Small but Mighty: Nanobodies in the Fight Against Infectious Diseases
Abstract
:1. Introduction
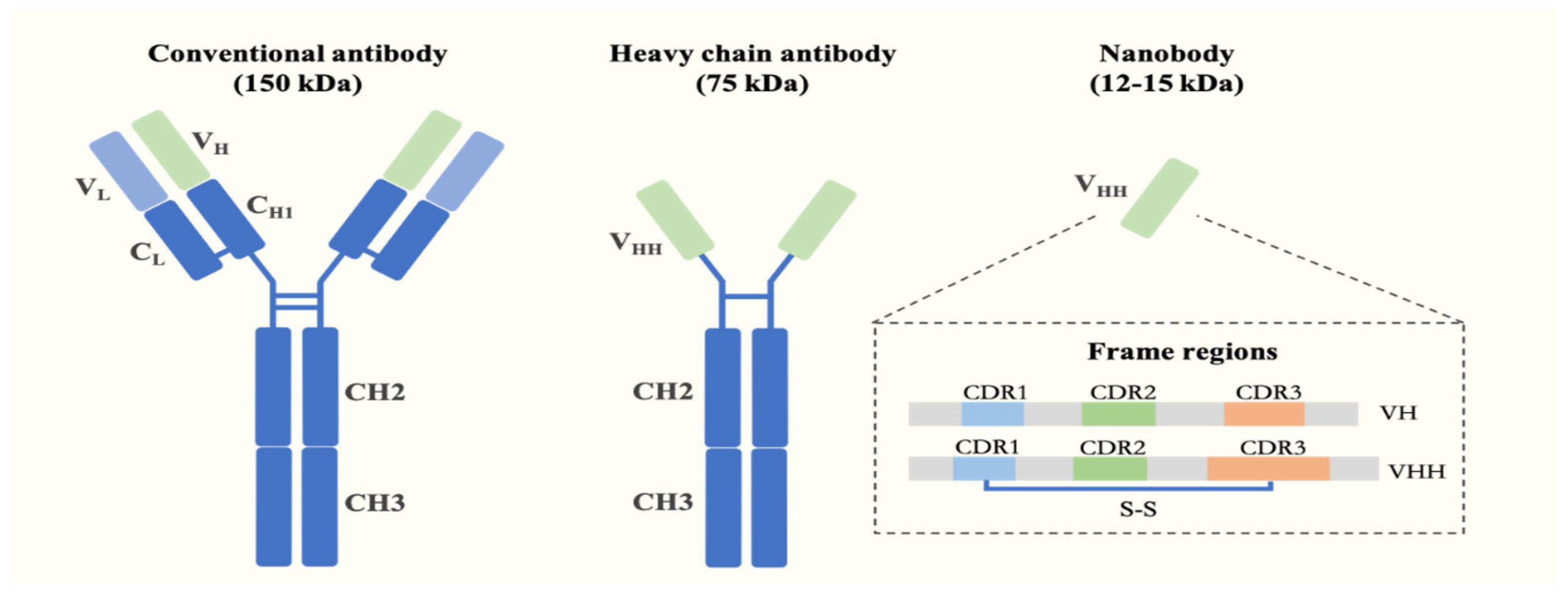
2. Characteristics and Preparation of Nanobodies
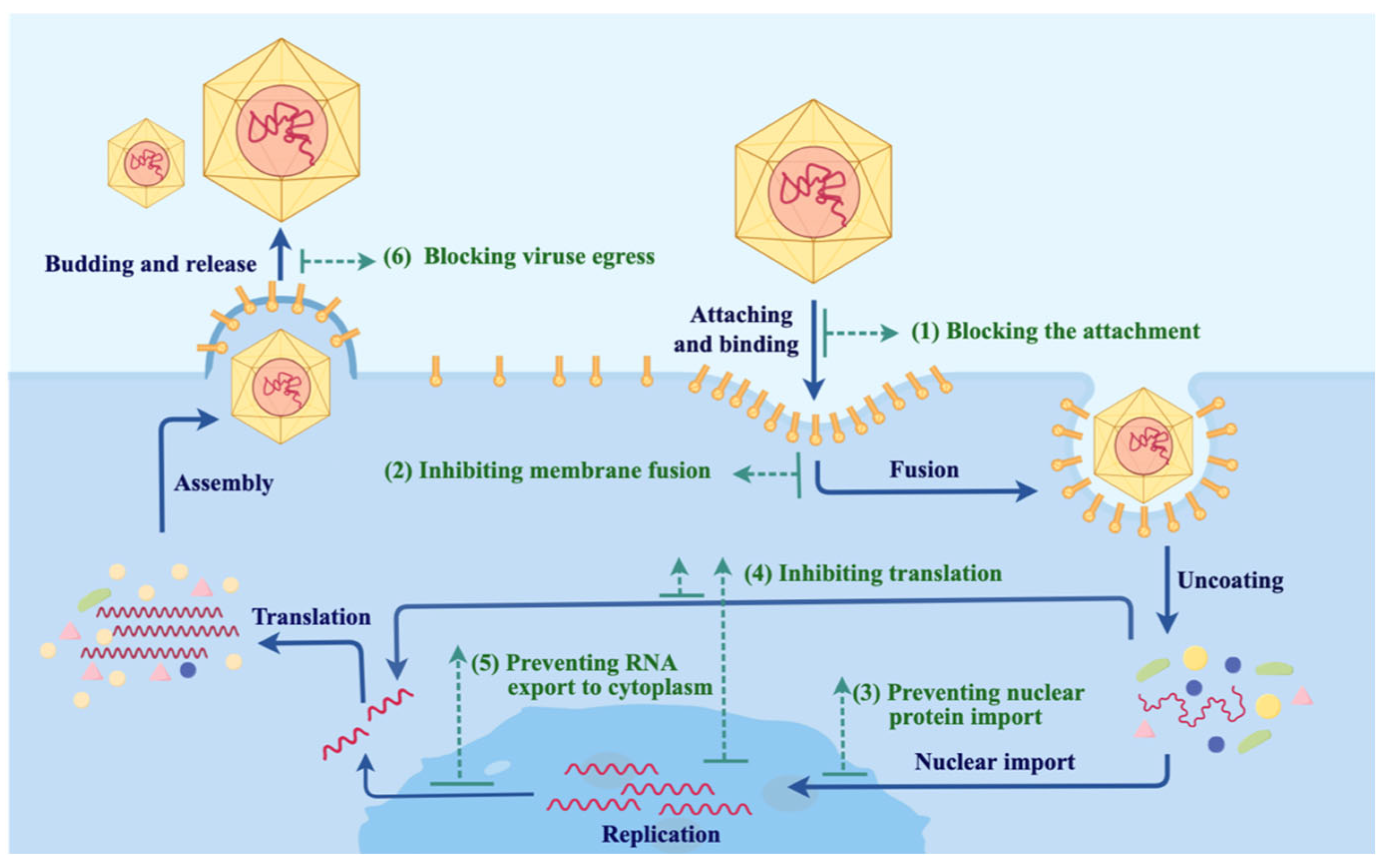
3. Viral Infection Pathways and Possible Intervention Strategies with Nbs
3.1. Blocking Virus–Host Recognition and Membrane Fusion
3.2. Inhibiting Intracellular Viral Replication and Transcription
3.3. Inhibiting the Egress of Newly Made Viruses
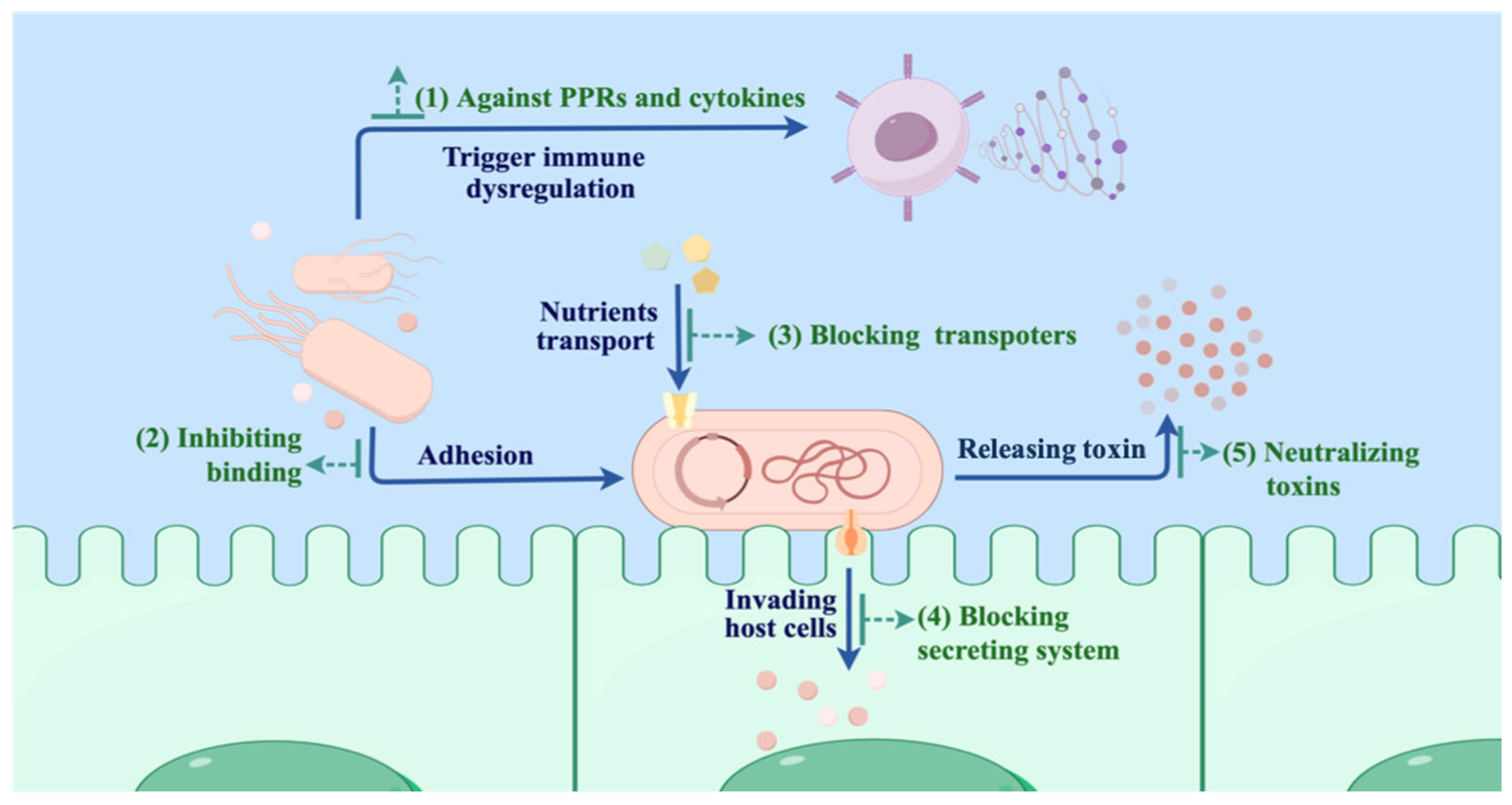
4. Mechanisms and Intervention Strategies of Nbs in Bacterial Infectious Treatment
4.1. Inhibiting Bacterial Adhesion, Colonization, and Growth
4.2. Blocking Bacterial Invasion
4.3. Neutralizing Bacterial Toxins
4.4. Blocking Pattern Recognition Receptors
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Mahon, M.B.; Sack, A.; Aleuy, O.A.; Barbera, C.; Brown, E.; Buelow, H.; Civitello, D.J.; Cohen, J.M.; de Wit, L.A.; Forstchen, M.; et al. A meta-analysis on global change drivers and the risk of infectious disease. Nature 2024, 629, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, E.; Ghasemian, A.; Abdinasir, A.; Nematollahi Mahani, S.A.; Rawaf, S.; Salehi Vaziri, M.; Gouya, M.M.; Minh Nhu Nguyen, T.; Al Awaidy, S.; Al Ariqi, L.; et al. Emerging and Re-emerging Infectious Diseases in the WHO Eastern Mediterranean Region, 2001-2018. Int. J. Health Policy Manag. 2022, 11, 1286–1300. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Schaible, K.; Gouveia-Pisano, J.A.; Yehoshua, A.; Malhotra, D.; Di Fusco, M.; Cha-Silva, A.S.; Andersen, K.M.; Nicholls, L.; Landi, S.N.; et al. Economic burden of COVID-19 for employers and employees in the United States. J. Med. Econ. 2024, 27, 267–278. [Google Scholar] [CrossRef]
- Casel, M.A.; Park, S.J.; Choi, Y.K. Severe fever with thrombocytopenia syndrome virus: Emerging novel phlebovirus and their control strategy. Exp. Mol. Med. 2021, 53, 713–722. [Google Scholar] [CrossRef]
- Killerby, M.E.; Biggs, H.M.; Midgley, C.M.; Gerber, S.I.; Watson, J.T. Middle East Respiratory Syndrome Coronavirus Transmission. Emerg. Infect. Dis. 2020, 26, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on Zika virus infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A.; Hewlett, A.; Kraft, C.S.; Vega, M.-A.d.L.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020-21. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: Development strategies and progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Chenoweth, A.; Crescioli, S.; Reichert, J.M. Antibodies to watch in 2022. mAbs 2022, 14, 2014296. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, H.; Wirth, T.; Schenkwein, D.; Räty, J.K.; Ylä-Herttuala, S. Challenges in monoclonal antibody-based therapies. Ann. Med. 2009, 41, 322–331. [Google Scholar] [CrossRef]
- Liu, M.M.; Li, L.; Jin, D.; Liu, Y.Z. Nanobody-A versatile tool for cancer diagnosis and therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1697. [Google Scholar] [CrossRef]
- Minatel, V.M.; Prudencio, C.R.; Barraviera, B.; Ferreira, R.S., Jr. Nanobodies: A promising approach to treatment of viral diseases. Front. Immunol. 2024, 14, 1303353. [Google Scholar] [CrossRef]
- Bruce, V.J.; Ta, A.N.; McNaughton, B.R. Minimalist Antibodies and Mimetics: An Update and Recent Applications. Chembiochem 2016, 17, 1892–1899. [Google Scholar] [CrossRef]
- Hamerscasterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally-occurring antibodies devoid of light-Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E.C.; Flajnik, M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 1995, 374, 168–173. [Google Scholar] [CrossRef]
- Wu, T.T.; Johnson, G.; Kabat, E.A. Length distribution of cdrh3 in antibodies. Proteins-Struct. Funct. Genet. 1993, 16, 1–7. [Google Scholar] [CrossRef]
- Muyldermans, S.; Atarhouch, T.; Saldanha, J.; Barbosa, J.A.R.G.; Hamers, R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. Des. Sel. 1994, 7, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Xun, G.J.; Song, X.P.; Hu, J.; Zhang, H.W.; Liu, L.; Zhang, Z.; Gong, R. Potent Human Single-Domain Antibodies Specific for a Novel Prefusion Epitope of Respiratory Syncytial Virus F Glycoprotein. J. Virol. 2021, 95, e0048521. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, M.; Conrath, K.; Meirhaeghe, A.V.; Meersman, F.; Heremans, K.; Frenken, L.G.J.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002, 11, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhan, W.Q.; Yang, Z.L.; Tu, C.; Hu, G.W.; Zhang, X.; Song, W.P.; Du, S.J.; Zhu, Y.F.; Huang, K.K.; et al. Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific single-domain antibody. Cell 2022, 185, 1389. [Google Scholar] [CrossRef]
- Wu, X.L.; Cheng, L.; Fu, M.; Huang, B.L.; Zhu, L.J.; Xu, S.J.; Shi, H.X.; Zhang, D.D.; Yuan, H.Y.; Nawaz, W.; et al. A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration. Cell Rep. 2021, 37, 109869. [Google Scholar] [CrossRef]
- Liu, B.Y.; Yang, D.W. Easily Established and Multifunctional Synthetic Nanobody Libraries as Research Tools. Int. J. Mol. Sci. 2022, 23, 1482. [Google Scholar] [CrossRef]
- Abbady, A.; Al-Mariri, A.; Zarkawi, M.; Al-Assad, A.; Muyldermans, S. Evaluation of a nanobody phage display library constructed from a Brucella-immunised camel. Vet. Immunol. Immunopathol. 2011, 142, 49–56. [Google Scholar] [CrossRef]
- Liu, W.; Song, H.; Chen, Q.; Yu, J.; Xian, M.; Nian, R.; Feng, D. Recent advances in the selection and identification of antigen-specific nanobodies. Mol. Immunol. 2018, 96, 37–47. [Google Scholar] [CrossRef]
- Wilton, E.E.; Opyr, M.P.; Kailasam, S.; Kothe, R.F.; Wieden, H.J. sdAb-DB: The Single Domain Antibody Database. ACS Synth. Biol. 2018, 7, 2480–2484. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Muyldermans, S. A guide to: Generation and design of nanobodies. FEBS J. 2021, 288, 2084–2102. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.-S. Molecular Virology of Human Pathogenic Viruses; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; McLellan, J.S. Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef]
- Cunningham, S.; Piedra, P.A.; Martinon-Torres, F.; Szymanski, H.; Brackeva, B.; Dombrecht, E.; Detalle, L.; Fleurinck, C.; Grp, R.S. Nebulised ALX-0171 for respiratory syncytial virus lower respiratory tract infection in hospitalised children: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 21–32. [Google Scholar] [CrossRef]
