Blockade of Intranigral and Systemic D3 Receptors Stimulates Motor Activity in the Rat Promoting a Reciprocal Interaction among Glutamate, Dopamine, and GABA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Microdialysis Coupled to Behavioral Measurements
2.3. Experimental Design and Statistical Analysis
2.4. Drugs
3. Results
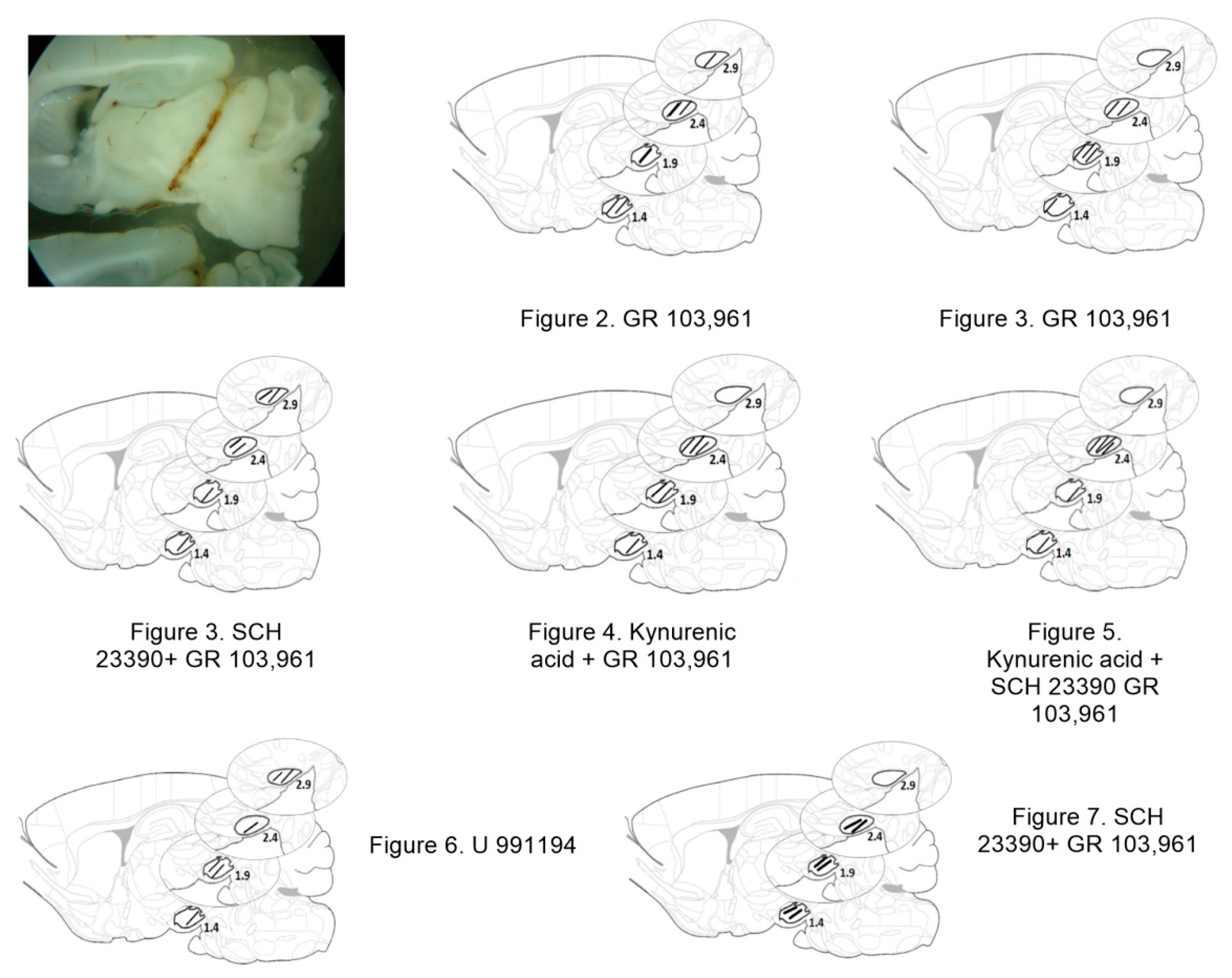
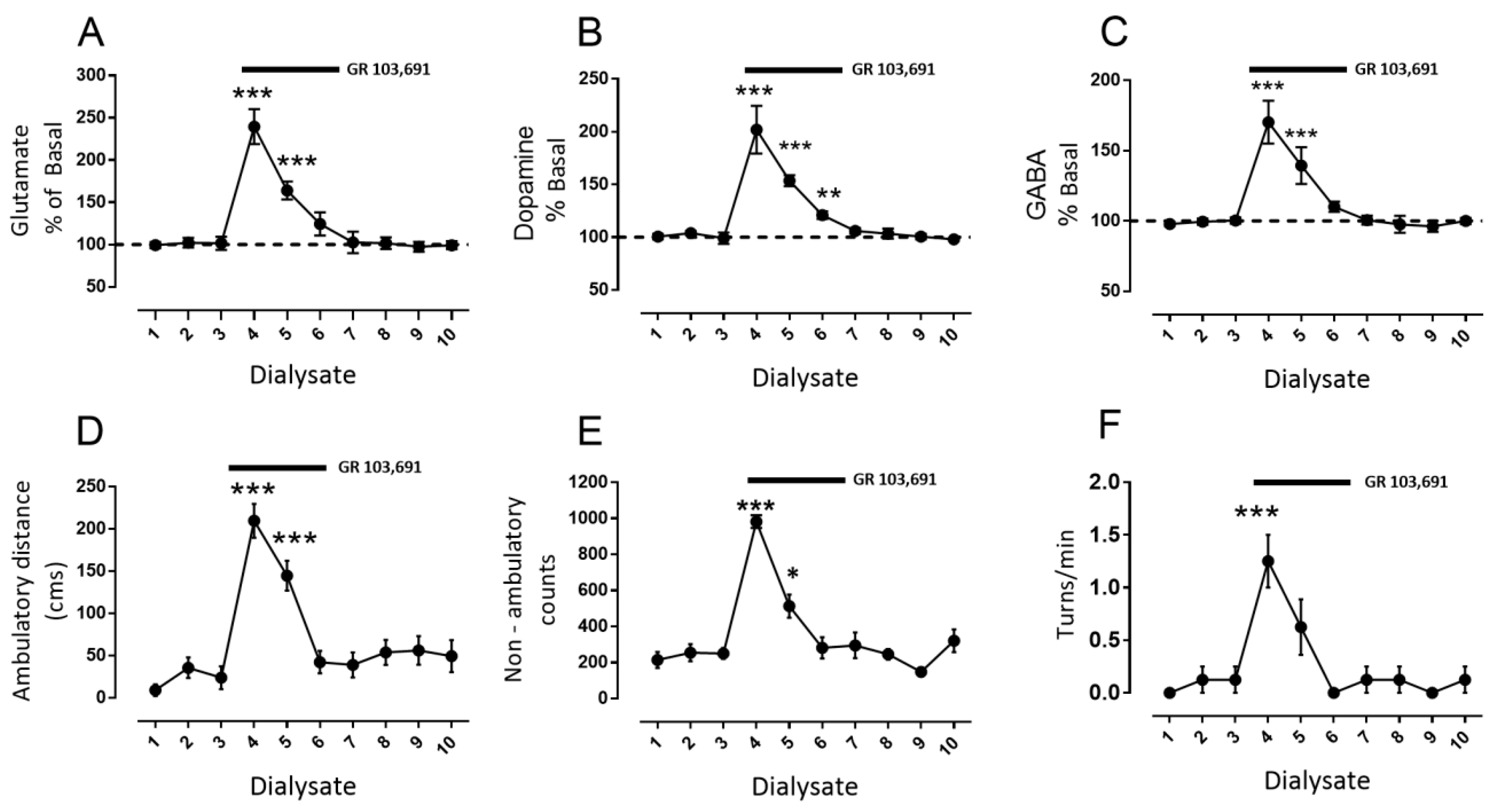
