Targeting the Electron Transport System for Enhanced Longevity
Abstract
1. Aging: Mitochondrial Dysfunction at the Center
1.1. Aging Hallmarks
1.2. The Aging Mitochondria
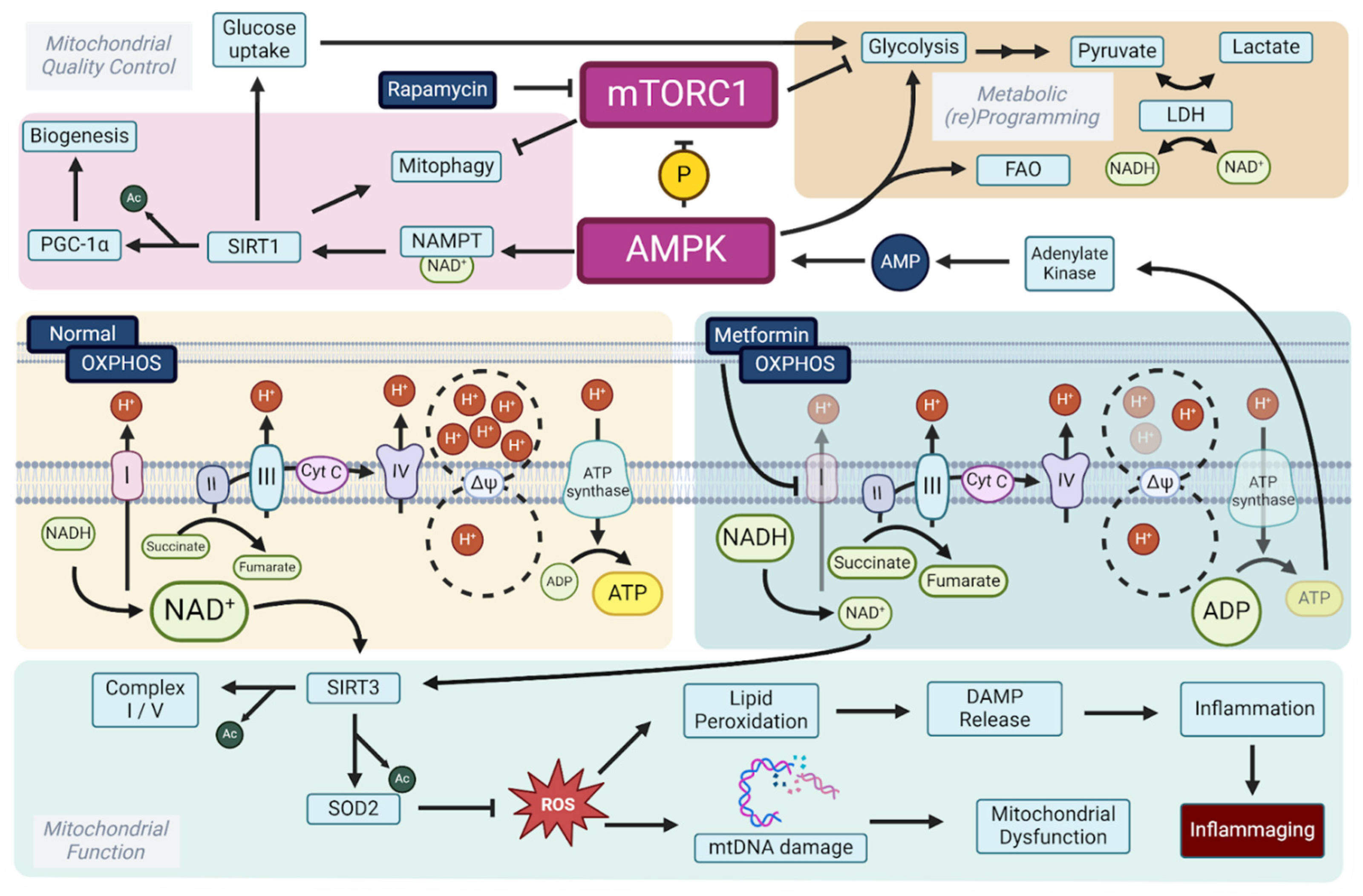
2. Longevity Drugs Modulate the mTOR–AMPK Axis, Converging on the Mitochondrial Level
3. Modulating Oxidative Phosphorylation Activity to Convey Longevity
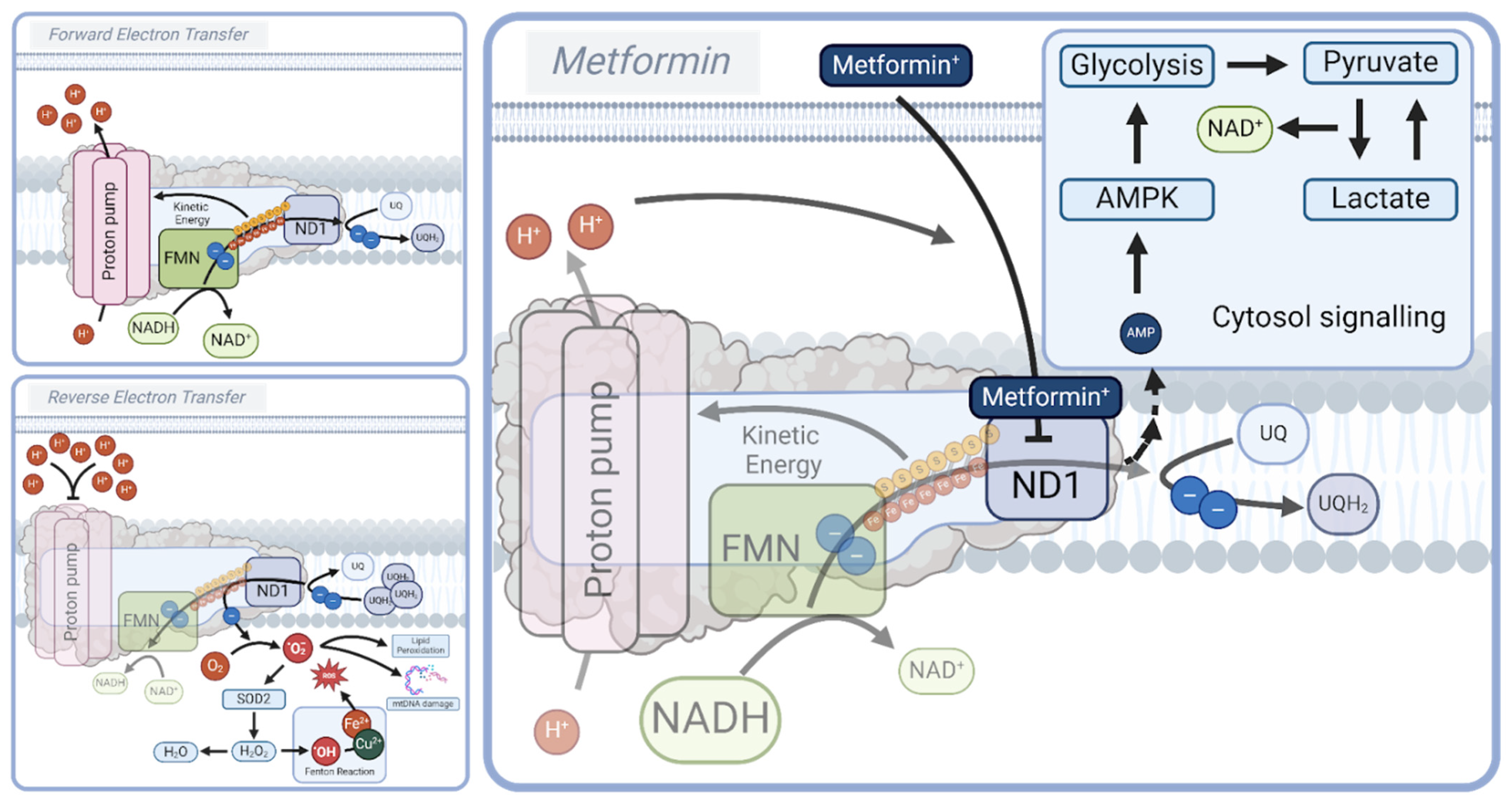
3.1. Respiratory Complex I: A Master Regulator of Longevity
3.2. NAD+ Boosters: NMN and NR
4. Redox Stress: (Targeted) Antioxidants and Senolytics
4.1. (Targeted) Antioxidants
4.2. Sonlicromanol (KH176)
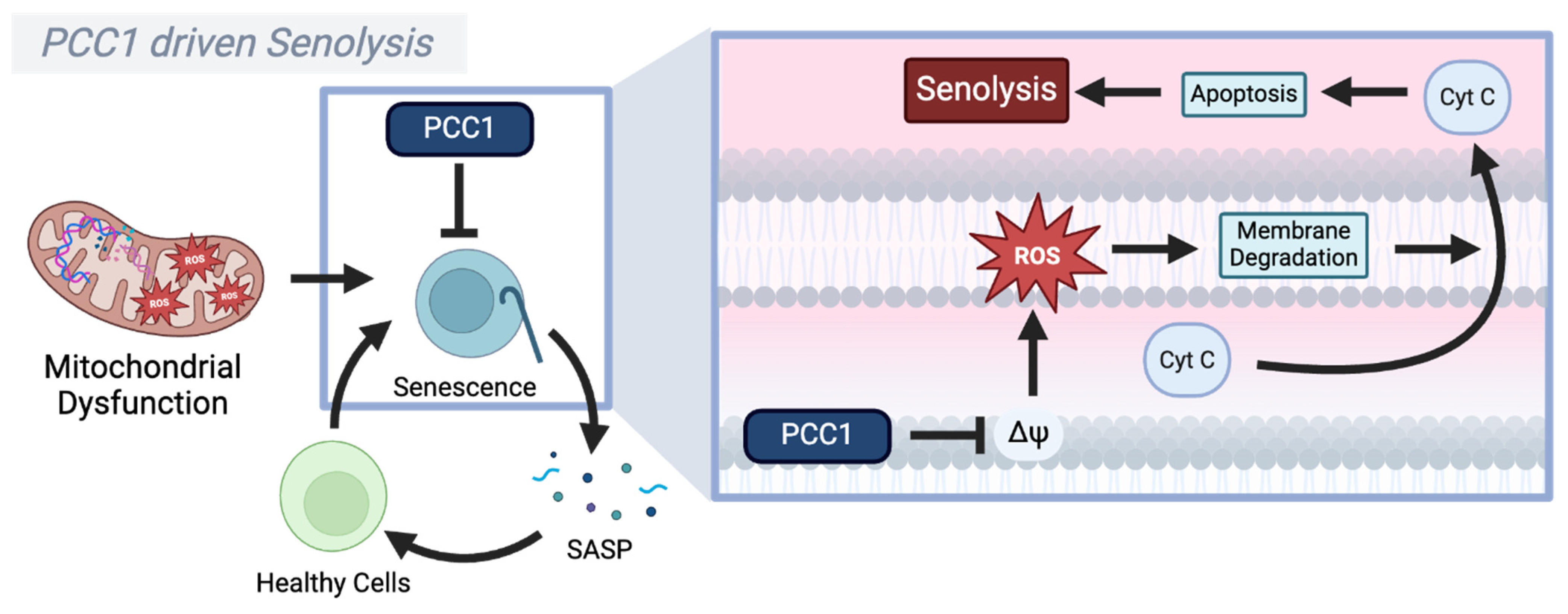
4.3. Procyanidin C1 (PCC1)
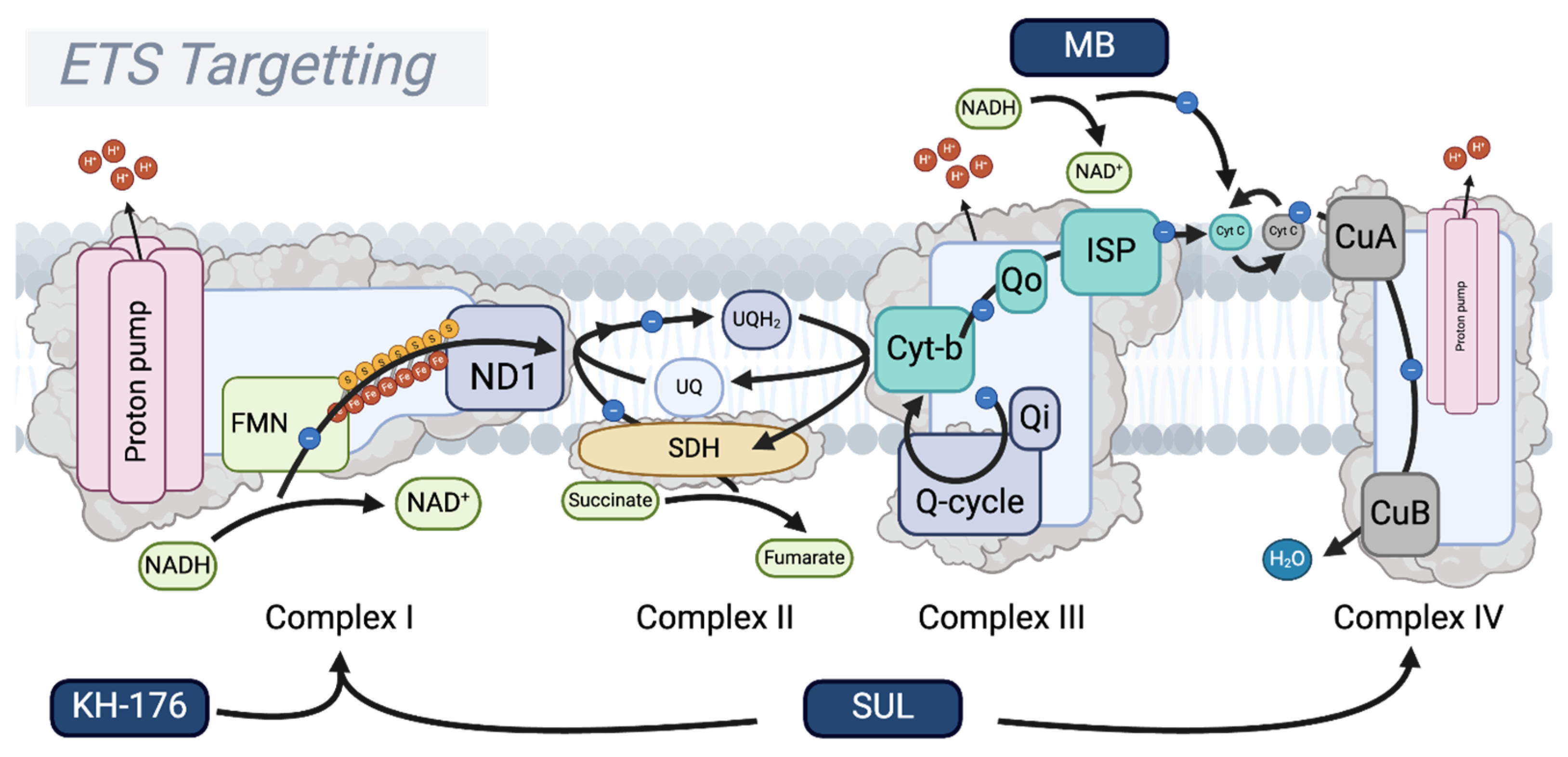
5. Oxidative Phosphorylation: Targeting the Respiratory Complexes
5.1. The Respiratory Complex III–Cytochrome C–Complex IV Supercomplex
5.2. Elamipretide (SS-31, MTP-131, and Bendavia)
5.3. Methylene Blue
5.4. SUL-138 (SUL-109/238)
6. Future Directions for Mitochondrial Anti-Aging Drugs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| ARE | Antioxidant response element |
| ATP | Adenosine triphosphate |
| CL | Cardiolipin |
| Cyt C | Cytochrome C |
| DAMPs | Damage-associated molecular patterns |
| DDR | DNA damage response |
| DMT2 | Diabetes mellitus type 2 |
| ETS | Electron transport system |
| FET | Forward electron transfer |
| FMN | Flavin mononucleotide |
| GSH | Glutathione |
| H2O2 | Hydrogen peroxide |
| IMM | Inner mitochondrial membrane |
| IMS | Intermembrane space |
| IRI | Ischemia–reperfusion injury |
| LDH | Lactate dehydrogenase |
| LOO• | Lipid peroxyl radicals |
| MB | Methylene Blue |
| MMP | Mitochondrial membrane permeability |
| MOMP | Mitochondrial outer membrane permeabilization |
| mPGES-1 | Microsomal prostaglandin E synthase-1 |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| mtDNA | Mitochondrial DNA |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADH | Reduced nicotinamide adenine dinucleotide |
| NAM | Nicotinamide |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| ND1 | NADH dehydrogenase 1 subunit |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain-containing 3 |
| NMN | Nicotinamide mononucleotide |
| NMNAT | Nicotinamide mononucleotide adenylyltransferase |
| NR | Nicotinamide riboside |
| NRF2 | NF-E2-related factor 2 |
| NRK | Nicotinamide riboside kinase |
| OH• | Hydroxyl radical |
| OMM | Outer mitochondrial membrane |
| OXPHOS | Oxidative phosphorylation |
| O2 | Oxygen |
| O2−• | Superoxide anion |
| PARPs | Poly(ADP-ribose) polymerases |
| PCC1 | Procyanidin C1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator-1 alpha |
| PGE2 | Prostaglandin E2 |
| PNP | Purine nucleoside phosphorylase |
| PRRs | Pattern recognition receptors |
| Prx | Peroxiredoxin |
| RET | Reverse electron transfer |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| SIRT1 | Sirtuin 1 |
| SIRT3 | Sirtuin 3 |
| SOD2 | Superoxide dismutase 2 |
| SS-31 | Elamipretide |
| TDZs | Thiazolidinediones |
| TPP | Triphenylphosphonium |
| Trx | Thioredoxin |
| TrxR | Thioredoxin reductase |
| UPRmt | Mitochondrial unfolded protein response |
| UQ | Ubiquinone |
| UQH2 | Ubiquinol |
References
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- López-Gil, L.; Pascual-Ahuir, A.; Proft, M. Genomic Instability and Epigenetic Changes during Aging. Int. J. Mol. Sci. 2023, 24, 14279. [Google Scholar] [CrossRef]
- Li, Z.; Anugula, S.; Rasmussen, L.J. Genomic instability and aging. In Aging; Elsevier: Amsterdam, The Netherlands, 2023; pp. 275–295. [Google Scholar]
- Kucab, J.E.; Zou, X.; Morganella, S.; Joel, M.; Nanda, A.S.; Nagy, E.; Gomez, C.; Degasperi, A.; Harris, R.; Jackson, S.P.; et al. A Compendium of Mutational Signatures of Environmental Agents. Cell 2019, 177, 821–836.e16. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, S.; Lee, M.; Maslov, A.Y.; Shi, M.; Waldman, S.; Marsh, A.; Siddiqui, T.; Dong, X.; Peter, Y.; et al. Single-cell analysis of somatic mutations in human bronchial epithelial cells in relation to aging and smoking. Nat. Genet. 2022, 54, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.A.; Wilke, C.O. The evolutionary consequences of erroneous protein synthesis. Nat. Rev. Genet. 2009, 10, 715–724. [Google Scholar] [CrossRef]
- Paull, T.T. DNA damage and regulation of protein homeostasis. DNA Repair 2021, 105, 103155. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.K.; Armanios, M. Telomere-mediated lung disease. Physiol. Rev. 2022, 102, 1703–1720. [Google Scholar] [CrossRef]
- Simm, A.; Campisi, J. Stress and Aging. Exp. Gerontol. 2014, 59, 1–2. [Google Scholar] [CrossRef]
- Dhar, P.; Moodithaya, S.S.; Patil, P. Epigenetic alterations—The silent indicator for early aging and age-associated health-risks. Aging Med. 2022, 5, 287–293. [Google Scholar] [CrossRef]
- Mishra, S.; Raval, M.; Kachhawaha, A.S.; Tiwari, B.S.; Tiwari, A.K. Aging: Epigenetic modifications. Prog. Mol. Biol. Transl. Sci. 2023, 197, 171–209. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, S. Role of autophagy in aging: The good, the bad, and the ugly. Aging Cell 2022, 22, e13753. [Google Scholar] [CrossRef]
- Barbosa, M.C.; Grosso, R.A.; Fader, C.M. Hallmarks of Aging: An Autophagic Perspective. Front. Endocrinol. 2019, 9, 790. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Maslov, A.Y.; Vijg, J. Genome instability, cancer and aging. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef]
- Ali, A.; Gioscia-Ryan, R.; Yang, D.; Sutton, N.R.; Tyrrell, D.J. Cardiovascular aging: Spotlight on mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H317–H333. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Zhang, M.; Beard, D.A.; Goldstein, D.R. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ. Res. 2020, 126, 298–314. [Google Scholar] [CrossRef]
- Rossmann, M.P.; Dubois, S.M.; Agarwal, S.; Zon, L.I. Mitochondrial function in development and disease. Dis. Model. Mech. 2021, 14, e048912. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Greaves, L.C.; Nooteboom, M.; Elson, J.L.; Tuppen, H.A.L.; Taylor, G.A.; Commane, D.M.; Arasaradnam, R.P.; Khrapko, K.; Taylor, R.W.; Kirkwood, T.B.L.; et al. Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing. PLOS Genet. 2014, 10, e1004620. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Sekiya, R.; Goto, S.; Li, T.-S. Chronic replication stress invokes mitochondria dysfunction via impaired parkin activity. Sci. Rep. 2024, 14, 7877. [Google Scholar] [CrossRef]
- Shpilka, T.; Haynes, C.M. The mitochondrial UPR: Mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 2017, 19, 109–120. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria, Bioenergetics, and the Epigenome in Eukaryotic and Human Evolution. Cold Spring Harb. Symp. Quant. Biol. 2009, 74, 383–393. [Google Scholar] [CrossRef]
- Quirós, P.M.; Mottis, A.; Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 2016, 17, 213–226. [Google Scholar] [CrossRef]
