D.; Mustea, A.; Sehouli, J.; Kristiansen, G.; Altevogt, P. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol. Oncol. 2007, 107, 563–571. [Google Scholar] [CrossRef]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.-D.; Abdel-Bakky, M.; Gutwein, P.; Altevogt, P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Ahmad, T.; Agrawal, A.; Ghosh, B. Proinflammatory role of epithelial cell–derived exosomes in allergic airway inflammation. J. Allergy Clin. Immunol. 2013, 131, 1194–1203.e1114. [Google Scholar] [CrossRef]
- Bozyk, N.; Tang, K.D.; Zhang, X.; Batstone, M.; Kenny, L.; Vasani, S.; Punyadeera, C. Salivary exosomes as biomarkers for early diagnosis of oral squamous cell carcinoma. Oral Oncol. Rep. 2023, 6, 100017. [Google Scholar] [CrossRef]
- Bano, A.; Vats, R.; Verma, D.; Yadav, P.; Kamboj, M.; Bhardwaj, R. Exploring salivary exosomes as early predictors of oral cancer in susceptible tobacco consumers: Noninvasive diagnostic and prognostic applications. J. Cancer Res. Clin. Oncol. 2023, 149, 15781–15793. [Google Scholar] [CrossRef]
- Rodríguez-Zorrilla, S.; Lorenzo-Pouso, A.I.; Fais, S.; Logozzi, M.A.; Mizzoni, D.; Di Raimo, R.; Giuliani, A.; García-García, A.; Pérez-Jardón, A.; Ortega, K.L.; et al. Increased Plasmatic Levels of Exosomes Are Significantly Related to Relapse Rate in Patients with Oral Squamous Cell Carcinoma: A Cohort Study. Cancers 2023, 15, 5693. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Santiago, M.; Priego-Capote, F.; Turck, N.; Robin, X.; Jurado-Gámez, B.; Sanchez, J.C.; Luque de Castro, M.D. Human sweat metabolomics for lung cancer screening. Anal. Bioanal. Chem. 2015, 407, 5381–5392. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Akram, M.R.; Kalsoom Khan, A.; Zia, M.A.; Siddiqi, A.R.; Murtaza, G. Recent developments in sweat analysis and its applications. Int. J. Anal. Chem. 2015, 2015, 164974. [Google Scholar] [CrossRef]
- Inubushi, S.; Kawaguchi, H.; Mizumoto, S.; Kunihisa, T.; Baba, M.; Kitayama, Y.; Takeuchi, T.; Hoffman, R.M.; Tanino, H.; Sasaki, R. Oncogenic miRNAs Identified in Tear Exosomes From Metastatic Breast Cancer Patients. Anticancer Res. 2020, 40, 3091–3096. [Google Scholar] [CrossRef]
- Ge, X.; Wang, Y.; Nie, J.; Li, Q.; Tang, L.; Deng, X.; Wang, F.; Xu, B.; Wu, X.; Zhang, X.; et al. The diagnostic/prognostic potential and molecular functions of long non-coding RNAs in the exosomes derived from the bile of human cholangiocarcinoma. Oncotarget 2017, 8, 69995–70005. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Angelini, D.F.; Di Raimo, R.; Falchi, M.; Battistini, L.; Fais, S. Microenvironmental pH and Exosome Levels Interplay in Human Cancer Cell Lines of Different Histotypes. Cancers 2018, 10, 370. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Chen, Y.; Song, S.; Huang, X.; Yang, Y.; Li, Y.; Tong, Y.; Xie, Y.; Li, J. Construction of exosome SORL1 detection platform based on 3D porous microfluidic chip and its application in early diagnosis of colorectal cancer. Small 2023, 19, 2207381. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-H.; Xue, L.; Hsu, C.-C.; Paez, J.S.P.; Pan, L.; Andaluz, H.; Wendt, M.K.; Iliuk, A.B.; Zhu, J.-K.; Tao, W.A. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Luan, X.; Jiang, G.; Yang, L.; Yan, K.; Li, S.; Xiang, W.; Zhou, J. The Dual effects of exosomes on Glioma: A Comprehensive Review. J. Cancer 2023, 14, 2707. [Google Scholar] [CrossRef]
- Shang, Z.; Wanyan, P.; Wang, M.; Zhang, B.; Cui, X.; Wang, X. Stem cell-derived exosomes for traumatic spinal cord injury: A systematic review and network meta-analysis based on a rat model. Cytotherapy 2024, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- De La Peña, H.; Madrigal, J.; Rusakiewicz, S.; Bencsik, M.; Cave, G.W.; Selman, A.; Rees, R.C.; Travers, P.J.; Dodi, I.A. Artificial exosomes as tools for basic and clinical immunology. J. Immunol. Methods 2009, 344, 121–132. [Google Scholar] [CrossRef]
- Kang, C.; He, H.; Liu, P.; Liu, Y.; Li, X.; Zhang, J.; Ran, H.; Zeng, X.; Zhao, H.; Liu, J. Role of dendritic cell-derived exosomes in allergic rhinitis. Int. J. Mol. Med. 2023, 52, 117. [Google Scholar] [CrossRef]
- Hergenreider, E.; Heydt, S.; Tréguer, K.; Boettger, T.; Horrevoets, A.J.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Yang, X.; Zhao, H. Harnessing exosomes as cutting-edge drug delivery systems for revolutionary osteoarthritis therapy. Biomed. Pharmacother. 2023, 165, 115135. [Google Scholar] [CrossRef] [PubMed]
- Gudbergsson, J.M.; Johnsen, K.B. Exosomes and autophagy: Rekindling the vesicular waste hypothesis. J. Cell Commun. Signal. 2019, 13, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D. A brief history of adherons: The discovery of brain exosomes. Int. J. Mol. Sci. 2020, 21, 7673. [Google Scholar] [CrossRef] [PubMed]
- Nik Mohamed Kamal, N.N.S.; Shahidan, W.N.S. Salivary exosomes: From waste to promising periodontitis treatment. Front. Physiol. 2022, 12, 798682. [Google Scholar] [CrossRef]
- Lai, Z.; Liang, J.; Zhang, J.; Mao, Y.; Zheng, X.; Shen, X.; Lin, W.; Xu, G. Exosomes as a delivery tool of exercise-induced beneficial factors for the prevention and treatment of cardiovascular disease: A systematic review and meta-analysis. Front. Physiol. 2023, 14, 1190095. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L. The role of exosomes in central nervous system tissue regeneration and repair. Biomed. Mater. 2023, 18, 052003. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, L.; Zou, H.; He, Y.; Pan, Y.; Ye, L.; Huang, Y.; Fan, W.; Zhang, J.; Ma, Y. Optogenetic engineered umbilical cord MSC-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J. Nanobiotechnol. 2023, 21, 176. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, P.; Zhong, L.; Wang, S.; Feng, Q.; Wei, X.; Zhou, L. Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines. Front. Bioeng. Biotechnol. 2022, 10, 1025138. [Google Scholar] [CrossRef]
- Hu, T.; Chang, S.; Qi, F.; Zhang, Z.; Chen, J.; Jiang, L.; Wang, D.; Deng, C.; Nie, K.; Xu, G. Neural grafts containing exosomes derived from Schwann cell-like cells promote peripheral nerve regeneration in rats. Burn Trauma 2023, 11, tkad013. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, J.; Wang, P.; Liu, X.; Liu, P.; Cheng, X.; Cao, L.; Wu, H.; Chen, J.; Zhou, L. Neural stem cell-derived exosomes and regeneration: Cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 198. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Gahan, P.B. Exosomes in immune regulation. Non-Coding RNA 2021, 7, 4. [Google Scholar] [CrossRef]
- Greening, D.W.; Gopal, S.K.; Xu, R.; Simpson, R.J.; Chen, W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015, 40, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, M.-y.; Guo, Y.; Zhao, X.; Lim, H.-m.C. Mast cell-derived exosomes at the stimulated acupoints activating the neuro-immune regulation. Chin. J. Integr. Med. 2017, 23, 878–880. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Peng, H.; Huyan, T.; Cacalano, N.A. Exosomes: Versatile nano mediators of immune regulation. Cancers 2019, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Charrier, M.; Viaud, S.; André, F.; Besse, B.; Chaput, N.; Zitvogel, L. Dendritic cell–derived exosomes as immunotherapies in the fight against cancer. J. Immunol. 2014, 193, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Anel, A.; Gallego-Lleyda, A.; de Miguel, D.; Naval, J.; Martínez-Lostao, L. Role of exosomes in the regulation of T-cell mediated immune responses and in autoimmune disease. Cells 2019, 8, 154. [Google Scholar] [CrossRef]
- Shen, Y.; Xue, C.; Li, X.; Ba, L.; Gu, J.; Sun, Z.; Han, Q.; Zhao, R.C. Effects of gastric cancer cell-derived exosomes on the immune regulation of mesenchymal stem cells by the NF-kB signaling pathway. Stem Cells Dev. 2019, 28, 464–476. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom. Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef]
- Kanninen, K.M.; Bister, N.; Koistinaho, J.; Malm, T. Exosomes as new diagnostic tools in CNS diseases. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 403–410. [Google Scholar] [CrossRef]
- Lee, M.; Ban, J.-J.; Im, W.; Kim, M. Influence of storage condition on exosome recovery. Biotechnol. Bioprocess. Eng. 2016, 21, 299–304. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, L.; Lv, D.; Zhu, X.; Tang, H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J. Hematol. Oncol. 2019, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jeon, H.; Yoo, S.M.; Lee, M.S. The effect of storage temperature on the biological activity of extracellular vesicles for the complement system. Vitr. Cell Dev. Biol. Anim. 2018, 54, 423–429. [Google Scholar] [CrossRef]
- Ghafarian, F.; Pashirzad, M.; Khazaei, M.; Rezayi, M.; Hassanian, S.M.; Ferns, G.A.; Avan, A. The clinical impact of exosomes in cardiovascular disorders: From basic science to clinical application. J. Cell. Physiol. 2019, 234, 12226–12236. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Colleran, R.; Braun, S.; Cassese, S.; Hieber, J.; Fusaro, M.; Kufner, S.; Ott, I.; Byrne, R.A.; Husser, O. High-sensitivity troponin T and mortality after elective percutaneous coronary intervention. J. Am. Coll. Cardiol. 2016, 68, 2259–2268. [Google Scholar] [CrossRef]
- Ibrahim, A.G.-E.; Cheng, K.; Marbán, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Saha, P.; Sharma, S.; Korutla, L.; Datla, S.R.; Shoja-Taheri, F.; Mishra, R.; Bigham, G.E.; Sarkar, M.; Morales, D.; Bittle, G. Circulating exosomes derived from transplanted progenitor cells aid the functional recovery of ischemic myocardium. Sci. Transl. Med. 2019, 11, eaau1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.; Hosen, M.R.; Zietzer, A.; Flender, A.; Levermann, P.; Schmitz, T.; Frühwald, D.; Goody, P.; Nickenig, G. Atherosclerotic conditions promote the packaging of functional microRNA-92a-3p into endothelial microvesicles. Circ. Res. 2019, 124, 575–587. [Google Scholar] [CrossRef]
- Li, H.; Liao, Y.; Gao, L.; Zhuang, T.; Huang, Z.; Zhu, H.; Ge, J. Coronary serum exosomes derived from patients with myocardial ischemia regulate angiogenesis through the miR-939-mediated nitric oxide signaling pathway. Theranostics 2018, 8, 2079. [Google Scholar] [CrossRef]
- Cheow, E.S.H.; Cheng, W.C.; Lee, C.N.; De Kleijn, D.; Sorokin, V.; Sze, S.K. Plasma-derived extracellular vesicles contain predictive biomarkers and potential therapeutic targets for myocardial ischemic (MI) injury. Mol. Cell. Proteom. 2016, 15, 2628–2640. [Google Scholar] [CrossRef]
- Bi, S.; Wang, C.; Jin, Y.; Lv, Z.; Xing, X.; Lu, Q. Correlation between serum exosome derived miR-208a and acute coronary syndrome. Int. J. Clin. Exp. Med. 2015, 8, 4275. [Google Scholar]
- Xue, R.; Tan, W.; Wu, Y.; Dong, B.; Xie, Z.; Huang, P.; He, J.; Dong, Y.; Liu, C. Role of exosomal miRNAs in heart failure. Front. Cardiovasc. Med. 2020, 7, 592412. [Google Scholar] [CrossRef] [PubMed]
- Goren, Y.; Kushnir, M.; Zafrir, B.; Tabak, S.; Lewis, B.S.; Amir, O. Serum levels of microRNAs in patients with heart failure. Eur. J. Heart Fail. 2012, 14, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, Y.; Du, Y.; Tao, J.; Li, W.; Zhou, Z.; Yang, Z. Circulating exosomal miR-92b-5p is a promising diagnostic biomarker of heart failure with reduced ejection fraction patients hospitalized for acute heart failure. J. Thorac. Dis. 2018, 10, 6211–6220. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Sakata, Y.; Suna, S.; Nakatani, D.; Usami, M.; Hara, M.; Kitamura, T.; Hamasaki, T.; Nanto, S.; Kawahara, Y.; et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ. Res. 2013, 113, 322–326. [Google Scholar] [CrossRef]
- Hir, S.R.; Alizadeh, Z.; Mazinani, M.; Rad, M.M.; Fazlollahi, M.R.; Kazemnejad, A.; Hosseini, A.Z.; Moin, M. Exosomal MICRORNAS as biomarkers in allergic asthma. Iran. J. Allergy Asthma Immunol. 2021, 20, 160–168. [Google Scholar]
- Nagano, T.; Katsurada, M.; Dokuni, R.; Hazama, D.; Kiriu, T.; Umezawa, K.; Kobayashi, K.; Nishimura, Y. Crucial role of extracellular vesicles in bronchial asthma. Int. J. Mol. Sci. 2019, 20, 2589. [Google Scholar] [CrossRef]
- Levänen, B.; Bhakta, N.R.; Paredes, P.T.; Barbeau, R.; Hiltbrunner, S.; Pollack, J.L.; Sköld, C.M.; Svartengren, M.; Grunewald, J.; Gabrielsson, S. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J. Allergy Clin. Immunol. 2013, 131, 894–903.e898. [Google Scholar] [CrossRef]
- Alhamdan, F.; Greulich, T.; Daviaud, C.; Marsh, L.M.; Pedersen, F.; Thölken, C.; Pfefferle, P.I.; Bahmer, T.; Potaczek, D.P.; Tost, J. Identification of extracellular vesicle microRNA signatures specifically linked to inflammatory and metabolic mechanisms in obesity-associated low type-2 asthma. Allergy 2023, 78, 2944–2958. [Google Scholar] [CrossRef]
- McDowell, P.J.; Heaney, L.G. Different endotypes and phenotypes drive the heterogeneity in severe asthma. Allergy 2020, 75, 302–310. [Google Scholar] [CrossRef]
- Vázquez-Mera, S.; Martelo-Vidal, L.; Miguéns-Suárez, P.; Saavedra-Nieves, P.; Arias, P.; González-Fernández, C.; Mosteiro-Añón, M.; Corbacho-Abelaira, M.D.; Blanco-Aparicio, M.; Méndez-Brea, P. Serum exosome inflamma-miRs are surrogate biomarkers for asthma phenotype and severity. Allergy 2023, 78, 141–155. [Google Scholar] [CrossRef]
- Cottin, V.; Hirani, N.A.; Hotchkin, D.L.; Nambiar, A.M.; Ogura, T.; Otaola, M.; Skowasch, D.; Park, J.S.; Poonyagariyagorn, H.K.; Wuyts, W. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018, 27, 180076. [Google Scholar] [CrossRef] [PubMed]
