Sex-Specific Inflammatory Profiles Affect Neuropsychiatric Issues in COVID-19 Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Data Collection
2.2. Neuropsychiatric Assessment
2.3. Laboratory Determinants
2.4. Statistical Analyses
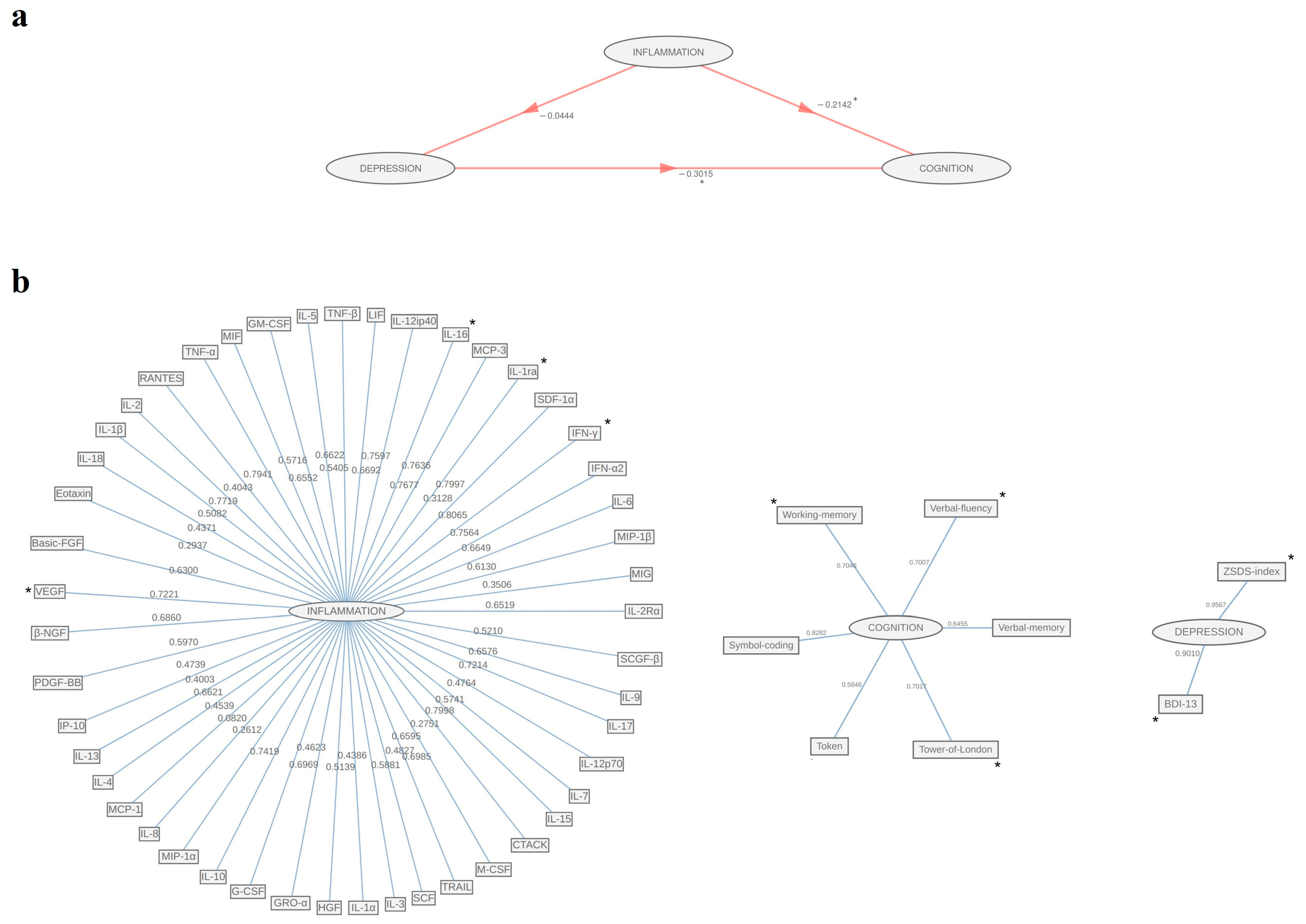
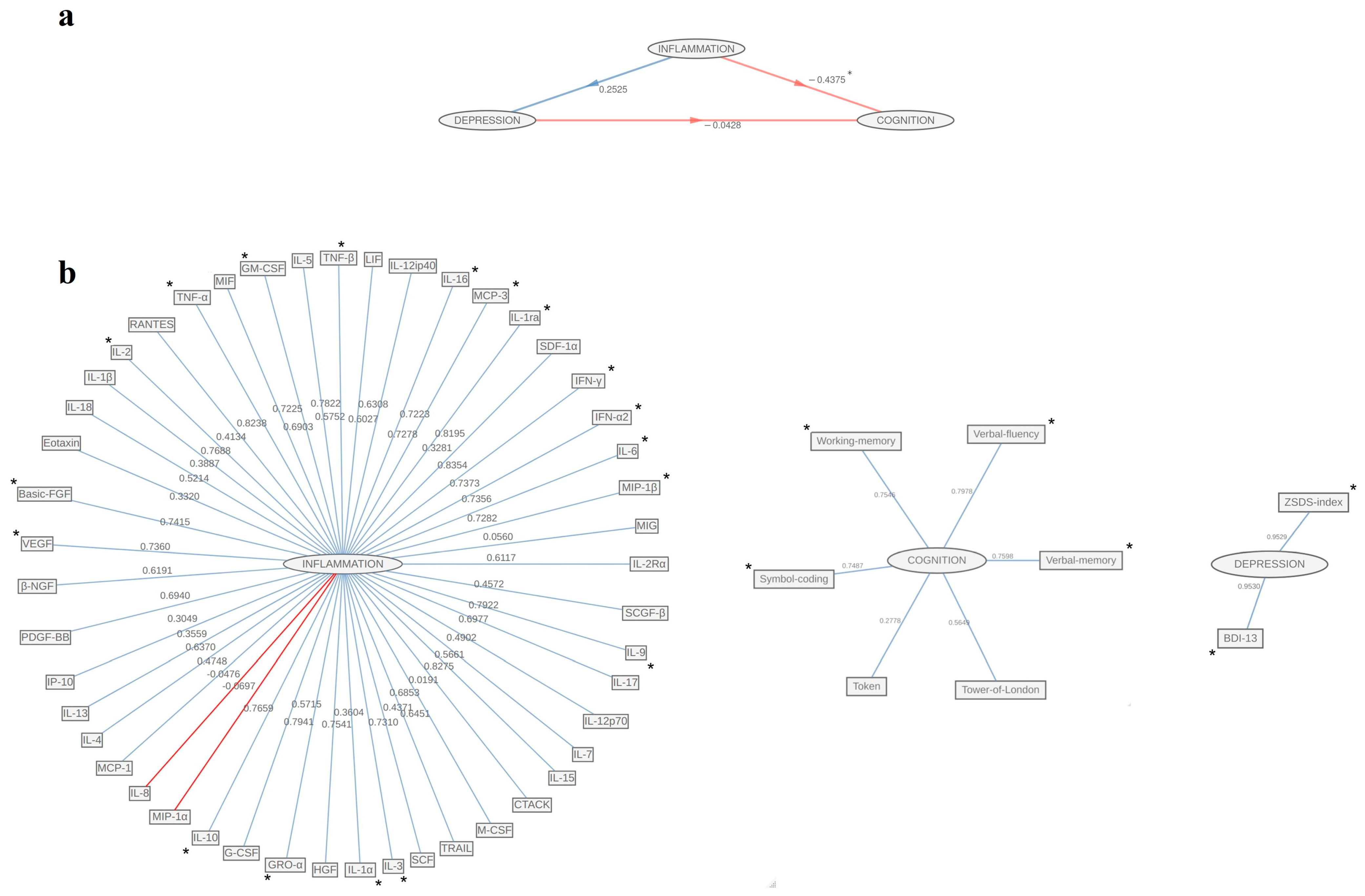
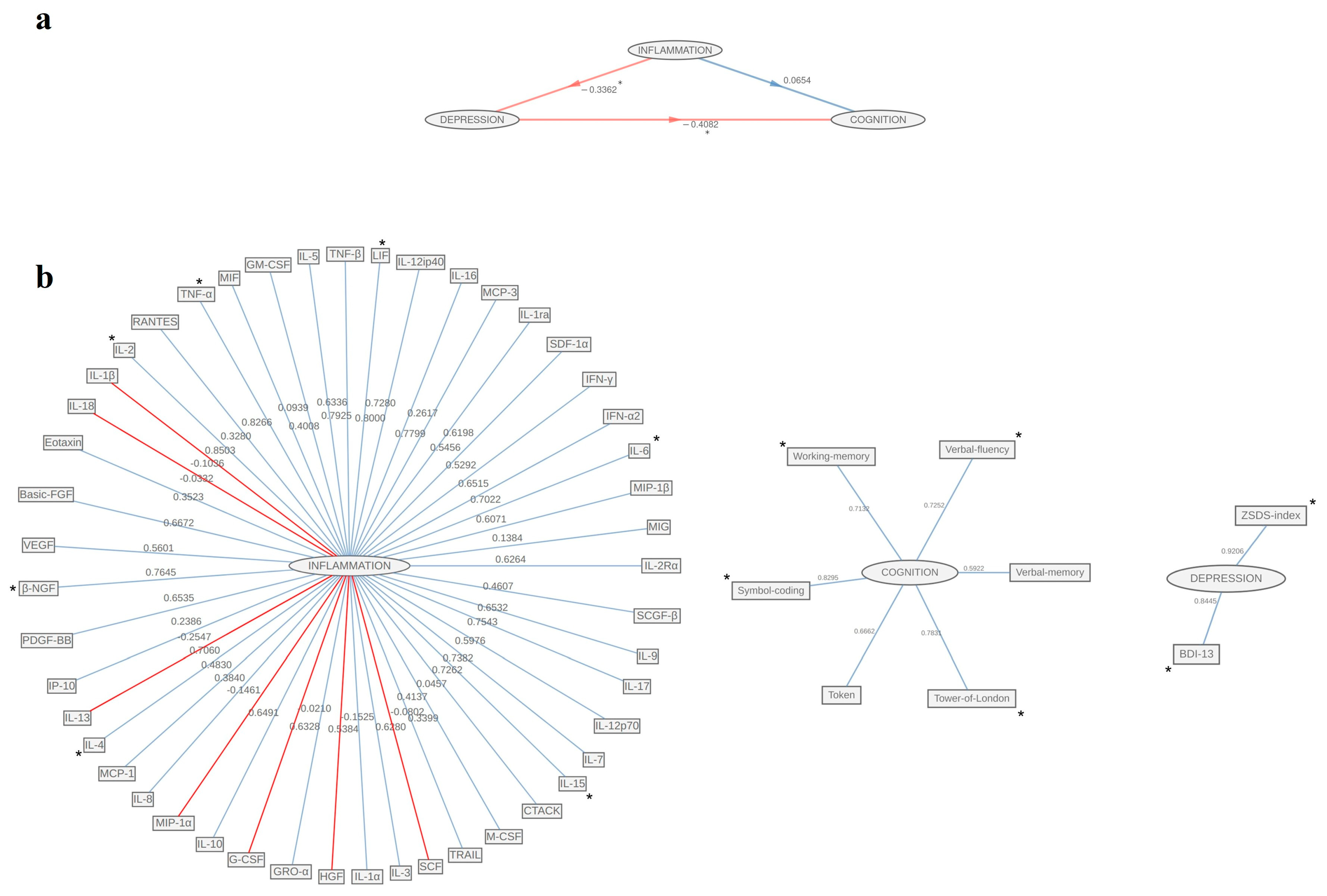
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allison, D.J.; Ditor, D.S. The common inflammatory etiology of depression and cognitive impairment: A therapeutic target. J. Neuroinflamm. 2014, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-alpha. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef]
- Banks, W.A.; Erickson, M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Poletti, S.; Mazza, M.G.; Benedetti, F. Inflammatory mediators in major depression and bipolar disorder. Transl. Psychiatry 2024, 14, 247. [Google Scholar] [CrossRef]
- Poletti, S.; Paolini, M.; Ernst, J.; Bollettini, I.; Melloni, E.; Vai, B.; Harrington, Y.; Bravi, B.; Calesella, F.; Lorenzi, C.; et al. Long-term effect of childhood trauma: Role of inflammation and white matter in mood disorders. Brain Behav. Immun. Health 2022, 26, 100529. [Google Scholar] [CrossRef]
- Poletti, S.; Vai, B.; Mazza, M.G.; Zanardi, R.; Lorenzi, C.; Calesella, F.; Cazzetta, S.; Branchi, I.; Colombo, C.; Furlan, R.; et al. A peripheral inflammatory signature discriminates bipolar from unipolar depression: A machine learning approach. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110136. [Google Scholar] [CrossRef]
- Vai, B.; Mazza, M.G.; Cazzetta, S.; Calesella, F.; Aggio, V.; Lorenzi, C.; Zanardi, R.; Poletti, S.; Colombo, C.; Benedetti, F. Higher Interleukin 13 differentiates patients with a positive history of suicide attempts in major depressive disorder. J. Affect. Disord. Rep. 2021, 6, 100254. [Google Scholar] [CrossRef]
- Benedetti, F.; Dallaspezia, S.; Melloni, E.M.T.; Lorenzi, C.; Zanardi, R.; Barbini, B.; Colombo, C. Effective Antidepressant Chronotherapeutics (Sleep Deprivation and Light Therapy) Normalize the IL-1beta:IL-1ra Ratio in Bipolar Depression. Front. Physiol 2021, 12, 740686. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Poletti, S.; Hoogenboezem, T.A.; Locatelli, C.; de Wit, H.; Wijkhuijs, A.J.M.; Colombo, C.; Drexhage, H.A. Higher Baseline Proinflammatory Cytokines Mark Poor Antidepressant Response in Bipolar Disorder. J. Clin. Psychiatry 2017, 78, e986–e993. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Zanardi, R.; Mazza, M.G. Antidepressant psychopharmacology: Is inflammation a future target? Int. Clin. Psychopharmacol. 2022, 37, 79–81. [Google Scholar] [CrossRef]
- Breit, S.; Mazza, E.; Poletti, S.; Benedetti, F. White matter integrity and pro-inflammatory cytokines as predictors of antidepressant response in MDD. J. Psychiatr. Res 2023, 159, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Bravi, B.; Melloni, E.M.T.; Paolini, M.; Palladini, M.; Calesella, F.; Servidio, L.; Agnoletto, E.; Poletti, S.; Lorenzi, C.; Colombo, C.; et al. Choroid plexus volume is increased in mood disorders and associates with circulating inflammatory cytokines. Brain Behav. Immun. 2024, 116, 52–61. [Google Scholar] [CrossRef]
- Vai, B.; Palladini, M.; Lorenzi, C.; Zanardi, R.; Poletti, S.; Aggio, V.; Benedetti, F. Interleukin 6 associates with reduced grey matter volume and resting-state connectivity in the anterior cingulate cortex in bipolar patients. Brain Behav. Immun. Health 2022, 26, 100522. [Google Scholar] [CrossRef]
