Proteomics Approaches for Discovering Novel Protein Biomarkers in Inflammatory Bowel Disease-Related Cancer
Abstract
1. Introduction
1.1. Inflammatory Bowel Disease
1.1.1. Symptoms
1.1.2. Intraintestinal and Extraintestinal Complications
1.1.3. Diagnosis
1.2. Epidemiology
1.3. Pathogenesis of IBD
1.4. Treatment of IBD
2. IBD-Related Cancer: Discovery of Biomarkers and Technological Approaches
2.1. The Medical Problem
2.1.1. IBD and Cancer: A Long-Known Correlation
2.1.2. Recent Observational Studies
2.1.3. Statistical Correlation Between IBD and Cancer Development
2.2. Proteomics Relevance in Personalized Medicine
2.3. Most-Used Proteomics Techniques in Clinical Studies
2.4. Proteomic Approaches in IBD
3. Proteomics Application in IBD-Related Cancer Studies
3.1. Inflammation-Related Cancer
3.1.1. Colorectal Cancer
3.1.2. Small Bowel Cancer
3.1.3. Cholangiocarcinoma
3.2. Immunosuppression-Related Cancer
3.2.1. Skin Cancer
3.2.2. Lymphoma
3.2.3. Glioblastoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | inflammatory bowel disease |
| UC | ulcerative colitis |
| CD | Crohn’s disease |
| IBD-U | unclassified IBD |
| GI | gastrointestinal |
| GWAS | genome-wide association studies |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| AIEC | Adherent-Invasive Escherichia coli |
| ELISA | enzyme-linked immunosorbent assay |
| CRP | C-reactive protein |
| ECCO | European Crohn’s and Colitis Organisation |
| 5-ASA | 5-Aminosalicylic acid |
| TNFα | tumor necrosis factor α |
| ADCC | antibody-dependent cell-mediated cytotoxicity |
| S1P | Sphingosine-1-phosphate |
| SIR | Standardized Incidence Ratio |
| CRC | colorectal cancer |
| SBC | small bowel cancer |
| SBA | small bowel adenocarcinoma |
| ALL | acute lymphocytic leukemia |
| 6-TGN | 6-thioguanine nucleotides |
| MMR | mismatch repair |
| MS | mass spectrometry |
| TOF | time-of-flight |
| ESI | electrospray ionization |
| MALDI | matrix-assisted laser desorption/ionization |
| SELDI | surface-enhanced laser desorption/ionization |
| MRM | multiple reaction monitoring |
| PSC | primary sclerosing cholangitis |
| CCA | cholangiocarcinoma |
| NMSC | non-melanoma skin cancer |
| BCC | basal cell carcinoma |
| EBV | Epstein–Barr virus |
References
- Bernstein, C.N.; Eliakim, A.; Fedail, S.; Fried, M.; Gearry, R.; Goh, K.-L.; Hamid, S.; Khan, A.G.; Khalif, I.; Ng, S.C.; et al. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2016, 50, 803–818. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Lophaven, S.N.; Lynge, E.; Burisch, J. The Incidence of Inflammatory Bowel Disease in Denmark 1980–2013: A Nationwide Cohort Study. Aliment. Pharmacol. Ther. 2017, 45, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Jin, Y.; Shao, X.; Xu, Y.; Ma, G.; Jiang, Y.; Xu, Y.; Jiang, Y.; Hu, D. Global, Regional, and National Burden of Inflammatory Bowel Disease, 1990–2021: Insights from the Global Burden of Disease 2021. Int. J. Color. Dis. 2024, 39, 139. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, G.H.; Song, M.; Yon, D.K.; Lee, S.W.; Koyanagi, A.; Jacob, L.; Kostev, K.; Dragioti, E.; Radua, J.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Diseases, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Dig. Liver Dis. 2023, 55, 1352–1359. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-Microbe Interactions Have Shaped the Genetic Architecture of Inflammatory Bowel Disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Hubbard, J.; Korzenik, J.; Sands, B.E.; Panaccione, R.; Ghosh, S.; Wheeler, A.J.; Villeneuve, P.J. The Inflammatory Bowel Diseases and Ambient Air Pollution: A Novel Association. Am. J. Gastroenterol. 2010, 105, 2412–2419. [Google Scholar] [CrossRef]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.L.; Barnich, N.; Bringer, M.A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.F. High Prevalence of Adherent-Invasive Escherichia Coli Associated with Ileal Mucosa in Crohn’s Disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef]
