A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer
Abstract
:1. Introduction
2. Tumor-Associated O-glycosylation
- IgA nephropathy (IgAN): IgAN is a very common glomerulonephritis that is characterized by deposition of IgA immune complexes in the glomerulus. The O-glycans in the hinge-region of IgA-1 antibodies isolated from glomeruli deposits lack galactose and, thus, display high amounts of Tn antigen [22,23,24]. The aberrantly glycosylated IgA antibodies form immune complexes with anti-glycan autoantibodies, thus classifying IgAN as an autoimmune disease. The high quantity of Tn antigen on the IgA may facilitate binding to and subsequent signaling of the C-type lectin macrophage galactose-type lectin (MGL) on antigen presenting cells (APCs). MGL triggering has been shown to augment production of IL-10 [25], which could result in stimulation of IgA-producing B cells, thus aggravating the disease. The role of IL-10 in disease progression is further supported by the finding that compared to healthy donors, whole blood cultures from IgAN patients are more prone to produce IL-10 after stimulation with lipopolysaccharide or phytohemagglutinin [26].
- Tn syndrome: Tn syndrome is characterized by Tn antigen expression on all major blood cell lineages. Patients with Tn syndrome are not clinically affected, except for minor signs of hemolysis or thrombocytopenia [27]. Tn syndrome is associated with a somatic mutation of COSMC, thereby preventing T-synthase function [28]. Interestingly, only 1–2% of T cells are affected. As binding of MGL to Tn antigen positive CD45 on T cells induces T cell apoptosis [29], it is tempting to speculate that Tn antigen positive T cells are cleared from the circulation and, thus, undetectable in these patients.
- Inflammatory bowel disease: also in an inflammatory autoimmune setting O-glycans appear to be important for disease progression. Mice with an intestinal epithelial cell-specific loss of Core 1-derived O-glycans spontaneously develop colitis, suggesting a protective role of Core 1 in preventing intestinal inflammation [30]. Indeed, also patients with active ulcerative colitis show impaired expression of intestinal glycans and an accompanying increase in truncated glycans. An increased amount of the sTn antigen could be detected in 18% of the patients, compared to 2% in the control patients. Interestingly, the aberrant glycosylation profile was shown to be reversible upon remission and was significantly correlated to the extent of inflammation [31].
3. The Interplay between Tumor Cells and the Immune System
4. Immune Receptors Involved in the Recognition of Tumor-Associated O-glycans
5. Effect of Aberrantly-Glycosylated Mucins on Immunity
5.1. Uptake and Processing of Mucins by DCs
5.2. Influence of Mucin Engagement on Adaptive Immunity
6. Effect of Tumor-Associated O-glycans on APCs and the Initiation of Adaptive Immunity
6.1. Immune Modulation by MGL+ APCs
6.2. Sialylated O-glycans and Immunity
7. Effect of Tumor-Associated O-glycans on Natural Killer Cells
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoseki, J.; Ushioda, R.; Nagata, K. Mechanism and components of endoplasmic reticulum-associated degradation. J. Biochem. 2010, 147, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Yang, J.; Lehotay, M.; Ogata, S.; Itzkowitz, S. Pathways of mucin O-glycosylation in normal and malignant rat colonic epithelial cells reveal a mechanism for cancer-associated Sialyl-Tn antigen expression. Biol. Chem. 2001, 382, 219–232. [Google Scholar] [PubMed]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 64, 7050–7057. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.J.; Tham, K.M.; Chia, J.; Wang, S.C.; Steentoft, C.; Clausen, H.; Bard-Chapeau, E.A.; Bard, F.A. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc. Natl. Acad. Sci. USA 2013, 110, E3152–E3161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ju, T.; Ding, X.; Xia, B.; Wang, W.; Xia, L.; He, M.; Cummings, R.D. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc. Natl. Acad. Sci. USA 2010, 107, 9228–9233. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Cummings, R.D.; Canfield, W.M. Purification, characterization, and subunit structure of rat core 1 Beta1,3-galactosyltransferase. J. Biol. Chem. 2002, 277, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Herzog, B.H.; Fu, J.; Sheng, M.; Bergstrom, K.; McDaniel, J.M.; Kondo, Y.; McGee, S.; Cai, X.; Li, P.; et al. Loss of Core 1-derived O-Glycans Decreases Breast Cancer Development in Mice. J. Biol. Chem. 2015, 290, 20159–20166. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Liu, C.H.; Huang, M.J.; Lai, H.S.; Lee, P.H.; Hu, R.H.; Huang, M.C. C1GALT1 enhances proliferation of hepatocellular carcinoma cells via modulating MET glycosylation and dimerization. Cancer Res. 2013, 73, 5580–5590. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, P.; Dabelsteen, S.; Madsen, F.B.; Francavilla, C.; Kopp, K.L.; Steentoft, C.; Vakhrushev, S.Y.; Olsen, J.V.; Hansen, L.; Bennett, E.P.; et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA 2014, 111, E4066–E4075. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Karsten, U.R.; Liebrich, W.; Haensch, W.; Springer, G.F.; Schlag, P.M. Expression of Thomsen-Friedenreich-related antigens in primary and metastatic colorectal carcinomas. A reevaluation. Cancer 1995, 76, 1700–1708. [Google Scholar] [CrossRef]
- Ghazizadeh, M.; Ogawa, H.; Sasaki, Y.; Araki, T.; Aihara, K. Mucin carbohydrate antigens (T, Tn, and sialyl-Tn) in human ovarian carcinomas: Relationship with histopathology and prognosis. Hum. Pathol. 1997, 28, 960–966. [Google Scholar] [CrossRef]
- Imai, J.; Ghazizadeh, M.; Naito, Z.; Asano, G. Immunohistochemical expression of T, Tn and sialyl-Tn antigens and clinical outcome in human breast carcinoma. Anticancer Res. 2001, 21, 1327–1334. [Google Scholar] [PubMed]
- Karsten, U.; Goletz, S. What makes cancer stem cell markers different? Springerplus 2013. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Carvalho, S.; Marcos-Pinto, R.; Magalhaes, A.; Oliveira, C.; Gu, J.; Dinis-Ribeiro, M.; Carneiro, F.; Seruca, R.; Reis, C.A. Gastric cancer: Adding glycosylation to the equation. Trends Mol. Med. 2013, 19, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Marcos, N.T.; Bennett, E.P.; Gomes, J.; Magalhaes, A.; Gomes, C.; David, L.; Dar, I.; Jeanneau, C.; DeFrees, S.; Krustrup, D.; et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front. Biosci. (Elite Ed) 2011, 3, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Videira, P.A.; Lima, L.; Pereira, S.; Silva, M.; Carrascal, M.; Severino, P.F.; Fernandes, E.; Almeida, A.; Costa, C.; et al. Overexpression of tumour-associated carbohydrate antigen sialyl-Tn in advanced bladder tumours. Mol. Oncol. 2013, 7, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Laack, E.; Nikbakht, H.; Peters, A.; Kugler, C.; Jasiewicz, Y.; Edler, L.; Hossfeld, D.K.; Schumacher, U. Lectin histochemistry of resected adenocarcinoma of the lung: Helix pomatia agglutinin binding is an independent prognostic factor. Am. J. Pathol. 2002, 160, 1001–1008. [Google Scholar] [CrossRef]
- Chia, J.; Goh, G.; Bard, F. Short O-GalNAc glycans: Regulation and role in tumor development and clinical perspectives. Biochim. Biophys. Acta 2016. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I. Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep. 2006, 7, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Goletz, S. What controls the expression of the core-1 (Thomsen-Friedenreich) glycotope on tumor cells? Biochemistry (Mosc) 2015, 80, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.C.; Bailey, E.M.; Barratt, J.; Buck, K.S.; Feehally, J. Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J. Am. Soc. Nephrol. 1999, 10, 1763–1771. [Google Scholar] [PubMed]