- Njock, M.-S.; Guiot, J.; Henket, M.A.; Nivelles, O.; Thiry, M.; Dequiedt, F.; Corhay, J.-L.; Louis, R.E.; Struman, I. Sputum exosomes: Promising biomarkers for idiopathic pulmonary fibrosis. Thorax 2019, 74, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Cambier, M.; Boeckx, A.; Henket, M.; Nivelles, O.; Gester, F.; Louis, E.; Malaise, M.; Dequiedt, F.; Louis, R. Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p. Thorax 2020, 75, 870–881. [Google Scholar] [CrossRef]
- Motawi, T.K.; Mohamed, M.R.; Shahin, N.N.; Ali, M.A.; Azzam, M.A. Time-course expression profile and diagnostic potential of a miRNA panel in exosomes and total serum in acute liver injury. Int. J. Biochem. Cell Biol. 2018, 100, 11–21. [Google Scholar] [CrossRef]
- Lv, X.-F.; Zhang, A.-Q.; Liu, W.-Q.; Zhao, M.; Li, J.; He, L.; Cheng, L.; Sun, Y.-F.; Qin, G.; Lu, P. Liver injury changes the biological characters of serum small extracellular vesicles and reprograms hepatic macrophages in mice. World J. Gastroenterol. 2021, 27, 7509. [Google Scholar] [CrossRef] [PubMed]
- Gim, J.-A.; Bang, S.M.; Lee, Y.-S.; Lee, Y.; Yim, S.Y.; Jung, Y.K.; Kim, H.; Kim, B.-H.; Kim, J.H.; Seo, Y.S. Evaluation of the severity of nonalcoholic fatty liver disease through analysis of serum exosomal miRNA expression. PLoS ONE 2021, 16, e0255822. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Pan, H.-T. microRNA profiles of serum exosomes derived from children with nonalcoholic fatty liver. Genes Genom. 2022, 44, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-Q.; Lee, D.; Kim, Y.; Bang, G.; Cho, K.; Lee, Y.-S.; Yeon, J.E.; Lubman, D.M.; Kim, J. Label-free quantitative proteomic analysis of serum extracellular vesicles differentiating patients of alcoholic and nonalcoholic fatty liver diseases. J. Proteom. 2021, 245, 104278. [Google Scholar] [CrossRef]
- Scavo, M.P.; Depalo, N.; Rizzi, F.; Carrieri, L.; Serino, G.; Franco, I.; Bonfiglio, C.; Pesole, P.L.; Cozzolongo, R.; Gianuzzi, V. Exosomal fzd-7 expression is modulated by different lifestyle interventions in patients with nafld. Nutrients 2022, 14, 1133. [Google Scholar] [CrossRef]
- Szabo, G.; Bala, S. Reply: To PMID 22684891. Hepatology 2013, 57, 2547. [Google Scholar] [CrossRef]
- Yang, Z.; Ross, R.A.; Zhao, S.; Tu, W.; Liangpunsakul, S.; Wang, L. LncRNA AK054921 and AK128652 are potential serum biomarkers and predictors of patient survival with alcoholic cirrhosis. Hepatol. Commun. 2017, 1, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Momen-Heravi, F.; Furi, I.; Kodys, K.; Catalano, D.; Gangopadhyay, A.; Haraszti, R.; Satishchandran, A.; Iracheta-Vellve, A.; Adejumo, A. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology 2018, 67, 1986–2000. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ree, M.H.; Jansen, L.; Kruize, Z.; Van Nuenen, A.C.; Van Dort, K.A.; Takkenberg, R.B.; Reesink, H.W.; Kootstra, N.A. Plasma microRNA levels are associated with hepatitis B e antigen status and treatment response in chronic hepatitis B patients. J. Infect. Dis. 2017, 215, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Duan, J.; Ma, Y.; Shen, Z.; Wei, L.; Cui, X.; Zhang, J.; Xie, Y.; Liu, J. Quantitative proteomic analysis of exosome protein content changes induced by hepatitis B virus in Huh-7 cells using SILAC labeling and LC–MS/MS. J. Proteome Res. 2014, 13, 5391–5402. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Q.; Huang, C.; Xu, W.; Li, Q.; Chen, L.; Huang, Y. Using next-generation sequencing to identify novel exosomal miRNAs as biomarkers for significant hepatic fibrosis. Discov. Med. 2021, 31, 147–159. [Google Scholar]
- Fan, Z.; Zhang, Q.; Chen, H.; He, P.; Li, Y.; Si, M.; Jiao, X. Circulating microRNAs as a biomarker to predict therapy efficacy in hepatitis C patients with different genotypes. Microb. Pathog. 2017, 112, 320–326. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Chang, Y.; Han, J.-A.; Kang, S.M.; Jeong, S.W.; Ryu, T.; Park, H.S.; Yoo, J.-J.; Lee, S.H.; Kim, S.G.; Kim, Y.S. Clinical impact of serum exosomal microRNA in liver fibrosis. PLoS ONE 2021, 16, e0255672. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, Z.; Qin, S.; Ruan, X.; Jiang, H. Serum-derived miR-574-5p-containing exosomes contribute to liver fibrosis by activating hepatic stellate cells. Mol. Biol. Rep. 2022, 49, 1945–1954. [Google Scholar] [CrossRef]
- Jiménez-Alesanco, A.; Marcuello, M.; Pastor-Jiménez, M.; López-Puerto, L.; Bonjoch, L.; Gironella, M.; Carrascal, M.; Abian, J.; de-Madaria, E.; Closa, D. Acute pancreatitis promotes the generation of two different exosome populations. Sci. Rep. 2019, 9, 19887. [Google Scholar] [CrossRef]
- Alemayehu, M. Participatory Forest Management for Balancing the Trade Off Between Livelihood Improvement and Forest Conservation. Master’s Thesis, Wondo Genet College of Forestry and Natural, Resources Hawassa University, Wondo Genet, Ethiopia, 2014. [Google Scholar]
- Jia, Y.-C.; Ding, Y.-X.; Mei, W.-T.; Wang, Y.-T.; Zheng, Z.; Qu, Y.-X.; Liang, K.; Li, J.; Cao, F.; Li, F. Extracellular vesicles and pancreatitis: Mechanisms, status and perspectives. Int. J. Biol. Sci. 2021, 17, 549. [Google Scholar] [CrossRef]
- Lai, X.; Wang, M.; McElyea, S.D.; Sherman, S.; House, M.; Korc, M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017, 393, 86–93. [Google Scholar] [CrossRef]
- Nakamura, S.; Sadakari, Y.; Ohtsuka, T.; Okayama, T.; Nakashima, Y.; Gotoh, Y.; Saeki, K.; Mori, Y.; Nakata, K.; Miyasaka, Y.; et al. Pancreatic juice exosomal microRNAs as biomarkers for detection of pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 2104–2111. [Google Scholar] [CrossRef]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torrado, R.; Rantsiou, K.; Perrone, B.; Navarro-Tapia, E.; Querol, A.; Cocolin, L. Ecological interactions among Saccharomyces cerevisiae strains: Insight into the dominance phenomenon. Sci. Rep. 2017, 7, 43603. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, L.; Parmar, N.; Kumar, S.; Nanjundan, J.; Singh, G.; Thakur, A.K. Molecular characterization and genetic diversity analysis in Indian mustard (Brassica juncea L. Czern & Coss.) varieties using SSR markers. PLoS ONE 2022, 17, e0272914. [Google Scholar]
- Sonoda, H.; Yokota-Ikeda, N.; Oshikawa, S.; Kanno, Y.; Yoshinaga, K.; Uchida, K.; Ueda, Y.; Kimiya, K.; Uezono, S.; Ueda, A. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am. J. Physiol.-Ren. Physiol. 2009, 297, F1006–F1016. [Google Scholar] [CrossRef]
- Asvapromtada, S.; Sonoda, H.; Kinouchi, M.; Oshikawa, S.; Takahashi, S.; Hoshino, Y.; Sinlapadeelerdkul, T.; Yokota-Ikeda, N.; Matsuzaki, T.; Ikeda, M. Characterization of urinary exosomal release of aquaporin-1 and-2 after renal ischemia-reperfusion in rats. Am. J. Physiol.-Ren. Physiol. 2018, 314, F584–F601. [Google Scholar] [CrossRef]
- Lv, L.-L.; Feng, Y.; Wu, M.; Wang, B.; Li, Z.-L.; Zhong, X.; Wu, W.-J.; Chen, J.; Ni, H.-F.; Tang, T.-T. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020, 27, 210–226. [Google Scholar] [CrossRef]
- Andersen, H.; Friis, U.G.; Hansen, P.B.; Svenningsen, P.; Henriksen, J.E.; Jensen, B.L. Diabetic nephropathy is associated with increased urine excretion of proteases plasmin, prostasin and urokinase and activation of amiloride-sensitive current in collecting duct cells. Nephrol. Dial. Transplant. 2015, 30, 781–789. [Google Scholar] [CrossRef]
- Zubiri, I.; Posada-Ayala, M.; Sanz-Maroto, A.; Calvo, E.; Martin-Lorenzo, M.; Gonzalez-Calero, L.; de la Cuesta, F.; Lopez, J.A.; Fernandez-Fernandez, B.; Ortiz, A. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J. Proteom. 2014, 96, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Nicoletti, M.C.; Carmosino, M.; Mastrofrancesco, L.; Di Franco, A.; Indrio, F.; Lella, R.; Laviola, L.; Giorgino, F.; Svelto, M. Urinary excretion of kidney aquaporins as possible diagnostic biomarker of diabetic nephropathy. J. Diabetes Res. 2017, 2017, 4360357. [Google Scholar] [CrossRef] [PubMed]
- Delić, D.; Eisele, C.; Schmid, R.; Baum, P.; Wiech, F.; Gerl, M.; Zimdahl, H.; Pullen, S.S.; Urquhart, R. Urinary exosomal miRNA signature in type II diabetic nephropathy patients. PLoS ONE 2016, 11, e0150154. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Forner, M.J.; Pinto, C.; Chaves, F.J.; Cortes, R.; Redon, J. Increased urinary exosomal microRNAs in patients with systemic lupus erythematosus. PLoS ONE 2015, 10, e0138618. [Google Scholar] [CrossRef]
- Solé, C.; Cortés-Hernández, J.; Felip, M.L.; Vidal, M.; Ordi-Ros, J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol. Dial. Transplant. 2015, 30, 1488–1496. [Google Scholar] [CrossRef]
- Tangtanatakul, P.; Klinchanhom, S.; Sodsai, P.; Sutichet, T.; Promjeen, C.; Avihingsanon, Y.; Hirankarn, N. Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac. J. Allergy Immunol. 2019, 37, 189–197. [Google Scholar] [PubMed]
- Li, Y.; Xu, X.; Tang, X.; Bian, X.; Shen, B.; Zhao, H.; Luo, S.; Chen, Z.; Zhang, K. MicroRNA expression profile of urinary exosomes in Type IV lupus nephritis complicated by cellular crescent. J. Biol. Res.-Thessalon. 2018, 25, 16. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015, 11, 600–607. [Google Scholar] [CrossRef]
- Dutta, S.; Hornung, S.; Kruayatidee, A.; Maina, K.N.; Del Rosario, I.; Paul, K.C.; Wong, D.Y.; Duarte Folle, A.; Markovic, D.; Palma, J.-A. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol. 2021, 142, 495–511. [Google Scholar] [CrossRef]
- Meloni, M.; Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bolognesi, E.; Picciolini, S.; Marano, M.; Magliozzi, A.; Di Fonzo, A.; Arighi, A. Oligomeric α-synuclein and tau aggregates in NDEVs differentiate Parkinson's disease from atypical parkinsonisms. Neurobiol. Dis. 2023, 176, 105947. [Google Scholar] [CrossRef]
- Citterio, L.A.; Mancuso, R.; Agostini, S.; Meloni, M.; Clerici, M. Serum and exosomal miR-7-1-5p and miR-223-3p as possible biomarkers for Parkinson’s disease. Biomolecules 2023, 13, 865. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Shim, K.H.; Kim, D.; Bae, H.; Jeong, D.-E.; Kang, M.J.; An, S.S.A. Assessment of acetylcholinesterase activity in CD9-positive exosomes from patients with Parkinson’s disease. Front. Aging Neurosci. 2024, 16, 1332455. [Google Scholar] [CrossRef]
- Shim, K.H.; Go, H.G.; Bae, H.; Jeong, D.-E.; Kim, D.; Youn, Y.C.; Kim, S.; An, S.S.A.; Kang, M.J. Decreased exosomal acetylcholinesterase activity in the plasma of patients with Parkinson’s disease. Front. Aging Neurosci. 2021, 13, 665400. [Google Scholar]
- Bhattacharyya, P.; Biswas, A.; Biswas, S.C. Brain-enriched miR-128: Reduced in exosomes from Parkinson’s patient plasma, improves synaptic integrity, and prevents 6-OHDA mediated neuronal apoptosis. Front. Cell. Neurosci. 2023, 16, 1037903. [Google Scholar] [CrossRef] [PubMed]
- Chiasserini, D.; van Weering, J.R.; Piersma, S.R.; Pham, T.V.; Malekzadeh, A.; Teunissen, C.E.; de Wit, H.; Jiménez, C.R. Proteomic analysis of cerebrospinal fluid extracellular vesicles: A comprehensive dataset. J. Proteom. 2014, 106, 191–204. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Kapogiannis, D. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 2015, 85, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef]
- Minagar, A.; Jy, W.; Jimenez, J.; Sheremata, W.; Mauro, L.; Mao, W.; Horstman, L.; Ahn, Y. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 2001, 56, 1319–1324. [Google Scholar] [CrossRef]
- Giovannelli, I.; Martelli, F.; Repice, A.; Massacesi, L.; Azzi, A.; Giannecchini, S. Detection of JCPyV microRNA in blood and urine samples of multiple sclerosis patients under natalizumab therapy. J. Neurovirol. 2015, 21, 666–670. [Google Scholar] [CrossRef]
- Barnett, M.; Beadnall, H.; Buckland, M.; Devenney, E.; Ebrahimkhani, S.; Hawke, S.; Vafaee, F.; Hur, S.S.; Suter, C.; Young, P. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci. Rep. 2017, 7, 14293. [Google Scholar]
- Kimura, K.; Hohjoh, H.; Fukuoka, M.; Sato, W.; Oki, S.; Tomi, C.; Yamaguchi, H.; Kondo, T.; Takahashi, R.; Yamamura, T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat. Commun. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Huang, J.; Qu, M.; Zhang, Y.; Geng, J.; Zhang, Z.; Liu, J.; Yang, G.Y. Increased Circulating Exosomal miRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shang, X.; Guo, M.; Su, L.; Wang, J. Exosomes in the diagnosis of neuropsychiatric diseases: A review. Biology 2024, 13, 387. [Google Scholar] [CrossRef]
- Spreafico, M.; Grillo, B.; Rusconi, F.; Battaglioli, E.; Venturin, M. Multiple layers of CDK5R1 regulation in Alzheimer’s disease implicate long non-coding RNAs. Int. J. Mol. Sci. 2018, 19, 2022. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yang, Y.; Zhao, G.; Zhang, Y.; Sun, Y.; Liao, Y.; Kang, Z.; Feng, X.; Sun, J.; Yue, W. The association of redox regulatory drug target genes with psychiatric disorders: A mendelian randomization study. Antioxidants 2024, 13, 398. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Q.; Zhang, Y.; Guan, X.; Xiu, M.; Zhang, X. Superoxide dismutase, BDNF, and cognitive improvement in drug-naive first-episode patients with schizophrenia: A 12-week longitudinal study. Int. J. Neuropsychopharmacol. 2022, 25, 128–135. [Google Scholar] [CrossRef]