- Morrens, M.; Overloop, C.; Coppens, V.; Loots, E.; Van Den Noortgate, M.; Vandenameele, S.; Leboyer, M.; De Picker, L. The relationship between immune and cognitive dysfunction in mood and psychotic disorder: A systematic review and a meta-analysis. Mol. Psychiatry 2022, 27, 3237–3246. [Google Scholar] [CrossRef]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. The relationship between inflammatory biomarkers and cognitive dysfunction in patients with schizophrenia: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 121, 110668. [Google Scholar] [CrossRef]
- Pan, J.; Hu, J.B.; Meng, D.; Chen, L.; Wei, X. Neuroinflammation in dementia: A meta-analysis of PET imaging studies. Medicine 2024, 103, e38086. [Google Scholar] [CrossRef]
- Su, C.; Zhao, K.; Xia, H.; Xu, Y. Peripheral inflammatory biomarkers in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Psychogeriatrics 2019, 19, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Lemche, E.; Killick, R.; Mitchell, J.; Caton, P.W.; Choudhary, P.; Howard, J.K. Molecular mechanisms linking type 2 diabetes mellitus and late-onset Alzheimer’s disease: A systematic review and qualitative meta-analysis. Neurobiol. Dis. 2024, 196, 106485. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T. Cognitive patterns in immune-mediated inflammatory diseases compared with age-matched controls: A systematic review and meta-analyses. Clin. Med. 2023, 23, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.; Sheehan, D.; Brown, S.; O’Flanagan, S.; Savinelli, S.; O’Keeffe, F.; Bramham, J. Immune Response and Cognitive Impairment in Post-COVID Syndrome: A Systematic Review. Am. J. Med. 2024, 138, 698–711.e2. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalová, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867. e20. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Y.; Zeng, D.; Wang, M.; Duan, X. Predictors of cognitive decline in older individuals without dementia: An updated meta-analysis. Ann. Clin. Transl. Neurol. 2023, 10, 497–506. [Google Scholar] [CrossRef]
- Baune, B.T.; Smith, E.; Reppermund, S.; Air, T.; Samaras, K.; Lux, O.; Brodaty, H.; Sachdev, P.; Trollor, J.N. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: The prospective Sydney Memory and Aging Study. Psychoneuroendocrinology 2012, 37, 1521–1530. [Google Scholar] [CrossRef]
- Wiels, W.; Baeken, C.; Engelborghs, S. Depressive Symptoms in the Elderly-An Early Symptom of Dementia? A Systematic Review. Front. Pharmacol. 2020, 11, 34. [Google Scholar] [CrossRef]
- Chalmers, R.A.; Cervin, M.; Choo, C.; Baune, B.T.; Trollor, J.N.; Numbers, K.; Sachdev, P.S.; Brodaty, H.; Kochan, N.A.; Medvedev, O.N. Networks of inflammation, depression, and cognition in aging males and females. Aging Clin. Exp. Res. 2022, 34, 2387–2398. [Google Scholar] [CrossRef]
- Niles, A.N.; Smirnova, M.; Lin, J.; O’Donovan, A. Gender differences in longitudinal relationships between depression and anxiety symptoms and inflammation in the health and retirement study. Psychoneuroendocrinology 2018, 95, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Palladini, M.; Villa, G.; Agnoletto, E.; Harrington, Y.; Vai, B.; Benedetti, F. Prevalence of depression in SARS-CoV-2 infected patients: An umbrella review of meta-analyses. Gen. Hosp. Psychiatry 2023, 80, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Benedetti, F.; Palladini, M.; Paolini, M.; Melloni, E.; Vai, B.; De Lorenzo, R.; Furlan, R.; Rovere-Querini, P.; Falini, A.; Mazza, M.G. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: A multimodal magnetic resonance imaging study. Brain Behav. Immun. Health 2021, 18, 100387. [Google Scholar] [CrossRef]
