The RNA Splicing Response to DNA Damage
Abstract
:1. Introduction
1.1. The DNA Damage Response

1.2. Splicing and Alternative Splicing


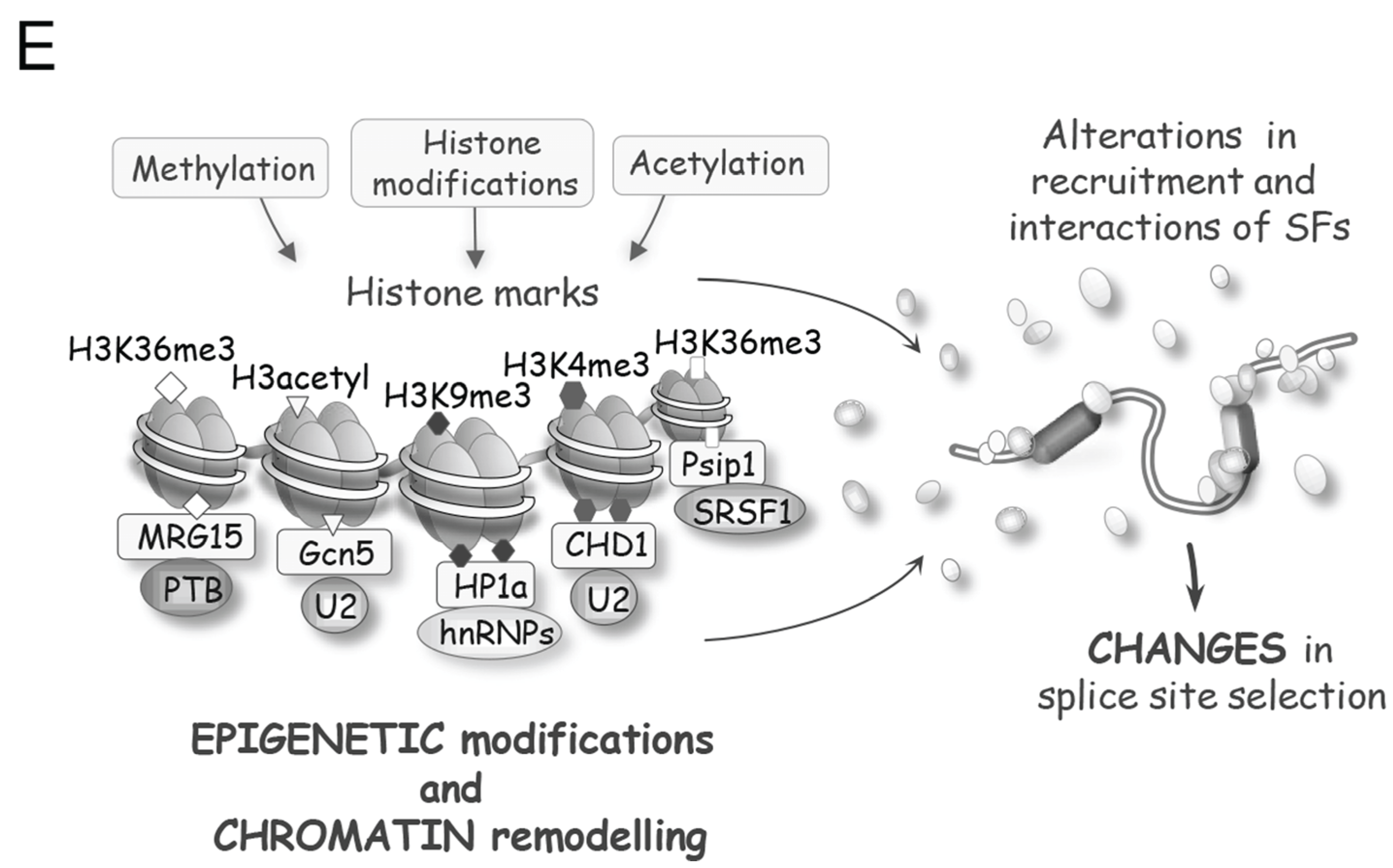
2. DNA Damage Modifies Splicing Proteins
2.1. PARylation
2.2. Arginine Methylation
2.3. Acetylation

2.4. Ubiquitylation and Sumoylation
2.5. Phosphorylation
3. The Depletion of Splicing Factors Causes DNA Damage

4. Exclusion and Recruitment of Splicing Factors at Sites of Damage
5. DNA Damage Relocalizes Splicing Factors
6. DNA Damage Alters the Expression of Splicing Factors
7. Impact of DNA Damage on Constitutive Splicing
8. DNA Damage Alters Transcription-Coupled Splicing Decisions
- •
- UV treatment of Hep3B and HCT116 cells changes the phosphorylation of the CTD of RNA polymerase II to affect transcription elongation and alternative splicing in an ATM/ATR-independent manner [79]. On the other hand, and as discussed above, lesions created by UV block transcription leading to the dissociation of late-stage spliceosomes [103].
- •
- SR proteins regulate splicing decisions by interacting directly with exon and intron sequence elements on pre-mRNAs [30,31,187]. More recently however, SRSF2 was implicated in the release of the transcription elongation factor P-TEFb from the repressor 7SK RNA at paused transcription sites [188]. A similar function for hnRNP A1/A2 in the release of P-TEFb and transcription elongation has been reported [189]. Since DNA damage promotes changes in the level of SRSF2 and in the localization of hnRNP A1 [56,146], splicing alterations may possibly occur through P-TEFb-mediated effects on transcription elongation.
- •
- The RNA polymerase II-associated protein EWS confers resistance to IR and UV light [78,190], and mice lacking EWS are hypersensitive to IR [123]. These phenotypes may be due, in part, by the fact that a deficiency in EWS affects the alternative splicing of cyclin D1 [191], Fas [192], Mdm2 [162], and the DNA repair genes Abl1, Chek2 and Map4k2 [78]. EWS interacts with spliceosome components, including the U1 snRNP protein U1C [193], the branch site protein SF1 [194] and YB-1 [162,195]. UV decreases the association of EWS with target RNAs [78]. Camptothecin impairs the interaction between EWS and YB-1, possibly affecting spliceosome assembly to provoke exon skipping in Mdm2 and other genes [162]. Although camptothecin treatment leads to hyperphosphorylation of the CTD of RNA polymerase II [162,196], the transcription elongation inhibitor DRB prevents camptothecin-mediated polymerase II phosphorylation, but not the impact of camptothecin on splicing, suggesting that a change in transcription elongation is not responsible for the observed shift in Mdm2 splicing. While the impact of camptothecin on Mdm2 splicing is independent of p53 [162], it is unclear how it promotes a loss of interaction between EWS and YB-1. UV irradiation partially relocalizes EWS to the nucleolus, but this effect is not seen with the TOP2 inhibitor etoposide [78].
- •
- The epigenetic histone mark H3K36me3 is required to recruit the mismatch repair machinery and for HR-mediated repair [197,198]. H3K36me3 is elevated in nucleosomes residing on exons relative to those found in introns [199,200,201,202], and a splicing inhibitor or splice site mutations alters the deposition of the H3K36me3 mark [203]. The preferential association of H3K36me3 with coding sequences may therefore be used to prioritize the repair of coding portions of the genome. Notably, the chromatin-associated protein PSIP1 interacts with H3K36me3 and with several splicing regulators including SRSF1, SRSF2, SRSF10, hnRNP proteins and snRNP helicases [204]. Moreover, silencing the expression of the short splice variant of PSIP1 changes the localization of SRSF1 and impacts alternative splicing [204]. DNA damage leads to the phosphorylation of PSIP1 [64], but it is not known if this alters the interaction of PSIP with H3K36me3 or with splicing regulators to affect splicing. Interestingly, the splicing factor protein SF3B1, whose expression is altered by DNA damage and whose activity is linked to DNA repair, preferentially associates with nucleosomes residing on exons to modulate splicing [205]. If DNA damage promotes the dissociation of splicing components from exon-specific nucleosomes, this could represent a strategy for the unobstructed sensing of local damage and the recruitment of the repair machinery.
- •
- Several large non-coding RNAs (lncRNAs) provide binding platforms for splicing regulators [206,207] and factors that modify chromatin to alter splice site selection [208]. DNA damage affects the transcription of the lncRNAs TUG1, Panda and lincRNA-p21 [209,210]. Panda sequesters transcription factors induced by DNA damage and prevents apoptosis when cells are treated with doxorubicin [211]. LincRNA-p21 recruits hnRNP K to control the transcription of p53-dependent genes [212], and enhances sensitivity to radiotherapy [210]. Whether the interaction of hnRNP K with lincRNA-p21 is affected by DNA damage to impact hnRNP K-mediated splicing events is not yet known.