- Zhao, P.; Shi, W.; Ye, Y.; Xu, K.; Hu, J.; Chao, H.; Tao, Z.; Xu, L.; Gu, W.; Zhang, L. Atox1 protects hippocampal neurons after traumatic brain injury via DJ-1 mediated anti-oxidative stress and mitophagy. Redox Biol. 2024, 72, 103156. [Google Scholar] [CrossRef]
- Nam, Y.; Na, J.; Ma, S.-X.; Park, H.; Park, H.; Lee, E.; Kim, H.; Jang, S.-M.; Ko, H.S.; Kim, S. DJ-1 protects cell death from a mitochondrial oxidative stress due to GBA1 deficiency. Genes Genom. 2024, 46, 519–529. [Google Scholar] [CrossRef]
- Tsoporis, J.N.; Ektesabi, A.M.; Gupta, S.; Izhar, S.; Salpeas, V.; Rizos, I.K.; Kympouropoulos, S.P.; Dos Santos, C.C.; Parker, T.G.; Rizos, E. A longitudinal study of alterations of circulating DJ-1 and miR203a-3p in association to olanzapine medication in a sample of first episode patients with schizophrenia. J. Psychiatr. Res. 2022, 146, 109–117. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Dobrowolny, H.; Tran, J.; Mustapic, M.; Frodl, T.; Meyer-Lotz, G.; Schiltz, K.; Schanze, D.; Rietschel, M.; Bernstein, H.-G. Insulin-signaling abnormalities in drug-naive first-episode schizophrenia: Transduction protein analyses in extracellular vesicles of putative neuronal origin. Eur. Psychiatry 2019, 62, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Prigerson, H.G.; Boelen, P.A.; Xu, J.; Smith, K.V.; Maciejewski, P.K. Validation of the new DSM-5-TR criteria for prolonged grief disorder and the PG-13-Revised (PG-13-R) scale. World Psychiatry 2021, 20, 96–106. [Google Scholar] [CrossRef]
- Jiang, M.; Gu, Y.-f.; Cai, J.-f.; Wang, A.; He, Y.; Feng, Y.-l. MiR-186-5p dysregulation leads to depression-like behavior by de-repressing SERPINF1 in hippocampus. Neuroscience 2021, 479, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Gong, P.; Han, S.; Zhang, J.; Zhang, S.; Zhang, B.; Lin, Y.; Xu, K.; Wen, G.; Liu, K. Reduced cerebral cortex thickness is related to overexpression of exosomal miR-146a-5p in medication-free patients with major depressive disorder. Psychol. Med. 2023, 53, 6253–6260. [Google Scholar] [CrossRef]
- Li, L.-D.; Naveed, M.; Du, Z.-W.; Ding, H.; Gu, K.; Wei, L.-L.; Zhou, Y.-P.; Meng, F.; Wang, C.; Han, F. Abnormal expression profile of plasma-derived exosomal microRNAs in patients with treatment-resistant depression. Hum. Genom. 2021, 15, 55. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Baniahmad, A.; Taheri, M.; Samadian, M. A review on the role of DANCR in the carcinogenesis. Cancer Cell Int. 2022, 22, 194. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef]
- Banigan, M.G.; Kao, P.F.; Kozubek, J.A.; Winslow, A.R.; Medina, J.; Costa, J.; Schmitt, A.; Schneider, A.; Cabral, H.; Cagsal-Getkin, O. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE 2013, 8, e48814. [Google Scholar] [CrossRef]
- Ceylan, D.; Tufekci, K.U.; Keskinoglu, P.; Genc, S.; Ozerdem, A. Circulating exosomal microRNAs in bipolar disorder. J. Affect. Dısorders 2020, 262, 99–107. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Salomon, C.; Kobayashi, M.; Ashman, K.; Sobrevia, L.; Mitchell, M.D.; Rice, G.E. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS ONE 2013, 8, e79636. [Google Scholar] [CrossRef]
- Salomon, C.; Torres, M.J.; Kobayashi, M.; Scholz-Romero, K.; Sobrevia, L.; Dobierzewska, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS ONE 2014, 9, e98667. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; Maharaj, N.; Moodley, J.; Mackraj, I. Placental exosomes and pre-eclampsia: Maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta 2016, 46, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Biró, O.; Alasztics, B.; Molvarec, A.; Joó, J.; Nagy, B.; Rigó Jr, J. Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension. Pregnancy Hypertens. 2017, 10, 207–212. [Google Scholar] [CrossRef]
- Nikolaeva, M.G.; Terekhina, V.U.; Kudinov, A.V.; Momot, A.P. The Role of Extracellular Vesicles of Various Origins in the Development of Preeclampsia. Ann. Russ. Acad. Med. Sci. 2021, 76, 237–243. [Google Scholar] [CrossRef]
- Gong, S.; Gaccioli, F.; Dopierala, J.; Sovio, U.; Cook, E.; Volders, P.-J.; Martens, L.; Kirk, P.D.; Richardson, S.; Smith, G.C. The RNA landscape of the human placenta in health and disease. Nat. Commun. 2021, 12, 2639. [Google Scholar] [CrossRef]
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.K.; Sheller-Miller, S.; Salomon, C.; Team, G.S. Circulating exosomal miRNA profile during term and preterm birth pregnancies: A longitudinal study. Endocrinology 2019, 160, 249–275. [Google Scholar] [CrossRef]
- Jiang, P.-Y.; Zhu, X.-J.; Jiang, R.-A.; Zhang, Y.-N.; Liu, L.; Yang, X.-F. MicroRNAs derived from urinary exosomes act as novel biomarkers in the diagnosis of intrahepatic cholestasis of pregnancy. Am. J. Transl. Res. 2019, 11, 6249. [Google Scholar]
- Nielsen, M.R.; Frederiksen-Møller, B.; Zachar, R.; Jørgensen, J.S.; Hansen, M.R.; Ydegaard, R.; Svenningsen, P.; Buhl, K.; Jensen, B.L. Urine exosomes from healthy and hypertensive pregnancies display elevated level of α-subunit and cleaved α-and γ-subunits of the epithelial sodium channel—ENaC. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 1107–1119. [Google Scholar] [CrossRef]
- Liu, Q.; Rojas-Canales, D.M.; Divito, S.J.; Shufesky, W.J.; Stolz, D.B.; Erdos, G.; Sullivan, M.L.; Gibson, G.A.; Watkins, S.C.; Larregina, A.T. Donor dendritic cell–derived exosomes promote allograft-targeting immune response. J. Clin. Investig. 2016, 126, 2805–2820. [Google Scholar] [CrossRef]
- Gonzalez-Nolasco, B.; Wang, M.; Prunevieille, A.; Benichou, G. Emerging role of exosomes in allorecognition and allograft rejection. Curr. Opin. Organ. Transplant. 2018, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.E.; Bracamonte-Baran, W.; Burlingham, W.J. Donor-derived exosomes: The trick behind the semidirect pathway of allorecognition. Curr. Opin. Organ. Transplant. 2017, 22, 46–54. [Google Scholar] [CrossRef]
- Vallabhajosyula, P.; Korutla, L.; Habertheuer, A.; Yu, M.; Rostami, S.; Yuan, C.-X.; Reddy, S.; Liu, C.; Korutla, V.; Koeberlein, B. Tissue-specific exosome biomarkers for noninvasively monitoring immunologic rejection of transplanted tissue. J. Clin. Investig. 2017, 127, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, M.; Xu, Z.; Nayak, D.; Sharma, M.; Hachem, R.; Walia, R.; Bremner, R.; Smith, M.; Mohanakumar, T. Donor-derived exosomes with lung self-antigens in human lung allograft rejection. Am. J. Transplant. 2017, 17, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Gregson, A.L.; Hoji, A.; Injean, P.; Poynter, S.T.; Briones, C.; Palchevskiy, V.; Sam Weigt, S.; Shino, M.Y.; Derhovanessian, A.; Sayah, D. Altered exosomal RNA profiles in bronchoalveolar lavage from lung transplants with acute rejection. Am. J. Respir. Crit. Care Med. 2015, 192, 1490–1503. [Google Scholar] [CrossRef]
- Kennel, P.J.; Saha, A.; Maldonado, D.A.; Givens, R.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Ji, R.; Yahi, A.; George, I. Serum exosomal protein profiling for the non-invasive detection of cardiac allograft rejection. J. Heart Lung Transplant. 2018, 37, 409–417. [Google Scholar] [CrossRef]
- Sukma Dewi, I.; Celik, S.; Karlsson, A.; Hollander, Z.; Lam, K.; McManus, J.-W.; Tebbutt, S.; Ng, R.; Keown, P.; McMaster, R. Exosomal miR-142-3p is increased during cardiac allograft rejection and augments vascular permeability through down-regulation of endothelial RAB11FIP2 expression. Cardiovasc. Res. 2017, 113, 440–452. [Google Scholar] [CrossRef]
- Lim, J.-H.; Lee, C.-H.; Kim, K.Y.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; Kim, Y.-L.; Baek, M.-C.; Park, J.B. Novel urinary exosomal biomarkers of acute T cell-mediated rejection in kidney transplant recipients: A cross-sectional study. PLoS ONE 2018, 13, e0204204. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, E.; Kahwaji, J.; Nast, C.C.; Li, P.; Mirocha, J.; Thomas, D.L.; Ge, S.; Vo, A.A.; Jordan, S.C. Plasma exosomes from HLA-sensitized kidney transplant recipients contain mRNA transcripts which predict development of antibody-mediated rejection. Transplantation 2017, 101, 2419–2428. [Google Scholar] [CrossRef]
- Farid, W.R.; Pan, Q.; van der Meer, A.J.; de Ruiter, P.E.; Ramakrishnaiah, V.; de Jonge, J.; Kwekkeboom, J.; Janssen, H.L.; Metselaar, H.J.; Tilanus, H.W. Hepatocyte-derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transplant. 2012, 18, 290–297. [Google Scholar] [CrossRef]
- Ma, J.; Xu, M.; Yin, M.; Hong, J.; Chen, H.; Gao, Y.; Xie, C.; Shen, N.; Gu, S.; Mo, X. Exosomal hsa-miR199a-3p promotes proliferation and migration in neuroblastoma. Front. Oncol. 2019, 9, 459. [Google Scholar] [CrossRef]
- Colletti, M.; Tomao, L.; Galardi, A.; Paolini, A.; Di Paolo, V.; De Stefanis, C.; Mascio, P.; Nazio, F.; Petrini, S.; Castellano, A. Neuroblastoma-secreted exosomes carrying miR-375 promote osteogenic differentiation of bone-marrow mesenchymal stromal cells. J. Extracell. Vesicles 2020, 9, 1774144. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Skog, J.; Nakano, I.; Pingle, S.; Kalinina, J.; Hua, W.; Kesari, S.; Mao, Y. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): A platform for glioblastoma biomarker development. PLoS ONE 2013, 8, e78115. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Bæk, R.; Jakobsen, K.; Meldgaard, P.; Folkersen, B.; Rasmussen, T.R.; Varming, K.; Jørgensen, M.; Sorensen, B. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 2016, 10, 1595–1602. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Qiao, Z.; Shang, Z.; Xia, Z.; Niu, X.; Qian, L.; Zhang, Y.; Fan, L.; Cao, C.-X. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal. Chim. Acta 2017, 982, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Hydbring, P.; De Petris, L.; Zhang, Y.; Brandén, E.; Koyi, H.; Novak, M.; Kanter, L.; Hååg, P.; Hurley, J.; Tadigotla, V. Exosomal RNA-profiling of pleural effusions identifies adenocarcinoma patients through elevated miR-200 and LCN2 expression. Lung Cancer 2018, 124, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, J.; Mei, S.; Wu, D.; Mu, Z.; Chen, B.; Xie, Y.; Ye, Y.; Liu, J. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. J. Cell. Mol. Med. 2017, 21, 1228–1236. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, W.; Bu, J.; Li, Y.; Li, R.; Nie, R.; Xiao, C.; Ma, K.; Huang, X.; Li, Y. Exosomal protein CD82 as a diagnostic biomarker for precision medicine for breast cancer. Mol. Carcinog. 2019, 58, 674–685. [Google Scholar] [CrossRef]
- Ando, W.; Kikuchi, K.; Uematsu, T.; Yokomori, H.; Takaki, T.; Sogabe, M.; Kohgo, Y.; Otori, K.; Ishikawa, S.; Okazaki, I. Novel breast cancer screening: Combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci. Rep. 2019, 9, 13595. [Google Scholar] [CrossRef]
- Fang, S.; Tian, H.; Li, X.; Jin, D.; Li, X.; Kong, J.; Yang, C.; Yang, X.; Lu, Y.; Luo, Y. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS ONE 2017, 12, e0175050. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.-G.; Lee, J.-E.; Cho, Y.-E.; Lee, S.J.; Jung, J.H.; Chae, Y.S.; Bae, H.-I.; Kim, Y.-B.; Kim, I.-S.; Park, H.Y. Identification of developmental endothelial locus-1 on circulating extracellular vesicles as a novel biomarker for early breast cancer detection. Clin. Cancer Res. 2016, 22, 1757–1766. [Google Scholar] [CrossRef]
- Moon, P.-G.; Lee, J.-E.; Cho, Y.-E.; Lee, S.J.; Chae, Y.S.; Jung, J.H.; Kim, I.-S.; Park, H.Y.; Baek, M.-C. Fibronectin on circulating extracellular vesicles as a liquid biopsy to detect breast cancer. Oncotarget 2016, 7, 40189. [Google Scholar] [CrossRef]
- Kibria, G.; Ramos, E.K.; Lee, K.E.; Bedoyan, S.; Huang, S.; Samaeekia, R.; Athman, J.J.; Harding, C.V.; Lötvall, J.; Harris, L. A rapid, automated surface protein profiling of single circulating exosomes in human blood. Sci. Rep. 2016, 6, 36502. [Google Scholar] [CrossRef]
- Chao, M.P.; Jaiswal, S.; Weissman-Tsukamoto, R.; Alizadeh, A.A.; Gentles, A.J.; Volkmer, J.; Weiskopf, K.; Willingham, S.B.; Raveh, T.; Park, C.Y. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2010, 2, 63ra94. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Gibbs, L.D.; Maji, S.; Lewis, C.M.; Suzuki, S.; Vishwanatha, J.K. Serum exosomal-annexin A2 is associated with African-American triple-negative breast cancer and promotes angiogenesis. Breast Cancer Res. 2020, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Hannafon, B.N.; Trigoso, Y.D.; Calloway, C.L.; Zhao, Y.D.; Lum, D.H.; Welm, A.L.; Zhao, Z.J.; Blick, K.E.; Dooley, W.C.; Ding, W. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016, 18, 90. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.; Conroy, T. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef]
- Kaur, S.; Smith, L.M.; Patel, A.; Menning, M.; Watley, D.C.; Malik, S.S.; Krishn, S.R.; Mallya, K.; Aithal, A.; Sasson, A.R. A combination of MUC5AC and CA19-9 improves the diagnosis of pancreatic cancer: A multicenter study. Off. J. Am. Coll. Gastroenterol.|ACG 2017, 112, 172–183. [Google Scholar] [CrossRef]
- Kitagawa, T.; Taniuchi, K.; Tsuboi, M.; Sakaguchi, M.; Kohsaki, T.; Okabayashi, T.; Saibara, T. Circulating pancreatic cancer exosomal RNA s for detection of pancreatic cancer. Mol. Oncol. 2019, 13, 212–227. [Google Scholar] [CrossRef]