- Druzhyna, N.M.; Wilson, G.L.; LeDoux, S.P. Mitochondrial DNA repair in aging and disease. Mech. Ageing Dev. 2008, 129, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N.; Guo, L.; Yang, Y. The Mitochondrial Response to DNA Damage. Front. Cell Dev. Biol. 2021, 9, 669379. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.C.; Uchiyama, L.F.; Nunnari, J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 2016, 353, aaf5549. [Google Scholar] [CrossRef] [PubMed]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef]
- Lemasters, J.J. Selective Mitochondrial Autophagy, or Mitophagy, as a Targeted Defense Against Oxidative Stress, Mitochondrial Dysfunction, and Aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef]
- Vermulst, M.; Bielas, J.H.; Kujoth, G.C.; Ladiges, W.C.; Rabinovitch, P.S.; A Prolla, T.; A Loeb, L. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 2007, 39, 540–543. [Google Scholar] [CrossRef]
- Wolf, A.M. MtDNA mutations and aging—Not a closed case after all? Signal Transduct. Target. Ther. 2021, 6, 56. [Google Scholar] [CrossRef]
- Zapico, S.C.; Ubelaker, D.H. mtDNA Mutations and Their Role in Aging, Diseases and Forensic Sciences. Aging Dis. 2013, 4, 364–380. [Google Scholar] [CrossRef]
- Mengel-From, J.; Thinggaard, M.; Dalgård, C.; Kyvik, K.O.; Christensen, K.; Christiansen, L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet. 2014, 133, 1149–1159. [Google Scholar] [CrossRef]
- Iqbal, T.; Nakagawa, T. The therapeutic perspective of NAD+ precursors in age-related diseases. Biochem. Biophys. Res. Commun. 2024, 702, 149590. [Google Scholar] [CrossRef]
- Wenz, T. Mitochondria and PGC-1α in Aging and Age-Associated Diseases. J. Aging Res. 2011, 2011, e810619. [Google Scholar] [CrossRef]
- Matysek, A.; Sun, L.; Kimmantudawage, S.P.; Feng, L.; Maier, A.B. Targeting impaired nutrient sensing via the sirtuin pathway with novel compounds to prevent or treat dementia: A systematic review. Ageing Res. Rev. 2023, 90, 102029. [Google Scholar] [CrossRef] [PubMed]
- Bakula, D.; Scheibye-Knudsen, M. MitophAging: Mitophagy in Aging and Disease. Front. Cell Dev. Biol. 2020, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, L.; Xie, J.; Zhang, Z.; Dong, G.; Liang, J.; Liu, L.; Zhou, H.; Luo, P. Resveratrol Ameliorates Mitochondrial Elongation via Drp1/Parkin/PINK1 Signaling in Senescent-Like Cardiomyocytes. Oxidative Med. Cell. Longev. 2017, 2017, 4175353. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Jiang, X.; Yang, J.; Zhang, J.; Yang, F.; Cao, H. Mitophagy: A potential therapeutic target for insulin resistance. Front. Physiol. 2022, 13, 957968. [Google Scholar] [CrossRef]
- Chen, A.; Kristiansen, C.K.; Hong, Y.; Kianian, A.; Fang, E.F.; Sullivan, G.J.; Wang, J.; Li, X.; Bindoff, L.A.; Liang, K.X. Nicotinamide Riboside and Metformin Ameliorate Mitophagy Defect in Induced Pluripotent Stem Cell-Derived Astrocytes with POLG Mutations. Front. Cell Dev. Biol. 2021, 9, 737304. [Google Scholar] [CrossRef]
- Grazioli, S.; Pugin, J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018, 9, 832. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef]
- Song, M.J.; Park, C.; Kim, H.; Han, S.; Lee, S.H.; Lee, D.H.; Chung, J.H. Carnitine acetyltransferase deficiency mediates mitochondrial dysfunction-induced cellular senescence in dermal fibroblasts. Aging Cell 2023, 22, e14000. [Google Scholar] [CrossRef]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Shah, R.B.; Singhal, S.; Dutta, S.B.; Bansal, S.; Sinha, S.; Haque, M. Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes. Drug Des. Dev. Ther. 2023, ume 17, 1907–1932. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Berstein, L.M.; Egormin, P.A.; Piskunova, T.S.; Popovich, I.G.; Zabezhinski, M.A.; Tyndyk, M.L.; Yurova, M.V.; Kovalenko, I.G.; Poroshina, T.E.; et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 2008, 7, 2769–2773. [Google Scholar] [CrossRef] [PubMed]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef]
- Strong, R.; Miller, R.A.; Antebi, A.; Astle, C.M.; Bogue, M.; Denzel, M.S.; Fernandez, E.; Flurkey, K.; Hamilton, K.L.; Lamming, D.W.; et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 2016, 15, 872–884. [Google Scholar] [CrossRef]
- Bannister, C.A.; Holden, S.E.; Jenkins-Jones, S.; Morgan, C.L.; Halcox, J.P.; Schernthaner, G.; Mukherjee, J.; Currie, C.J. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes. Metab. 2014, 16, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Landman, G.W.D.; Kleefstra, N.; Van Hateren, K.J.J.; Groenier, K.H.; Gans, R.O.B.; Bilo, H.J.G. Metformin Associated With Lower Cancer Mortality in Type 2 Diabetes: ZODIAC-16. Diabetes Care 2009, 33, 322–326. [Google Scholar] [CrossRef]
- Cheng, C.; Lin, C.-H.; Tsai, Y.-W.; Tsai, C.-J.; Chou, P.-H.; Lan, T.-H. Type 2 Diabetes and Antidiabetic Medications in Relation to Dementia Diagnosis. J. Gerontol. Ser. A 2014, 69, 1299–1305. [Google Scholar] [CrossRef]
- E Tinetti, M.; McAvay, G.; Trentalange, M.; Cohen, A.B.; Allore, H.G. Association between guideline recommended drugs and death in older adults with multiple chronic conditions: Population based cohort study. BMJ 2015, 351, h4984. [Google Scholar] [CrossRef]
- Stevenson-Hoare, J.; Leonenko, G.; Escott-Price, V. Comparison of long-term effects of metformin on longevity between people with type 2 diabetes and matched non-diabetic controls. BMC Public Health 2023, 23, 804. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Brutsaert, E.F.; Anghel, V.; Zhang, K.; Bloomgarden, N.; Pollak, M.; Mar, J.C.; Hawkins, M.; Crandall, J.P.; Barzilai, N. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 2018, 17, e12723. [Google Scholar] [CrossRef] [PubMed]
- Nefs, G.; Bot, M.; Browne, J.L.; Speight, J.; Pouwer, F. Diabetes MILES—The Netherlands: Rationale, design and sample characteristics of a national survey examining the psychosocial aspects of living with diabetes in Dutch adults. BMC Public Health 2012, 12, 925. [Google Scholar] [CrossRef]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Padki, M.M.; Stambler, I. Targeting Aging with Metformin (TAME). In Encyclopedia of Gerontology and Population Aging; Gu, D., Dupre, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 4908–4910. [Google Scholar]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Wilkinson, J.E.; Burmeister, L.; Brooks, S.V.; Chan, C.; Friedline, S.; Harrison, D.E.; Hejtmancik, J.F.; Nadon, N.; Strong, R.; Wood, L.K.; et al. Rapamycin slows aging in mice. Aging Cell 2012, 11, 675–682. [Google Scholar] [CrossRef]
- Mannick, J.B.; Morris, M.; Hockey, H.-U.P.; Roma, G.; Beibel, M.; Kulmatycki, K.; Watkins, M.; Shavlakadze, T.; Zhou, W.; Quinn, D.; et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 2018, 10, eaaq1564. [Google Scholar] [CrossRef]
