Abstract
Cardiometabolic risk and associated dysfunctions contribute largely to the recent rise in mortality globally. Advancements in multi-omics in recent years promise a better understanding of potential biomarkers that enable an early diagnosis of cardiometabolic dysfunction. However, the molecular mechanisms driving the onset and progression of cardiometabolic disorders remain poorly understood. Adipokines are adipocyte-specific cytokines that are central to deleterious cardiometabolic alterations. They exhibit both pro-inflammatory and anti-inflammatory effects, complicating their association with cardiometabolic disturbances. Thus, understanding the cardiometabolic association of adipokines from a molecular and signaling perspective assumes great importance. This review presents a comprehensive outline of the most prominent adipokines exhibiting pro-inflammatory and/or anti-inflammatory functions in cardiometabolic dysfunction. The review also presents an insight into the pathophysiological implications of such adipokines in different cardiometabolic dysfunction conditions, the status of adipokine druggability, and future studies that can be undertaken to address the existing scientific gap. A clear understanding of the functional and mechanistic role of adipokines can potentially improve our understanding of cardiovascular disease pathophysiology and enhance our current therapeutic regimen in the years to come.
1. Introduction
Metabolic syndrome (MetS) is clinically defined as a cluster of biochemical anomalies that are associated with a broad array of cardiometabolic risk factors such as abdominal obesity, augmented blood pressure, increased triglyceride levels, and/or enhanced fasting blood sugar [1,2]. Studies over the past few years have shown a steep rise in the global prevalence of MetS or cardiometabolic dysfunction [3,4,5]. This has primarily been attributed to a spectrum of metabolic and cardiovascular symptoms such as an increase in childhood obesity and type II diabetes (T2D), hypertension, dyslipidemia, and atherosclerosis [6,7]. In the United States, 29% of youth aged between 2 and 19 years and 19% of the adult population exhibit stable cardiometabolic conditions [8,9]. The subjects at cardiometabolic risk are susceptible to a broad array of suboptimal risk factors over an extended timeline and eventually culminate in reliance on therapy that can control but not reverse the altered cardiometabolic condition [10]. Recent courses of meta-analysis studies largely associate prediabetes (non-diabetic hyperglycemia), non-alcoholic fatty liver disease (NAFLD), and other high-risk metabolic states with an increased risk of MetS occurrence and/or progression [11,12,13]. This presents the dire need to identify novel biomarkers of MetS that ensure the early diagnosis and mitigation of cardiometabolic risk to augment healthy life expectancy beyond current estimates [14]. Despite multi-omics and genomic approaches promising to identify potential biomarkers and a better understanding of MetS occurrence [15], associated molecular mechanisms remain poorly understood.
The adipose tissue is primarily responsible for energy storage and secretes a broad spectrum of regulatory molecules that exhibit autocrine, paracrine, and endocrine functions to maintain metabolic stability [16,17]. Adipokines are immunomodulatory molecules secreted by adipocytes [18]. Studies report that alterations in adipocyte levels are closely associated with chronic inflammatory responses, culminating in cardiometabolic dysfunction [19]. The secretory profile of adipokines is altered in metabolic dysfunction, with an overall increase in the production of pro-inflammatory adipokines such as tumor necrosis factor (TNF)-α, leptin, resistin, angiopoietin-related protein 2 (ANGPTL2), and interleukins (ILs) [20]. This stimulates obesity-associated stress via the activation of the autonomic nervous system (ANS) and the hypothalamus–pituitary–adrenal (HPA) axis [21]. Prolonged ANS and HPA axis stimulation induces a rise in glucocorticoid levels that, in turn, increase white adipose tissue (WAT) mass [22]. The secretion of pro-inflammatory cytokines from increased WAT mass induces pre-adipocyte differentiation and further contributes to maintaining the activation status of the HPA axis [22]. This promotes a deleterious cascade of metabolic and immune responses that induce chronic inflammatory condition and ensure the onset of cardiometabolic dysfunction [22].
Although the majority of the adipokines are pro-inflammatory, studies also identify anti-inflammatory adipokines, chiefly adiponectin, and their beneficial impact against complications associated with MetS [23]. The dysregulation of anti-inflammatory adipokines strongly contributes to local or systemic inflammatory induction and MetS onset [24]. Clinical studies strongly suggest a negative correlation between adiponectin profile and pro-inflammatory C-reactive protein (CRP) and IL-6 levels [25,26]. Adiponectin reduces TNF-α expression through NF-ƘB signaling downregulation [27], attenuates class A scavenger receptor (SR)-A, prevents macrophage foam cell formation [28], and decreases CD4+ T lymphocyte infiltration into atherosclerotic lesions via the regulation of macrophage-derived T lymphocyte chemoattractants [29]. However, a clear mechanistic understanding of the role of anti-inflammatory adipokines in MetS conditions remains poorly addressed.
This review attempts to focus on the functional and mechanistic role of pro-inflammatory and anti-inflammatory adipokines in cardiometabolic dysfunction from a molecular signaling perspective. The review also highlights the specific influence of adipokines in regulating the onset and progression of some of the prominent cardiometabolic dysfunctional conditions, such as hypertension, T2D, dyslipidemia, and renal damage, among others. Such functional and mechanistic elucidation of the role of adipokines can enable a better understanding of MetS pathophysiology and potentially improve therapeutic strategies in the years to come.
2. Adipokines
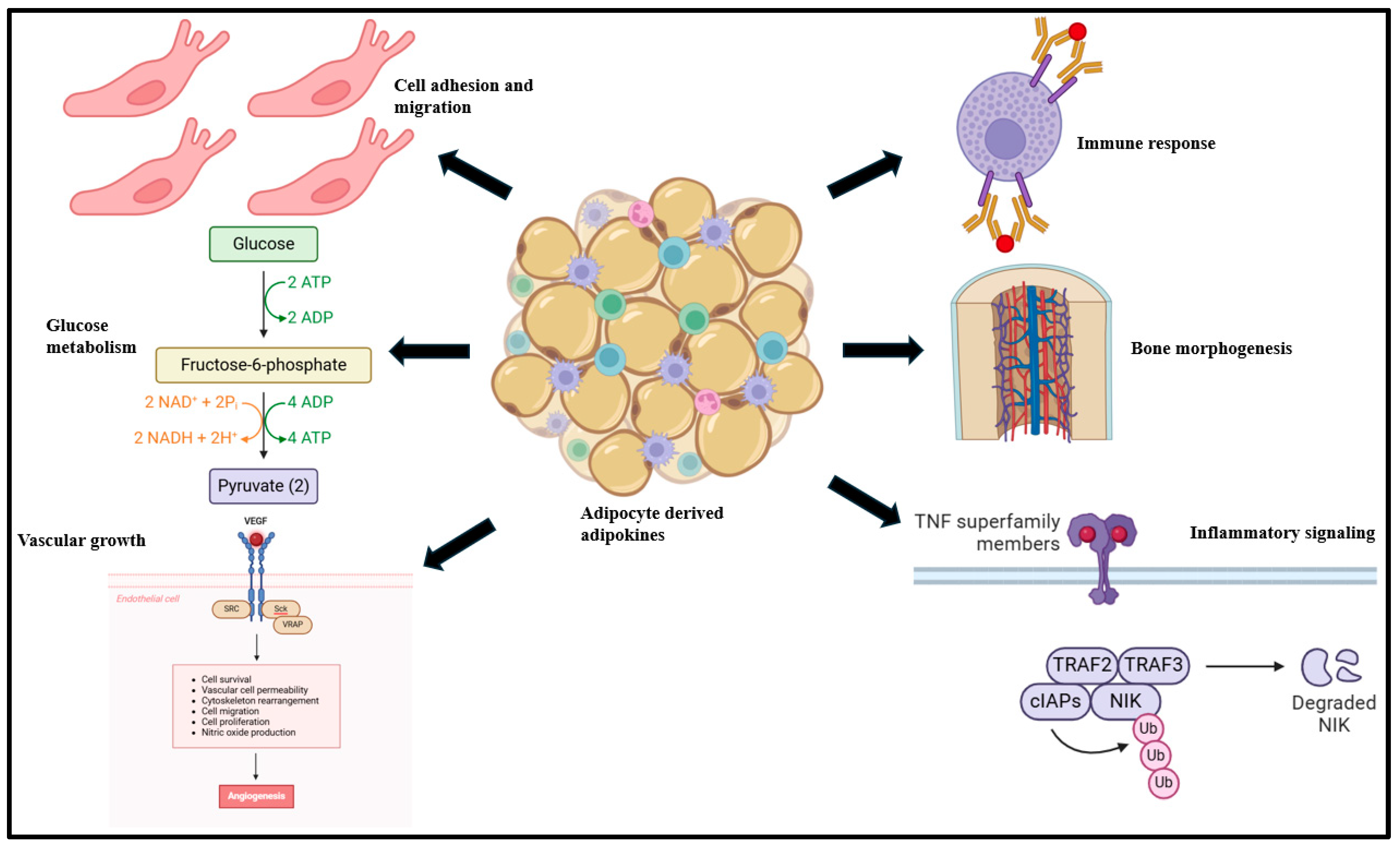
Adipokines are bioactive peptides derived from adipocytes that regulate a broad array of physiological functions, such as body fat distribution, insulin sensitivity, endothelial function, and blood pressure, among others [30,31,32]. In adipose tissue, adipokines contribute to adipogenesis, immune cell migration, and metabolism [33,34,35]. At the systemic level, adipokines influence the biological functioning of the heart, liver, brain, and vasculature [33] and worsen immune response, inflammatory signaling, glucose metabolism, bone morphogenesis, myocardial contractility, and cell adhesion, among others (Figure 1) [35].
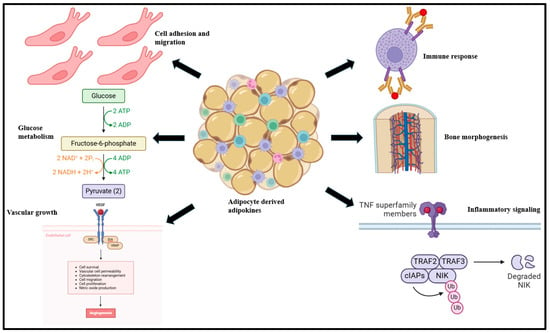
Figure 1.
Adipokines, derived primarily from adipocyte tissue, influence a broad variety of biological functions such as immune response, bone morphogenesis, inflammation, cellular adhesion, migration, glucose metabolism, and vascular growth functions [36].
2.1. Pro-Inflammatory Adipokines
2.1.1. Tumor Necrosis Factor (TNF)-α
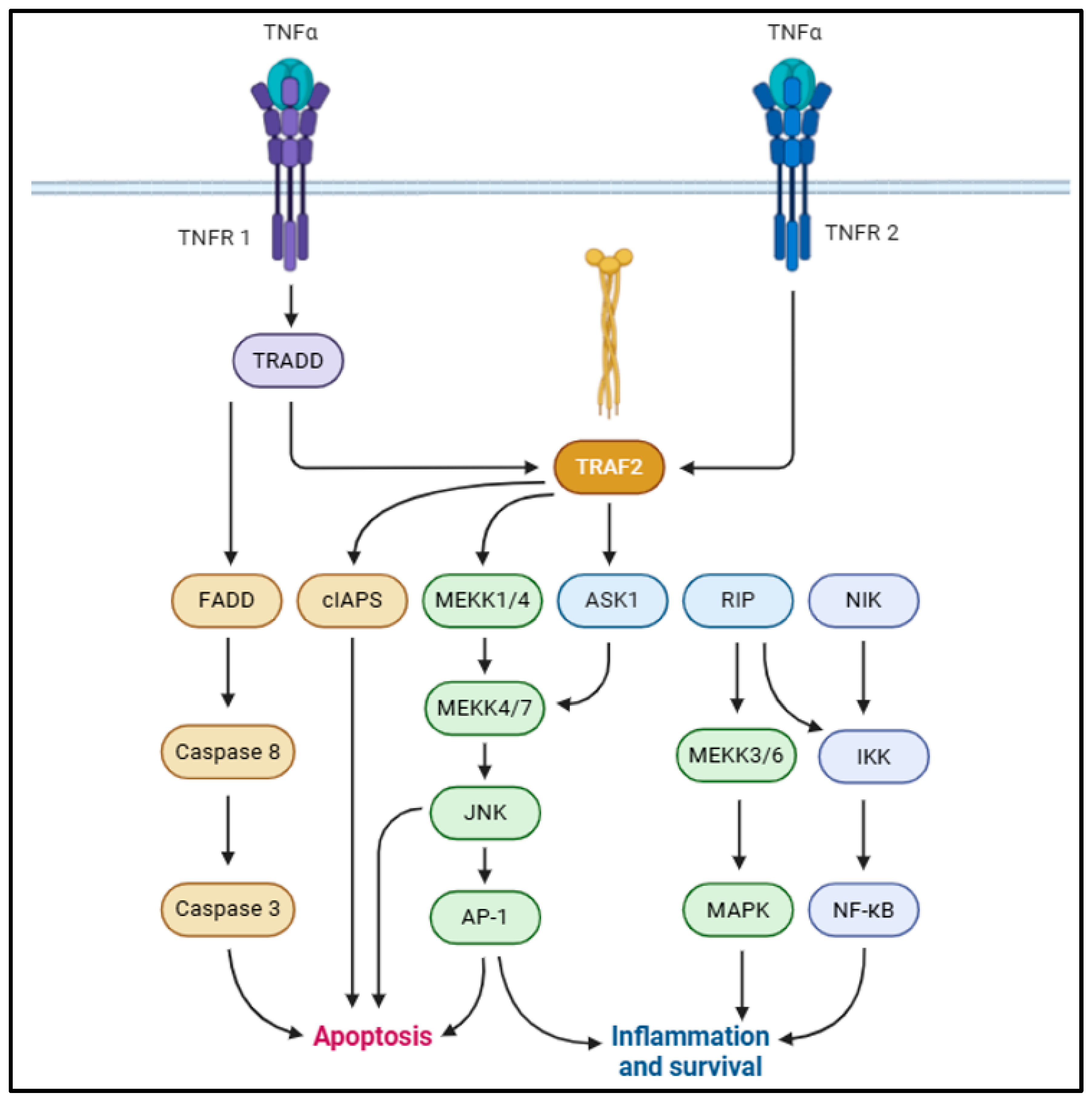
Tumor necrosis factor (TNF)-α is a 17 kDa proteolytic cleavage product from a 26 kD transmembrane monomer, typically driven by TNF-α-converting enzyme (TACE) [37,38]. TNF-α is associated with a broad array of physiological functions such as immune response, cellular proliferation, differentiation, apoptosis, and energy homeostasis [39,40]. TNF-α is typically derived from macrophages, adipocytes, and visceral fat and bears a positive correlation with hyperinsulinemia, body mass index (BMI), and insulin resistance [41,42]. Studies suggest that TNF-α augments IL-6 production and impairs insulin signaling in adipocytes and hepatocytes [43,44,45]. TNF-α typically correlates with adiposity, increased adipocyte lipolysis, fatty acid release into the bloodstream from adipocytes, and insulin resistance [46,47,48]. This is typically mediated via binding with TNF-α receptor (TNFR)-1 or TNFR-2 and the activation of downstream signaling cascades [49]. The adipogenic effects of TNF-α are majorly mediated via TNFR1 binding, although some studies also suggest regulation via TNFR2 binding [50,51,52]. Upon TNFR1 binding, TNFR-associated death domain protein (TRADD) recruits Fas-associated death domain protein (FADD), TNFR-associated factor 2 (TRAF2), receptor-interacting protein (RIP)-1, and mitogen-activated protein kinase (MAPK), activating death domain protein (MADD) [53,54]. In adipocytes, TNF-α activates extracellular signal-regulated kinase (ERK)-1/2, p38 MAPK, and c-Jun N-terminal kinase (JNK) via MADD [49,55,56]. This drives the phosphorylation of insulin receptor substrate (IRS)-1 and compromises insulin signaling [57,58]. TRAF2 and RIP1 recruit inhibitors of NF-ƘB (IƘB) kinase (IKK) to TNFR1 and activate the NF-ƘB pro-inflammatory signaling pathway (Figure 2) [59,60]. In addition to suppressing IR, IRS-1, and glucose transporter type IV (GLUT4) expression [45,61], TNF-α also suppresses peroxisome proliferator-activated receptor (PPAR)-γ transcription factor in adipocytes [62]. This is mediated primarily via serine phosphorylation of PPAR-γ N-terminal residues, chiefly driven by JNK/ERK1/2 signaling activation [63,64].
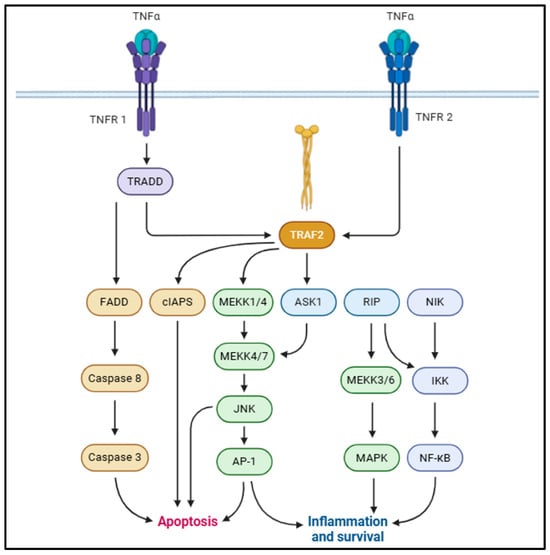
Figure 2.
Schematic representation of pro-inflammatory TNF-α signaling, contributing to MetS onset [36].
2.1.2. Leptin
Leptin is a peptide hormone encoded on chromosome 7 locus that is primarily derived from WAT [65,66]. It is a 16 kDa protein of 167 amino acids that possesses the capabilities to circulate in its free state as well as bound to proteins [67]. It possesses great potential to cross the blood–brain barrier and exercise a significant impact on hypothalamus functioning via the hypothalamic leptin receptor (LEPR) [68,69,70,71]. It regulates satiety, appetite, reproductive functions, fertility, puberty, atherogenesis, and energy expenditure [72]. Leptin plays a significant role in lipogenesis and the expansion of the total fatty acid content of the body and exhibits sex differences in its synthesis potential, where females exhibit a higher rate and extent of leptin synthesis relative to males [48,73,74]. Additionally, leptin reduces insulin secretion and modulates hematopoiesis, β-cell functions of the pancreas, peripheral adiposity, and central nervous system (CNS) functions [75,76,77,78,79]. Cumulatively, these effects are associated with cardiometabolic stability [80,81]. In fact, CNS/leptin signaling disruption has been reported to contribute to cardiometabolic dysfunction such as obesity, hypertension, and T2D [82,83,84]. This is primarily driven by systemic leptin resistance and tolerance in obese patients [85,86].
As a pro-inflammatory adipokine, leptin promotes inflammatory cytokine upsurge in macrophages [87,88] and T lymphocytes and triggers inflammatory signaling via the Janus kinase/signal transducer and the activator of transcription-3 (JAK-STAT3) [89,90], MAPK [91,92], and phosphatidylinositol-4,5-bisphosphate3-kinase (PI3K) signaling cascades (Figure 3) [93,94]. The binding of leptin to LEPR activates JAK2, and this phosphorylates Tyr985, Tyr1077, and Tyr1138 in the LEPR cytoplasmic domain [95,96]. Upon phosphorylation, STAT3 translocates from the cytoplasm to the nucleus, binds to the pomc promoter, and stimulates proopiomelanocortin (POMC) [90,97]. Leptin-associated PI3K/Akt activation is typically mediated via IRS phosphorylation [98,99]. This stimulates the mammalian target of rapamycin (mTOR), typically via p70S6 kinase phosphorylation [100,101].
Randomized controlled trials (RCTs) in hypothalamic amenorrhea (HA) women (low circulating leptin) suggested controversial perspectives on leptin administration [102]. One RCT suggested that leptin augments osteocalcin and urine-N-terminal telopeptide but had no impact on bone mineral density [103]. However, another RCT suggested that leptin administration improved lumbar spine bone mineral density in hypoleptinemic women [104]. Thirty-six weeks of leptin administration in HA women culminated in parathyroid hormonal and RANKL/OPG ratio downregulation, pointing to reduced osteoclastic activity [105,106]. These studies keep the central and peripheral impact of leptin on bone mineral density highly debatable.