- Makler, A.; Narayanan, R. Mining exosomal genes for pancreatic cancer targets. Cancer Genom. Proteom. 2017, 14, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Im, E.-J.; Moon, P.-G.; Baek, M.-C. Discovery of a diagnostic biomarker for colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer 2018, 18, 1058. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Guo, X.; Zhou, L.; Jia, Z.; Peng, Z.; Tang, Y.; Liu, W.; Zhu, B.; Wang, L. GPC 1 exosome and its regulatory mi RNA s are specific markers for the detection and target therapy of colorectal cancer. J. Cell. Mol. Med. 2017, 21, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, J.-Z.; Luo, J.-J.; Wang, Y.-Q.; Pan, Q. Exosomes in the oncobiology, diagnosis, and therapy of hepatic carcinoma: A new player of an old game. BioMed Res. Int. 2018, 2018, 2747461. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef]
- Li, Q.; Shao, Y.; Zhang, X.; Zheng, T.; Miao, M.; Qin, L.; Wang, B.; Ye, G.; Xiao, B.; Guo, J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumor Biol. 2015, 36, 2007–2012. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Liu, X.; Xu, M.; Chen, X.; Zhu, Y.; Guo, Z.; Bai, T.; Dong, L.; Wei, C. Serum and exosome long non coding RNAs as potential biomarkers for hepatocellular carcinoma. J. Cancer 2018, 9, 2631. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Wang, C.-Y.; Chen, K.-Y.; Huang, L.-T. Urinary exosomal thyroglobulin in thyroid cancer patients with post-ablative therapy: A new biomarker in thyroid cancer. Front. Endocrinol. 2020, 11, 382. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Cipolla, C.; Graceffa, G.; Barone, R.; Bucchieri, F.; Bulone, D.; Cabibi, D.; Campanella, C.; Marino Gammazza, A.; Pitruzzella, A. Immunomorphological pattern of molecular chaperones in normal and pathological thyroid tissues and circulating exosomes: Potential use in clinics. Int. J. Mol. Sci. 2019, 20, 4496. [Google Scholar] [CrossRef]
- Lee, J.C.; Zhao, J.-T.; Gundara, J.; Serpell, J.; Bach, L.A.; Sidhu, S. Papillary thyroid cancer–derived exosomes contain miRNA-146b and miRNA-222. J. Surg. Res. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Pan, Q.; Zhao, J.; Li, M.; Liu, X.; Xu, Y.; Li, W.; Wu, S.; Su, Z. Exosomal miRNAs are potential diagnostic biomarkers between malignant and benign thyroid nodules based on next-generation sequencing. Carcinogenesis 2020, 41, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, R.; Burdakov, V.; Shtam, T.; Radzhabova, Z.; Vasilyev, D.; Tsyrlina, E.; Titov, S.; Ivanov, M.; Berstein, L.; Filatov, M. Plasma exosomal miR-21 and miR-181a differentiates follicular from papillary thyroid cancer. Tumor Biol. 2016, 37, 12011–12021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lv, J.; Zou, X.; Huang, Z.; Zhang, H.; Liu, Q.; Jiang, L.; Zhou, X.; Zhu, W. A three plasma microRNA signature for papillary thyroid carcinoma diagnosis in Chinese patients. Gene 2019, 693, 37–45. [Google Scholar] [CrossRef]
- Yang, C.; Wei, Y.; Yu, L.; Xiao, Y. Identification of altered circular RNA expression in serum exosomes from patients with papillary thyroid carcinoma by high-throughput sequencing. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 2785. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Tan, Y.; Guo, L.; Tang, A.; Zhao, Y. Identification of exosomal miRNA biomarkers for diagnosis of papillary thyroid cancer by small RNA sequencing. Eur. J. Endocrinol. 2020, 182, 111–121. [Google Scholar] [CrossRef]
- Rappa, G.; Puglisi, C.; Santos, M.F.; Forte, S.; Memeo, L.; Lorico, A. Extracellular vesicles from thyroid carcinoma: The new frontier of liquid biopsy. Int. J. Mol. Sci. 2019, 20, 1114. [Google Scholar] [CrossRef]
- Liang, M.; Yu, S.; Tang, S.; Bai, L.; Cheng, J.; Gu, Y.; Li, S.; Zheng, X.; Duan, L.; Wang, L. A panel of plasma exosomal miRNAs as potential biomarkers for differential diagnosis of thyroid nodules. Front. Genet. 2020, 11, 449. [Google Scholar] [CrossRef]
- Kawakami, K.; Fujita, Y.; Matsuda, Y.; Arai, T.; Horie, K.; Kameyama, K.; Kato, T.; Masunaga, K.; Kasuya, Y.; Tanaka, M. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer 2017, 17, 316. [Google Scholar] [CrossRef]
- Øverbye, A.; Skotland, T.; Koehler, C.J.; Thiede, B.; Seierstad, T.; Berge, V.; Sandvig, K.; Llorente, A. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget 2015, 6, 30357. [Google Scholar] [CrossRef]
- Liu, T.; Mendes, D.E.; Berkman, C.E. Functional prostate-specific membrane antigen is enriched in exosomes from prostate cancer cells. Int. J. Oncol. 2014, 44, 918–922. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015, 67, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Joncas, F.H.; Lucien, F.; Rouleau, M.; Morin, F.; Leong, H.S.; Pouliot, F.; Fradet, Y.; Gilbert, C.; Toren, P. Plasma extracellular vesicles as phenotypic biomarkers in prostate cancer patients. Prostate 2019, 79, 1767–1776. [Google Scholar] [CrossRef]
- Bhagirath, D.; Yang, T.L.; Bucay, N.; Sekhon, K.; Majid, S.; Shahryari, V.; Dahiya, R.; Tanaka, Y.; Saini, S. microRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res. 2018, 78, 1833–1844. [Google Scholar] [CrossRef]
- Logozzi, M.; Angelini, D.F.; Giuliani, A.; Mizzoni, D.; Di Raimo, R.; Maggi, M.; Gentilucci, A.; Marzio, V.; Salciccia, S.; Borsellino, G.; et al. Increased Plasmatic Levels of PSA-Expressing Exosomes Distinguish Prostate Cancer Patients from Benign Prostatic Hyperplasia: A Prospective Study. Cancers 2019, 11, 1449. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Capasso, C.; Del Prete, S.; Di Raimo, R.; Falchi, M.; Angelini, D.F.; Sciarra, A.; Maggi, M.; Supuran, C.T.; et al. Plasmatic exosomes from prostate cancer patients show increased carbonic anhydrase IX expression and activity and low pH. J. Enzym. Inhib. Med. Chem. 2020, 35, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Giuliani, A.; Maggi, M.; Sciarra, A.; Fais, S. Plasmatic Exosome Number and Size Distinguish Prostate Cancer Patients From Healthy Individuals: A Prospective Clinical Study. Front. Oncol. 2021, 11, 727317. [Google Scholar] [CrossRef]
- Huang, H.; Du, J.; Jin, B.; Pang, L.; Duan, N.; Huang, C.; Hou, J.; Yu, W.; Hao, H.; Li, H. Combination of urine exosomal mRNAs and lncRNAs as novel diagnostic biomarkers for bladder cancer. Front. Oncol. 2021, 11, 667212. [Google Scholar] [CrossRef]
- Yazarlou, F.; Modarressi, M.H.; Mowla, S.J.; Oskooei, V.K.; Motevaseli, E.; Tooli, L.F.; Nekoohesh, L.; Eghbali, M.; Ghafouri-Fard, S.; Afsharpad, M. Urinary exosomal expression of long non-coding RNAs as diagnostic marker in bladder cancer. Cancer Manag. Res. 2018, 10, 6357–6365. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Fujita, K.; Jingushi, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Ueda, Y.; Tanigawa, G.; Yoshioka, I.; Ueda, K. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget 2017, 8, 24668. [Google Scholar] [CrossRef]
- Piao, X.M.; Jeong, P.; Kim, Y.H.; Byun, Y.J.; Xu, Y.; Kang, H.W.; Ha, Y.S.; Kim, W.T.; Lee, J.Y.; Woo, S.H. Urinary cell-free microRNA biomarker could discriminate bladder cancer from benign hematuria. Int. J. Cancer 2019, 144, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gimple, R.C.; Lau, W.B.; Lau, B.; Fei, F.; Shen, Q.; Liao, X.; Li, Y.; Wang, W.; He, Y. The present and future of the mass spectrometry-based investigation of the exosome landscape. Mass. Spectrom. Rev. 2020, 39, 745–762. [Google Scholar] [CrossRef]
- De Palma, G.; Sallustio, F.; Curci, C.; Galleggiante, V.; Rutigliano, M.; Serino, G.; Ditonno, P.; Battaglia, M.; Schena, F.P. The three-gene signature in urinary extracellular vesicles from patients with clear cell renal cell carcinoma. J. Cancer 2016, 7, 1960–1967. [Google Scholar] [CrossRef]
- Zhang, W.; Ni, M.; Su, Y.; Wang, H.; Zhu, S.; Zhao, A.; Li, G. MicroRNAs in serum exosomes as potential biomarkers in clear-cell renal cell carcinoma. Eur. Urol. Focus 2018, 4, 412–419. [Google Scholar] [CrossRef]
- Xiao, C.-T.; Lai, W.-J.; Zhu, W.-A.; Wang, H. MicroRNA derived from circulating exosomes as noninvasive biomarkers for diagnosing renal cell carcinoma. OncoTargets Ther. 2020, 13, 10765–10774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Zhou, F. Biological functions and clinical applications of exosomal long non-coding RNAs in cancer. J. Cell. Mol. Med. 2020, 24, 11656–11666. [Google Scholar] [CrossRef]
- Qu, L.; Ding, J.; Chen, C.; Wu, Z.-J.; Liu, B.; Gao, Y.; Chen, W.; Liu, F.; Sun, W.; Li, X.-F. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell 2016, 29, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, M.C.; Jeong, J.-Y.; Hwang, S.; Jung, S.G.; Joo, W.D.; Park, H.; Song, S.H.; Lee, C.; Kim, T.H. Serum exosomal miRNA-145 and miRNA-200c as promising biomarkers for preoperative diagnosis of ovarian carcinomas. J. Cancer 2019, 10, 1958. [Google Scholar] [CrossRef]
- Meng, X.; Müller, V.; Milde-Langosch, K.; Trillsch, F.; Pantel, K.; Schwarzenbach, H. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget 2016, 7, 16923. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, X.; Ying, X.; Zhu, Q.; Wang, X.; Jiang, L.; Chen, X.; Wu, Y.; Wang, X. Suppression of endothelial cell migration by tumor associated macrophage-derived exosomes is reversed by epithelial ovarian cancer exosomal lncRNA. Cancer Cell Int. 2017, 17, 62. [Google Scholar] [CrossRef]
- Li, X.; Wang, X. The emerging roles and therapeutic potential of exosomes in epithelial ovarian cancer. Mol. Cancer 2017, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Zavesky, L.; Jandakova, E.; Turyna, R.; Langmeierova, L.; Weinberger, V.; Minar, L. Supernatant versus exosomal urinary microRNAs. Two fractions with different outcomes in gynaecological cancers. Neoplasma 2016, 63, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Galbo Jr, P.M.; Ciesielski, M.J.; Figel, S.; Maguire, O.; Qiu, J.; Wiltsie, L.; Minderman, H.; Fenstermaker, R.A. Circulating CD9+/GFAP+/survivin+ exosomes in malignant glioma patients following survivin vaccination. Oncotarget 2017, 8, 114722. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, W.; Yang, B.; Tian, H. ATF1 and RAS in exosomes are potential clinical diagnostic markers for cervical cancer. Cell Biochem. Funct. 2017, 35, 477–483. [Google Scholar] [CrossRef]
- Rolih, V.; Barutello, G.; Iussich, S.; De Maria, R.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. CSPG4: A prototype oncoantigen for translational immunotherapy studies. J. Transl. Med. 2017, 15, 151. [Google Scholar] [CrossRef]
- Uranowska, K.; Samadaei, M.; Kalic, T.; Pinter, M.; Breiteneder, H.; Hafner, C. A chondroitin sulfate proteoglycan 4-specific monoclonal antibody inhibits melanoma cell invasion in a spheroid model. Int. J. Oncol. 2021, 59, 70. [Google Scholar] [CrossRef]
- Pietrowska, M.; Zebrowska, A.; Gawin, M.; Marczak, L.; Sharma, P.; Mondal, S.; Mika, J.; Polańska, J.; Ferrone, S.; Kirkwood, J.M. Proteomic profile of melanoma cell-derived small extracellular vesicles in patients’ plasma: A potential correlate of melanoma progression. J. Extracell. Vesicles 2021, 10, e12063. [Google Scholar] [CrossRef]
- Xiao, D.; Ohlendorf, J.; Chen, Y.; Taylor, D.D.; Rai, S.N.; Waigel, S.; Zacharias, W.; Hao, H.; McMasters, K.M. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS ONE 2012, 7, e46874. [Google Scholar] [CrossRef]
- Guan, M.; Chen, X.; Ma, Y.; Tang, L.; Guan, L.; Ren, X.; Yu, B.; Zhang, W.; Su, B. MDA-9 and GRP78 as potential diagnostic biomarkers for early detection of melanoma metastasis. Tumor Biol. 2015, 36, 2973–2982. [Google Scholar] [CrossRef]
- Logozzi, M.; De Milito, A.; Lugini, L.; Borghi, M.; Calabrò, L.; Spada, M.; Perdicchio, M.; Marino, M.L.; Federici, C.; Iessi, E.; et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE 2009, 4, e5219. [Google Scholar] [CrossRef]
- Lutherborrow, M.; Bryant, A.; Jayaswal, V.; Agapiou, D.; Palma, C.; Yang, Y.H.; Ma, D.D. Expression profiling of cytogenetically normal acute myeloid leukemia identifies microRNAs that target genes involved in monocytic differentiation. Am. J. Hematol. 2011, 86, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.; Whiteside, T.L. Plasma-derived exosomes in acute myeloid leukemia for detection of minimal residual disease: Are we ready? Expert. Rev. Mol. Diagn. 2016, 16, 623–629. [Google Scholar] [CrossRef]
- Harshman, S.W.; Canella, A.; Ciarlariello, P.D.; Agarwal, K.; Branson, O.E.; Rocci, A.; Cordero, H.; Phelps, M.A.; Hade, E.M.; Dubovsky, J.A. Proteomic characterization of circulating extracellular vesicles identifies novel serum myeloma associated markers. J. Proteom. 2016, 136, 89–98. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Huang, Z.; Keramat, S.; Izadirad, M.; Li, Y.-D.; Bo, L.; Gharehbaghian, A.; Chen, Z.-S. The roles of exosomal microRNAs in diffuse large B-cell lymphoma: Diagnosis, prognosis, clinical application, and biomolecular mechanisms. Front. Oncol. 2022, 12, 904637. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Cao, X.; Jiang, Y.; Xu, J.; Zheng, Y.; Kang, D.; Xu, C. Circulating exosomal microRNAs as diagnostic and prognostic biomarkers in patients with diffuse large B-cell lymphoma. Hematol. Oncol. 2022, 40, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Riadi, A.; Widiyanto, S.D.; Ayuni, A.Q. Gestational Diabetes Mellitus (GDM) Screening In Pregnant Women And Diabetes In The Elderly Getasan. Jar. Lab. Medis 2023, 5, 54–61. [Google Scholar]
- Nik Mohamed Kamal, N.N.S.B.; Shahidan, W.N.S. Non-exosomal and exosomal circulatory microRNAs: Which are more valid as biomarkers? Front. Pharmacol. 2020, 10, 1500. [Google Scholar] [CrossRef]