- Putignani, L.; Oliva, S.; Isoldi, S.; Del Chierico, F.; Carissimi, C.; Laudadio, I.; Cucchiara, S.; Stronati, L. Fecal and Mucosal Microbiota Profiling in Pediatric Inflammatory Bowel Diseases. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1376–1386. [Google Scholar] [CrossRef]
- Zhou, L.; Ivanov, I.I.; Spolski, R.; Min, R.; Shenderov, K.; Egawa, T.; Levy, D.E.; Leonard, W.J.; Littman, D.R. IL-6 Programs TH-17 Cell Differentiation by Promoting Sequential Engagement of the IL-21 and IL-23 Pathways. Nat. Immunol. 2007, 8, 967–974. [Google Scholar] [CrossRef]
- Gu, C.; Wu, L.; Li, X. IL-17 Family: Cytokines, Receptors and Signaling. Cytokine 2013, 64, 477–485. [Google Scholar] [CrossRef]
- Kotla, N.G.; Rochev, Y. IBD Disease-Modifying Therapies: Insights from Emerging Therapeutics. Trends Mol. Med. 2023, 29, 241–253. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef] [PubMed]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef]
- Craigle, V. MedWatch: The FDA Safety Information and Adverse Event Reporting Program. J. Med. Libr. Assoc. 2007, 95, 224. [Google Scholar] [CrossRef]
- Noor, N.M.; Bourke, A.; Subramanian, S. Review Article: Novel Therapies in Inflammatory Bowel Disease—An Update for Clinicians. Aliment. Pharmacol. Ther. 2024, 60, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Bargen, J.A. Chronic Ulcerative Colitis Associated with Malignant Disease. Arch. Surg. 1928, 17, 561–576. [Google Scholar] [CrossRef]
- Ekbom, A. Clinical Review Risk Factors and Distinguishing Features of Cancer in IBD. Inflamm. Bowel Dis. 1998, 4, 235–243. [Google Scholar] [CrossRef]
- Scharl, S.; Barthel, C.; Rossel, J.B.; Biedermann, L.; Misselwitz, B.; Schoepfer, A.M.; Straumann, A.; Vavricka, S.R.; Rogler, G.; Scharl, M.; et al. Malignancies in Inflammatory Bowel Disease: Frequency, Incidence and Risk Factors—Results from the Swiss IBD Cohort Study. Am. J. Gastroenterol. 2019, 114, 116–126. [Google Scholar] [CrossRef]
- Biancone, L.; Armuzzi, A.; Scribano, M.L.; Castiglione, F.; D’Incà, R.; Orlando, A.; Papi, C.; Daperno, M.; Vecchi, M.; Riegler, G.; et al. Cancer Risk in Inflammatory Bowel Disease: A 6-Year Prospective Multicenter Nested Case-Control IG-IBD Study. Inflamm. Bowel Dis. 2020, 26, 450–459. [Google Scholar] [CrossRef]
- Fotoohi, A.K.; Coulthard, S.A.; Albertioni, F. Thiopurines: Factors Influencing Toxicity and Response. Biochem. Pharmacol. 2010, 79, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Ledder, O. The Question That Doesn’t Seem to Go Away: Cancer Risk of Anti-TNF Therapy. Dig. Dis. Sci. 2022, 67, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xie, S.; Yuan, C.; Yang, Z.; Liu, S.; Zhang, Q.; Sun, F.; Wu, J.; Zhan, S.; Zhu, S.; et al. Inflammatory Bowel Disease and Long-Term Risk of Cancer: A Prospective Cohort Study among Half a Million Adults in UK Biobank. Inflamm. Bowel Dis. 2023, 29, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Mann, M. From Genomics to Proteomics. Nature 2003, 422, 193–197. [Google Scholar] [CrossRef]
- Maes, E.; Mertens, I.; Valkenborg, D.; Pauwels, P.; Rolfo, C.; Baggerman, G. Proteomics in Cancer Research: Are We Ready for Clinical Practice? Crit. Rev. Oncol. Hematol. 2015, 96, 437–448. [Google Scholar] [CrossRef]
- Ahsan, H. Monoplex and Multiplex Immunoassays: Approval, Advancements, and Alternatives. Comp. Clin. Path 2022, 31, 333–345. [Google Scholar] [CrossRef]