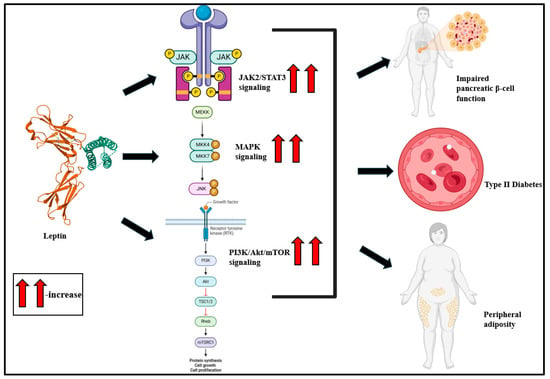
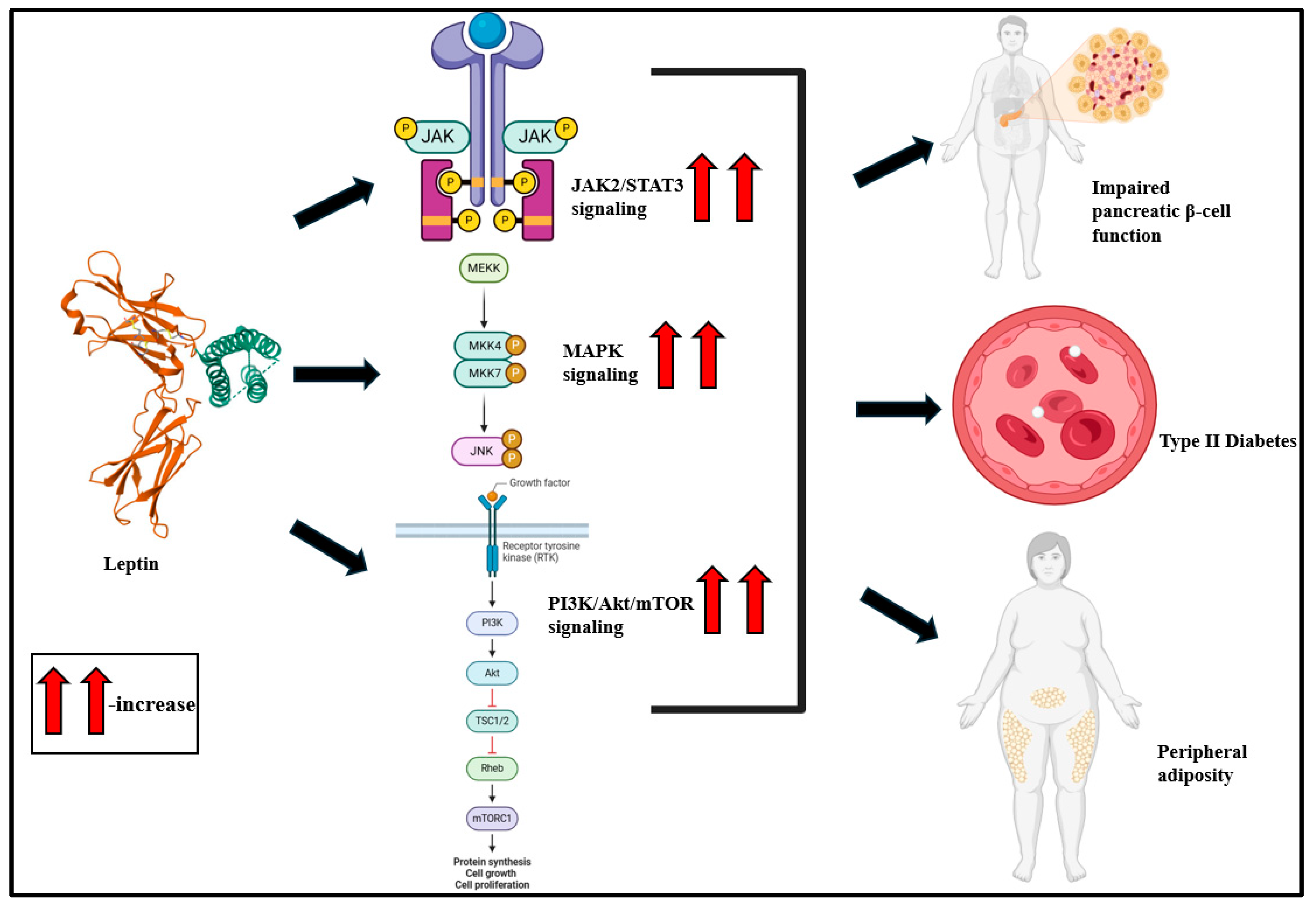
Figure 3.
Leptin, a pro-inflammatory cytokine, activates JAK2/STAT3, MAPK, and PI3K/Akt/mTOR signaling and is predisposed to impaired pancreatic β-cell function, T2D, and peripheral adiposity [36,107].
Figure 3.
Leptin, a pro-inflammatory cytokine, activates JAK2/STAT3, MAPK, and PI3K/Akt/mTOR signaling and is predisposed to impaired pancreatic β-cell function, T2D, and peripheral adiposity [36,107].
2.1.3. Visfatin
Visfatin, a 52 kDa pre-B-cell colony-enhancing factor (PBEF) protein, is primarily derived from visceral adipose tissue macrophages [108,109]. Its intracellular form, nicotinamide phosphoribosyl transferase (NAMPT), is a key regulator of nicotinamide adenine dinucleotide (NAD) biosynthesis [110]. Visfatin drives the release of pro-inflammatory mediators and upregulates vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF)-2, matrix metalloproteinase, and monocyte chemoattractant protein-1 (MCP-1) production [111]. This culminates in NF-ƘB, MAPK, and PI3K signaling activation [112]. Studies over the years suggest high plasma circulatory levels of visfatin in patients experiencing MetS, such as obesity and T2D [113,114,115]. High circulatory levels of visfatin have been associated with B-cell deterioration [116,117]. Visfatin plays a role in the pathogenesis of T2D, potentially via interacting with insulin receptors, and it is typically driven by the phosphorylation of insulin substrate-1 and insulin substrate-2 [118]. Studies suggest that visfatin is central to NOD, LRR, and pyrin domain-containing protein 3 (NLRP3) inflammasome activation [119]. The NLRP3 inflammasome activation promotes the extracellular release of damage-associated molecular pattern (DAMP) molecules, like high-mobility group box-1 (HMGB1), and drives podocyte and inter-endothelial injury through the paracrine and autocrine signaling cascade [4,120,121]. This holds the key to arterial inflammation and endothelial damage in a TLR4-dependent manner and culminates in obesity (Figure 4) [122,123].
Clinical studies with 12 healthy men suggested that visfatin levels are elevated by microgravity and remain increased post-recovery [124]. A small cohort study suggested a correlation between visfatin and male lumbar spine bone mineral density [125]. A large group of clinical studies, however, report augmented visfatin tissue expression in sepsis, psoriasis, and other inflammatory states [126,127]. Clinical studies also highlight enhanced serum visfatin in atherosclerosis and coronary artery disease (CAD) patients [128,129]. Clinical studies also show that visfatin levels are raised significantly in gestational T2D patients [130]. This has been confirmed further at different gestational stages, such as the first trimester [131], second trimester [132], and third trimester [133].
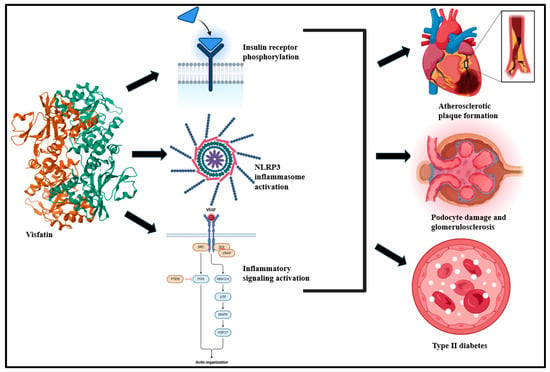
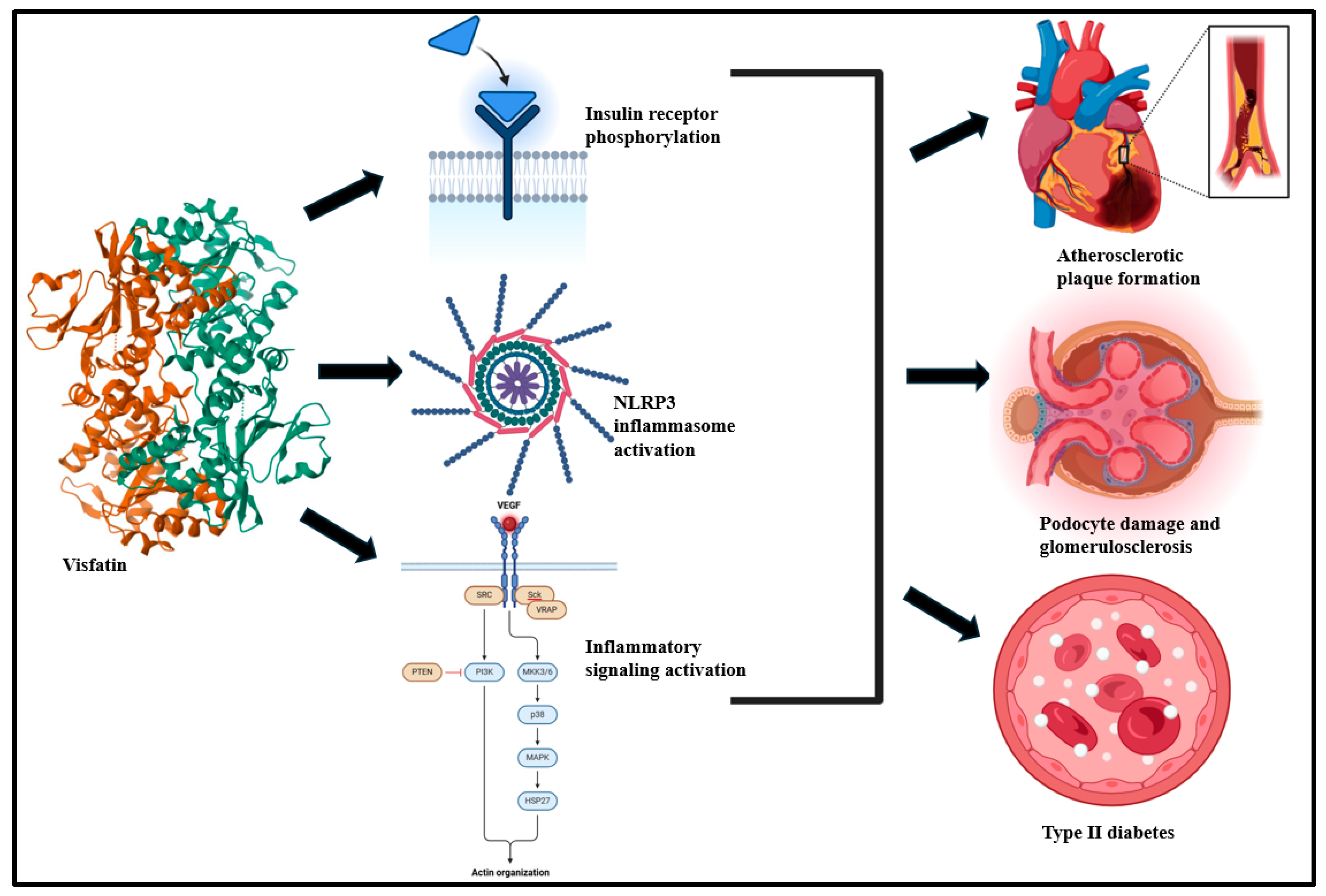
Figure 4.
Visceral adipose tissue-derived visfatin drives insulin receptor phosphorylation, NLRP3 inflammasome, and inflammatory signaling activation and culminates in atherosclerosis, podocyte damage, and T2D [36,134].
Figure 4.
Visceral adipose tissue-derived visfatin drives insulin receptor phosphorylation, NLRP3 inflammasome, and inflammatory signaling activation and culminates in atherosclerosis, podocyte damage, and T2D [36,134].
2.1.4. Resistin
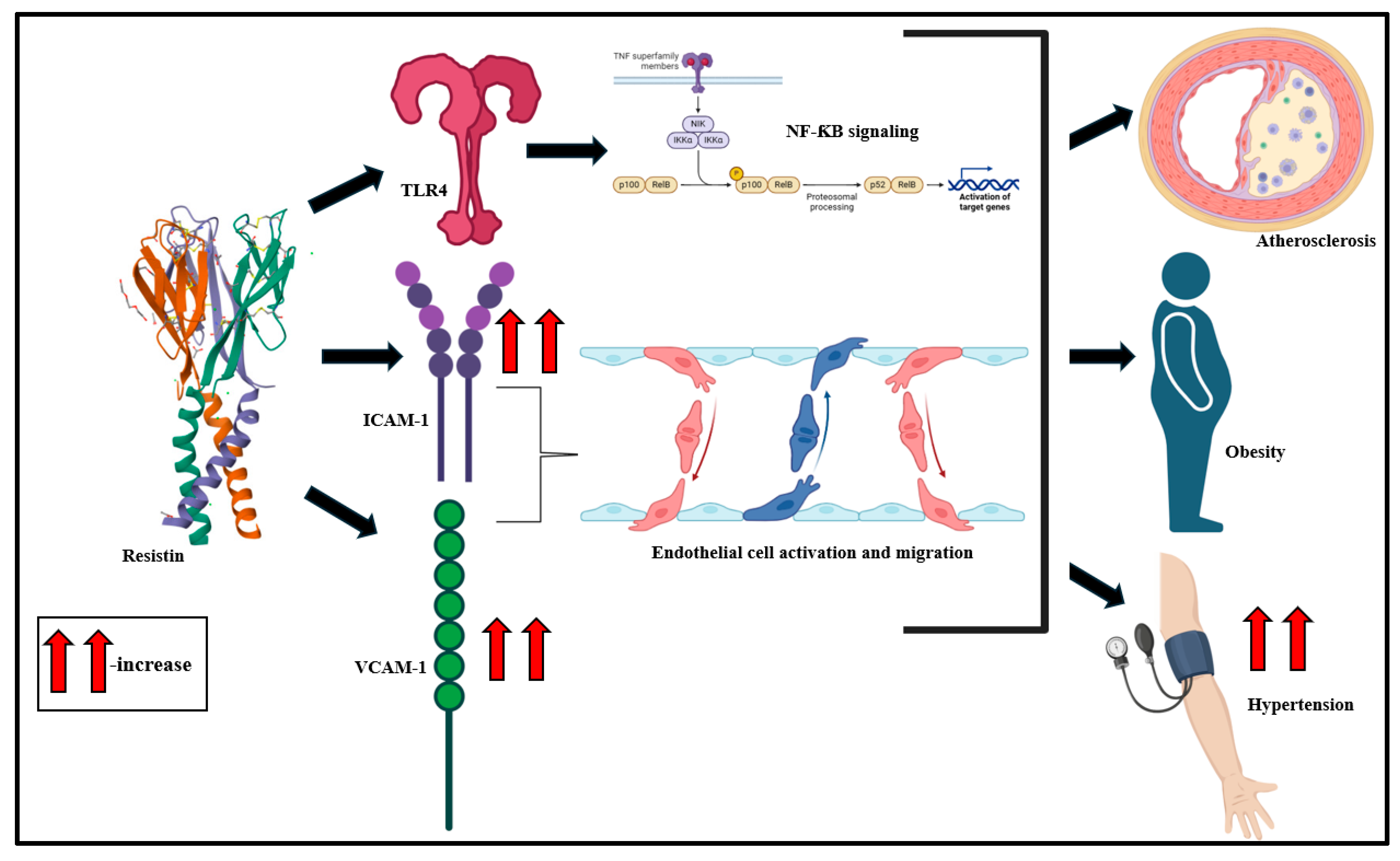
Resistin, primarily derived from macrophages, monocytes, and pre-adipocytes, is a polypeptide of 108 amino acids with a molecular weight of 11.3 kDa [135,136,137]. Resistin modulates insulin homeostasis and selectively disables the inhibitory impact of insulin on hepatic glucose generation [138,139,140,141]. Investigations propose that the 420C/G polymorphism of the resistin gene is associated with the high circulatory potential of resistin [142]. Studies suggest that resistin downregulates adenosine monophosphate-activated protein kinase (AMPK) signaling in the liver and skeletal muscle sites, which interferes with insulin signaling [143,144]. Resistin also binds to toll-like receptor (TLR) 4 in the hypothalamus and mediates inflammatory stimulation via the classical NF-ƘB signaling cascade [145,146]. Endothelial cell stimulation is also driven by resistin, typically via the increase in expression of endothelin-1, intercellular adhesion molecule (ICAM)-1, and vascular cell adhesion molecule (VCAM)-1 [147]. Cumulatively, these serve as precursors to atherosclerosis, hypertension, and MetS onset (Figure 5) [148,149,150].
Clinical studies, over the years, highlight a strong association of resistin with insulin resistance in obese individuals [151]. Plasma resistin levels are significantly altered with CVD risk factors and correlate with vascular smooth muscle cellular dysfunction in advanced atherosclerosis and also with intimal hyperplasia [152]. This clinical inflammatory state also involves macrophage infiltration in adipose tissue and the production of VCAM-1, ICAM-1, and MCP-1 [137,153].
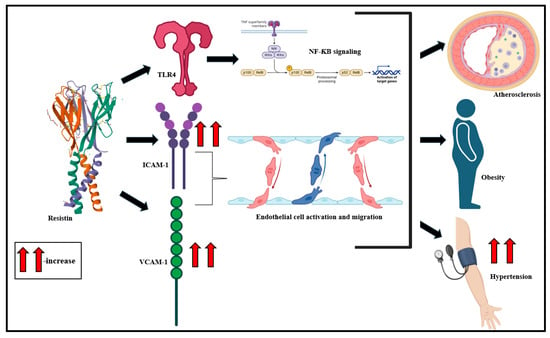
Figure 5.
Resistin mediates TLR4-driven NF-ƘB signaling activation, ICAM-1- and VCAM-1-driven endothelial cell stimulation, and migration and culminates in atherosclerosis, obesity, and hypertension [36,154].
Figure 5.
Resistin mediates TLR4-driven NF-ƘB signaling activation, ICAM-1- and VCAM-1-driven endothelial cell stimulation, and migration and culminates in atherosclerosis, obesity, and hypertension [36,154].
2.1.5. Angiopoietin-like Protein (ANGPTL)
Angiopoietin-like protein (ANGPTL) is a family of proteins that exhibit an N-terminal coiled coil domain and a C-terminal fibrinogen-like domain [155,156]. They act differently as compared to structurally similar angiopoietin, as they do not bind to angiopoietin receptors Tie1 or Tie2 [157,158].
ANGPTL2
ANGPTL2 is a 57 kDa glycosylated protein member of the ANGPTL family derived from adipose tissue [159,160]. Existing studies suggest that ANGPTL2 expression and circulation are augmented in high-fat diet conditions and associated with local inflammation, an increase in macrophage accumulation, endothelial injury, adipose tissue-specific pro-inflammatory M1 polarization, and systemic insulin resistance [159,161,162]. An ANGPTL2-based inflammatory response is typically guided by the activation of the NF-ƘB signaling pathway [159]. ANGPTL2 binds to α5β1, activates Rac1, degrades IƘB, and promotes the translocation of NF-ƘB to the nucleus [163,164]. Extended NF-ƘB signaling activation by ANGPTL2 affects pancreatic and hepatic functioning and accounts for the development of T2D and insulin resistance [159]. ANGPTL2 also accelerates ICAM-1 and P-selectin expression and mediates atherosclerotic plaque formation [165]. In fact, a rise in circulating ANGPTL2 levels bears a close association with C-reactive protein expression in obesity and an increase in expression of pro-inflammatory TNF-α and its receptor TNFR1 in the onset and progression of T2D, obesity, and heart failure [159,166,167].
ANGPTL4
Classified as a pro-inflammatory adipokine owing to its increased expression in adipose tissue and the liver, ANGPTL4 is central to obesity and MetS onset, chiefly via lipoprotein lipase inhibition [168,169]. This, in turn, negatively regulates triglyceride metabolism and culminates in lipid accumulation and atherosclerosis onset [170,171]. Studies suggest that the deletion of ANGPTL4 in adipose tissue reduces circulating cholesterol and triglyceride concentrations, vascular inflammation, and aortic endothelial activation [172]. ANGPTL4 positively regulates IL-1β production and associated NF-Ƙβ signaling [172,173]. ANGPTL4 is chiefly triggered by factors, such as glucocorticoids and hypoxia-inducible factor-1α (HIF-1α), augmenting ANGPTL4 levels in WAT mass [173]. Additionally, ANGPTL4 is associated with serine palmitoyl transferase long chain base subunit 2 (SPTLC2) expressional upregulation, which is a key enzyme associated with ceramide production [174,175]. The activation of ceramides induces protein kinase C (PKC)-ζ dependent lipolysis, promotes pro-inflammatory mediator secretion, and leads to MetS onset [175,176,177].
ANGPTL8
Also known as betatrophin and lipasin, ANGPTL8 is primarily localized in the liver and adipose tissue [178]. It lacks the ANGPTL-typical fibrinogen-like domain [179]. Circulating ANGPTL8 has been reported to be augmented in systemic inflammatory response syndrome (SIRS), T2D, atherosclerosis, and NAFLD [180,181]. ANGPTL8, in combination with leukocyte immunoglobulin-like receptor B (LILRB)-2, acts as a pro-inflammatory inducer of hepatic stellate cells [182]. This, in turn, augments ERK signaling and increases genes associated with liver fibrosis [182]. ANGPTL8/LILRB2 binding on macrophages enhances the conversion of hepatic macrophages to the pro-inflammatory M1 subtype, which is primarily driven by p38/Akt/p65 phosphorylation [183]. This promotes lipid accumulation and enhances progression from simple hepatic steatosis to steatohepatitis [179,183].
2.1.6. IL-6
Interleukin (IL)-6 is derived from T-cells, B-cells, and keratinocytes and is central to the regulation of a broad spectrum of biological functions [184,185]. It is a 21–28 kDa protein (exact molecular weight depends on the extent of post-translational modifications) consisting of 212 amino acids and a signal peptide of 27 amino acids [186]. Studies suggest that adipose tissue contributes 15–35% of basal circulating IL-6 [187]. IL-6 primarily functions via the activation of two signaling cascades, the JAK/STAT pathway and the MAPK pathway [188]. IL-6 binds to the IL-6 receptor (IL-6R) and leads to IL-6/IL-6R/gp130 complex formation [189]. The cytoplasmic domain of gp130 does not possess kinase activity, and the oligomerized receptor leads to the activation of tyrosine kinases of the JAK family of proteins [189,190]. This phosphorylates tyrosine residues of gp130 and opens binding sites for STAT transcription factors and Src-homology 2 (SH2) domain-containing tyrosine phosphatase 2 [191,192]. This, in turn, activates MAPK signaling [193]. The existing paradigm of studies suggests that elevated plasma IL-6 levels are associated with obesity, insulin resistance, and MetS [194,195]. In hepatocytes, a rise in IL-6 bears a close association with the onset of insulin resistance, typically via the downregulation of IRS-1 tyrosine phosphorylation and SOCS-3 expressional upsurge [196]. Adipose tissue-specific IL-6 expression has been observed to induce an inhibitory impact on insulin signaling, accompanied by the downregulation of IRS-1 and GLUT4 gene functions [197,198].
The summary of the deleterious mechanisms of some of the prominent pro-inflammatory adipokines are summarized in Table 1.

Table 1.
Summary of the deleterious mechanisms of some of the prominent pro-inflammatory adipokines.
Table 1.