- Liu, J.; Han, Y.; Hu, S.; Cai, Y.; Yang, J.; Ren, S.; Zhao, Y.; Lu, T.; Zhou, X.; Wang, X. Circulating exosomal MiR-107 restrains tumorigenesis in diffuse large B-cell lymphoma by targeting 14-3-3η. Front. Cell Dev. Biol. 2021, 9, 667800. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Ozer, H.G.; Lehman, A.M.; Maddocks, K.; Yu, L.; Johnson, A.J.; Byrd, J.C. Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood J. Am. Soc. Hematol. 2015, 125, 3297–3305. [Google Scholar] [CrossRef]
- Eassa, H.A. Exosomes: Double-edged weapon in cancer therapy. Curr. Pharm. Des. 2023, 29, 2366–2368. [Google Scholar] [CrossRef]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L. Monitoring HSP70 exosomes in cancer patients’ follow up: A clinical prospective pilot study. J. Extracell. Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zuñiga, F.; Rice, G.E.; Perrin, L.C.; Hooper, J.D.; Salomon, C. Tumor-derived exosomes in ovarian cancer–liquid biopsies for early detection and real-time monitoring of cancer progression. Oncotarget 2017, 8, 104687. [Google Scholar] [CrossRef]
- Armstrong, E.A.; Beal, E.W.; Chakedis, J.; Paredes, A.Z.; Moris, D.; Pawlik, T.M.; Schmidt, C.R.; Dillhoff, M.E. Exosomes in pancreatic cancer: From early detection to treatment. J. Gastrointest. Surg. 2018, 22, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Salciccia, S.; Frisenda, M.; Bevilacqua, G.; Gobbi, L.; Bucca, B.; Moriconi, M.; Viscuso, P.; Gentilucci, A.; Mariotti, G.; Cattarino, S. Exosome analysis in prostate cancer: How they can improve biomarkers’ performance. Curr. Issues Mol. Biol. 2023, 45, 6085–6096. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.G.; Beyenal, H.; Dong, W.-J. Exosomes as powerful engines in cancer: Isolation, characterization and detection techniques. Biosensors 2021, 11, 518. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, W.; Lv, M.; Fan, S.; Lu, Y.; Wu, Q.; Pi, J. Potentiality of exosomal proteins as novel cancer biomarkers for liquid biopsy. Front. Immunol. 2022, 13, 792046. [Google Scholar] [CrossRef]
- Li, J.; Liang, Y.; Zhao, X.; Wu, C. Integrating machine learning algorithms to systematically assess reactive oxygen species levels to aid prognosis and novel treatments for triple-negative breast cancer patients. Front. Immunol. 2023, 14, 1196054. [Google Scholar] [CrossRef]
- Li, B.; Kugeratski, F.G.; Kalluri, R. A novel machine learning algorithm selects proteome signature to specifically identify cancer exosomes. Elife 2024, 12, RP90390. [Google Scholar] [CrossRef]
- Wang, Z.; Du, X.; Lian, W.; Chen, J.; Hong, C.; Li, L.; Chen, D. A novel disulfidptosis-associated expression pattern in breast cancer based on machine learning. Front. Genet. 2023, 14, 1193944. [Google Scholar] [CrossRef]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.-M.; Xu, Y.-M.; Huang, L.-F.; Wang, X.-Z. Exosomes: Novel biomarkers for clinical diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Wu, Y. Recent advances in exosomal protein detection via liquid biopsy biosensors for cancer screening, diagnosis, and prognosis. AAPS J. 2018, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Shegekar, T.; Vodithala, S.; Juganavar, A. The emerging role of liquid biopsies in revolutionising cancer diagnosis and therapy. Cureus 2023, 15, e43650. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Janku, F.; Zhan, Q.; Fan, J.-B. Accessing genetic information with liquid biopsies. Trends Genet. 2015, 31, 564–575. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.; Enderle, D.; Noerholm, M.; Breakefield, X.; Skog, J. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Cheruvanky, A.; Zhou, H.; Pisitkun, T.; Kopp, J.B.; Knepper, M.A.; Yuen, P.S.; Star, R.A. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1657–F1661. [Google Scholar] [CrossRef]
- Sher, M.; Asghar, W. Development of a multiplex fully automated assay for rapid quantification of CD4+ T cells from whole blood. Biosens. Bioelectron. 2019, 142, 111490. [Google Scholar] [CrossRef]
- Boriachek, K.; Islam, M.N.; Möller, A.; Salomon, C.; Nguyen, N.T.; Hossain, M.S.A.; Yamauchi, Y.; Shiddiky, M.J. Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small 2018, 14, 1702153. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, S.; Draz, M.S.; Zarghooni, M.; Sanati-Nezhad, A.; Ghavami, S.; Shafiee, H.; Akbari, M. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosens. Bioelectron. 2017, 91, 588–605. [Google Scholar] [CrossRef] [PubMed]
- Salvati, E.; Stellacci, F.; Krol, S. Nanosensors for early cancer detection and for therapeutic drug monitoring. Nanomedicine 2015, 10, 3495–3512. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Zhao, Z.; Gao, H.; Liu, C.; Zhu, L.; Wang, C.; Yang, Y. Emerging nanotechnologies for liquid biopsy: The detection of circulating tumor cells and extracellular vesicles. Adv. Mater. 2019, 31, 1805344. [Google Scholar] [CrossRef]
- Mathew, D.G.; Beekman, P.; Lemay, S.G.; Zuilhof, H.; Le Gac, S.; van der Wiel, W.G. Electrochemical detection of tumor-derived extracellular vesicles on nanointerdigitated electrodes. Nano Lett. 2019, 20, 820–828. [Google Scholar] [CrossRef]
- Fais, S.; Logozzi, M. The Diagnostic and Prognostic Value of Plasmatic Exosome Count in Cancer Patients and in Patients with Other Pathologies. Int. J. Mol. Sci. 2024, 25, 1049. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Orefice, N.S.; Di Raimo, R.; Mizzoni, D.; Fais, S. The Importance of Detecting, Quantifying, and Characterizing Exosomes as a New Diagnostic/Prognostic Approach for Tumor Patients. Cancers 2023, 15, 2878. [Google Scholar] [CrossRef]
- Zhi, F.; Zhou, G.; Wang, S.; Shi, Y.; Peng, Y.; Shao, N.; Guan, W.; Qu, H.; Zhang, Y.; Wang, Q. A microRNA expression signature predicts meningioma recurrence. Int. J. Cancer 2013, 132, 128–136. [Google Scholar] [CrossRef]
- Drusco, A.; Bottoni, A.; Lagana, A.; Acunzo, M.; Fassan, M.; Cascione, L.; Antenucci, A.; Kumchala, P.; Vicentini, C.; Gardiman, M.P. A differentially expressed set of microRNAs in cerebro-spinal fluid (CSF) can diagnose CNS malignancies. Oncotarget 2015, 6, 20829. [Google Scholar] [CrossRef]
- Masoudi, M.S.; Mehrabian, E.; Mirzaei, H. MiR-21: A key player in glioblastoma pathogenesis. J. Cell. Biochem. 2018, 119, 1285–1290. [Google Scholar] [CrossRef]
- Jiang, L.; Mao, P.; Song, L.; Wu, J.; Huang, J.; Lin, C.; Yuan, J.; Qu, L.; Cheng, S.-Y.; Li, J. miR-182 as a prognostic marker for glioma progression and patient survival. Am. J. Pathol. 2010, 177, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Lin, C.; Gong, H.; Wang, C.; Liu, L.; Wu, J.; Tao, S.; Hu, B.; Cheng, S.-Y.; Li, M. miR-486 sustains NF-κB activity by disrupting multiple NF-κB-negative feedback loops. Cell Res. 2013, 23, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zheng, Z.; Zheng, Y.; Lu, X.; Xu, L.; Lin, L. microRNA-328 is a favorable prognostic marker in human glioma via suppressing invasive and proliferative phenotypes of malignant cells. Int. J. Neurosci. 2016, 126, 145–153. [Google Scholar] [CrossRef]
- Lan, F.; Yue, X.; Xia, T. Exosomal microRNA-210 is a potentially non-invasive biomarker for the diagnosis and prognosis of glioma. Oncol. Lett. 2020, 19, 1967–1974. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Z.; Gareev, I.; Yan, T.; Chen, X.; Ahmad, A.; Zhang, D.; Zhao, B.; Beylerli, O.; Yang, G. Exosomal miR-2276-5p in plasma is a potential diagnostic and prognostic biomarker in glioma. Front. Cell Dev. Biol. 2021, 9, 671202. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.S.; Mahjabeen, I.; Hussain, M.Z.; Rizwan, M.; Arshad, M.; Mehmood, A.; Haris, M.S.; Kayani, M.A. Role of exosomal miRNA-19a/19b and PTEN in brain tumor diagnosis. Future Oncol. 2023, 19, 1563–1576. [Google Scholar] [CrossRef]
- Bao, Z.; Zhang, N.; Niu, W.; Mu, M.; Zhang, X.; Hu, S.; Niu, C. Exosomal miR-155-5p derived from glioma stem-like cells promotes mesenchymal transition via targeting ACOT12. Cell Death Dis. 2022, 13, 725. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, B.; Peng, C.; Wang, L.; Li, C. Identification of serum exosomal miR-98–5p, miR-183–5p, miR-323–3p and miR-19b-3p as potential biomarkers for glioblastoma patients and investigation of their mechanisms. Curr. Res. Transl. Med. 2022, 70, 103315. [Google Scholar] [CrossRef]
- Zottel, A.; Šamec, N.; Kump, A.; Dall’Olio, L.R.; Pužar Dominkuš, P.; Romih, R.; Hudoklin, S.; Mlakar, J.; Nikitin, D.; Sorokin, M. Analysis of mir-9-5p, mir-124-3p, mir-21-5p, mir-138-5p, and mir-1-3p in glioblastoma cell lines and extracellular vesicles. Int. J. Mol. Sci. 2020, 21, 8491. [Google Scholar] [CrossRef]
- Zhong, F.; Huang, T.; Leng, J. Serum miR-29b as a novel biomarker for glioblastoma diagnosis and prognosis. Int. J. Clin. Exp. Pathol. 2019, 12, 4106. [Google Scholar]
- Shao, N.; Xue, L.; Wang, R.; Luo, K.; Zhi, F.; Lan, Q. miR-454-3p is an exosomal biomarker and functions as a tumor suppressor in glioma. Mol. Cancer Ther. 2019, 18, 459–469. [Google Scholar] [CrossRef]
- Santangelo, A.; Imbrucè, P.; Gardenghi, B.; Belli, L.; Agushi, R.; Tamanini, A.; Munari, S.; Bossi, A.M.; Scambi, I.; Benati, D. A microRNA signature from serum exosomes of patients with glioma as complementary diagnostic biomarker. J. Neuro-Oncol. 2018, 136, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, R.; Iinuma, H.; Dejima, H.; Sakai, T.; Uehara, H.; Matsutani, N.; Kawamura, M. Usefulness of plasma exosomal microRNA-451a as a noninvasive biomarker for early prediction of recurrence and prognosis of non-small cell lung cancer. Oncology 2018, 94, 311–323. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, W.; Shan, X.; Zhu, W.; Xu, J.; Guo, R.; Cheng, W.; Wang, F.; Qi, L.-W.; Chen, Y. A six-microRNA panel in plasma was identified as a potential biomarker for lung adenocarcinoma diagnosis. Oncotarget 2016, 8, 6513. [Google Scholar] [CrossRef] [PubMed]
- Grimolizzi, F.; Monaco, F.; Leoni, F.; Bracci, M.; Staffolani, S.; Bersaglieri, C.; Gaetani, S.; Valentino, M.; Amati, M.; Rubini, C. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci. Rep. 2017, 7, 15277. [Google Scholar] [CrossRef] [PubMed]
- Shikeeva, A.; Kekeeva, T.; Zavalishina, L.; Andreeva, Y.Y.; Zaletaev, D.; Frank, G. Expression of microRNA let-7a, miR-155, and miR-205 in tumor and tumor-adjacent histologically normal tissue in patients with non-small cell lung cancer. Arkhiv Patol. 2016, 78, 3–10. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Y.; Chen, H.; Fei, S.; Chen, D.; Cai, X.; Liu, L.; Lin, B.; Su, H.; Zhao, L. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non–small cell lung cancer using next-generation sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Chen, G.; Yan, G.; Zhang, X.; Wan, Y.; Li, Q.; Zhu, B.; Zhuo, W. Identification of a serum microRNA expression signature for detection of lung cancer, involving miR-23b, miR-221, miR-148b and miR-423-3p. Lung Cancer 2017, 114, 6–11. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2016, 8, 13048. [Google Scholar] [CrossRef]
- Gonzalez-Villasana, V.; Rashed, M.H.; Gonzalez-Cantú, Y.; Bayraktar, R.; Menchaca-Arredondo, J.L.; Vazquez-Guillen, J.M.; Rodriguez-Padilla, C.; Lopez-Berestein, G.; Resendez-Perez, D. Presence of circulating miR-145, miR-155, and miR-382 in exosomes isolated from serum of breast cancer patients and healthy donors. Dis. Markers 2019, 2019, 6852917. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Iinuma, H.; Umemoto, Y.; Yanagisawa, T.; Matsumoto, A.; Jinno, H. Exosome-encapsulated microRNA-223-3p as a minimally invasive biomarker for the early detection of invasive breast cancer. Oncol. Lett. 2018, 15, 9584–9592. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Stevic, I.; Pan, C.; Müller, V.; Oliveira-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Different signatures of miR-16, miR-30b and miR-93 in exosomes from breast cancer and DCIS patients. Sci. Rep. 2018, 8, 12974. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, M.; Huang, Z.; Zhou, X.; Liu, Q.; Xia, T.; Zhu, W. Circulating miR-532-502 cluster derived from chromosome X as biomarkers for diagnosis of breast cancer. Gene 2020, 722, 144104. [Google Scholar] [CrossRef]
- Li, M.; Zou, X.; Xia, T.; Wang, T.; Liu, P.; Zhou, X.; Wang, S.; Zhu, W. A five-miRNA panel in plasma was identified for breast cancer diagnosis. Cancer Med. 2019, 8, 7006–7017. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, Y.; Xia, T.; Zhou, X.; Huang, Z.; Zhang, H.; Zhu, W.; Ding, Q.; Wang, S. Circulating microRNAs from the miR-106a–363 cluster on chromosome X as novel diagnostic biomarkers for breast cancer. Breast Cancer Res. Treat. 2018, 170, 257–270. [Google Scholar] [CrossRef]
- Zou, X.; Xia, T.; Li, M.; Wang, T.; Liu, P.; Zhou, X.; Huang, Z.; Zhu, W. MicroRNA profiling in serum: Potential signatures for breast cancer diagnosis. Cancer Biomark. 2021, 30, 41–53. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Ma, L.-J.; Yang, H.-B.; Jing, J.-F.; Jia, M.-M.; Zhang, X.-J.; Guo, F.; Gao, J.-N. Identification of serum exosomal miR-148a as a novel prognostic biomarker for breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7303–7309. [Google Scholar]
- Rodríguez-Martínez, A.; de Miguel-Pérez, D.; Ortega, F.G.; García-Puche, J.L.; Robles-Fernández, I.; Exposito, J.; Martorell-Marugan, J.; Carmona-Sáez, P.; Garrido-Navas, M.d.C.; Rolfo, C. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res. 2019, 21, 21. [Google Scholar] [CrossRef]