- Hiki, Y.; Odani, H.; Takahashi, M.; Yasuda, Y.; Nishimoto, A.; Iwase, H.; Shinzato, T.; Kobayashi, Y.; Maeda, K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001, 59, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Tomana, M.; Novak, J.; Julian, B.A.; Matousovic, K.; Konecny, K.; Mestecky, J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J. Clin. Investig. 1999, 104, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; Bay, S.; Vuist, I.M.; Kalay, H.; Garcia-Vallejo, J.J.; Leclerc, C.; van Kooyk, Y. MGL signaling augments TLR2-mediated responses for enhanced IL-10 and TNF-alpha secretion. J. Leukoc. Biol. 2013, 94, 315–323. [Google Scholar] [CrossRef] [PubMed]
- De Fijter, J.W.; Daha, M.R.; Schroeijers, W.E.; van Es, L.A.; Van Kooten, C. Increased IL-10 production by stimulated whole blood cultures in primary IgA nephropathy. Clin. Exp. Immunol. 1998, 111, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.G. Tn-syndrome. Biochim. Biophys. Acta 1999, 1455, 255–268. [Google Scholar] [CrossRef]
- Ju, T.; Cummings, R.D. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature 2005, 437. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; Gringhuis, S.I.; Geijtenbeek, T.B.; van Kooyk, Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat. Immunol. 2006, 7, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wei, B.; Wen, T.; Johansson, M.E.; Liu, X.; Bradford, E.; Thomsson, K.A.; McGee, S.; Mansour, L.; Tong, M.; et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Investig. 2011, 121, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.M.; Karlsson, H.; Crespo, J.G.; Johansson, M.E.; Eklund, L.; Sjovall, H.; Hansson, G.C. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel. Dis. 2011, 17, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Goedert, J.J.; Cote, T.R.; Virgo, P.; Scoppa, S.M.; Kingma, D.W.; Gail, M.H.; Jaffe, E.S.; Biggar, R.J. Spectrum of AIDS-associated malignant disorders. Lancet 1998, 351, 1833–1839. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Shankaran, V.; Dighe, A.S.; Stockert, E.; Aguet, M.; Old, L.J.; Schreiber, R.D. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7556–7561. [Google Scholar] [CrossRef] [PubMed]
- Burnet, F.M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970, 13, 1–27. [Google Scholar] [PubMed]
- Burnet, M. Immunological Factors in the Process of Carcinogenesis. Br. Med. Bull. 1964, 20, 154–158. [Google Scholar] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Pages, F.; Marincola, F.M.; Thurin, M.; Trinchieri, G.; Fox, B.A.; Gajewski, T.F.; Ascierto, P.A. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 2012, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.Y.; Zang, R.Y.; Tang, M.Q.; Chen, J. Significance of systematic retroperitoneal lymphadenectomy at second-look laparotomy for ovarian cancer. Zhonghua Fu Chan Ke Za Zhi 2003, 38, 69–71. [Google Scholar] [PubMed]
- Marrogi, A.J.; Munshi, A.; Merogi, A.J.; Ohadike, Y.; El-Habashi, A.; Marrogi, O.L.; Freeman, S.M. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int. J. Cancer 1997, 74, 492–501. [Google Scholar] [CrossRef]
- Naito, Y.; Saito, K.; Shiiba, K.; Ohuchi, A.; Saigenji, K.; Nagura, H.; Ohtani, H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998, 58, 3491–3494. [Google Scholar] [PubMed]
- Nakano, O.; Sato, M.; Naito, Y.; Suzuki, K.; Orikasa, S.; Aizawa, M.; Suzuki, Y.; Shintaku, I.; Nagura, H.; Ohtani, H. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001, 61, 5132–5136. [Google Scholar] [PubMed]
- Schumacher, K.; Haensch, W.; Roefzaad, C.; Schlag, P.M. Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res. 2001, 61, 3932–3936. [Google Scholar] [PubMed]