Summary of the deleterious mechanisms of some of the prominent pro-inflammatory adipokines.
| Adipokine | Pro-Inflammatory Mechanisms | References |
|---|---|---|
| TNF-α | Adipogenic effects are majorly mediated via TNFR1 binding; activates ERK-1/2, p38 MAPK, and c-JNK via MADD; activates NF-ƘB pro-inflammatory signaling and suppresses PPAR-γ (via serine phosphorylation) in adipocytes | [52,55,59] |
| Leptin | Promotes inflammatory cytokine upsurge in macrophages; triggers inflammatory signaling via JAK-STAT3/MAPK/PI3K signaling | [87,91,93] |
| Visfatin | Upregulates VEGF, FGF-2, matrix metalloproteinase, and MCP-1 production; central to NLRP3 inflammasome activation; drives arterial inflammation and endothelial damage and leads to obesity | [111,119,123] |
| Resistin | Downregulates AMPK signaling in the liver and skeletal muscle and interferes with insulin signaling; binds to TLR4 in the hypothalamus and mediates NF-ƘB signaling activation | [143,146] |
| ANGPTL2 | Binds to α5β1, activates Rac1, degrades IƘB, and promotes NF-ƘB signaling activation; accelerates ICAM-1 and P-selectin expression and mediates atherosclerotic plaque formation | [164,165] |
| ANGPTL4 | Inhibits lipoprotein lipase and culminates in lipid accumulation and atherosclerosis; positively regulates IL-1β production and NF-ƘB signaling; upregulates SPTLC2-induced ceramide production | [168,172,174] |
| ANGPTL8 | Combines with LILRB-2 on macrophages and enhances the conversion of hepatic macrophages to M1 subtype; primarily driven by p38/Akt/p65 phosphorylation; promotes lipid accumulation and enhances progression from simple hepatic steatosis to steatohepatitis | [179,181,182] |
| IL-6 | Activates JAK/STAT and MAPK pathways; induces insulin resistance via the downregulation of IRS-1 tyrosine phosphorylation and SOCS-3 expressional upsurge | [188,196] |
2.2. Anti-Inflammatory Adipokines
2.2.1. Adiponectin
Adiponectin is a 30 kDa adipocyte-specific adipokine that exhibits a collagen domain and a globular domain bearing homologous sequences to complement factor C1q [199,200]. As an anti-inflammatory adipokine, adipokine levels have been reported across studies to be negatively associated with plasma concentrations of pro-inflammatory CRP and IL-6 [25,26]. Adiponectin downregulates lipopolysaccharide (LPS)-induced TNF-α expression, vascular inflammation, metalloproteinase (MMP)-12 expression, and class A scavenger receptor (SR)-A in human monocyte-derived macrophages via NF-ƘB signaling inhibition [27,201,202]. This also attenuates macrophage foam cell formation [28]. Adiponectin has also been reported to reduce infiltrating CD4+ T lymphocyte population into atherosclerotic lesions [29]. This is driven by the downregulation of T lymphocyte chemoattractants like IF-inducing protein, IF-inducible T-cell α chemoattractant (I-TAC), and CXCl11 [23,29]. From a cardioprotection perspective, adiponectin decreases oxidative and nitrative stress and prevents inflammatory signaling post-ischemia/reperfusion injury [203]. Adiponectin is central to macrophage phenotypic switching from activated M1 phenotypes in obese and MetS subjects to anti-inflammatory M2 [204,205]. This is driven primarily via PPAR-γ-dependent signaling and holds the key to guiding macrophage ability to remove early apoptotic bodies through the calreticulin/CD91-regulating mechanism [206,207].
A large cohort of clinical studies highlights lower adiponectin among osteoporosis patients [208]. In chronic kidney disease (CKD) patients, adiponectin has been reported to be inversely correlated with cortical thickness and trabecular volumetric BMD [209]. In fact, a large body of clinical evidence shows low adiponectin levels to exhibit a strong correlation with cardiovascular complications, atherosclerosis, and MetS onset [210,211]. Low adiponectin clinically correlates with peripheral arterial stiffness in hypertension patients and compromised glycemic control in pregnancy [212,213].
2.2.2. C1q/TNF-Related Protein (CTRP) Family
Primarily recognized as adiponectin structural paralogs, the CTRP family possesses a signal sequence, a C1q globular domain, and a collagen domain [214].
CTRP3
CTRP3, also known as cartductin or cartonectin, is primarily derived from adipocytes and mesenteric adipose tissue [215,216]. CTRP3 exhibits two alternatively spliced isoforms, CTRP3A and CTRP3B, and they differ in glycosylation and overall length [217]. It is chiefly responsible for attenuating macrophage migration inhibitory factor (MIF), MCP-1 and C-C motif chemokine ligand 4 (CCL4), TLR stimulation, and downregulating LPS-induced pro-inflammatory signaling [215,218]. Downregulated CTRP3 has been correlated with increased CCL2 and reduced adiponectin in pre-adipocytes of MetS subjects [215]. CTRP3 also improves cardiac function post-ischemia via augmenting revascularization and apoptotic reduction in an ischemic heart [219].
CTRP6
CTRP6, predominantly expressed in adipose tissue, circulates in the blood as heteromeric, oligomeric, or homotrimer [220,221]. CTRP6 augments anti-inflammatory IL-10 expression in monocyte-derived macrophages via p42/44 MAPK signaling activation [221]. Studies also suggest that CTRP6 activates AMPK and promotes fatty acid oxidation, regulating metabolism and inflammatory status in obese conditions [222].
CTRP9
CTRP9, another adipose tissue-specific adipokine, forms a heterotrimer with adiponectin and shares an AdipoR1 receptor with adiponectin [223,224]. Studies suggest that CTRP9 augments endothelial vasorelaxation in aortic rings [225]. This is driven primarily via AdipoR1/AMPK/endothelial nitric oxide synthase (eNOS) signaling [23,226]. It prevents vascular smooth muscle proliferation via protein kinase A-associated signaling [226]. CTRP9 also activates AMPK and Akt phosphorylation and increases insulin-mediated glucose uptake [223]. This attenuates the onset of insulin resistance, hepatic steatosis, and other metabolic dysfunctions [227].
CTRP12
Also known as adipolin, CTRP12 is a recently identified adipose tissue-derived adipokine that betters insulin sensitivity and glucose tolerance under obese conditions [228,229]. Molecular investigations reveal that CTRP12 is transcriptionally modulated by Krüppel-like factor (KLF) [230,231]. KLF3 negatively binds with the CTRP12 promoter and downregulates CTRP12 activity, outlining KLF3 as a potentially druggable target for manipulating CTRP12 transcription [232]. On the other hand, KLF-15 positively regulates CTRP12 adipocyte activity, reversing TNF-α-induced JNK dependence in obesity [233]. CTRP12 attenuates pro-inflammatory macrophage infiltration and an upsurge in the expression of TNF-α, IL-1β, and MCP-1 [229]. This is mediated primarily via Akt signaling and culminates in the downregulation of gluconeogenesis and enhances glucose uptake [23,229].
2.2.3. Omentin-1
Omentin-1, a galactofuranose-binding lectin, is primarily expressed in visceral adipose tissue [234]. Studies reveal that omentin-1 is downregulated in patients exhibiting glucose intolerance, T2D, obesity, and/or in women with polycystic ovary syndrome [235,236,237]. Omentin-1 has been reported across studies to be negatively associated with metabolic dysfunction phenotypes such as dyslipidemia, hypertension, and glucose intolerance [238]. Studies suggest that omentin-1 promotes glucose uptake in adipocytes [239]. From a cardioprotective perspective, omentin-1 prevents arterial stiffness and a rise in carotid intima-media thickness [240]. This is further substantiated by reports of decreased omentin-1 in CAD [241]. Omentin-1 enhances endothelial cellular differentiation and vasodilation and reduces endothelial cell apoptosis in an AMPK/eNOS-dependent manner [242,243,244]. Omentin-1 recovers blood flow and capillary density in ischemic conditions via eNOS activation [245,246,247]. On the other hand, omentin-1 attenuates pro-inflammatory JNK signaling and avoids THP-1 monocyte adhesion to endothelial cells in response to TNF-α stimulation [248]. Omentin-1-mediated ERK/NF-ƘB signaling downregulation reduces ICAM-1 expression and attenuates p38/JNK-mediated VCAM-1 expression in vascular smooth muscle cells [249]. This avoids vascular inflammation and accounts for the cardioprotective functions of omentin-1 [250,251].
Clinical studies suggest omentin-1 as a potential biomarker for irritable bowel syndrome (IBS) patients. Beyond IBS, clinical cohort studies suggest synovial fluid-specific downregulated omentin-1 as a hallmark of rheumatoid arthritis and osteoarthritis onset [252]. In vivo studies suggest that omentin-1 supplementation betters intestinal inflammation in colitis mice, osteoporosis, and atherosclerosis [253]. Clinical cohort studies suggest that omentin-1 levels are alarmingly raised in sepsis patients [254]. Patients experiencing septic shock also exhibit higher omentin-1 and lower omentin-1 kinetics, suggesting that omentin-1 is a clinically relevant biomarker of sepsis onset and progression [254].
2.2.4. Secreted Frizzled-Related Proteins (SFRPs)
Constituting a class of Wnt antagonists, SFRPs bind directly with Wnt ligands in extracellular space and intervene in both canonical and non-canonical Wnt pathways [255].
SFRP2
SFRP2 is an anti-inflammatory adipokine that resides in the cell matrix as well as the cytoplasm [256]. It is a 34 kDa adipokine expressed largely in adipose tissue and modulates cellular differentiation, proliferation, myocardial fibrosis, hypertrophy, and angiogenesis [257,258]. As a cardioprotective adipokine, SFRP2 attenuates cardiomyocyte apoptosis and oxidative stress and reduces ventricular fibrosis and associated myocardial infarction [259,260]. Cardioprotective activity of SFRP2 is mediated typically via Wnt/β-catenin pathway inhibition and promoting calcineurin/TFEB-driven autophagy in diabetic mice model studies [261,262]. SFRP2 also attenuates mitochondrial dysfunction in diabetic hearts and augments FUNDC1, a mitochondrial membrane protein, which binds with LC3II and activates mitophagy-induced cardiac function improvement [262,263].
SFRP5
SFRP5 is an anti-inflammatory adipokine that regulates metabolic dysfunction conditions [264]. Over the years, a broad range of studies suggest that SFRP5 is central to downregulating MetS conditions, such as obesity and insulin resistance, restoring endothelial nitric oxide levels in T2D [265,266,267,268]. SFRP5 exhibits anti-inflammatory activity primarily driven via Wnt5a-c-JNK (Jun N-terminal kinase) signaling inhibition [269]. Multicenter cohort studies suggest that circulating SFRP5 is lowered and correlates with worsened prognosis in heart failure and T2D patients [270]. Upregulated SFRP5 attenuates cardiac inflammation and cardiomyocyte apoptosis [271]. SFRP5 is a crucial predictor of heart failure prognosis, and lowered SFRP5 levels are corrected with vascular calcification and coronary atherosclerosis [272,273].
2.2.5. Myeloid-Derived Growth Factor (MYDGF)
MYDGF, derived from chromosome 19 ORF 10, is a cardiomyocyte-protective angiogenic protein [274]. It augments the phosphorylation of BAD (at S136), Akt (at T308 and S473), and BAX (at S184) [274,275]. This downregulates cytosolic cytochrome C, cleaved caspase-9, and effector caspase-3 and caspase-7 [275]. MYDGF promotes MAPK1/MAPK3/STAT3 phosphorylation-driven endothelial cell proliferation and upregulates c-Myc/FoxM1 signaling-mediated cardiomyocyte proliferation post-heart failure [276,277,278]. MYDGF has also been reported to prevent cardiac microvascular endothelial cell apoptosis, post-ischemia/reperfusion (IR) [279]. MYDGF-ablated mice exhibit enlarged infarct size, augmented cardiomyocyte apoptosis, downregulated endothelial cell proliferation, and angiogenic responses [274]. The overexpression of MYDGF in bone marrow attenuates leukocyte influx into the aorta and prevents atherosclerosis onset via the downregulation of PKCδ/MAP4K4/NF-κB signaling [280]. MYDGF inhibits MAP4K4 phosphorylation and decreases FOXO3a signaling and LDL transcytosis, rendering protection against atherosclerosis onset [281]. In T2D, MYDGF enhances intestinal GLP-1 secretion via MAPK/MEK/ERK and PKA/GSK-3β/β-catenin signaling activation and improves insulin resistance, lipid metabolism, and glucose tolerance levels [282]. In addition, MYDGF attenuates renal oxidative stress and downregulates renal tubular cell apoptosis, inflammation, and podocyte injury via RUNX2/p27/cyclin A/CDK2 signaling activation [283,284].
Cumulatively, the metabolically protective functions of anti-inflammatory adipokines are summarized in Table 2.

Table 2.
Summary of the metabolically protective functions of some of the prominent anti-inflammatory adipokines.
3. Targeting Adipokines in MetS Treatment
Clinical manifestations of MetS, such as obesity, hypertension, and dyslipidemia, among others, exhibit deleterious impacts on cardiovascular homeostasis [285,286]. Adipokines, secreted primarily from adipose tissue, exhibit a substantial role in regulating metabolic and cardiovascular functions [287]. Therefore, targeting one or more of these bioactive peptides promises to design an effective therapeutic regimen against MetS conditions (Table 2).
3.1. Atherosclerosis and Dyslipidemia
MetS-characterized obesity is largely associated with atherosclerosis onset and is predisposed to CAD and cardiovascular disease-associated mortality [288,289,290]. Adipocyte hypertrophy without hyperplasia accounts for multiple dysfunctions such as lipid overload, adipocyte necrosis, and inflammation and culminates in atherogenic dyslipidemia onset, which is specifically characterized by triglyceride upsurge and lowered high-density lipoprotein (HDL) levels [291,292,293]. A vast majority of adipokines have been reported across studies to initiate the onset of atherosclerosis symptoms such as decreased fibrinolysis (Plasminogen Activator Inhibitor (PAI)-1), enhanced inflammation (TNF-α, IL-6), and significant insulin resistance (resistin) [294,295,296,297,298]. On the other hand, anti-inflammatory adipokine adiponectin is well recognized for its anti-atherogenic and anti-inflammatory regulatory impact [299,300]. Multiple studies over the years also suggest that dyslipidemia onset and progression are governed largely by circulating leptin and TNF-α adipokines [301]. Both leptin and TNF-α enhance the transmigration potential of low-density lipoprotein (LDL), oxidize LDL, and associate with a rise in MCP-1, VCAM-1, and ICAM-1 in endothelium [302]. Leptin-associated MCP-1 upsurge augments atherosclerotic plaque rupture and thrombus formation, culminates in coronary artery occlusion and, reduces blood supply to the heart [303]. Thus, adipokine-directed drug development assumes importance in atherosclerosis treatment.
Statins, the most widely accepted and utilized class of drugs against atherosclerosis and dyslipidemia, downregulate IL-6 expression and release, adipocyte differentiation via PPAR-γ/422Ap inhibition, C/EBPa, sterol regulatory element-binding protein (SREBP)-1, serum TNF-α, and leptin levels in adipocytes [304,305,306,307,308,309,310]. In fact, statins have also been reported to augment circulatory adiponectin concentration [311,312,313]. Fibrates, well identified as PPAR-γ activators, downregulate TNF-α mRNA and TNF-α serum concentrations in hypercholesterolemic subjects [314,315,316]. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors attenuate PCSK9 from binding with LDLR [317]. This promotes a high absorption of oxidized LDLs from the bloodstream and reduces plasma LDL levels [318]. Fibrates also inhibit adipocyte-specific leptin secretion and significantly augment fatty acid oxidation and the serum concentration of anti-inflammatory adiponectin and reduce visceral adipose mass and the upregulation of uncoupling protein (UCP)-1, Positive Regulatory Domain zinc finger region protein 16 (PRDM16), peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α, nuclear respiratory factor 1, and mitochondrial transcription factor A [319,320,321,322].
3.2. Obesity and Type 2 Diabetes (T2D)
As an increasingly serious risk factor predisposed to a broad variety of dysfunctions, obesity is a highly complicated disease condition that promotes a chronic inflammatory state and enhances insulin resistance, MetS, and the onset of T2D [323,324]. WAT expansion in obesity is characterized by ectopic fat deposition, extracellular matrix (ECM) and vascular alteration, sustained inflammation and fibrosis, damaged mitochondria, and disturbed adipokine secretion [325,326,327]. Circulating adipokines, chiefly leptin and TNF-α, are reported across studies to bear a direct correlation with dysregulated body fat deposition [328,329]. Therefore, targeting adipokines against obesity and associated T2D promises to greatly improve therapy.
Dietary and lifestyle modifications constitute the first line of therapy against obesity and correlated T2D [330]. Lifestyle interventions have been reported to reduce adiposity, mitigate T2D, augment mitochondrial biogenesis, and improve the inflammatory status of WAT [331,332]. Routine courses of aerobic and resistance exercise augment lipolysis via AMPK phosphorylation and SREBP-1c decrease [333,334]. This downregulates hepatic triglyceride synthesis, suppresses Acetyl Co-A carboxylase, and augments fatty acid oxidation and the reduction in fat mass and associated inflammatory adipokine levels [335,336]. Regular exercise promotes peripheral glucose uptake via GLUT4 translocation to skeletal muscle cell membranes [337]. This accelerates AMPK activity and, consequently, promotes insulin sensitivity [338]. Studies also suggest that chronic exercise decreases pro-inflammatory TNF-α, leptin, and MCP-1 levels [339,340]. Anti-obesity and associated T2D pharmacotherapy are currently constituted by glucagon-like peptide-1 receptor (GLP1R) agonists, biguanides (primarily metformin), thiazolidinediones, sulfonylureas, α- glucosidase inhibitors, lipase inhibitors, 5-hydroxytryptamine receptor agonists, and noradrenaline agonist/carbonic anhydrase inhibitor combination therapy, among others [341,342,343,344,345,346,347]. GLP-1R agonists typically include liraglutide and semaglutide and have been reported of late to reduce VAT adipose tissue mass [348]. GLP1R agonists also enhance adiponectin secretion and downregulate leptin levels, improving anti-inflammatory and insulin sensitivity effects [349]. Biguanides, like metformin, augment adiponectin and lower pro-inflammatory leptin and resistin and activate AMPK signaling that promotes energy metabolism and free fatty acid oxidation [350]. This downregulates ectopic fat accumulation, which enhances insulin sensitivity and avoids MetS onset [351]. To target adipokines in obesity and correlated T2D, the majority class of drugs are currently in developmental and/or clinical trial stages. These chiefly include leptin analogs, adiponectin and adiponectin receptor agonists, fibroblast growth factor (FGF)-21 analogs, IL-1R antagonists, anti-IL-1β antibodies, and palmitic acid hydroxy stearic acid (PAHSA) analogs [352,353,354,355,356]. These candidates propose to potentially lower mTOR activation, improve pancreatic β-cell function, promote gut GLP1 secretion via GPR120, and improve ceramidase activity through AdipoR1 or AdipoR2 stimulation [357,358,359].
3.3. Hypertension
Visceral fat accumulation constitutes one of the most prominent risk factors that is predisposed to hypertension onset [360,361,362]. Resistant hypertension is chiefly characterized by an obesity-associated rise in blood pressure despite the continued administration of multiple classes of anti-hypertensive drugs [363,364]. This has been chiefly reported to be mediated via increased aldosterone and pro-inflammatory adipokine levels [365,366]. Clinical trials of late suggest that obese subjects exhibit upregulated leptin, resistin, partial leptin resistance, and lowered adiponectin levels that cumulatively account for hypertension onset and progression [367,368,369,370]. Studies suggest proximity between resistin levels and renin–angiotensin–aldosterone system (RAAS) activity, and subjects with hypoaldosteronism are associated with increased resistin, leptin, body mass index (BMI), and deleterious modifications of cardiac morphology [371,372]. Alongside increased resistin and leptin, lowered plasma adiponectin levels are correlated with MetS cardiovascular outcomes [373]. Raised aldosterone levels downregulate adiponectin expression in adipocytes and outline RAAS/adiponectin interaction in MetS condition [374].
Existing therapeutic regimens against MetS-induced hypertension majorly target and intervene with RAAS activity [375]. Such classes of drugs primarily include angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers and have been reported to better the adipokine secretion profile in hypertensive subjects [376,377]. In fact, studies suggest that mineralocorticoid receptor (MR) antagonism enhances adiponectin secretion and downregulates pro-inflammatory adipokine and inflammatory mediator(s) secretion [378]. Hyperleptinemia and associated leptin resistance, one of the precursors of MetS-induced hypertension, is associated with dysregulated sympathetic hyperactivation [377]. This suggests that the leptin receptor is a strong potential drug target for alleviating hypertension.
3.4. Renal Dysfunction
MetS-associated hyperlipemia and CAD cumulatively activate a complex signaling network and account for kidney damage [379]. An adipocyte-specific rise in oxidative stress drives an increase in pro-inflammatory leptin levels and hyperleptinemia, TNF-α, IL-6, IL-12, and vascular smooth muscle cell calcification [380,381]. Together, they contribute to a rise in glomerular TGF-β1 expression, endothelial cell proliferation, and collagen type IV mRNA generation and lead to focal glomerulosclerosis, proteinuria, and mesangial glucose uptake [382,383].
Although no clinically approved adipokine-directed drugs currently exist for treating renal dysfunction, ongoing developmental studies raise significant hope. MetS has been typically associated with lowered plasma adiponectin levels and increased senescent cell load, renal fibrosis, and functional impairment [384,385]. The administration of senolytics, such as Quercetin, results in a better adiponectin secretory profile, attenuates renal fibrosis, augments renal cortical oxygenation, and reduces plasma creatinine levels [386,387]. Pharmacological targeting of the renin–angiotensin system (RAS) driven by an angiotensin receptor blockade exhibits renoprotection by lowering visceral fat and WAT-specific leptin secretion [388,389]. Combining spironolactone with ACE inhibitors promises to decrease albuminuria and obesity-associated renal dysfunction [390]. Studies also suggest that sodium/glucose co-transporter (SGLT)-2 inhibitors have potential to better MetS-induced kidney injury [391]. They attenuate glomerular hyperfiltration and impede renal injury progression [392]. This can potentially improve glomerular and tubular hemodynamics and culminate in renoprotection [393,394]. However, further mechanistic understanding of SGLT2 inhibition needs to be undertaken to potentially define their interaction (if any) with the adipokine profile and better explain associated renoprotection.
3.5. Osteoporosis
Osteoporosis, a global health concern for the elderly population, is largely characterized by reduced bone density and dysregulated bone structural integrity [395,396]. The existing paradigm of studies suggests that adipokines influence bone mineral density, cortical thickness, and fracture [396]. Pro-inflammatory adipokines, like leptin and resistin, bear a strong association with poor bone strength [397]. Increased circulating resistin increases osteoclastic activity and promotes bone remodeling and resorption via NF-Ƙβ signaling activation [398,399]. Clinical studies suggest that resistin levels, along with IL-1 and IL-6, are augmented in post-menopausal women and serve as predisposing factors for osteoporosis onset [400]. Leptin mRNA levels have been reported to be augmented in the cartilage and synovial fluid of osteoporosis and osteoarthritis patients [401]. Leptin activates c-Myc and PKA/ATF4/RANKL signaling that downregulates osteoblast proliferation and promotes bone resorption of osteoclasts [402]. This culminates in chondrocyte hypertrophy and excessive ossification [403]. Similarly, inflammatory adipokine visfatin has been reported to promote bone catabolism and negatively influence osteoblastic glucose uptake and collagen synthesis [404].