- Shuken, S.R. An Introduction to Mass Spectrometry-Based Proteomics. J. Proteome Res. 2023, 22, 2151–2171. [Google Scholar] [CrossRef]
- Duarte, T.T.; Spencer, C.T. Personalized Proteomics: The Future of Precision Medicine. Proteomes 2016, 4, 29. [Google Scholar] [CrossRef]
- Frantzi, M.; Metzger, J.; Banks, R.E.; Husi, H.; Klein, J.; Dakna, M.; Mullen, W.; Cartledge, J.J.; Schanstra, J.P.; Brand, K.; et al. Discovery and Validation of Urinary Biomarkers for Detection of Renal Cell Carcinoma. J. Proteom. 2014, 98, 44–58. [Google Scholar] [CrossRef]
- Frantzi, M.; Van Kessel, K.E.; Zwarthoff, E.C.; Marquez, M.; Rava, M.; Malats, N.; Merseburger, A.S.; Katafigiotis, I.; Stravodimos, K.; Mullen, W.; et al. Development and Validation of Urine-Based Peptide Biomarker Panels for Detecting Bladder Cancer in a Multi-Center Study. Clin. Cancer Res. 2016, 22, 4077–4086. [Google Scholar] [CrossRef]
- Njoku, K.; Pierce, A.; Geary, B.; Campbell, A.E.; Kelsall, J.; Reed, R.; Armit, A.; Da Sylva, R.; Zhang, L.; Agnew, H.; et al. Quantitative SWATH-Based Proteomic Profiling of Urine for the Identification of Endometrial Cancer Biomarkers in Symptomatic Women. Br. J. Cancer 2023, 128, 1723–1732. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, X.; Ren, J.; Huang, S.; Xiao, Z.; Jiang, H.; Tan, Y. Quantitative Proteomic Analysis of Cervical Cancer Based on TMT-Labeled Quantitative Proteomics. J. Proteom. 2022, 252, 104453. [Google Scholar] [CrossRef] [PubMed]
- Whiteaker, J.R.; Lin, C.; Kennedy, J.; Hou, L.; Trute, M.; Sokal, I.; Yan, P.; Schoenherr, R.M.; Zhao, L.; Voytovich, U.J.; et al. A Targeted Proteomics-Based Pipeline for Verification of Biomarkers in Plasma. Nat. Biotechnol. 2011, 29, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Asonuma, K.; Kobayashi, T.; Kikkawa, N.; Nakano, M.; Sagami, S.; Morikubo, H.; Miyatani, Y.; Hojo, A.; Fukuda, T.; Hibi, T. Optimal Use of Serum Leucine-Rich Alpha-2 Glycoprotein as a Biomarker for Small Bowel Lesions of Crohn’s Disease. Inflamm. Intest. Dis. 2023, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.D.; Codrici, E.; Albulescu, L.; Mihai, S.; Enciu, A.M.; Albulescu, R.; Tanase, C.P. Potential Serum Biomarkers for Glioblastoma Diagnostic Assessed by Proteomic Approaches. Proteome Sci. 2014, 12, 47. [Google Scholar] [CrossRef]
- Salomon, B.; Sudhakar, P.; Bergemalm, D.; Andersson, E.; Grännö, O.; Carlson, M.; Hedin, C.R.H.; Söderholm, J.D.; Öhman, L.; Ungaro, R.C.; et al. Characterization of Inflammatory Bowel Disease Heterogeneity Using Serum Proteomics: A Multicenter Study. J. Crohns Colitis 2024, 19, jjae169. [Google Scholar] [CrossRef]
- Basso, D.; Padoan, A.; D’Incà, R.; Arrigoni, G.; Scapellato, M.L.; Contran, N.; Franchin, C.; Lorenzon, G.; Mescoli, C.; Moz, S.; et al. Peptidomic and Proteomic Analysis of Stool for Diagnosing IBD and Deciphering Disease Pathogenesis. Clin. Chem. Lab. Med. 2020, 58, 968–979. [Google Scholar] [CrossRef]
- Park, J.M.; Han, N.Y.; Han, Y.M.; Chung, M.K.; Lee, H.K.; Ko, K.H.; Kim, E.H.; Hahm, K.B. Predictive Proteomic Biomarkers for Inflammatory Bowel Disease-Associated Cancer: Where Are We Now in the Era of the next Generation Proteomics? World J. Gastroenterol. 2014, 20, 13466–13476. [Google Scholar] [CrossRef] [PubMed]
- Angriman, I.; Scarpa, M.; D’Incà, R.; Basso, D.; Ruffolo, C.; Polese, L.; Sturniolo, G.C.; D’Amico, D.F.; Plebani, M. Enzymes in Feces: Useful Markers of Chronic Inflammatory Bowel Disease. Clin. Chim. Acta 2007, 381, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Vaiopoulou, A.; Gazouli, M.; Theodoropoulos, G.; Zografos, G. Current Advantages in the Application of Proteomics in Inflammatory Bowel Disease. Dig. Dis. Sci. 2012, 57, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, N.; Kushima, R.; Vieth, M.; Mukaisho, K.I.; Kakinoki, R.; Okabe, H.; Borchard, F.; Stolte, M.; Okanoue, T.; Hattori, T. Cytokeratin 7/20 and Mucin Core Protein Expression in Ulcerative Colitis-Associated Colorectal Neoplasms. Virchows Arch. 2006, 448, 756–762. [Google Scholar] [CrossRef]