9. DNA Damage Modulates the Alternative Splicing of Genes Involved in the DDR
| Treatment | Cell Line | Affected Gene | PMID Number |
|---|---|---|---|
| 5-Aza dC | MCF-7 | FN1, SYNE2 | 25313066 |
| Aclarubicin | SMA fibroblasts | SMN2 | 11734549 |
| Amsacrine | U-937 | CASP2 | 14757846 |
| Arsenic (III) chloride | BEAS-2B | GADD45 | 18942077 |
| Arsenite | AGS | CD44, SFRS10 (TRA2B) | 19439532 |
| Bleomycin (BLM) | HLE, HLF | FIR | 24811221 |
| BN80927 (TOP1, TOP2 inhibitor) | U-937 | CASP2 | 14757846 |
| Cadmium dichloride | RKO, EB-1 | PA26 | 9926927 |
| Camptothecin | HCT116 | AASDHPPT, APTX, BAT1, CASP2, CCT2, DDX17, EIF2S2, PNN, PPFIA1, PSMD12, RBM8A, RIOK1, RTN4, SF1, TCP1, WHSC1, ZRANB2 | 20817775 |
| HeLa, U-937 | CASP2 | 14757846, 18166155 | |
| MCF-7 | CDC25C, FN1, SYNE2, HRAS, CHD2, EED, KIAA0232, MDM2, PAPOLG, RC3H2, THUMPD2, ZCCHC8, VEGF-A, RBM8A, SF1 | 22871320, 25313066, 17709397, 20972445, 18086921, 20817775 | |
| A431 | FOS (generic splicing) | 16921380 | |
| Jurkat T lymphoma | HNRPDL, IVNS1ABP, SF3B3, RUNX1, HMGXb4, SNRPB, SRSF2 | 21163941 | |
| HaCat, MDA-MB-231 | VEGF-A | 18086921 | |
| Capecitabine | MCF-7, HeLa S3, PA-1 | BCL2L1 (Bcl-x) | 18566212 |
| Carboplatin | RTC | BCL2L1 (Bcl-x) | 21198546 |
| Chlorambucil | EcR293 | BCL2L1 (Bcl-x) | 18566212 |
| Cisplatin | SH-SY5Y | APAF1, H-RAS | 23613995 |
| Hep3B | BCL2L1 (Bcl-x) | 19450518 | |
| EcR293, MCF-7, HeLa S3, PC3, PA-1, SKOV-3 | BCL2L1 (Bcl-x) | 18566212 | |
| HT1080 | BCL2L1 (Bcl-x), PIG3, Smac/DIABLO, MDM2 | 21327085, 25884497 | |
| H358 | CASP8 | 21157427 | |
| MCF-7 | CDC25C, MDM4, MAGOH, AMZ2, CSDE1, EIF4A2, MDM2, MTA1, NFE2L1, STRAP, TMPO, VEGF, MDM2 | 22871320, 18711402, 25884497, 25845590, 17018606 | |
| AT5BIVA, MO59J | HNRNPDL | 25884497 | |
| HeLa S3, BT549, HDF1, MDA-MB-231, MG-63, MSU, RD, U2OS | MDM2 | 25845590, 25884497, 17018606 | |
| H1299 | MDM2, MDM4 | 17018606, 18711402 | |
| Ishikawa | MDM2, VEGF | 25884497 | |
| HCT116, IMR90 | MDM4 | 18711402 | |
| Cyclohexamide | U937 | CASP2, FAS | 15746654, 16131458 |
| Cyclophosphamide | SKOV3 | BCL2L1 (Bcl-x) | 18566212 |
| H358 | BCL2L1 (Bcl-x), CASP9 | 18806759 | |
| Cytarabine | PC3 | BCL2L1 (Bcl-x) | 18566212 |
| Dacarbazine | EcR293 | BCL2L1 (Bcl-x) | 18566212 |
| Dactinomycin | EcR293, MCF-7, HeLa S3, PC3 | BCL2L1 (Bcl-x) | 18566212 |
| NIH3T3 | MDM2 | 18469520 | |
| Daunorubicin | MCF-7, PC3, PA1 | BCL2L1 (Bcl-x) | 18566212 |
| Diflomotecan | U-937 | CASP2 | 14757846 |
| Docetacel | HeLa S3 | BCL2L1 (Bcl-x) | 18566212 |
| Doxorubicin | EU-3 | BIRC5 | 15334064 |
| U-937, HCT116 | CASP2 | 14757846, 20817775 | |
| MDA-MB-231 | CDC25C | 22871320 | |
| MCF-7 | CDC25C, PPM1D | 2287132, 18845566 | |
| NIH3T3 | MDM2 | 18469520 | |
| EB-1, RKO | PA26 | 9926927 | |
| T47D | PPM1D | 18845566 | |
| Epirubicin | EcR293, MCF-7, HeLa S3, PC3 | BCL2L1 (Bcl-x) | 18566212 |
| HeLa, HL-60 | CASP2 | 14757846, 12169392 | |
| U937 | CASP2, FAS | 15746654, 16131458, 12169392, 14757846 | |
| MCF-7 | CDC25C | 22871320 | |
| U2OS | NOXA, GADD45 (generic splicing) | 21460037 | |
| Fluorouracil (5FU) | EcR293 | BCL2L1 (Bcl-x) | 18566212 |
| Gemcitabine | A549 | BCL2L1 (Bcl-x), CASP9 | 11801602 |
| EcR293, MCF-7, PA-1, SKOV3, MiaPaCa2, PT45P1 | BCL2L1 (Bcl-x), MKNK2 | 18566212, 22797067 | |
| H2O2 | Saos2 | ATF-3 | 12034827 |
| HCT116 | SRSF3 | 24284797 | |
| IDC92 (Indole derivative) | MDA-MB-435S | RON | 20864806 |
| Indolocarbazole derivative (NB-506) | P388 | BCL2L1 (Bcl-x), CD44, SC35, STY | 11559564 |
| Ionizing radiation (IR) | MCF-7 | BCL2L1 (Bcl-x), CLU | 16465415, 15530543 |
| SH-SY5Y | APAF1, H-RAS | 23613995 | |
| LCL lymphoblastoid | ASPM, FBXW7, GADD45G, MDM2, VWCE | 22039421 | |
| Ionizing radiation (IR) | Primary fibroblasts | ATF-2 | 12833146 |
| PBMCs | NBS1 | 18582154 | |
| NF AG1519 | RAD17 | 11602352 | |
| Irinotecan | U-937 | CASP2 | 14757846 |
| L-mimosin | MCF-7 | VEGF-A | 18086921 |
| Methotrexate | EcR293, MCF-7, HeLa S3 | BCL2L1 (Bcl-x) | 18566212 |
| Mitomycin C | PC3, U2OS, HTC116 | CD44, KSR1, IL24 | 20110258, 17699766 |
| U2OS | FAS | 18571879 | |
| MCF-7, OVCAR3, SKOV3 | VEGF-A | 18086921, 25990504 | |
| Mitoxantrone | U-937 | CASP2 | 14757846 |
| Oxaliplatin | EcR293, MCF-7, HeLa S3, PC3, PA-1, SKOV-3 | BCL2L1 (Bcl-x) | 18566212, 20980256 |
| MCF-7 | MDM2 | 25884497 | |
| Paclitaxel | U937 | CASP2, FAS | 15746654, 16131458 |
| Paraquat | SH-SY5Y | APAF1, BIN1, CASP9, CHN1, ERRC1, GNAO1, H-RAS, LMO3, NRG1, SKP2, SMN1, RPRD1A | 23613995, 21120952 |
| Sodium arsenite | HeLa | ABCG2, MGP, NCAM2 | 25879800 |
| HCT116 | SFRS10, SRSF3 | 24865968, 24284797 | |
| TAS-103 (TOP1, TOP2 inhibitor) | U-937 | CASP2 | 14757846 |
| Topotecan | EcR293, PC3 | BCL2L1 (Bcl-x) | 18566212 |
| UV irradiation | HeLa | ABL1, CHEK2, MAP4K2, MDM2, PIG3 | 21816343, 25845590, 18801469 |
| HT1080 | BCL2L1 (Bcl-x), PIG3, Smac/DIABLO | 21327085 | |
| Human skin | ELN | 19054052 | |
| H1299 | MDM2, MDM4 | 17018606 | |
| MCF-7 | MDM2, MDM4, VEGF-A, PIG3 | 25845590, 17018606, 18086921, 18801469 | |
| MRC-5V1 | SRSF1 | 21984412 | |
| UV-B irradiation | HaCat | VEGF-A | 18086921 |
| MDA-MB-231 | VEGF-A | 18086921 | |
| UV-C irradiation | HeLa | ADAR2, DDO | 15728250 |
| Hep3B | BCL2L1 (Bcl-x), CASP9 | 19450518 |
| Treatment | Cell Line | Reference for Web Link | Source | PMID Number |
|---|---|---|---|---|
| Twenty chemotherapeutic drugs | EcR293, MCF-7, HeLa, PC3, PA-1, SKOV-3 | [230] | Supplementary Information | 18566212 |
| Camptothecin | HCT116 | [231] | Table S3 | 20817775 |
| Camptothecin | Jurkat T lymphoma | [232] | Table S2 | 21163941 |
| Camptothecin | MCF-7 | [233] | Table S1 | 20972445 |
| Cisplatin | MCF-7 | [234] | File S4 | 25884497 |
| Ionizing radiation (IR) | Lymphoblastoid cell lines, Primary fibroblasts | [235] | Table S8, Table S11 | 22039421 |
| Sodium arsenite | HeLa | [236] | File S12, Table 6 | 25879800 |
| UV-C irradiation | Hep3B | [237] | Table S2 | 19450518 |
| UV-irradiation | Human dermal fibroblasts | [238] | Table S2 | 26106861 |
| UV-B irradiation | Several | [239] | Table 2 | 18086921 |
| GO Biological Process Complete | Number of Genes | Fold Enrichment | p Value |
|---|---|---|---|
| DNA repair | 94 | 2.29 | 4.04E−09 |
| cell cycle checkpoint | 58 | 2.4 | 2.09E−05 |
| cell cycle phase transition | 64 | 2.18 | 1.19E−04 |
| mitotic cell cycle phase transition | 63 | 2.18 | 1.70E−04 |
| mitotic cell cycle phase | 57 | 2.05 | 5.09E−04 |
| mitotic cell cycle process | 138 | 2.04 | 1.00E−10 |
| cell cycle phase | 57 | 2.04 | 6.26E−04 |
| mitotic cell cycle | 51 | 2.03 | 6.87E−12 |
| intrinsic apoptotic signaling pathway | 41 | 2.49 | 1.72E−04 |
| apoptotic signaling pathway | 75 | 2.25 | 1.85E−06 |
| RNA splicing | 93 | 2.92 | 3.69E−15 |
| RNA splicing, via transesterification reactions | 68 | 3.19 | 3.00E−12 |
| mRNA splicing, via spliceosome | 67 | 3.19 | 5.26E−12 |
| chromatin modification | 117 | 2.42 | 1.29E−13 |
| covalent chromatin modification | 75 | 2.55 | 6.99E−09 |
| chromatin organization | 123 | 2.1 | 4.28E−10 |
| chromosome organization | 167 | 2.04 | 1.31E−13 |
| histone modification | 74 | 2.54 | 1.18E−08 |
| regulation of gene expression, epigenetic | 49 | 2.39 | 4.28E−04 |
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lenzken, S.C.; Loffreda, A.; Barabino, S.M. RNA splicing: A new player in the DNA damage response. Int. J. Cell Biol. 2013. [Google Scholar] [CrossRef]
- Dutertre, M.; Lambert, S.; Carreira, A.; Amor-Gueret, M.; Vagner, S. DNA damage: RNA-binding proteins protect from near and far. Trends Biochem. Sci. 2014, 39, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Busa, R.; Sette, C. An emerging role for nuclear RNA-mediated responses to genotoxic stress. RNA Biol. 2010, 7, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Naro, C.; Bielli, P.; Pagliarini, V.; Sette, C. The interplay between DNA damage response and RNA processing: The unexpected role of splicing factors as gatekeepers of genome stability. Front. Genet. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, M.; Sanchez, G.; Barbier, J.; Corcos, L.; Auboeuf, D. The emerging role of pre-messenger RNA splicing in stress responses: Sending alternative messages and silent messengers. RNA Biol. 2011, 8, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes Dev. 2011, 25, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, E.; Loewer, A.; Lahav, G. The ups and downs of p53: Understanding protein dynamics in single cells. Nat. Rev. Cancer 2009, 9, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Boucas, J.; Riabinska, A.; Jokic, M.; Herter-Sprie, G.S.; Chen, S.; Hopker, K.; Reinhardt, H.C. Posttranscriptional regulation of gene expression-adding another layer of complexity to the DNA damage response. Front. Genet. 2012. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiao, X.; Wang, C.; Shirley, L.A.; Elsaleh, H.; Dahl, O.; Wang, M.; Soutoglou, E.; Knudsen, E.S.; Pestell, R.G. Alternative cyclin D1 splice forms differentially regulate the DNA damage response. Cancer Res. 2010, 70, 8802–8811. [Google Scholar] [CrossRef] [PubMed]
- Boise, L.H.; Gonzalez-Garcia, M.; Postema, C.E.; Ding, L.; Lindsten, T.; Turka, L.A.; Mao, X.; Nunez, G.; Thompson, C.B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993, 74, 597–608. [Google Scholar] [CrossRef]
- Munding, E.M.; Shiue, L.; Katzman, S.; Donohue, J.P.; Ares, M., Jr. Competition between pre-mRNAs for the splicing machinery drives global regulation of splicing. Mol. Cell 2013, 51, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Jurica, M.S.; Moore, M.J. Pre-mRNA splicing: Awash in a sea of proteins. Mol. Cell 2003, 12, 5–14. [Google Scholar] [CrossRef]