A clear understanding of the link between adipokines and bone health promises to improve therapeutic regimens against osteoporosis-associated fracture risk and bone degeneration [405]. Pre-clinical studies propose that the ablation of chemerin or its receptor chemokine-like receptor 1 (CMKLR1) attenuates adipocyte differentiation, mesenchymal stem cell proliferation, and augmented osteoblast gene expression and mineralization with an osteoblastogenic stimulus [406]. Such studies also highlight that chemerin/CMKLR1 modulates alterations in adipogenic and osteoblastogenic transcription factors that interfere with Wnt signaling [407]. Moreover, pre-clinical studies have shown that anti-inflammatory adipokine omentin-1 attenuates estrogen deficiency-induced bone loss by RANK/OPG downregulation and augments osteoblast differentiation via TGF-β/Smad signaling. This, in turn, also increases the expression of transcription factors collagen1, osteopontin, osteocalcin, and osterix [408,409]. Currently, ongoing studies involving human subjects shall better explain the therapeutic potential of adipokines in osteoporosis management.
3.6. Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD is chiefly characterized by upregulated hepatic fat mass (steatosis) in the absence of alcohol intake [408,409]. Genetic alterations, gut microbiome dysregulation, and metabolic abnormalities are some of the key predisposing factors of NAFLD onset and culminate in hypertriglyceridemia, reduced HDL, hypertension, and/or high fasting glucose levels [410]. Adipose tissue and the liver exhibit a critically coordinated role in glucose and lipid metabolism and immunological and energy homeostasis in the body [411,412]. In obese conditions, ectopic lipid accumulation starts in the liver since WAT reaches its limit of lipid storage [413].
A large body of studies suggest the potential role of adipokines in NAFLD onset and progression [412]. Pre-clinical studies show that high plasma leptin is strongly correlated with hepatic inflammatory and fibrogenetic processes that culminate in NAFLD onset [414]. NAFLD murine model studies also reveal high plasma resistin that alters mitochondrial physiology and upregulates pro-inflammatory mediators that aggravate NAFLD-associated steatosis by AMPK/PGC-1α signaling [415]. Similarly, visfatin exacerbates NAFLD-induced hepatic steatosis, inflammatory cytokine levels, and cellular infiltration mediated by increased ER stress [416]. On the contrary, anti-inflammatory adipokine adiponectin attenuates NAFLD-induced hepatic inflammation, steatosis, and fibrosis [417]. Adiponectin reduces NAFLD-associated pro-inflammatory signaling via TLR4 modulation [417]. Pharmacological studies aimed at developing NAFLD therapy identified biguanides, chiefly metformin, in preventing hepatic triglyceride accumulation and NAFLD onset [418]. Randomized controlled trials with thiazolidinediones suggest that thiazolidinediones (chiefly Rosiglitazone and Pioglitazone) downregulate hepatic steatosis and lobular inflammation in non-diabetic NAFLD subjects [419]. Thiazolidinediones augment adiponectin secretion that controls the visceral/subcutaneous fat mass ratio. Additionally, thiazolidinediones also reduce leptin levels in NAFLD subjects [420]. However, further pathophysiological studies with enhanced clinical insights shall enable a better comprehension of adipokine therapeutic potential for NAFLD treatment.
Cumulatively, the summary of the existing adipokine-targeted therapeutic regimens for MetS treatment are summarized in Table 3.

Table 3.
Brief summary of the existing adipokine-targeted therapeutic regimens for MetS treatment.
Table 3.
Brief summary of the existing adipokine-targeted therapeutic regimens for MetS treatment.
| MetS Condition | Adipokine-Targeted Therapeutic Intervention | References |
|---|---|---|
| Atherosclerosis and Dyslipidemia | (a) Statins—downregulate IL-6, adipocyte differentiation, adipocyte leptin, and TNF-α (b) Fibrates—downregulate visceral adipose mass, TNF-α serum concentration, and mRNA and promote fatty acid oxidation, serum adiponectin levels, and UCP-1 (c) PCSK9 inhibitors—attenuate PCSK9 from binding with LDLR, promote a high absorption of oxidized LDLs from the bloodstream, and reduce plasma LDL levels | [305,308,314,316,318] |
| Obesity and T2D | (a) Lifestyle modifications—reduce adiposity, promote mitochondrial biogenesis, and improve WAT inflammatory status (b) Adiponectin receptor agonists—improve pancreatic β-cell function (c) Palmitic acid hydroxy stearic acid (PAHSA) analogs—improve ceramidase activity via AdipoR1 or AdipoR2 stimulation (d) GLP1R agonists—enhance adiponectin secretion and downregulate leptin levels; improve anti-inflammatory and insulin sensitivity effects (e) Biguanides—augment adiponectin and lower pro-inflammatory leptin and resistin; activate AMPK signaling that promotes energy metabolism and free fatty acid oxidation | [347,348,350,356,357,359] |
| Hypertension | (a) ACE inhibitors—improve adipokine profile (b) MR antagonists—promote adiponectin secretion; downregulate pro-inflammatory adipokine release | [376,377,378] |
| Renal Dysfunction | (a) Senolytics—improve adiponectin profile, attenuate renal fibrosis, and augment renal cortical oxygenation (b) RAS inhibitors—lower visceral fat and WAT leptin secretion (c) SGLT2 inhibitors—prevent glomerular hyperfiltration and hinder renal damage progression | [386,388,392] |
| Osteoporosis | (a) Omentin-1 supplementation—attenuate estrogen deficiency-induced bone loss by RANK/OPG downregulation; augment osteoblast differentiation via TGF-β/Smad signaling (b) Chemerin inhibition—attenuate adipocyte differentiation, mesenchymal stem cell proliferation, and augmented osteoblast gene expression and mineralization | [406,408] |
| Non-Alcoholic Fatty Liver Disease (NAFLD) | (a) Biguanides—prevent hepatic triglyceride accumulation (b) Thiazolidinediones—downregulate hepatic steatosis and lobular inflammation; promote adiponectin secretion that controls the visceral/subcutaneous fat mass ratio | [418,420] |
4. Current Limitations and Future Landscape
Obesity and associated metabolic disorders are well-established precursors to a broad variety of neurodegenerative, respiratory, and cardiovascular complications [1,421,422]. Aspiring to improve MetS therapy, the role of adipokines assumes great significance in better explaining the mechanisms via which MetS initiates and amplifies associated dysfunction [423,424]. The manipulation of the adipokine secretion profile promises great potential for ameliorating MetS [299,425]. However, adipokine-directed therapy currently faces significant challenges. In developing novel drug candidates to better adiponectin secretion profiles, adiponectin protein stability, restricted circulatory half-life, and susceptibility to gastrointestinal enzymatic degradation constitute major concerns [426,427,428]. A lack of tissue specificity and diverse physiological adipokine activities are predisposed to unprecedented adverse effects of adipokine-directed therapy [429,430]. The determination of the optimal drug dose and the understanding of the long-term physiological impact of adipokine-targeted therapy remain largely unclear to date [431,432]. The genetic polymorphisms of the ADPOQ gene and associated inter-individual differences, the potential for drug–drug interactions, and comorbid health status complicate our understanding even further [433,434,435,436].
Addressing the above-mentioned concerns assumes importance and constitutes the future landscape of adipokine-directed therapy development. Liposome-mediated drug delivery can be focused on augmenting circulatory half-life and improving the therapeutic profile of the designed drug [437]. Existing genetic polymorphisms point towards developing personalized therapy, relying on individual adiponectin secretion profiles [438]. Extensive pharmacokinetic and pharmacodynamic studies need to be undertaken to better understand drug–drug interactions and the impact of dysfunctional metabolism on the adipokine profile [439]. Multicentric clinical trials can potentially better explain adipokine-directed therapeutic efficacy across patient cohorts [440]. Additionally, developing adiponectin analogs promises to be a great step toward confronting existing limitations with the adiponectin profile [429].
5. Conclusions
The existing paradigm of studies suggests that the adipokine secretory profile is a strong mediator of MetS onset and correlated physiological damage. The signaling networks regulated by pro-inflammatory and anti-inflammatory adipokines constitute a uniquely complicated cascade for driving or hindering inflammatory response. Understanding these signaling cross-talks can potentially empower translational investigations to better comprehend adipokine-directed therapy in MetS conditions. Studies propose adipokine-targeted therapy as a promising strategy for mitigating MetS and associated complications. However, existing limitations pose major challenges for the clinical implication of such therapy. Addressing these scientific gaps and amalgamating them with advanced genomics and metabolomic investigations coupled with greater courses of translational research can potentially enable clinical implications and ensure a better understanding of precision-driven adipokine-targeted MetS therapy in the near future.
Author Contributions
Conceptualization, S.D., K.M.B. and S.K.; writing—original draft preparation, S.D., writing—review and editing, K.M.B. and S.K.; funding acquisition, S.K. and K.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Health, grant number R01HL148711, and the American Heart Association, grant number 24IPA127635.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- Rus, M.; Crisan, S.; Andronie-Cioara, F.L.; Indries, M.; Marian, P.; Pobirci, O.L.; Ardelean, A.I. Prevalence and risk factors of metabolic syndrome: A prospective study on cardiovascular health. Medicina 2023, 59, 1711. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D. Assessing and managing the metabolic syndrome in children and adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Rahman, M.A.; Koka, S.; Boini, K.M. High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies. Cells 2024, 13, 1946. [Google Scholar] [CrossRef]
- Datta, S.; Pasham, S.; Inavolu, S.; Boini, K.M.; Koka, S. Role of gut microbial metabolites in cardiovascular diseases—Current insights and the road ahead. Int. J. Mol. Sci. 2024, 25, 10208. [Google Scholar] [CrossRef]
- Frisardi, V.; Matrone, C.; Street, M.E. Metabolic syndrome and autophagy: Focus on HMGB1 protein. Front. Cell Dev. Biol. 2021, 9, 654913. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A.; Farkhondeh, T. Evaluation of antidiabetic activity of carnosol (phenolic diterpene in rosemary) in streptozotocin-induced diabetic rats. Cardiovasc. Haematol. Disord.-Drug Targets (Former. Curr. Drug Targets-Cardiovasc. Hematol. Disord.) 2017, 17, 11–17. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Ning, H.; Labarthe, D.; Brewer, L.; Sharma, G.; Rosamond, W.; Foraker, R.E.; Black, T.; Grandner, M.A.; Allen, N.B. Status of cardiovascular health in US adults and children using the American Heart Association’s new “Life’s Essential 8” metrics: Prevalence estimates from the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation 2022, 146, 822–835. [Google Scholar] [CrossRef]
- Mietus-Snyder, M.; Perak, A.M.; Cheng, S.; Hayman, L.L.; Haynes, N.; Meikle, P.J.; Shah, S.H.; Suglia, S.F. Next generation, modifiable cardiometabolic biomarkers: Mitochondrial adaptation and metabolic resilience: A scientific statement from the American Heart Association. Circulation 2023, 148, 1827–1845. [Google Scholar] [CrossRef]
- Bjornstad, P.; Drews, K.; Zeitler, P.S. Long-term complications in youth-onset type 2 diabetes. Reply. N. Engl. J. Med. 2021, 385, 2016. [Google Scholar]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ 2020, 370, i5953. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, X.; Sun, L.; He, Y.; Zheng, S.; Zhang, Y.; Huang, Y. Prediabetes and the risk of heart failure: A meta-analysis. Diabetes Obes. Metab. 2021, 23, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wen, W.; Xie, D.; Qiu, M.; Cai, X.; Zheng, S.; Huang, Y. Association between non-alcoholic fatty liver disease and risk of incident heart failure: A meta-analysis of observational studies. Ther. Adv. Chronic Dis. 2022, 13, 20406223221119626. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Sidney, S.; Fairchild, A.L.; Howard, V.J.; Labarthe, D.R.; Shay, C.M.; Tiner, A.C.; Whitsel, L.P.; Rosamond, W.D.; Committee, A.H.A.A.C. Recommendations for cardiovascular health and disease surveillance for 2030 and beyond: A policy statement from the American Heart Association. Circulation 2020, 141, e104–e119. [Google Scholar] [CrossRef]
- Musunuru, K.; Hershberger, R.E.; Day, S.M.; Klinedinst, N.J.; Landstrom, A.P.; Parikh, V.N.; Prakash, S.; Semsarian, C.; Sturm, A.C.; Genomic, A.H.A.C.o.; et al. Genetic testing for inherited cardiovascular diseases: A scientific statement from the American Heart Association. Circ. Genom. Precis. Med. 2020, 13, e000067. [Google Scholar] [CrossRef]
- Johnston, E.K.; Abbott, R.D. Adipose tissue paracrine-, autocrine-, and matrix-dependent signaling during the development and progression of obesity. Cells 2023, 12, 407. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Su, Z.; Yang, J.; Sun, W.; Kong, X. The potential mechanisms underlying the modulating effect of perirenal adipose tissue on hypertension: Physical compression, paracrine, and neurogenic regulation. Life Sci. 2024, 342, 122511. [Google Scholar] [CrossRef]
- Bradley, D.; Xu, A.; Hsueh, W.A. The immunomodulatory roles of adipocytes. Front. Media SA 2021, 12, 827281. [Google Scholar]
- Liu, X.; Jiang, X.; Hu, J.; Ding, M.; Lee, S.K.; Korivi, M.; Qian, Y.; Li, T.; Wang, L.; Li, W. Exercise attenuates high-fat diet-induced PVAT dysfunction through improved inflammatory response and BMP4-regulated adipose tissue browning. Front. Nutr. 2024, 11, 1393343. [Google Scholar] [CrossRef]
- Tilg, H.; Ianiro, G.; Gasbarrini, A.; Adolph, T.E. Adipokines: Masterminds of metabolic inflammation. Nat. Rev. Immunol. 2024, 25, 250–265. [Google Scholar] [CrossRef]
- Gianotti, L.; Belcastro, S.; D’Agnano, S.; Tassone, F. The stress axis in obesity and diabetes mellitus: An update. Endocrines 2021, 2, 334–347. [Google Scholar] [CrossRef]
- Bel, J.S.; Tai, T.; Khaper, N.; Lees, S.J. Chronic glucocorticoid exposure causes brown adipose tissue whitening, alters whole-body glucose metabolism and increases tissue uncoupling protein-1. Physiol. Rep. 2022, 10, e15292. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014, 25, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, hepatokines and myokines: Focus on their role and molecular mechanisms in adipose tissue inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Funahashi, T.; Nakamura, T.; Nishida, M.; Kumada, M.; Okamoto, Y.; Ohashi, K.; Nagaretani, H.; Kishida, K. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation 2003, 107, 671–674. [Google Scholar] [CrossRef]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA 2003, 289, 1799–1804. [Google Scholar] [CrossRef]
- Yokota, T.; Oritani, K.; Takahashi, I.; Ishikawa, J.; Matsuyama, A.; Ouchi, N.; Kihara, S.; Funahashi, T.; Tenner, A.J.; Tomiyama, Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood J. Am. Soc. Hematol. 2000, 96, 1723–1732. [Google Scholar]
- Ouchi, N.; Kihara, S.; Arita, Y.; Nishida, M.; Matsuyama, A.; Okamoto, Y.; Ishigami, M.; Kuriyama, H.; Kishida, K.; Nishizawa, H. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 2001, 103, 1057–1063. [Google Scholar] [CrossRef]
- Okamoto, Y.; Folco, E.J.; Minami, M.; Wara, A.; Feinberg, M.W.; Sukhova, G.K.; Colvin, R.A.; Kihara, S.; Funahashi, T.; Luster, A.D. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ. Res. 2008, 102, 218–225. [Google Scholar] [CrossRef]
- Blüher, M.; Mantzoros, C.S. From leptin to other adipokines in health and disease: Facts and expectations at the beginning of the 21st century. Metabolism 2015, 64, 131–145. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Blüher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Adipokines–removing road blocks to obesity and diabetes therapy. Mol. Metab. 2014, 3, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Clinical relevance of adipokines. Diabetes Metab. J. 2012, 36, 317–327. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Biorender. Create Professional Science Figures in Minutes. 2024. Available online: https://www.biorender.com/ (accessed on 18 April 2025).