- Que, R.; Ding, G.; Chen, J.; Cao, L. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J. Surg. Oncol. 2013, 11, 219. [Google Scholar] [CrossRef]
- Ali, S.; Dubaybo, H.; Brand, R.E.; Sarkar, F.H. Differential expression of microRNAs in tissues and plasma co-exists as a biomarker for pancreatic cancer. J. Cancer Sci. Ther. 2015, 7, 336. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, W.-B.; Zhou, J.; Wei, Y.; Wang, H.-M.; Liu, X.-D.; Chen, X.-C.; Wang, W.; Ye, L.; Yao, L.C. Circulating exosomal microRNAs as novel potential detection biomarkers in pancreatic cancer. Oncol. Lett. 2020, 20, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, B.; Yue, S.; Galli, U.; Rana, S.; Gross, W.; Müller, M.; Giese, N.A.; Kalthoff, H.; Becker, T.; Büchler, M.W. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int. J. Cancer 2015, 136, 2616–2627. [Google Scholar] [CrossRef]
- Li, Z.; Tao, Y.; Wang, X.; Jiang, P.; Li, J.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Zhen, P. Tumor-secreted exosomal miR-222 promotes tumor progression via regulating P27 expression and re-localization in pancreatic cancer. Cell. Physiol. Biochem. 2018, 51, 610–629. [Google Scholar] [CrossRef]
- Kawamura, S.; Iinuma, H.; Wada, K.; Takahashi, K.; Minezaki, S.; Kainuma, M.; Shibuya, M.; Miura, F.; Sano, K. Exosome-encapsulated microRNA-4525, microRNA-451a and microRNA-21 in portal vein blood is a high-sensitive liquid biomarker for the selection of high-risk pancreatic ductal adenocarcinoma patients. J. Hepato-Biliary-Pancreat. Sci. 2019, 26, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Mikamori, M.; Yamada, D.; Eguchi, H.; Hasegawa, S.; Kishimoto, T.; Tomimaru, Y.; Asaoka, T.; Noda, T.; Wada, H.; Kawamoto, K. MicroRNA-155 controls exosome synthesis and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci. Rep. 2017, 7, 42339. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Tomofuji, T.; Maruyama, T.; Yoneda, T.; Ekuni, D.; Azuma, T.; Miyai, H.; Mizuno, H.; Kato, H.; Tsutsumi, K. miR-1246 and miR-4644 in salivary exosome as potential biomarkers for pancreatobiliary tract cancer. Oncol. Rep. 2016, 36, 2375–2381. [Google Scholar] [CrossRef]
- Karimi, N.; Feizi, M.A.H.; Safaralizadeh, R.; Hashemzadeh, S.; Baradaran, B.; Shokouhi, B.; Teimourian, S. Serum overexpression of miR-301a and miR-23a in patients with colorectal cancer. J. Chin. Med. Assoc. 2019, 82, 215–220. [Google Scholar] [CrossRef]
- Cho, W.-C.; Kim, M.; Park, J.W.; Jeong, S.-Y.; Ku, J.-L. Exosomal miR-193a and let-7g accelerate cancer progression on primary colorectal cancer and paired peritoneal metastatic cancer. Transl. Oncol. 2021, 14, 101000. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Gu, R.H.; Yan, B. Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J. Cell. Biochem. 2019, 120, 1457–1463. [Google Scholar] [CrossRef]
- Min, L.; Chen, L.; Liu, S.; Yu, Y.; Guo, Q.; Li, P.; Zhu, S. Loss of circulating exosomal miR-92b is a novel biomarker of colorectal cancer at early stage. Int. J. Med. Sci. 2019, 16, 1231. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Y.; Song, X.; Song, X.; Niu, L.; Xie, L. Tumor-derived exosomal miRNA-320d as a biomarker for metastatic colorectal cancer. J. Clin. Lab. Anal. 2019, 33, e23004. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, Y.; Liang, C.; Cheng, M.; Jin, C.; Duan, Q.; Xu, D.; Yang, L.; Zhang, X.; Ren, B. Exosomal miR-6803-5p as potential diagnostic and prognostic marker in colorectal cancer. J. Cell. Biochem. 2018, 119, 4113–4119. [Google Scholar] [CrossRef]
- Liu, X.; Pan, B.; Sun, L.; Chen, X.; Zeng, K.; Hu, X.; Xu, T.; Xu, M.; Wang, S. Circulating exosomal miR-27a and miR-130a act as novel diagnostic and prognostic biomarkers of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, X.; Pan, B.; Hu, X.; Zhu, Y.; Su, Y.; Guo, Z.; Zhang, G.; Xu, M.; Xu, X. Serum exosomal miR-122 as a potential diagnostic and prognostic biomarker of colorectal cancer with liver metastasis. J. Cancer 2020, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Song, X.; Niu, L.; Tang, Y.; Song, X.; Xie, L. Circulating exosomal miR-150-5p and miR-99b-5p as diagnostic biomarkers for colorectal cancer. Front. Oncol. 2019, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Zhao, Y.; Wang, X.; Qin, L.; Hu, R. Development and validation of serum exosomal microRNAs as diagnostic and prognostic biomarkers for hepatocellular carcinoma. J. Cell. Biochem. 2019, 120, 135–142. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Q.; An, L.; Fang, G.; Hong, D.; Jiao, T.; Yang, H.; Wang, Z. Serum exosomal microRNA-370-3p and microRNA-196a-5p are potential biomarkers for the diagnosis and prognosis of hepatocellular carcinoma. Folia Histochem. Cytobiol. 2022, 60, 215–225. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zhang, P.; Guo, G.; Jiang, T.; Zhao, X.; Jiang, J.; Huang, X.; Tong, H.; Tian, Y. Serum exosomal micro RNA s combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018, 7, 1670–1679. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhowmik, S.; Majumdar, S.; Goswami, A.; Chakraborty, J.; Gupta, S.; Aggarwal, S.; Ray, S.; Chatterjee, R.; Bhattacharyya, S. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha-fetoprotein. Int. J. Cancer 2020, 147, 2934–2947. [Google Scholar] [CrossRef]
- Huang, C.; Tang, S.; Shen, D.; Li, X.; Liang, L.; Ding, Y.; Xu, B. Circulating plasma exosomal miRNA profiles serve as potential metastasis-related biomarkers for hepatocellular carcinoma. Oncol. Lett. 2021, 21, 168. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.S.; Park, N.R.; Nam, H.; Sung, P.S.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Hur, W.; Jang, J.W. Exosomal miR-125b exerts anti-metastatic properties and predicts early metastasis of hepatocellular carcinoma. Front. Oncol. 2021, 11, 637247. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-J.; Chong, Y.; Guo, Z.-W.; Xie, C.; Yang, X.-J.; Zhang, Q.; Li, S.-P.; Xiong, Y.; Yuan, Y.; Min, J. A serum microRNA classifier for early detection of hepatocellular carcinoma: A multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015, 16, 804–815. [Google Scholar] [CrossRef]
- Pu, C.; Huang, H.; Wang, Z.; Zou, W.; Lv, Y.; Zhou, Z.; Zhang, Q.; Qiao, L.; Wu, F.; Shao, S. Extracellular vesicle-associated mir-21 and mir-144 are markedly elevated in serum of patients with hepatocellular carcinoma. Front. Physiol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Hu, Z.; You, L.; Hu, S.; Yu, L.; Gao, Y.; Li, L.; Zhang, S. Hepatocellular carcinoma cell-derived exosomal miR-21-5p promotes the polarization of tumor-related macrophages (TAMs) through SP1/XBP1 and affects the progression of hepatocellular carcinoma. Int. Immunopharmacol. 2024, 126, 111149. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Feng, X.; Liu, H.; Tong, R.; Wu, J.; Li, C.; Yu, H.; Chen, Y.; Cheng, Q.; Chen, J. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene 2020, 39, 6529–6543. [Google Scholar] [CrossRef]
- Cho, H.J.; Baek, G.O.; Seo, C.W.; Ahn, H.R.; Sung, S.; Son, J.A.; Kim, S.S.; Cho, S.W.; Jang, J.W.; Nam, S.W. Exosomal microRNA-4661-5p–based serum panel as a potential diagnostic biomarker for early-stage hepatocellular carcinoma. Cancer Med. 2020, 9, 5459–5472. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Eun, J.W.; Baek, G.O.; Seo, C.W.; Ahn, H.R.; Kim, S.S.; Cho, S.W.; Cheong, J.Y. Serum exosomal microRNA, miR-10b-5p, as a potential diagnostic biomarker for early-stage hepatocellular carcinoma. J. Clin. Med. 2020, 9, 281. [Google Scholar] [CrossRef]
- Lin, J.; Lin, W.; Bai, Y.; Liao, Y.; Lin, Q.; Chen, L.; Wu, Y. Identification of exosomal hsa-miR-483-5p as a potential biomarker for hepatocellular carcinoma via microRNA expression profiling of tumor-derived exosomes. Exp. Cell Res. 2022, 417, 113232. [Google Scholar] [CrossRef]
- Sun, L.; Xu, M.; Zhang, G.; Dong, L.; Wu, J.; Wei, C.; Xu, K.; Zhang, L. Identification of Circulating Exosomal miR-101 and miR-125b Panel Act as a Potential Biomarker for Hepatocellular Carcinoma. Int. J. Genom. 2021, 2021, 1326463. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, H.-F.; Liu, M.-Y.; Xu, Y.-J.; He, J.-C.; Zhou, Y.; Cang, S.-D. Mechanism of exosomal microRNA-224 in development of hepatocellular carcinoma and its diagnostic and prognostic value. World J. Gastroenterol. 2019, 25, 1890. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, J.; Wu, J.; Ji, A.; Qiang, G.; Jiang, Y.; Jiang, C.; Ding, Y. Exosomal miR-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget 2017, 8, 80666. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Kim, J.; Kang, S.H.; Yang, S.R.; Cho, J.-Y.; Cho, H.C.; Shim, S.G.; Paik, Y.-H. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp. Mol. Med. 2015, 47, e184. [Google Scholar] [CrossRef]
- Deng, P.; Li, M.; Wu, Y. The Predictive Efficacy of Serum Exosomal microRNA-122 and microRNA-148a for Hepatocellular Carcinoma Based on Smart Healthcare. J. Healthc. Eng. 2022, 2022, 5914541. [Google Scholar] [CrossRef]
- Lee, H.; Quek, C.; Silva, I.; Tasker, A.; Batten, M.; Rizos, H.; Lim, S.Y.; Nur Gide, T.; Shang, P.; Attrill, G.H. Integrated molecular and immunophenotypic analysis of NK cells in anti-PD-1 treated metastatic melanoma patients. Oncoimmunology 2019, 8, e1537581. [Google Scholar] [CrossRef]
- Nakano, T.; Chen, I.-H.; Wang, C.-C.; Chen, P.-J.; Tseng, H.-P.; Huang, K.-T.; Hu, T.-H.; Li, L.-C.; Goto, S.; Cheng, Y.-F. Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am. J. Transplant. 2019, 19, 3250–3262. [Google Scholar] [CrossRef]
- Li, W.; Ding, X.; Wang, S.; Xu, L.; Yin, T.; Han, S.; Geng, J.; Sun, W. Downregulation of serum exosomal miR-320d predicts poor prognosis in hepatocellular carcinoma. J. Clin. Lab. Anal. 2020, 34, e23239. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Jiang, Y.; Yang, L.; Yan, S.; Wang, Y.G.; Lu, X.J. Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma. J. Cell. Biochem. 2018, 119, 4711–4716. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, J.; Zhou, K.; Chen, F.; Wang, Z.; Liao, B.; Dai, Z.; Cao, Y.; Fan, J.; Zhou, J. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. OncoTargets Ther. 2017, 10, 3843–3851. [Google Scholar] [CrossRef]
- Li, M.; Rai, A.J.; DeCastro, G.J.; Zeringer, E.; Barta, T.; Magdaleno, S.; Setterquist, R.; Vlassov, A.V. An optimized procedure for exosome isolation and analysis using serum samples: Application to cancer biomarker discovery. Methods 2015, 87, 26–30. [Google Scholar] [CrossRef]
- Endzeliņš, E.; Berger, A.; Melne, V.; Bajo-Santos, C.; Soboļevska, K.; Ābols, A.; Rodriguez, M.; Šantare, D.; Rudņickiha, A.; Lietuvietis, V. Detection of circulating miRNAs: Comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer 2017, 17, 730. [Google Scholar] [CrossRef]
- Samsonov, R.; Shtam, T.; Burdakov, V.; Glotov, A.; Tsyrlina, E.; Berstein, L.; Nosov, A.; Evtushenko, V.; Filatov, M.; Malek, A. Lectin-induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: Application for prostate cancer diagnostic. Prostate 2016, 76, 68–79. [Google Scholar] [CrossRef]
- Foj, L.; Ferrer, F.; Serra, M.; Arévalo, A.; Gavagnach, M.; Giménez, N.; Filella, X. Exosomal and non-exosomal urinary miRNAs in prostate cancer detection and prognosis. Prostate 2017, 77, 573–583. [Google Scholar] [CrossRef]
- Danarto, R.; Astuti, I.; Umbas, R.; Haryana, S.M. Urine miR-21-5p and miR-200c-3p as potential non-invasive biomarkers in patients with prostate cancer. Turk. J. Urol. 2019, 46, 26. [Google Scholar] [CrossRef] [PubMed]
- Koppers-Lalic, D.; Hackenberg, M.; De Menezes, R.; Misovic, B.; Wachalska, M.; Geldof, A.; Zini, N.; de Reijke, T.; Wurdinger, T.; Vis, A. Non-invasive prostate cancer detection by measuring miRNA variants (isomiRs) in urine extracellular vesicles. Oncotarget 2016, 7, 22566. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Y.-Y.; Wang, J.; Zeng, X.-F.; Li, R.; Kang, W.; Hao, X.-K. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. OncoTargets Ther. 2015, 9, 139–148. [Google Scholar]
- Bryant, R.; Pawlowski, T.; Catto, J.; Marsden, G.; Vessella, R.; Rhees, B.; Kuslich, C.; Visakorpi, T.; Hamdy, F. Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer 2012, 106, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Kaul, D.; Mavuduru, R.; Kakkar, N.; Bhatia, A. Urinary-exosomal miR-2909: A novel pathognomonic trait of prostate cancer severity. J. Biotechnol. 2017, 259, 135–139. [Google Scholar] [CrossRef]
- Rodríguez, M.; Bajo-Santos, C.; Hessvik, N.P.; Lorenz, S.; Fromm, B.; Berge, V.; Sandvig, K.; Linē, A.; Llorente, A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer 2017, 16, 156. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Fujita, K.; Tomiyama, E.; Hatano, K.; Hayashi, Y.; Wang, C.; Ishizuya, Y.; Yamamoto, Y.; Hayashi, T.; Kato, T. MiR-30b-3p and miR-126-3p of urinary extracellular vesicles could be new biomarkers for prostate cancer. Transl. Androl. Urol. 2021, 10, 1918. [Google Scholar] [CrossRef]
- Saleeb, R.; Kim, S.S.; Ding, Q.; Scorilas, A.; Lin, S.; Khella, H.W.; Boulos, C.; Ibrahim, G.; Yousef, G.M. The miR-200 family as prognostic markers in clear cell renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 955–963. [Google Scholar] [CrossRef]
- Kurahashi, R.; Kadomatsu, T.; Baba, M.; Hara, C.; Itoh, H.; Miyata, K.; Endo, M.; Morinaga, J.; Terada, K.; Araki, K. MicroRNA-204-5p: A novel candidate urinary biomarker of Xp11. 2 translocation renal cell carcinoma. Cancer Sci. 2019, 110, 1897–1908. [Google Scholar] [CrossRef]