- Wahl, M.C.; Will, C.L.; Luhrmann, R. The spliceosome: Design principles of a dynamic RNP machine. Cell 2009, 136, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Matera, A.G.; Wang, Z. A day in the life of the spliceosome. Nat. Rev. Mol. Cell Biol. 2014, 15, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.C.; Luhrmann, R. SnapShot: Spliceosome dynamics II. Cell 2015, 162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wahl, M.C.; Luhrmann, R. SnapShot: Spliceosome Dynamics I. Cell 2015, 161. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Steitz, J.A. Splicing double: Insights from the second spliceosome. Nat. Rev. Mol. Cell Biol. 2003, 4, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Dufu, K.; Romney, B.; Feldt, M.; Elenko, M.; Reed, R. Functional coupling of RNAP II transcription to spliceosome assembly. Genes Dev. 2006, 20, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.L. Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 2014, 15, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, M.; Long, M. Intron-exon structures of eukaryotic model organisms. Nucleic Acids Res. 1999, 27, 3219–3228. [Google Scholar] [PubMed]
- Schmucker, D.; Clemens, J.C.; Shu, H.; Worby, C.A.; Xiao, J.; Muda, M.; Dixon, J.E.; Zipursky, S.L. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell 2000, 101, 671–684. [Google Scholar] [CrossRef]
- Kelemen, O.; Convertini, P.; Zhang, Z.; Wen, Y.; Shen, M.; Falaleeva, M.; Stamm, S. Function of alternative splicing. Gene 2013, 514, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, B.J. Alternative splicing: New insights from global analyses. Cell 2006, 126, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Shkreta, L.; Bell, B.; Revil, T.; Venables, J.P.; Prinos, P.; Elela, S.A.; Chabot, B. Cancer-associated perturbations in alternative pre-messenger RNA splicing. In RNA and Cancer; Wu, J., Ed.; Springer Berlin: Heidelberg, Germany, 2013; pp. 41–94. [Google Scholar]
- Zhang, J.; Manley, J.L. Misregulation of pre-mRNA alternative splicing in cancer. Cancer Discov. 2013, 3, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Tazi, J.; Bakkour, N.; Stamm, S. Alternative splicing and disease. Biochim. Biophys. Acta 2009, 1792, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.D.; Ares, M., Jr. Context-dependent control of alternative splicing by RNA-binding proteins. Nat. Rev. Genet. 2014, 15, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Rio, D.C. Mechanisms and regulation of alternative pre-mRNA splicing. Ann. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.L.; Green, M.R. Pre-mRNA splicing of IgM exons M1 and M2 is directed by a juxtaposed splicing enhancer and inhibitor. Genes Dev. 1999, 13, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mayeda, A.; Krainer, A.R. Exon identity established through differential antagonism between exonic splicing silencer-bound hnRNP A1 and enhancer-bound SR proteins. Mol. Cell 2001, 8, 1351–1361. [Google Scholar] [CrossRef]
- Izquierdo, J.M.; Majos, N.; Bonnal, S.; Martinez, C.; Castelo, R.; Guigo, R.; Bilbao, D.; Valcarcel, J. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell 2005, 19, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Sebestyen, E.; Zawisza, M.; Eyras, E. Detection of recurrent alternative splicing switches in tumor samples reveals novel signatures of cancer. Nucleic Acids Res. 2015, 43, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Chiou, N.T.; Shankarling, G.; Lynch, K.W. hnRNP L and hnRNP A1 induce extended U1 snRNA interactions with an exon to repress spliceosome assembly. Mol. Cell 2013, 49, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Kanopka, A.; Muhlemann, O.; Akusjarvi, G. Inhibition by SR proteins of splicing of a regulated adenovirus pre-mRNA. Nature 1996, 381, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Contreras, R.; Fisette, J.F.; Nasim, F.U.; Madden, R.; Cordeau, M.; Chabot, B. Intronic binding sites for hnRNP A/B and hnRNP F/H proteins stimulate pre-mRNA splicing. PLoS Biol. 2006, 4, e21. [Google Scholar] [CrossRef] [PubMed]
- Naftelberg, S.; Schor, I.E.; Ast, G.; Kornblihtt, A.R. Regulation of alternative splicing through coupling with transcription and chromatin structure. Ann. Rev. Biochem. 2015, 84, 165–198. [Google Scholar] [CrossRef] [PubMed]
- D’Amours, D.; Desnoyers, S.; D’Silva, I.; Poirier, G.G. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 1999, 342, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Kraus, W.L. On PAR with PARP: Cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012, 26, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Haince, J.F.; McDonald, D.; Rodrigue, A.; Dery, U.; Masson, J.Y.; Hendzel, M.J.; Poirier, G.G. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J. Biol. Chem. 2008, 283, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Krietsch, J.; Caron, M.C.; Gagne, J.P.; Ethier, C.; Vignard, J.; Vincent, M.; Rouleau, M.; Hendzel, M.J.; Poirier, G.G.; Masson, J.Y. PARP activation regulates the RNA-binding protein NONO in the DNA damage response to DNA double-strand breaks. Nucleic Acids Res. 2012, 40, 10287–10301. [Google Scholar] [CrossRef] [PubMed]
- Hottiger, M.O. SnapShot: ADP-ribosylation signaling. Mol. Cell 2015. [Google Scholar] [CrossRef] [PubMed]
- Malanga, M.; Czubaty, A.; Girstun, A.; Staron, K.; Althaus, F.R. Poly(ADP-ribose) binds to the splicing factor ASF/SF2 and regulates its phosphorylation by DNA topoisomerase I. J. Biol. Chem. 2008, 283, 19991–19998. [Google Scholar] [CrossRef] [PubMed]
- Gagne, J.P.; Hunter, J.M.; Labrecque, B.; Chabot, B.; Poirier, G.G. A proteomic approach to the identification of heterogeneous nuclear ribonucleoproteins as a new family of poly(ADP-ribose)-binding proteins. Biochem. J. 2003, 371, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Tulin, A.V. Post-transcriptional regulation by poly(ADP-ribosyl)ation of the RNA-binding proteins. Int. J. Mol. Sci. 2013, 14, 16168–16183. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Tulin, A.V. Poly(ADP-ribosyl)ation of heterogeneous nuclear ribonucleoproteins modulates splicing. Nucleic Acids Res. 2009, 37, 3501–3513. [Google Scholar] [CrossRef] [PubMed]
- Jungmichel, S.; Rosenthal, F.; Altmeyer, M.; Lukas, J.; Hottiger, M.O.; Nielsen, M.L. Proteome-wide identification of poly(ADP-Ribosyl)ation targets in different genotoxic stress responses. Mol. Cell 2013, 52, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Beli, P.; Lukashchuk, N.; Wagner, S.A.; Weinert, B.T.; Olsen, J.V.; Baskcomb, L.; Mann, M.; Jackson, S.P.; Choudhary, C. Proteomic investigations reveal a role for RNA processing factor THRAP3 in the DNA damage response. Mol. Cell 2012, 46, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Richard, S. Arginine methylation an emerging regulator of protein function. Mol. Cell 2005, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Chiou, Y.Y.; Fu, S.L.; Shih, I.Y.; Weng, T.H.; Lin, W.J.; Lin, C.H. Arginine methylation of hnRNPK negatively modulates apoptosis upon DNA damage through local regulation of phosphorylation. Nucleic Acids Res. 2014, 42, 9908–9924. [Google Scholar] [CrossRef] [PubMed]
- Revil, T.; Pelletier, J.; Toutant, J.; Cloutier, A.; Chabot, B. Heterogeneous nuclear ribonucleoprotein K represses the production of pro-apoptotic Bcl-xS splice isoform. J. Biol. Chem. 2009, 284, 21458–21467. [Google Scholar] [PubMed]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Nakka, K.K.; Chaudhary, N.; Joshi, S.; Bhat, J.; Singh, K.; Chatterjee, S.; Malhotra, R.; De, A.; Santra, M.K.; Dilworth, F.J.; et al. Nuclear matrix-associated protein SMAR1 regulates alternative splicing via HDAC6-mediated deacetylation of Sam68. Proc. Natl. Acad. Sci. USA 2015, 112, E3374–E3383. [Google Scholar] [CrossRef] [PubMed]
- Edmond, V.; Moysan, E.; Khochbin, S.; Matthias, P.; Brambilla, C.; Brambilla, E.; Gazzeri, S.; Eymin, B. Acetylation and phosphorylation of SRSF2 control cell fate decision in response to cisplatin. EMBO J. 2011, 30, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Bennetzen, M.V.; Larsen, D.H.; Dinant, C.; Watanabe, S.; Bartek, J.; Lukas, J.; Andersen, J.S. Acetylation dynamics of human nuclear proteins during the ionizing radiation-induced DNA damage response. Cell Cycle 2013, 12, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Durocher, D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol. Cell 2013, 49, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.Y.; Yu, Y.; Shen, Z.; Beck, W.T. Nucleolar delocalization of human topoisomerase I in response to topotecan correlates with sumoylation of the protein. J. Biol. Chem. 2002, 277, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, M.T.; Matunis, M.J. SUMO modification of heterogeneous nuclear ribonucleoproteins. Mol. Cell. Biol. 2004, 24, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Pelisch, F.; Gerez, J.; Druker, J.; Schor, I.E.; Munoz, M.J.; Risso, G.; Petrillo, E.; Westman, B.J.; Lamond, A.I.; Arzt, E.; et al. The serine/arginine-rich protein SF2/ASF regulates protein sumoylation. Proc. Natl. Acad. Sci. USA 2010, 107, 16119–16124. [Google Scholar] [CrossRef] [PubMed]
- Moumen, A.; Masterson, P.; O’Connor, M.J.; Jackson, S.P. hnRNP K: An HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell 2005, 123, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Pelisch, F.; Pozzi, B.; Risso, G.; Munoz, M.J.; Srebrow, A. DNA damage-induced heterogeneous nuclear ribonucleoprotein K sumoylation regulates p53 transcriptional activation. J. Biol. Chem. 2012, 287, 30789–30799. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Smolka, M.B.; Albuquerque, C.P.; Chen, S.H.; Zhou, H. Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. USA 2007, 104, 10364–10369. [Google Scholar] [CrossRef] [PubMed]
- Chanarat, S.; Burkert-Kautzsch, C.; Meinel, D.M.; Strasser, K. Prp19C and TREX: Interacting to promote transcription elongationand mRNA export. Transcription 2012, 3, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bennetzen, M.V.; Larsen, D.H.; Bunkenborg, J.; Bartek, J.; Lukas, J.; Andersen, J.S. Site-specific phosphorylation dynamics of the nuclear proteome during the DNA damage response. Mol. Cell. Proteomics 2010, 9, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Bensimon, A.; Schmidt, A.; Ziv, Y.; Elkon, R.; Wang, S.Y.; Chen, D.J.; Aebersold, R.; Shiloh, Y. ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci. Signal. 2010. [Google Scholar] [CrossRef] [PubMed]