- Rossey, I.; Gilman, M.S.A.; Kabeche, S.C.; Sedeyn, K.; Wrapp, D.; Kanekiyo, M.; Chen, M.; Mas, V.; Spitaels, J.; Melero, J.A.; et al. Potent single-domain antibodies that arrest respiratory syncytial virus fusion protein in its prefusion state. Nat. Commun. 2017, 8, 14158. [Google Scholar] [CrossRef]
- Rossey, I.; Hsieh, C.L.; Sedeyn, K.; Ballegeer, M.; Schepens, B.; McLellan, J.S.; Saelens, X. A Vulnerable, Membrane-Proximal Site in Human Respiratory Syncytial Virus F Revealed by a Prefusion-Specific Single-Domain Antibody. J. Virol. 2021, 95, 10.1128. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020, 295, 12910–12934. [Google Scholar] [CrossRef]
- Wrapp, D.; De Vlieger, D.; Corbett, K.S.; Torres, G.M.; Wang, N.S.; Van Breedam, W.; Roose, K.; van Schie, L.; Hoffmann, M.; Pöhlmann, S.; et al. Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell 2020, 181, 1436–1441. [Google Scholar] [CrossRef]
- Nambulli, S.; Xiang, Y.; Tilston-Lunel, N.L.; Rennick, L.J.; Sang, Z.; Klimstra, W.B.; Reed, D.S.; Crossland, N.A.; Shi, Y.; Duprex, W.P. Inhalable Nanobody (PiN-21) prevents and treats SARS-CoV-2 infections in Syrian hamsters at ultra-low doses. Sci. Adv. 2021, 7, eabh0319. [Google Scholar] [CrossRef]
- Sun, D.; Sang, Z.; Kim, Y.J.; Xiang, Y.; Cohen, T.; Belford, A.K.; Huet, A.; Conway, J.F.; Sun, J.; Taylor, D.J.; et al. Potent neutralizing nanobodies resist convergent circulating variants of SARS-CoV-2 by targeting diverse and conserved epitopes. Nat. Commun. 2021, 12, 4676. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, K.; Jung, S.; Conte, A.; Lieberman, J.; Muecksch, F.; Lorenzi, J.C.C.; Park, S.; Schmidt, F.; Wang, Z.; et al. Nanobodies from camelid mice and llamas neutralize SARS-CoV-2 variants. Nature 2021, 595, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Esparza, T.J.; Martin, N.P.; Anderson, G.P.; Goldman, E.R.; Brody, D.L. High affinity nanobodies block SARS-CoV-2 spike receptor binding domain interaction with human angiotensin converting enzyme. Sci. Rep. 2020, 10, 22370. [Google Scholar] [CrossRef] [PubMed]
- Güttler, T.; Aksu, M.; Dickmanns, A.; Stegmann, K.M.; Gregor, K.; Rees, R.; Taxer, W.; Rymarenko, O.; Schünemann, J.; Dienemann, C.; et al. Neutralization of SARS-CoV-2 by highly potent, hyperthermostable, and mutation-tolerant nanobodies. EMBO J. 2021, 40, e107985. [Google Scholar] [CrossRef]
- Gai, J.; Ma, L.; Li, G.; Zhu, M.; Qiao, P.; Li, X.; Zhang, H.; Zhang, Y.; Chen, Y.; Ji, W.; et al. A potent neutralizing nanobody against SARS-CoV-2 with inhaled delivery potential. MedComm 2021, 2, 101–113. [Google Scholar] [CrossRef]
- Li, T.T.; Cai, H.M.; Yao, H.B.; Zhou, B.J.; Zhang, N.; van Vlissingen, M.F.; Kuiken, T.; Han, W.Y.; GeurtsvanKessel, C.H.; Gong, Y.H.; et al. A synthetic nanobody targeting RBD protects hamsters from SARS-CoV-2 infection. Nat. Commun. 2021, 12, 4635. [Google Scholar] [CrossRef]
- Wu, Y.L.; Li, C.; Xia, S.; Tian, X.L.; Kong, Y.; Wang, Z.; Gu, C.J.; Zhang, R.; Tu, C.; Xie, Y.H.; et al. Identification of Human Single-Domain Antibodies against SARS-CoV-2. Cell Host Microbe 2020, 27, 891. [Google Scholar] [CrossRef]
- Custódio, T.F.; Das, H.; Sheward, D.J.; Hanke, L.; Pazicky, S.; Pieprzyk, J.; Sorgenfrei, M.; Schroer, M.A.; Gruzinov, A.Y.; Jeffries, C.M.; et al. Selection, biophysical and structural analysis of synthetic nanobodies that effectively neutralize SARS-CoV-2. Nat. Commun. 2020, 11, 5588. [Google Scholar] [CrossRef]
- Chi, X.; Liu, X.; Wang, C.; Zhang, X.; Li, X.; Hou, J.; Ren, L.; Jin, Q.; Wang, J.; Yang, W. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat. Commun. 2020, 11, 4528. [Google Scholar] [CrossRef]
- Chen, X.; Gentili, M.; Hacohen, N.; Regev, A. A cell-free nanobody engineering platform rapidly generates SARS-CoV-2 neutralizing nanobodies. Nat. Commun. 2021, 12, 5506. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, W.; Meng, X.; Huang, X.; Yang, Y.; Zhao, D.; Zhou, P.; Wang, X.; Zhao, C.; Sun, Y.; et al. Potent Neutralization of SARS-CoV-2 by Hetero-Bivalent Alpaca Nanobodies Targeting the Spike Receptor-Binding Domain. J. Virol. 2021, 95, e02438-20. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, Z.; Li, H.; Zhong, K.; Zhao, Q.; Wang, Z.; Wu, Z.; Yang, D.; Sun, S.; Yang, N.; et al. Development of multivalent nanobodies blocking SARS-CoV-2 infection by targeting RBD of spike protein. J. Nanobiotechnol. 2021, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Mikolajek, H.; Le Bas, A.; Clark, J.J.; Sharma, P.; Kipar, A.; Dormon, J.; Norman, C.; Weckener, M.; Clare, D.K.; et al. A potent SARS-CoV-2 neutralising nanobody shows therapeutic efficacy in the Syrian golden hamster model of COVID-19. Nat. Commun. 2021, 12, 5469. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Huang, B.; Jia, Z.; Wang, B.; Gallolu Kankanamalage, S.; Titong, A.; Liu, Y. Development of multi-specific humanized llama antibodies blocking SARS-CoV-2/ACE2 interaction with high affinity and avidity. Emerg. Microbes Infect. 2020, 9, 1034–1036. [Google Scholar] [CrossRef]
- Schoof, M.; Faust, B.; Saunders, R.A.; Sangwan, S.; Rezelj, V.; Hoppe, N.; Boone, M.; Billesbølle, C.B.; Puchades, C.; Azumaya, C.M.; et al. An ultrapotent synthetic nanobody neutralizes SARS-CoV-2 by stabilizing inactive Spike. Science 2020, 370, 1473–1479. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Zheng, P.; Dube, P.H.; Zeng, W.; Chen, S.; Cheng, Q.; Yang, Y.; Wu, Y.; Zhou, J.; et al. Hetero-bivalent nanobodies provide broad-spectrum protection against SARS-CoV-2 variants of concern including Omicron. Cell Res. 2022, 32, 831–842. [Google Scholar] [CrossRef]
- Haga, K.; Takai-Todaka, R.; Matsumura, Y.; Song, C.; Takano, T.; Tojo, T.; Nagami, A.; Ishida, Y.; Masaki, H.; Tsuchiya, M.; et al. Nasal delivery of single-domain antibody improves symptoms of SARS-CoV-2 infection in an animal model. PLOS Pathog. 2021, 17, e1009542. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Feng, B.; Li, C.; Zhang, Z.; Huang, Y.; Liu, B.; Zhang, Z.; Luo, J.; Wang, Q.; Yin, L.; Chen, S.; et al. A shark-derived broadly neutralizing nanobody targeting a highly conserved epitope on the S2 domain of sarbecoviruses. J. Nanobiotechnol. 2025, 23, 110. [Google Scholar] [CrossRef]