- Hashimoto, K.; Saigusa, S.; Araki, T.; Tanaka, K.; Okita, Y.; Fujikawa, H.; Kawamura, M.; Okugawa, Y.; Toiyama, Y.; Inoue, Y.; et al. Correlation of CCL20 Expression in Rectal Mucosa with the Development of Ulcerative Colitis-Associated Neoplasia. Oncol. Lett. 2013, 6, 1271–1276. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, D.K.; Park, H.J.; Oh, T.Y.; Kim, J.H.; Cho, S.W.; Paik, Y.K.; Nahm, K.B. Loss of Transgelin in Repeated Bouts of Ulcerative Colitis-Induced Colon Carcinogenesis. Proteomics 2006, 6, 1158–1165. [Google Scholar] [CrossRef]
- May, D.; Pan, S.; Crispin, D.A.; Lai, K.; Bronner, M.P.; Hogan, J.; Hockenbery, D.M.; McIntosh, M.; Brentnall, T.A.; Chen, R. Investigating Neoplastic Progression of Ulcerative Colitis with Label-Free Comparative Proteomics. J. Proteome Res. 2011, 10, 200–209. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and Cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Jewel Samadder, N.; Valentine, J.F.; Guthery, S.; Singh, H.; Bernstein, C.N.; Wan, Y.; Wong, J.; Boucher, K.; Pappas, L.; Rowe, K.; et al. Colorectal Cancer in Inflammatory Bowel Diseases: A Population-Based Study in Utah. Dig. Dis. Sci. 2017, 62, 2126–2132. [Google Scholar] [CrossRef]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The Risk of Colorectal Cancer in Ulcerative Colitis: A Meta-Analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Lepage, C.; Jooste, V.; Guéant, J.L.; Faivre, J.; Bouvier, A.M. Colorectal Cancer in Inflammatory Bowel Diseases: A Population-Based Study (1976–2008). Inflamm. Bowel Dis. 2012, 18, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Selinger, C.P.; Andrews, J.M.; Titman, A.; Norton, I.; Jones, D.B.; McDonald, C.; Barr, G.; Selby, W.; Leong, R.; Andrews, J.; et al. Long-Term Follow-up Reveals Low Incidence of Colorectal Cancer, but Frequent Need for Resection, Among Australian Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Sharma, S.; Mohamedali, A.; Mahboob, S.; Redmond, W.J.; Pascovici, D.; Wu, J.X.; Zaw, T.; Adhikari, S.; Vaibhav, V.; et al. Potential Early Clinical Stage Colorectal Cancer Diagnosis Using a Proteomics Blood Test Panel. Clin. Proteom. 2019, 16, 34. [Google Scholar] [CrossRef]
- Boehm, D.; Krzystek-Korpacka, M.; Neubauer, K.; Matusiewicz, M.; Berdowska, I.; Zielinski, B.; Paradowski, L.; Gamian, A. Paraoxonase-1 Status in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2009, 15, 93–99. [Google Scholar] [CrossRef]
- Devarajan, A.; Shih, D.; Reddy, S.T. Inflammation, Infection, Cancer 5 and All That…the Role of Paraoxonases. Adv. Exp. Med. Biol. 2014, 824, 33–41. [Google Scholar] [CrossRef]
- Tratenšek, A.; Locatelli, I.; Grabnar, I.; Drobne, D.; Vovk, T. Oxidative Stress-Related Biomarkers as Promising Indicators of Inflammatory Bowel Disease Activity: A Systematic Review and Meta-Analysis. Redox Biol. 2024, 77, 103380. [Google Scholar] [CrossRef]
- Contran, N.; Arrigoni, G.; Battisti, I.; D’Incà, R.; Angriman, I.; Franchin, C.; Scapellato, M.L.; Padoan, A.; Moz, S.; Aita, A.; et al. Colorectal Cancer and Inflammatory Bowel Diseases Share Common Salivary Proteomic Pathways. Sci. Rep. 2024, 14, 17711. [Google Scholar] [CrossRef]
- Chan, M.; Bennick, A. Proteolytic Processing of a Human Salivary Proline-Rich Protein Precursor by Proprotein Convertases. Eur. J. Biochem. 2001, 268, 3423–3431. [Google Scholar] [CrossRef]
