Detoxification and Immune Transcriptomic Response of the Gill Tissue of Bay Scallop (Argopecten irradians) Following Exposure to the Algicide Palmitoleic Acid
Abstract
:1. Introduction
2. Results
2.1. Analysis of Differentially Expressed Genes Libraries
2.2. Analysis of Sequences Mapping
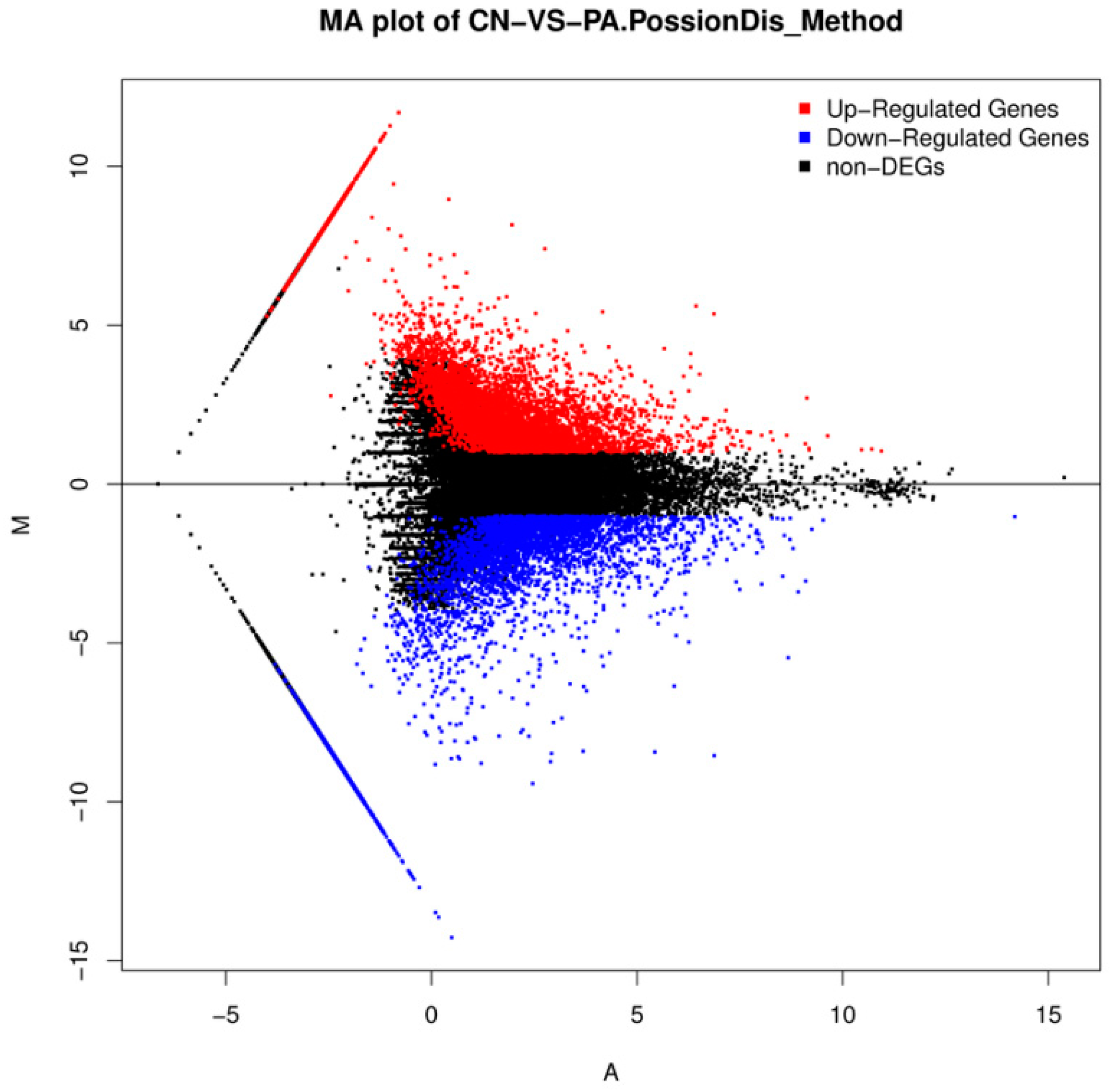
2.3. Differential Gene Expression Analysis
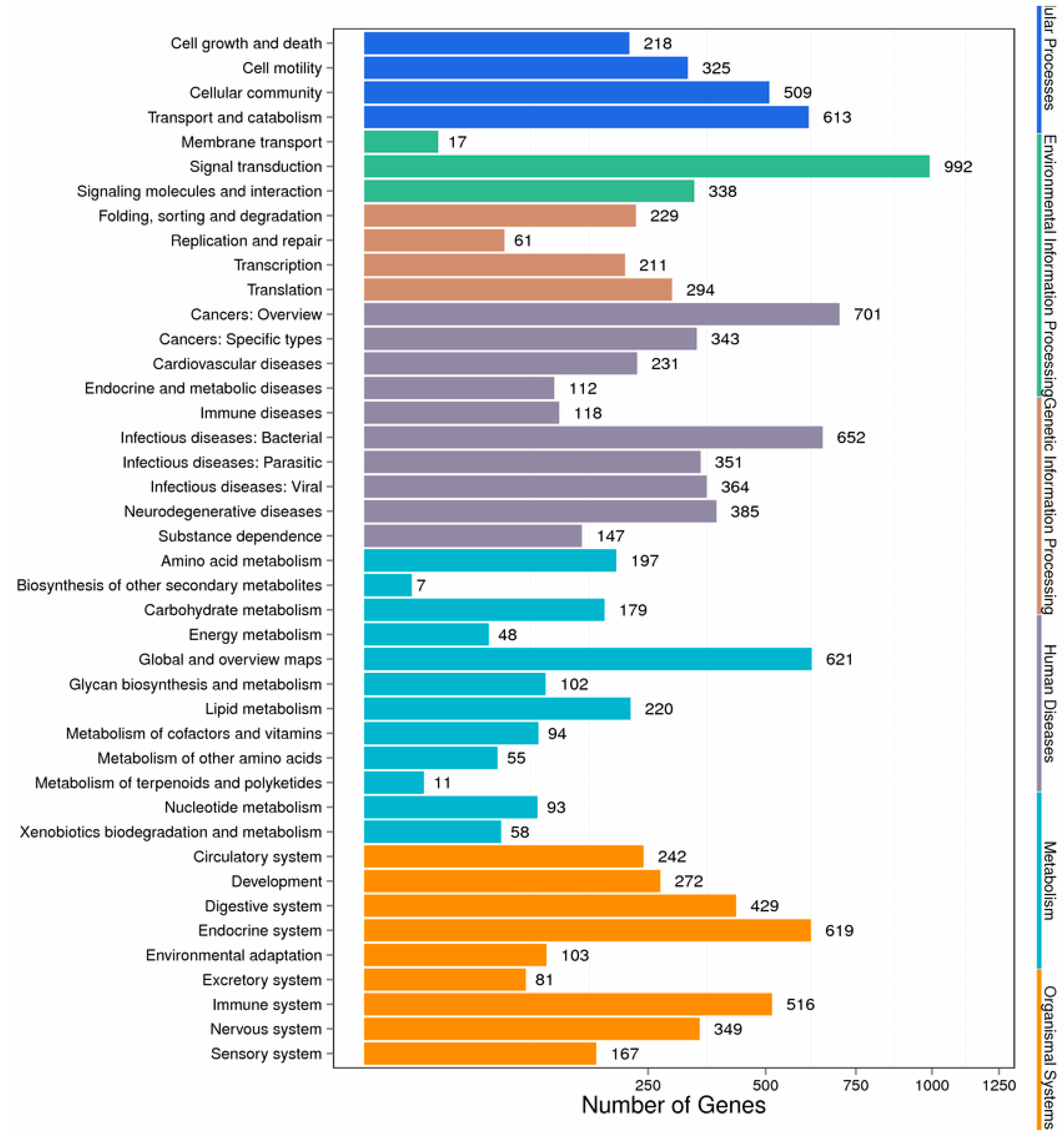
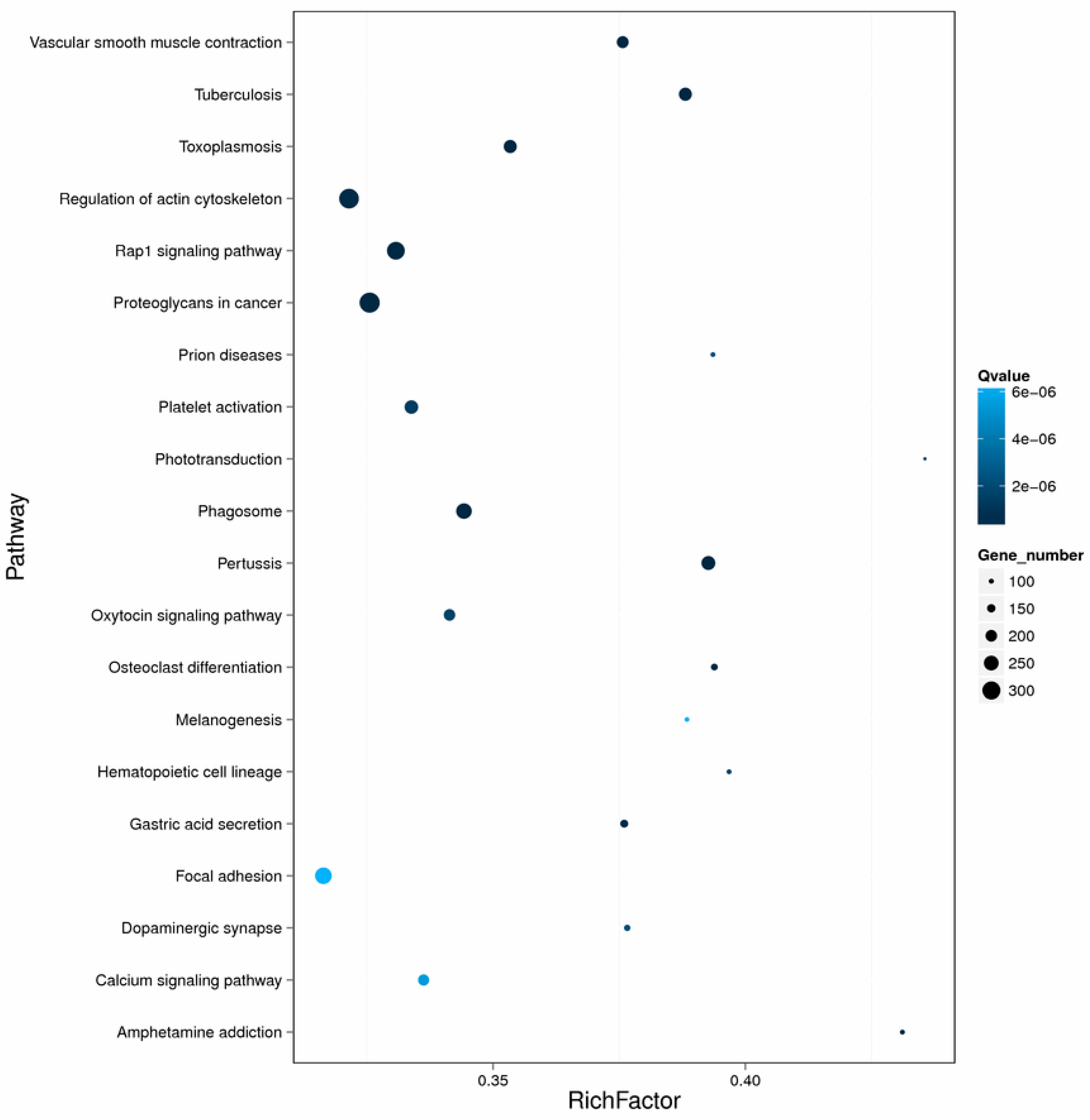
2.4. Enrichment and Pathway Analysis
2.5. Identification of Genes Related to Palmitoleic Acid-Induced Stress Response
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance and Palmitoleic Acid Exposure
4.2. RNA Preparation
4.3. Library Preparation and Illumina Sequencing
4.4. De Novo Transcriptome Assembly
4.5. Gene Annotation and Analysis
4.6. Enrichment Analysis of Differentially Expressed Genes
4.7. Quantitative Real-Time PCR Verification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cai, Y.; Pan, L.; Hu, F.; Jin, Q.; Liu, T. Deep sequencing-based transcriptome profiling analysis of Chlamys farreri exposed to benzo[a]pyrene. Gene 2014, 551, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.D.; Bowen, V.T.; Farrington, J.W.; Harvey, G.; Martin, J.H.; Parker, P.L.; Risebrough, R.W.; Robertson, W.; Schneider, E.; Gamble, E. The mussel watch. Environ. Conserv. 1978, 5, 101–125. [Google Scholar] [CrossRef]
- Hu, F.; Pan, L.; Cai, Y.; Liu, T.; Jin, Q. Deep sequencing of the scallop Chlamys farreri transcriptome response to tetrabromobisphenol A (TBBPA) stress. Mar. Genom. 2015, 19, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Miao, J.; Wang, J.; Liu, J. AHH activity, tissue dose and DNA damage in different tissues of the scallop Chlamys farreri exposed to benzo[a]pyrene. Environ. Pollut. 2008, 153, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, L.Q.; Zhang, L.; Miao, J.; Wang, J. Immune responses, ROS generation and the haemocyte damage of scallop Chlamys farreri exposed to Aroclor 1254. Fish Shellfish Immunol. 