- Mannick, J.B.; Teo, G.; Bernardo, P.; Quinn, D.; Russell, K.; Klickstein, L.; Marshall, W.; Shergill, S. Targeting the biology of ageing with mTOR inhibitors to improve immune function in older adults: Phase 2b and phase 3 randomised trials. Lancet Healthy Longev. 2021, 2, e250–e262. [Google Scholar] [CrossRef]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Mitchell, S.E. Caloric restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar] [CrossRef]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brûlé, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, Independent of AMPK, Inhibits mTORC1 in a Rag GTPase-Dependent Manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef]
- Hoong, C.W.S.; Chua, M.W.J. SGLT2 Inhibitors as Calorie Restriction Mimetics: Insights on Longevity Pathways and Age-Related Diseases. Endocrinology 2021, 162, bqab079. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Wilmanski, T.; Baloni, P.; Robinson, M.; Garcia, G.G.; Hoopmann, M.R.; Midha, M.K.; Baxter, D.H.; Maes, M.; Morrone, S.R.; et al. Author Correction: Lifespan-extending interventions induce consistent patterns of fatty acid oxidation in mouse livers. Commun. Biol. 2023, 6, 768. [Google Scholar] [CrossRef] [PubMed]
- Chiao, Y.A.; Kolwicz, S.C.; Basisty, N.; Gagnidze, A.; Zhang, J.; Gu, H.; Djukovic, D.; Beyer, R.P.; Raftery, D.; MacCoss, M.; et al. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging 2016, 8, 314–327. [Google Scholar] [CrossRef]
- Kim, H.-S.; Ren, G.; Kim, T.; Bhatnagar, S.; Yang, Q.; Bahk, Y.Y.; Kim, J.-A. Metformin reduces saturated fatty acid-induced lipid accumulation and inflammatory response by restoration of autophagic flux in endothelial cells. Sci. Rep. 2020, 10, 13523. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Westbrook, R.; Hill, C.; Boparai, R.K.; Arum, O.; Spong, A.; Wang, F.; Javors, M.A.; Chen, J.; Sun, L.Y.; et al. Duration of Rapamycin Treatment Has Differential Effects on Metabolism in Mice. Cell Metab. 2013, 17, 456–462. [Google Scholar] [CrossRef]
- Selman, C.; Tullet, J.M.A.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal Protein S6 Kinase 1 Signaling Regulates Mammalian Life Span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef]
- Lamming, D.W.; Ye, L.; Katajisto, P.; Goncalves, M.D.; Saitoh, M.; Stevens, D.M.; Davis, J.G.; Salmon, A.B.; Richardson, A.; Ahima, R.S.; et al. Rapamycin-Induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science 2012, 335, 1638–1643. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chakrabarty, R.P.; D’alessandro, K.B.; Sebo, Z.L.; Grant, R.A.; Gao, P.; Budinger, G.R.; Chandel, N.S. Metformin targets mitochondrial complex I to lower blood glucose levels. Sci. Adv. 2024, 10, eads5466. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar] [CrossRef]
- El-Mir, M.-Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef]
- Maurer, J.; Zhao, X.; Irmler, M.; Gudiksen, A.; Pilmark, N.S.; Li, Q.; Goj, T.; Beckers, J.; de Angelis, M.H.; Birkenfeld, A.L.; et al. Redox state and altered pyruvate metabolism contribute to a dose-dependent metformin-induced lactate production of human myotubes. Am. J. Physiol. Cell Physiol. 2023, 325, C1131–C1143. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Brandauer, J.; Vienberg, S.G.; Andersen, M.A.; Ringholm, S.; Risis, S.; Larsen, P.S.; Kristensen, J.M.; Frøsig, C.; Leick, L.; Fentz, J.; et al. AMP-activated protein kinase regulates nicotinamide phosphoribosyl transferase expression in skeletal muscle. J. Physiol. 2013, 591, 5207–5220. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Huang, X.; Li, X.; Qiu, X.; Li, M.; Liu, R.; He, T.; Tang, Q. AMPK phosphorylates NAMPT to regulate NAD + homeostasis under ionizing radiation. Open Biol. 2022, 12, 220213. [Google Scholar] [CrossRef] [PubMed]
- Traba, J.; Kwarteng-Siaw, M.; Okoli, T.C.; Li, J.; Huffstutler, R.D.; Bray, A.; Waclawiw, M.A.; Han, K.; Pelletier, M.; Sauve, A.A.; et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J. Clin. Investig. 2015, 125, 4592–4600. [Google Scholar] [CrossRef]
- Yerra, V.G.; Kalvala, A.K.; Kumar, A. Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J. Nutr. Biochem. 2017, 47, 41–52. [Google Scholar] [CrossRef]
- Kickstein, E.; Krauss, S.; Thornhill, P.; Rutschow, D.; Zeller, R.; Sharkey, J.; Williamson, R.; Fuchs, M.; Koehler, A.; Glossmann, H.; et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 21830–21835. [Google Scholar] [CrossRef]
- Hirst, J. Mitochondrial Complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Wikström, M.; Djurabekova, A.; Sharma, V. On the role of ubiquinone in the proton translocation mechanism of respiratory complex I. FEBS Lett. 2023, 597, 224–236. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.S.; D’Imprima, E.; Vonck, J. Mitochondrial Respiratory Chain Complexes. In Membrane Protein Complexes: Structure and Function; Harris, J., Boekema, E., Eds.; Subcellular Biochemistry; Springer: Singapore, 2018; Volume 87, pp. 167–227. [Google Scholar] [CrossRef]
- Wikström, M.; Hummer, G. Stoichiometry of proton translocation by respiratory complex I and its mechanistic implications. Proc. Natl. Acad. Sci. USA 2012, 109, 4431–4436. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Plourde, A.; Bueler, S.A.; Liu, J.; Brzezinski, P.; Vahidi, S.; Rubinstein, J.L. Structure of mycobacterial respiratory complex I. Proc. Natl. Acad. Sci. USA 2023, 120, e2214949120. [Google Scholar] [CrossRef]
- Iii, R.O.; Friday, E.; Turturro, F.; Welbourne, T. Troglitazone Induced Cytosolic Acidification via Extracellular Signal-Response Kinase Activation and Mitochondrial Depolarization: Complex I Proton Pumping Regulates Ammoniagenesis in Proximal Tubule-like LLC-PK1 Cells. Cell. Physiol. Biochem. 2008, 22, 475–486. [Google Scholar] [CrossRef]
- Roberts, P.G.; Hirst, J. The Deactive Form of Respiratory Complex I from Mammalian Mitochondria Is a Na+/H+ Antiporter. J. Biol. Chem. 2012, 287, 34743–34751. [Google Scholar] [CrossRef]
- Nicholls, D.G. Does a transmembrane sodium gradient control membrane potential in mammalian mitochondria? Cell Calcium 2024, 124, 102962. [Google Scholar] [CrossRef]
- Shivaprakash, P.; Beeraka, N.M.; Madhunapantula, S.R.V.; Nikolenko, V.N.; Basalingappa, K.M. Metformin Effects on SHIP2, AMPKs and Gut Microbiota: Recent Updates on Pharmacology. Curr. Med. Chem. 2025, 32, 1732–1754. [Google Scholar] [CrossRef] [PubMed]
- Ishima, T.; Kimura, N.; Kobayashi, M.; Watanabe, C.; Jimbo, E.F.; Kobayashi, R.; Horii, T.; Hatada, I.; Murayama, K.; Ohtake, A.; et al. NADH Reductive Stress and Its Correlation with Disease Severity in Leigh Syndrome: A Pilot Study Using Patient Fibroblasts and a Mouse Model. Biomolecules 2024, 15, 38. [Google Scholar] [CrossRef]
- Babot, M.; Birch, A.; Labarbuta, P.; Galkin, A. Characterisation of the active/de-active transition of mitochondrial complex I. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1837, 1083–1092. [Google Scholar] [CrossRef]