- Zhao, S.; Zeng, W.; Yu, F.; Xu, P.; Chen, C.-Y.; Chen, W.; Dong, Y.; Wang, F.; Ma, L. Visual and High-Efficiency Secretion of SARS-CoV-2 Nanobodies with Escherichia coli. Biomolecules 2025, 15, 111. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. The Pathology of Influenza Virus Infections. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Hu, D.; Wu, Y.L.; Ying, T.L. Recent advances in “universal” influenza virus antibodies: The rise of a hidden trimeric interface in hemagglutinin globular head. Front. Med. 2020, 14, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Gaiotto, T.; Ramage, W.; Ball, C.; Risley, P.; Carnell, G.W.; Temperton, N.; Engelhardt, O.G.; Hufton, S.E. Nanobodies mapped to cross-reactive and divergent epitopes on A(H7N9) influenza hemagglutinin using yeast display. Sci. Rep. 2021, 11, 3126. [Google Scholar] [CrossRef]
- Ramage, W.; Gaiotto, T.; Ball, C.; Risley, P.; Carnell, G.W.; Temperton, N.; Cheung, C.Y.; Engelhardt, O.G.; Hufton, S.E. Cross-Reactive and Lineage-Specific Single Domain Antibodies against Influenza B Hemagglutinin. Antibodies 2019, 8, 14. [Google Scholar] [CrossRef]
- Tillib, S.V.; Ivanova, T.I.; Vasilev, L.A.; Rutovskaya, M.V.; Saakyan, S.A.; Gribova, I.Y.; Tutykhina, I.L.; Sedova, E.S.; Lysenko, A.A.; Shmarov, M.M.; et al. Formatted single-domain antibodies can protect mice against infection with influenza virus (H5N2). Antivir. Res. 2013, 97, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Shcheblyakov, D.V.; Voronina, D.V.; Favorskaya, I.A.; Esmagambetov, I.B.; Alekseeva, I.A.; Korobkova, A.I.; Ryabova, E.I.; Derkaev, A.A.; Kan, V.Y.; Dzharullaeva, A.S.; et al. Broadly Reactive Nanobody Targeting the H3 Hemagglutinin of the Influenza A Virus. Acta Naturae 2024, 16, 101–110. [Google Scholar] [CrossRef]
- Guthmiller, J.J.; Han, J.; Utset, H.A.; Li, L.; Lan, L.Y.-L.; Henry, C.; Stamper, C.T.; McMahon, M.; O’Dell, G.; Fernández-Quintero, M.L.; et al. Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature 2022, 602, 314–320. [Google Scholar] [CrossRef]
- Barbieri, E.S.; Sosa-Holt, C.; Ibaez, L.I.; Baztarrica, J.; Garaicoechea, L.; Gay, C.L.; Caceres, C.J.; Aduriz, M.; Baumeister, E.; Escribano, J.A. Anti-hemagglutinin monomeric nanobody provides prophylactic immunity against H1 subtype influenza A viruses. PLoS ONE 2024, 19, 24. [Google Scholar] [CrossRef]
- Chen, Z.-S.; Huang, H.-C.; Wang, X.; Schön, K.; Jia, Y.; Lebens, M.; Besavilla, D.F.; Murti, J.R.; Ji, Y.; Sarshad, A.A.; et al. Influenza A Virus H7 nanobody recognizes a conserved immunodominant epitope on hemagglutinin head and confers heterosubtypic protection. Nat. Commun. 2025, 16, 432. [Google Scholar] [CrossRef]
- Voronina, D.V.; Shcheblyakov, D.V.; Esmagambetov, I.B.; Derkaev, A.A.; Popova, O.; Shcherbinin, D.N. Development of Neutralizing Nanobodies to the Hemagglutinin Stem Domain of Influenza A Viruses. Acta Naturae 2021, 13, 33–41. [Google Scholar] [CrossRef]
- Hufton, S.E.; Risley, P.; Ball, C.R.; Major, D.; Engelhardt, O.G.; Poole, S. The Breadth of Cross Sub-Type Neutralisation Activity of a Single Domain Antibody to Influenza Hemagglutinin Can Be Increased by Antibody Valency. PLoS ONE 2014, 9, e103294. [Google Scholar] [CrossRef] [PubMed]
- Jähnichen, S.; Blanchetot, C.; Maussang, D.; Gonzalez-Pajuelo, M.; Chow, K.Y.; Bosch, L.; De Vrieze, S.; Serruys, B.; Ulrichts, H.; Vandevelde, W.; et al. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 20565–20570. [Google Scholar] [CrossRef] [PubMed]
- Bobkov, V.; Zarca, A.M.; Van Hout, A.; Arimont, M.; Doijen, J.; Bialkowska, M.; Toffoli, E.; Klarenbeek, A.; van der Woning, B.; van der Vliet, H.J.; et al. Nanobody-Fc constructs targeting chemokine receptor CXCR4 potently inhibit signaling and CXCR4-mediated HIV-entry and induce antibody effector functions. Biochem. Pharmacol. 2018, 158, 413–424. [Google Scholar] [CrossRef]
- Van Hout, A.; Klarenbeek, A.; Bobkov, V.; Doijen, J.; Arimont, M.; Zhao, C.; Heukers, R.; Rimkunas, R.; de Graaf, C.; Verrips, T.; et al. CXCR4-targeting nanobodies differentially inhibit CXCR4 function and HIV entry. Biochem. Pharmacol. 2018, 158, 402–412. [Google Scholar] [CrossRef]
- Cunha-Santos, C.; Perdigao, P.R.L.; Martin, F.; Oliveira, J.G.; Cardoso, M.; Manuel, A.; Taveira, N.; Goncalves, J. Inhibition of HIV replication through siRNA carried by CXCR4-targeted chimeric nanobody. Cell. Mol. Life Sci. 2020, 77, 2859–2870. [Google Scholar] [CrossRef]
- Strauss, M.; Schotte, L.; Thys, B.; Filman, D.J.; Hogle, J.M. Five of Five VHHs Neutralizing Poliovirus Bind the Receptor-Binding Site. J. Virol. 2016, 90, 3496–3505. [Google Scholar] [CrossRef]
- Schotte, L.; Strauss, M.; Thys, B.; Halewyck, H.; Filman, D.J.; Bostina, M.; Hogle, J.M.; Rombaut, B. Mechanism of action and capsid-stabilizing properties of VHHs with an in-vitro antipolioviral activity. J. Virol. 2014, 88, 4403–4413. [Google Scholar] [CrossRef]
- Thys, B.; Schotte, L.; Muyldermans, S.; Wernery, U.; Hassanzadeh-Ghassabeh, G.; Rombaut, B. In vitro antiviral activity of single domain antibody fragments against poliovirus. Antivir. Res. 2010, 87, 257–264. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, Y.; Ao, Z.; Chen, Y.; Jiang, Y.; Chen, X.; Qi, R.; Fu, B.; Tang, J.; Fang, M.; et al. A broad-spectrum nanobody targeting the C-terminus of the hepatitis B surface antigen for chronic hepatitis B infection therapy. Antivir. Res. 2022, 199, 105265. [Google Scholar] [CrossRef]
- Tarr, A.W.; Lafaye, P.; Meredith, L.; Damier-Piolle, L.; Urbanowicz, R.A.; Meola, A.; Jestin, J.L.; Brown, R.J.P.; McKeating, J.A.; Rey, F.A.; et al. An Alpaca Nanobody Inhibits Hepatitis C Virus Entry and Cell-to-Cell Transmission. Hepatology 2013, 58, 932–939. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Jiang, K.; Shan, H.; Shi, M.; Chen, B.; Hua, Z. Nanobody against the E7 oncoprotein of human papillomavirus 16. Mol. Immunol. 2019, 109, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Maffey, L.; Vega, C.G.; Miño, S.; Garaicoechea, L.; Parreño, V. Anti-VP6 VHH: An Experimental Treatment for Rotavirus A-Associated Disease. PLoS ONE 2016, 11, e0162351. [Google Scholar] [CrossRef] [PubMed]
- Esmagambetov, I.B.; Shcheblyakov, D.V.; Egorova, D.A.; Voronina, O.L.; Derkaev, A.A.; Voronina, D.V.; Popova, O.; Ryabova, E.I.; Shcherbinin, D.N.; Aksenova, E.I.; et al. Nanobodies Are Potential Therapeutic Agents for the Ebola Virus Infection. Acta Naturae 2021, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Webb, E.M.; Zabetakis, D.; Burke, C.W.; Gardner, C.L.; Glass, P.J.; Legler, P.M.; Weger-Lucarelli, J.; Anderson, G.P.; Goldman, E.R. Stabilization of a Broadly Neutralizing Anti-Chikungunya Virus Single Domain Antibody. Front. Med. 2021, 8, 626028. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Shriver-Lake, L.C.; Zabetakis, D.; Anderson, G.P.; Goldman, E.R. Selection and characterization of protective anti-chikungunya virus single domain antibodies. Mol. Immunol. 2019, 105, 190–197. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Lu, Y.; Hu, H.; Wang, J.; Guo, C.; Deng, Q.; Liao, C.; Wu, Q.; Hu, T.; et al. Highly potent multivalent VHH antibodies against Chikungunya isolated from an alpaca naïve phage display library. J. Nanobiotechnol. 2022, 20, 231. [Google Scholar] [CrossRef]