- Leva, V.; Giuliano, S.; Bardoni, A.; Camerini, S.; Crescenzi, M.; Lisa, A.; Biamonti, G.; Montecucco, A. Phosphorylation of SRSF1 is modulated by replicational stress. Nucleic Acids Res. 2012, 40, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Moumen, A.; Magill, C.; Dry, K.L.; Jackson, S.P. ATM-dependent phosphorylation of heterogeneous nuclear ribonucleoprotein K promotes p53 transcriptional activation in response to DNA damage. Cell Cycle 2013, 12, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Blasius, M.; Forment, J.V.; Thakkar, N.; Wagner, S.A.; Choudhary, C.; Jackson, S.P. A phospho-proteomic screen identifies substrates of the checkpoint kinase Chk1. Genome Biol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Katzenberger, R.J.; Marengo, M.S.; Wassarman, D.A. ATM and ATR pathways signal alternative splicing of Drosophila TAF1 pre-mRNA in response to DNA damage. Mol. Cell. Biol. 2006, 26, 9256–9267. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.H.; Choi, H.K.; Jung, S.Y.; Hyle, J.; Kim, B.J.; Yoon, K.; Cho, E.J.; Youn, H.D.; Lahti, J.M.; Qin, J.; et al. CHK2 kinase promotes pre-mRNA splicing via phosphorylating CDK11(p110). Oncogene 2014, 33, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Shkreta, L.; Michelle, L.; Toutant, J.; Tremblay, M.L.; Chabot, B. The DNA damage response pathway regulates the alternative splicing of the apoptotic mediator Bcl-x. J. Biol. Chem. 2011, 286, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Katzenberger, R.J.; Marengo, M.S.; Wassarman, D.A. Control of alternative splicing by signal-dependent degradation of splicing-regulatory proteins. J. Biol. Chem. 2009, 284, 10737–10746. [Google Scholar] [CrossRef] [PubMed]
- Akaike, Y.; Masuda, K.; Kuwano, Y.; Nishida, K.; Kajita, K.; Kurokawa, K.; Satake, Y.; Shoda, K.; Imoto, I.; Rokutan, K. HuR regulates alternative splicing of the TRA2beta gene in human colon cancer cells under oxidative stress. Mol. Cell. Biol. 2014, 34, 2857–2873. [Google Scholar] [CrossRef] [PubMed]
- Best, A.; James, K.; Dalgliesh, C.; Hong, E.; Kheirolahi-Kouhestani, M.; Curk, T.; Xu, Y.; Danilenko, M.; Hussain, R.; Keavney, B.; et al. Human Tra2 proteins jointly control a CHEK1 splicing switch among alternative and constitutive target exons. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Paronetto, M.P.; Minana, B.; Valcarcel, J. The Ewing sarcoma protein regulates DNA damage-induced alternative splicing. Mol. Cell 2011, 43, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.J.; Perez Santangelo, M.S.; Paronetto, M.P.; de la Mata, M.; Pelisch, F.; Boireau, S.; Glover-Cutter, K.; Ben-Dov, C.; Blaustein, M.; Lozano, J.J.; et al. DNA damage regulates alternative splicing through inhibition of RNA polymerase II elongation. Cell 2009, 137, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Lukas, J.; Lukas, C.; Bartek, J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat. Cell Biol. 2011, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Gómez Acuña, L.I.; Fiszbein, A.; Allo, M.; Schor, I.E.; Kornblihtt, A.R. Connections between chromatin signatures and splicing. Wiley Interdiscip. Rev. RNA 2013, 4, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, R.D.; Soni, D.V.; Wollman, R.; Hahn, A.T.; Yee, M.C.; Guan, A.; Hesley, J.A.; Miller, S.C.; Cromwell, E.F.; Solow-Cordero, D.E.; et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol. Cell 2009, 35, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, S.; Rondon, A.G.; Luna, R.; Aguilera, A. The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability. EMBO J. 2002, 21, 3526–3535. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Manley, J.L. Cotranscriptional processes and their influence on genome stability. Genes Dev. 2006, 20, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Garcia-Muse, T. R loops: From transcription byproducts to threats to genome stability. Mol. Cell 2012, 46, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Sanchez, M.S.; Barroso, S.; Gomez-Gonzalez, B.; Luna, R.; Aguilera, A. Genome instability and transcription elongation impairment in human cells depleted of THO/TREX. PLoS Genet. 2011, 7, e1002386. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Manley, J.L. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell 2005, 122, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Sun, Y.; Ding, J.H.; Lin, S.; Rose, D.W.; Rosenfeld, M.G.; Fu, X.D.; Li, X. Splicing regulator SC35 is essential for genomic stability and cell proliferation during mammalian organogenesis. Mol. Cell. Biol. 2007, 27, 5393–5402. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Manley, J.L. Alternative splicing and control of apoptotic DNA fragmentation. Cell Cycle 2006, 5, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Sordet, O.; Redon, C.E.; Guirouilh-Barbat, J.; Smith, S.; Solier, S.; Douarre, C.; Conti, C.; Nakamura, A.J.; Das, B.B.; Nicolas, E.; et al. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO Rep. 2009, 10, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Niu, T.; Manley, J.L. The RNA binding protein RNPS1 alleviates ASF/SF2 depletion-induced genomic instability. RNA 2007, 13, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Tuduri, S.; Crabbe, L.; Conti, C.; Tourriere, H.; Holtgreve-Grez, H.; Jauch, A.; Pantesco, V.; de Vos, J.; Thomas, A.; Theillet, C.; et al. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nat. Cell Biol. 2009, 11, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Labourier, E.; Forne, T.; Divita, G.; Derancourt, J.; Riou, J.F.; Antoine, E.; Cathala, G.; Brunel, C.; Tazi, J. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 1996, 381, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Czubaty, A.; Girstun, A.; Kowalska-Loth, B.; Trzcinska, A.M.; Purta, E.; Winczura, A.; Grajkowski, W.; Staron, K. Proteomic analysis of complexes formed by human topoisomerase I. Biochim. Biophys. Acta 2005, 1749, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zheng, X.; Chen, H.; Guo, Y.; Jiang, H.; He, X.; Zhu, X.; Zheng, Y. Splicing function of mitotic regulators links R-loop-mediated DNA damage to tumor cell killing. J. Cell Biol. 2015, 209, 235–246. [Google Scholar] [CrossRef] [PubMed]
- LaBranche, H.; Dupuis, S.; Ben-David, Y.; Bani, M.R.; Wellinger, R.J.; Chabot, B. Telomere elongation by hnRNP A1 and a derivative that interacts with telomeric repeats and telomerase. Nat. Genet. 1998, 19, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Fiset, S.; Chabot, B. hnRNP A1 may interact simultaneously with telomeric DNA and the human telomerase RNA in vitro. Nucleic Acids Res. 2001, 29, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Manche, L.; Xu, R.M.; Krainer, A.R. hnRNP A1 associates with telomere ends and stimulates telomerase activity. RNA 2006, 12, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.L.; Centore, R.C.; O’Sullivan, R.J.; Rai, R.; Tse, A.; Songyang, Z.; Chang, S.; Karlseder, J.; Zou, L. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature 2011, 471, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, N.M.; Rafalska-Metcalf, I.U.; Balane-Bolivar, C.; Janicki, S.M.; Greenberg, R.A. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell 2010, 141, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.P.; Wall, S.J.; Barre, B.; Panov, K.I.; Ajuh, P.M.; Perkins, N.D. Regulation of cyclin D1 RNA stability by SNIP1. Cancer Res. 2008, 68, 7621–7628. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Hsu, I.W.; Tarn, W.Y. TRAP150 activates pre-mRNA splicing and promotes nuclear mRNA degradation. Nucleic Acids Res. 2010, 38, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Tresini, M.; Warmerdam, D.O.; Kolovos, P.; Snijder, L.; Vrouwe, M.G.; Demmers, J.A.; van IJcken, W.F.; Grosveld, F.G.; Medema, R.H.; Hoeijmakers, J.H.; et al. The core spliceosome as target and effector of non-canonical ATM signalling. Nature 2015, 523, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Allemand, E.; Hastings, M.L.; Murray, M.V.; Myers, M.P.; Krainer, A.R. Alternative splicing regulation by interaction of phosphatase PP2Cgamma with nucleic acid-binding protein YB-1. Nat. Struct. Mol. Biol. 2007, 14, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.V.; Kobayashi, R.; Krainer, A.R. The type 2C Ser/Thr phosphatase PP2Cgamma is a pre-mRNA splicing factor. Genes Dev. 1999, 13, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Mandusic, V.; Nikolic-Vukosavljevic, D.; Tanic, N.; Kanjer, K.; Neskovic-Konstantinovic, Z.; Celeketic, D.; Dimitrijevic, B. Expression of estrogen receptor beta wt isoform (ERbeta1) and ERbetaDelta5 splice variant mRNAs in sporadic breast cancer. J. Cancer Res. Clin. Oncol. 2007, 133, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.H.; Kim, R.H.; Kang, M.K.; Kim, R.H.; Kim, S.G.; Lim, P.K.; Yochim, J.M.; Baluda, M.A.; Park, N.H. p53 promotes the fidelity of DNA end-joining activity by, in part, enhancing the expression of heterogeneous nuclear ribonucleoprotein G. DNA Repair 2007, 6, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Adamson, B.; Smogorzewska, A.; Sigoillot, F.D.; King, R.W.; Elledge, S.J. A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response. Nat. Cell Biol. 2012, 14, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, B.; Zhang, Z.; Raitskin, O.; Hiller, M.; Benderska, N.; Hartmann, A.M.; Bracco, L.; Elliott, D.; Ben-Ari, S.; Soreq, H.; et al. Heterogeneous nuclear ribonucleoprotein G regulates splice site selection by binding to CC(A/C)-rich regions in pre-mRNA. J. Biol. Chem. 2009, 284, 14303–14315. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, Y.; Lorson, C.L.; Stamm, S.; Androphy, E.J.; Wirth, B. Htra2-beta 1 stimulates an exonic splicing enhancer and can restore full-length SMN expression to survival motor neuron 2 (SMN2). Proc. Natl. Acad. Sci. USA 2000, 97, 9618–9623. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, Y.; Wirth, B. hnRNP-G promotes exon 7 inclusion of survival motor neuron (SMN) via direct interaction with Htra2-β1. Hum. Mol. Genet. 2002, 11, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Venables, J.P.; Elliott, D.J.; Makarova, O.V.; Makarov, E.M.; Cooke, H.J.; Eperon, I.C. RBMY, a probable human spermatogenesis factor, and other hnRNP G proteins interact with Tra2β and affect splicing. Hum. Mol. Genet. 2000, 9, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Nasim, M.T.; Chernova, T.K.; Chowdhury, H.M.; Yue, B.G.; Eperon, I.C. HnRNP G and Tra2β: Opposite effects on splicing matched by antagonism in RNA binding. Hum. Mol. Genet. 2003, 12, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.C.; Patton, J.G. Identification and characterization of a novel serine-arginine-rich splicing regulatory protein. Mol. Cell. Biol. 2000, 20, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.C.; Li, J.; Peng, R.; Patton, J.G. Regulation of alternative splicing by SRrp86 through coactivation and repression of specific SR proteins. RNA 2002, 8, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Barnard, D.C.; Patton, J.G. A unique glutamic acid-lysine (EK) domain acts as a splicing inhibitor. J. Biol. Chem. 2002, 277, 39485–39492. [Google Scholar] [CrossRef] [PubMed]
- Dardenne, E.; Pierredon, S.; Driouch, K.; Gratadou, L.; Lacroix-Triki, M.; Espinoza, M.P.; Zonta, E.; Germann, S.; Mortada, H.; Villemin, J.P.; et al. Splicing switch of an epigenetic regulator by RNA helicases promotes tumor-cell invasiveness. Nat. Struct. Mol. Biol. 2012, 19, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Dardenne, E.; Espinoza, M.P.; Fattet, L.; Germann, S.; Lambert, M.P.; Neil, H.; Zonta, E.; Mortada, H.; Gratadou, L.; Deygas, M.; et al. RNA helicases DDX5 and DDX17 dynamically orchestrate transcription, miRNA, and splicing programs in cell differentiation. Cell Rep. 2014, 7, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Samaan, S.; Tranchevent, L.C.; Dardenne, E.; Espinoza, M.P.; Zonta, E.; Germann, S.; Gratadou, L.; Dutertre, M.; Auboeuf, D. The Ddx5 and Ddx17 RNA helicases are cornerstones in the complex regulatory array of steroid hormone-signaling pathways. Nucleic Acids Res. 2014, 42, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Rogelj, B.; Easton, L.E.; Bogu, G.K.; Stanton, L.W.; Rot, G.; Curk, T.; Zupan, B.; Sugimoto, Y.; Modic, M.; Haberman, N.; et al. Widespread binding of FUS along nascent RNA regulates alternative splicing in the brain. Sci. Rep. 2012. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, A.S.; Kim, S.H.; Trinh, A.T.; Rodenkirch, L.A.; Tibbetts, R.S. The RNA-binding protein fused in sarcoma (FUS) functions downstream of poly(ADP-ribose) polymerase (PARP) in response to DNA damage. J. Biol. Chem. 2013, 288, 24731–24741. [Google Scholar] [CrossRef] [PubMed]
- Rulten, S.L.; Rotheray, A.; Green, R.L.; Grundy, G.J.; Moore, D.A.; Gomez-Herreros, F.; Hafezparast, M.; Caldecott, K.W. PARP-1 dependent recruitment of the amyotrophic lateral sclerosis-associated protein FUS/TLS to sites of oxidative DNA damage. Nucleic Acids Res. 2014, 42, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Watford, W.; Li, C.; Parmelee, A.; Bryant, M.A.; Deng, C.; O’Shea, J.; Lee, S.B. Ewing sarcoma gene EWS is essential for meiosis and B lymphocyte development. J. Clin. Investig. 2007, 117, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Pan, L.; Su, S.C.; Quinn, E.J.; Sasaki, M.; Jimenez, J.C.; Mackenzie, I.R.; Huang, E.J.; Tsai, L.H. Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat. Neurosci. 2013, 16, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chi, B.; Xia, W.; Gangopadhyay, J.; Yamazaki, T.; Winkelbauer-Hurt, M.E.; Yin, S.; Eliasse, Y.; Adams, E.; Shaw, C.E.; et al. U1 snRNP is mislocalized in ALS patient fibroblasts bearing NLS mutations in FUS and is required for motor neuron outgrowth in zebrafish. Nucleic Acids Res. 2015, 43, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Mai, R.T.; Fang, W.H.; Lin, C.C.; Chiu, C.C.; Wu Lee, Y.H. YB-1 disrupts mismatch repair complex formation, interferes with MutSalpha recruitment on mismatch and inhibits mismatch repair through interacting with PCNA. Oncogene 2014, 33, 5065–5077. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, I.; Guay, D.; Lebel, M. YB-1 promotes strand separation in vitro of duplex DNA containing either mispaired bases or cisplatin modifications, exhibits endonucleolytic activities and binds several DNA repair proteins. Nucleic Acids Res. 2004, 32, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Selyutina, A.A.; Buldakov, I.A.; Evdokimova, V.; Ovchinnikov, L.P.; Sorokin, A.V. The proteolytic YB-1 fragment interacts with DNA repair machinery and enhances survival during DNA damaging stress. Cell Cycle 2013, 12, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Anantha, R.W.; Alcivar, A.L.; Ma, J.; Cai, H.; Simhadri, S.; Ule, J.; Konig, J.; Xia, B. Requirement of heterogeneous nuclear ribonucleoprotein C for BRCA gene expression and homologous recombination. PLoS ONE 2013, 8, e61368. [Google Scholar] [CrossRef] [PubMed]
- Zarnack, K.; Konig, J.; Tajnik, M.; Martincorena, I.; Eustermann, S.; Stevant, I.; Reyes, A.; Anders, S.; Luscombe, N.M.; Ule, J. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell 2013, 152, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Venables, J.P.; Koh, C.S.; Froehlich, U.; Lapointe, E.; Couture, S.; Inkel, L.; Bramard, A.; Paquet, E.R.; Watier, V.; Durand, M.; et al. Multiple and specific mRNA processing targets for the major human hnRNP proteins. Mol. Cell. Biol. 2008, 28, 6033–6043. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.C.; Narlikar, G.J.; Boyapaty, G.; Kingston, R.E.; Weissman, S.M. Heterogeneous nuclear ribonucleoprotein C1/C2, MeCP1, and SWI/SNF form a chromatin remodeling complex at the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 2005, 102, 15012–15017. [Google Scholar] [CrossRef] [PubMed]
- Batsche, E.; Yaniv, M.; Muchardt, C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat. Struct. Mol. Biol. 2006, 13, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Eberhart, C.G.; Kai, M. RNA binding protein RBM14 promotes radio-resistance in glioblastoma by regulating DNA repair and cell differentiation. Oncotarget 2014, 5, 2820–2826. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.; Dusterhoft, A.; Henriques, J.A.; Brendel, M. Allelism of PSO4 and PRP19 links pre-mRNA processing with recombination and error-prone DNA repair in Saccharomyces cerevisiae. Nucleic Acids Res. 1996, 24, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Kaur, R.; Lu, X.; Shen, X.; Li, L.; Legerski, R.J. The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J. Biol. Chem. 2005, 280, 40559–40567. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Legerski, R.J. The Prp19/Pso4 core complex undergoes ubiquitylation and structural alterations in response to DNA damage. Biochem. Biophys. Res. Commun. 2007, 354, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Marechal, A.; Li, J.M.; Ji, X.Y.; Wu, C.S.; Yazinski, S.A.; Nguyen, H.D.; Liu, S.; Jimenez, A.E.; Jin, J.; Zou, L. PRP19 transforms into a sensor of RPA-ssDNA after DNA damage and drives ATR activation via a ubiquitin-mediated circuitry. Mol. Cell 2014, 53, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Huang, J. The PSO4 protein complex associates with replication protein A (RPA) and modulates the activation of ataxia telangiectasia-mutated and Rad3-related (ATR). J. Biol. Chem. 2014, 289, 6619–6626. [Google Scholar] [CrossRef] [PubMed]
- Salton, M.; Lerenthal, Y.; Wang, S.Y.; Chen, D.J.; Shiloh, Y. Involvement of Matrin 3 and SFPQ/NONO in the DNA damage response. Cell Cycle 2010, 9, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Udayakumar, D.; Takeda, Y.; Dynan, W.S. Identification of the polypyrimidine tract binding protein-associated splicing factor.p54(nrb) complex as a candidate DNA double-strand break rejoining factor. J. Biol. Chem. 2005, 280, 5205–5210. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, A.; Schmidt, U.; Monajembashi, S.; Franke, C.; Schlott, B.; Grosse, F.; Greulich, K.O.; Saluz, H.P.; Hanel, F. Proteomic identification of PSF and p54(nrb) as TopBP1-interacting proteins. J. Cell. Biochem. 2012, 113, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, C.; Baker, D.K.; Pierce, A.J.; Pittman, D.L. The splicing-factor related protein SFPQ/PSF interacts with RAD51D and is necessary for homology-directed repair and sister chromatid cohesion. Nucleic Acids Res. 2011, 39, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kuhne, W.W.; Kulharya, A.; Hudson, F.Z.; Ha, K.; Cao, Z.; Dynan, W.S. Involvement of p54(nrb), a PSF partner protein, in DNA double-strand break repair and radioresistance. Nucleic Acids Res. 2009, 37, 6746–6753. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.B.; Attig, J.; Bellora, N.; Konig, J.; Hallegger, M.; Kayikci, M.; Eyras, E.; Ule, J.; Smith, C.W. Nuclear matrix protein Matrin3 regulates alternative splicing and forms overlapping regulatory networks with PTB. EMBO J. 2015, 34, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Van Oordt, W.H.; Diaz-Meco, M.T.; Lozano, J.; Krainer, A.R.; Moscat, J.; Caceres, J.F. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 2000, 149, 307–316. [Google Scholar] [CrossRef]
- Guil, S.; Long, J.C.; Caceres, J.F. hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol. Cell. Biol. 2006, 26, 5744–5758. [Google Scholar] [CrossRef] [PubMed]