2009, 26, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pan, L.; Wang, J.; Yang, H.; Liu, D. Application of the biomarker responses in scallop (Chlamys farreri) to assess metals and PAHs pollution in Jiaozhou Bay, China. Mar. Environ. Res. 2012, 80, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Fu, L.; An, X.; Zhang, B.; Li, Y.; Chen, Z.; Zheng, W.; Yi, L.; Zheng, T. A novel algicide: Evidence of the effect of a fatty acid compound from the marine bacterium, Vibrio sp. BS02 on the harmful dinoflagellate, Alexandrium tamarense. PLoS ONE 2014, 9, e91201. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Zhou, Y.; Lv, J.; Su, J.; Tian, Y.; Zhang, J.; Lin, G.; Zheng, T. An algicidal protein produced by bacterium isolated from the Donghai Sea, China. Harmful Algae 2012, 13, 83–88. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, B.; Zhang, J.; Huang, L.; Lin, J.; Li, X.; Zhou, Y.; Wang, H.; Yang, X.; Su, J. A marine algicidal actinomycete and its active substance against the harmful algal bloom species Phaeocystis globosa. Appl. Microbiol. Biotechnol. 2013, 97, 9207–9215. [Google Scholar] [CrossRef] [PubMed]
- Gainey, L.; Shumway, S.; Shumway, S. A compendium of the responses of bivalve molluscs to toxic dinoflagellates. J. Shellfish Res. 1988, 7, 623–628. [Google Scholar]
- Zheng, T.; Su, J.; Maskaoui, K.; Yu, Z.; Hu, Z.; Xu, J.; Hong, H. Microbial modulation in the biomass and toxin production of a red-tide causing alga. Mar. Pollut. Bull. 2005, 51, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhou, M.; Fu, M.; Yu, R.; Wang, Y.; Li, J. Effects of the dinoflagellate Alexandrium tamarense on early development of the scallop Argopecten irradians concentricus. Aquaculture 2003, 217, 167–178. [Google Scholar] [CrossRef]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Kim, S.G.; Yun, S.; Park, S.C. Effect of the algaecide palmitoleic acid on the immune function of the bay scallop Argopecten irradians. Molecules 2016, 21, 610. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Yun, S.; Kim, H.J.; Kim, S.G.; Park, S.C. Immune response of the bay scallop, Argopecten irradians, after exposure to the algicide palmitoleic acid. Fish Shellfish Immunol. 2016, 57, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.A.; Spacek, D.V.; Snyder, M.P. High-throughput sequencing technologies. Mol. Cell 2015, 58, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Dong, S.; Fang, C.; Wu, X.; Ye, T.; Lin, Y. Deep sequencing-based transcriptome profiling analysis of Oryzias melastigma exposed to PFOS. Aquat. Toxicol. 2012, 120, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, H.; Kong, L.; Li, Q. Transcriptomic responses to salinity stress in the Pacific oyster Crassostrea gigas. PLoS ONE 2012, 7, e46244. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Sun, Y.; Wang, J.; Xing, Q.; Zou, J.; Li, R.; Wang, Z.; Wang, S.; Hu, X.; Zhang, L. Sequencing-based gene network analysis provides a core set of gene resource for understanding thermal adaptation in Zhikong scallop Chlamys farreri. Mol. Ecol. Resour. 2014, 14, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Philipp, E.E.; Kraemer, L.; Melzner, F.; Poustka, A.J.; Thieme, S.; Findeisen, U.; Schreiber, S.; Rosenstiel, P. Massively parallel RNA sequencing identifies a complex immune gene repertoire in the lophotrochozoan Mytilus edulis. PLoS ONE 2012, 7, e33091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannam, M.L.; Bamber, S.D.; Moody, J.A.; Galloway, T.S.; Jones, M.B. Immune function in the Arctic Scallop, Chlamys islandica, following dispersed oil exposure. Aquat. Toxicol. 2009, 92, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Marioni, J.C.; Mason, C.E.; Mane, S.M.; Stephens, M.; Gilad, Y. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008, 18, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, P. Q-Gene: Processing quantitative real-time RT–PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pan, L.; Li, Z.; Cai, Y.; Miao, J. Metabolites analysis, metabolic enzyme activities and bioaccumulation in the clam Ruditapes philippinarum exposed to benzo[a]pyrene. Ecotox. Environ. Saf. 2014, 107, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Pan, L.; Liu, T.; Hu, F. RNA-seq based on transcriptome reveals differ genetic expressing in Chlamys farreri exposed to carcinogen PAHs. Environ. Toxicol. Pharmacol. 2015, 39, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Favreau, L.; Pickett, C. Transcriptional regulation of the rat NAD(P)H: Quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J. Biol. Chem. 1991, 266, 4556–4561. [Google Scholar] [PubMed]
- Jaiswal, A.K. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic. Biol. Med. 2000, 29, 254–262. [Google Scholar] [CrossRef]
- Anzenbacher, P.; Anzenbacherová, E. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci. 2001, 58, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zou, Y.; Weng, H.W.; Li, H.Y.; Liu, J.S.; Yang, W.D. Proteomic profile in Perna viridis after exposed to Prorocentrum lima, a dinoflagellate producing DSP toxins. Environ. Pollut. 2015, 196, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, J.; Chen, W.C.; Li, H.Y.; Liu, J.S.; Jiang, T.; Yang, W.D. P-glycoprotein expression in Perna viridis after exposure to Prorocentrum lima, a dinoflagellate producing DSP toxins. Fish Shellfish Immunol. 2014, 39, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Yang, H.S.; Delaporte, M.; Zhao, S.J.; Xing, K. Immune responses of the scallop Chlamys farreri after air exposure to different temperatures. J. Exp. Mar. Biol. Ecol. 2007, 345, 52–60. [Google Scholar] [CrossRef]