3.1. D3R Blockade Increases Glutamate, Dopamine, and GABA in the SNr of Freely Moving Rats
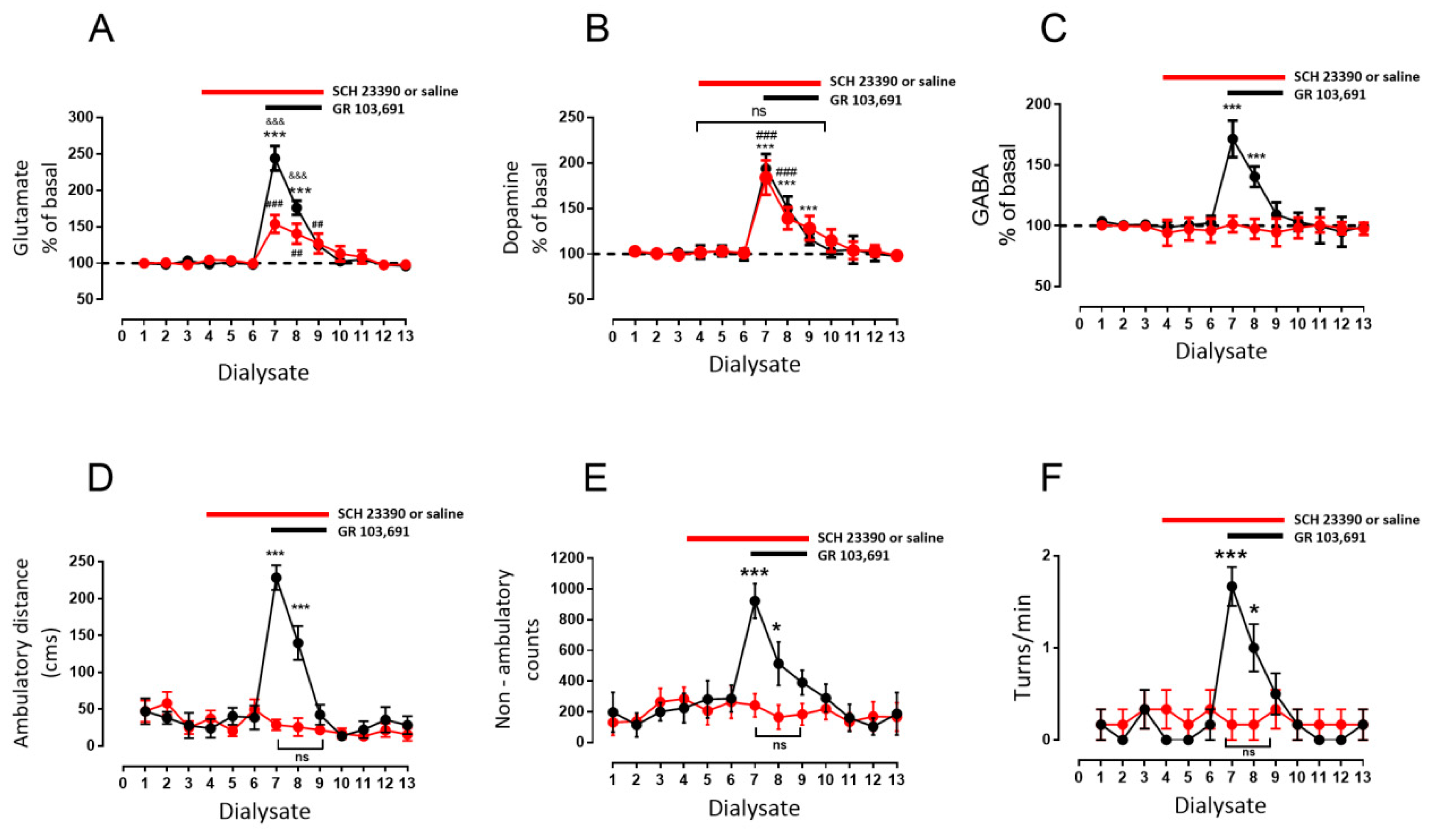