- Pages, F.; Berger, A.; Camus, M.; Sanchez-Cabo, F.; Costes, A.; Molidor, R.; Mlecnik, B.; Kirilovsky, A.; Nilsson, M.; Damotte, D.; et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 2005, 353, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Balsamo, M.; Manzini, C.; Pietra, G.; Raggi, F.; Blengio, F.; Mingari, M.C.; Varesio, L.; Moretta, L.; Bosco, M.C.; Vitale, M. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur. J. Immunol. 2013, 43, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Almand, B.; Resser, J.R.; Lindman, B.; Nadaf, S.; Clark, J.I.; Kwon, E.D.; Carbone, D.P.; Gabrilovich, D.I. Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 2000, 6, 1755–1766. [Google Scholar] [PubMed]
- Muenst, S.; Laubli, H.; Soysal, S.D.; Zippelius, A.; Tzankov, A.; Hoeller, S. The immune system and cancer evasion strategies: Therapeutic concepts. J. Intern Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, A.; Matsumoto, Y.; Fukushi, J.; Iura, K.; Matsunobu, T.; Endo, M.; Fujiwara, T.; Iida, K.; Fujiwara, Y.; Hatano, M.; et al. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3K/Akt pathways. Br. J. Cancer 2015, 112, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, C.C.; Maurer, M.J.; Oberg, A.L.; Visscher, D.W.; Kalli, K.R.; Hartmann, L.C.; Goode, E.L.; Knutson, K.L. The ratios of CD8+ T cells to CD4+ CD25+ FOXP3+ and FOXP3− T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS ONE 2013, 8, e80063. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhou, S.; Wang, Y.; Li, R.L.; Zhong, C.; Liang, C.; Sun, Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; Paessens, L.C.; Broks-van den Berg, V.C.; Geijtenbeek, T.B.; van Kooyk, Y. The C-type lectin macrophage galactose-type lectin impedes migration of immature APCs. J. Immunol. 2008, 181, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Jegouzo, S.A.; Quintero-Martinez, A.; Ouyang, X.; dos Santos, A.; Taylor, M.E.; Drickamer, K. Organization of the extracellular portion of the macrophage galactose receptor: A trimeric cluster of simple binding sites for N-acetylgalactosamine. Glycobiology 2013, 23, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Mortezai, N.; Behnken, H.N.; Kurze, A.K.; Ludewig, P.; Buck, F.; Meyer, B.; Wagener, C. Tumor-associated Neu5Ac-Tn and Neu5Gc-Tn antigens bind to C-type lectin CLEC10A (CD301, MGL). Glycobiology 2013, 23, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Streng-Ouwehand, I.; Litjens, M.; Weelij, D.R.; Garcia-Vallejo, J.J.; van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Characterization of murine MGL1 and MGL2 C-type lectins: Distinct glycan specificities and tumor binding properties. Mol. Immunol. 2009, 46, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; van Liempt, E.; Geijtenbeek, T.B.; van Kooyk, Y. Differential regulation of C-type lectin expression on tolerogenic dendritic cell subsets. Immunobiology 2006, 211, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Beatson, R.; Maurstad, G.; Picco, G.; Arulappu, A.; Coleman, J.; Wandell, H.H.; Clausen, H.; Mandel, U.; Taylor-Papadimitriou, J.; Sletmoen, M.; et al. The Breast Cancer-Associated Glycoforms of MUC1, MUC1-Tn and sialyl-Tn, Are Expressed in COSMC Wild-Type Cells and Bind the C-Type Lectin MGL. PLoS ONE 2015, 10, e0125994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raes, G.; Brys, L.; Dahal, B.K.; Brandt, J.; Grooten, J.; Brombacher, F.; Vanham, G.; Noel, W.; Bogaert, P.; Boonefaes, T.; et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J. Leukoc. Biol. 2005, 77, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Schiarea, S.; Liguori, M.; Fabbri, M.; Pesce, S.; Zammataro, L.; Pasqualini, F.; Nebuloni, M.; Chiabrando, C.; Mantovani, A.; et al. Tumor-conditioned macrophages secrete migration-stimulating factor: A new marker for M2-polarization, influencing tumor cell motility. J. Immunol. 2010, 185, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Chieppa, M.; Bianchi, G.; Solinas, G.; Fabbri, M.; Laskarin, G.; Mantovani, A. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin. Dev. Immunol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Lenos, K.; Goos, J.A.; Vuist, I.M.; den Uil, S.H.; Delis-van Diemen, P.M.; Belt, E.J.; Stockmann, H.B.; Bril, H.; de Wit, M.; Carvalho, B.; et al. MGL ligand expression is correlated to BRAF mutation and associated with poor survival of stage III colon cancer patients. Oncotarget 2015, 6, 26278–26290. [Google Scholar] [CrossRef] [PubMed]