- Li, D.Y.; Lin, F.F.; Li, G.P.; Zeng, F.C. Exosomal microRNA-15a from ACHN cells aggravates clear cell renal cell carcinoma via the BTG2/PI3K/AKT axis. Kaohsiung J. Med. Sci. 2021, 37, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Giridhar, K.V.; Tian, Y.; Tschannen, M.R.; Zhu, J.; Huang, C.-C.; Kilari, D.; Kohli, M.; Wang, L. Plasma exosomal miRNAs-based prognosis in metastatic kidney cancer. Oncotarget 2017, 8, 63703. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Chen, C.; Wu, Z.; Bai, P.; Li, S.; Chen, B.; Liu, R.; Zhang, K.; Li, W. Serum exosomal miR-210 as a potential biomarker for clear cell renal cell carcinoma. J. Cell. Biochem. 2019, 120, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Hirata, H.; Ueno, K.; Mori, J.; Oka, S.; Shimizu, K.; Kawai, Y.; Inoue, R.; Yamamoto, Y.; Matsumoto, H. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget 2017, 8, 109877. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wu, Q.; Wu, X.; Zhu, Q.; Wang, X.; Jiang, L.; Chen, X.; Wang, X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget 2016, 7, 43076. [Google Scholar] [CrossRef]
- Alshamrani, A.A. Roles of microRNAs in ovarian cancer tumorigenesis: Two decades later, what have we learned? Front. Oncol. 2020, 10, 1084. [Google Scholar] [CrossRef]
- Su, Y.Y.; Sun, L.; Guo, Z.R.; Li, J.C.; Bai, T.T.; Cai, X.X.; Li, W.H.; Zhu, Y.F. Upregulated expression of serum exosomal miR-375 and miR-1307 enhance the diagnostic power of CA125 for ovarian cancer. J. Ovarian Res. 2019, 12, 6. [Google Scholar] [CrossRef]
- Maeda, K.; Sasaki, H.; Ueda, S.; Miyamoto, S.; Terada, S.; Konishi, H.; Kogata, Y.; Ashihara, K.; Fujiwara, S.; Tanaka, Y. Serum exosomal microRNA-34a as a potential biomarker in epithelial ovarian cancer. J. Ovarian Res. 2020, 13, 47. [Google Scholar] [CrossRef]
- Pan, C.; Stevic, I.; Müller, V.; Ni, Q.; Oliveira-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Exosomal micro RNA s as tumor markers in epithelial ovarian cancer. Mol. Oncol. 2018, 12, 1935–1948. [Google Scholar] [CrossRef]
- Zhou, J.; Gong, G.; Tan, H.; Dai, F.; Zhu, X.; Chen, Y.; Wang, J.; Liu, Y.; Chen, P.; Wu, X. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma. Oncol. Rep. 2015, 33, 2915–2923. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Z.; Wang, M.; Zhang, M.; Chen, Y.; Yang, X.; Zhou, C.; Liu, Y.; Hong, L.; Zhang, L. Detection of plasma exosomal miRNA-205 as a biomarker for early diagnosis and an adjuvant indicator of ovarian cancer staging. J. Ovarian Res. 2022, 15, 27. [Google Scholar] [CrossRef]
- Liu, J.; Yoo, J.; Ho, J.Y.; Jung, Y.; Lee, S.; Hur, S.Y.; Choi, Y.J. Plasma-derived exosomal miR-4732-5p is a promising noninvasive diagnostic biomarker for epithelial ovarian cancer. J. Ovarian Res. 2021, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Seo, S.M.; Kim, T.W.; Ryu, J.; Kong, H.; Jang, S.H.; Jang, Y.S.; Kim, K.S.; Kim, J.H.; Ryu, S. Circulating exosomal miR-1290 for diagnosis of epithelial ovarian cancer. Curr. Issues Mol. Biol. 2022, 44, 288–300. [Google Scholar] [CrossRef]
- Chen, L.; Wang, K.; Li, L.; Zheng, B.; Zhang, Q.; Zhang, F.; Chen, J.; Wang, S. Plasma exosomal miR-1260a, miR-7977 and miR-192-5p as diagnostic biomarkers in epithelial ovarian cancer. Future Oncol. 2022, 18, 2919–2931. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Hou, L.; Ma, Y.; Zhou, L.; Wang, F.; Cheng, B.; Wang, W.; Lu, B.; Liu, P.; Lu, W. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol. Cancer 2019, 18, 76. [Google Scholar] [CrossRef]
- Cafforio, P.; Palmirotta, R.; Lovero, D.; Cicinelli, E.; Cormio, G.; Silvestris, E.; Porta, C.; D’Oronzo, S. Liquid biopsy in cervical cancer: Hopes and pitfalls. Cancers 2021, 13, 3968. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.-X.; Zhang, X.-Y.; Chen, S.-R.; Li, C.-Z. Upregulated exosomal miR-221/222 promotes cervical cancer via repressing methyl-CpG-binding domain protein 2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3645–3653. [Google Scholar]
- Liu, J.; Sun, H.; Wang, X.; Yu, Q.; Li, S.; Yu, X.; Gong, W. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int. J. Mol. Sci. 2014, 15, 758–773. [Google Scholar] [CrossRef]
- Roman-Canal, B.; Moiola, C.P.; Gatius, S.; Bonnin, S.; Ruiz-Miró, M.; González, E.; González-Tallada, X.; Llordella, I.; Hernández, I.; Porcel, J.M. EV-associated miRNAs from peritoneal lavage are a source of biomarkers in endometrial cancer. Cancers 2019, 11, 839. [Google Scholar] [CrossRef]
- Lv, A.; Tu, Z.; Huang, Y.; Lu, W.; Xie, B. Circulating exosomal miR-125a-5p as a novel biomarker for cervical cancer. Oncol. Lett. 2021, 21, 54. [Google Scholar] [CrossRef]
- Ma, G.; Song, G.; Zou, X.; Shan, X.; Liu, Q.; Xia, T.; Zhou, X.; Zhu, W. Circulating plasma microRNA signature for the diagnosis of cervical cancer. Cancer Biomark. 2019, 26, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Movahedi, M.; Rejali, M.; Maleki, F.; Moetamani-Ahmadi, M.; Seifi, S.; Hosseini, Z.; Khazaei, M.; Amerizadeh, F.; Ferns, G.A. The potential prognostic and therapeutic application of tissue and circulating microRNAs in cervical cancer. J. Cell. Physiol. 2019, 234, 1289–1294. [Google Scholar] [CrossRef]
- Mendaza, S.; Fernández-Irigoyen, J.; Santamaría, E.; Arozarena, I.; Guerrero-Setas, D.; Zudaire, T.; Guarch, R.; Vidal, A.; Salas, J.-S.; Matias-Guiu, X. Understanding the molecular mechanism of miR-877-3p could provide potential biomarkers and therapeutic targets in squamous cell carcinoma of the cervix. Cancers 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, D.; Li, H.; She, H.; Yang, Y.; Zhang, H.; Miao, G. Significance of high YKL-40 expression regulated by miR-24 in cervical cancer progression and prognosis. Int. J. Clin. Exp. Pathol. 2016, 9, 5128–5137. [Google Scholar]
- Zhang, J.; Wu, H.; Li, P.; Zhao, Y.; Liu, M.; Tang, H. NF-κB-modulated miR-130a targets TNF-α in cervical cancer cells. J. Transl. Med. 2014, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.-C.; Zhang, Z.-S.; Chen, F. MicroRNA-155 regulates cervical cancer via inducing Th17/Treg imbalance. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3719–3726. [Google Scholar]
- Srivastava, A.; Moxley, K.; Ruskin, R.; Dhanasekaran, D.N.; Zhao, Y.D.; Ramesh, R. A non-invasive liquid biopsy screening of urine-derived exosomes for miRNAs as biomarkers in endometrial cancer patients. AAPS J. 2018, 20, 82. [Google Scholar] [CrossRef]
- Fogli, S.; Polini, B.; Carpi, S.; Pardini, B.; Naccarati, A.; Dubbini, N.; Lanza, M.; Breschi, M.C.; Romanini, A.; Nieri, P. Identification of plasma microRNAs as new potential biomarkers with high diagnostic power in human cutaneous melanoma. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Stark, M.S.; Klein, K.; Weide, B.; Haydu, L.E.; Pflugfelder, A.; Tang, Y.H.; Palmer, J.M.; Whiteman, D.C.; Scolyer, R.A.; Mann, G.J. The prognostic and predictive value of melanoma-related microRNAs using tissue and serum: A microRNA expression analysis. EBioMedicine 2015, 2, 671–680. [Google Scholar] [CrossRef]
- Li, P.; He, Q.-y.; Luo, C.-q.; Qian, L.-y. Circulating miR-221 expression level and prognosis of cutaneous malignant melanoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 2472. [Google Scholar]
- Guo, W.; Wang, H.; Yang, Y.; Guo, S.; Zhang, W.; Liu, Y.; Yi, X.; Ma, J.; Zhao, T.; Liu, L. Down-regulated miR-23a contributes to the metastasis of cutaneous melanoma by promoting autophagy. Theranostics 2017, 7, 2231. [Google Scholar] [CrossRef]
- Tembe, V.; Schramm, S.J.; Stark, M.S.; Patrick, E.; Jayaswal, V.; Tang, Y.H.; Barbour, A.; Hayward, N.K.; Thompson, J.F.; Scolyer, R.A. MicroRNA and mRNA expression profiling in metastatic melanoma reveal associations with BRAF mutation and patient prognosis. Pigment. Cell Melanoma Res. 2015, 28, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Liu, T.; Qiao, L.; Gao, M.; Li, J. Decreased serum microRNA-206 level predicts unfavorable prognosis in patients with melanoma. Int. J. Clin. Exp. Pathol. 2015, 8, 3097. [Google Scholar] [PubMed]
- Meng, F.; Zhang, Y.; Li, X.; Wang, J.; Wang, Z. Clinical significance of miR-138 in patients with malignant melanoma through targeting of PDK1 in the PI3K/AKT autophagy signaling pathway. Oncol. Rep. 2017, 38, 1655–1662. [Google Scholar] [CrossRef]
- Svedman, F.C.; Lohcharoenkal, W.; Bottai, M.; Brage, S.E.; Sonkoly, E.; Hansson, J.; Pivarcsi, A.; Eriksson, H. Extracellular microvesicle microRNAs as predictive biomarkers for targeted therapy in metastastic cutaneous malignant melanoma. PLoS ONE 2018, 13, e0206942. [Google Scholar] [CrossRef]
- Pegoraro, A.; De Marchi, E.; Ferracin, M.; Orioli, E.; Zanoni, M.; Bassi, C.; Tesei, A.; Capece, M.; Dika, E.; Negrini, M. P2X7 promotes metastatic spreading and triggers release of miRNA-containing exosomes and microvesicles from melanoma cells. Cell Death Dis. 2021, 12, 1088. [Google Scholar] [CrossRef]
- Pfeffer, S.R.; Grossmann, K.F.; Cassidy, P.B.; Yang, C.H.; Fan, M.; Kopelovich, L.; Leachman, S.A.; Pfeffer, L.M. Detection of exosomal miRNAs in the plasma of melanoma patients. J. Clin. Med. 2015, 4, 2012–2027. [Google Scholar] [CrossRef]
- Tengda, L.; Shuping, L.; Mingli, G.; Jie, G.; Yun, L.; Weiwei, Z.; Anmei, D. Serum exosomal microRNAs as potent circulating biomarkers for melanoma. Melanoma Res. 2018, 28, 295–303. [Google Scholar] [CrossRef]
- Alegre, E.; Sanmamed, M.F.; Rodriguez, C.; Carranza, O.; Martín-Algarra, S.; Gonzalez, A. Study of circulating microRNA-125b levels in serum exosomes in advanced melanoma. Arch. Pathol. Lab. Med. 2014, 138, 828–832. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Wang, L.; Li, M.; Shen, M.; Zhou, Z.; Zhu, S.; Li, K.; Fang, Z.; Yan, B. The plasma exosomal miR-1180-3p serves as a novel potential diagnostic marker for cutaneous melanoma. Cancer Cell Int. 2021, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Levati, L.; Bassi, C.; Mastroeni, S.; Lupini, L.; Antonini Cappellini, G.C.; Bonmassar, L.; Alvino, E.; Caporali, S.; Lacal, P.M.; Narducci, M.G. Circulating miR-1246 and miR-485-3p as promising biomarkers of clinical response and outcome in melanoma patients treated with targeted therapy. Cancers 2022, 14, 3706. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Wu, J.; Xiao, R.; Liu, J. High serum extracellular vesicle miR-10b expression predicts poor prognosis in patients with acute myeloid leukemia. Cancer Biomark. 2020, 27, 1–9. [Google Scholar] [CrossRef]
- Jiang, L.; Deng, T.; Wang, D.; Xiao, Y. Elevated serum exosomal miR-125b level as a potential marker for poor prognosis in intermediate-risk acute myeloid leukemia. Acta Haematol. 2018, 140, 183–192. [Google Scholar] [CrossRef]
- Sun, L.-H.; Tian, D.; Yang, Z.-C.; Li, J.-L. Exosomal miR-21 promotes proliferation, invasion and therapy resistance of colon adenocarcinoma cells through its target PDCD4. Sci. Rep. 2020, 10, 8271. [Google Scholar] [CrossRef]
- Tickner, J.A.; Richard, D.J.; O’Byrne, K.J. EV, microvesicles/microRNAs and stem cells in cancer. In Exosomes, Stem Cells and MicroRNA. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; pp. 123–135. [Google Scholar]
- Park, B.; Choi, M.E.; Ryu, K.J.; Park, C.; Choi, M.; Yoon, S.E.; Kim, W.S.; Kim, H.H.; Hong, J.Y.; Kim, S.J. Exosomal miR-155-5p drives ibrutinib resistance in B-cell lymphoma. Exp. Cell Res. 2024, 442, 114248. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liu, K.-K.; Wang, N.-L.; Xie, Z.-W.; Chu, J.-H. Expression and Clinical Significance of Exosome Derived MiR-181b-5p in Children with Acute Lymphoblastic Leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2023, 31, 643–648. [Google Scholar]
- Gao, X.; Wan, Z.; Wei, M.; Dong, Y.; Zhao, Y.; Chen, X.; Li, Z.; Qin, W.; Yang, G.; Liu, L. Chronic myelogenous leukemia cells remodel the bone marrow niche via exosome-mediated transfer of miR-320. Theranostics 2019, 9, 5642. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Marchesi, F.; Palombi, F.; Pelosi, A.; Di Pace, A.L.; Sacconi, A.; Terrenato, I.; Annibali, O.; Tomarchio, V.; Marino, M. MiR-22, a serum predictor of poor outcome and therapy response in diffuse large B-cell lymphoma patients. Br. J. Haematol. 2021, 195, 399–404. [Google Scholar] [CrossRef]
- Caner, V.; Cetin, G.O.; Hacioglu, S.; Baris, I.C.; Tepeli, E.; Turk, N.S.; Bagci, G.; Yararbas, K.; Cagliyan, G. The miRNA content of circulating exosomes in DLBCL patients and in vitro influence of DLBCL-derived exosomes on miRNA expression of healthy B-cells from peripheral blood. Cancer Biomark. 2021, 32, 519–529. [Google Scholar] [CrossRef]
- Khare, D.; Goldschmidt, N.; Bardugo, A.; Gur-Wahnon, D.; Ben-Dov, I.Z.; Avni, B. Plasma microRNA profiling: Exploring better biomarkers for lymphoma surveillance. PLoS ONE 2017, 12, e0187722. [Google Scholar] [CrossRef] [PubMed]
- Inada, K.; Okoshi, Y.; Cho, Y.; Saito, H.; Iijima, T.; Hori, M.; Kojima, H. Availability of circulating microRNAs as a biomarker for early diagnosis of diffuse large B-cell lymphoma. Open J. Blood Dis. 2015, 5, 48–58. [Google Scholar] [CrossRef]
- Feng, Y.; Zhong, M.; Zeng, S.; Wang, L.; Liu, P.; Xiao, X.; Liu, Y. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics 2019, 11, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Yi, X.; Chen, X.; Wu, Z.; You, H.; Chen, X.; Zhang, G.; Sun, Y.; Bu, X.; Wu, X. Warburg effect-promoted exosomal circ_0072083 releasing up-regulates NANGO expression through multiple pathways and enhances temozolomide resistance in glioma. J. Exp. Clin. Cancer Res. 2021, 40, 164. [Google Scholar] [CrossRef]