- Llorian, M.; Beullens, M.; Lesage, B.; Nicolaescu, E.; Beke, L.; Landuyt, W.; Ortiz, J.M.; Bollen, M. Nucleocytoplasmic shuttling of the splicing factor SIPP1. J. Biol. Chem. 2005, 280, 38862–38869. [Google Scholar] [CrossRef] [PubMed]
- Busa, R.; Geremia, R.; Sette, C. Genotoxic stress causes the accumulation of the splicing regulator Sam68 in nuclear foci of transcriptionally active chromatin. Nucleic Acids Res. 2010, 38, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Busa, R.; Paronetto, M.P.; Farini, D.; Pierantozzi, E.; Botti, F.; Angelini, D.F.; Attisani, F.; Vespasiani, G.; Sette, C. The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene 2007, 26, 4372–4382. [Google Scholar] [CrossRef] [PubMed]
- Shkreta, L.; Froehlich, U.; Paquet, E.R.; Toutant, J.; Elela, S.A.; Chabot, B. Anticancer drugs affect the alternative splicing of Bcl-x and other human apoptotic genes. Mol. Cancer Ther. 2008, 7, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Paronetto, M.P.; Achsel, T.; Massiello, A.; Chalfant, C.E.; Sette, C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J. Cell Biol. 2007, 176, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Cai, L.; Zhu, J.; Chen, M.; Chen, J.; Li, Z.H.; Liu, X.D.; Wang, S.G.; Bie, P.; Jiang, P.; et al. Fyn requires HnRNPA2B1 and Sam68 to synergistically regulate apoptosis in pancreatic cancer. Carcinogenesis 2011, 32, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Tissier, A.; Janel-Bintz, R.; Coulon, S.; Klaile, E.; Kannouche, P.; Fuchs, R.P.; Cordonnier, A.M. Crosstalk between replicative and translesional DNA polymerases: PDIP38 interacts directly with Poleta. DNA Repair 2010, 9, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Zhang, S.; Mordue, D.; Wu, J.M.; Zhang, Z.; Darzynkiewicz, Z.; Lee, E.Y.; Lee, M.Y. PDIP38 is translocated to the spliceosomes/nuclear speckles in response to UV-induced DNA damage and is required for UV-induced alternative splicing of MDM2. Cell Cycle 2013, 12, 3184–3193. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Lenzken, S.C.; Ruepp, M.D.; Ranzini, F.; Maffioletti, A.; Alvarez, R.; Muhlemann, O.; Barabino, S.M. Paraquat modulates alternative pre-mRNA splicing by modifying the intracellular distribution of SRPK2. PLoS ONE 2013, 8, e61980. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, E.; Endo, H. SR and SR-related proteins redistribute to segregated fibrillar components of nucleoli in a response to DNA damage. Nucleus 2010, 1, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.M. Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J. Biol. Chem. 2008, 283, 19077–19084. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Abdelmohsen, K.; Gorospe, M. Regulation of HuR by DNA damage response kinases. J. Nucleic Acids 2010. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Abdelmohsen, K.; Kim, M.M.; Srikantan, S.; Lee, E.K.; Tominaga, K.; Selimyan, R.; Martindale, J.L.; Yang, X.; Lehrmann, E.; et al. Global dissociation of HuR-mRNA complexes promotes cell survival after ionizing radiation. EMBO J. 2011, 30, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Mazan-Mamczarz, K.; Hagner, P.R.; Zhang, Y.; Dai, B.; Lehrmann, E.; Becker, K.G.; Keene, J.D.; Gorospe, M.; Liu, Z.; Gartenhaus, R.B. ATM regulates a DNA damage response posttranscriptional RNA operon in lymphocytes. Blood 2011, 117, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, M.; Sanchez, G.; de Cian, M.C.; Barbier, J.; Dardenne, E.; Gratadou, L.; Dujardin, G.; le Jossic-Corcos, C.; Corcos, L.; Auboeuf, D. Cotranscriptional exon skipping in the genotoxic stress response. Nat. Struct. Mol. Biol. 2010, 17, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Vichi, P.; Coin, F.; Renaud, J.P.; Vermeulen, W.; Hoeijmakers, J.H.; Moras, D.; Egly, J.M. Cisplatin- and UV-damaged DNA lure the basal transcription factor TFIID/TBP. EMBO J. 1997, 16, 7444–7456. [Google Scholar] [CrossRef] [PubMed]
- Rockx, D.A.; Mason, R.; van Hoffen, A.; Barton, M.C.; Citterio, E.; Bregman, D.B.; van Zeeland, A.A.; Vrieling, H.; Mullenders, L.H. UV-induced inhibition of transcription involves repression of transcription initiation and phosphorylation of RNA polymerase II. Proc. Natl. Acad. Sci. USA 2000, 97, 10503–10508. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.E.; Chu, G. Portrait of transcriptional responses to ultraviolet and ionizing radiation in human cells. Nucleic Acids Res. 2004, 32, 4786–4803. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, H.C.; Cannell, I.G.; Morandell, S.; Yaffe, M.B. Is post-transcriptional stabilization, splicing and translation of selective mRNAs a key to the DNA damage response? Cell Cycle 2011, 10, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Long, J.C.; Caceres, J.F. The SR protein family of splicing factors: Master regulators of gene expression. Biochem. J. 2009, 417, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Perry, W.L., 3rd; Shepard, R.L.; Sampath, J.; Yaden, B.; Chin, W.W.; Iversen, P.W.; Jin, S.; Lesoon, A.; O’Brien, K.A.; Peek, V.L.; et al. Human splicing factor SPF45 (RBM17) confers broad multidrug resistance to anticancer drugs when overexpressed—A phenotype partially reversed by selective estrogen receptor modulators. Cancer Res. 2005, 65, 6593–6600. [Google Scholar] [PubMed]
- Haley, B.; Paunesku, T.; Protic, M.; Woloschak, G.E. Response of heterogeneous ribonuclear proteins (hnRNP) to ionising radiation and their involvement in DNA damage repair. Int. J. Radiat. Biol. 2009, 85, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yan, C.; Gan, T.; Chen, Z.; Lu, X.; Duerksen-Hughes, P.J.; Zhu, X.; Yang, J. Nuclear proteome analysis of cisplatin-treated HeLa cells. Mutat. Res. 2010, 691, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Decorsiere, A.; Cayrel, A.; Vagner, S.; Millevoi, S. Essential role for the interaction between hnRNP H/F and a G quadruplex in maintaining p53 pre-mRNA 3'-end processing and function during DNA damage. Genes Dev. 2011, 25, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Shin, W.K.; Caudill, M.A.; Stover, P.J. A UV-responsive internal ribosome entry site enhances serine hydroxymethyltransferase 1 expression for DNA damage repair. J. Biol. Chem. 2009, 284, 31097–31108. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.D.; Zhang, Y.; Cocklin, R.R.; Witzmann, F.A.; Wang, M. Proteomic analysis of differential protein expression induced by ultraviolet light radiation in HeLa cells. Proteomics 2003, 3, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Filippov, V.; Filippova, M.; Duerksen-Hughes, P.J. The early response to DNA damage can lead to activation of alternative splicing activity resulting in CD44 splice pattern changes. Cancer Res. 2007, 67, 7621–7630. [Google Scholar] [CrossRef] [PubMed]
- Comiskey, D.F., Jr.; Jacob, A.G.; Singh, R.K.; Tapia-Santos, A.S.; Chandler, D.S. Splicing factor SRSF1 negatively regulates alternative splicing of MDM2 under damage. Nucleic Acids Res. 2015, 43, 4202–4218. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Z.; Sinha, R.; Karni, R.; Krainer, A.R. SF2/ASF autoregulation involves multiple layers of post-transcriptional and translational control. Nat. Struct. Mol. Biol. 2010, 17, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Solier, S.; Barb, J.; Zeeberg, B.R.; Varma, S.; Ryan, M.C.; Kohn, K.W.; Weinstein, J.N.; Munson, P.J.; Pommier, Y. Genome-wide analysis of novel splice variants induced by topoisomerase I poisoning shows preferential occurrence in genes encoding splicing factors. Cancer Res. 2010, 70, 8055–8065. [Google Scholar] [CrossRef] [PubMed]
- Colla, S.; Ong, D.S.; Ogoti, Y.; Marchesini, M.; Mistry, N.A.; Clise-Dwyer, K.; Ang, S.A.; Storti, P.; Viale, A.; Giuliani, N.; et al. Telomere dysfunction drives aberrant hematopoietic differentiation and myelodysplastic syndrome. Cancer Cell. 2015, 27, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.Z.; Grate, L.; Donohue, J.P.; Preston, C.; Nobida, N.; O’Brien, G.; Shiue, L.; Clark, T.A.; Blume, J.E.; Ares, M., Jr. Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay. Genes Dev. 2007, 21, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.Y.; Schmidt, D.; Pan, Q.; Ramani, A.K.; Fraser, A.G.; Odom, D.T.; Blencowe, B.J. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res. 2011, 21, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.I.; Gorski, J.J.; Barros, E.M.; Irwin, G.W.; Manti, L.; Powell, A.J.; Pellagatti, A.; Lukashchuk, N.; McCance, D.J.; McCluggage, W.G.; et al. Identification of a BRCA1-mRNA splicing complex required for efficient DNA repair and maintenance of genomic stability. Mol. Cell 2014, 54, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.I.; Harkin, D.P. BRCA1, a “complex” protein involved in the maintenance of genomic stability. FEBS J. 2015, 282, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Cortez, D.; Wang, Y.; Qin, J.; Elledge, S.J. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 1999, 286, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Otomo, T.; Hishii, M.; Arai, H.; Sato, K.; Sasai, K. Microarray analysis of temporal gene responses to ionizing radiation in two glioblastoma cell lines: Up-regulation of DNA repair genes. J. Radiat. Res. 2004, 45, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Jones, K.A. SKIP counteracts p53-mediated apoptosis via selective regulation of p21Cip1 mRNA splicing. Genes Dev. 2011, 25, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.; Urlaub, H.; Will, C.L.; Luhrmann, R. Protein composition of human mRNPs spliced in vitro and differential requirements for mRNP protein recruitment. RNA 2007, 13, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Fu, X.D. Regulation of splicing by SR proteins and SR protein-specific kinases. Chromosoma 2013, 122, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhou, Y.; Pandit, S.; Huang, J.; Li, H.; Lin, C.Y.; Xiao, R.; Burge, C.B.; Fu, X.D. SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. Cell 2013, 153, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, B.; Blanchette, M.; Monette, A.; Mouland, A.J.; Wellinger, R.J.; Chabot, B. A Function for the hnRNP A1/A2 Proteins in Transcription Elongation. PLoS ONE 2015, 10, e0126654. [Google Scholar] [CrossRef] [PubMed]