- De Lorenzo, R.; Mazza, M.G.; Sciorati, C.; Leone, R.; Scavello, F.; Palladini, M.; Merolla, A.; Ciceri, F.; Bottazzi, B.; Garlanda, C.; et al. Post-COVID Trajectory of Pentraxin 3 Plasma Levels Over 6 Months and Their Association with the Risk of Developing Post-Acute Depression and Anxiety. CNS Drugs 2024, 38, 459–472. [Google Scholar] [CrossRef]
- Lorkiewicz, P.; Waszkiewicz, N. Biomarkers of Post-COVID Depression. J. Clin. Med. 2021, 10, 4142. [Google Scholar] [CrossRef]
- Palladini, M.; Mazza, M.G.; De Lorenzo, R.; Spadini, S.; Aggio, V.; Bessi, M.; Calesella, F.; Bravi, B.; Rovere-Querini, P.; Benedetti, F. Circulating inflammatory markers predict depressive symptomatology in COVID-19 survivors. Cytokine 2025, 186, 156839. [Google Scholar] [CrossRef]
- Poletti, S.; Palladini, M.; Mazza, M.G.; De Lorenzo, R.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: Role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 773–782. [Google Scholar] [CrossRef]
- Jokelainen, J.; Timonen, M.; Keinänen-Kiukaanniemi, S.; Härkönen, P.; Jurvelin, H.; Suija, K. Validation of the Zung self-rating depression scale (SDS) in older adults. Scand. J. Prim. Health Care 2019, 37, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Alexandrowicz, R.W.; Fritzsche, S.; Keller, F. A psychometric view on the applicability of the BDI-II in non-clinical populations. Neuropsychiatrie 2014, 28, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Anselmetti, S.; Poletti, S.; Ermoli, E.; Bechi, M.; Cappa, S.; Venneri, A.; Smeraldi, E.; Cavallaro, R. The Brief Assessment of Cognition in Schizophrenia. Normative data for the Italian population. Neurol. Sci. 2008, 29, 85–92. [Google Scholar]
- Sanchez, G. PLS path modeling with R. Berkeley Trowchez Ed. 2013, 383, 551. [Google Scholar]
- Reinartz, W.; Haenlein, M.; Henseler, J. An empirical comparison of the efficacy of covariance-based and variance-based SEM. Int. J. Res. Mark. 2009, 26, 332–344. [Google Scholar] [CrossRef]
- Hair, J.F.; Astrachan, C.B.; Moisescu, O.I.; Radomir, L.; Sarstedt, M.; Vaithilingam, S.; Ringle, C.M. Executing and interpreting applications of PLS-SEM: Updates for family business researchers. J. Fam. Bus. Strategy 2021, 12, 100392. [Google Scholar] [CrossRef]
- Mostafavi, N.; Jeong, A.; Vlaanderen, J.; Imboden, M.; Vineis, P.; Jarvis, D.; Kogevinas, M.; Probst-Hensch, N.; Vermeulen, R. The mediating effect of immune markers on the association between ambient air pollution and adult-onset asthma. Sci. Rep. 2019, 9, 8818. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef]
- Kropp, D.R.; Hodes, G.E. Sex differences in depression: An immunological perspective. Brain Res. Bull. 2023, 196, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, G.M.; Greene, J.; Vertes, P.E.; Drevets, W.C.; Bullmore, E.T. Major Depressive Disorder Is Associated with Differential Expression of Innate Immune and Neutrophil-Related Gene Networks in Peripheral Blood: A Quantitative Review of Whole-Genome Transcriptional Data From Case-Control Studies. Biol. Psychiatry 2020, 88, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Derry, H.M.; Padin, A.C.; Kuo, J.L.; Hughes, S.; Kiecolt-Glaser, J.K. Sex Differences in Depression: Does Inflammation Play a Role? Curr. Psychiatry Rep. 2015, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Moieni, M.; Irwin, M.R.; Jevtic, I.; Olmstead, R.; Breen, E.C.; Eisenberger, N.I. Sex differences in depressive and socioemotional responses to an inflammatory challenge: Implications for sex differences in depression. Neuropsychopharmacology 2015, 40, 1709–1716. [Google Scholar] [CrossRef]