- Saeland, E.; van Vliet, S.J.; Backstrom, M.; van den Berg, V.C.; Geijtenbeek, T.B.; Meijer, G.A.; van Kooyk, Y. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol. Immunother. 2007, 56, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Napoletano, C.; Rughetti, A.; Agervig Tarp, M.P.; Coleman, J.; Bennett, E.P.; Picco, G.; Sale, P.; Denda-Nagai, K.; Irimura, T.; Mandel, U.; et al. Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Res. 2007, 67, 8358–8367. [Google Scholar] [CrossRef] [PubMed]
- Saeland, E.; Belo, A.I.; Mongera, S.; van Die, I.; Meijer, G.A.; van Kooyk, Y. Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. Int. J. Cancer 2012, 131, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan "self-associated molecular patterns" dampen innate immunity, but pathogens can mimic them. Glycobiology 2011, 21, 1121–1124. [Google Scholar] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Schwandt, T.; Greuter, M.; Oosting, M.; Jungerkes, F.; Tuting, T.; Boon, L.; O'Toole, T.; Kraal, G.; Limmer, A.; et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Veninga, H.; Borg, E.G.; Vreeman, K.; Taylor, P.R.; Kalay, H.; van Kooyk, Y.; Kraal, G.; Martinez-Pomares, L.; den Haan, J.M. Antigen targeting reveals splenic CD169+ macrophages as promoters of germinal center B-cell responses. Eur. J. Immunol. 2015, 45, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Hartnell, A.; Happerfield, L.; Miles, D.W.; Burchell, J.; Taylor-Papadimitriou, J.; Crocker, P.R. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology 1999, 98, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Akita, K.; Inoue, M.; Taketani, S.; Nakada, H. Down-modulation of B cell signal transduction by ligation of mucins to CD22. Biochem. Biophys. Res. Commun. 2008, 372, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Ohta, M.; Toda, M.; Murata, T.; Usui, T.; Akita, K.; Inoue, M.; Nakada, H. Mucin-induced apoptosis of monocyte-derived dendritic cells during maturation. Proteomics 2008, 8, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Ishida, A.; Toda, M.; Akita, K.; Inoue, M.; Yamashita, K.; Watanabe, M.; Murata, T.; Usui, T.; Nakada, H. Immunomodulation of monocyte-derived dendritic cells through ligation of tumor-produced mucins to Siglec-9. Biochem. Biophys. Res. Commun. 2010, 402, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.O.; Burchell, J.; Kudryashov, V.; Yin, B.W.; Taylor-Papadimitriou, J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J. Biol. Chem. 1996, 271, 33325–33334. [Google Scholar] [CrossRef] [PubMed]
- Michaelsson, E.; Broddefalk, J.; Engstrom, A.; Kihlberg, J.; Holmdahl, R. Antigen processing and presentation of a naturally glycosylated protein elicits major histocompatibility complex class II-restricted, carbohydrate-specific T cells. Eur. J. Immunol. 1996, 26, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Haurum, J.S.; Arsequell, G.; Lellouch, A.C.; Wong, S.Y.; Dwek, R.A.; McMichael, A.J.; Elliott, T. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic T lymphocytes. J. Exp. Med. 1994, 180, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Vlad, A.M.; Muller, S.; Cudic, M.; Paulsen, H.; Otvos, L., Jr.; Hanisch, F.G.; Finn, O.J. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J. Exp. Med. 2002, 196, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, T.; Hanisch, F.G. O-glycosylated human MUC1 repeats are processed in vitro by immunoproteasomes. J. Immunol. 2007, 179, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.G.; Schwientek, T.; von Bergwelt-Baildon, M.S.; Schultze, J.L.; Finn, O. O-Linked glycans control glycoprotein processing by antigen-presenting cells: A biochemical approach to the molecular aspects of MUC1 processing by dendritic cells. Eur. J. Immunol. 2003, 33, 3242–3254. [Google Scholar] [CrossRef] [PubMed]