- Kriegler, M.; Perez, C.; DeFay, K.; Albert, I.; Lu, S. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: Ramifications for the complex physiology of TNF. Cell 1988, 53, 45–53. [Google Scholar] [CrossRef]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef]
- Xu, H.; Sethi, J.K.; Hotamisligil, G.S. Transmembrane tumor necrosis factor (TNF)-α inhibits adipocyte differentiation by selectively activating TNF receptor 1. J. Biol. Chem. 1999, 274, 26287–26295. [Google Scholar] [CrossRef]
- Perez, C.; Albert, I.; DeFay, K.; Zachariades, N.; Gooding, L.; Kriegler, M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell 1990, 63, 251–258. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Jellema, A.; Plat, J.; Mensink, R. Weight reduction, but not a moderate intake of fish oil, lowers concentrations of inflammatory markers and PAI-1 antigen in obese men during the fasting and postprandial state. Eur. J. Clin. Investig. 2004, 34, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Klein, J.; Lossner, U.; Paschke, R. Interleukin (IL)-6 mRNA expression is stimulated by insulin, isoproterenol, tumour necrosis factor alpha, growth hormone, and IL-6 in 3T3-L1 adipocytes. Horm. Metab. Res. 2003, 35, 147–152. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Ruan, H.; Miles, P.D.; Ladd, C.M.; Ross, K.; Golub, T.R.; Olefsky, J.M.; Lodish, H.F. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-α: Implications for insulin resistance. Diabetes 2002, 51, 3176–3188. [Google Scholar] [CrossRef]
- Souza, S.C.; Palmer, H.J.; Kang, Y.H.; Yamamoto, M.T.; Muliro, K.V.; Eric Paulson, K.; Greenberg, A.S. TNF-α induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. J. Cell. Biochem. 2003, 89, 1077–1086. [Google Scholar] [CrossRef]
- Zhang, H.H.; Halbleib, M.; Ahmad, F.; Manganiello, V.C.; Greenberg, A.S. Tumor necrosis factor-α stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 2002, 51, 2929–2935. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, S.; Kant, R. An update on metabolic syndrome: Metabolic risk markers and adipokines in the development of metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2409–2417. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Sethi, J.K. TNF-α and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Atkinson, R.L.; Spiegelman, B.M. Differential regulation of the p80 tumor necrosis factor receptor in human obesity and insulin resistance. Diabetes 1997, 46, 451–455. [Google Scholar] [CrossRef]
- Good, M.; Newell, F.M.; Haupt, L.M.; Whitehead, J.P.; Hutley, L.J.; Prins, J.B. TNF and TNF receptor expression and insulin sensitivity in human omental and subcutaneous adipose tissue—Influence of BMI and adipose distribution. Diabetes Vasc. Dis. Res. 2006, 3, 26–33. [Google Scholar] [CrossRef]
- Pandey, M.; Tuncman, G.; Hotamisligil, G.S.; Samad, F. Divergent roles for p55 and p75 TNF-α receptors in the induction of plasminogen activator inhibitor-1. Am. J. Pathol. 2003, 162, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yuan, W.; Fan, J.-S.; Lin, Z. Structure of the C-terminal domain of TRADD reveals a novel fold in the death domain superfamily. Sci. Rep. 2017, 7, 7073. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Shu, H.-B.; Pan, M.-G.; Goeddel, D.V. TRADD–TRAF2 and TRADD–FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996, 84, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef]
- Gountopoulou, A.; Leondaritis, G.; Galanopoulou, D.; Mavri-Vavayanni, M. TNFα is a potent inducer of platelet-activating factor synthesis in adipocytes but not in preadipocytes. Differential regulation by PI3K. Cytokine 2008, 41, 174–181. [Google Scholar] [CrossRef]
- Alipourfard, I.; Datukishvili, N.; Mikeladze, D. TNF-α Downregulation Modifies Insulin Receptor Substrate 1 (IRS-1) in Metabolic Signaling of Diabetic Insulin-Resistant Hepatocytes. Mediat. Inflamm. 2019, 2019, 3560819. [Google Scholar] [CrossRef]
- Rui, L.; Aguirre, V.; Kim, J.K.; Shulman, G.I.; Lee, A.; Corbould, A.; Dunaif, A.; White, M.F. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser 307 via distinct pathways. J. Clin. Investig. 2001, 107, 181–189. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Morgan, M.; Kim, D.-G.; Lee, J.-Y.; Bai, L.; Lin, Y.; Liu, Z.-g.; Kim, Y.-S. TNFα-induced noncanonical NF-κB activation is attenuated by RIP1 through stabilization of TRAF2. J. Cell Sci. 2011, 124, 647–656. [Google Scholar] [CrossRef]
- Jackson-Bernitsas, D.; Ichikawa, H.; Takada, Y.; Myers, J.; Lin, X.; Darnay, B.; Chaturvedi, M.; Aggarwal, B. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-κB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene 2007, 26, 1385–1397. [Google Scholar] [CrossRef]
- Stephens, J.M.; Lee, J.; Pilch, P.F. Tumor necrosis factor-α-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 1997, 272, 971–976. [Google Scholar] [CrossRef]
- Ruan, H.; Pownall, H.J.; Lodish, H.F. Troglitazone antagonizes tumor necrosis factor-α-induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NF-κB. J. Biol. Chem. 2003, 278, 28181–28192. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Kim, J.B.; Sarraf, P.; Spiegelman, B.M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science 1996, 274, 2100–2103. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Reginato, M.J.; Shao, D.; Lazar, M.A.; Chatterjee, V.K. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J. Biol. Chem. 1997, 272, 5128–5132. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-D.; Li, D.; Wang, S.; Zhang, S.; Zhao, H.; Price, R.A. Linkage and linkage disequilibrium mapping of genes influencing human obesity in chromosome region 7q22. 1–7q35. Diabetes 2003, 52, 1557–1561. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Liu, L.; Chen, Y.; Zhang, X.; Zhou, H.; Hu, S.; Li, X.; Li, M.; Li, J.; Cheng, S. Cancer-cell-secreted miR-204-5p induces leptin signalling pathway in white adipose tissue to promote cancer-associated cachexia. Nat. Commun. 2023, 14, 5179. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Shi, Y.; Kim, H.; Hamann, C.A.; Rhea, E.M.; Brunger, J.M.; Lippmann, E.S. Nuclear receptor ligand screening in an iPSC-derived in vitro blood–brain barrier model identifies new contributors to leptin transport. Fluids Barriers CNS 2022, 19, 77. [Google Scholar] [CrossRef]
- González-García, I.; García-Clavé, E.; Cebrian-Serrano, A.; Le Thuc, O.; Contreras, R.E.; Xu, Y.; Gruber, T.; Schriever, S.C.; Legutko, B.; Lintelmann, J. Estradiol regulates leptin sensitivity to control feeding via hypothalamic Cited1. Cell Metab. 2023, 35, 438–455.e437. [Google Scholar] [CrossRef]
- Martins, F.F.; Santos-Reis, T.; Marinho, T.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Hypothalamic anorexigenic signaling pathways (leptin, amylin, and proopiomelanocortin) are semaglutide (GLP-1 analog) targets in obesity control in mice. Life Sci. 2023, 313, 121268. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, M.-S. Molecular mechanisms of central leptin resistance in obesity. Arch. Pharmacal Res. 2013, 36, 201–207. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.-Y.; Hamnvik, O.-P.R.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. -Endocrinol. Metab. 2011, 301, E567–E584. [Google Scholar] [CrossRef] [PubMed]
- Assinder, S.J.; Boumelhem, B.B. Oxytocin stimulates lipolysis, prostaglandin E2 synthesis, and leptin secretion in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2021, 534, 111381. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Netting, M.J.; Lai, C.T.; Narayanan, A.; Dymock, M.; Rea, A.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, B.S. Reduction in maternal energy intake during lactation decreased maternal body weight and concentrations of leptin, insulin and adiponectin in human milk without affecting milk production, milk macronutrient composition or infant growth. Nutrients 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Badoer, E. Cardiovascular and metabolic crosstalk in the brain: Leptin and resistin. Front. Physiol. 2021, 12, 639417. [Google Scholar] [CrossRef]
- Duquenne, M.; Folgueira, C.; Bourouh, C.; Millet, M.; Silva, A.; Clasadonte, J.; Imbernon, M.; Fernandois, D.; Martinez-Corral, I.; Kusumakshi, S. Leptin brain entry via a tanycytic LepR–EGFR shuttle controls lipid metabolism and pancreas function. Nat. Metab. 2021, 3, 1071–1090. [Google Scholar] [CrossRef]
- Trinh, T.; Broxmeyer, H.E. Role for leptin and leptin receptors in stem cells during health and diseases. Stem Cell Rev. Rep. 2021, 17, 511–522. [Google Scholar] [CrossRef]
- Picó, C.; Palou, M.; Pomar, C.A.; Rodríguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef]
- Da Silva, A.A.; Pinkerton, M.A.; Spradley, F.T.; Palei, A.C.; Hall, J.E.; do Carmo, J.M. Chronic CNS-mediated cardiometabolic actions of leptin: Potential role of sex differences. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2021, 320, R173–R181. [Google Scholar] [CrossRef]
- Shah, N.; Khadilkar, A.; Oza, C.; Bhor, S.; Ladkat, D.; Gondhalekar, K.; More, C.; Khadilkar, V. Adiponectin–leptin ratio as a marker of cardio-metabolic risk in Indian children and youth with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2023, 36, 561–567. [Google Scholar] [CrossRef]
- Tahir, U.A.; Gerszten, R.E. Molecular Biomarkers for Cardiometabolic Disease: Risk Assessment in Young Individuals. Circ. Res. 2023, 132, 1663–1673. [Google Scholar] [CrossRef]
- Hayden, M.R.; Banks, W.A. Deficient leptin cellular signaling plays a key role in brain ultrastructural remodeling in obesity and type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 5427. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cordero, J.A.; Pérez-Pérez, A.; Jiménez-Cortegana, C.; Alba, G.; Flores-Barragán, A.; Sánchez-Margalet, V. Obesity as a risk factor for dementia and Alzheimer’s disease: The role of leptin. Int. J. Mol. Sci. 2022, 23, 5202. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Kwitek, A.E.; Sigmund, C.D.; Morselli, L.L.; Grobe, J.L. Recent advances in hypertension: Intersection of metabolic and blood pressure regulatory circuits in the central nervous system. Hypertension 2021, 77, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.D.; Roland, B.L.; Cole, R.L.; Trevaskis, J.L.; Weyer, C.; Koda, J.E.; Anderson, C.M.; Parkes, D.G.; Baron, A.D. Leptin responsiveness restored by amylin agonism in diet-induced obesity: Evidence from nonclinical and clinical studies. Proc. Natl. Acad. Sci. USA 2008, 105, 7257–7262. [Google Scholar] [CrossRef]
- Ravussin, E.; Smith, S.R.; Mitchell, J.A.; Shringarpure, R.; Shan, K.; Maier, H.; Koda, J.E.; Weyer, C. Enhanced weight loss with pramlintide/metreleptin: An integrated neurohormonal approach to obesity pharmacotherapy. Obesity 2009, 17, 1736–1743. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, R.; Hu, C. Leptin/obR signaling exacerbates obesity-related neutrophilic airway inflammation through inflammatory M1 macrophages. Mol. Med. 2023, 29, 100. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.; Chen, Z.; Liang, K.; Gao, X.; Wang, X.; Makota, F.V.; Ong, H.S.; Wan, Y.; Luo, K. The metabolic hormone leptin promotes the function of TFH cells and supports vaccine responses. Nat. Commun. 2021, 12, 3073. [Google Scholar] [CrossRef]
- Jiang, M.; He, J.; Sun, Y.; Dong, X.; Yao, J.; Gu, H.; Liu, L. Leptin induced TLR4 expression via the JAK2-STAT3 pathway in obesity-related osteoarthritis. Oxidative Med. Cell. Longev. 2021, 2021, 7385160. [Google Scholar] [CrossRef]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 phosphorylation in central leptin resistance. Nutr. Metab. 2021, 18, 39. [Google Scholar] [CrossRef]
- Kamareddine, L.; Ghantous, C.M.; Allouch, S.; Al-Ashmar, S.A.; Anlar, G.; Kannan, S.; Djouhri, L.; Korashy, H.M.; Agouni, A.; Zeidan, A. Between inflammation and autophagy: The role of leptin-adiponectin axis in cardiac remodeling. J. Inflamm. Res. 2021, 14, 5349–5365. [Google Scholar] [CrossRef]
- Tan, R.; Hu, X.; Wang, X.; Sun, M.; Cai, Z.; Zhang, Z.; Fu, Y.; Chen, X.; An, J.; Lu, H. Leptin promotes the proliferation and neuronal differentiation of neural stem cells through the cooperative action of MAPK/ERK1/2, JAK2/STAT3 and PI3K/AKT signaling pathways. Int. J. Mol. Sci. 2023, 24, 15151. [Google Scholar] [CrossRef] [PubMed]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, F.; Li, F.; Wang, B.; Hu, Y.; Li, X. Autocrined leptin promotes proliferation of non-small cell lung cancer (NSCLC) via PI3K/AKT and p53 pathways. Ann. Transl. Med. 2021, 9, 568. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Z.; Rui, L. Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. J. Biol. Chem. 2008, 283, 28066–28073. [Google Scholar] [CrossRef]
- Villanueva, E.C.; Myers, M. Leptin receptor signaling and the regulation of mammalian physiology. Int. J. Obes. 2008, 32, S8–S12. [Google Scholar] [CrossRef]
- Gao, Q.; Wolfgang, M.J.; Neschen, S.; Morino, K.; Horvath, T.L.; Shulman, G.I.; Fu, X.-Y. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl. Acad. Sci. USA 2004, 101, 4661–4666. [Google Scholar] [CrossRef]
- Könner, A.C.; Brüning, J.C. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012, 16, 144–152. [Google Scholar] [CrossRef]
- Tong, K.-M.; Shieh, D.-C.; Chen, C.-P.; Tzeng, C.-Y.; Wang, S.-P.; Huang, K.-C.; Chiu, Y.-C.; Fong, Y.-C.; Tang, C.-H. Leptin induces IL-8 expression via leptin receptor, IRS-1, PI3K, Akt cascade and promotion of NF-κB/p300 binding in human synovial fibroblasts. Cell. Signal. 2008, 20, 1478–1488. [Google Scholar] [CrossRef]
- Cota, D.; Proulx, K.; Smith, K.A.B.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef]
- Dagon, Y.; Hur, E.; Zheng, B.; Wellenstein, K.; Cantley, L.C.; Kahn, B.B. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metab. 2012, 16, 104–112. [Google Scholar] [CrossRef]
- Deepika, F.; Bathina, S.; Armamento-Villareal, R. Novel adipokines and their role in bone metabolism: A narrative review. Biomedicines 2023, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Chamberland, J.P.; Liu, X.; Matarese, G.; Gao, C.; Stefanakis, R.; Brinkoetter, M.T.; Gong, H.; Arampatzi, K.; Mantzoros, C.S. Leptin is an effective treatment for hypothalamic amenorrhea. Proc. Natl. Acad. Sci. USA 2011, 108, 6585–6590. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, E.; Magkos, F.; Aronis, K.N.; Brinkoetter, M.; Chamberland, J.P.; Chou, S.; Arampatzi, K.M.; Gao, C.; Koniaris, A.; Mantzoros, C.S. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism 2011, 60, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.-P.; Polyzos, S.A.; Anastasilakis, A.D.; Chou, S.; Mantzoros, C.S. The effect of leptin replacement on parathyroid hormone, RANKL-osteoprotegerin axis, and Wnt inhibitors in young women with hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 2014, 99, E2252–E2258. [Google Scholar] [CrossRef]
- Conroy, R.; Girotra, M.; Shane, E.; McMahon, D.J.; Pavlovich, K.H.; Leibel, R.L.; Rosenbaum, M.; Korner, J. Leptin administration does not prevent the bone mineral metabolism changes induced by weight loss. Metabolism 2011, 60, 1222–1226. [Google Scholar] [CrossRef][Green Version]
- Bank RPD. 7Z3Q-Crystal Structure of the Human Leptin:LepR-CRH2 Encounter Complex to 3.6 A Resolution: RCSB Protein Data Bank. 2025. Available online: https://www.rcsb.org/structure/7Z3Q (accessed on 18 April 2025).
- Stastny, J.; Bienertova-Vasku, J.; Vasku, A. Visfatin and its role in obesity development. Diabetes Metab. Syndr. Clin. Res. Rev. 2012, 6, 120–124. [Google Scholar] [CrossRef]
- Berndt, J.; Kloting, N.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schon, M.R.; Stumvoll, M.; Bluher, M. Plasma visfatin concentrations and fat depot–specific mRNA expression in humans. Diabetes 2005, 54, 2911–2916. [Google Scholar] [CrossRef]
- Rongvaux, A.; Galli, M.; Denanglaire, S.; Van Gool, F.; Dreze, P.L.; Szpirer, C.; Bureau, F.; Andris, F.; Leo, O. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J. Immunol. 2008, 181, 4685–4695. [Google Scholar] [CrossRef]
- Bae, Y.-H.; Bae, M.-K.; Kim, S.-R.; Lee, J.H.; Wee, H.-J.; Bae, S.-K. Upregulation of fibroblast growth factor-2 by visfatin that promotes endothelial angiogenesis. Biochem. Biophys. Res. Commun. 2009, 379, 206–211. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef]
- Zahorska-Markiewicz, B.; Olszanecka-Glinianowicz, M.; Janowska, J.; Kocełak, P.; Semik-Grabarczyk, E.; Holecki, M.; Dąbrowski, P.; Skorupa, A. Serum concentration of visfatin in obese women. Metabolism 2007, 56, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, B.; Tang, J.; Lu, W.; Wang, W.; Zhou, L.; Shang, W.; Li, F.; Ma, Q.; Yang, Y. Serum visfatin concentrations in obese adolescents and its correlation with age and high-density lipoprotein cholesterol. Diabetes Res. Clin. Pract. 2008, 79, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bermejo, A.; Chico-Julia, B.; Fernandez-Balsells, M.; Recasens, M.n.; Esteve, E.; Casamitjana, R.; Ricart, W.; Fernández-Real, J.-M. Serum visfatin increases with progressive β-cell deterioration. Diabetes 2006, 55, 2871–2875. [Google Scholar] [CrossRef]
- Dakroub, A.; Nasser, S.A.; Younis, N.; Bhagani, H.; Al-Dhaheri, Y.; Pintus, G.; Eid, A.A.; El-Yazbi, A.F.; Eid, A.H. Visfatin: A possible role in cardiovasculo-metabolic disorders. Cells 2020, 9, 2444. [Google Scholar] [CrossRef]
- Boini, K.M.; Zhang, C.; Xia, M.; Han, W.; Brimson, C.; Poklis, J.L.; Li, P.L. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim. Biophys. Acta 2010, 1801, 1294–1304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Koka, S.; Xia, M.; Zhang, C.; Zhang, Y.; Li, P.-L.; Boini, K.M. Podocyte NLRP3 inflammasome activation and formation by adipokine visfatin. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019, 53, 355. [Google Scholar]
- Chen, Y.; Pitzer, A.L.; Li, X.; Li, P.L.; Wang, L.; Zhang, Y. Instigation of endothelial Nlrp3 inflammasome by adipokine visfatin promotes inter-endothelial junction disruption: Role of HMGB 1. J. Cell. Mol. Med. 2015, 19, 2715–2727. [Google Scholar] [CrossRef]
- Datta, S.; Rahman, M.A.; Koka, S.; Boini, K.M. High Mobility Group Box 1 (HMGB1) Mediates Nicotine-Induced Podocyte Injury. Front. Pharmacol. 2025, 15, 1540639. [Google Scholar] [CrossRef]
- Xia, M.; Boini, K.M.; Abais, J.M.; Xu, M.; Zhang, Y.; Li, P.-L. Endothelial NLRP3 inflammasome activation and enhanced neointima formation in mice by adipokine visfatin. Am. J. Pathol. 2014, 184, 1617–1628. [Google Scholar] [CrossRef]
- Romacho, T.; Valencia, I.; Ramos-González, M.; Vallejo, S.; López-Esteban, M.; Lorenzo, O.; Cannata, P.; Romero, A.; San Hipólito-Luengo, A.; Gómez-Cerezo, J.F. Visfatin/eNampt induces endothelial dysfunction in vivo: A role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci. Rep. 2020, 10, 5386. [Google Scholar] [CrossRef] [PubMed]
- Linossier, M.-T.; Amirova, L.E.; Thomas, M.; Normand, M.; Bareille, M.-P.; Gauquelin-Koch, G.; Beck, A.; Costes-Salon, M.-C.; Bonneau, C.; Gharib, C. Effects of short-term dry immersion on bone remodeling markers, insulin and adipokines. PLoS ONE 2017, 12, e0182970. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Iorio, M.; Napoli, N.; Cotesta, D.; Zinnamosca, L.; Marinelli, C.; Petramala, L.; Minisola, S.; D’erasmo, E.; Letizia, C. Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome. J. Endocrinol. Investig. 2011, 34, e12–e15. [Google Scholar] [CrossRef] [PubMed]
- Koczan, D.; Guthke, R.; Thiesen, H.-J.; Ibrahim, S.M.; Kundt, G.; Krentz, H.; Gross, G.; Kunz, M. Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur. J. Dermatol. 2005, 15, 251–257. [Google Scholar]
- Jia, S.H.; Li, Y.; Parodo, J.; Kapus, A.; Fan, L.; Rotstein, O.D.; Marshall, J.C. Pre–B cell colony–enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Investig. 2004, 113, 1318–1327. [Google Scholar] [CrossRef]
- Duman, H.; Özyıldız, A.G.; Bahçeci, İ.; Duman, H.; Uslu, A.; Ergül, E. Serum visfatin level is associated with complexity of coronary artery disease in patients with stable angina pectoris. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719880448. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Xu, X.; Wan, R.-H.; Xia, S.; Lu, J.; Huang, Q. Association between serum visfatin levels and atherosclerotic plaque in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2019, 11, 60. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, D.; Meng, Z.; Wang, H.; Zhao, K.; Feng, X.; Li, Y.; Dun, A.; Jin, X.; Hou, H. Association between circulating visfatin and gestational diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2018, 55, 1113–1120. [Google Scholar] [CrossRef]
- Ferreira, A.F.A.; Rezende, J.C.; Vaikousi, E.; Akolekar, R.; Nicolaides, K.H. Maternal serum visfatin at 11–13 weeks of gestation in gestational diabetes mellitus. Clin. Chem. 2011, 57, 609–613. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, Y.; Xu, J.; Fang, Q.; Chen, D. Correlations of serum visfatin and metabolisms of glucose and lipid in women with gestational diabetes mellitus. J. Diabetes Investig. 2016, 7, 247–252. [Google Scholar] [CrossRef]
- Souvannavong-Vilivong, X.; Sitticharoon, C.; Klinjampa, R.; Keadkraichaiwat, I.; Sripong, C.; Chatree, S.; Sririwichitchai, R.; Lertbunnaphong, T. Placental expressions and serum levels of adiponectin, visfatin, and omentin in GDM. Acta Diabetol. 2019, 56, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Bank RPD. 2G96-Crystal Structure of Visfatin/Pre-B Cell Colony Enhancing Factor 1/Nicotinamide Phosphoribosyltransferase in Complex with Niconamide Mononucleotide: RCS Protein Data Bank. 2025. Available online: https://www.rcsb.org/structure/2g96 (accessed on 18 April 2025).
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Singh, A.; Pant, A. Could resistin be a noble marker for metabolic syndrome? Diabetes Metab. Syndr. Clin. Res. Rev. 2010, 4, 239–244. [Google Scholar] [CrossRef]
- Al Hannan, F.; Culligan, K.G. Human resistin and the RELM of Inflammation in diabesity. Diabetol. Metab. Syndr. 2015, 7, 54. [Google Scholar] [CrossRef]
- Onuma, H.; Tabara, Y.; Kawamura, R.; Ohashi, J.; Nishida, W.; Takata, Y.; Ochi, M.; Nishimiya, T.; Kawamoto, R.; Kohara, K. Plasma resistin is associated with single nucleotide polymorphisms of a possible resistin receptor, the decorin gene, in the general Japanese population. Diabetes 2013, 62, 649–652. [Google Scholar] [CrossRef][Green Version]
- Singh, A.K.; Tiwari, S.; Gupta, A.; Natu, S.M.; Mittal, B.; Pant, A.B. Association of resistin with metabolic syndrome in Indian subjects. Metab. Syndr. Relat. Disord. 2012, 10, 286–291. [Google Scholar] [CrossRef]
- Rajala, M.W.; Qi, Y.; Patel, H.R.; Takahashi, N.; Banerjee, R.; Pajvani, U.B.; Sinha, M.K.; Gingerich, R.L.; Scherer, P.E.; Ahima, R.S. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes 2004, 53, 1671–1679. [Google Scholar] [CrossRef]
- Banerjee, R.R.; Rangwala, S.M.; Shapiro, J.S.; Rich, A.S.; Rhoades, B.; Qi, Y.; Wang, J.; Rajala, M.W.; Pocai, A.; Scherer, P.E. Regulation of fasted blood glucose by resistin. Science 2004, 303, 1195–1198. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, V.; Srivastava, N.; Gupta, V.; Mishra, S.; Mishra, S.; Shankar, M.N.; Roy, U.; Chandra, A.; Negi, M. Resistin 420C/G gene polymorphism on circulating resistin, metabolic risk factors and insulin resistance in adult women. Immunol. Lett. 2014, 162, 287–291. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Y.; Li, F.; He, J.; Ding, H.; Yan, L.; Cheng, H. Resistin induces insulin resistance by both AMPK-dependent and AMPK-independent mechanisms in HepG2 cells. Endocrine 2009, 36, 60–69. [Google Scholar] [CrossRef]
- Li, F.-P.; He, J.; Li, Z.-Z.; Luo, Z.-F.; Yan, L.; Li, Y. Effects of resistin expression on glucose metabolism and hepatic insulin resistance. Endocrine 2009, 35, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.R.; Lazar, M.A. Human resistin: Found in translation from mouse to man. Trends Endocrinol. Metab. 2011, 22, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Benomar, Y.; Taouis, M. Molecular mechanisms underlying obesity-induced hypothalamic inflammation and insulin resistance: Pivotal role of resistin/TLR4 pathways. Front. Endocrinol. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Tsiotra, P.C.; Boutati, E.; Dimitriadis, G.; Raptis, S.A. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. BioMed Res. Int. 2013, 2013, 487081. [Google Scholar] [CrossRef]
- Khera, A.V.; Qamar, A.; Murphy, S.A.; Cannon, C.P.; Sabatine, M.S.; Rader, D.J. On-statin resistin, leptin, and risk of recurrent coronary events after hospitalization for an acute coronary syndrome (from the pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 study). Am. J. Cardiol. 2015, 116, 694–698. [Google Scholar] [CrossRef]
- Bik, W.; Ostrowski, J.; Baranowska-Bik, A.; Wolinska-Witort, E.; Bialkowska, M.; Martynska, L.; Baranowska, B. Adipokines and genetic factors in overweight or obese but metabolically healthy Polish women. Neuroendocrinol. Lett. 2010, 31, 497. [Google Scholar]
- Zhang, Y.; Li, Y.; Yu, L.; Zhou, L. Association between serum resistin concentration and hypertension: A systematic review and meta-analysis. Oncotarget 2017, 8, 41529. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Alonso-Iglesias, E. Resistin: Insulin resistance to malignancy. Clin. Chim. Acta 2015, 438, 46–54. [Google Scholar] [CrossRef]
- Askin, L.; Abus, S.; Tanriverdi, O. Resistin and cardiovascular disease: A review of the current literature regarding clinical and pathological relationships. Curr. Cardiol. Rev. 2022, 18, 70–76. [Google Scholar] [CrossRef]
- Deb, A.; Deshmukh, B.; Ramteke, P.; Bhati, F.K.; Bhat, M.K. Resistin: A journey from metabolism to cancer. Transl. Oncol. 2021, 14, 101178. [Google Scholar] [CrossRef]
- Bank RPD. RFX-Crystal Structure of Resisitin: RCS Protein Data Bank. 2025. Available online: https://www.rcsb.org/structure/1rfx (accessed on 18 April 2025).