- Odchimar, N.M.O.; Dulay, A.N.G.; Orosco, F.L. Molecular modelling and optimization of a high-affinity nanobody targeting the nipah virus fusion protein through in silico site-directed mutagenesis. Comput. Biol. Chem. 2025, 115, 108354. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Huang, B.; Ma, X.; Zhu, L.; Zheng, N.; Xu, S.; Nawaz, W.; Xu, C.; Wu, Z. A single-domain antibody inhibits SFTSV and mitigates virus-induced pathogenesis in vivo. JCI Insight 2020, 5, e136855. [Google Scholar] [CrossRef]
- Detalle, L.; Stohr, T.; Palomo, C.; Piedra, P.A.; Gilbert, B.E.; Mas, V.; Millar, A.; Power, U.F.; Stortelers, C.; Allosery, K.; et al. Generation and Characterization of ALX-0171, a Potent Novel Therapeutic Nanobody for the Treatment of Respiratory Syncytial Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 6–13. [Google Scholar] [CrossRef]
- Hultberg, A.; Temperton, N.J.; Rosseels, V.; Koenders, M.; Gonzalez-Pajuelo, M.; Schepens, B.; Ibañez, L.I.; Vanlandschoot, P.; Schillemans, J.; Saunders, M. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS ONE 2011, 6, e17665. [Google Scholar] [CrossRef]
- Schepens, B.; Ibañez, L.I.; De Baets, S.; Hultberg, A.; Bogaert, P.; De Bleser, P.; Vervalle, F.; Verrips, T.; Melero, J.; Vandevelde, W.; et al. Nanobodies® Specific for Respiratory Syncytial Virus Fusion Protein Protect Against Infection by Inhibition of Fusion. J. Infect. Dis. 2011, 204, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Brecher, M.; Soufal, A.; Gaiotto, T.; Tian, S.; Chandramouli, S.; Dewar, V.; Ferrant, L.; Zhang, M.; Zhou, X.; et al. Structural interrogation of a trimeric prefusion RSV fusion protein vaccine candidate by a camelid nanobody. Vaccine 2023, 41, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Koenig, P.-A.; Das, H.; Liu, H.; Kümmerer, B.M.; Gohr, F.N.; Jenster, L.-M.; Schiffelers, L.D.J.; Tesfamariam, Y.M.; Uchima, M.; Wuerth, J.D.; et al. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science 2021, 371, eabe6230. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Le Bas, A.; Ruza, R.R.; Duyvesteyn, H.M.E.; Mikolajek, H.; Malinauskas, T.; Tan, T.K.; Rijal, P.; Dumoux, M.; Ward, P.N.; et al. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020, 27, 846–854. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Jin, Y.; Zhu, Y.; Wu, Y.; Li, C.; Kong, Y.; Song, W.; Tian, X.; Zhan, W.; et al. A non-ACE2 competing human single-domain antibody confers broad neutralization against SARS-CoV-2 and circulating variants. Signal Transduct. Target. Ther. 2021, 6, 378. [Google Scholar] [CrossRef]
- Zupancic, J.M.; Desai, A.A.; Schardt, J.S.; Pornnoppadol, G.; Makowski, E.K.; Smith, M.D.; Kennedy, A.A.; Barbosa, M.G.D.; Cascalho, M.; Lanigan, T.M.; et al. Directed evolution of potent neutralizing nanobodies against SARS-CoV-2 using CDR-swapping mutagenesis. Cell Chem. Biol. 2021, 28, 1379. [Google Scholar] [CrossRef]
- Dong, J.; Huang, B.; Wang, B.; Titong, A.; Gallolu Kankanamalage, S.; Jia, Z.; Wright, M.; Parthasarathy, P.; Liu, Y. Development of humanized tri-specific nanobodies with potent neutralization for SARS-CoV-2. Sci. Rep. 2020, 10, 17806. [Google Scholar] [CrossRef]
- Liu, H.; Wu, L.; Liu, B.; Xu, K.; Lei, W.; Deng, J.; Rong, X.; Du, P.; Wang, L.; Wang, D. Two pan-SARS-CoV-2 nanobodies and their multivalent derivatives effectively prevent Omicron infections in mice. Cell Rep. Med. 2023, 4, 100918. [Google Scholar] [CrossRef]
- Casasnovas, J.M.; Margolles, Y.; Noriega, M.A.; Guzman, M.; Arranz, R.; Melero, R.; Casanova, M.; Corbera, J.A.; Jimenez-de-Oya, N.; Gastaminza, P.; et al. Nanobodies Protecting From Lethal SARS-CoV-2 Infection Target Receptor Binding Epitopes Preserved in Virus Variants Other Than Omicron. Front. Immunol. 2022, 13, 863831. [Google Scholar] [CrossRef]
- Gaiotto, T.; Hufton, S.E. Cross-Neutralising Nanobodies Bind to a Conserved Pocket in the Hemagglutinin Stem Region Identified Using Yeast Display and Deep Mutational Scanning. PLoS ONE 2016, 11, 0164296. [Google Scholar] [CrossRef]
- Laursen, N.S.; Friesen, R.H.E.; Zhu, X.; Jongeneelen, M.; Blokland, S.; Vermond, J.; van Eijgen, A.; Tang, C.; van Diepen, H.; Obmolova, G.; et al. Universal protection against influenza infection by a multidomain antibody to influenza hemagglutinin. Science 2018, 362, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.M.; Ibañez, L.I.; Van den Hoecke, S.; De Baets, S.; Smet, A.; Roose, K.; Schepens, B.; Descamps, F.J.; Fiers, W.; Muyldermans, S.; et al. Single-Domain Antibodies Targeting Neuraminidase Protect against an H5N1 Influenza Virus Challenge. J. Virol. 2014, 88, 8278–8296. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.W.; Meng, W.X.; Guo, H.J.; Pan, W.Q.; Liu, J.S.; Peng, T.; Chen, L.; Chen, C.Y. Potent Neutralization of Influenza A Virus by a Single-Domain Antibody Blocking M2 Ion Channel Protein. PLoS ONE 2011, 6, e28309. [Google Scholar] [CrossRef]
- Ashour, J.; Schmidt, F.I.; Hanke, L.; Cragnolini, J.; Cavallari, M.; Altenburg, A.; Brewer, R.; Ingram, J.; Shoemaker, C.; Ploegh, H.L. Intracellular Expression of Camelid Single-Domain Antibodies Specific for Influenza Virus Nucleoprotein Uncovers Distinct Features of Its Nuclear Localization. J. Virol. 2015, 89, 2792–2800. [Google Scholar] [CrossRef]
- Hanke, L.; Knockenhauer, K.E.; Brewer, R.C.; Diest, E.v.; Schmidt, F.I.; Schwartz, T.U.; Ploegh, H.L. The Antiviral Mechanism of an Influenza A Virus Nucleoprotein-Specific Single-Domain Antibody Fragment. mBio 2016, 7, e01569-16. [Google Scholar] [CrossRef]
- Jittavisutthikul, S.; Thanongsaksrikul, J.; Thueng-in, K.; Chulanetra, M.; Srimanote, P.; Seesuay, W.; Malik, A.A.; Chaicumpa, W. Humanized-VHH Transbodies that Inhibit HCV Protease and Replication. Viruses 2015, 7, 2030–2056. [Google Scholar] [CrossRef]
- Phalaphol, A.; Thueng-in, K.; Thanongsaksrikul, J.; Poungpair, O.; Bangphoomi, K.; Sookrung, N.; Srimanote, P.; Chaicumpa, W. Humanized-VH/VHH that inhibit HCV replication by interfering with the virus helicase activity. J. Virol. Methods 2013, 194, 289–299. [Google Scholar] [CrossRef]