- Hurov, K.E.; Cotta-Ramusino, C.; Elledge, S.J. A genetic screen identifies the Triple T complex required for DNA damage signaling and ATM and ATR stability. Genes Dev. 2010, 24, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Bittencourt, D.; Laud, K.; Barbier, J.; Delattre, O.; Auboeuf, D.; Dutertre, M. Alteration of cyclin D1 transcript elongation by a mutated transcription factor up-regulates the oncogenic D1b splice isoform in cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 6004–6009. [Google Scholar] [CrossRef] [PubMed]
- Paronetto, M.P.; Bernardis, I.; Volpe, E.; Bechara, E.; Sebestyen, E.; Eyras, E.; Valcarcel, J. Regulation of FAS exon definition and apoptosis by the Ewing sarcoma protein. Cell Rep. 2014, 7, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Knoop, L.L.; Baker, S.J. The splicing factor U1C represses EWS/FLI-mediated transactivation. J. Biol. Chem. 2000, 275, 24865–24871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Paley, A.J.; Childs, G. The transcriptional repressor ZFM1 interacts with and modulates the ability of EWS to activate transcription. J. Biol. Chem. 1998, 273, 18086–18091. [Google Scholar] [CrossRef] [PubMed]
- Chansky, H.A.; Hu, M.; Hickstein, D.D.; Yang, L. Oncogenic TLS/ERG and EWS/Fli-1 fusion proteins inhibit RNA splicing mediated by YB-1 protein. Cancer Res. 2001, 61, 3586–3590. [Google Scholar] [PubMed]
- Sordet, O.; Larochelle, S.; Nicolas, E.; Stevens, E.V.; Zhang, C.; Shokat, K.M.; Fisher, R.P.; Pommier, Y. Hyperphosphorylation of RNA polymerase II in response to topoisomerase I cleavage complexes and its association with transcription- and BRCA1-dependent degradation of topoisomerase I. J. Mol. Biol. 2008, 381, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mao, G.; Tong, D.; Huang, J.; Gu, L.; Yang, W.; Li, G.M. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell 2013, 153, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.X.; Ahrabi, S.; Zalmas, L.P.; Sarkar, S.; Aymard, F.; Bachrati, C.Z.; Helleday, T.; Legube, G.; La Thangue, N.B.; Porter, A.C.; et al. SETD2-dependent histone H3K36 trimethylation is required for homologous recombination repair and genome stability. Cell Rep. 2014, 7, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Dhami, P.; Saffrey, P.; Bruce, A.W.; Dillon, S.C.; Chiang, K.; Bonhoure, N.; Koch, C.M.; Bye, J.; James, K.; Foad, N.S.; et al. Complex exon-intron marking by histone modifications is not determined solely by nucleosome distribution. PLoS ONE 2010, 5, e12339. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Enroth, S.; Rada-Iglesias, A.; Wadelius, C.; Komorowski, J. Nucleosomes are well positioned in exons and carry characteristic histone modifications. Genome Res. 2009, 19, 1732–1741. [Google Scholar] [CrossRef]
- Spies, N.; Nielsen, C.B.; Padgett, R.A.; Burge, C.B. Biased chromatin signatures around polyadenylation sites and exons. Mol. Cell 2009, 36, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kolasinska-Zwierz, P.; Down, T.; Latorre, I.; Liu, T.; Liu, X.S.; Ahringer, J. Differential chromatin marking of introns and expressed exons by H3K36me3. Nat. Genet. 2009, 41, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Fong, N.; Erickson, B.; Bentley, D.L. Pre-mRNA splicing is a determinant of histone H3K36 methylation. Proc. Natl. Acad. Sci. USA 2011, 108, 13564–13569. [Google Scholar] [CrossRef] [PubMed]
- Pradeepa, M.M.; Sutherland, H.G.; Ule, J.; Grimes, G.R.; Bickmore, W.A. Psip1/Ledgf p52 binds methylated histone H3K36 and splicing factors and contributes to the regulation of alternative splicing. PLoS Genet. 2012, 8, e1002717. [Google Scholar] [CrossRef] [PubMed]
- Kfir, N.; Lev-Maor, G.; Glaich, O.; Alajem, A.; Datta, A.; Sze, S.K.; Meshorer, E.; Ast, G. SF3B1 association with chromatin determines splicing outcomes. Cell Rep. 2015, 11, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, Q.C.; da Rocha, S.T.; Flynn, R.A.; Bharadwaj, M.; Calabrese, J.M.; Magnuson, T.; Heard, E.; Chang, H.Y. Systematic discovery of Xist RNA binding proteins. Cell 2015, 161, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, I.; Munita, R.; Agirre, E.; Dittmer, T.A.; Gysling, K.; Misteli, T.; Luco, R.F. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat. Struct. Mol. Biol. 2015, 22, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Peng, G. Non-coding RNAs: An emerging player in DNA damage response. Mutat. Res. Rev. Mutat. Res. 2015, 763, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Z.; Zhao, Q.; Zhu, Y.; Zhao, C.; Li, X.; Ma, Z.; Li, X.; Zhang, Y. LincRNA-p21 enhances the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/beta-catenin signaling pathway. Oncol. Rep. 2014, 31, 1839–1845. [Google Scholar] [PubMed]
- Hung, T.; Wang, Y.; Lin, M.F.; Koegel, A.K.; Kotake, Y.; Grant, G.D.; Horlings, H.M.; Shah, N.; Umbricht, C.; Wang, P.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011, 43, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, N.; Zamudio, J.R.; Jong, R.M.; Soukup, D.; Resnick, R.; Sarma, K.; Ward, A.J.; Raj, A.; Lee, J.T.; Sharp, P.A.; et al. LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol. Cell 2014, 54, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Sevcik, J.; Falk, M.; Macurek, L.; Kleiblova, P.; Lhota, F.; Hojny, J.; Stefancikova, L.; Janatova, M.; Bartek, J.; Stribrna, J.; et al. Expression of human BRCA1Delta17–19 alternative splicing variant with a truncated BRCT domain in MCF-7 cells results in impaired assembly of DNA repair complexes and aberrant DNA damage response. Cell. Signal. 2013, 25, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, T.; Ma, K.; Tian, Z.; Zhu, Y.; Chen, F.; Hu, G. The impacts of ERCC1 gene exon VIII alternative splicing on cisplatin-resistance in ovarian cancer cells. Cancer Investig. 2009, 27, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Berge, E.O.; Staalesen, V.; Straume, A.H.; Lillehaug, J.R.; Lonning, P.E. Chk2 splice variants express a dominant-negative effect on the wild-type Chk2 kinase activity. Biochim. Biophys. Acta 2010, 1803, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Baldin, V.; Cans, C.; Knibiehler, M.; Ducommun, B. Phosphorylation of human CDC25B phosphatase by CDK1-cyclin A triggers its proteasome-dependent degradation. J. Biol. Chem. 1997, 272, 32731–32734. [Google Scholar] [CrossRef] [PubMed]
- Schwerk, C.; Schulze-Osthoff, K. Regulation of apoptosis by alternative pre-mRNA splicing. Mol. Cell 2005, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sigalas, I.; Calvert, A.H.; Anderson, J.J.; Neal, D.E.; Lunec, J. Alternatively spliced mdm2 transcripts with loss of p53 binding domain sequences: Transforming ability and frequent detection in human cancer. Nat. Med. 1996, 2, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Eijkelenboom, A.; Burgering, B.M. FOXOs: Signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 2013, 14, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, H.C.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in aging and cancer. Trends Genet. 2012, 28, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Wang, Q.; Kennedy, C.J.; Silver, P.A. An alternative splicing network links cell-cycle control to apoptosis. Cell 2010, 142, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Anczukow, O.; Rosenberg, A.Z.; Akerman, M.; Das, S.; Zhan, L.; Karni, R.; Muthuswamy, S.K.; Krainer, A.R. The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat. Struct. Mol. Biol. 2012, 19, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Olshavsky, N.A.; Comstock, C.E.; Schiewer, M.J.; Augello, M.A.; Hyslop, T.; Sette, C.; Zhang, J.; Parysek, L.M.; Knudsen, K.E. Identification of ASF/SF2 as a critical, allele-specific effector of the cyclin D1b oncogene. Cancer Res. 2010, 70, 3975–3984. [Google Scholar] [CrossRef] [PubMed]
- Paronetto, M.P.; Cappellari, M.; Busa, R.; Pedrotti, S.; Vitali, R.; Comstock, C.; Hyslop, T.; Knudsen, K.E.; Sette, C. Alternative splicing of the cyclin D1 proto-oncogene is regulated by the RNA-binding protein Sam68. Cancer Res. 2010, 70, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, J.H.; Back, S.H.; Jang, S.K. Polypyrimidine tract-binding protein enhances the internal ribosomal entry site-dependent translation of p27Kip1 mRNA and modulates transition from G1 to S phase. Mol. Cell. Biol. 2005, 25, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Sakamoto, S.; Sakai, T.; Yasunaga, J.; Komatsu, K.; Matsuoka, M. A potential link between alternative splicing of the NBS1 gene and DNA damage/environmental stress. Radiat. Res. 2008, 170, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Merdzhanova, G.; Edmond, V.; de Seranno, S.; van den Broeck, A.; Corcos, L.; Brambilla, C.; Brambilla, E.; Gazzeri, S.; Eymin, B. E2F1 controls alternative splicing pattern of genes involved in apoptosis through upregulation of the splicing factor SC35. Cell. Death Differ. 2008, 15, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.S.; Singh, R.K.; Caldwell, L.C.; Bitler, J.L.; Lozano, G. Genotoxic stress induces coordinately regulated alternative splicing of the p53 modulators MDM2 and MDM4. Cancer Res. 2006, 66, 9502–9508. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Delforge, Y.; Deward, A.; Habraken, Y.; Hennuy, B.; Piette, J.; Klinck, R.; Chabot, B.; Colige, A.; Lambert, C. Role of the splicing factor SRSF4 in cisplatin-induced modifications of pre-mRNA splicing and apoptosis. BMC Cancer 2015. [Google Scholar] [CrossRef] [PubMed]
- Index of /annotate/luli. Available online: http://lgfus.ca/annotate/luli/ (accessed on 23 October 2015).