- Kubota, Y.; Oike, Y.; Satoh, S.; Tabata, Y.; Niikura, Y.; Morisada, T.; Akao, M.; Urano, T.; Ito, Y.; Miyamoto, T. Cooperative interaction of Angiopoietin-like proteins 1 and 2 in zebrafish vascular development. Proc. Natl. Acad. Sci. USA 2005, 102, 13502–13507. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Moon, S.-O.; Koh, K.N.; Kim, H.; Uhm, C.-S.; Kwak, H.J.; Kim, N.-G.; Koh, G.Y. Molecular cloning, expression, and characterization of angiopoietin-related protein: Angiopoietin-related protein induces endothelial cell sprouting. J. Biol. Chem. 1999, 274, 26523–26528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Umikawa, M.; Cui, C.; Li, J.; Chen, X.; Zhang, C.; Huynh, H.; Kang, X.; Silvany, R.; Wan, X. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature 2012, 485, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Oike, Y.; Yasunaga, K.; Suda, T. Angiopoietin-related/angiopoietin-like proteins regulate angiogenesis. Int. J. Hematol. 2004, 80, 21–28. [Google Scholar] [CrossRef]
- Tabata, M.; Kadomatsu, T.; Fukuhara, S.; Miyata, K.; Ito, Y.; Endo, M.; Urano, T.; Zhu, H.J.; Tsukano, H.; Tazume, H. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009, 10, 178–188. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.K.; Jang, Y.J.; Park, H.S.; Kim, J.-H.; Hong, J.P.; Lee, Y.J.; Heo, Y.-S. Enhanced ANGPTL2 expression in adipose tissues and its association with insulin resistance in obese women. Sci. Rep. 2018, 8, 13976. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ohta, M.; Desai, D.; Figueiredo, J.-L.; Whelan, M.C.; Sugano, T.; Yamabi, M.; Yano, W.; Faits, T.; Yabusaki, K. Angiopoietin like protein 2 (ANGPTL2) promotes adipose tissue macrophage and T lymphocyte accumulation and leads to insulin resistance. PLoS ONE 2015, 10, e0131176. [Google Scholar] [CrossRef]
- Meng, Q.-X.; Wen, L.; Chen, X.-Y.; Zhong, H.-J. Association of serum angiopoietin-like protein 2 and epinephrine levels in metabolically healthy but obese individuals: In vitro and in vivo evidence. Exp. Ther. Med. 2013, 5, 1631–1636. [Google Scholar] [CrossRef]
- Oike, Y.; Tabata, M. Angiopoietin-like proteins potential therapeutic targets for metabolic syndrome and cardiovascular disease. Circ. J. 2009, 73, 2192–2197. [Google Scholar] [CrossRef]
- Thorin-Trescases, N.; Thorin, E. High circulating levels of ANGPTL2: Beyond a clinical marker of systemic inflammation. Oxidative Med. Cell. Longev. 2017, 2017, 1096385. [Google Scholar] [CrossRef]
- Farhat, N.; Thorin-Trescases, N.; Mamarbachi, M.; Villeneuve, L.; Yu, C.; Martel, C.; Duquette, N.; Gayda, M.; Nigam, A.; Juneau, M. Angiopoietin-like 2 promotes atherogenesis in mice. J. Am. Heart Assoc. 2013, 2, e000201. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, W.J.; Lee, M.J.; Kang, Y.M.; Jang, J.E.; Leem, J.; La Lee, Y.; Seol, S.M.; Yoon, H.K.; Park, J.-Y. Association of serum angiopoietin-like protein 2 with carotid intima-media thickness in subjects with type 2 diabetes. Cardiovasc. Diabetol. 2015, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-L.; Wu, Y.-W.; Wu, C.-C.; Hwang, J.-J.; Yang, W.-S. Serum angiopoietin-like protein 2 concentrations are independently associated with heart failure. PLoS ONE 2015, 10, e0138678. [Google Scholar] [CrossRef] [PubMed]
- Dewey, F.E.; Gusarova, V.; O’Dushlaine, C.; Gottesman, O.; Trejos, J.; Hunt, C.; Van Hout, C.V.; Habegger, L.; Buckler, D.; Lai, K.-M.V. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med. 2016, 374, 1123–1133. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Busquets, S. Mediators of cachexia in cancer patients. Nutrition 2019, 66, 11–15. [Google Scholar] [CrossRef]
- Janssen, A.W.; Katiraei, S.; Bartosinska, B.; Eberhard, D.; Willems van Dijk, K.; Kersten, S. Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia 2018, 61, 1447–1458. [Google Scholar] [CrossRef]
- Katanasaka, Y.; Saito, A.; Sunagawa, Y.; Sari, N.; Funamoto, M.; Shimizu, S.; Shimizu, K.; Akimoto, T.; Ueki, C.; Kitano, M. ANGPTL4 expression is increased in epicardial adipose tissue of patients with coronary artery disease. J. Clin. Med. 2022, 11, 2449. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Zhang, X.; Varela, L.; Rotllan, N.; Goedeke, L.; Chaube, B.; Camporez, J.-P.; Vatner, D.F.; Horvath, T.L. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight 2018, 3, e97918. [Google Scholar] [CrossRef]
- Guo, L.; Li, S.; Zhao, Y.; Qian, P.; Ji, F.; Qian, L.; Wu, X.; Qian, G. Silencing angiopoietin-like protein 4 (ANGPTL4) protects against lipopolysaccharide-induced acute lung injury via regulating SIRT1/NF-kB pathway. J. Cell. Physiol. 2015, 230, 2390–2402. [Google Scholar] [CrossRef]
- Chen, T.-C.; Lee, R.A.; Tsai, S.L.; Kanamaluru, D.; Gray, N.E.; Yiv, N.; Cheang, R.T.; Tan, J.H.; Lee, J.Y.; Fitch, M.D. An ANGPTL4–ceramide–protein kinase Cζ axis mediates chronic glucocorticoid exposure–induced hepatic steatosis and hypertriglyceridemia in mice. J. Biol. Chem. 2019, 294, 9213–9224. [Google Scholar] [CrossRef]
- Chaurasia, B.; Kaddai, V.A.; Lancaster, G.I.; Henstridge, D.C.; Sriram, S.; Galam, D.L.A.; Gopalan, V.; Prakash, K.B.; Velan, S.S.; Bulchand, S. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 2016, 24, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Bini, S.; D’Erasmo, L.; Di Costanzo, A.; Minicocci, I.; Pecce, V.; Arca, M. The interplay between angiopoietin-like proteins and adipose tissue: Another piece of the relationship between adiposopathy and cardiometabolic diseases? Int. J. Mol. Sci. 2021, 22, 742. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Datta, S.; Lakkakula, H.; Koka, S.; Boini, K.M. Acid Sphingomyelinase and Ceramide Signaling Pathway Mediates Nicotine-Induced NLRP3 Inflammasome Activation and Podocyte Injury. Biomedicines 2025, 13, 416. [Google Scholar] [CrossRef]
- Guo, C.; Wang, C.; Deng, X.; He, J.; Yang, L.; Yuan, G. ANGPTL8 in metabolic homeostasis: More friend than foe? Open Biol. 2021, 11, 210106. [Google Scholar] [CrossRef]
- Ye, H.; Zong, Q.; Zou, H.; Zhang, R. Emerging insights into the roles of ANGPTL8 beyond glucose and lipid metabolism. Front. Physiol. 2023, 14, 1275485. [Google Scholar] [CrossRef]
- Jiao, X.; He, J.; Yang, Y.; Yang, S.; Li, J.; Qin, Y. Associations between circulating full-length angiopoietin-like protein 8 levels and severity of coronary artery disease in Chinese non-diabetic patients: A case–control study. Cardiovasc. Diabetol. 2018, 17, 92. [Google Scholar] [CrossRef]
- Vatner, D.F.; Goedeke, L.; Camporez, J.-P.G.; Lyu, K.; Nasiri, A.R.; Zhang, D.; Bhanot, S.; Murray, S.F.; Still, C.D.; Gerhard, G.S. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia 2018, 61, 1435–1446. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Hu, L.; Tang, J.; Meng, Z.; Dai, L.; Gao, Y.; Ma, S.; Wang, X.; Yuan, Y. ANGPTL8 accelerates liver fibrosis mediated by HFD-induced inflammatory activity via LILRB2/ERK signaling pathways. J. Adv. Res. 2023, 47, 41–56. [Google Scholar] [CrossRef]
- Li, D.-P.; Huang, L.; Kan, R.-R.; Meng, X.-Y.; Wang, S.-Y.; Zou, H.-J.; Guo, Y.-M.; Luo, P.-Q.; Pan, L.-M.; Xiang, Y.-X. LILRB2/PirB mediates macrophage recruitment in fibrogenesis of nonalcoholic steatohepatitis. Nat. Commun. 2023, 14, 4436. [Google Scholar] [CrossRef]
- Keller, P.; Keller, C.; Carey, A.L.; Jauffred, S.; Fischer, C.P.; Steensberg, A.; Pedersen, B.K. Interleukin-6 production by contracting human skeletal muscle: Autocrine regulation by IL-6. Biochem. Biophys. Res. Commun. 2003, 310, 550–554. [Google Scholar] [CrossRef]
- Akira, S.; Taga, T.; Kishimoto, T. Interleukin-6 in biology and medicine. Adv. Immunol. 1993, 54, 1–78. [Google Scholar] [PubMed]
- Kim, J.H.; Bachmann, R.A.; Chen, J. Interleukin-6 and insulin resistance. Vitam. Horm. 2009, 80, 613–633. [Google Scholar] [PubMed]
- Tiwari, S.; Gupta, V.; Paul, B.N.; Kumar, S.; Chandra, A.; Dhananjai, S.; Negi, M.P.S.; Ghatak, A. IL-6 gene expression in adipose tissue of postmenopausal women and its association with metabolic risk factors. Mol. Cell. Endocrinol. 2015, 399, 87–94. [Google Scholar]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Schindler, C.; Darnell Jr, J. Transcriptional responses to polypeptide ligands: The JAK-STAT pathway. Annu. Rev. Biochem. 1995, 64, 621–651. [Google Scholar] [CrossRef]
- Taga, T. Gp130, a shared signal transducing receptor component for hematopoietic and neuropoietic cytokines. J. Neurochem. 1996, 67, 1–10. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334, 297–314. [Google Scholar] [CrossRef]
- Garbers, C.; Aparicio-Siegmund, S.; Rose-John, S. The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Curr. Opin. Immunol. 2015, 34, 75–82. [Google Scholar] [CrossRef]
- Terstegen, L.; Gatsios, P.; Bode, J.G.; Schaper, F.; Heinrich, P.C.; Graeve, L. The inhibition of interleukin-6-dependent STAT activation by mitogen-activated protein kinases depends on tyrosine 759 in the cytoplasmic tail of glycoprotein 130. J. Biol. Chem. 2000, 275, 18810–18817. [Google Scholar] [CrossRef]
- Hu, F.B.; Meigs, J.B.; Li, T.Y.; Rifai, N.; Manson, J.E. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004, 53, 693–700. [Google Scholar] [CrossRef]
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes. Res. 2001, 9, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Klover, P.J.; Zimmers, T.A.; Koniaris, L.G.; Mooney, R.A. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 2003, 52, 2784–2789. [Google Scholar] [CrossRef] [PubMed]
- Senn, J.J.; Klover, P.J.; Nowak, I.A.; Mooney, R.A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002, 51, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Lagathu, C.; Bastard, J.-P.; Auclair, M.; Maachi, M.; Capeau, J.; Caron, M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: Prevention by rosiglitazone. Biochem. Biophys. Res. Commun. 2003, 311, 372–379. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.-i.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Ryo, M.; Nakamura, T.; Kihara, S.; Kumada, M.; Shibazaki, S.; Takahashi, M.; Nagai, M.; Matsuzawa, Y.; Funahashi, T. Adiponectin as a biomarker of the metabolic syndrome. Circ. J. 2004, 68, 975–981. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Argueta, J.G.M.; Masuhiro, Y.; Kagishita, M.; Nonaka, K.; Saito, T.; Hanazawa, S.; Yamashita, Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005, 579, 6821–6826. [Google Scholar] [CrossRef]
- Summer, R.; Little, F.; Ouchi, N.; Takemura, Y.; Aprahamian, T.; Dwyer, D.; Fitzsimmons, K.; Suki, B.; Parameswaran, H.; Fine, A. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2008, 294, L1035–L1042. [Google Scholar] [CrossRef]
- Shibata, R.; Sato, K.; Pimentel, D.R.; Takemura, Y.; Kihara, S.; Ohashi, K.; Funahashi, T.; Ouchi, N.; Walsh, K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK-and COX-2–dependent mechanisms. Nat. Med. 2005, 11, 1096–1103. [Google Scholar] [CrossRef]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Szmitko, P.E.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Chan, L.; Al-Omran, M.; Teoh, H. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am. J. Physiol. -Heart Circ. Physiol. 2010, 299, H656–H663. [Google Scholar] [CrossRef] [PubMed]
- Takemura, Y.; Ouchi, N.; Shibata, R.; Aprahamian, T.; Kirber, M.T.; Summer, R.S.; Kihara, S.; Walsh, K. Adiponectin modulates inflammatory reactions via calreticulin receptor–dependent clearance of early apoptotic bodies. J. Clin. Investig. 2007, 117, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, S.; Arsenijevic, N.; Djukic, A.; Djukic, S.; Simonovic, S.Z.; Jovanovic, M.; Pejnovic, N.; Nikolic, V.; Zivanovic, S.; Stefanovic, M. Adiponectin as a potential biomarker of low bone mineral density in postmenopausal women with metabolic syndrome. Acta Endocrinol. 2018, 14, 201. [Google Scholar] [CrossRef]
- Bacchetta, J.; Boutroy, S.; Guebre-Egziabher, F.; Juillard, L.; Drai, J.; Pelletier, S.; Richard, M.; Charrié, A.; Carlier, M.C.; Chapurlat, R. The relationship between adipokines, osteocalcin and bone quality in chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 3120–3125. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lee, C.-J.; Yang, C.-F.; Chen, Y.-C.; Wang, J.-H.; Hsu, B.-G. Low serum adiponectin level is associated with metabolic syndrome and is an independent marker of peripheral arterial stiffness in hypertensive patients. Diabetol. Metab. Syndr. 2017, 9, 49. [Google Scholar] [CrossRef]
- Fujishima, Y.; Kita, S.; Nishizawa, H.; Maeda, N.; Shimomura, I. Cardiovascular significance of adipose-derived adiponectin and liver-derived xanthine oxidoreductase in metabolic syndrome. Endocr. J. 2023, 70, 663–675. [Google Scholar] [CrossRef]
- Akhtar, Y.; Nawaz, S.; Khan, M.S.; Habib, S.H.; Malik, M.O.; Fatima, S. Relationship of serum adiponectin levels with glycaemic status in pregnant women. J. Ayub Med. Coll. Abbottabad 2022, 34, 235–238. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Mendez-Sanchez, N.; Laurindo, L.F. AdipoRon and ADP355, adiponectin receptor agonists, in Metabolic-associated Fatty Liver Disease (MAFLD) and Nonalcoholic Steatohepatitis (NASH): A systematic review. Biochem. Pharmacol. 2023, 218, 115871. [Google Scholar] [CrossRef]
- Wong, G.W.; Wang, J.; Hug, C.; Tsao, T.-S.; Lodish, H.F. A family of Acrp30/adiponectin structural and functional paralogs. Proc. Natl. Acad. Sci. USA 2004, 101, 10302–10307. [Google Scholar] [CrossRef]
- Kopp, A.; Bala, M.; Buechler, C.; Falk, W.; Gross, P.; Neumeier, M.; Scholmerich, J.; Schaffler, A. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 2010, 151, 5267–5278. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Chen, N.; Obermeier, F.; Paul, G.; Büchler, C.; Kopp, A.; Falk, W.; Schäffler, A. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm. Bowel Dis. 2011, 17, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Wei, Z.; Wong, G.W. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J. Biol. Chem. 2010, 285, 39691–39701. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Schäffler, A.; Fleck, M.; Schölmerich, J.; Schütz, C.; Buechler, C. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett. 2005, 579, 5565–5570. [Google Scholar] [CrossRef]
- Yi, W.; Sun, Y.; Yuan, Y.; Lau, W.B.; Zheng, Q.; Wang, X.; Wang, Y.; Shang, X.; Gao, E.; Koch, W.J. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation 2012, 125, 3159–3169. [Google Scholar] [CrossRef]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Revett, T.; Gimeno, R.; Lodish, H.F. Molecular, biochemical and functional characterizations of C1q/TNF family members: Adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem. J. 2008, 416, 161–177. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lee, W.; Park, E.-J.; Park, S.-Y. C1qTNF-related protein-6 increases the expression of interleukin-10 in macrophages. Mol. Cells 2010, 30, 59–64. [Google Scholar] [CrossRef]
- Lee, W.; Kim, M.-J.; Park, E.-J.; Choi, Y.-J.; Park, S.-Y. C1qTNF-related protein-6 mediates fatty acid oxidation via the activation of the AMP-activated protein kinase. FEBS Lett. 2010, 584, 968–972. [Google Scholar] [CrossRef]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Ge, G.; Spooner, E.; Hug, C.; Gimeno, R.; Lodish, H.F. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009, 23, 241. [Google Scholar] [CrossRef]
- Kambara, T.; Ohashi, K.; Shibata, R.; Ogura, Y.; Maruyama, S.; Enomoto, T.; Uemura, Y.; Shimizu, Y.; Yuasa, D.; Matsuo, K. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J. Biol. Chem. 2012, 287, 18965–18973. [Google Scholar] [CrossRef]
- Zheng, Q.; Yuan, Y.; Yi, W.; Lau, W.B.; Wang, Y.; Wang, X.; Sun, Y.; Lopez, B.L.; Christopher, T.A.; Peterson, J.M. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Shibata, R.; Ohashi, K.; Enomoto, T.; Kambara, T.; Yamamoto, T.; Ogura, Y.; Yuasa, D.; Joki, Y.; Matsuo, K. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013, 27, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Wei, Z.; Seldin, M.M.; Byerly, M.S.; Aja, S.; Wong, G.W. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2013, 305, R522–R533. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Ohashi, K.; Shibata, R.; Higuchi, A.; Maruyama, S.; Izumiya, Y.; Walsh, K.; Murohara, T.; Ouchi, N. Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J. Biol. Chem. 2011, 286, 34552–34558. [Google Scholar] [CrossRef]
- Wei, Z.; Peterson, J.M.; Lei, X.; Cebotaru, L.; Wolfgang, M.J.; Baldeviano, G.C.; Wong, G.W. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J. Biol. Chem. 2012, 287, 10301–10315. [Google Scholar] [CrossRef]
- Wei, Z.; Lei, X.; Seldin, M.M.; Wong, G.W. Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J. Biol. Chem. 2012, 287, 35804–35814. [Google Scholar] [CrossRef]
- Enomoto, T.; Shibata, R.; Ohashi, K.; Kambara, T.; Kataoka, Y.; Uemura, Y.; Yuasa, D.; Murohara, T.; Ouchi, N. Regulation of adipolin/CTRP12 cleavage by obesity. Biochem. Biophys. Res. Commun. 2012, 428, 155–159. [Google Scholar] [CrossRef]
- Bell-Anderson, K.S.; Funnell, A.P.; Williams, H.; Mat Jusoh, H.; Scully, T.; Lim, W.F.; Burdach, J.G.; Mak, K.S.; Knights, A.J.; Hoy, A.J. Loss of Krüppel-like factor 3 (KLF3/BKLF) leads to upregulation of the insulin-sensitizing factor adipolin (FAM132A/CTRP12/C1qdc2). Diabetes 2013, 62, 2728–2737. [Google Scholar] [CrossRef]
- Enomoto, T.; Ohashi, K.; Shibata, R.; Kambara, T.; Uemura, Y.; Yuasa, D.; Kataoka, Y.; Miyabe, M.; Matsuo, K.; Joki, Y. Transcriptional regulation of an insulin-sensitizing adipokine adipolin/CTRP12 in adipocytes by Krüppel-like factor 15. PLoS ONE 2013, 8, e83183. [Google Scholar] [CrossRef]
- Tsuji, S.; Uehori, J.; Matsumoto, M.; Suzuki, Y.; Matsuhisa, A.; Toyoshima, K.; Seya, T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 2001, 276, 23456–23463. [Google Scholar] [CrossRef]
- de Souza Batista, C.M.; Yang, R.-Z.; Lee, M.-J.; Glynn, N.M.; Yu, D.-Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Adya, R.; Farhatullah, S.; Lewandowski, K.C.; O’Hare, P.; Lehnert, H.; Randeva, H.S. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: Ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes 2008, 57, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Mahde, A.; Shaker, M.; Al-Mashhadani, Z. Study of omentin1 and other adipokines and hormones in PCOS patients. Oman Med. J. 2009, 24, 108. [Google Scholar] [PubMed]
- Shibata, R.; Ouchi, N.; Takahashi, R.; Terakura, Y.; Ohashi, K.; Ikeda, N.; Higuchi, A.; Terasaki, H.; Kihara, S.; Murohara, T. Omentin as a novel biomarker of metabolic risk factors. Diabetol. Metab. Syndr. 2012, 4, 37. [Google Scholar] [CrossRef]
- Yang, R.-Z.; Lee, M.-J.; Hu, H.; Pray, J.; Wu, H.-B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.-W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- Shibata, R.; Takahashi, R.; Kataoka, Y.; Ohashi, K.; Ikeda, N.; Kihara, S.; Murohara, T.; Ouchi, N. Association of a fat-derived plasma protein omentin with carotid artery intima-media thickness in apparently healthy men. Hypertens. Res. 2011, 34, 1309–1312. [Google Scholar] [CrossRef]
- Shibata, R.; Ouchi, N.; Kikuchi, R.; Takahashi, R.; Takeshita, K.; Kataoka, Y.; Ohashi, K.; Ikeda, N.; Kihara, S.; Murohara, T. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis 2011, 219, 811–814. [Google Scholar] [CrossRef]
- Maruyama, S.; Shibata, R.; Kikuchi, R.; Izumiya, Y.; Rokutanda, T.; Araki, S.; Kataoka, Y.; Ohashi, K.; Daida, H.; Kihara, S. Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J. Biol. Chem. 2012, 287, 408–417. [Google Scholar] [CrossRef]
- Zhui, L.; Yuling, C.; Hansheng, W.; Xiangjie, L. Omentin reduces venous neointimal hyperplasia in arteriovenous fistula through hypoxia-inducible factor-1 alpha inhibition. Microvasc. Res. 2024, 154, 104688. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, L.; Zhong, N.; Wen, D.; Liu, L. Multifaced roles of adipokines in endothelial cell function. Front. Endocrinol. 2024, 15, 1490143. [Google Scholar] [CrossRef]