- Bojesen, R.D.; Riis, L.B.; Høgdall, E.; Nielsen, O.H.; Jess, T. Inflammatory Bowel Disease and Small Bowel Cancer Risk, Clinical Characteristics, and Histopathology: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1900–1907.e2. [Google Scholar] [CrossRef]
- Lin, M.; Liu, J.; Zhang, F.; Qi, G.; Tao, S.; Fan, W.; Chen, M.; Ding, K.; Zhou, F. The Role of Leucine-Rich Alpha-2-Glycoprotein-1 in Proliferation, Migration, and Invasion of Tumors. J. Cancer Res. Clin. Oncol. 2022, 148, 283–291. [Google Scholar] [CrossRef]
- Shimoyama, T.; Yamamoto, T.; Yoshiyama, S.; Nishikawa, R.; Umegae, S. Leucine-Rich Alpha-2 Glycoprotein Is a Reliable Serum Biomarker for Evaluating Clinical and Endoscopic Disease Activity in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2023, 29, 1399–1408. [Google Scholar] [CrossRef]
- Huai, J.P.; Ding, J.; Ye, X.H.; Chen, Y.P. Inflammatory Bowel Disease and Risk of Cholangiocarcinoma: Evidence from a Meta-Analysis of Population-Based Studies. Asian Pac. J. Cancer Prev. 2014, 15, 3477–3482. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Refsum, E.; Helsingen, L.M.; Folseraas, T.; Ploner, A.; Wieszczy, P.; Barua, I.; Jodal, H.C.; Melum, E.; Løberg, M.; et al. Risk of Hepato-Pancreato-Biliary Cancer Is Increased by Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A Population-Based Cohort Study. United Eur. Gastroenterol. J. 2022, 10, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, L.; He, Q.; Wang, S.; Pan, Z.; Wang, T.; Zhao, Y. Diagnostic Accuracy of Serum CA19-9 in Patients with Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2015, 21, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, K.M.; Hosseinzadeh, Y.; Nourpanah, Z.; Shirazinezhad, A.M.; Nikniaz, Z. The Value of Serum CA19-9 in Predicting Primary Sclerosing Cholangitis in Patients with Ulcerative Colitis. Adv. Dig. Med. 2020, 7, 147–151. [Google Scholar] [CrossRef]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in Pancreatic Cancer: Biomarker, Predictor and Promoter. Biochim. Biophys Acta Rev Cancer 2021, 1875, 188409. [Google Scholar] [CrossRef]
- Shi, Y.; Deng, X.; Zhan, Q.; Shen, B.; Jin, X.; Zhu, Z.; Chen, H.; Li, H.; Peng, C. A Prospective Proteomic-Based Study for Identifying Potential Biomarkers for the Diagnosis of Cholangiocarcinoma. J. Gastrointest. Surg. 2013, 17, 1584–1591. [Google Scholar] [CrossRef]
- Ma, J.; Grant, C.E.; Plagens, R.N.; Barrett, L.N.; Guisbert, K.S.K.; Guisbert, E. Cellular Proteomes Drive Tissue-Specific Regulation of the Heat Shock Response. G3 Genes. Genomes Genet. 2017, 7, 1011–1018. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front Immunol 2018, 9, 1298. [Google Scholar] [CrossRef]
- Long, M.D.; Martin, C.F.; Pipkin, C.A.; Herfarth, H.H.; Sandler, R.S.; Kappelman, M.D. Risk of Melanoma and Nonmelanoma Skin Cancer among Patients with Inflammatory Bowel Disease. Gastroenterology 2012, 143, 390–399.e1. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Liu, Z.C.; Liao, W.X.; Wei, J.X.; Huang, X.W.; Yang, C.; Xia, Y.H.; Li, L.; Ye, C.; Dai, S.X. Risk of Skin Cancers in Thiopurines-Treated and Thiopurines-Untreated Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2019, 34, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.T.; Sandborn, W.J.; Colombel, J.F.; Robinson, A.M.; Lau, W.; Huang, B.; Pollack, P.F.; Thakkar, R.B.; Lewis, J.D. Increased Risk of Malignancy with Adalimumab Combination Therapy, Compared with Monotherapy, for Crohn’s Disease. Gastroenterology 2014, 146, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Berl, A.; Shir-Az, O.; Genish, I.; Biran, H.; Mann, D.; Singh, A.; Wise, J.; Kravtsov, V.; Kidron, D.; Golberg, A.; et al. Exploring Multisite Heterogeneity of Human Basal Cell Carcinoma Proteome and Transcriptome. PLoS ONE 2023, 18, e0293744. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, D.; Yang, J.; Hammock, B.D.; De Montellano, P.R.O. Catalytic Activities of Tumor-Specific Human Cytochrome P450 CYP2W1 toward Endogenous Substrates. Drug Metab. Dispos. 2016, 44, 771–780. [Google Scholar] [CrossRef]