- Forsman, A.; Beirnaert, E.; Aasa-Chapman, M.M.I.; Hoorelbeke, B.; Hijazi, K.; Koh, W.; Tack, V.; Szynol, A.; Kelly, C.; McKnight, Á.; et al. Llama Antibody Fragments with Cross-Subtype Human Immunodeficiency Virus Type 1 (HIV-1)-Neutralizing Properties and High Affinity for HIV-1 gp120. J. Virol. 2008, 82, 12069–12081. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Prabakaran, P.; Ying, T.; Wang, Y.; Sun, J.; Macedo Camila, D.S.; Zhu, Z.; He, Y.; Polonis Victoria, R.; et al. Exceptionally Potent and Broadly Cross-Reactive, Bispecific Multivalent HIV-1 Inhibitors Based on Single Human CD4 and Antibody Domains. J. Virol. 2014, 88, 1125–1139. [Google Scholar] [CrossRef]
- Weissenhorn, W.; Hulsik, D.L.; Hock, M.; Liu, Y.Y.; Strokappe, N.M.; Khattabi, M.E.; Langedijk, J.P.; McCoy, L.E.; Forsman-Quigley, A.; Aasa-Chapman, M.M.; et al. A gp41 MPER-specific llama VHH requires a hydrophobic CDR3 determinant for neutralization but not for antigen recognition. Retrovirology 2012, 9, e1003202. [Google Scholar] [CrossRef]
- Vercruysse, T.; Pardon, E.; Vanstreels, E.; Steyaert, J.; Daelemans, D. An Intrabody Based on a Llama Single-domain Antibody Targeting the N-terminal α-Helical Multimerization Domain of HIV-1 Rev Prevents Viral Production. J. Biol. Chem. 2010, 285, 21768–21780. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, J.; Hérate, C.; Guenzel, C.A.; Vérollet, C.; Järviluoma, A.; Mazzolini, J.; Rafie, S.; Chames, P.; Baty, D.; Saksela, K.; et al. Single-Domain Antibody-SH3 Fusions for Efficient Neutralization of HIV-1 Nef Functions. J. Virol. 2012, 86, 4856–4867. [Google Scholar] [CrossRef] [PubMed]
- Wichgers Schreur, P.J.; van de Water, S.; Harmsen, M.; Bermúdez-Méndez, E.; Drabek, D.; Grosveld, F.; Wernike, K.; Beer, M.; Aebischer, A.; Daramola, O.; et al. Multimeric single-domain antibody complexes protect against bunyavirus infections. eLife 2020, 9, e52716. [Google Scholar] [CrossRef] [PubMed]
- Boons, E.; Li, G.; Vanstreels, E.; Vercruysse, T.; Pannecouque, C.; Vandamme, A.-M.; Daelemans, D. A stably expressed llama single-domain intrabody targeting Rev displays broad-spectrum anti-HIV activity. Antivir. Res. 2014, 112, 91–102. [Google Scholar] [CrossRef]
- Foster, J.L.; Garcia, J.V. HIV-1 Nef: At the crossroads. Retrovirology 2008, 5, 84. [Google Scholar] [CrossRef]
- Bouchet, J.; Basmaciogullari, S.E.; Chrobak, P.; Stolp, B.; Bouchard, N.; Fackler, O.T.; Chames, P.; Jolicoeur, P.; Benichou, S.; Baty, D. Inhibition of the Nef regulatory protein of HIV-1 by a single-domain antibody. Blood 2011, 117, 3559–3568. [Google Scholar] [CrossRef]
- Gong, J.Z.; Xu, W.F.; Zhang, J. Structure and functions of influenza virus neuraminidase. Curr. Med. Chem. 2007, 14, 113–122. [Google Scholar] [CrossRef]
- Saelens, X. The Role of Matrix Protein 2 Ectodomain in the Development of Universal Influenza Vaccines. J. Infect. Dis. 2019, 219, S68–S74. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 400, 1102. [Google Scholar] [CrossRef]
- Deusenbery, C.; Wang, Y.; Shukla, A. Recent Innovations in Bacterial Infection Detection and Treatment. ACS Infect. Dis. 2021, 7, 695–720. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, L.; Wang, A.; Jin, Y.; Zhou, D. Nanobodies: The potential application in bacterial treatment and diagnosis. Biochem. Pharmacol. 2023, 214, 115640. [Google Scholar] [CrossRef] [PubMed]
- Polidoro, R.B.; Hagan, R.S.; Santiago, R.D.; Schmidt, N.W. Overview: Systemic Inflammatory Response Derived From Lung Injury Caused by SARS-CoV-2 Infection Explains Severe Outcomes in COVID-19. Front. Immunol. 2020, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.L.; Mao, J.H. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Kumar, V. Sepsis roadmap: What we know, what we learned, and where we are going. Clin. Immunol. 2020, 210, 108264. [Google Scholar] [CrossRef]
- Amcheslavsky, A.; Wallace, A.L.; Ejemel, M.; Li, Q.; McMahon, C.T.; Stoppato, M.; Giuntini, S.; Schiller, Z.A.; Pondish, J.R.; Toomey, J.R.; et al. Anti-CfaE nanobodies provide broad cross-protection against major pathogenic enterotoxigenic Escherichia coli strains, with implications for vaccine design. Sci. Rep. 2021, 11, 2751. [Google Scholar] [CrossRef]
- Payandeh, Z.; Rasooli, I.; Mousavi Gargari, S.L.; Rajabi Bazl, M.; Ebrahimizadeh, W. Immunoreaction of a recombinant nanobody from camelid single domain antibody fragment with Acinetobacter baumannii. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 92–98. [Google Scholar] [CrossRef]
- Knauf, G.A.; Groover, K.E.; O’Donnell, A.C.; Davies, B.W. Generation of Synthetic Acinetobacter baumannii-Specific Nanobodies. Acs Infect. Dis. 2023, 9, 1190–1195. [Google Scholar] [CrossRef]
- Adams, H.; Horrevoets, W.M.; Adema, S.M.; Carr, H.E.V.; van Woerden, R.E.; Koster, M.; Tommassen, J. Inhibition of biofilm formation by Camelid single-domain antibodies against the flagellum of Pseudomonas aeruginosa. J. Biotechnol. 2014, 191, 131–138. [Google Scholar] [CrossRef]
- Mireku, S.A.; Sauer, M.M.; Glockshuber, R.; Locher, K.P. Structural basis of nanobody-mediated blocking of BtuF, the cognate substrate-binding protein of the Escherichia coli vitamin B12 transporter BtuCD. Sci. Rep. 2017, 7, 14296. [Google Scholar] [CrossRef]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. In Virulence Mechanisms of Bacterial Pathogens; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 213–239. [Google Scholar] [CrossRef]
- Hotinger, J.A.; May, A.E. Antibodies inhibiting the type III secretion system of Gram-negative pathogenic bacteria. Antibodies 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Kühne Sarah, A.; Hawes William, S.; La Ragione Roberto, M.; Woodward Martin, J.; Whitelam Garry, C.; Gough Kevin, C. Isolation of Recombinant Antibodies against EspA and Intimin of Escherichia coli O157:H7. J. Clin. Microbiol. 2004, 42, 2966–2976. [Google Scholar] [CrossRef] [PubMed]
- Cascales, E.; Christie, P.J. The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 2003, 1, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Lin, M.Q.; Yan, Q.; Budachetri, K.; Hou, L.B.; Sahni, A.; Liu, H.Y.; Han, N.C.; Lakritz, J.; Pei, D.H.; et al. An intracellular nanobody targeting T4SS effector inhibits Ehrlichia infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2024102118. [Google Scholar] [CrossRef]