- Kohler-Forsberg, O.; Buttenschon, H.N.; Tansey, K.E.; Maier, W.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; Rietschel, M.; et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 2017, 62, 344–350. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Chang, K.A. Sex Difference in Peripheral Inflammatory Biomarkers in Drug-Naive Patients with Major Depression in Young Adulthood. Biomedicines 2021, 9, 708. [Google Scholar] [CrossRef]
- Lombardo, G.; Mondelli, V.; Worrell, C.; Sforzini, L.; Mariani, N.; Nikkheslat, N.; Nettis, M.A.; Kose, M.; Zajkowska, Z.; Cattaneo, A.; et al. Disturbed sex hormone milieu in males and females with major depressive disorder and low-grade inflammation. J. Affect. Disord. 2024, 356, 167–176. [Google Scholar] [CrossRef]
- Zito, S.; Nosari, G.; Pigoni, A.; Moltrasio, C.; Delvecchio, G. Association between testosterone levels and mood disorders: A minireview. J. Affect. Disord. 2023, 330, 48–56. [Google Scholar] [CrossRef]
- Harrington, Y.A.; Fortaner-Uya, L.; Paolini, M.; Poletti, S.; Lorenzi, C.; Spadini, S.; Melloni, E.M.T.; Agnoletto, E.; Zanardi, R.; Colombo, C.; et al. Disentangling the Genetic Landscape of Peripartum Depression: A Multi-Polygenic Machine Learning Approach on an Italian Sample. Genes 2024, 15, 1517. [Google Scholar] [CrossRef]
- Harrington, Y.A.; Paolini, M.; Fortaner-Uyà, L.; Maccario, M.; Melloni, E.M.; Poletti, S.; Lorenzi, C.; Zanardi, R.; Colombo, C.; Benedetti, F. History of Peripartum Depression Moderates the Association Between Estradiol Polygenic Risk Scores and Basal Ganglia Volumes in Major Depressive Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2025, 10, 7–16. [Google Scholar] [CrossRef]
- Grasshoff, H.; Comduhr, S.; Monne, L.R.; Muller, A.; Lamprecht, P.; Riemekasten, G.; Humrich, J.Y. Low-Dose IL-2 Therapy in Autoimmune and Rheumatic Diseases. Front. Immunol. 2021, 12, 648408. [Google Scholar] [CrossRef] [PubMed]
- Klatzmann, D.; Abbas, A.K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 2015, 15, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Rosenzwajg, M.; Lorenzon, R.; Cacoub, P.; Pham, H.P.; Pitoiset, F.; El Soufi, K.; Ribet, C.; Bernard, C.; Aractingi, S.; Banneville, B.; et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 2019, 78, 209–217. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, E.G.; da Silva, G.M.; Dos Santos, A.A. Neuronal cell survival: The role of interleukins. Ann. N. Y Acad. Sci. 2009, 1153, 57–64. [Google Scholar] [CrossRef]
- Poletti, S.; Zanardi, R.; Mandelli, A.; Aggio, V.; Finardi, A.; Lorenzi, C.; Borsellino, G.; Carminati, M.; Manfredi, E.; Tomasi, E.; et al. Low-dose interleukin 2 antidepressant potentiation in unipolar and bipolar depression: Safety, efficacy, and immunological biomarkers. Brain Behav. Immun. 2024, 118, 52–68. [Google Scholar] [CrossRef]