- Wood, L.D.; Calhoun, E.S.; Silliman, N.; Ptak, J.; Szabo, S.; Powell, S.M.; Riggins, G.J.; Wang, T.L.; Yan, H.; Gazdar, A.; et al. Somatic Mutations of GUCY2F, EPHA3, and NTRK3 in Human Cancers. Hum. Mutat. 2006, 27, 1060–1061. [Google Scholar] [CrossRef]
- Beaugerie, L.; Brousse, N.; Marie Bouvier, A.; Frédéric Colombel, J.; Lémann, M.; Cosnes, J.; Hébuterne, X.; Cortot, A.; Bouhnik, Y.; Pierre Gendre, J.; et al. Lymphoproliferative Disorders in Patients Receiving Thiopurines for Infl Ammatory Bowel Disease: A Prospective Observational Cohort Study. Lancet 2009, 374, 1617–1625. [Google Scholar] [CrossRef]
- Khan, N.; Abbas, A.M.; Lichtenstein, G.R.; Loftus, E.V.; Bazzano, L.A. Risk of Lymphoma in Patients with Ulcerative Colitis Treated with Thiopurines: A Nationwide Retrospective Cohort Study. Gastroenterology 2013, 145, 1007–1015.e3. [Google Scholar] [CrossRef]
- Vos, A.C.W.; Bakkal, N.; Minnee, R.C.; Casparie, M.K.; De Jong, D.J.; Dijkstra, G.; Stokkers, P.; Van Bodegraven, A.A.; Pierik, M.; Van Der Woude, C.J.; et al. Risk of Malignant Lymphoma in Patients with Inflammatory Bowel Diseases: A Dutch Nationwide Study. Inflamm. Bowel Dis. 2011, 17, 1837–1845. [Google Scholar] [CrossRef]
- Marchesi, F.; Martin, A.P.; Thirunarayanan, N.; Devany, E.; Mayer, L.; Grisotto, M.G.; Furtado, G.C.; Lira, S.A. CXCL13 Expression in the Gut Promotes Accumulation of IL-22-Producing Lymphoid Tissue-Inducer Cells, and Formation of Isolated Lymphoid Follicles. Mucosal Immunol. 2009, 2, 486–494. [Google Scholar] [CrossRef]
- Spåth, F.; Wibom, C.; Krop, E.J.M.; Johansson, A.S.; Bergdahl, I.A.; Vermeulen, R.; Melin, B. Biomarker Dynamics in B-Cell Lymphoma: A Longitudinal Prospective Study of Plasma Samples up to 25 Years before Diagnosis. Cancer Res. 2017, 77, 1408–1415. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.; Ao, D.; Wei, X. CXCL13-CXCR5 Axis: Regulation in Inflammatory Diseases and Cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877. [Google Scholar] [CrossRef]
- Lapsia, S.; Koganti, S.; Spadaro, S.; Rajapakse, R.; Chawla, A.; Bhaduri-Mcintosh, S. Anti-TNFα Therapy for Inflammatory Bowel Diseases Is Associated with Epstein-Barr Virus Lytic Activation. J. Med. Virol. 2016, 88, 312–318. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The Microbiota-Gut-Brain Axis in Gastrointestinal Disorders: Stressed Bugs, Stressed Brain or Both? J. Physiol. 2014, 592, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.M.; Yasin, R.; Mohammad, I.S.; Fan, Y.; Li, H.; Shahzad, M.; Xu, J. The Gut-Brain-Axis: A Positive Relationship between Gut Microbial Dysbiosis and Glioblastoma Brain Tumour. Heliyon 2024, 10, e30494. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Luo, H.; Samii, A.; Etminan, M. The Risk of Glioblastoma with TNF Inhibitors. Pharmacotherapy 2016, 36, 449–454. [Google Scholar] [CrossRef]
- Tichy, J.; Spechtmeyer, S.; Mittelbronn, M.; Hattingen, E.; Rieger, J.; Senft, C.; Foerch, C. Prospective Evaluation of Serum Glial Fibrillary Acidic Protein (GFAP) as a Diagnostic Marker for Glioblastoma. J. Neurooncol. 2015, 126, 361–369. [Google Scholar] [CrossRef]
- Kimura, A.; Takemura, M.; Yamamoto, Y.; Hayashi, Y.; Saito, K.; Shimohata, T. Cytokines and Biological Markers in Autoimmune GFAP Astrocytopathy: The Potential Role for Pathogenesis and Therapeutic Implications. J. Neuroimmunol. 2019, 334, 576999. [Google Scholar] [CrossRef]