- Dröse, S.; Stepanova, A.; Galkin, A. Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1857, 946–957. [Google Scholar] [CrossRef]
- Gorenkova, N.; Robinson, E.; Grieve, D.J.; Galkin, A. Conformational Change of Mitochondrial Complex I Increases ROS Sensitivity During Ischemia. Antioxidants Redox Signal. 2013, 19, 1459–1468. [Google Scholar] [CrossRef]
- Hirst, J.; King, M.S.; Pryde, K.R. The production of reactive oxygen species by complex I. Biochem. Soc. Trans. 2008, 36 Pt 5, 976–980. [Google Scholar] [CrossRef]
- Saura, P.; Kaila, V.R.I. Energetics and Dynamics of Proton-Coupled Electron Transfer in the NADH/FMN Site of Respiratory Complex I. J. Am. Chem. Soc. 2019, 141, 5710–5719. [Google Scholar] [CrossRef] [PubMed]
- Grivennikova, V.G.; Kozlovsky, V.S.; Vinogradov, A.D. Respiratory complex II: ROS production and the kinetics of ubiquinone reduction. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1858, 109–117. [Google Scholar] [CrossRef]
- Dhingra, R.; A Kirshenbaum, L. Succinate dehydrogenase/complex II activity obligatorily links mitochondrial reserve respiratory capacity to cell survival in cardiac myocytes. Cell Death Dis. 2015, 6, e1956. [Google Scholar] [CrossRef] [PubMed]
- Maklashina, E.; Cecchini, G. The quinone-binding and catalytic site of complex II. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 1877–1882. [Google Scholar] [CrossRef]
- Mazat, J.-P.; Devin, A.; Ransac, S. Modelling mitochondrial ROS production by the respiratory chain. Cell. Mol. Life Sci. 2019, 77, 455–465. [Google Scholar] [CrossRef]
- Robb, E.L.; Hall, A.R.; Prime, T.A.; Eaton, S.; Szibor, M.; Viscomi, C.; James, A.M.; Murphy, M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018, 293, 9869–9879. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Niatsetskaya, Z.V.; Sosunov, S.A.; Matsiukevich, D.; Utkina-Sosunova, I.V.; Ratner, V.I.; Starkov, A.A.; Ten, V.S. The Oxygen Free Radicals Originating from Mitochondrial Complex I Contribute to Oxidative Brain Injury Following Hypoxia–Ischemia in Neonatal Mice. J. Neurosci. 2012, 32, 3235–3244. [Google Scholar] [CrossRef]
- Sahni, P.V.; Zhang, J.; Sosunov, S.; Galkin, A.; Niatsetskaya, Z.; Starkov, A.; Brookes, P.S.; Ten, V.S. Krebs cycle metabolites and preferential succinate oxidation following neonatal hypoxic-ischemic brain injury in mice. Pediatr. Res. 2017, 83, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Zheng, J.-J.; Shen, X.; Yan, L.; Wei, H.; Gao, X.; Zhao, Y. Accelerated discovery of superoxide-dismutase nanozymes via high-throughput computational screening. Nat. Commun. 2021, 12, 6866. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Garrido, A.; Hernández-Rodríguez, M.; Zamorano-Ulloa, R.; Correa-Basurto, J.; Mendieta-Wejebe, J.E.; Ramírez-Rosales, D.; Rosales-Hernández, M.C. In Vitro Effect of H2O2, Some Transition Metals and Hydroxyl Radical Produced Via Fenton and Fenton-Like Reactions, on the Catalytic Activity of AChE and the Hydrolysis of ACh. Neurochem. Res. 2014, 39, 2093–2104. [Google Scholar] [CrossRef]
- Mohsin, A.A.; Chen, Q.; Quan, N.; Rousselle, T.; Maceyka, M.W.; Samidurai, A.; Thompson, J.; Hu, Y.; Li, J.; Lesnefsky, E.J. Mitochondrial Complex I Inhibition by Metformin Limits Reperfusion Injury. J. Pharmacol. Exp. Ther. 2019, 369, 282–290. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014, 3, e02242. [Google Scholar] [CrossRef]
- Yang, M.; Darwish, T.; Larraufie, P.; Rimmington, D.; Cimino, I.; Goldspink, D.A.; Jenkins, B.; Koulman, A.; Brighton, C.A.; Ma, M.; et al. Inhibition of mitochondrial function by metformin increases glucose uptake, glycolysis and GDF-15 release from intestinal cells. Sci. Rep. 2021, 11, 2529. [Google Scholar] [CrossRef]
- Yerra, V.G.; Kalvala, A.K.; Sherkhane, B.; Areti, A.; Kumar, A. Adenosine monophosphate-activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy. Neuropharmacology 2018, 131, 256–270. [Google Scholar] [CrossRef]
- Salama, A.A.; Yassen, N.N.; Mansour, H.M. Naringin protects mice from D-galactose-induced lung aging and mitochondrial dysfunction: Implication of SIRT1 pathways. Life Sci. 2023, 324, 121471. [Google Scholar] [CrossRef]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of Toxicity in Rotenone Models of Parkinson’s Disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Lapointe, N.; St-Hilaire, M.; Martinoli, M.; Blanchet, J.; Gould, P.; Rouillard, C.; Cicchetti, F. Rotenone induces non-specific central nervous system and systemic toxicity. FASEB J. 2004, 18, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, A.D.; Webb, K.R.; Keeney, M.T.; Van Laar, V.S.; Zharikov, A.; Burton, E.A.; Hastings, T.G.; Glajch, K.E.; Hirst, W.D.; Greenamyre, J.T.; et al. Transient exposure to rotenone causes degeneration and progressive parkinsonian motor deficits, neuroinflammation, and synucleinopathy. NPJ Park. Dis. 2023, 9, 121. [Google Scholar] [CrossRef]
- Rodríguez-Nuevo, A.; Torres-Sanchez, A.; Duran, J.M.; De Guirior, C.; Martínez-Zamora, M.A.; Böke, E. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 2022, 607, 756–761. [Google Scholar] [CrossRef]
- de Marañón, A.M.; Díaz-Pozo, P.; Canet, F.; Díaz-Morales, N.; Abad-Jiménez, Z.; López-Domènech, S.; Vezza, T.; Apostolova, N.; Morillas, C.; Rocha, M.; et al. Metformin modulates mitochondrial function and mitophagy in peripheral blood mononuclear cells from type 2 diabetic patients. Redox Biol. 2022, 53, 102342. [Google Scholar] [CrossRef]
- Binyamin, O.; Frid, K.; Keller, G.; Saada, A.; Gabizon, R. Comparing anti–aging hallmark activities of Metformin and Nano-PSO in a mouse model of genetic Creutzfeldt-Jakob Disease. Neurobiol. Aging 2021, 110, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arriola, E.; EL Hafidi, M.; Ortega-Cuéllar, D.; Carvajal, K. AMP-Activated Protein Kinase Regulates Oxidative Metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 Transcriptional Regulators. PLoS ONE 2016, 11, e0148089. [Google Scholar] [CrossRef]
- Luján, L.M.L.; McCarty, M.F.; Di Nicolantonio, J.J.; Ruiz, J.C.G.; Rosas-Burgos, E.C.; Plascencia-Jatomea, M.; Assanga, S.B.I. Nutraceuticals/Drugs Promoting Mitophagy and Mitochondrial Biogenesis May Combat the Mitochondrial Dysfunction Driving Progression of Dry Age-Related Macular Degeneration. Nutrients 2022, 14, 1985. [Google Scholar] [CrossRef]
- Nunn, A.V.; Bell, J.D.; Guy, G.W. Lifestyle-induced metabolic inflexibility and accelerated ageing syndrome: Insulin resistance, friend or foe? Nutr. Metab. 2009, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Brunmair, B.; Staniek, K.; Gras, F.; Scharf, N.; Althaym, A.; Clara, R.; Roden, M.; Gnaiger, E.; Nohl, H.; Waldhäusl, W.; et al. Thiazolidinediones, Like Metformin, Inhibit Respiratory Complex I: A common mechanism contributing to their antidiabetic actions? Diabetes 2004, 53, 1052–1059. [Google Scholar] [CrossRef]