- Whang, I.; De Zoysa, M.; Nikapitiya, C.; Lee, Y.; Kim, Y.; Lee, S.; Oh, C.; Jung, S.J.; Oh, M.J.; Choi, C.Y.; et al. Molecular characterization and expression analysis of Cathepsin B and L cysteine proteases from rock bream (Oplegnathus fasciatus). Fish Shellfish Immunol. 2011, 30, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.K.; Dixit, P.; Sharma, R.L. Immunodiagnostic/protective role of cathepsin L cysteine proteinases secreted by Fasciola species. Vet. Parasitol. 2008, 154, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Chwieralski, C.E.; Welte, T.; Buhling, F. Cathepsin-regulated apoptosis. Apoptosis 2006, 11, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Jin, K.; Wang, L.; Sun, F.; Li, J. Identification of cathepsin B in the razor clam Sinonovacula constricta and its role in innate immune responses. Dev. Comp. Immunol. 2013, 41, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, Q.; Chen, H.; Yang, C.; Fan, Y. Identification of the spinal expression profile of non-coding RNAs involved in neuropathic pain following spared nerve injury by sequence analysis. Front. Mol. Neurosci. 2017, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Cock, P.J.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Audic, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Kim, S.W.; Yun, S.; Park, S.C. Effects of algal toxin okadaic acid on the non-specific immune and antioxidant response of bay scallop (Argopecten irradians). Fish Shellfish Immunol. 2017, 65, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J. Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





| Sample | Total Raw Reads (Mb) | Total Clean Reads (Mb) | Total Clean Bases (Gb) | Clean Reads Q20 (%) | Clean Reads Q30 (%) | Clean Reads Ratio (%) |
|---|---|---|---|---|---|---|
| CN | 45.92 | 45.76 | 4.58 | 98.21 | 95.30 | 99.65 |
| PA | 45.92 | 45.76 | 4.58 | 98.40 | 95.69 | 99.65 |
| Sample | Total Number | Total Length | Mean Length | N50 | N70 | N90 | GC (%) |
|---|---|---|---|---|---|---|---|
| CN | 78,434 | 52,871,582 | 674 | 1230 | 568 | 252 | 39.13 |