- Brown, M.A.; Hural, J. Functions of IL-4 and control of its expression. Crit. Rev. Immunol. 1997, 17, 1–32. [Google Scholar] [CrossRef]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the brain: A cytokine to remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Caligiuri, M.A. Interleukin 15: Biology and relevance to human disease. Blood 2001, 97, 14–32. [Google Scholar] [CrossRef]
- Pan, W.; Wu, X.; He, Y.; Hsuchou, H.; Huang, E.Y.-K.; Mishra, P.K.; Kastin, A.J. Brain interleukin-15 in neuroinflammation and behavior. Neurosci. Biobehav. Rev. 2013, 37, 184–192. [Google Scholar] [CrossRef]
- Zacchigna, S.; Lambrechts, D.; Carmeliet, P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Alleva, E.; Fiore, M. Stress and nerve growth factor: Findings in animal models and humans. Pharmacol. Biochem. Behav. 2002, 73, 159–166. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lin, P.Y.; Tu, K.Y.; Cheng, Y.S.; Wu, C.K.; Tseng, P.T. Significantly lower nerve growth factor levels in patients with major depressive disorder than in healthy subjects: A meta-analysis and systematic review. Neuropsychiatr. Dis. Treat 2015, 11, 925–933. [Google Scholar] [PubMed]
- Davis, S.M.; Pennypacker, K.R. The role of the leukemia inhibitory factor receptor in neuroprotective signaling. Pharmacol. Ther. 2018, 183, 50–57. [Google Scholar] [CrossRef]
- Balschun, D.; Wetzel, W.; Del Rey, A.; Pitossi, F.; Schneider, H.; Zuschratter, W.; Besedovsky, H.O. Interleukin-6: A cytokine to forget. FASEB J. 2004, 18, 1788–1790. [Google Scholar] [CrossRef] [PubMed]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef]
- Oppenheim, J.J. Cytokines: Past, present, and future. Int. J. Hematol. 2001, 74, 3–8. [Google Scholar] [CrossRef]
- Silva, R.; Travassos, L.H.; Dutra, F.F. The dichotomic role of single cytokines: Fine-tuning immune responses. Cytokine 2024, 173, 156408. [Google Scholar] [CrossRef]
- Acciai, F.; Hardy, M. Depression in later life: A closer look at the gender gap. Soc. Sci. Res. 2017, 68, 163–175. [Google Scholar] [CrossRef]
- Auerbach, R.P.; Alonso, J.; Axinn, W.G.; Cuijpers, P.; Ebert, D.D.; Green, J.G.; Hwang, I.; Kessler, R.C.; Liu, H.; Mortier, P.; et al. Mental disorders among college students in the World Health Organization World Mental Health Surveys. Psychol. Med. 2016, 46, 2955–2970. [Google Scholar] [CrossRef]
| Socio-Demographics and Clinical Features | Whole Sample (n = 101) | Females (n = 45) | Males (n = 56) | t or χ2 | p-Values |
|---|---|---|---|---|---|
| Males (females) | 56 (45) | - | - | - | - |
| Age | 53.23 ± 10.3 | 51.78 ± 11.46 | 54.39 ± 9.2 | −1.27 | 0.206 |
| Education (years) | 12.98 ± 3.60 | 12.67 ± 3.88 | 12.73 ± 3.59 | 0.09 | 0.93 |
| BDI-13 | 4 ± 5.57 | 5.76 ± 6.39 | 2.68 ± 4.41 | 2.86 | 0.005 ** |
| BDI-13 ≥ 9 yes (%) | 13 (12.87%) | 9 (20%) | 4 (7.14%) | 3.68 | 0.055 |
| ZSDS index | 46.18 ± 12.61 | 52.34 ± 12.64 | 41.23 ± 10.26 | 4.88 | <0.001 *** |
| ZSDS index ≥ 50 (%) | 37 (36.63%) | 24 (53.34%) | 13 (23.21%) | 9.75 | 0.002 ** |
| BACS—verbal memory | 49.64 ± 9.75 | 51.59 ± 10.33 | 48.07 ± 9.05 | 1.82 | 0.071 |
| BACS—verbal fluency | 46.8 ± 12.16 | 43.99 ± 9.42 | 47.76 ± 13.84 | −1.56 | 0.121 |
| BACS—working memory | 21.16 ± 5.17 | 20.08 ± 4.89 | 22.03 ± 5.27 | −1.92 | 0.058 |
| BACS—selective attention | 50.97 ± 11.97 | 50.88 ± 12.06 | 51.04 ± 12 | −0.06 | 0.949 |
| BACS—psychomotor coordination | 72.63 ± 19.34 | 71.99 ± 17.23 | 73.14 ± 21.03 | −0.3 | 0.768 |
| BACS—executive functions | 14.75 ± 4.55 | 14.4 ± 4.59 | 15.03 ± 4.55 | −0.7 | 0.489 |
| Immune-Inflammatory Biomarkers | Whole Sample (n = 101) | Females (n = 45) | Males (n = 56) | Z | p |