- Russell, A.B.; Peterson, S.B.; Mougous, J.D. Type VI secretion system effectors: Poisons with a purpose. Nat. Rev. Microbiol. 2014, 12, 137–148. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Logger, L.; Spinelli, S.; Desmyter, A.; Le, T.T.H.; Kellenberger, C.; Douzi, B.; Durand, E.; Roussel, A.; Cascales, E.; et al. Inhibition of Type VI Secretion by an Anti-TssM Llama Nanobody. PLoS ONE 2015, 10, e0122187. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Spinelli, S.; Desmyter, A.; Le, T.T.H.; Kellenberger, C.; Cascales, E.; Cambillau, C.; Roussel, A. Production, crystallization and X-ray diffraction analysis of a complex between a fragment of the TssM T6SS protein and a camelid nanobody. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 266–271. [Google Scholar] [CrossRef]
- Johannes, L.; Römer, W. Shiga toxins—from cell biology to biomedical applications. Nat. Rev. Microbiol. 2010, 8, 105–116. [Google Scholar] [CrossRef]
- Lo, A.W.H.; Moonens, K.; De Kerpel, M.; Brys, L.; Pardon, E.; Remaut, H.; De Greve, H. The Molecular Mechanism of Shiga Toxin Stx2e Neutralization by a Single-domain Antibody Targeting the Cell Receptor-binding Domain. J. Biol. Chem. 2014, 289, 25374–25381. [Google Scholar] [CrossRef]
- Tremblay, J.M.; Mukherjee, J.; Leysath, C.E.; Debatis, M.; Ofori, K.; Baldwin, K.; Boucher, C.; Peters, R.; Beamer, G.; Sheoran, A.; et al. A Single VHH-Based Toxin-Neutralizing Agent and an Effector Antibody Protect Mice Against Challenge with Shiga Toxins 1 and 2. Infect. Immun. 2013, 81, 4592–4603. [Google Scholar] [CrossRef]
- Robinson Sally, R.; Dayao Denise, A.; Medina Jhon, A.; Martone Cara, J.; Yauch Anne, K.; Hinkley, T.; Erasmus Jesse, H.; Shoemaker Charles, B.; Tzipori, S. An anti-Shiga toxin VHH nanobody multimer protects mice against fatal toxicosis when administered intramuscularly as repRNA. Infect. Immun. 2024, 92, e00239-00224. [Google Scholar] [CrossRef] [PubMed]
- Banaei, N.; Anikst, V.; Schroeder, L.F. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 2368–2369. [Google Scholar] [CrossRef] [PubMed]
- Bella, S.D.; Sanson, G.; Monticelli, J.; Zerbato, V.; Principe, L.; Giuffrè, M.; Pipitone, G.; Luzzati, R. Clostridioides difficile infection: History, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options. Clin. Microbiol. Rev. 2024, 37, e00135-23. [Google Scholar] [CrossRef] [PubMed]
- Hussack, G.; Ryan, S.; van Faassen, H.; Rossotti, M.; MacKenzie, C.R.; Tanha, J. Neutralization of Clostridium difficile toxin B with VHH-Fc fusions targeting the delivery and CROPs domains. PLoS ONE 2018, 13, 0208978. [Google Scholar] [CrossRef]
- Orth, P.; Xiao, L.; Hernandez, L.D.; Reichert, P.; Sheth, P.R.; Beaumont, M.; Yang, X.Y.; Murgolo, N.; Ermakov, G.; DiNunzio, E.; et al. Mechanism of Action and Epitopes of Clostridium difficile Toxin B-neutralizing Antibody Bezlotoxumab Revealed by X-ray Crystallography. J. Biol. Chem. 2014, 289, 18008–18021. [Google Scholar] [CrossRef]
- Yang, Z.; Schmidt, D.; Liu, W.; Li, S.; Shi, L.; Sheng, J.; Chen, K.; Yu, H.; Tremblay, J.M.; Chen, X.; et al. A Novel Multivalent, Single-Domain Antibody Targeting TcdA and TcdB Prevents Fulminant Clostridium difficile Infection in Mice. J. Infect. Dis. 2014, 210, 964–972. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, H.; Cai, C.; Zhu, S.; Zhou, Y.; Yang, X.; He, R.; Li, C.; Guo, S.; Li, S.; et al. Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 2015, 25, 157–168. [Google Scholar] [CrossRef]
- Kordus, S.L.; Kroh, H.K.; Rodríguez, R.C.; Shrem, R.A.; Peritore-Galve, F.C.; Shupe, J.A.; Wadzinski, B.E.; Lacy, D.B.; Spiller, B.W. Nanobodies against C. difficile TcdA and TcdB reveal unexpected neutralizing epitopes and provide a toolkit for toxin quantitation in vivo. PLoS Pathog. 2023, 19, e1011496. [Google Scholar] [CrossRef]
- Schmidt, D.J.; Beamer, G.; Tremblay, J.M.; Steele, J.A.; Kim, H.B.; Wang, Y.; Debatis, M.; Sun, X.; Kashentseva, E.A.; Dmitriev, I.P.; et al. A Tetraspecific VHH-Based Neutralizing Antibody Modifies Disease Outcome in Three Animal Models of Clostridium difficile Infection. Clin. Vaccine Immunol. 2016, 23, 774–784. [Google Scholar] [CrossRef]
- Hussack, G.; Arbabi-Ghahroudi, M.; van Faassen, H.; Songer, J.G.; Ng, K.K.S.; MacKenzie, R.; Tanha, J. Neutralization of Clostridium difficile Toxin A with Single-domain Antibodies Targeting the Cell Receptor Binding Domain. J. Biol. Chem. 2011, 286, 8961–8976. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, L.; Yu, H.; Zhang, Y.; Chen, K.; Saint Fleur, A.; Bai, G.; Feng, H. Intravenous adenovirus expressing a multi-specific, single-domain antibody neutralizing TcdA and TcdB protects mice from Clostridium difficile infection. Pathog. Dis. 2016, 74, ftw078. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.K.; Strokappe, N.M.; Hultberg, A.; Truusalu, K.; Smidt, I.; Mikelsaar, R.-H.; Mikelsaar, M.; Verrips, T.; Hammarström, L.; Marcotte, H. Neutralization of Clostridium difficile Toxin B Mediated by Engineered Lactobacilli That Produce Single-Domain Antibodies. Infect. Immun. 2016, 84, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhu, Y.; Zhang, Y.; Hamza, T.; Yu, H.; Saint Fleur, A.; Galen, J.; Yang, Z.; Feng, H. A probiotic yeast-based immunotherapy against Clostridioides difficile infection. Sci. Transl. Med. 2020, 12, eaax4905. [Google Scholar] [CrossRef]
- Unger, M.; Eichhoff, A.M.; Schumacher, L.; Strysio, M.; Menzel, S.; Schwan, C.; Alzogaray, V.; Zylberman, V.; Seman, M.; Brandner, J.; et al. Selection of Nanobodies that Block the Enzymatic and Cytotoxic Activities of the Binary Clostridium Difficile Toxin CDT. Sci. Rep. 2015, 5, 7850. [Google Scholar] [CrossRef]
- Collier, R.J.; Young, J.A. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 2003, 19, 45–70. [Google Scholar] [CrossRef]
- Shali, A.; Hasannia, S.; Gashtasbi, F.; Abdous, M.; Shahangian, S.S.; Jalili, S. Generation and screening of efficient neutralizing single domain antibodies (VHHs) against the critical functional domain of anthrax protective antigen (PA). Int. J. Biol. Macromol. 2018, 114, 1267–1278. [Google Scholar] [CrossRef]
- Moayeri, M.; Leysath, C.E.; Tremblay, J.M.; Vrentas, C.; Crown, D.; Leppla, S.H.; Shoemaker, C.B. A Heterodimer of a VHH (Variable Domains of Camelid Heavy Chain-only) Antibody That Inhibits Anthrax Toxin Cell Binding Linked to a VHH Antibody That Blocks Oligomer Formation Is Highly Protective in an Anthrax Spore Challenge Model. J. Biol. Chem. 2015, 290, 6584–6595. [Google Scholar] [CrossRef]