| PA | 80,539 | 56,824,660 | 705 | 1308 | 610 | 261 | 39.60 |
| Sample | Total Number | Total Length | Mean Length | N50 | N70 | N90 | GC (%) |
|---|---|---|---|---|---|---|---|
| CN | 51,495 | 41,085,320 | 797 | 1412 | 702 | 302 | 39.47 |
| PA | 55,973 | 46,742,302 | 835 | 1503 | 753 | 313 | 39.86 |
| All unigenes | 62,099 | 57,266,803 | 922 | 1735 | 897 | 338 | 39.51 |
| Description | Transcript | Log2 Fold Change (RNA-seq) | Regulation |
|---|---|---|---|
| Down regulated | |||
| Immune system | CLEC4E | −4.278 | Down |
| CLEC4F | −9.069 | Down | |
| FIBCD 1 | −2.224 | Down | |
| ACP | −1.088 | Down | |
| Apoptosis | CTS-B | −1.25 | Down |
| BIRC7 | −14.271 | Down | |
| Transmembrane proteins | ABCB1 | −1.481 | Down |
| ABCC1 | −1.167 | Down | |
| ABCG2 | −1.514 | Down | |
| Oxidative stress | NQR2 | −8.406 | Down |
| Metabolism of xenobiotics | CYP 4F22 | −7.988 | Down |
| CYP 4B1 | −8.778 | Down | |
| CYP 2C8 | −3.918 | Down | |
| CREB 3 | −1.147 | Down | |
| Antioxidant | GR | −5.628 | Down |
| Nrf2 | −1.035 | Down | |
| Up regulated | |||
| Antioxidant | Cu/Zn SOD | 2.107 | Up |
| Mn SOD | 1.728 | Up | |
| GST 1 | 2.860 | Up | |
| GST 2 | 10.276 | Up | |
| GST 3 | 3.237 | Up | |
| GST ω | 1.198 | Up | |
| GST A | 2.648 | Up | |
| GST κ | 5.319 | Up | |
| Apoptosis | CTS-A | 1.07 | Up |
| Immune system | TLR 2 | 1.780 | Up |
| TLR 4 | 4.268 | Up | |
| TLR 13 | 5.349 | Up | |
| Metabolism of xenobiotics | CYP 2C14 | 8.683 | Up |
| CYP 2U1 | 4.241 | Up | |
| CYP 3A24 | 1.219 | Up | |
| CYP 4A2 | 1.786 | Up | |
| mo-A | 1.86 | Up | |
| Primer | Products | |
|---|---|---|
| Primers Used for Fe/Mn SOD | ||
| Mn SOD-F | 5’GTGCCTCTACTGCTGTCC3’ | 103 bp |
| Mn SOD-R | 5’TGAAGTGGGTCCTGGTTA3’ | |
| Primers used for GST-Omega | ||
| GST ω-F | 5’GGCAAACCCGCTTCTGTA3’ | 213 bp |
| GST ω-R | 5’CCCGTGCTGTGGGATAAA3’ | |
| Primers used for GST-Kappa | ||
| GST κ-F | 5’GACCAAGGGATTTCTGATGAG3’ | 183 bp |
| GST κ-R | 5’ATGAGCGACAATAATAGGGGAT3’ | |