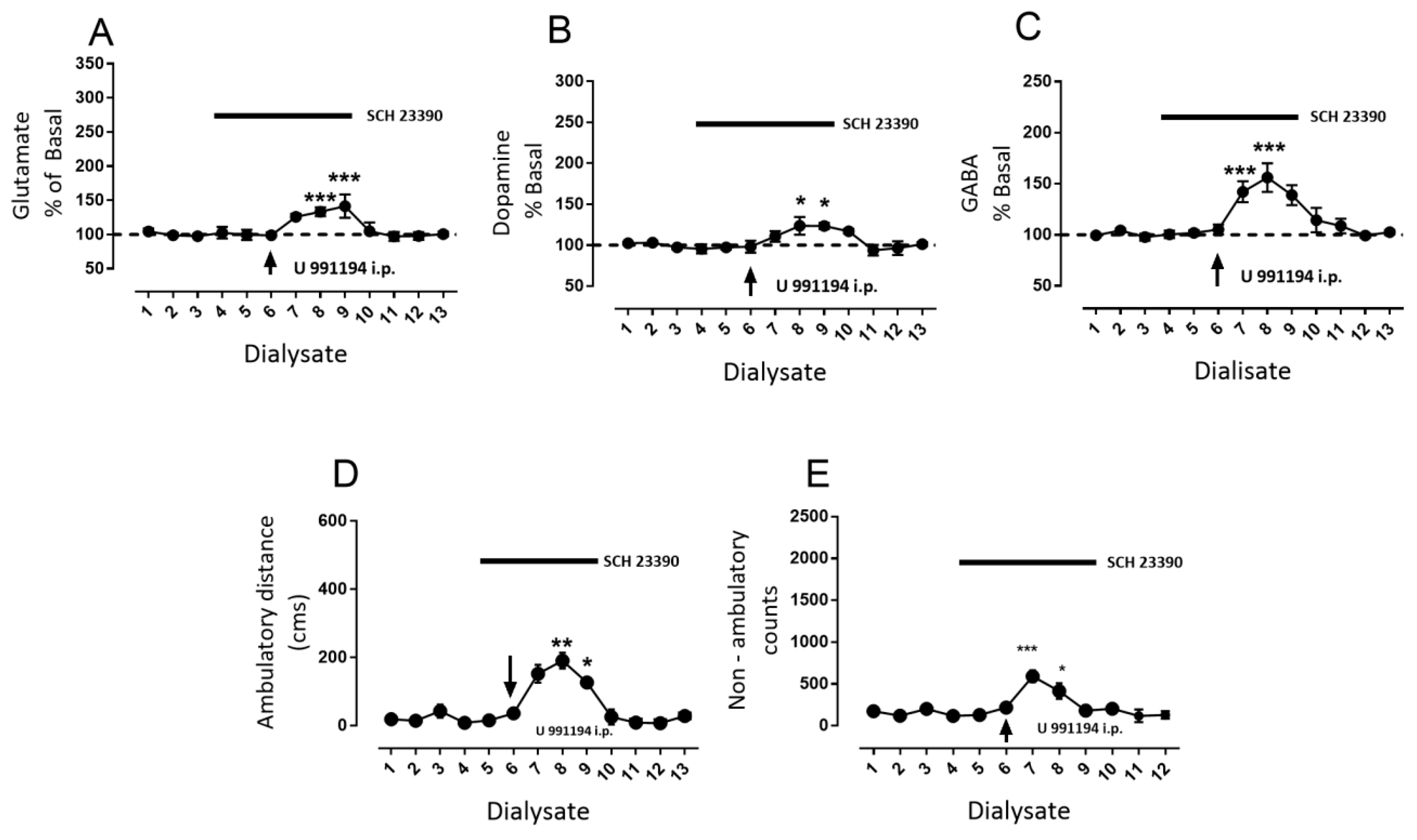
3.2. D1R-Dependent Effects of D3R Blockade on GABA and Glutamate Release
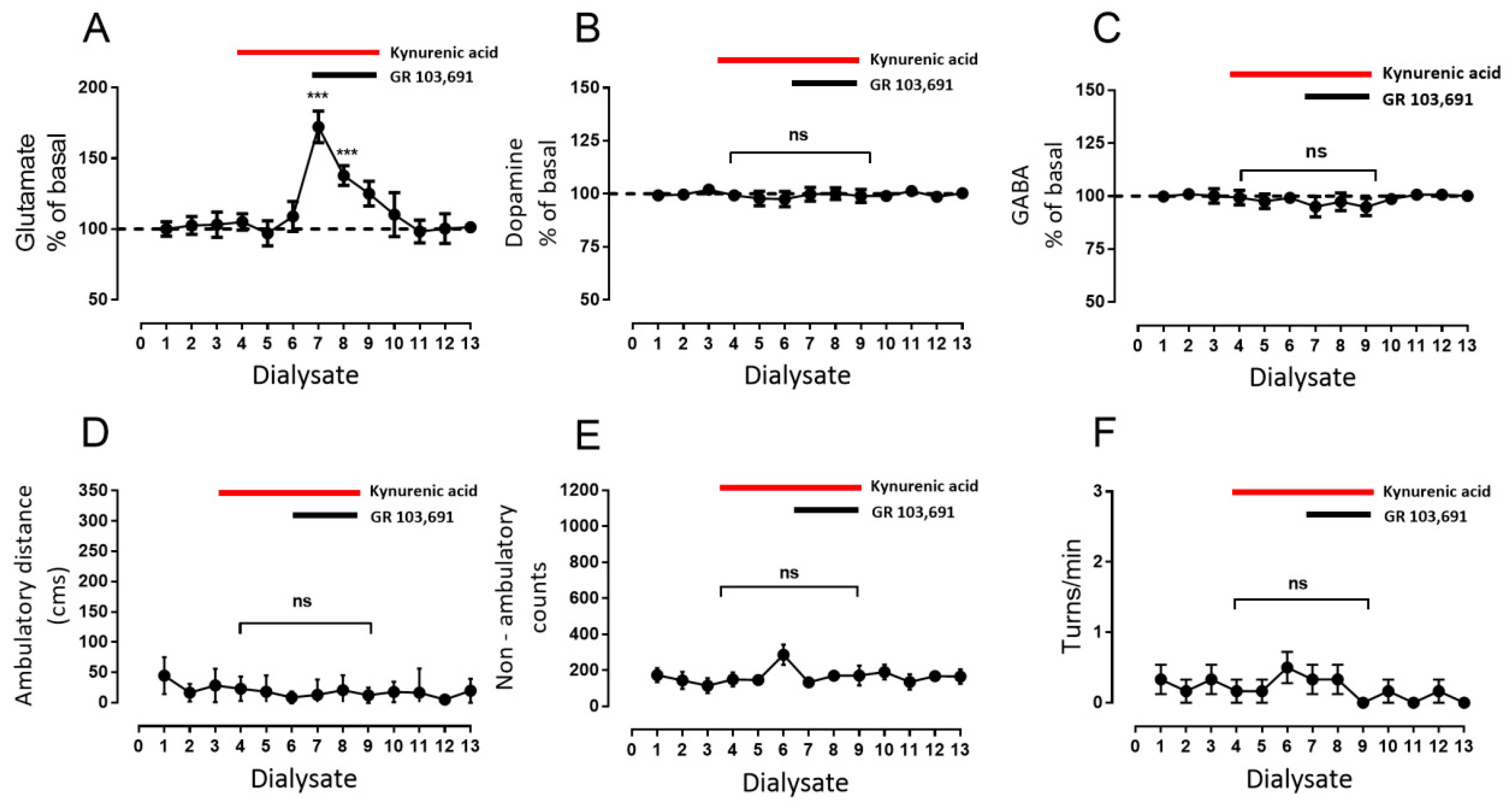