- Vrentas, C.E.; Moayeri, M.; Keefer, A.B.; Greaney, A.J.; Tremblay, J.; O’Mard, D.; Leppla, S.H.; Shoemaker, C.B. A Diverse Set of Single-domain Antibodies (VHHs) against the Anthrax Toxin Lethal and Edema Factors Provides a Basis for Construction of a Bispecific Agent That Protects against Anthrax Infection. J. Biol. Chem. 2016, 291, 21596–21606. [Google Scholar] [CrossRef]
- Ardekani, L.S.; Gargari, S.L.M.; Rasooli, I.; Bazl, M.R.; Mohammadi, M.; Ebrahimizadeh, W.; Bakherad, H.; Zare, H. A novel nanobody against urease activity of Helicobacter pylori. Int. J. Infect. Dis. 2013, 17, e723–e728. [Google Scholar] [CrossRef]
- Hoseinpoor, R.; Mousavi Gargari, S.L.; Rasooli, I.; Rajabibazl, M.; Shahi, B. Functional Mutations in and Characterization of VHH Against Helicobacter pylori Urease. Appl. Biochem. Biotechnol. 2014, 172, 3079–3091. [Google Scholar] [CrossRef]
- Vanmarsenille, C.; Díaz del Olmo, I.; Elseviers, J.; Hassanzadeh Ghassabeh, G.; Moonens, K.; Vertommen, D.; Martel, A.; Haesebrouck, F.; Pasmans, F.; Hernalsteens, J.-P.; et al. Nanobodies targeting conserved epitopes on the major outer membrane protein of Campylobacter as potential tools for control of Campylobacter colonization. Vet. Res. 2017, 48, 86. [Google Scholar] [CrossRef] [PubMed]
- Mejías, M.P.; Hiriart, Y.; Lauché, C.; Fernández-Brando, R.J.; Pardo, R.; Bruballa, A.; Ramos, M.V.; Goldbaum, F.A.; Palermo, M.S.; Zylberman, V. Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS). Sci. Rep. 2016, 6, 24913. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, H.; Hussack, G.; Aubry, A.; van Faassen, H.; Guan, Y.; Arbabi-Ghahroudi, M.; MacKenzie, R.; Logan, S.M.; Tanha, J. Targeting surface-layer proteins with single-domain antibodies: A potential therapeutic approach against Clostridium difficile-associated disease. Appl. Microbiol. Biotechnol. 2015, 99, 8549–8562. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Van Hauwermeiren, F.; Van der Verren, S.E.; Jonckheere, W.; Goncalves, A.; Pardon, E.; Steyaert, J.; De Greve, H.; Lamkanfi, M.; Remaut, H. Structure of S-layer protein Sap reveals a mechanism for therapeutic intervention in anthrax. Nat. Microbiol. 2019, 4, 1805–1814. [Google Scholar] [CrossRef]
- Liao, Z.; Su, J. Progresses on three pattern recognition receptor families (TLRs, RLRs and NLRs) in teleost. Dev. Comp. Immunol. 2021, 122, 104131. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Wills-Karp, M. Allergen-specific pattern recognition receptor pathways. Curr. Opin. Immunol. 2010, 22, 777–782. [Google Scholar] [CrossRef]
- O’neill, L.A.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Liao, S.; Liu, S.; Zhang, Y. Preparation of anti toll-like receptor-4 nano-antibody and its effect on gram negative sepsis. J. Nanosci. Nanotechnol. 2021, 21, 1048–1053. [Google Scholar] [CrossRef]
- Scott, C.L.; Zheng, F.; De Baetselier, P.; Martens, L.; Saeys, Y.; De Prijck, S.; Lippens, S.; Abels, C.; Schoonooghe, S.; Raes, G.; et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 2016, 7, 10321. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Chen, J.-B.; Tsai, T.-F.; Tsai, Y.-C.; Tsai, C.-Y.; Liang, P.-H.; Hsu, T.-L.; Wu, C.-Y.; Netea, M.G.; Wong, C.-H.; et al. CLEC4F Is an Inducible C-Type Lectin in F4/80-Positive Cells and Is Involved in Alpha-Galactosylceramide Presentation in Liver. PLoS ONE 2013, 8, e65070. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhou, J.; Ouyang, Z.; Zhang, J.; Wang, X.; Muyldermans, S.; Van Ginderachter, J.; Devoogdt, N.; Wen, Y.; Schoonooghe, S. Development and characterization of nanobodies targeting the kupffer cell. Front. Immunol. 2021, 12, 641819. [Google Scholar] [CrossRef]
- Zheng, F.; Sparkes, A.; De Baetselier, P.; Schoonooghe, S.; Stijlemans, B.; Muyldermans, S.; Flamand, V.; Van Ginderachter, J.A.; Devoogdt, N.; Raes, G. Molecular imaging with Kupffer cell-targeting nanobodies for diagnosis and prognosis in mouse models of liver pathogenesis. Mol. Imaging Biol. 2017, 19, 49–58. [Google Scholar] [CrossRef]
- Sparkes, A.; De Baetselier, P.; Brys, L.; Cabrito, I.; Sterckx, Y.G.; Schoonooghe, S.; Muyldermans, S.; Raes, G.; Bucala, R.; Vanlandschoot, P.; et al. Novel half-life extended anti-MIF nanobodies protect against endotoxic shock. FASEB J. 2018, 32, 3411–3422. [Google Scholar] [CrossRef]
| Virus | Form | Target | Affinity (KD) | Function | Reference |
|---|---|---|---|---|---|
| Respiratory syncytial virus | Trimer | F protein | 0.1 nM | Inhibiting viral fusion | [90] |
| Dimer | F protein | 1.8 nM | [91,92] | ||
| Monomer | PreF protein | 0.4–0.5 nM | [22] | ||
| Monomer | PreF protein | <18.0 pM | [36] | ||
| Monomer | PreF protein | 84.0 pM | [37] | ||
| Monomer | PreF protein | EC50: ~18 ng/mL | [93] | ||
| SARS-CoV-2 | Monomer | RBD | 38.6 nM | Inhibiting binding | [40] |
| Monomer | RBD | <1.0 pM | [41] | ||
| Monomer | RBD | 3.3–8.2 nM | [43] | ||
| Monomer | RBD | 4.9 nM | [44] | ||
| Monomer | RBD | 4.0 nM | [45] | ||
| Monomer | RBD | 21.6 nM | [46] | ||
| Monomer | RBD | 1.9–22.2 nM | [94] | ||
| Monomer | RBD | 12.0 nM | [95] | ||
| Monomer | RBD | 57.0 nM | [48] | ||
| Monomer | RBD | 10.6 nM | [49] | ||
| Monomer | RBD | 1.0–35.5 nM | [50] | ||
| Monomer | RBD | 2.2 nM | [51] | ||
| Monomer | RBD | 64 nM | [96] | ||
| Monomer | RBD | 1.0 nM | [47] | ||
| Monomer | RBD | 1.4 nM | [58] | ||
| Dimer | RBD | <0.1 nM | [52] | ||
| Dimer | RBD | IC50: 1.5 nM | [53] | ||
| Dimer | RBD | <1 nM | [24] | ||
| Dimer | RBD | 34.0 nM | [97] | ||
| Trimer | RBD | 0.5 nM | [25] | ||
| Trimer | RBD | 18 pM | [54] | ||
| Trimer | RBD | <0.1 nM | [98] | ||
| Trimer | RBD | 41.0 nM | [56] | ||
| Monomer Dimer Trimer Decameric | RBD | IC50: 2.4–6626 ng/mL IC50: 2.5–682.8 ng/mL IC50: 0.33–8202 ng/mL IC50: 0.18–18.7 ng/mL | [99] | ||
| Monomer | RBD | 0.5–3 nM | [100] | ||
| Fc fused dimer | Conserved RBD residues | 1.2–7.96 nM | [57] | ||
| Monomer | N-terminal domain of S protein | 1.4 nM | Inhibiting viral fusion | [58] | |
| Monomer Dimer Trimer | S2 domain | 19–46 nM 5–10 nM 0.8–1.8 nM | [60] | ||
| Influenza virus | Monomer | H7 head region of HA | 2.6 nM | Inhibiting binding | [64] |
| Trimer | H5 head region of HA | IC50: 4.2 nM | [66] | ||
| Monomer | IBV head region of HA | 0.1–0.3 nM | [65] | ||
| Monomer | H1 stem domain of HA | 3.7–15.7 nM | [71] | ||
| Monomer | H1 stem domain of HA | 0.7 nM | [72] | ||
| Monomer | H9 stem region of HA | 6.9 nM | [101] | ||
| Fc fused Dimer | H3 head region of HA | EC50: 0.02–0.92 nM | [67] | ||