- Scatena, R.; Martorana, G.; Bottoni, P.; Giardina, B. Mitochondrial Dysfunction by Synthetic Ligands of Peroxisome Proliferator Activated Receptors (PPARs). IUBMB Life 2004, 56, 477–482. [Google Scholar] [CrossRef]
- Home, P.D.; Pocock, S.J.; Beck-Nielsen, H.; Curtis, P.S.; Gomis, R.; Hanefeld, M.; Jones, N.P.; Komajda, M.; McMurray, J.J. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. Lancet 2009, 373, 2125–2135. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. New Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef]
- Sanz, M.-N.; Sánchez-Martín, C.; Detaille, D.; Vial, G.; Rigoulet, M.; El-Mir, M.-Y.; Rodríguez-Villanueva, G. Acute Mitochondrial Actions of Glitazones on the Liver: A Crucial Parameter for their Antidiabetic Properties. Cell. Physiol. Biochem. 2011, 28, 899–910. [Google Scholar] [CrossRef]
- Landry, A.P.; Ding, H. Redox Control of Human Mitochondrial Outer Membrane Protein MitoNEET [2Fe-2S] Clusters by Biological Thiols and Hydrogen Peroxide. J. Biol. Chem. 2014, 289, 4307–4315. [Google Scholar] [CrossRef]
- Zuris, J.A.; Ali, S.S.; Yeh, H.; Nguyen, T.A.; Nechushtai, R.; Paddock, M.L.; Jennings, P.A. NADPH Inhibits [2Fe-2S] Cluster Protein Transfer from Diabetes Drug Target MitoNEET to an Apo-acceptor Protein. J. Biol. Chem. 2012, 287, 11649–11655. [Google Scholar] [CrossRef]
- Tan, G.; Landry, A.P.; Dai, R.; Wang, L.; Lu, J.; Ding, H. Competition of zinc ion for the [2Fe–2S] cluster binding site in the diabetes drug target protein mitoNEET. BioMetals 2012, 25, 1177–1184. [Google Scholar] [CrossRef]
- Landry, A.P.; Cheng, Z.; Ding, H. Reduction of mitochondrial protein mitoNEET [2Fe–2S] clusters by human glutathione reductase. Free. Radic. Biol. Med. 2015, 81, 119–127. [Google Scholar] [CrossRef]
- Landry, A.P.; Wang, Y.; Cheng, Z.; Crochet, R.B.; Lee, Y.-H.; Ding, H. Flavin nucleotides act as electron shuttles mediating reduction of the [2Fe-2S] clusters in mitochondrial outer membrane protein mitoNEET. Free. Radic. Biol. Med. 2016, 102, 240–247. [Google Scholar] [CrossRef]
- Lan, T.; Bi, F.; Xu, Y.; Yin, X.; Chen, J.; Han, X.; Guo, W. PPAR-γ activation promotes xenogenic bioroot regeneration by attenuating the xenograft induced-oxidative stress. Int. J. Oral Sci. 2023, 15, 10. [Google Scholar] [CrossRef]
- Kohan-Ghadr, H.-R.; A Kilburn, B.; Kadam, L.; Johnson, E.; Kolb, B.L.; Rodriguez-Kovacs, J.; Hertz, M.; Armant, D.R.; Drewlo, S. Rosiglitazone augments antioxidant response in the human trophoblast and prevents apoptosis. Biol. Reprod. 2019, 100, 479–494. [Google Scholar] [CrossRef]
- Borrelli, A.; Bonelli, P.; Tuccillo, F.M.; Goldfine, I.D.; Evans, J.L.; Buonaguro, F.M.; Mancini, A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.I.; Taegtmeyer, H. Insulin Sensitizers and Heart Failure: An Engine Flooded with Fuel. Curr. Hypertens. Rep. 2010, 12, 399–401. [Google Scholar] [CrossRef][Green Version]
- Tenenbaum, A.; Fisman, E.Z. Balanced pan-PPAR activator bezafibrate in combination with statin: Comprehensive lipids control and diabetes prevention? Cardiovasc. Diabetol. 2012, 11, 140. [Google Scholar] [CrossRef]
- Athyros, V.G.; Imprialos, K.; Stavropoulos, K.; Sahinidis, A.; Doumas, M. Understanding the cardiovascular risk with non-insulin antidiabetic drugs. Expert Opin. Drug Saf. 2019, 18, 241–251. [Google Scholar] [CrossRef]
- Honka, H.; Solis-Herrera, C.; Triplitt, C.; Norton, L.; Butler, J.; DeFronzo, R.A. Therapeutic Manipulation of Myocardial Metabolism: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2022–2039. [Google Scholar] [CrossRef]
- Peluso, A.; Damgaard, M.V.; Mori, M.A.S.; Treebak, J.T. Age-Dependent Decline of NAD+—Universal Truth or Confounded Consensus? Nutrients 2021, 14, 101. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, C.; Li, T.; Zhang, W.; Zong, Z.; Liu, X.; Deng, H. Reduced Nicotinamide Mononucleotide (NMNH) Potently Enhances NAD+ and Suppresses Glycolysis, the TCA Cycle, and Cell Growth. J. Proteome Res. 2021, 20, 2596–2606. [Google Scholar] [CrossRef]
- Mehmel, M.; Jovanović, N.; Spitz, U. Nicotinamide Riboside—The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef]
- Li, H.-Y.; Cai, Z.-Y. SIRT3 regulates mitochondrial biogenesis in aging-related diseases. J. Biomed. Res. 2023, 37, 77. [Google Scholar] [CrossRef]
- Imai, S.-I.; Guarente, L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech. Dis. 2016, 2, 16017. [Google Scholar] [CrossRef]
- Imai, S.-I.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Anderson, R.M.; Bitterman, K.J.; Wood, J.G.; Medvedik, O.; Cohen, H.; Lin, S.S.; Manchester, J.K.; Gordon, J.I.; Sinclair, D.A. Manipulation of a Nuclear NAD+ Salvage Pathway Delays Aging without Altering Steady-state NAD+ Levels. J. Biol. Chem. 2002, 277, 18881–18890. [Google Scholar] [CrossRef]
- Belenky, P.; Racette, F.G.; Bogan, K.L.; McClure, J.M.; Smith, J.S.; Brenner, C. Nicotinamide Riboside Promotes Sir2 Silencing and Extends Lifespan via Nrk and Urh1/Pnp1/Meu1 Pathways to NAD+. Cell 2007, 129, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD + Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016, 24, 566–581. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Lautrup, S.; Jensen, M.B.; Yang, B.; SenGupta, T.; Caponio, D.; Khezri, R.; Demarest, T.G.; Aman, Y.; et al. NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat. Commun. 2019, 10, 5284. [Google Scholar] [CrossRef]
- Lin, S.S.; Manchester, J.K.; Gordon, J.I. Enhanced Gluconeogenesis and Increased Energy Storage as Hallmarks of Aging in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 36000–36007. [Google Scholar] [CrossRef]
- Beas, A.O.; Gordon, P.B.; Prentiss, C.L.; Olsen, C.P.; Kukurugya, M.A.; Bennett, B.D.; Parkhurst, S.M.; Gottschling, D.E. Independent regulation of age associated fat accumulation and longevity. Nat. Commun. 2020, 11, 279. [Google Scholar] [CrossRef]
- Orlandi, I.; Stamerra, G.; Vai, M. Altered Expression of Mitochondrial NAD+ Carriers Influences Yeast Chronological Lifespan by Modulating Cytosolic and Mitochondrial Metabolism. Front. Genet. 2018, 9, 676. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD+/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef]
- Fang, E.F.; Scheibye-Knudsen, M.; Brace, L.E.; Kassahun, H.; SenGupta, T.; Nilsen, H.; Mitchell, J.R.; Croteau, D.L.; Bohr, V.A. Defective Mitophagy in XPA via PARP-1 Hyperactivation and NAD+/SIRT1 Reduction. Cell 2014, 157, 882–896. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Guillemin, G.J.; Smythe, G.; Sachdev, P. Mapping NAD+ metabolism in the brain of ageing Wistar rats: Potential targets for influencing brain senescence. Biogerontology 2013, 15, 177–198. [Google Scholar] [CrossRef]
- Ma, C.; Pi, C.; Yang, Y.; Lin, L.; Shi, Y.; Li, Y.; Li, Y.; He, X. Nampt Expression Decreases Age-Related Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Sirt1. PLoS ONE 2017, 12, e0170930. [Google Scholar] [CrossRef] [PubMed]