- Genome-wide analysis of novel splice variants induced by topoisomerase I poisoning shows preferential occurrence in genes encoding splicing factors. Supplemental Table 3. Supplementary Material. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992871/#SD1 (accessed on 23 October 2015).
- Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Supplemental Material. Supp. Table S3.xls. Available online: http://genome.cshlp.org/content/21/3/390/suppl/DC1 (accessed on 23 October 2015).
- Cotranscriptional exon skipping in the genotoxic stress response. Supplementary Table 1. Available online: http://www.nature.com/nsmb/journal/v17/n11/extref/nsmb.1912-S1.pdf (accessed on 23 October 2015).
- Additional file 4. Post-transcriptional events, from RNA-Seq in control and cisplatin-treated MCF7 cells. Available online: http://www.biomedcentral.com/1471-2407/15/227/suppl/S4 (accessed on 23 October 2015).
- PLOS ONE: Alternative Transcript Initiation and Splicing as a Response to DNA Damage. Supporting Information. Table S8. Genes predicted to be alternatively spliced in LCLs at 4 hours post 10 Gy IR based on SI. Table S11. Genes predicted to be alternatively spliced in fibroblasts at 4 hours post 10 Gy IR based on SI. Available online: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0025758#pone.0025758.s010 (accessed on 23 October 2015).
- Additional file 12: Table S6.Genes with iAs-mediated alternatively spliced events. Available online: http://www.biomedcentral.com/1471-2164/16/212/suppl/S12 (accessed on 23 October 2015).
- DNA Damage Regulates Alternative Splicing through Inhibition of RNA Polymerase II Elongation: Supplemental Data. Document S2. Supplemental Spreadsheet. Available online: http://www.sciencedirect.com/science/article/pii/S0092867409002700 (accessed on 23 October 2015).
- Nature14512-s1.pdf. Supplementary Information. SI Table 2: UV-triggered and ATM-dependent alternative splicing events. Available online: http://www.nature.com/nature/journal/v523/n7558/extref/nature14512-s1.pdf (accessed on 23 October 2015).
- Newly identified biologically active and proteolysis-resistant VEGF-A isoform VEGF111 is induced by genotoxic agents. Table II. Induction of VEGF111 and Bcl-Xs/Bcl-Xl ratio by UV-B. Available online: http://jcb.rupress.org/content/179/6/1261.full (accessed on 23 October 2015).
- Rahmutulla, B.; Matsushita, K.; Satoh, M.; Seimiya, M.; Tsuchida, S.; Kubo, S.; Shimada, H.; Ohtsuka, M.; Miyazaki, M.; Nomura, F. Alternative splicing of FBP-interacting repressor coordinates c-Myc, P27Kip1/cyclinE and Ku86/XRCC5 expression as a molecular sensor for bleomycin-induced DNA damage pathway. Oncotarget 2014, 5, 2404–2417. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Kajiwara, T.; Tamura, M.; Satoh, M.; Tanaka, N.; Tomonaga, T.; Matsubara, H.; Shimada, H.; Yoshimoto, R.; Ito, A.; et al. SAP155-mediated splicing of FUSE-binding protein-interacting repressor serves as a molecular switch for c-myc gene expression. Mol. Cancer Res. 2012, 10, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Massiello, A.; Roesser, J.R.; Chalfant, C.E. SAP155 Binds to ceramide-responsive RNA cis-element 1 and regulates the alternative 5' splice site selection of Bcl-x pre-mRNA. J. Fed. Am. Soc. Exp. Biol. 2006, 20, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protocols 2013, 8, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer 2012, 12, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.H.; Schierz, A.C.; Ward, S.E.; Al-Lazikani, B.; Pearl, F.M. Therapeutic opportunities within the DNA damage response. Nature Rev. Cancer 2015, 15, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.E.; Kennedy, R.D.; Mullan, P.B.; Gilmore, P.M.; Carty, M.; Johnston, P.G.; Harkin, D.P. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003, 63, 6221–6228. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Damm, F.; Kosmider, O.; Gelsi-Boyer, V.; Renneville, A.; Carbuccia, N.; Hidalgo-Curtis, C.; Valle, V.D.; Couronne, L.; Scourzic, L.; Chesnais, V.; et al. Mutations affecting mRNA splicing define distinct clinical phenotypes and correlate with patient outcome in myelodysplastic syndromes. Blood 2012, 119, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Makishima, H.; Visconte, V.; Sakaguchi, H.; Jankowska, A.M.; Abu Kar, S.; Jerez, A.; Przychodzen, B.; Bupathi, M.; Guinta, K.; Afable, M.G.; et al. Mutations in the spliceosome machinery, a novel and ubiquitous pathway in leukemogenesis. Blood 2012, 119, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Karni, R.; de Stanchina, E.; Lowe, S.W.; Sinha, R.; Mu, D.; Krainer, A.R. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat. Struct. Mol. Biol. 2007, 14, 185–193. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Chen, M.; Assanah, M.; Canoll, P.; Manley, J.L. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 2009, 463, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, S.; Bielli, P.; Passacantilli, I.; Pilozzi, E.; Fendrich, V.; Capurso, G.; Fave, G.D.; Sette, C. Modulation of PKM alternative splicing by PTBP1 promotes gemcitabine resistance in pancreatic cancer cells. Oncogene 2015. [Google Scholar] [CrossRef] [PubMed]
- Bonnal, S.; Vigevani, L.; Valcarcel, J. The spliceosome as a target of novel antitumour drugs. Nat. Rev. Drug Discov. 2012, 11, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Bakkour, N.; Lin, Y.L.; Maire, S.; Ayadi, L.; Mahuteau-Betzer, F.; Nguyen, C.H.; Mettling, C.; Portales, P.; Grierson, D.; Chabot, B.; et al. Small-molecule inhibition of HIV pre-mRNA splicing as a novel antiretroviral therapy to overcome drug resistance. PLoS Pathog. 2007, 3, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Yankner, B.A. The aging stress response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Sulli, G.; di Micco, R.; d’Adda di Fagagna, F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat. Rev. Cancer 2012, 12, 709–720. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.J.; Karlseder, J. The great unravelling: Chromatin as a modulator of the aging process. Trends Biochem. Sci. 2012, 37, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Holly, A.C.; Melzer, D.; Pilling, L.C.; Fellows, A.C.; Tanaka, T.; Ferrucci, L.; Harries, L.W. Changes in splicing factor expression are associated with advancing age in man. Mech. Ageing Dev. 2013, 134, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Blair, C.D.; Faddah, D.A.; Kieckhaefer, J.E.; Olive, M.; Erdos, M.R.; Nabel, E.G.; Collins, F.S. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Investig. 2011, 121, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkreta, L.; Chabot, B. The RNA Splicing Response to DNA Damage. Biomolecules 2015, 5, 2935-2977. https://doi.org/10.3390/biom5042935
Shkreta L, Chabot B. The RNA Splicing Response to DNA Damage. Biomolecules. 2015; 5(4):2935-2977. https://doi.org/10.3390/biom5042935
Chicago/Turabian StyleShkreta, Lulzim, and Benoit Chabot. 2015. "The RNA Splicing Response to DNA Damage" Biomolecules 5, no. 4: 2935-2977. https://doi.org/10.3390/biom5042935
APA StyleShkreta, L., & Chabot, B. (2015). The RNA Splicing Response to DNA Damage. Biomolecules, 5(4), 2935-2977. https://doi.org/10.3390/biom5042935