| IL-2Rα | 32 ± 11 | 30 ± 9 | 35 ± 11.75 | −2.34702 | 0.019 * |
| MIG | 45 ± 39 | 41 ± 45 | 52 ± 32.5 | −1.32895 | 0.183864 |
| MIP-1β | 430 ± 90 | 405 ± 85 | 437.25 ± 86 | −1.56468 | 0.117659 |
| IL-6 | 15 ± 4 | 14.5 ± 4 | 15.75 ± 4.25 | −1.34262 | 0.179396 |
| IFN-α2 | 15 ± 2 | 15 ± 3 | 16 ± 2.75 | −1.41778 | 0.156256 |
| IFN-gamma | 38 ± 9 | 36 ± 9 | 39.25 ± 7.625 | −1.81066 | 0.070195 |
| SDF-1α | 283.5 ± 143.5 | 255 ± 137 | 307.5 ± 147.5 | −1.94048 | 0.052323 |
| IL-1ra | 10 ± 3.5 | 10 ± 4 | 10 ± 3 | −0.50562 | 0.613126 |
| MCP-3 | 18 ± 3 | 18 ± 2 | 18 ± 3 | −0.84383 | 0.398763 |
| IL-16 | 22 ± 6 | 21 ± 5 | 24 ± 7 | −2.18645 | 0.028783 * |
| IL-12(p40) | 14 ± 2 | 14 ± 1.75 | 14 ± 3 | −1.52368 | 0.127588 |
| LIF | 17 ± 3 | 16 ± 3 | 17 ± 2.875 | −1.77308 | 0.076217 |
| TNF-β | 163 ± 38.5 | 158 ± 29 | 164.75 ± 36.5 | −1.45877 | 0.144628 |
| IL-5 | 26.5 ± 8 | 24 ± 7 | 27.5 ± 8 | −2.10446 | 0.035339 * |
| GM-CSF | 16 ± 3 | 16 ± 3 | 16.75 ± 3.75 | −0.37921 | 0.704530 |
| MIF | 409 ± 153 | 394 ± 147.5 | 451 ± 149.75 | −0.96341 | 0.335345 |
| TNF-α | 21 ± 5.5 | 21 ± 6 | 21 ± 4.25 | −0.77551 | 0.438040 |
| RANTES | 2463 ± 772 | 2389 ± 657.5 | 2564.25 ± 853.625 | −1.07615 | 0.281863 |
| IL-2 | 12 ± 3 | 12 ± 2 | 12 ± 3 | −0.74134 | 0.458485 |
| IL-1β | 13 ± 5 | 12 ± 6 | 13 ± 5 | −0.98390 | 0.325163 |
| IL-18 | 52 ± 24.5 | 47.75 ± 21 | 57.5 ± 25.25 | −2.32994 | 0.019810 * |
| Eotaxin | 223.5 ± 106.5 | 199 ± 106.5 | 238.75 ± 124 | −2.48367 | 0.013004 * |
| Basic_FGF | 13 ± 2.5 | 12.5 ± 3 | 13 ± 3 | −1.99514 | 0.046029 * |
| VEGF | 28 ± 6.25 | 28 ± 6 | 29 ± 6.75 | −1.08981 | 0.275797 |
| β-NGF | 15.5 ± 3 | 15.5 ± 3 | 15.75 ± 3.5 | −0.11957 | 0.904823 |
| PDGF-BB | 35 ± 15.5 | 31.5 ± 14 | 38 ± 13.25 | −1.91315 | 0.055730 |
| IP-10 | 142 ± 97 | 123 ± 99.5 | 154.25 ± 115 | −2.35044 | 0.018752 * |
| IL-13 | 18 ± 8 | 18 ± 11 | 18 ± 5.5 | 0.02733 | 0.978196 |
| IL-4 | 14 ± 3.5 | 14 ± 3.5 | 14.25 ± 3.25 | −1.40411 | 0.160286 |
| MCP-1 | 32 ± 16 | 30 ± 11 | 34 ± 16.25 | −2.09421 | 0.036242 * |
| IL-8 | 15 ± 4 | 14.5 ± 5.25 | 15 ± 3.75 | −0.49878 | 0.617931 |
| MIP-1α | 16 ± 5 | 16.5 ± 4 | 16 ± 6 | −0.60811 | 0.543117 |
| IL-10 | 10 ± 3 | 10 ± 2 | 10 ± 3 | −1.07273 | 0.283394 |
| G-CSF | 20 ± 3.5 | 19.5 ± 3 | 20 ± 4.25 | −0.71743 | 0.473109 |
| GRO-α | 46 ± 13.5 | 46.5 ± 15 | 45.5 ± 12.5 | −0.04441 | 0.964576 |
| HGF | 37 ± 12.5 | 37 ± 12 | 37 ± 13 | −0.45779 | 0.647105 |
| IL-1α | 12.5 ± 2 | 12 ± 2 | 13 ± 2 | −1.42803 | 0.153285 |
| IL-3 | 17 ± 4 | 17 ± 3 | 17 ± 3 | −0.93608 | 0.349235 |
| SCF | 29 ± 9.5 | 29 ± 11 | 29.5 ± 9.5 | −0.89508 | 0.370745 |
| TRAIL | 29 ± 6.5 | 29 ± 8.5 | 28 ± 6.5 | 0.43046 | 0.666863 |
| M-CSF | 34 ± 7.5 | 32 ± 6 | 35.75 ± 7.25 | −2.26161 | 0.023722 * |
| CTACK | 99 ± 43 | 98 ± 49 | 100 ± 43.75 | −0.91558 | 0.359889 |
| IL-15 | 20 ± 5 | 20 ± 4 | 21 ± 4.5 | −1.19572 | 0.231808 |
| IL-7 | 14 ± 3 | 13 ± 2 | 14 ± 3.75 | −2.43243 | 0.014998 * |
| IL-12(p70) | 19 ± 4 | 18 ± 3 | 19 ± 5.25 | −1.82091 | 0.068622 |
| IL-17 | 15 ± 2 | 14 ± 3 | 15 ± 2 | −1.63984 | 0.101039 |
| IL-9 | 120 ± 25.25 | 113 ± 24 | 121.75 ± 25.75 | −1.04881 | 0.294264 |
| SCGF-β | 68 ± 28.5 | 62.5 ± 29 | 72 ± 28.5 | −1.55102 | 0.120899 |
| Whole Sample (n = 101) | |||
|---|---|---|---|
| Inflammation | |||
| Variables | Original Loadings | Boot Lower CI | Boot Upper CI |
| IL-8 | 0.082 | −0.137 | 0.673 |
| MIP-1α | 0.261 | −0.099 | 0.437 |
| CTACK | 0.275 | −0.14 | 0.566 |
| Eotaxin | 0.294 | −0.236 | 0.511 |