- Lin, S.; Li, X.; Zhang, J.; Zhang, Y. Omentin-1: Protective impact on ischemic stroke via ameliorating atherosclerosis. Clin. Chim. Acta 2021, 517, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Binti Kamaruddin, N.A.; Fong, L.Y.; Tan, J.J.; Abdullah, M.N.H.; Singh Cheema, M.; Bin Yakop, F.; Yong, Y.K. Cytoprotective role of omentin against oxidative stress-induced vascular endothelial cells injury. Molecules 2020, 25, 2534. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Huang, D.; Liu, X.; Wang, Y.; Liu, J.; Liu, F.; Yu, B. Omentin-1 effects on mesenchymal stem cells: Proliferation, apoptosis, and angiogenesis in vitro. Stem Cell Res. Ther. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, X.; Liu, F.; Tan, H.; Shang, D. Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NF-κB pathway. Biochem. Biophys. Res. Commun. 2012, 425, 401–406. [Google Scholar] [CrossRef]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur. J. Pharmacol. 2012, 686, 116–123. [Google Scholar] [CrossRef]
- Zhao, A.; Xiao, H.; Zhu, Y.; Liu, S.; Zhang, S.; Yang, Z.; Du, L.; Li, X.; Niu, X.; Wang, C. Omentin-1: A newly discovered warrior against metabolic related diseases. Expert Opin. Ther. Targets 2022, 26, 275–289. [Google Scholar] [CrossRef]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T.-a.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T. Counteractive effects of omentin-1 against atherogenesis. Cardiovasc. Res. 2016, 110, 118–128. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, C.; Huang, X.; Liu, Y.; Zhou, Z.; Pan, Y. Omentin-1, a Protective Adipokine for Irritable Bowel Syndrome. J. Inflamm. Res. 2025, 18, 1689–1701. [Google Scholar] [CrossRef]
- Tao, M.; Yan, W.; Chen, C.; Tang, M.; Zhao, X.; Feng, Q.; Fei, X.; Fu, Y. Omentin-1 ameliorates experimental inflammatory bowel disease via Nrf2 activation and redox regulation. Life Sci. 2023, 328, 121847. [Google Scholar] [CrossRef]
- Karampela, I.; Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; Dalamaga, M. Diagnostic and prognostic value of serum omentin-1 in sepsis: A prospective study in critically Ill patients. Medicina 2023, 59, 833. [Google Scholar] [CrossRef]
- Tong, S.; Ji, Q.; Du, Y.; Zhu, X.; Zhu, C.; Zhou, Y. Sfrp5/Wnt pathway: A protective regulatory system in atherosclerotic cardiovascular disease. J. Interferon Cytokine Res. 2019, 39, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, X.; Zheng, H.; Huang, G.; Li, W.; Liu, X.; Liang, J.; Cao, Y.; Hu, Y.; Huang, Y. SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxidative Med. Cell. Longev. 2021, 2021, 9265016. [Google Scholar] [CrossRef] [PubMed]
- Vatner, D.E.; Oydanich, M.; Zhang, J.; Babici, D.; Vatner, S.F. Secreted frizzled-related protein 2, a novel mechanism to induce myocardial ischemic protection through angiogenesis. Basic Res. Cardiol. 2020, 115, 48. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.M.; Abou-Leisa, R.; Stafford, N.; Maqsood, A.; Zi, M.; Prehar, S.; Baudoin-Stanley, F.; Wang, X.; Neyses, L.; Cartwright, E.J. The plasma membrane calcium ATPase 4 signalling in cardiac fibroblasts mediates cardiomyocyte hypertrophy. Nat. Commun. 2016, 7, 11074. [Google Scholar] [CrossRef]
- He, W.; Zhang, L.; Ni, A.; Zhang, Z.; Mirotsou, M.; Mao, L.; Pratt, R.E.; Dzau, V.J. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc. Natl. Acad. Sci. USA 2010, 107, 21110–21115. [Google Scholar] [CrossRef]
- Ni, J.; Liu, X.; Yin, Y.; Zhang, P.; Xu, Y.-W.; Liu, Z. Exosomes derived from TIMP2-modified human umbilical cord mesenchymal stem cells enhance the repair effect in rat model with myocardial infarction possibly by the Akt/Sfrp2 pathway. Oxidative Med. Cell. Longev. 2019, 2019, 1958941. [Google Scholar] [CrossRef]
- Wang, X.; Cui, T. Autophagy modulation: A potential therapeutic approach in cardiac hypertrophy. Am. J. Physiol. -Heart Circ. Physiol. 2017, 313, H304–H319. [Google Scholar] [CrossRef]
- Zheng, H.; Li, W.; Huang, G.; Zhu, H.; Wen, W.; Liu, X.; Sun, L.; Ma, T.; Huang, X.; Hu, Y. Secreted frizzled-related protein 2 ameliorates diabetic cardiomyopathy by activating mitophagy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 166989. [Google Scholar] [CrossRef]
- Zhang, W.; Siraj, S.; Zhang, R.; Chen, Q. Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy 2017, 13, 1080–1081. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Sanna, F.; Margaritis, M.; Badi, I.; Akawi, N.; Herdman, L.; Coutinho, P.; Fagan, H.; Antonopoulos, A.S.; Oikonomou, E.K. Adipose tissue–derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci. Transl. Med. 2019, 11, eaav5055. [Google Scholar] [CrossRef]
- Carstensen-Kirberg, M.; Kannenberg, J.M.; Huth, C.; Meisinger, C.; Koenig, W.; Heier, M.; Peters, A.; Rathmann, W.; Roden, M.; Herder, C. Inverse associations between serum levels of secreted frizzled-related protein-5 (SFRP5) and multiple cardiometabolic risk factors: KORA F4 study. Cardiovasc. Diabetol. 2017, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, H.; Li, Y.; Wang, J.; Lai, Y.; Gao, L.; Lei, L.; Yang, G.; Liao, X.; Fang, X. Plasma Sfrp5 levels correlate with determinants of the metabolic syndrome in Chinese adults. Diabetes/Metab. Res. Rev. 2017, 33, e2896. [Google Scholar] [CrossRef] [PubMed]
- Teliewubai, J.; Bai, B.; Zhou, Y.; Lu, Y.; Yu, S.; Chi, C.; Li, J.; Blacher, J.; Xu, Y.; Zhang, Y. Association of asymptomatic target organ damage with secreted frizzled related protein 5 in the elderly: The Northern Shanghai Study. Clin. Interv. Aging 2018, 13, 389–395. [Google Scholar] [CrossRef]
- Cho, Y.K.; Kang, Y.M.; Lee, S.E.; Lee, Y.L.; Seol, S.M.; Lee, W.J.; Park, J.-Y.; Jung, C.H. Effect of SFRP5 (Secreted Frizzled–Related protein 5) on the WNT5A (wingless-type family member 5a)-induced endothelial dysfunction and its relevance with arterial stiffness in human subjects. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1358–1367. [Google Scholar] [CrossRef]
- Ouchi, N.; Higuchi, A.; Ohashi, K.; Oshima, Y.; Gokce, N.; Shibata, R.; Akasaki, Y.; Shimono, A.; Walsh, K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 2010, 329, 454–457. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, H.; Liu, X.; Chen, P.; Zhang, Y.; Luo, J.; Kuang, J.; Li, J.; Yang, Y.; Ma, T. Prognostic value of secreted frizzled-related protein 5 in heart failure patients with and without type 2 diabetes mellitus. Circ. Heart Fail. 2020, 13, e007054. [Google Scholar] [CrossRef]
- Nakamura, K.; Sano, S.; Fuster, J.J.; Kikuchi, R.; Shimizu, I.; Ohshima, K.; Katanasaka, Y.; Ouchi, N.; Walsh, K. Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury *♦. J. Biol. Chem. 2016, 291, 2566–2575. [Google Scholar] [CrossRef]
- Deng, D.; Diao, Z.; Han, X.; Liu, W. Secreted frizzled-related protein 5 attenuates high phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt/ß-catenin pathway. Calcif. Tissue Int. 2016, 99, 66–75. [Google Scholar] [CrossRef]
- Jin, X.; Guo, B.; Yan, J.; Yang, R.; Chang, L.; Wang, Y.; Miao, C.; Liu, S.; Zhang, H.; Li, Y. Angiotensin II increases secreted frizzled-related protein 5 (sFRP5) expression through AT1 receptor/Rho/ROCK1/JNK signaling in cardiomyocytes. Mol. Cell. Biochem. 2015, 408, 215–222. [Google Scholar] [CrossRef]
- Korf-Klingebiel, M.; Reboll, M.R.; Klede, S.; Brod, T.; Pich, A.; Polten, F.; Napp, L.C.; Bauersachs, J.; Ganser, A.; Brinkmann, E. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat. Med. 2015, 21, 140–149. [Google Scholar] [CrossRef]
- Chen, P.; Huang, X.; Li, W.; Wen, W.; Cao, Y.; Li, J.; Huang, Y.; Hu, Y. Myeloid-derived growth factor in diseases: Structure, function and mechanisms. Mol. Med. 2024, 30, 103. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Wang, T.; Wang, H.; Yang, M.; Yin, H. Mucin-fused myeloid-derived growth factor (MYDGF164) exhibits a prolonged serum half-life and alleviates fibrosis in chronic kidney disease. Br. J. Pharmacol. 2022, 179, 4136–4156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, S.; Wang, S.; Fan, M.; Jin, W.; Li, X.; Wang, C.; Yang, Y. Production of bioactive recombinant human myeloid-derived growth factor in Escherichia coli and its mechanism on vascular endothelial cell proliferation. J. Cell. Mol. Med. 2020, 24, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Feng, J.; Liu, W.; Li, Y.; Liu, J.; Yin, Q.; Lian, H.; Liu, L.; Nie, Y. Mydgf promotes Cardiomyocyte proliferation and Neonatal Heart regeneration. Theranostics 2020, 10, 9100. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, J.; Zhao, R.; Long, X.; Li, C.; Liu, W.; Chen, W.; Shi, B. Role of Mydgf in the regulation of hypoxia/reoxygenation-induced apoptosis in cardiac microvascular endothelial cells. In Vitro Cell. Dev. Biol. -Anim. 2022, 58, 669–678. [Google Scholar] [CrossRef]
- Meng, B.; Li, Y.; Ding, Y.; Xu, X.; Wang, L.; Guo, B.; Zhu, B.; Zhang, J.; Xiang, L.; Dong, J. Myeloid-derived growth factor inhibits inflammation and alleviates endothelial injury and atherosclerosis in mice. Sci. Adv. 2021, 7, eabe6903. [Google Scholar] [CrossRef]
- Xu, J.; Ma, H.; Shi, L.; Zhou, H.; Cheng, Y.; Tong, J.; Meng, B.; Xu, X.; He, K.; Ding, S. Inflammatory cell–derived MYDGF attenuates endothelial LDL transcytosis to protect against atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e443–e467. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Guo, B.; Zhang, J.; Zhu, B.; Li, H.; Ding, Y.; Meng, B.; Zhao, H.; Xiang, L. Myeloid-derived growth factor promotes intestinal glucagon-like peptide-1 production in male mice with type 2 diabetes. Endocrinology 2020, 161, bqaa003. [Google Scholar] [CrossRef]
- Wang, Y.; Lifshitz, L.; Silverstein, N.J.; Mintzer, E.; Luk, K.; StLouis, P.; Brehm, M.A.; Wolfe, S.A.; Deeks, S.G.; Luban, J. Transcriptional and chromatin profiling of human blood innate lymphoid cell subsets sheds light on HIV-1 pathogenesis. EMBO J. 2023, 42, e114153. [Google Scholar] [CrossRef]
- Zhan, P.; Zhang, Y.; Shi, W.; Liu, X.; Qiao, Z.; Wang, Z.; Wang, X.; Wu, J.; Tang, W.; Sun, Y. Myeloid-derived growth factor deficiency exacerbates mitotic catastrophe of podocytes in glomerular disease. Kidney Int. 2022, 102, 546–559. [Google Scholar] [CrossRef]
- Lakka, H.-M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Neeland, I.J.; Berry, J.D.; Ayers, C.R.; Rohatgi, A.; Das, S.R.; Khera, A.; McGuire, D.K.; de Lemos, J.A.; Turer, A.T. The relationship of body mass and fat distribution with incident hypertension: Observations from the Dallas Heart Study. J. Am. Coll. Cardiol. 2014, 64, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 272. [Google Scholar] [CrossRef]
- Ostrom, M.P.; Gopal, A.; Ahmadi, N.; Nasir, K.; Yang, E.; Kakadiaris, I.; Flores, F.; Mao, S.S.; Budoff, M.J. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J. Am. Coll. Cardiol. 2008, 52, 1335–1343. [Google Scholar] [CrossRef]
- Agnelli, G.; Belch, J.J.; Baumgartner, I.; Giovas, P.; Hoffmann, U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis 2020, 293, 94–100. [Google Scholar] [CrossRef]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Fuster, J.J.; Zuriaga, M.A.; Ngo, D.T.-M.; Farb, M.G.; Aprahamian, T.; Yamaguchi, T.P.; Gokce, N.; Walsh, K. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes 2015, 64, 1235–1248. [Google Scholar] [CrossRef]
- Fernando, S.; Bursill, C.A.; Nicholls, S.J.; Psaltis, P.J. Pathophysiology of atherosclerosis. Mech. Vasc. Dis. A Textb. Vasc. Spec. 2020, 23, 19–45. [Google Scholar]
- Dandona, P.; Weinstock, R.; Thusu, K.; Abdel-Rahman, E.; Aljada, A.; Wadden, T. Tumor necrosis factor-α in sera of obese patients: Fall with weight loss. J. Clin. Endocrinol. Metab. 1998, 83, 2907–2910. [Google Scholar]
- Khoukaz, H.B.; Vadali, M.; Schoenherr, A.; Ramirez-Perez, F.I.; Morales-Quinones, M.; Sun, Z.; Fujie, S.; Foote, C.A.; Lyu, Z.; Zeng, S. PAI-1 Regulates the Cytoskeleton and Intrinsic Stiffness of Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2191–2203. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, Y.; Wu, G.; Niu, L.; Fang, C.; Li, Y.; Jiang, L.; Yuan, C.; Huang, M. Specific inhibition on PAI-1 reduces the dose of Alteplase for ischemic stroke treatment. Int. J. Biol. Macromol. 2024, 257, 128618. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Adeyanju, O.; Cai, D.; Tucker, T.A.; Idell, S.; Chen, S.-Y.; Guo, X. DOCK2 Promotes Atherosclerosis by Mediating the Endothelial Cell Inflammatory Response. Am. J. Pathol. 2024, 194, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.N.M.; Yamazaki, F.; Konrad, R.J.; Nishikawa, Y.; Tanaka, A.; Son, Y.; Ozaki, Y.; Takehana, K.; Tanizaki, H. Circulating CD31 and resistin levels reflect different stages of coronary atherosclerosis in patients with psoriasis. J. Dermatol. 2025, 52, 67–78. [Google Scholar] [CrossRef]
- Jung, H.N.; Jung, C.H. The role of anti-inflammatory adipokines in cardiometabolic disorders: Moving beyond adiponectin. Int. J. Mol. Sci. 2021, 22, 13529. [Google Scholar] [CrossRef]
- Pan, S.; Wang, Y.; Zhang, Y.; Ma, X.; Peng, J.; Li, F.; Qian, W.; Zong, J. The Relationship Between the Serum NLRP3 and Adiponectin Levels and Coronary Lesions in Patients with Unstable Angina with Type 2 Diabetes. Clin. Interv. Aging 2024, 19, 1301–1308. [Google Scholar] [CrossRef]
- Stephen, S.L.; Freestone, K.; Dunn, S.; Twigg, M.W.; Homer-Vanniasinkam, S.; Walker, J.H.; Wheatcroft, S.B.; Ponnambalam, S. Scavenger receptors and their potential as therapeutic targets in the treatment of cardiovascular disease. Int. J. Hypertens. 2010, 2010, 646929. [Google Scholar] [CrossRef]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef]
- Freitas Lima, L.C.; Braga, V.d.A.; do Socorro de França Silva, M.; Cruz, J.d.C.; Sousa Santos, S.H.; de Oliveira Monteiro, M.M.; Balarini, C.d.M. Adipokines, diabetes and atherosclerosis: An inflammatory association. Front. Physiol. 2015, 6, 304. [Google Scholar] [CrossRef]
- Van Harmelen, V.; Skurk, T.; Röhrig, K.; Hauner, H. HMG-CoA reductase inhibitor cerivastatin inhibits interleukin-6 expression and secretion in human adipocytes. Horm. Metab. Res. 2003, 35, 466–470. [Google Scholar]
- Arnaud, C.; Burger, F.; Steffens, S.; Veillard, N.R.; Nguyen, T.H.; Trono, D.; Mach, F. Statins reduce interleukin-6–induced C-reactive protein in human hepatocytes: New evidence for direct antiinflammatory effects of statins. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Rezaie-Majd, A.; Maca, T.; Bucek, R.A.; Valent, P.; Müller, M.R.; Husslein, P.; Kashanipour, A.; Minar, E.; Baghestanian, M. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Abbasifard, M.; Kandelouei, T.; Aslani, S.; Razi, B.; Imani, D.; Fasihi, M.; Cicero, F.; Sahebkar, A. Effect of statins on the plasma/serum levels of inflammatory markers in patients with cardiovascular disease; a systematic review and meta-analysis of randomized clinical trials. Inflammopharmacology 2022, 30, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, F.; Karav, S.; Jamialahmadi, T.; Sahebkar, A. Impact of statin therapy on CD40: CD40L signaling: Mechanistic insights and therapeutic opportunities. Pharmacol. Rep. 2024, 77, 1–29. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Velidakis, N.; Khattab, E.; Kassimis, G.; Patsourakos, N. The interplay between statins and adipokines. Is this another explanation of statins’ ‘pleiotropic’ effects? Cytokine 2021, 148, 155698. [Google Scholar] [CrossRef]
- Dias, S.; Paredes, S.; Ribeiro, L. Drugs involved in dyslipidemia and obesity treatment: Focus on adipose tissue. Int. J. Endocrinol. 2018, 2018, 2637418. [Google Scholar] [CrossRef]
- Koh, K.K.; Quon, M.J.; Han, S.H.; Chung, W.-J.; Ahn, J.Y.; Seo, Y.-H.; Kang, M.H.; Ahn, T.H.; Choi, I.S.; Shin, E.K. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation 2004, 110, 3687–3692. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, S.; Li, Z.; Zhou, Y.; Wang, R.; Wang, Y.; Chen, Y. Short-Term Statin Therapy Induces Hepatic Insulin Resistance Through HNF4α/PAQR9/PPM1α Axis Regulated AKT Phosphorylation. Adv. Sci. 2024, 11, 2403451. [Google Scholar] [CrossRef]
- Lei, Y.; Cui, Q.; Yang, G.; Piao, L.; Inoue, A.; Wu, H.; Li, X.; Kuzuya, M.; Cheng, X.W. Statins mitigate stress-related vascular aging and atherosclerosis in apoE-deficient mice fed high fat-diet: The role of glucagon-like peptide-1/adiponectin axis. Front. Cell Dev. Biol. 2021, 9, 687868. [Google Scholar] [CrossRef]
- Cabrero, A.; Alegret, M.; Sanchez, R.M.; Adzet, T.; Laguna, J.C.; Vázquez, M. Bezafibrate reduces mRNA levels of adipocyte markers and increases fatty acid oxidation in primary culture of adipocytes. Diabetes 2001, 50, 1883–1890. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Yang, S.; Liu, G.; Pan, L.; Gu, C.; Wang, Y.; Li, D.; Zhao, R.; Wu, M. Mechanisms of atherosclerosis induced by postprandial lipemia. Front. Cardiovasc. Med. 2021, 8, 636947. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bai, L.; Qiao, H.; Li, Y.; Yang, L.; Zhang, J.; Lin, R.; Ren, F.; Zhang, J.; Ji, M. The protective effect of fenofibrate against TNF-α-induced CD40 expression through SIRT1-mediated deacetylation of NF-κB in endothelial cells. Inflammation 2014, 37, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ortona, S.; Barisione, C.; Ferrari, P.F.; Palombo, D.; Pratesi, G. PCSK9 and other metabolic targets to counteract ischemia/reperfusion injury in acute myocardial infarction and visceral vascular surgery. J. Clin. Med. 2022, 11, 3638. [Google Scholar] [CrossRef] [PubMed]
- Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J. Am. Coll. Cardiol. 2013, 62, 1401–1408. [Google Scholar] [CrossRef]
- Damci, T.; Tatliagac, S.; Osar, Z.; Ilkova, H. Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. Eur. J. Intern. Med. 2003, 14, 357–360. [Google Scholar] [CrossRef]
- Sahebkar, A.; Watts, G.F. Fibrate therapy and circulating adiponectin concentrations: A systematic review and meta-analysis of randomized placebo-controlled trials. Atherosclerosis 2013, 230, 110–120. [Google Scholar] [CrossRef]
- Rachid, T.L.; Penna-de-Carvalho, A.; Bringhenti, I.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. PPAR-α agonist elicits metabolically active brown adipocytes and weight loss in diet-induced obese mice. Cell Biochem. Funct. 2015, 33, 249–256. [Google Scholar] [CrossRef]
- Rachid, T.L.; Penna-de-Carvalho, A.; Bringhenti, I.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Fenofibrate (PPARalpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol. Cell. Endocrinol. 2015, 402, 86–94. [Google Scholar] [CrossRef]
- Pu, X.; Chen, D. Targeting adipokines in obesity-related tumors. Front. Oncol. 2021, 11, 685923. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Scherer, P.E. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 2012, 23, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H.; Scherer, P.E. Gluttony, sloth and the metabolic syndrome: A roadmap to lipotoxicity. Trends Endocrinol. Metab. 2010, 21, 345–352. [Google Scholar] [CrossRef]
- Farooqi, I.S.; O’Rahilly, S. Leptin: A pivotal regulator of human energy homeostasis. Am. J. Clin. Nutr. 2009, 89, 980S–984S. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Linden, M.A.; Pincu, Y.; Martin, S.A.; Woods, J.A.; Baynard, T. Moderate exercise training provides modest protection against adipose tissue inflammatory gene expression in response to high-fat feeding. Physiol. Rep. 2014, 2, e12071. [Google Scholar] [CrossRef]
- Reinehr, T. Lifestyle intervention in childhood obesity: Changes and challenges. Nat. Rev. Endocrinol. 2013, 9, 607–614. [Google Scholar] [CrossRef]
- Yoon, K.J.; Zhang, D.; Kim, S.-J.; Lee, M.-C.; Moon, H.Y. Exercise-induced AMPK activation is involved in delay of skeletal muscle senescence. Biochem. Biophys. Res. Commun. 2019, 512, 604–610. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Zhu, Y.; Li, Z.; Zhu, Y.T.; Wu, J.C.; Qin, Z.H.; Xiang, M.; Lin, F. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci. Ther. 2019, 25, 796–807. [Google Scholar] [CrossRef]