- Hiltbold, E.M.; Vlad, A.M.; Ciborowski, P.; Watkins, S.C.; Finn, O.J. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J. Immunol. 2000, 165, 3730–3741. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.B.; Petersen, C.; Lavrsen, K.; Harndahl, M.; Buus, S.; Clausen, H.; Pedersen, A.E.; Wandall, H.H. Cancer associated aberrant protein O-glycosylation can modify antigen processing and immune response. PLoS ONE 2012, 7, e50139. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Streng-Ouwehand, I.; Litjens, M.; Kalay, H.; Saeland, E.; van Kooyk, Y. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int. J. Cancer 2011, 128, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Leone, B.E.; Zerbi, A.; Balzano, G.; Cainarca, S.; Sordi, V.; Pontillo, M.; Mercalli, A.; di Carlo, V.; Allavena, P.; et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J. Immunol. 2004, 172, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- Rughetti, A.; Pellicciotta, I.; Biffoni, M.; Backstrom, M.; Link, T.; Bennet, E.P.; Clausen, H.; Noll, T.; Hansson, G.C.; Burchell, J.M.; et al. Recombinant tumor-associated MUC1 glycoprotein impairs the differentiation and function of dendritic cells. J. Immunol. 2005, 174, 7764–7772. [Google Scholar] [CrossRef] [PubMed]
- Von Mensdorff-Pouilly, S.; Petrakou, E.; Kenemans, P.; van Uffelen, K.; Verstraeten, A.A.; Snijdewint, F.G.; van Kamp, G.J.; Schol, D.J.; Reis, C.A.; Price, M.R.; et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and n-acetylgalactosamine (GalNAc) peptides. Int. J. Cancer. 2000, 86, 702–712. [Google Scholar] [CrossRef]
- Blixt, O.; Bueti, D.; Burford, B.; Allen, D.; Julien, S.; Hollingsworth, M.; Gammerman, A.; Fentiman, I.; Taylor-Papadimitriou, J.; Burchell, J.M. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011, 13, R25. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, Y.; Linehan, M.; Weinstein, J.S.; Laidlaw, B.J.; Craft, J.E.; Iwasaki, A. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 2013, 39, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Freire, T.; Zhang, X.; Deriaud, E.; Ganneau, C.; Vichier-Guerre, S.; Azria, E.; Launay, O.; Lo-Man, R.; Bay, S.; Leclerc, C. Glycosidic Tn-based vaccines targeting dermal dendritic cells favor germinal center B-cell development and potent antibody response in the absence of adjuvant. Blood 2010, 116, 3526–3536. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.D.; Zhou, X.; Yu, G.; Zhao, Y.L.; Ren, Y.; Zhou, Y.D.; Li, Q.; Zhang, X.L. Knockdown of core 1 beta 1, 3-galactosyltransferase prolongs skin allograft survival with induction of galectin-1 secretion and suppression of CD8+ T cells: T synthase knockdown effects on galectin-1 and CD8+ T cells. J. Clin. Immunol. 2012, 32, 820–836. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Geijtenbeek, T.B. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol. 2009, 10, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; Vuist, I.M.; Lenos, K.; Tefsen, B.; Kalay, H.; Garcia-Vallejo, J.J.; van Kooyk, Y. Human T cell activation results in extracellular signal-regulated kinase (ERK)-calcineurin-dependent exposure of Tn antigen on the cell surface and binding of the macrophage galactose-type lectin (MGL). J. Biol. Chem. 2013, 288, 27519–27532. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Romain, G.; Flamar, A.L.; Duluc, D.; Dullaers, M.; Li, X.H.; Zurawski, S.; Bosquet, N.; Palucka, A.K.; Le Grand, R.; et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J. Exp. Med. 2012, 209, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Wandall, H.H.; Belleudi, F.; Bellati, F.; Pierelli, L.; et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 2012, 42, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, M.A.; Severino, P.F.; Guadalupe Cabral, M.; Silva, M.; Ferreira, J.A.; Calais, F.; Quinto, H.; Pen, C.; Ligeiro, D.; Santos, L.L.; et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol. Oncol. 2014, 8, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Perdicchio, M.; Cornelissen, L.A.; Streng-Ouwehand, I.; Engels, S.; Verstege, M.I.; Boon, L.; Geerts, D.; van Kooyk, Y.; Unger, W.W. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget 2016, 7, 8771–8782. [Google Scholar] [PubMed]