- Pi, C.; Yang, Y.; Sun, Y.; Wang, H.; Sun, H.; Ma, M.; Lin, L.; Shi, Y.; Li, Y.; Li, Y.; et al. Nicotinamide phosphoribosyltransferase postpones rat bone marrow mesenchymal stem cell senescence by mediating NAD+–Sirt1 signaling. Aging 2019, 11, 3505–3522. [Google Scholar] [CrossRef]
- Johnson, S.; Wozniak, D.F.; Imai, S. CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ Aging Mech. Dis. 2018, 4, 10. [Google Scholar] [CrossRef]
- Martin, S.A.; DeMuth, T.M.; Miller, K.N.; Pugh, T.D.; Polewski, M.A.; Colman, R.J.; Eliceiri, K.W.; Beasley, T.M.; Johnson, S.C.; Anderson, R.M. Regional metabolic heterogeneity of the hippocampus is nonuniformly impacted by age and caloric restriction. Aging Cell 2015, 15, 100–110. [Google Scholar] [CrossRef]
- Blacher, E.; Dadali, T.; Bespalko, A.; Haupenthal, V.J.; Grimm, M.O.W.; Hartmann, T.; Lund, F.E.; Stein, R.; Levy, A. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann. Neurol. 2015, 78, 88–103. [Google Scholar] [CrossRef]
- Scheibye-Knudsen, M.; Mitchell, S.J.; Fang, E.F.; Iyama, T.; Ward, T.; Wang, J.; Dunn, C.A.; Singh, N.; Veith, S.; Hasan-Olive, M.; et al. A High-Fat Diet and NAD + Activate Sirt1 to Rescue Premature Aging in Cockayne Syndrome. Cell Metab. 2014, 20, 840–855. [Google Scholar] [CrossRef]
- McReynolds, M.R.; Chellappa, K.; Chiles, E.; Jankowski, C.; Shen, Y.; Chen, L.; Descamps, H.C.; Mukherjee, S.; Bhat, Y.R.; Lingala, S.R.; et al. NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Syst. 2021, 12, 1160–1172.e4. [Google Scholar] [CrossRef] [PubMed]
- Bagga, P.; Hariharan, H.; Wilson, N.E.; Beer, J.C.; Shinohara, R.T.; Elliott, M.A.; Baur, J.A.; Marincola, F.M.; Witschey, W.R.; Haris, M.; et al. Single-Voxel 1H MR spectroscopy of cerebral nicotinamide adenine dinucleotide (NAD+) in humans at 7T using a 32-channel volume coil. Magn. Reson. Med. 2019, 83, 806–814. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Lu, M.; Lee, B.-Y.; Ugurbil, K.; Chen, W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA 2015, 112, 2876–2881. [Google Scholar] [CrossRef]
- Brakedal, B.; Dölle, C.; Riemer, F.; Ma, Y.; Nido, G.S.; Skeie, G.O.; Craven, A.R.; Schwarzlmüller, T.; Brekke, N.; Diab, J.; et al. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 2022, 34, 396–407.e6. [Google Scholar] [CrossRef]
- Ahmadi, A.; Begue, G.; Valencia, A.P.; Norman, J.E.; Lidgard, B.; Bennett, B.J.; Van Doren, M.P.; Marcinek, D.J.; Fan, S.; Prince, D.K.; et al. Randomized crossover clinical trial of coenzyme Q10 and nicotinamide riboside in chronic kidney disease. JCI Insight 2023, 8, e167274. [Google Scholar] [CrossRef]
- Steinhubl, S.R. Why Have Antioxidants Failed in Clinical Trials? Am. J. Cardiol. 2008, 101, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.M.; Holt, A.G. Why antioxidant therapies have failed in clinical trials. J. Theor. Biol. 2018, 457, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Spychalowicz, A.; Wilk, G.; Sliwa, T.; Ludew, D.; Guzik, T.J. Novel Therapeutic Approaches in Limiting Oxidative Stress and Inflammation. Curr. Pharm. Biotechnol. 2012, 13, 2456–2466. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Targeting Antioxidants to Mitochondria by Conjugation to Lipophilic Cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- McCormick, B.; Lowes, D.; Colvin, L.; Torsney, C.; Galley, H. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br. J. Anaesth. 2016, 117, 659–666. [Google Scholar] [CrossRef]
- Dhanasekaran, A.; Kotamraju, S.; Kalivendi, S.V.; Matsunaga, T.; Shang, T.; Keszler, A.; Joseph, J.; Kalyanaraman, B. Supplementation of Endothelial Cells with Mitochondria-targeted Antioxidants Inhibit Peroxide-induced Mitochondrial Iron Uptake, Oxidative Damage, and Apoptosis. J. Biol. Chem. 2004, 279, 37575–37587. [Google Scholar] [CrossRef]
- Minter, B.E.; Lowes, D.A.; Webster, N.R.; Galley, H.F. Differential Effects of MitoVitE, α-Tocopherol and Trolox on Oxidative Stress, Mitochondrial Function and Inflammatory Signalling Pathways in Endothelial Cells Cultured under Conditions Mimicking Sepsis. Antioxidants 2020, 9, 195. [Google Scholar] [CrossRef]
- Hegarty, K.J.; Byrne, F.L. Comment on “Differential Effects of MitoVitE, α-Tocopherol and Trolox on Oxidative Stress, Mitochondrial Function and Inflammatory Signalling Pathways in Endothelial Cells Cultured under Conditions Mimicking Sepsis. Antioxidants 2020, 9(3), 195”. Antioxidants 2020, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Minter, B.E.; Lowes, D.A.; Webster, N.R.; Galley, H.F. Reply to Comment on “Differential Effects of MitoVitE, α-Tocopherol and Trolox on Oxidative Stress, Mitochondrial Function and Inflammatory Signalling Pathways in Endothelial Cells Cultured under Conditions Mimicking Sepsis. Antioxidants 2020, 9(3), 195”. Antioxidants 2020, 9, 464. [Google Scholar] [CrossRef]
- Covey, M.V.; Murphy, M.P.; Hobbs, C.E.; Smith, R.A.; Oorschot, D.E. Effect of the mitochondrial antioxidant, Mito Vitamin E, on hypoxic–ischemic striatal injury in neonatal rats: A dose–response and stereological study. Exp. Neurol. 2006, 199, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Adlam, V.J.; Harrison, J.C.; Porteous, C.M.; James, A.M.; Smith, R.A.J.; Murphy, M.P.; Sammut, I.A. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005, 19, 1088–1095. [Google Scholar] [CrossRef]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M.; Protect Study Group. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Cao, T.; Xiong, S.; Ma, J.; Fan, G.-C.; Lacefield, J.C.; Lu, Y.; Le Tissier, S.; Peng, T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free. Radic. Biol. Med. 2016, 90, 12–23. [Google Scholar] [CrossRef]
- Yang, S.-G.; Park, H.-J.; Kim, J.-W.; Jung, J.-M.; Kim, M.-J.; Jegal, H.-G.; Kim, I.-S.; Kang, M.-J.; Wee, G.; Yang, H.-Y.; et al. Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci. Rep. 2018, 8, 10130. [Google Scholar] [CrossRef]
- Shetty, S.; Anushree, U.; Kumar, R.; Bharati, S. Mitochondria-targeted antioxidant, mito-TEMPO mitigates initiation phase of N-Nitrosodiethylamine-induced hepatocarcinogenesis. Mitochondrion 2021, 58, 123–130. [Google Scholar] [CrossRef]
- Hu, H.; Li, M. Mitochondria-targeted antioxidant mitotempo protects mitochondrial function against amyloid beta toxicity in primary cultured mouse neurons. Biochem. Biophys. Res. Commun. 2016, 478, 174–180. [Google Scholar] [CrossRef]
- Chen, J.-W.; Ma, P.-W.; Yuan, H.; Wang, W.-L.; Lu, P.-H.; Ding, X.-R.; Lun, Y.-Q.; Yang, Q.; Lu, L.-J. mito-TEMPO Attenuates Oxidative Stress and Mitochondrial Dysfunction in Noise-Induced Hearing Loss via Maintaining TFAM-mtDNA Interaction and Mitochondrial Biogenesis. Front. Cell. Neurosci. 2022, 16, 803718. [Google Scholar] [CrossRef] [PubMed]
- Study Details. Effects of an Antioxidant Supplement on Blood Vessel Health. Available online: https://clinicaltrials.gov/study/NCT06424756?intr=MitoTempo&rank=1 (accessed on 17 March 2025).