- Xia, D.; Gu, X. Plasmatic exosome-derived circRNAs panel act as fingerprint for glioblastoma. Aging 2021, 13, 19575. [Google Scholar] [CrossRef]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Uziel, O.; Kanner, A.A.; Beery, E.; Lev, S.; Lahav, M.; Horn-Fichman, S.; Nof, S.H.; Laviv, Y.; Yust-Katz, S.; Amiel, A. Is serum-derived exosomal hTERT transcript a marker of oncogenic activity in primary brain tumors? An exploratory study. Cancer Med. 2024, 13, e6784. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Qiu, F.; Qiu, Z. Proteomic identification of exosomal LRG1: A potential urinary biomarker for detecting NSCLC. Electrophoresis 2011, 32, 1976–1983. [Google Scholar] [CrossRef]
- Galindo-Hernandez, O.; Villegas-Comonfort, S.; Candanedo, F.; Gonzalez-Vazquez, M.-C.; Chavez-Ocana, S.; Jimenez-Villanueva, X.; Sierra-Martinez, M.; Salazar, E.P. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch. Med. Res. 2013, 44, 208–214. [Google Scholar] [CrossRef]
- Rupp, A.-K.; Rupp, C.; Keller, S.; Brase, J.C.; Ehehalt, R.; Fogel, M.; Moldenhauer, G.; Marmé, F.; Sültmann, H.; Altevogt, P. Loss of EpCAM expression in breast cancer derived serum exosomes: Role of proteolytic cleavage. Gynecol. Oncol. 2011, 122, 437–446. [Google Scholar] [CrossRef]
- Khan, S.; Bennit, H.F.; Turay, D.; Perez, M.; Mirshahidi, S.; Yuan, Y.; Wall, N.R. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer 2014, 14, 176. [Google Scholar] [CrossRef]
- Ciravolo, V.; Huber, V.; Ghedini, G.C.; Venturelli, E.; Bianchi, F.; Campiglio, M.; Morelli, D.; Villa, A.; Mina, P.D.; Menard, S. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J. Cell. Physiol. 2012, 227, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol. Cancer Res. 2017, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Wang, T.; Sun, X.; Zhang, P.; Chen, Y.; Jin, J.; Hua, D. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J. Surg. Oncol. 2017, 115, 932–940. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, L.; Yue, Z.; Jia, M.; Wang, H.; Cao, L.-L. The potential of serum exosomal hsa_circ_0028861 as the novel diagnostic biomarker of HBV-derived hepatocellular cancer. Front. Genet. 2021, 12, 703205. [Google Scholar] [CrossRef]
- Chen, W.; Quan, Y.; Fan, S.; Wang, H.; Liang, J.; Huang, L.; Chen, L.; Liu, Q.; He, P.; Ye, Y. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020, 475, 119–128. [Google Scholar] [CrossRef]
- Lyu, L.; Yang, W.; Yao, J.; Wang, H.; Zhu, J.; Jin, A.; Liu, T.; Wang, B.; Zhou, J.; Fan, J. The diagnostic value of plasma exosomal hsa_circ_0070396 for hepatocellular carcinoma. Biomark. Med. 2021, 15, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, W.; Zhuo, H.; Zhu, D.; Rong, D.; Sun, J.; Song, J. Cancer-associated fibroblast exosomes promote chemoresistance to cisplatin in hepatocellular carcinoma through circZFR targeting signal transducers and activators of transcription (STAT3)/nuclear factor-kappa B (NF-κB) pathway. Bioengineered 2022, 13, 4786–4797. [Google Scholar] [CrossRef]
- Yao, Z.; Jia, C.; Tai, Y.; Liang, H.; Zhong, Z.; Xiong, Z.; Deng, M.; Zhang, Q. Serum exosomal long noncoding RNAs lnc-FAM72D-3 and lnc-EPC1-4 as diagnostic biomarkers for hepatocellular carcinoma. Aging 2020, 12, 11843. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhang, G.M. Serum exosomal long noncoding RNA CRNDE level for hepatocellular carcinoma diagnosis. J. Clin. Lab. Anal. 2022, 36, e24144. [Google Scholar] [CrossRef]
- Wang, G.; He, L.; Wang, S.; Zhang, M.; Li, Y.; Liu, Q.; Sun, N.; Zhang, X.; Liu, Y.; Zhang, J. EV PD-L1 is correlated with clinical features and contributes to T cell suppression in pediatric thyroid cancer. J. Clin. Endocrinol. Metab. 2020, 105, e2970–e2981. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Angelini, D.F.; Iessi, E.; Mizzoni, D.; Di Raimo, R.; Federici, C.; Lugini, L.; Borsellino, G.; Gentilucci, A.; Pierella, F. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017, 403, 318–329. [Google Scholar] [CrossRef]
- Soekmadji, C.; Riches, J.D.; Russell, P.J.; Ruelcke, J.E.; McPherson, S.; Wang, C.; Hovens, C.M.; Corcoran, N.M.; BioResource, T.A.P.C.C.; Hill, M.M. Modulation of paracrine signaling by CD9 positive small extracellular vesicles mediates cellular growth of androgen deprived prostate cancer. Oncotarget 2016, 8, 52237. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jutzy, J.M.; Valenzuela, M.M.A.; Turay, D.; Aspe, J.R.; Ashok, A.; Mirshahidi, S.; Mercola, D.; Lilly, M.B.; Wall, N.R. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS ONE 2012, 7, e46737. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Chen, W.; Yin, L.; Zhu, J.; Zhang, H.; Cai, C.; Li, P.; Huang, L.; Ma, P. Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer. J. Cancer 2018, 9, 2659. [Google Scholar] [CrossRef]
- Bijnsdorp, I.V.; Geldof, A.A.; Lavaei, M.; Piersma, S.R.; van Moorselaar, R.J.A.; Jimenez, C.R. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J. Extracell. Vesicles 2013, 2, 22097. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gui, R. Circulating exosomal circFoxp1 confers cisplatin resistance in epithelial ovarian cancer cells. J. Gynecol. Oncol. 2020, 31, e75. [Google Scholar] [CrossRef]
- Qiu, J.-J.; Lin, X.-J.; Tang, X.-Y.; Zheng, T.-T.; Lin, Y.-Y.; Hua, K.-Q. Exosomal metastasis-associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int. J. Biol. Sci. 2018, 14, 1960. [Google Scholar] [CrossRef]
- Yin, J.; Yan, X.; Yao, X.; Zhang, Y.; Shan, Y.; Mao, N.; Yang, Y.; Pan, L. Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients. J. Cell. Mol. Med. 2012, 16, 337–348. [Google Scholar] [CrossRef]
- Wyciszkiewicz, A.; Kalinowska-Łyszczarz, A.; Nowakowski, B.; Kaźmierczak, K.; Osztynowicz, K.; Michalak, S. Expression of small heat shock proteins in exosomes from patients with gynecologic cancers. Sci. Rep. 2019, 9, 9817. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Qing, Y.; Li, D.; Cui, M.; Jin, P.; Xu, T. Proteomic and lipidomic analysis of exosomes derived from ovarian cancer cells and ovarian surface epithelial cells. J. Ovarian Res. 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sherman-Baust, C.A.; Tsai-Turton, M.; Bristow, R.E.; Roden, R.B.; Morin, P.J. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer 2009, 9, 244. [Google Scholar] [CrossRef]
- Szajnik, M.; Derbis, M.; Lach, M.; Patalas, P.; Michalak, M.; Drzewiecka, H.; Szpurek, D.; Nowakowski, A.; Spaczynski, M.; Baranowski, W. Exosomes in plasma of patients with ovarian carcinoma: Potential biomarkers of tumor progression and response to therapy. Gynecol. Obstet. 2013, 003. [Google Scholar] [CrossRef]
- Li, N.; Lin, G.; Zhang, Y.; Zhang, Q.; Zhang, H. Exosome-related protein CRABP2 is upregulated in ovarian carcinoma and enhances cell proliferation. Discov. Oncol. 2022, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Carbotti, G.; Orengo, A.M.; Mezzanzanica, D.; Bagnoli, M.; Brizzolara, A.; Emionite, L.; Puppo, A.; Centurioni, M.G.; Bruzzone, M.; Marroni, P. Activated leukocyte cell adhesion molecule soluble form: A potential biomarker of epithelial ovarian cancer is increased in type II tumors. Int. J. Cancer 2013, 132, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gong, Z.; Shen, Y.; Fang, Y.; Zhong, S. Circular RNA expression in extracellular vesicles isolated from serum of patients with endometrial cancer. Epigenomics 2018, 10, 187–197. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, T.; Lin, Z.; Gao, Y. Aberrant methylation of MEG3 functions as a potential plasma-based biomarker for cervical cancer. Sci. Rep. 2017, 7, 6271. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Zhang, L.; Cong, J.; Hou, J.; Liu, C. LncRNA PVT1 promotes the growth of HPV positive and negative cervical squamous cell carcinoma by inhibiting TGF-β1. Cancer Cell Int. 2018, 18, 70. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.C.; Luo, X.H.; Tao, G.X.; Guan, M.; Yuan, H.; Hu, D.K. Exosomal Long noncoding RNA s are differentially expressed in the Cervicovaginal lavage samples of cervical cancer patients. J. Clin. Lab. Anal. 2016, 30, 1116–1121. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Wang, K.; He, Y. Appraising the Value of Serum and Serum-Derived Exosomal LncRNA-EXOC7 as a Promising Biomarker in Cervical Cancer. Clin. Lab. 2020, 66, 1357–1363. [Google Scholar] [CrossRef]
- Dziechciowski, M.; Zapala, B.; Skotniczny, K.; Gawlik, K.; Pawlica-Gosiewska, D.; Piwowar, M.; Balajewicz-Nowak, M.; Basta, P.; Solnica, B.; Pitynski, K. Diagnostic and prognostic relevance of microparticles in peripheral and uterine blood of patients with endometrial cancer. Ginekol. Pol. 2018, 89, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Lin, C.; Peng, M.; Ren, J.; Jing, Y.; Lei, L.; Tao, Y.; Huang, J.; Yang, J.; Sun, M. Circulating plasma exosomal long non-coding RNAs LINC00265, LINC00467, UCA1, and SNHG1 as biomarkers for diagnosis and treatment monitoring of acute myeloid leukemia. Front. Oncol. 2022, 12, 1033143. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, M.J.; Szajnik, M.; Welsh, A.; Whiteside, T.L.; Boyiadzis, M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-β1. Haematologica 2011, 96, 1302. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS ONE 2014, 9, e103310. [Google Scholar] [CrossRef]

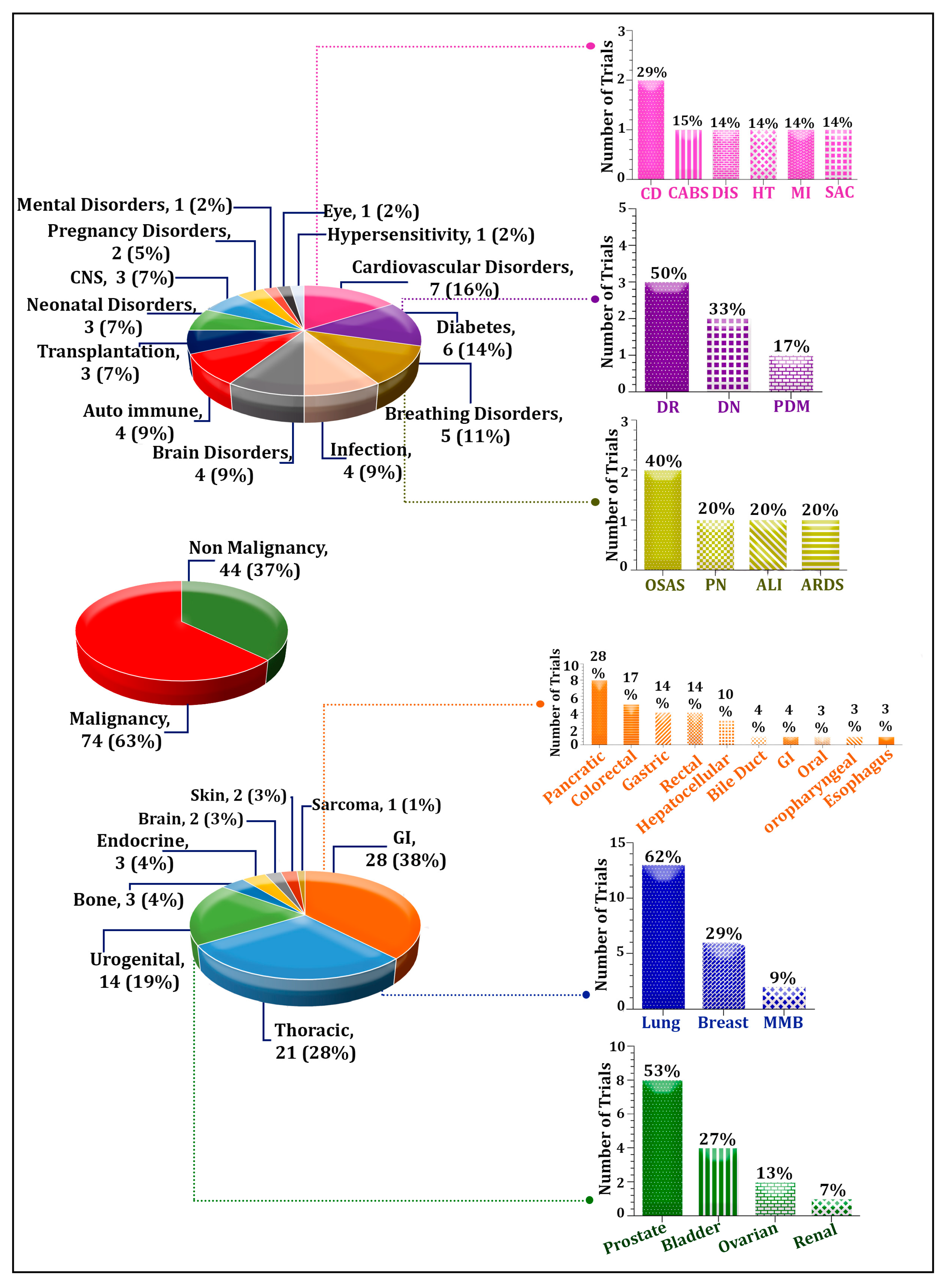
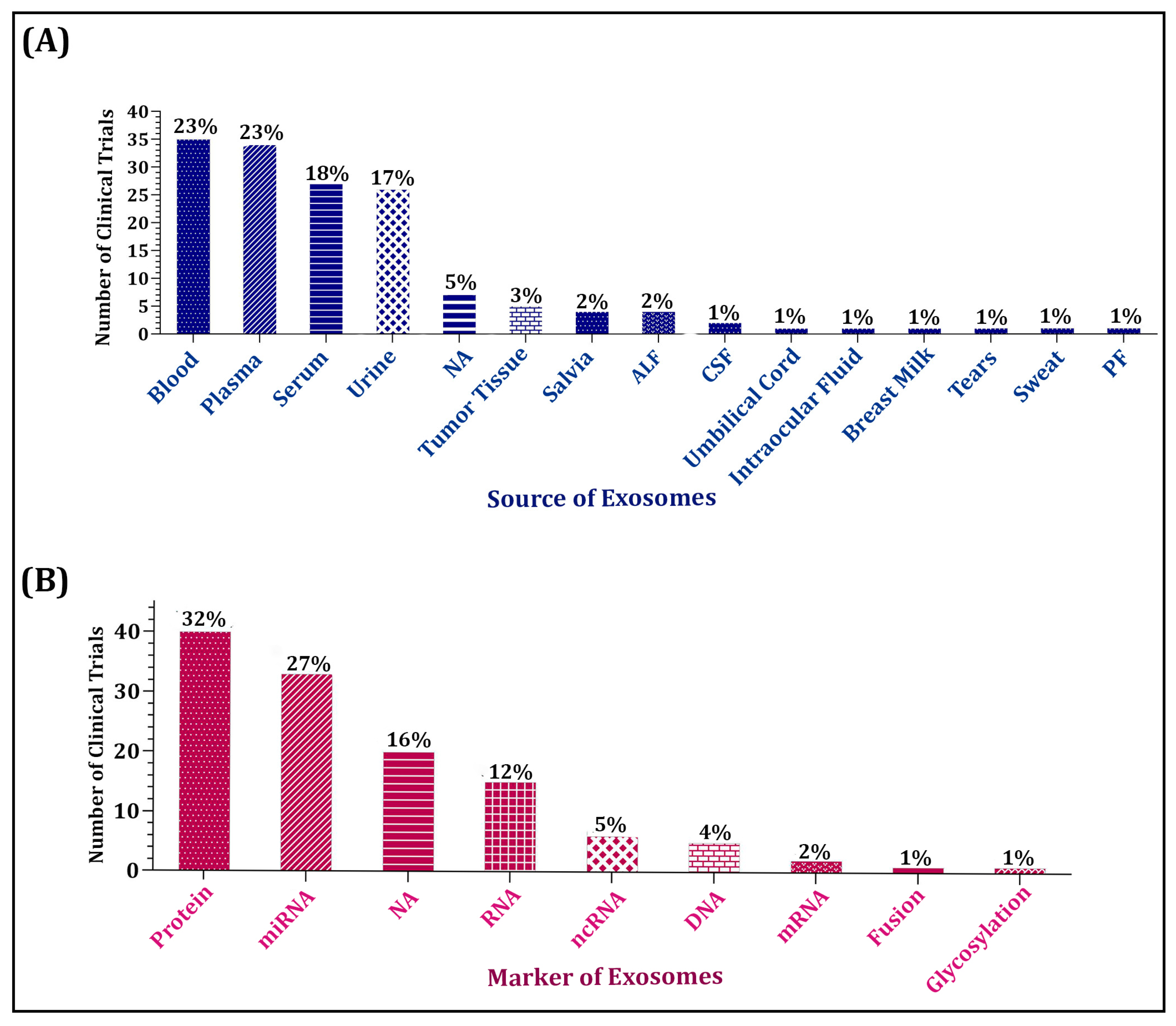
| No. | NCT Number | St. Date | Sex | Age | Enrollment | Disease Type | Source of Exosome | Associated Marker |
|---|---|---|---|---|---|---|---|---|
| Malignancy | ||||||||
| GI Cancer | ||||||||
| 1 | NCT06342427 | 2023 | ALL | Adult | 809 | Gastric Cancer | Serum | miRNA |
| 2 | NCT06023121 | 2018 | ALL | Adult | 800 | Gastric Cancer | Blood | LncRNA |
| 3 | NCT06469892 | 2020 | ALL | Adult | 225 | Oral Cancer | Plasma & Saliva | miR-185 |
| 4 | NCT03032913 | 2017 | ALL | Adult | 52 | Pancreatic Cancer | Plasma | NA |
| 5 | NCT04394572 | 2021 | ALL | Adult | 80 | Colorectal Cancer | Serum | Protein |
| Thoracic Cancer | ||||||||
| 6 | NCT02890849 | 2016 | ALL | All | 60 | Lung Cancer | Plasma | mRNA |
| 7 | NCT03830619 | 2017 | ALL | Adult | 1000 | Lung Cancer | Serum | lncRNA |
| 8 | NCT02869685 | 2017 | ALL | Adult | 60 | Lung Cancer | Plasma | miRNA |
| 9 | NCT03228277 | 2017 | ALL | Adult | 25 | Lung Cancer | Serum | DNA |
| Urogenital Cancer | ||||||||
| 10 | NCT02702856 | 2014 | MALE | Adult | 2000 | Prostate Cancer | Urine | RNA |
| 11 | NCT04720599 | 2020 | MALE | Adult | 120 | Prostate Cancer | NA | NA |
| 12 | NCT06193941 | 2023 | ALL | Adult | 400 | Bladder Cancer/Urothelial Carcinoma | Urine | RNA |
| Bone Cancer | ||||||||
| 13 | NCT05101655 | 2020 | ALL | Child | 60 | Osteosarcoma Lung Recurrence | Plasma | Protein |
| 14 | NCT03895216 | 2018 | ALL | Adult | 34 | Bone Metastases | Plasma | miRNA |
| 15 | NCT03488134 | 2018 | ALL | Adult | 74 | Thyroid Cancer | Urine | Protein |
| Non-Malignancy | ||||||||
| Cardiovascular Disorders | ||||||||
| 1 | NCT03034265 | 2016 | ALL | Adult | 24 | Hypertension | Urine | Protein |
| 2 | NCT02226055 | 2014 | ALL | Adult | 200 | Cardiovascular | Serum & Urine | NA |
| Breathing disorders | ||||||||
| 3 | NCT03811600 | 2019 | ALL | Adult | 90 | Obstructive Sleep Apnea Syndrome | Plasma & Serum | Protein |
| 4 | NCT04459182 | 2021 | ALL | Adult | 99 | Obstructive Sleep Apneas Hypopneas Syndrome | NA | miRNA |
| Autoimmune Disorder | ||||||||
| 5 | NCT03984006 | 2019 | ALL | Adult | 5 | Autoimmune Thyroid Heart Disease | Urine | Protein |
| Brain Disorder | ||||||||
| 6 | NCT03419000 | 2018 | ALL | Adult | 75 | Drug-Resistant Epilepsy | Blood | miRNA |
| CNS Disorder | ||||||||
| 7 | NCT01860118 | 2023 | ALL | Adult | 601 | Parkinson’s Disease | Blood & Urine | Protein |
| Infection | ||||||||
| 8 | NCT03267160 | 2017 | ALL | Adult | 30 | Hemodynamic Instability | Blood & Urine | Protein |
| Pregnancy disorder | ||||||||
| 9 | NCT03562715 | 2016 | FEMALE | Adult | 200 | Preeclampsia | Umbilical Cord | miRNA |
| Transplantation | ||||||||
| 10 | NCT03503461 | 2018 | ALL | Adult | 67 | Kidney Transplantation | Urine | Protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delshad, M.; Sanaei, M.-J.; Mohammadi, M.H.; Sadeghi, A.; Bashash, D. Exosomal Biomarkers: A Comprehensive Overview of Diagnostic and Prognostic Applications in Malignant and Non-Malignant Disorders. Biomolecules 2025, 15, 587. https://doi.org/10.3390/biom15040587
Delshad M, Sanaei M-J, Mohammadi MH, Sadeghi A, Bashash D. Exosomal Biomarkers: A Comprehensive Overview of Diagnostic and Prognostic Applications in Malignant and Non-Malignant Disorders. Biomolecules. 2025; 15(4):587. https://doi.org/10.3390/biom15040587
Chicago/Turabian StyleDelshad, Mahda, Mohammad-Javad Sanaei, Mohammad Hossein Mohammadi, Amir Sadeghi, and Davood Bashash. 2025. "Exosomal Biomarkers: A Comprehensive Overview of Diagnostic and Prognostic Applications in Malignant and Non-Malignant Disorders" Biomolecules 15, no. 4: 587. https://doi.org/10.3390/biom15040587
APA StyleDelshad, M., Sanaei, M.-J., Mohammadi, M. H., Sadeghi, A., & Bashash, D. (2025). Exosomal Biomarkers: A Comprehensive Overview of Diagnostic and Prognostic Applications in Malignant and Non-Malignant Disorders. Biomolecules, 15(4), 587. https://doi.org/10.3390/biom15040587