| Monomer | H1 head region of HA | EC50: 0.18–1.09 nM | [69] | ||
| Monomer | Conserved lateral region of H7-HA head | IC50: 9 ng/mL | [70] | ||
| Monomer | IAV and IBV stem region of HA | IC50: 10–100 nM | [102] | ||
| Dimer | IAV and IBV stem region of HA | IC50: 1–100 nM | |||
| Dimer | H5 NA | 0.4 nM | Inhibiting egress | [103] | |
| Monomer | M2 | 39.5 nM | Inhibiting uncoating | [104] | |
| Monomer | NP protein | N/A | Inhibiting replication | [105,106] | |
| Poliovirus | Monomer | Capsid protein | 6–84 nM | Inhibiting binding | [77,78,79] |
| Hepatitis B virus | Monomer | B surface antigen | EC50: 17.9–66.9 ng/mL | Inhibiting binding | [80] |
| Hepatitis C virus | Monomer | E2 glycoprotein | 1–10 μg/mL | Inhibiting binding and transmission | [81] |
| Monomer | NS3 Protease | N/A | Inhibiting replication | [107,108] | |
| Human immunodeficiency virus | Monomer | gp120 | IC50: <3 ng/mL | Inhibiting binding | [109] |
| Monomer Multimer | gp120 | 0.2–4.4 nM IC50: 0.5–21.0 nM | [110] | ||
| Dimer | gp41 | 0.15–29 nM | [111] | ||
| Monomer Dimer | CXCR4 | IC50: 13.6–82.0 nM IC50: 0.1–0.35 nM | [73] | ||
| Monomer | CXCR4 | 6.2–7.7 nM | [74,75] | ||
| Monomer | Rev protein | N/A | Inhibiting transcription | [112] | |
| Monomer | Nef protein | 2 nM | Inhibiting critical biological activities of Nef. | [113] | |
| Papillomavirus | Monomer | E7 oncoprotein | 750 nM | Inhibiting binding | [82] |
| Rotavirus | Monomer | VP6 capsid protein | EC50: 1.6 ng/ml | Inhibiting binding | [83] |
| Ebola Virus | Monomer | Glycoprotein | 55.3 nM | Inhibiting binding | [84] |
| Bunya virus | Multimer | Glycoprotein | ND50: <10 nM | Inhibiting binding | [114] |
| Chikungunya virus | Monomer | Glycoprotein E2 | PRNT50: 2.4 ng/mL | Inhibiting binding | [85] |
| Monomer | Glycoprotein E2 | PRNT50: 45.6 nM | [86] | ||
| Multimer | Glycoprotein E2 | 2.59–20.7 nM | [87] | ||
| Thrombocytopenia syndrome virus | Fc fused Nb | Glycoprotein N | IC50: 1.05 μg/mL | Inhibiting binding | [89] |
| Nipah virus | Monomer | Fusion protein | Binding energies: −3.73 kcal/mol | Inhibiting viral fusion | [88] |
| Bacteria | Form | Target | Affinity (KD) | Function | Reference |
|---|---|---|---|---|---|
| Enterotoxigenic Escherichia coli | Monomer | CfaE | IC100: 2.4–8 µM | Inhibiting colonization. | [127] |
| Monomer | TssM | 2–67 nM | Inhibiting toxin secretion | [138,139] | |
| Monomer | BtuCD-F | 1–770 nM | Inhibiting nutrients uptake | [131] | |
| Acinetobacter baumannii | Monomer | Bap | 38 nM | Inhibiting colonization. | [128] |
| Pseudomonas aeruginosa | Monomer | Flagellin | 2–5 nM | Inhibiting biofilm formation | [130] |
| Campylobacter | Monomer | Outer membrane protein | 118 nM | Inhibiting colonization | [163] |
| Shiga toxin-producing Escherichia coli | Monomer | B subunit of toxin Stx2e | IC50: 8 nM | Neutralizing toxin | [141] |
| Monomer Multimer | Stx2B | IC50: 0.5 nM IC50: 0.05 nM | Neutralizing toxin | [164] | |
| Multimer | Stx1B and Stx2B | Stx1 KD: 0.5 nM Stx2 KD: 0.004 nM | Neutralizing toxin | [142] | |
| Clostridium difficile | Multimer | GTD, CPD and RBD of TcdA and TcdB | TcdA IC50: 100 pM TcdB IC50: 10 pM | Neutralizing toxin | [151] |
| Multimer | RBD of TcdA and TcdB | TcdA KD: 2–290 nM TcdB KD: 2–24 nM | Neutralizing toxin | [152] | |
| Monomer | GTD CPD-DRBD DRBD CROPs | EC50: 110–350 nM EC50: 1 nM EC50: 1 nM EC50: 15.6–677.5 nM | Neutralizing toxin | [150] | |
| Monomer | CDTa ad CDTb | 0.5–15 nM | Neutralizing toxin and inhibiting adherence | [156] | |
| Monomer | Surface layer proteins | 3–6 nM | Inhibiting motility | [165] | |
| Bacillus anthracis | Monomer | Domain 4 of PA | N/A | Neutralizing toxin | [158] |
| Dimer | Domain 4 of PA and PA63 oligomer | IC50: 200 pM | [159] | ||
| Dimer | LF and EF | 10 pM | [160] | ||
| Dimer | EA1 | N/A | Inhibiting bacteria growth | [166] | |
| Helicobacter pylori | Dimer | UreC | 50 nM | Neutralizing toxin | [161] |
| Dimer | UreC | 20 nM | [162] |
| Category | Disease | Identifier | Biological | Clinical Trial Status | FDA Approval Dates |
|---|---|---|---|---|---|
| Nanobody | Campylobacter infection | NCT04182490 | LMN-101 | Phase II (completed) | |
| Rotaviral diarrhoea | NCT01259765 | VHH 203027 | Phase II (completed) | ||
| Respiratory syncytial virus | NCT02979431 | ALX-0171 | Phase II (completed) | ||
| Antibody | Respiratory syncytial virus | NCT05118386 | RSM01 | Phase I (completed) | |
| NCT06551506 | Nirsevimab | Phase IV (completed) | 2023 | ||
| NCT00233064 | Palivizumab | Phase IV (completed) | 1998 | ||
| NCT04767373 | Clesrovimab | Phase III (completed) | |||
| SARS-CoV-2 | NCT05982704 | Tixagevimab/Cilgavimab | Phase IV | 2021 | |
| NCT05780268 | LY3819253 | Phase III (completed) | 2020 | ||
| NCT05502081 | Casirivimab/Imdevimab | Phase IV (completed) | 2020 | ||
| Influenza virus | NCT02603952 | MEDI8852 | Phase II (completed) | ||
| NCT04780321 | JS016 | Phase II (completed) | |||
| Hepatitis B virus | NCT05856890 | HepB mAb19 | Phase I | ||
| Human immunodeficiency virus | NCT02707861 | Ibalizumab | Phase III (completed) | 2018 | |
| NCT02664415 | VRC01 | Phase II (completed) | |||
| NCT02588586 | 3BNC117 | Phase II (completed) | |||
| Ebola virus | NCT06841614 | Inmazeb | Phase III | 2020 | |
| NCT03719586 | MAb114 | Phase III (completed) | 2020 | ||
| NCT03719586 | ZMapp | Phase III (completed) | 2017 | ||
| Clostridium difficile infection | NCT03880539 | Bezlotoxumab | Phase IV (completed) | 2016 | |
| Bacillus anthracis infection | NCT02177721 | Raxibacumab | Phase IV | 2012 | |
| NCT03088111 | Obiltoxaximab | Phase IV | 2016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Huang, C.; Muyldermans, S.; Jia, L. Small but Mighty: Nanobodies in the Fight Against Infectious Diseases. Biomolecules 2025, 15, 610. https://doi.org/10.3390/biom15050610
Jiang W, Huang C, Muyldermans S, Jia L. Small but Mighty: Nanobodies in the Fight Against Infectious Diseases. Biomolecules. 2025; 15(5):610. https://doi.org/10.3390/biom15050610
Chicago/Turabian StyleJiang, Wenning, Chundong Huang, Serge Muyldermans, and Lingyun Jia. 2025. "Small but Mighty: Nanobodies in the Fight Against Infectious Diseases" Biomolecules 15, no. 5: 610. https://doi.org/10.3390/biom15050610
APA StyleJiang, W., Huang, C., Muyldermans, S., & Jia, L. (2025). Small but Mighty: Nanobodies in the Fight Against Infectious Diseases. Biomolecules, 15(5), 610. https://doi.org/10.3390/biom15050610