| Primers used for ACP | ||
| ACP-F | 5’AGACAGAACCCGACAACTC3’ | 266 bp |
| ACP-R | 5’GCTATGAGGCTGATTAGAAGG3’ | |
| Primers used for CTS-A | ||
| CTS-A-F | 5’ACCGCCCTTGACAGTAAC3’ | 203 bp |
| CTS-A-R | 5’CACGAAAGAAACGAGTAA3’ | |
| Primers used for CTS-B | ||
| CTS-B-F | 5’ACCCTCCGTCACATCCCA3’ | 205 bp |
| CTS-B-R | 5’GACTCCCGTAAGGCGTGGT3’ | |
| Primers used for mo-A | ||
| mo-A-F | 5’GGGGAGCATCTTTAATCG3’ | 136 bp |
| mo-A-R | 5’ACTGGCTGGTTCTTCACA3’ | |
| Primers used for NQR2 | ||
| NQR2-A-F | 5’CCAAGTACAACTACGGCTCT3’ | 270 bp |
| NQR2-A-R | 5’AAGGTCGGGAAACTATGC3’ | |
| Primers used for CYP3A24 | ||
| CYP3A24-F | 5’AGCAGCCAATGAGGAGTGT3’ | 198 bp |
| CYP3A24-R | 5’TGTAGGACCATATCGCAGAC3’ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Park, S.C. Detoxification and Immune Transcriptomic Response of the Gill Tissue of Bay Scallop (Argopecten irradians) Following Exposure to the Algicide Palmitoleic Acid. Biomolecules 2018, 8, 139. https://doi.org/10.3390/biom8040139
Chi C, Giri SS, Jun JW, Kim HJ, Kim SW, Kang JW, Park SC. Detoxification and Immune Transcriptomic Response of the Gill Tissue of Bay Scallop (Argopecten irradians) Following Exposure to the Algicide Palmitoleic Acid. Biomolecules. 2018; 8(4):139. https://doi.org/10.3390/biom8040139
Chicago/Turabian StyleChi, Cheng, Sib Sankar Giri, Jin Woo Jun, Hyoun Joong Kim, Sang Wha Kim, Jeong Woo Kang, and Se Chang Park. 2018. "Detoxification and Immune Transcriptomic Response of the Gill Tissue of Bay Scallop (Argopecten irradians) Following Exposure to the Algicide Palmitoleic Acid" Biomolecules 8, no. 4: 139. https://doi.org/10.3390/biom8040139
APA StyleChi, C., Giri, S. S., Jun, J. W., Kim, H. J., Kim, S. W., Kang, J. W., & Park, S. C. (2018). Detoxification and Immune Transcriptomic Response of the Gill Tissue of Bay Scallop (Argopecten irradians) Following Exposure to the Algicide Palmitoleic Acid. Biomolecules, 8(4), 139. https://doi.org/10.3390/biom8040139