- Jun, J.; Gim, J.; Kim, Y.; Kim, H.; Yu, S.J.; Yeo, I.; Park, J.; Yoo, J.J.; Cho, Y.Y.; Lee, D.H.; et al. Analysis of Significant Protein Abundance from Multiple Reaction-Monitoring Data. BMC Syst. Biol. 2018, 12, 123. [Google Scholar] [CrossRef]

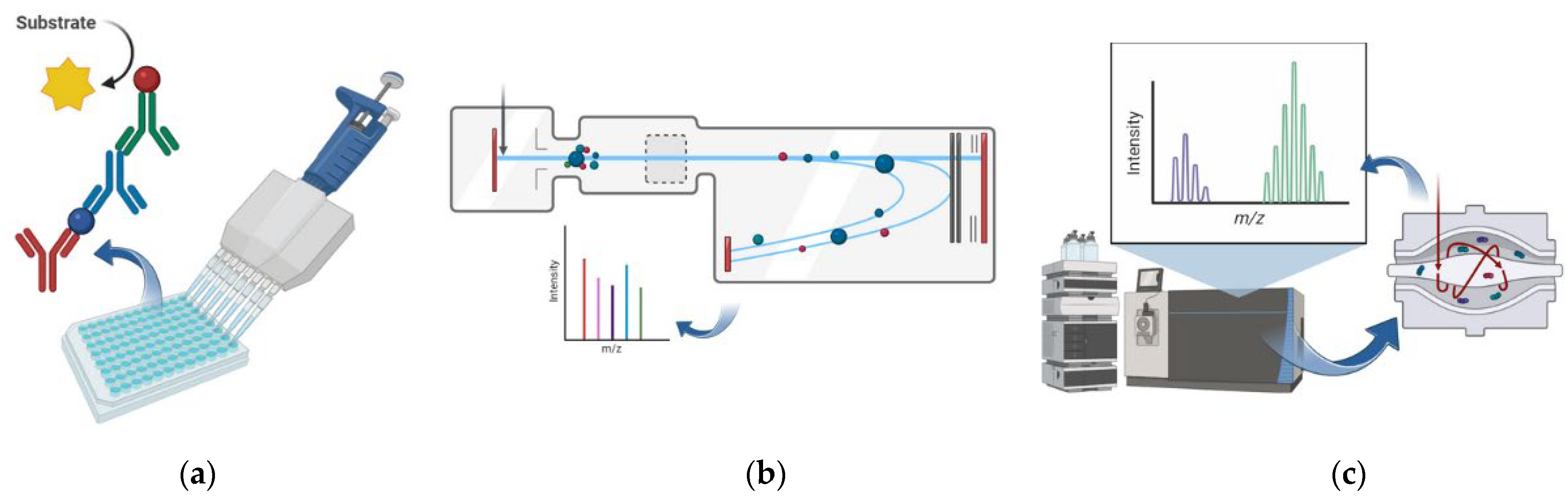
| Stool Examination | |
|---|---|
| Lactoferrin | Used to exclude intestinal inflammation, it is a negative diagnostic test |
| Calprotectin | Used to measure the activity of IBD |
| Blood Examination | |
|---|---|
| Erythrocyte sedimentation rate | Levels correlate with inflammation activity |
| C-reactive protein | Levels correlate with inflammation activity |
| Antibody tests (with ELISA) | Mostly for microbial agents that increase probability of IBD |
| UC: Highest Annual Incidence (per 100,000 Person-Years) | CD: Highest Annual Incidence (per 100,000 Person-Years) | Study Period | |
|---|---|---|---|
| Europe | 24.3 | 12.7 | 1930–2008 |
| Asia/Middle East | 6.3 | 5.0 | 1950–2008 |
| North America | 19.2 | 20.2 | 1920–2004 |
| Australia | 11.2 | 17.4 | 1967–2008 |
| Drug Class | Drug Compound | Mechanism of Action | Adverse Effects | Increased Cancer Risk |
|---|---|---|---|---|
| Amino salicylate | 5-Aminosalicylic acid (5-ASA) | Not fully understood. Possible inhibition of COX enzyme. | Nausea, vomiting, abdominal pain, tachypnea, hyperpnea, neurologic symptoms | No |
| Local corticosteroids | Budesonide | Binding to glucocorticoid receptor inhibits gene expression (i.e., NF-kB, IL-10) | Hypercorticism and adrenal axis suppression | No |
| Systemic corticosteroids | Prednisolone | Binding to glucocorticoid receptor inhibits gene expression (i.e., NF-kB, IL-10) | Gastrointestinal disturbances, insomnia, and restlessness | Not certain |
| Immuno- modulators | 6-mercaptopurine | Interferes with nucleic acid synthesis by inhibiting purine metabolism | Nausea, vomiting, and diarrhea; myelosuppression, liver dysfunction, gastroenteritis | Risk of developing skin cancer and leukemia under investigation |
| Azathioprine (prodrug of 6-mercaptopurine) | Immunosuppressive: modulation of rac1 induces T cell apoptosis | Bone marrow hypoplasia, bleeding, and infection, which may progress to death | Risk of developing skin cancer and lymphoma under investigation | |
| Methotrexate | Inhibition of cell division by inhibiting nucleotide synthesis | Nausea, vomiting, bone marrow suppression, gastrointestinal ulceration & bleeding | Not certain | |
| TNFα antagonists | Infliximab | Binding to TNFα: disruption of the proinflammatory cascade signaling. Can activate ADCC | Recurrent infections, hepatotoxicity, infusion reactions, allergic reactions | Risk of developing lymphoma and other tumors under investigation |
| Adalimumab | Binding to TNFα: disruption of the proinflammatory cascade signaling. Can activate ADCC | Skin rash, swelling, difficulty breathing or swallowing, dyspnea, allergic reactions | Risk of developing leukemia and other tumors under investigation | |
| Certolizumab | Binding to TNFα: disruption of the proinflammatory cascade signaling. Cannot activate ADCC | Recurrent infections, skin rash, fatigue, hepatotoxicity, allergic reactions | Risk of developing lymphoma and skin cancer under investigation | |
| IL-12 & IL-23 antagonists | Ustekinumab | Binding to IL-12 and IL-23 prevents receptor-mediated responses | Recurrent infections, allergic reactions, itching, diarrhea, nausea, fatigue, bleeding | No |
| Risankizumab | Binding to IL-23 inhibits the differentiation of Th17 cells, preventing inflammation | Recurrent infections, headache, itching, fatigue | No | |
| Integrin blockers | Vedolizumab | Binding to the α4β7 integrin prevents homing of T-lymphocytes to gut lymph tissue | Recurrent infections, rash, gastrointestinal symptoms (stypsis, anal abscess, etc.) | No |