- Magwere, T.; West, M.; Riyahi, K.; Murphy, M.P.; Smith, R.A.; Partridge, L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 2006, 127, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Bogue, M.; Fernandez, E.; Javors, M.A.; Libert, S.; Marinez, P.A.; Murphy, M.P.; Musi, N.; Nelson, J.F.; et al. Rapamycin-mediated mouse lifespan extension: Late-life dosage regimes with sex-specific effects. Aging Cell 2020, 19, e13269. [Google Scholar] [CrossRef]
- Beyrath, J.; Pellegrini, M.; Renkema, H.; Houben, L.; Pecheritsyna, S.; van Zandvoort, P.; Broek, P.v.D.; Bekel, A.; Eftekhari, P.; Smeitink, J.A.M. KH176 Safeguards Mitochondrial Diseased Cells from Redox Stress-Induced Cell Death by Interacting with the Thioredoxin System/Peroxiredoxin Enzyme Machinery. Sci. Rep. 2018, 8, 6577. [Google Scholar] [CrossRef]
- Koene, S.; Spaans, E.; Van Bortel, L.; Van Lancker, G.; Delafontaine, B.; Badilini, F.; Beyrath, J.; Smeitink, J. KH176 under development for rare mitochondrial disease: A first in man randomized controlled clinical trial in healthy male volunteers. Orphanet J. Rare Dis. 2017, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.C.; Koene, S.; de Laat, P.; Hemelaar, P.; Pickkers, P.; Spaans, E.; Beukema, R.; Beyrath, J.; Groothuis, J.; Verhaak, C.; et al. The KHENERGY Study: Safety and Efficacy of KH176 in Mitochondrial m.3243A>G Spectrum Disorders. Clin. Pharmacol. Ther. 2018, 105, 101–111. [Google Scholar] [CrossRef]
- Detienne, G.; De Haes, W.; Mergan, L.; Edwards, S.L.; Temmerman, L.; Van Bael, S. Beyond ROS clearance: Peroxiredoxins in stress signaling and aging. Ageing Res. Rev. 2018, 44, 33–48. [Google Scholar] [CrossRef]
- Jiang, X.; Renkema, H.; Pennings, B.; Pecheritsyna, S.; Schoeman, J.C.; Hankemeier, T.; Smeitink, J.; Beyrath, J. Mechanism of action and potential applications of selective inhibition of microsomal prostaglandin E synthase-1-mediated PGE2 biosynthesis by sonlicromanol’s metabolite KH176m. Sci. Rep. 2021, 11, 880. [Google Scholar] [CrossRef]
- Chen, J.; Deng, J.C.; Zemans, R.L.; Bahmed, K.; Kosmider, B.; Zhang, M.; Peters-Golden, M.; Goldstein, D.R. Age-induced prostaglandin E2 impairs mitochondrial fitness and increases mortality to influenza infection. Nat. Commun. 2022, 13, 679. [Google Scholar] [CrossRef]
- Liao, K.; Huang, F.; Xie, X.; Li, L. Targeting AMPK/eNOS pathway and mitochondria by sonlicromanol protects myocardial cells against ischemia-reperfusion injury. Gen. Physiol. Biophys. 2023, 42, 33–382. [Google Scholar] [CrossRef] [PubMed]
- Smeitink, J.; van Es, J.; Bosman, B.; Janssen, M.C.H.; Klopstock, T.; Gorman, G.; Vissing, J.; Ruiterkamp, G.; Edgar, C.J.; Abbink, E.J.; et al. Phase 2b program with sonlicromanol in patients with mitochondrial disease due to m.3243A>G mutation. Brain 2024, 148, 896–907. [Google Scholar] [CrossRef]
- Study Details. KHENERFIN Study: A Trial to Evaluate the Efficacy and Safety of Sonlicromanol in Primary Mitochondrial Diseases. Available online: https://clinicaltrials.gov/study/NCT06451757 (accessed on 19 February 2025).
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021, 3, 1706–1726. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Qiu, Y.; Shen, M.; Liu, W.; Feng, D.; Luo, Z.; Zhou, Y. Procyanidin C1 inhibits bleomycin-induced pulmonary fibrosis in mice by selective clearance of senescent myofibroblasts. FASEB J. 2024, 38, e23749. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Chen, X.; Yang, Z.; Chen, J.; Zhu, W.; Li, Y.; Wen, Y.; Deng, C.; Gu, C.; et al. Senolytic and senomorphic agent procyanidin C1 alleviates structural and functional decline in the aged retina. Proc. Natl. Acad. Sci. USA 2024, 121, e2311028121. [Google Scholar] [CrossRef]
- Cao, X.; Wu, Y.; Hong, H.; Tian, X.Y. Sirtuin 3 Dependent and Independent Effects of NAD+ to Suppress Vascular Inflammation and Improve Endothelial Function in Mice. Antioxidants 2022, 11, 706. [Google Scholar] [CrossRef]
- Hua, W.; Xie, L.; Dong, C.; Yang, G.; Chi, S.; Xu, Z.; Yang, C.; Wang, H.; Wu, X. Procyanidin C1 ameliorates acidic pH stress induced nucleus pulposus degeneration through SIRT3/FOXO3-mediated mitochondrial dynamics. J. Transl. Med. 2024, 22, 1071. [Google Scholar] [CrossRef]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Gluing the Respiratory Chain Together: Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002, 277, 43553–43556. [Google Scholar] [CrossRef]
- Wilkinson, J.A.; Silvera, S.; LeBlanc, P.J. The effect of cardiolipin side chain composition on cytochrome c protein conformation and peroxidase activity. Physiol. Rep. 2021, 9, e14772. [Google Scholar] [CrossRef] [PubMed]
- Snider, E.J.; Muenzner, J.; Toffey, J.R.; Hong, Y.; Pletneva, E.V. Multifaceted Effects of ATP on Cardiolipin-Bound Cytochrome c. Biochemistry 2013, 52, 993–995. [Google Scholar] [CrossRef]
- Sinibaldi, F.; Droghetti, E.; Polticelli, F.; Piro, M.C.; Di Pierro, D.; Ferri, T.; Smulevich, G.; Santucci, R. The effects of ATP and sodium chloride on the cytochrome c–cardiolipin interaction: The contrasting behavior of the horse heart and yeast proteins. J. Inorg. Biochem. 2011, 105, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Borisenko, G.G.; Tyurina, Y.Y.; Tyurin, V.A.; Jiang, J.; Potapovich, A.I.; Kini, V.; Amoscato, A.A.; Fujii, Y. Oxidative lipidomics of apoptosis: Redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free. Radic. Biol. Med. 2004, 37, 1963–1985. [Google Scholar] [CrossRef]
- Iyer, S.S.; He, Q.; Janczy, J.R.; Elliott, E.I.; Zhong, Z.; Olivier, A.K.; Sadler, J.J.; Knepper-Adrian, V.; Han, R.; Qiao, L.; et al. Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-M.; Liu, N.; Qin, Z.-H.; Wang, Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol. Sin. 2022, 43, 2439–2447. [Google Scholar] [CrossRef]
- Zanini, G.; Selleri, V.; Domenech, S.L.; Malerba, M.; Nasi, M.; Mattioli, A.V.; Pinti, M. Mitochondrial DNA as inflammatory DAMP: A warning of an aging immune system? Biochem. Soc. Trans. 2023, 51, 735–745. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxidants Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 2015, 22, 1399–1401. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef]
- Rasola, A.; Bernardi, P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 2007, 12, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Krauskopf, A.; Eriksson, O.; Craigen, W.J.; Forte, M.A.; Bernardi, P. Properties of the permeability transition in VDAC1−/− mitochondria. Biochim. Biophys. Acta (BBA) Bioenerg. 2006, 1757, 590–595. [Google Scholar] [CrossRef]
- Precht, T.A.; Phelps, R.A.; Linseman, D.A.; Butts, B.D.; Le, S.S.; Laessig, T.A.; Bouchard, R.J.; Heidenreich, K.A. The permeability transition pore triggers Bax translocation to mitochondria during neuronal apoptosis. Cell Death Differ. 2005, 12, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Figueiredo, P.A.; Powers, S.K.; Ferreira, R.M.; Appell, H.J.; Duarte, J.A. Aging Impairs Skeletal Muscle Mitochondrial Bioenergetic Function. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64A, 21–33. [Google Scholar] [CrossRef]
- Szeto, H.H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014, 171, 2029–2050. [Google Scholar] [CrossRef]
- Birk, A.V.; Chao, W.M.; Bracken, C.; Warren, J.D.; Szeto, H.H. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br. J. Pharmacol. 2014, 171, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; De Rasmo, D.; Signorile, A.; Corcelli, A.; Lobasso, S. Beneficial effects of SS-31 peptide on cardiac mitochondrial dysfunction in tafazzin knockdown mice. Sci. Rep. 2022, 12, 987. [Google Scholar] [CrossRef]