| SDF-1α | 0.313 | −0.292 | 0.605 |
| MIG | 0.351 | −0.057 | 0.55 |
| IL-13 | 0.401 | −0.215 | 0.75 |
| RANTES | 0.404 | 0.0731 | 0.632 |
| IL-18 | 0.437 | 0.032 | 0.653 |
| HGF | 0.439 | −0.172 | 0.731 |
| MCP-1 | 0.454 | −0.198 | 0.606 |
| G-CSF | 0.462 | 0.051 | 0.809 |
| IP-10 | 0.474 | −0.005 | 0.641 |
| IL-12p70 | 0.476 | −0.383 | 0.72 |
| SCF | 0.483 | −0.044 | 0.69 |
| IL-1β | 0.508 | −0.081 | 0.721 |
| IL-1α | 0.514 | −0.221 | 0.851 |
| SCGF-β | 0.521 | −0.073 | 0.685 |
| IL-5 | 0.54 | −0.346 | 0.779 |
| GM-CSF | 0.572 | 0.069 | 0.781 |
| IL-7 | 0.575 | −0.345 | 0.785 |
| IL-3 | 0.588 | −0.29 | 0.795 |
| PDGF-BB | 0.597 | 0.02 | 0.776 |
| MIP-1β | 0.613 | 0.035 | 0.778 |
| Basic-FGF | 0.63 | −0.247 | 0.842 |
| IL-2Rα | 0.652 | −0.015 | 0.838 |
| MIF | 0.655 | 0.145 | 0.842 |
| IL-9 | 0.658 | 0.021 | 0.814 |
| M-CSF | 0.659 | 0.042 | 0.819 |
| IL-4 | 0.662 | −0.246 | 0.796 |
| TNF-β | 0.662 | 0.074 | 0.815 |
| IL-6 | 0.665 | −0.248 | 0.834 |
| IL-12ip40 | 0.669 | −0.334 | 0.83 |
| β-NGF | 0.686 | −0.038 | 0.85 |
| GRO-α | 0.697 | 0.121 | 0.856 |
| TRAIL | 0.699 | 0.129 | 0.813 |
| IL-17 | 0.721 | −0.212 | 0.844 |
| VEGF | 0.722 | 0.008 | 0.845 |
| IL-10 | 0.742 | −0.109 | 0.826 |
| IFN-α2 | 0.756 | −0.13 | 0.841 |
| LIF | 0.76 | −0.18 | 0.837 |
| IL-16 | 0.763 | 0.192 | 0.839 |
| MCP-3 | 0.768 | −0.173 | 0.858 |
| IL-2 | 0.772 | −0.244 | 0.889 |
| TNF-α | 0.794 | −0.05 | 0.883 |
| IL-1ra | 0.8 | 0.036 | 0.855 |
| IL-15 | 0.8 | −0.058 | 0.883 |
| IFN-γ | 0.806 | 0.12 | 0.881 |
| Cognition | |||
| BACS—Psychomotor coordination | 0.585 | 0.211 | 0.756 |
| BACS—Verbal memory | 0.646 | 0.219 | 0.779 |
| BACS—Verbal fluency | 0.701 | 0.485 | 0.81 |
| BACS—Executive functions | 0.702 | 0.494 | 0.854 |
| BACS—Working memory | 0.705 | 0.521 | 0.818 |
| BACS—Selective attention | 0.828 | 0.691 | 0.883 |
| Depression | |||
| BDI-13 | 0.901 | 0.826 | 0.961 |
| ZSDS | 0.957 | 0.912 | 0.984 |
| Females (n = 45) | Males (n = 56) | |||||
|---|---|---|---|---|---|---|
| Inflammation | ||||||
| Variables | Original Loadings | Boot Lower CI | Boot Upper CI | Original Loadings | Boot Lower CI | Boot Upper CI |
| Basic-FGF | 0.742 | 0.387 | 0.872 | 0.667 | −0.093 | 0.891 |
| CTACK | 0.019 | −0.387 | 0.339 | 0.046 | −0.34 | 0.721 |
| Eotaxin | 0.332 | −0.121 | 0.701 | 0.352 | −0.151 | 0.572 |
| G-CSF | 0.571 | 0.235 | 0.787 | −0.021 | −0.308 | 0.803 |
| GM-CSF | 0.723 | 0.499 | 0.883 | 0.094 | −0.357 | 0.705 |
| GRO-α | 0.794 | 0.561 | 0.895 | 0.633 | 0.083 | 0.854 |
| HGF | 0.360 | −0.065 | 0.673 | −0.153 | −0.585 | 0.787 |
| IFN-α2 | 0.737 | 0.424 | 0.872 | 0.651 | 0.082 | 0.838 |
| IFN-γ | 0.835 | 0.569 | 0.907 | 0.529 | 0.053 | 0.885 |
| IL-10 | 0.766 | 0.471 | 0.902 | 0.649 | 0.016 | 0.832 |
| IL-12ip40 | 0.603 | 0.258 | 0.801 | 0.8 | −0.136 | 0.839 |
| IL-12p70 | 0.490 | −0.039 | 0.745 | 0.598 | −0.164 | 0.772 |
| IL-13 | 0.356 | −0.020 | 0.672 | −0.255 | −0.683 | 0.732 |
| IL-15 | 0.827 | 0.449 | 0.915 | 0.726 | 0.116 | 0.908 |
| IL-16 | 0.722 | 0.475 | 0.817 | 0.262 | −0.276 | 0.851 |
| IL-17 | 0.698 | 0.303 | 0.868 | 0.754 | −0.007 | 0.855 |
| IL-18 | 0.521 | 0.096 | 0.819 | −0.033 | −0.475 | 0.636 |
| IL-1ra | 0.820 | 0.545 | 0.902 | 0.62 | 0.085 | 0.854 |
| IL-1α | 0.754 | 0.389 | 0.876 | 0.538 | −0.064 | 0.895 |
| IL-1β | 0.389 | 0.075 | 0.720 | −0.104 | −0.594 | 0.737 |
| IL-2 | 0.769 | 0.364 | 0.887 | 0.85 | 0.005 | 0.903 |
| IL-2Rα | 0.612 | 0.088 | 0.865 | 0.626 | 0.036 | 0.894 |