| Janus kinase inhibitors | Upadacitinib | Inhibition of proinflammatory tyrosine-protein kinase JAK1 | Infections of upper airways, neutropenia, nausea, cough, hypercholesterolemia | Risk of developing non-melanoma skin cancer under investigation |
| Cancer | Incidence (New Cases of Cancer per 100,000 Men and Women per Year) | Death Rate (Deaths per 100,000 Men and Women per Year) | 5-Year Relative Survival (%) |
|---|---|---|---|
| Colorectal cancer | 37.1 | 12.9 | 65.4 |
| Small bowel cancer | 2.6 | 0.4 | 71.1 |
| Cholangiocarcinoma | 9.4 | 6.6 | 22 |
| Melanoma skin cancer | 21.9 | 2.0 | 94.7 |
| Non-Hodgkin lymphoma | 5.6 | 1.7 | 64.8 |
| Glioblastoma | 6.1 | 4.4 | 33 |
| Breast cancer | 130.8 | 19.2 | 91.7 |
| Lung cancer | 47.8 | 31.5 | 28.1 |
| Proteomic Technique | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| ELISA | High specificity; widely used in diagnostics; cost-effective; easy to automate | Limited to known targets; not suitable for biomarker discovery | [30] |
| LATEX TURBIDIMETRIC ASSAY | Rapid and inexpensive; good for routine diagnostics | Low sensitivity and specificity; limited to known proteins | [38] |
| Orbitrap MS | High resolution and mass accuracy; excellent for biomarker discovery | High cost; requires expert handling; lower throughput than some other MS types | [31] |
| MALDI-TOF MS | High-throughput; low cost per sample; rapid analysis | Requires pure samples; less effective for complex mixtures | [31] |
| ESI-TOF MS | High sensitivity; good for complex mixtures and quantitative analysis | More expensive than MALDI; lower throughput | [31] |
| SELDI-TOF MS | Suitable for biomarker profiling in diagnostics | Lower resolution and reproducibility; limited to surface-bound proteins | [39] |
| SWATH-MS | High reproducibility; quantitative; ideal for biomarker discovery; high-throughput | Requires spectral libraries; complex data analysis; high cost | [31] |
| Cancer | Potential Biomarker | Biological Matrix | Technique of Detection | Already Used in Diagnostics | Ref. |
|---|---|---|---|---|---|
| Colorectal Cancer | PON1 | Blood serum | SWATH-MS | No | [54] |
| PRB1 (fragment GG-17) | Feces | Orbitrap MS | No | [58] | |
| Small Bowel Cancer | LRG | Blood serum | Latex turbidimetric immunoassay | Yes | [38] |
| Cholangiocarcinoma | CA19-9 | Blood serum | ELISA | Yes | [65,66] |
| CCTγ and S100A9 | Liver biopsy | MALDI-TOF | No | [68] | |
| Skin Cancer (nodular BCC subtype) | CYP2W1 and NTRK3 | Skin biopsy | Mass Spectrometry | No | [74] |
| Lymphoma | CXCL13 | Blood | ELISA | No | [80] |
| EBV-related lymphoma | ZEBRA and RTA | Blood | PCR | No | [83] |
| Glioblastoma | GFAP | Blood serum | ELISA | No | [87] |
| S100A8/A9 | Blood serum | SELDI-TOF | No | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saccon, T.; Bergamo, M.; Franchin, C. Proteomics Approaches for Discovering Novel Protein Biomarkers in Inflammatory Bowel Disease-Related Cancer. Biomolecules 2025, 15, 1328. https://doi.org/10.3390/biom15091328
Saccon T, Bergamo M, Franchin C. Proteomics Approaches for Discovering Novel Protein Biomarkers in Inflammatory Bowel Disease-Related Cancer. Biomolecules. 2025; 15(9):1328. https://doi.org/10.3390/biom15091328
Chicago/Turabian StyleSaccon, Tommaso, Matilde Bergamo, and Cinzia Franchin. 2025. "Proteomics Approaches for Discovering Novel Protein Biomarkers in Inflammatory Bowel Disease-Related Cancer" Biomolecules 15, no. 9: 1328. https://doi.org/10.3390/biom15091328
APA StyleSaccon, T., Bergamo, M., & Franchin, C. (2025). Proteomics Approaches for Discovering Novel Protein Biomarkers in Inflammatory Bowel Disease-Related Cancer. Biomolecules, 15(9), 1328. https://doi.org/10.3390/biom15091328