- Marwarha, G.; Claycombe-Larson, K.; Lund, J.; Ghribi, O. Palmitate-Induced SREBP1 expression and activation underlies the increased BACE 1 activity and amyloid beta genesis. Mol. Neurobiol. 2019, 56, 5256–5269. [Google Scholar] [CrossRef] [PubMed]
- Olivier, S.; Foretz, M.; Viollet, B. Promise and challenges for direct small molecule AMPK activators. Biochem. Pharmacol. 2018, 153, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.B.; Konhilas, J.P.; Kelly, D.P.; Leinwand, L.A. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 2017, 25, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P.; Hoseini, R. Exercise training modulates adipokine dysregulations in metabolic syndrome. Sports Med. Health Sci. 2022, 4, 18–28. [Google Scholar] [CrossRef]
- Görgens, S.W.; Eckardt, K.; Jensen, J.; Drevon, C.A.; Eckel, J. Exercise and regulation of adipokine and myokine production. Prog. Mol. Biol. Transl. Sci. 2015, 135, 313–336. [Google Scholar]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Pryor, R.; Cabreiro, F. Repurposing metformin: An old drug with new tricks in its binding pockets. Biochem. J. 2015, 471, 307–322. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Thiazolidinediones. N. Engl. J. Med. 2004, 351, 1106–1118. [Google Scholar] [CrossRef]
- Turner, R.C.; Cull, C.A.; Frighi, V.; Holman, R.R.; Group, U.P.D.S.; Group, U.P.D.S. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). JAMA 1999, 281, 2005–2012. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015, 2, e000327. [Google Scholar] [CrossRef]
- Sumithran, P.; Proietto, J. Benefit-risk assessment of orlistat in the treatment of obesity. Drug Saf. 2014, 37, 597–608. [Google Scholar] [CrossRef]
- Smith, S.R.; Weissman, N.J.; Anderson, C.M.; Sanchez, M.; Chuang, E.; Stubbe, S.; Bays, H.; Shanahan, W.R.; Modification, B.; Overweight, L.f.; et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010, 363, 245–256. [Google Scholar] [CrossRef]
- Greenway, F.L.; Fujioka, K.; Plodkowski, R.A.; Mudaliar, S.; Guttadauria, M.; Erickson, J.; Kim, D.D.; Dunayevich, E. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010, 376, 595–605. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Zagaliotis, A.; Konstantaraki, M.; Filippatos, T.D. GLP-1 analogs and regional adiposity: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13574. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Mokgalaboni, K.; Ziqubu, K.; Cirilli, I.; Marcheggiani, F.; Louw, J. Adipokines as a therapeutic target by metformin to improve metabolic function: A systematic review of randomized controlled trials. Pharmacol. Res. 2021, 163, 105219. [Google Scholar] [CrossRef]
- Zhao, D.; Sohouli, M.H.; Rohani, P.; Fotros, D.; Velu, P.; Ziamanesh, F.; Fatahi, S.; Shojaie, S.; Li, Y. The effect of metformin on adipokines levels: A systematic review and meta-analysis of randomized-controlled trials. Diabetes Res. Clin. Pract. 2024, 207, 111076. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Leibel, R.L. 20 years of leptin: Role of leptin in energy homeostasis in humans. J. Endocrinol. 2014, 223, T83–T96. [Google Scholar] [CrossRef]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.-i.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Dong, J.Q.; Rossulek, M.; Somayaji, V.R.; Baltrukonis, D.; Liang, Y.; Hudson, K.; Hernandez-Illas, M.; Calle, R.A. Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br. J. Clin. Pharmacol. 2015, 80, 1051–1063. [Google Scholar] [CrossRef]
- Rissanen, A.; Howard, C.; Botha, J.; Thuren, T.; Investigators, G. Effect of anti-IL-1β antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: Results of a randomized, placebo-controlled trial. Diabetes Obes. Metab. 2012, 14, 1088–1096. [Google Scholar] [CrossRef]
- van Asseldonk, E.J.; van Poppel, P.C.; Ballak, D.B.; Stienstra, R.; Netea, M.G.; Tack, C.J. One week treatment with the IL-1 receptor antagonist anakinra leads to a sustained improvement in insulin sensitivity in insulin resistant patients with type 1 diabetes mellitus. Clin. Immunol. 2015, 160, 155–162. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Whang, P.G.; Patel, T.; Phillips, F.M.; Anderson, D.G.; Albert, T.J.; Hilibrand, A.S.; Brower, R.S.; Kurd, M.F.; Appannagari, A. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft for posterolateral lumbar arthrodesis: Minimum 4-year follow-up of a pilot study. Spine J. 2008, 8, 457–465. [Google Scholar] [CrossRef]
- Tanabe, H.; Fujii, Y.; Okada-Iwabu, M.; Iwabu, M.; Nakamura, Y.; Hosaka, T.; Motoyama, K.; Ikeda, M.; Wakiyama, M.; Terada, T. Crystal structures of the human adiponectin receptors. Nature 2015, 520, 312–316. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef]
- Busetto, L.; Tregnaghi, A.; De Marchi, F.; Segato, G.; Foletto, M.; Sergi, G.; Favretti, F.; Lise, M.; Enzi, G. Liver volume and visceral obesity in women with hepatic steatosis undergoing gastric banding. Obes. Res. 2002, 10, 408–411. [Google Scholar] [CrossRef]
- Fontana, L. The scientific basis of caloric restriction leading to longer life. Curr. Opin. Gastroenterol. 2009, 25, 144–150. [Google Scholar] [CrossRef]
- Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Jones, D.; Textor, S.; Goff, D.C.; Murphy, T.P.; Toto, R.D.; White, A.; Cushman, W.C.; White, W.; Sica, D. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008, 51, 1403–1419. [Google Scholar] [CrossRef]
- De Faria, A.P.; Modolo, R.; Fontana, V.; Moreno, H. Adipokines: Novel players in resistant hypertension. J. Clin. Hypertens. 2014, 16, 754–759. [Google Scholar] [CrossRef]
- Kurukulasuriya, L.R.; Stas, S.; Lastra, G.; Manrique, C.; Sowers, J.R. Hypertension in obesity. Endocrinol. Metab. Clin. N. Am. 2008, 37, 647–662. [Google Scholar] [CrossRef]
- Salles, G.F.; Fiszman, R.; Cardoso, C.R.; Muxfeldt, E.S. Relation of left ventricular hypertrophy with systemic inflammation and endothelial damage in resistant hypertension. Hypertension 2007, 50, 723–728. [Google Scholar] [CrossRef]
- Collins, S.; Kuhn, C.M. Role of leptin in fat regulation. Nature 1996, 380, 677. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef]
- McTernan, P.G.; Fisher, F.M.; Valsamakis, G.; Chetty, R.; Harte, A.; McTernan, C.L.; Clark, P.M.; Smith, S.A.; Barnett, A.H.; Kumar, S. Resistin and type 2 diabetes: Regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J. Clin. Endocrinol. Metab. 2003, 88, 6098–6106. [Google Scholar] [CrossRef]
- Koenig, W.; Khuseyinova, N.; Baumert, J.; Meisinger, C.; Löwel, H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: Results from the 18-year follow-up of a large cohort from southern Germany. J. Am. Coll. Cardiol. 2006, 48, 1369–1377. [Google Scholar] [CrossRef]
- Iacobellis, G.; Petramala, L.; Cotesta, D.; Pergolini, M.; Zinnamosca, L.; Cianci, R.; De Toma, G.; Sciomer, S.; Letizia, C. Adipokines and cardiometabolic profile in primary hyperaldosteronism. J. Clin. Endocrinol. Metab. 2010, 95, 2391–2398. [Google Scholar] [CrossRef]
- Yaturu, S.; Daberry, R.P.; Rains, J.; Jain, S. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine 2006, 34, 219–223. [Google Scholar] [CrossRef]
- Youn, J.-C.; Kim, C.; Park, S.; Lee, S.-H.; Kang, S.-M.; Choi, D.; Son, N.H.; Shin, D.-J.; Jang, Y. Adiponectin and progression of arterial stiffness in hypertensive patients. Int. J. Cardiol. 2013, 163, 316–319. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.-N.; Pan, C.-M.; Sun, F.; Zhu, D.-L.; Song, H.-D.; Chen, M.-D. Aldosterone perturbs adiponectin and PAI-1 expression and secretion in 3T3-L1 adipocytes. Horm. Metab. Res. 2011, 43, 464–469. [Google Scholar] [CrossRef]
- de Souza, F.; Muxfeldt, E.; Fiszman, R.; Salles, G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension 2010, 55, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Fontana, V.; de Faria, A.P.C.; Oliveira-Paula, G.H.; Silva, P.S.; Biagi, C.; Tanus-Santos, J.E.; Moreno, H. Effects of angiotensin-converting enzyme inhibition on leptin and adiponectin levels in essential hypertension. Basic Clin. Pharmacol. Toxicol. 2014, 114, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Nedogoda, S.V.; Ledyaeva, A.A.; Chumachok, E.V.; Tsoma, V.V.; Mazina, G.; Salasyuk, A.S.; Barykina, I.N. Randomized trial of perindopril, enalapril, losartan and telmisartan in overweight or obese patients with hypertension. Clin. Drug Investig. 2013, 33, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ricchiuti, V.; Lian, B.Q.; Yao, T.M.; Coutinho, P.; Romero, J.R.; Li, J.; Williams, G.H.; Adler, G.K. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-γ, and proinflammatory adipokines. Circulation 2008, 117, 2253–2261. [Google Scholar] [CrossRef]
- Tesauro, M.; Canale, M.P.; Rodia, G.; Di Daniele, N.; Lauro, D.; Scuteri, A.; Cardillo, C. Metabolic syndrome, chronic kidney, and cardiovascular diseases: Role of adipokines. Cardiol. Res. Pract. 2011, 2011, 653182. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Loffreda, S.; Yang, S.; Lin, H.; Karp, C.; Brengman, M.L.; Wang, D.; Klein, A.; Bulkley, G.B.; Bao, C.; Noble, P. Leptin regulates proinflammatory immune responses. FASEB J. 1998, 12, 57–65. [Google Scholar] [CrossRef]
- Papafragkaki, D.-K.; Tolis, G. Obesity and renal disease: A possible role of leptin. Hormones 2005, 4, 90–95. [Google Scholar]
- Adelman, R.D. Obesity and renal disease. Curr. Opin. Nephrol. Hypertens. 2002, 11, 331–335. [Google Scholar] [CrossRef]
- Kim, S.R.; Jiang, K.; Ogrodnik, M.; Chen, X.; Zhu, X.-Y.; Lohmeier, H.; Ahmed, L.; Tang, H.; Tchkonia, T.; Hickson, L.J. Increased renal cellular senescence in murine high-fat diet: Effect of the senolytic drug quercetin. Transl. Res. 2019, 213, 112–123. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Harris, R.A.; Hatahet, Z.; Chou, K.-m. Ablation of XP-V gene causes adipose tissue senescence and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 2015, 112, E4556–E4564. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Xu, M.; Zhu, Y.; Pirtskhalava, T.; Weivoda, M.M.; Hachfeld, C.M.; Prata, L.G.; van Dijk, T.H.; Verkade, E.; Casaclang-Verzosa, G. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 2019, 18, e12950. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Ramirez, A.; López-Aceituno, J.L.; Costa-Machado, L.F.; Plaza, A.; Barradas, M.; Fernandez-Marcos, P.J. Transient metabolic improvement in obese mice treated with navitoclax or dasatinib/quercetin. Aging 2020, 12, 11337. [Google Scholar] [CrossRef] [PubMed]
- Arabi, T.; Shafqat, A.; Sabbah, B.N.; Fawzy, N.A.; Shah, H.; Abdulkader, H.; Razak, A.; Sabbah, A.N.; Arabi, Z. Obesity-related kidney disease: Beyond hypertension and insulin-resistance. Front. Endocrinol. 2023, 13, 1095211. [Google Scholar] [CrossRef]
- Mallamaci, F.; Ruggenenti, P.; Perna, A.; Leonardis, D.; Tripepi, R.; Tripepi, G.; Remuzzi, G.; Zoccali, C.; Group, R.S. ACE inhibition is renoprotective among obese patients with proteinuria. J. Am. Soc. Nephrol. 2011, 22, 1122–1128. [Google Scholar] [CrossRef]
- Garg, R.; Adler, G.K. Aldosterone and the mineralocorticoid receptor: Risk factors for cardiometabolic disorders. Curr. Hypertens. Rep. 2015, 17, 52. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Chen, Y.; Dong, Y. Kidney damage caused by obesity and its feasible treatment drugs. Int. J. Mol. Sci. 2022, 23, 747. [Google Scholar] [CrossRef]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.D.; Fredericks, S. The role of adipokines in osteoporosis management: A mini review. Front. Endocrinol. 2024, 15, 1336543. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Tariq, S.; Khaliq, S.; Lone, K.P. Serum resistin levels as predictor of low bone mineral density in postmenopausal women. Health Care Women Int. 2021, 42, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Yakout, S.; Aljohani, N.; Al-Saleh, Y.; Al-Attas, O.S.; McTernan, P.G.; Alokail, M.S. Changes in serum cytokines and vitamin D in Saudi postmenopausal women with osteoporosis. Int. J. Clin. Exp. Med. 2017, 10, 1179–1185. [Google Scholar]
- Thommesen, L.; Stunes, A.K.; Monjo, M.; Grøsvik, K.; Tamburstuen, M.V.; Kjøbli, E.; Lyngstadaas, S.P.; Reseland, J.E.; Syversen, U. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J. Cell. Biochem. 2006, 99, 824–834. [Google Scholar] [CrossRef]
- Tariq, S.; Tariq, S.; Khaliq, S.; Baig, M.; Murad, M.A.; Lone, K.P. Association between vitamin D and resistin in postmenopausal females with altered bone health. Front. Endocrinol. 2021, 11, 615440. [Google Scholar] [CrossRef]
- Ben-Eliezer, M.; Phillip, M.; Gat-Yablonski, G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine 2007, 32, 235–244. [Google Scholar] [CrossRef]
- Astudillo, P.; Ríos, S.; Pastenes, L.; Pino, A.M.; Rodríguez, J.P. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J. Cell. Biochem. 2008, 103, 1054–1065. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Q. Adipokines: New therapeutic target for osteoarthritis? Curr. Rheumatol. Rep. 2019, 21, 71. [Google Scholar] [CrossRef]
- Liang, H.; Wang, H.; Luo, L.; Fan, S.; Zhou, L.; Liu, Z.; Yao, S.; Zhang, X.; Zhong, K.; Zhao, H. Toll-like receptor 4 promotes high glucose-induced catabolic and inflammatory responses in chondrocytes in an NF-κB-dependent manner. Life Sci. 2019, 228, 258–265. [Google Scholar] [CrossRef]
- Tariq, S.; Tariq, S.; Baig, M.; Valjevac, A. Osteoporosis and adipokines: The potential for future treatment. Front. Media SA 2024, 15, 1405412. [Google Scholar] [CrossRef] [PubMed]
- Biver, E.; Salliot, C.; Combescure, C.; Gossec, L.; Hardouin, P.; Legroux-Gerot, I.; Cortet, B. Influence of adipokines and ghrelin on bone mineral density and fracture risk: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2703–2713. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Govindarajan, R.; McMullen, N.M.; Sinal, C.J. Chemokine-like receptor 1 is a novel Wnt target gene that regulates mesenchymal stem cell differentiation. Stem Cells 2017, 35, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xie, P.-L.; Luo, X.-H.; Wu, X.-P.; Zhou, H.-D.; Tang, S.-Y.; Liao, E.-Y. Omentin-1 exerts bone-sparing effect in ovariectomized mice. Osteoporos. Int. 2012, 23, 1425–1436. [Google Scholar] [CrossRef]
- Tang, C.; Liang, D.; Qiu, Y.; Zhu, J.; Tang, G. Omentin-1 induces osteoblast viability and differentiation via the TGF-β/Smad signaling pathway in osteoporosis. Mol. Med. Rep. 2022, 25, 132. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride metabolism in the liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Francisco, V.; Sanz, M.J.; Real, J.T.; Marques, P.; Capuozzo, M.; Ait Eldjoudi, D.; Gualillo, O. Adipokines in non-alcoholic fatty liver disease: Are we on the road toward new biomarkers and therapeutic targets? Biology 2022, 11, 1237. [Google Scholar] [CrossRef]
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology 2020, 158, 1899–1912. [Google Scholar] [CrossRef]
- Martínez-Uña, M.; López-Mancheño, Y.; Diéguez, C.; Fernández-Rojo, M.A.; Novelle, M.G. Unraveling the role of leptin in liver function and its relationship with liver diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef]
- Wen, F.; Shi, Z.; Liu, X.; Tan, Y.; Wei, L.; Zhu, X.; Zhang, H.; Zhu, X.; Meng, X.; Ji, W. Acute elevated resistin exacerbates mitochondrial damage and aggravates liver steatosis through AMPK/PGC-1α signaling pathway in male NAFLD mice. Horm. Metab. Res. 2021, 53, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.J.; Choi, S.E.; Lee, N.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Visfatin exacerbates hepatic inflammation and fibrosis in a methionine-choline-deficient diet mouse model. J. Gastroenterol. Hepatol. 2021, 36, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Cornide-Petronio, M.E.; Jiménez-Castro, M.B.; Peralta, C. Data on adiponectin from 2010 to 2020: Therapeutic target and prognostic factor for liver diseases? Int. J. Mol. Sci. 2020, 21, 5242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An old drug with new applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1062–1079. [Google Scholar] [CrossRef]
- Fakih, W.; Zeitoun, R.; AlZaim, I.; Eid, A.H.; Kobeissy, F.; Abd-Elrahman, K.S.; El-Yazbi, A.F. Early metabolic impairment as a contributor to neurodegenerative disease: Mechanisms and potential pharmacological intervention. Obesity 2022, 30, 982–993. [Google Scholar] [CrossRef]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’Oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose tissue inflammation and pulmonary dysfunction in obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef]
- Varra, F.-N.; Varras, M.; Varra, V.-K.; Theodosis-Nobelos, P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options. Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Li, M.; Zhu, D.; Tian, G. Epicardial Adipose Tissue–Derived Leptin Promotes Myocardial Injury in Metabolic Syndrome Rats Through PKC/NADPH Oxidase/ROS Pathway. J. Am. Heart Assoc. 2023, 12, e029415. [Google Scholar] [CrossRef]
- Dai, M.; Yang, X.; Yu, Y.; Pan, W. Helminth and host crosstalk: New insight into treatment of obesity and its associated metabolic syndromes. Front. Immunol. 2022, 13, 827486. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Naimo, G.D.; Paolì, A.; Giordano, F.; Forestiero, M.; Panno, M.L.; Andò, S.; Mauro, L. Unraveling the role of adiponectin receptors in obesity-related breast cancer. Int. J. Mol. Sci. 2023, 24, 8907. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.; Kwon, D.; Yang, H. Long-term central infusion of adiponectin improves energy and glucose homeostasis by decreasing fat storage and suppressing hepatic gluconeogenesis without changing food intake. J. Neuroendocrinol. 2011, 23, 687–698. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Therapeutic potential of adiponectin in prediabetes: Strategies, challenges, and future directions. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222371. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Garaulet, M.; Hernandez-Morante, J.J.; de Heredia, F.P.; Tébar, F.J. Adiponectin, the controversial hormone. Public Health Nutr. 2007, 10, 1145–1150. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Fosse, V.; Oldoni, E.; Bietrix, F.; Budillon, A.; Daskalopoulos, E.P.; Fratelli, M.; Gerlach, B.; Groenen, P.M.; Hölter, S.M.; Menon, J.M. Recommendations for robust and reproducible preclinical research in personalised medicine. BMC Med. 2023, 21, 14. [Google Scholar] [CrossRef]
- Al-Jumaili, W.S.; Kadhim, A.H.; Al-Thuwaini, T.M. Polymorphism of the ADIPOQ gene and its association with productive traits in Awassi Ewes. Mol. Biol. Rep. 2023, 50, 913–917. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.; Scherer, P. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Mojarad-Jabali, S.; Mahdinloo, S.; Farshbaf, M.; Sarfraz, M.; Fatahi, Y.; Atyabi, F.; Valizadeh, H. Transferrin receptor-mediated liposomal drug delivery: Recent trends in targeted therapy of cancer. Expert Opin. Drug Deliv. 2022, 19, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Guerrero, A.; Pallàs, M. Advancing personalized medicine in neurodegenerative diseases: The role of epigenetics and pharmacoepigenomics in pharmacotherapy. Pharmacol. Res. 2024, 205, 107247. [Google Scholar] [CrossRef]
- Legere, R.M.; Taylor, D.R.; Davis, J.L.; Bello, K.; Parker, C.; Judd, R.L.; Wooldridge, A.A. Pharmacodynamic effects of pioglitazone on high molecular weight adiponectin concentrations and insulin response after oral sugar in equids. J. Equine Vet. Sci. 2019, 82, 102797. [Google Scholar] [CrossRef]
- Lysaght, J.; Conroy, M.J. The multifactorial effect of obesity on the effectiveness and outcomes of cancer therapies. Nat. Rev. Endocrinol. 2024, 20, 701–714. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).