- Petrosillo, G.; Moro, N.; Ruggiero, F.M.; Paradies, G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free. Radic. Biol. Med. 2009, 47, 969–974. [Google Scholar] [CrossRef]
- Karadas, O.; Ozpinar, N.; Bilgic, E.; Ozcelik, F.; Karadas, S. The physiological and lifespan alterations in Caenorhabditis elegans exposed to different dosages of melatonin. Pak. J. Pharm. Sci. 2019, 32, 625–630. [Google Scholar]
- Yang, S.-H.; Li, W.; Sumien, N.; Forster, M.; Simpkins, J.W.; Liu, R. Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: Methylene blue connects the dots. Prog. Neurobiol. 2017, 157, 273–291. [Google Scholar] [CrossRef]
- Sváb, G.; Kokas, M.; Sipos, I.; Ambrus, A.; Tretter, L. Methylene Blue Bridges the Inhibition and Produces Unusual Respiratory Changes in Complex III-Inhibited Mitochondria. Studies on Rats, Mice and Guinea Pigs. Antioxidants 2021, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Bouillaud, F.; Ransy, C.; Moreau, M.; Benhaim, J.; Lombès, A.; Haouzi, P. Methylene blue induced O2 consumption is not dependent on mitochondrial oxidative phosphorylation: Implications for salvage pathways during acute mitochondrial poisoning. Respir. Physiol. Neurobiol. 2022, 304, 103939. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Xue, H.; Thaivalappil, A.; Cao, K. The Potentials of Methylene Blue as an Anti-Aging Drug. Cells 2021, 10, 3379. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-M.; O’donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef] [PubMed]
- Stack, C.; Jainuddin, S.; Elipenahli, C.; Gerges, M.; Starkova, N.; Starkov, A.A.; Jové, M.; Portero-Otin, M.; Launay, N.; Pujol, A.; et al. Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum. Mol. Genet. 2014, 23, 3716–3732. [Google Scholar] [CrossRef]
- Poudel, S.B.; Frikha-Benayed, D.; Ruff, R.R.; Yildirim, G.; Dixit, M.; Korstanje, R.; Robinson, L.; Miller, R.A.; Harrison, D.E.; Strong, J.R.; et al. Targeting mitochondrial dysfunction using methylene blue or mitoquinone to improve skeletal aging. Aging 2024, 16, 4948–4964. [Google Scholar] [CrossRef]
- Gauthier, S.; Feldman, H.H.; Schneider, L.S.; Wilcock, G.K.; Frisoni, G.B.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Wischik, D.J.; et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: A randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 2016, 388, 2873–2884. [Google Scholar] [CrossRef]
- Wilcock, G.K.; Gauthier, S.; Frisoni, G.B.; Jia, J.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Schelter, B.O.; Wischik, D.J.; et al. Potential of Low Dose Leuco-Methylthioninium Bis(Hydromethanesulphonate) (LMTM) Monotherapy for Treatment of Mild Alzheimer’s Disease: Cohort Analysis as Modified Primary Outcome in a Phase III Clinical Trial. J. Alzheimer’s Dis. 2017, 61, 435–457. [Google Scholar] [CrossRef]
- Wischik, C.M.; Staff, R.T.; Wischik, D.J.; Bentham, P.; Murray, A.D.; Storey, J.M.; Kook, K.A.; Harrington, C.R. Tau Aggregation Inhibitor Therapy: An Exploratory Phase 2 Study in Mild or Moderate Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 44, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Hajmousa, G.; Vogelaar, P.; Brouwer, L.A.; van der Graaf, A.C.; Henning, R.H.; Krenning, G. The 6-chromanol derivate SUL-109 enables prolonged hypothermic storage of adipose tissue-derived stem cells. Biomaterials 2017, 119, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vogelaar, P.C.; Roorda, M.; de Vrij, E.L.; Houwertjes, M.C.; Goris, M.; Bouma, H.; van der Graaf, A.C.; Krenning, G.; Henning, R.H. The 6-hydroxychromanol derivative SUL-109 ameliorates renal injury after deep hypothermia and rewarming in rats. Nephrol. Dial. Transplant. 2018, 33, 2128–2138. [Google Scholar] [CrossRef]
- Star, B.S.; van der Slikke, E.C.; van Buiten, A.; Henning, R.H.; Bouma, H.R. The Novel Compound SUL-138 Counteracts Endothelial Cell and Kidney Dysfunction in Sepsis by Preserving Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 6330. [Google Scholar] [CrossRef] [PubMed]
- Tolouee, M.; Hendriks, K.D.W.; Lie, F.F.; Gartzke, L.P.; Goris, M.; Hoogstra-Berends, F.; Bergink, S.; Henning, R.H. Cooling of Cells and Organs Confers Extensive DNA Strand Breaks Through Oxidative Stress and ATP Depletion. Cell Transplant. 2022, 31, 1–12. [Google Scholar] [CrossRef]
- Gartzke, L.P.; Hendriks, K.D.W.; Hoogstra-Berends, F.; Joschko, C.P.; Strandmoe, A.-L.; Vogelaar, P.C.; Krenning, G.; Henning, R.H. Inhibition of Ferroptosis Enables Safe Rewarming of HEK293 Cells following Cooling in University of Wisconsin Cold Storage Solution. Int. J. Mol. Sci. 2023, 24, 10939. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Mestdagh, C.F.d.V.; Koopmans, F.; Breiter, J.C.; Timmerman, J.A.; Vogelaar, P.C.; Krenning, G.; Mansvelder, H.D.; Smit, A.B.; Henning, R.H.; van Kesteren, R.E. The hibernation-derived compound SUL-138 shifts the mitochondrial proteome towards fatty acid metabolism and prevents cognitive decline and amyloid plaque formation in an Alzheimer’s disease mouse model. Alzheimer’s Res. Ther. 2022, 14, 183. [Google Scholar] [CrossRef]
- Jüttner, A.A.; Jouabadi, S.M.; van der Linden, J.; de Vries, R.; Barnhoorn, S.; Garrelds, I.M.; Goos, Y.; van Veghel, R.; van der Pluijm, I.; Danser, A.H.J.; et al. The modified 6-chromanol SUL-238 protects against accelerated vascular aging in vascular smooth muscle Ercc1-deficient mice. J. Cardiovasc. Aging 2024, 4, 20. [Google Scholar] [CrossRef]
- Study Details. A First-In-Human Study of Single and Multiple Ascending Doses of Oral SUL-238 in Healthy Subjects. Available online: https://clinicaltrials.gov/study/NCT06277492?intr=SUL-238&rank=1 (accessed on 17 March 2025).
- Jiang, W.; He, F.; Ding, G.; Wu, J. Elamipretide reduces pyroptosis and improves functional recovery after spinal cord injury. CNS Neurosci. Ther. 2023, 29, 2843–2856. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.; Han, H.; Lu, Q.; Shen, Y. SS-31@Fer-1 Alleviates ferroptosis in hypoxia/reoxygenation cardiomyocytes via mitochondrial targeting. Biomed. Pharmacother. 2025, 183, 117832. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Johnson, M.A.; Vapniarsky, N.; Van Brocklin, M.W.; Williams, T.K.; Youngquist, S.T.; Ford, R.; Ewer, N.; Neff, L.P.; Hoareau, G.L. Elamipretide mitigates ischemia-reperfusion injury in a swine model of hemorrhagic shock. Sci. Rep. 2023, 13, 4496. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radovic, M.; Gartzke, L.P.; Wink, S.E.; van der Kleij, J.A.; Politiek, F.A.; Krenning, G. Targeting the Electron Transport System for Enhanced Longevity. Biomolecules 2025, 15, 614. https://doi.org/10.3390/biom15050614
Radovic M, Gartzke LP, Wink SE, van der Kleij JA, Politiek FA, Krenning G. Targeting the Electron Transport System for Enhanced Longevity. Biomolecules. 2025; 15(5):614. https://doi.org/10.3390/biom15050614
Chicago/Turabian StyleRadovic, Marko, Lucas P. Gartzke, Simon E. Wink, Joris A. van der Kleij, Frouwkje A. Politiek, and Guido Krenning. 2025. "Targeting the Electron Transport System for Enhanced Longevity" Biomolecules 15, no. 5: 614. https://doi.org/10.3390/biom15050614
APA StyleRadovic, M., Gartzke, L. P., Wink, S. E., van der Kleij, J. A., Politiek, F. A., & Krenning, G. (2025). Targeting the Electron Transport System for Enhanced Longevity. Biomolecules, 15(5), 614. https://doi.org/10.3390/biom15050614