- Perdicchio, M.; Ilarregui, J.M.; Verstege, M.I.; Cornelissen, L.A.; Schetters, S.T.; Engels, S.; Ambrosini, M.; Kalay, H.; Veninga, H.; den Haan, J.M.; et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl. Acad. Sci. USA 2016, 113, 3329–3334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, M.; Tu, W.; Nishijima, K.; Iijima, S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem. Biophys. Res. Commun. 2008, 369, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, R.; Ohtsubo, K.; Takamatsu, S.; Taniguchi, N.; Angata, T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-beta secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 2013, 23, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Morizane, T.; Watanabe, T.; Tsuchimoto, K.; Tsuchiya, M. Impaired T cell function and decreased natural killer activity in patients with liver cirrhosis and their significance in the development of hepatocellular carcinoma. Gastroenterol. Jpn. 1980, 15, 226–232. [Google Scholar] [PubMed]
- Sullivan, J.L.; Byron, K.S.; Brewster, F.E.; Purtilo, D.T. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science 1980, 210, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Van Rinsum, J.; Smets, L.A.; van Rooy, H.; van den Eijnden, D.H. Specific inhibition of human natural killer cell-mediated cytotoxicity by sialic acid and sialo-oligosaccharides. Int. J. Cancer 1986, 38, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Ogata, S.; Maimonis, P.J.; Itzkowitz, S.H. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Res. 1992, 52, 4741–4746. [Google Scholar] [PubMed]
- Cohen, M.; Elkabets, M.; Perlmutter, M.; Porgador, A.; Voronov, E.; Apte, R.N.; Lichtenstein, R.G. Sialylation of 3-methylcholanthrene-induced fibrosarcoma determines antitumor immune responses during immunoediting. J. Immunol. 2010, 185, 5869–5878. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Demoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Invest. 2014, 124, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef] [PubMed]



| Species | Receptor | O-glycan structure | |
|---|---|---|---|
| human | hMGL | Tn antigen |  |
| Sialyl-Tn antigen | 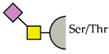 | ||
| mouse | mMGL1 | Lewis X | 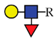 |
| mMGL2 | Tn antigen |  | |
| T antigen | 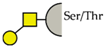 | ||
| Core-2 | 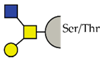 | ||
| human | hSiglec-3 | Sialyl-Tn antigen | 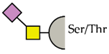 |
| hSiglec-9 | Sialyl-Tn antigen | 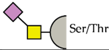 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornelissen, L.A.M.; Van Vliet, S.J. A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer. Biomolecules 2016, 6, 26. https://doi.org/10.3390/biom6020026
Cornelissen LAM, Van Vliet SJ. A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer. Biomolecules. 2016; 6(2):26. https://doi.org/10.3390/biom6020026
Chicago/Turabian StyleCornelissen, Lenneke A.M., and Sandra J. Van Vliet. 2016. "A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer" Biomolecules 6, no. 2: 26. https://doi.org/10.3390/biom6020026
APA StyleCornelissen, L. A. M., & Van Vliet, S. J. (2016). A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer. Biomolecules, 6(2), 26. https://doi.org/10.3390/biom6020026