| IL-3 | 0.731 | 0.268 | 0.888 | 0.628 | −0.089 | 0.793 |
| IL-4 | 0.637 | 0.331 | 0.791 | 0.706 | 0.001 | 0.841 |
| IL-5 | 0.575 | 0.020 | 0.823 | 0.793 | −0.2 | 0.874 |
| IL-6 | 0.736 | 0.234 | 0.887 | 0.702 | 0.004 | 0.869 |
| IL-7 | 0.566 | 0.190 | 0.763 | 0.738 | −0.091 | 0.821 |
| IL-8 | −0.048 | −0.281 | 0.744 | 0.384 | −0.04 | 0.707 |
| IL-9 | 0.792 | 0.530 | 0.886 | 0.653 | 0.032 | 0.827 |
| IP-10 | 0.305 | 0.003 | 0.677 | 0.239 | −0.232 | 0.682 |
| LIF | 0.631 | 0.308 | 0.818 | 0.728 | 0.062 | 0.873 |
| MCP-1 | 0.475 | 0.098 | 0.684 | 0.483 | −0.044 | 0.635 |
| MCP-3 | 0.728 | 0.466 | 0.870 | 0.78 | −0.032 | 0.877 |
| M-CSF | 0.685 | 0.197 | 0.883 | 0.414 | −0.089 | 0.814 |
| MIF | 0.690 | 0.381 | 0.828 | 0.401 | −0.129 | 0.855 |
| MIG | 0.056 | −0.200 | 0.412 | 0.138 | −0.292 | 0.724 |
| MIP-1α | −0.070 | −0.354 | 0.290 | −0.146 | −0.347 | 0.549 |
| MIP-1β | 0.728 | 0.485 | 0.837 | 0.607 | 0.027 | 0.78 |
| PDGF-BB | 0.694 | 0.432 | 0.832 | 0.653 | 0.034 | 0.798 |
| RANTES | 0.413 | 0.087 | 0.669 | 0.328 | −0.091 | 0.653 |
| SCF | 0.437 | 0.068 | 0.683 | −0.08 | −0.559 | 0.684 |
| SCGF-β | 0.457 | 0.051 | 0.710 | 0.461 | 0.019 | 0.742 |
| SDF-1α | 0.328 | −0.193 | 0.641 | 0.546 | −0.16 | 0.735 |
| TNF-α | 0.824 | 0.556 | 0.898 | 0.827 | 0.014 | 0.918 |
| TNF-β | 0.782 | 0.552 | 0.882 | 0.634 | 0.029 | 0.808 |
| TRAIL | 0.645 | 0.370 | 0.780 | 0.34 | −0.183 | 0.814 |
| VEGF | 0.736 | 0.321 | 0.876 | 0.56 | 0.064 | 0.871 |
| β-NGF | 0.619 | 0.305 | 0.839 | 0.765 | 0.087 | 0.919 |
| Cognition | ||||||
| BACS—Executive functions | 0.565 | −0.311 | 0.839 | 0.783 | 0.613 | 0.871 |
| BACS—Psychomotor coordination | 0.278 | −0.333 | 0.716 | 0.666 | 0.282 | 0.844 |
| BACS—Selective attention | 0.749 | 0.050 | 0.883 | 0.829 | 0.667 | 0.892 |
| BACS—Verbal fluency | 0.798 | 0.145 | 0.880 | 0.725 | 0.399 | 0.847 |
| BACS—Verbal memory | 0.760 | 0.068 | 0.871 | 0.592 | 0.182 | 0.826 |
| BACS—Working memory | 0.755 | 0.185 | 0.881 | 0.713 | 0.44 | 0.832 |
| Depression | ||||||
| BDI-13 | 0.953 | 0.674 | 0.998 | 0.844 | 0.554 | 0.939 |
| ZSDS | 0.953 | 0.701 | 0.998 | 0.921 | 0.855 | 0.992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palladini, M.; Mazza, M.G.; Bravi, B.; Bessi, M.; Lorenzi, M.C.; Spadini, S.; De Lorenzo, R.; Rovere-Querini, P.; Furlan, R.; Benedetti, F. Sex-Specific Inflammatory Profiles Affect Neuropsychiatric Issues in COVID-19 Survivors. Biomolecules 2025, 15, 600. https://doi.org/10.3390/biom15040600
Palladini M, Mazza MG, Bravi B, Bessi M, Lorenzi MC, Spadini S, De Lorenzo R, Rovere-Querini P, Furlan R, Benedetti F. Sex-Specific Inflammatory Profiles Affect Neuropsychiatric Issues in COVID-19 Survivors. Biomolecules. 2025; 15(4):600. https://doi.org/10.3390/biom15040600
Chicago/Turabian StylePalladini, Mariagrazia, Mario Gennaro Mazza, Beatrice Bravi, Margherita Bessi, Maria Cristina Lorenzi, Sara Spadini, Rebecca De Lorenzo, Patrizia Rovere-Querini, Roberto Furlan, and Francesco Benedetti. 2025. "Sex-Specific Inflammatory Profiles Affect Neuropsychiatric Issues in COVID-19 Survivors" Biomolecules 15, no. 4: 600. https://doi.org/10.3390/biom15040600
APA StylePalladini, M., Mazza, M. G., Bravi, B., Bessi, M., Lorenzi, M. C., Spadini, S., De Lorenzo, R., Rovere-Querini, P., Furlan, R., & Benedetti, F. (2025). Sex-Specific Inflammatory Profiles Affect Neuropsychiatric Issues in COVID-19 Survivors. Biomolecules, 15(4), 600. https://doi.org/10.3390/biom15040600