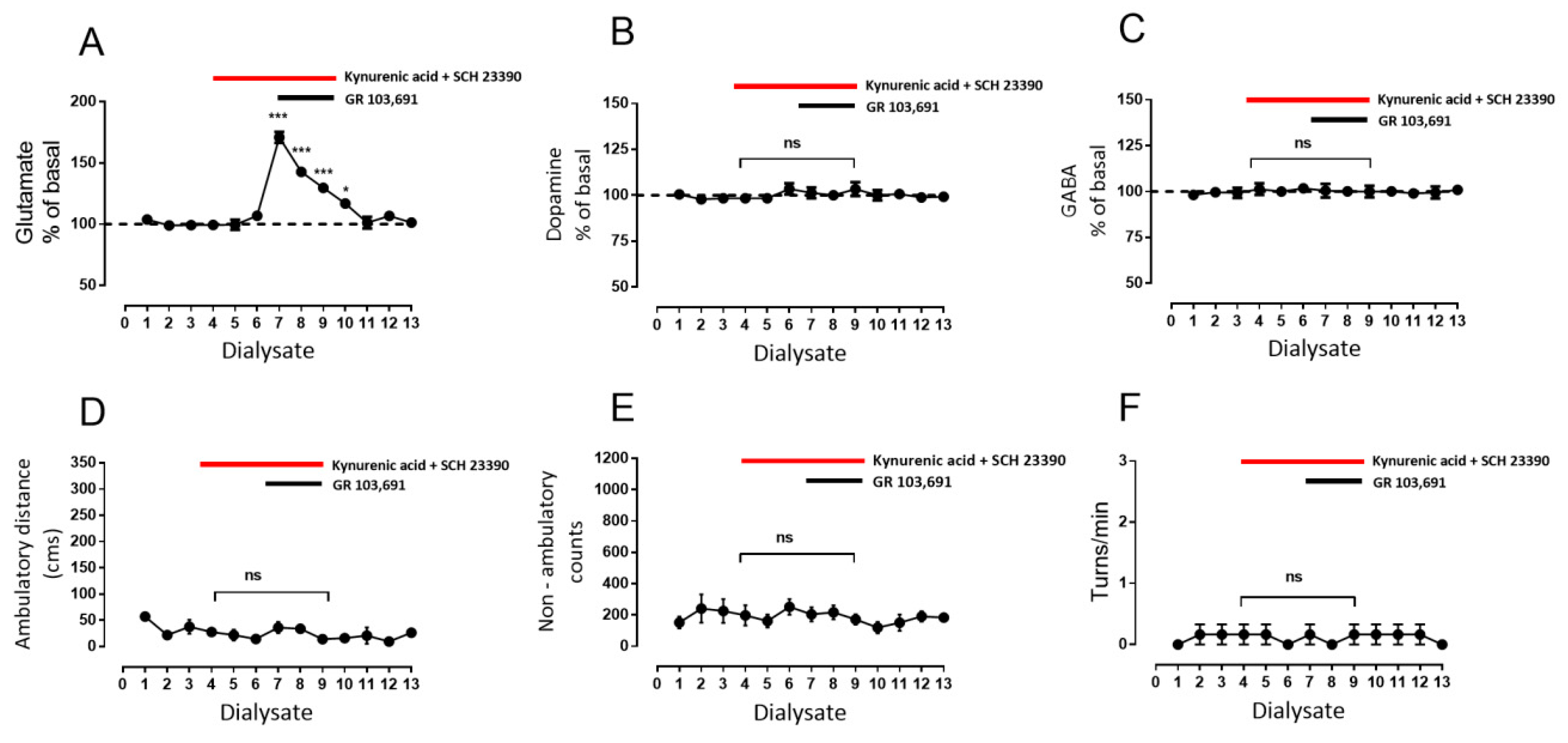
3.3. Increments in Glutamate and GABA Release by D3R Blockade Depend on Endogenous Dopamine
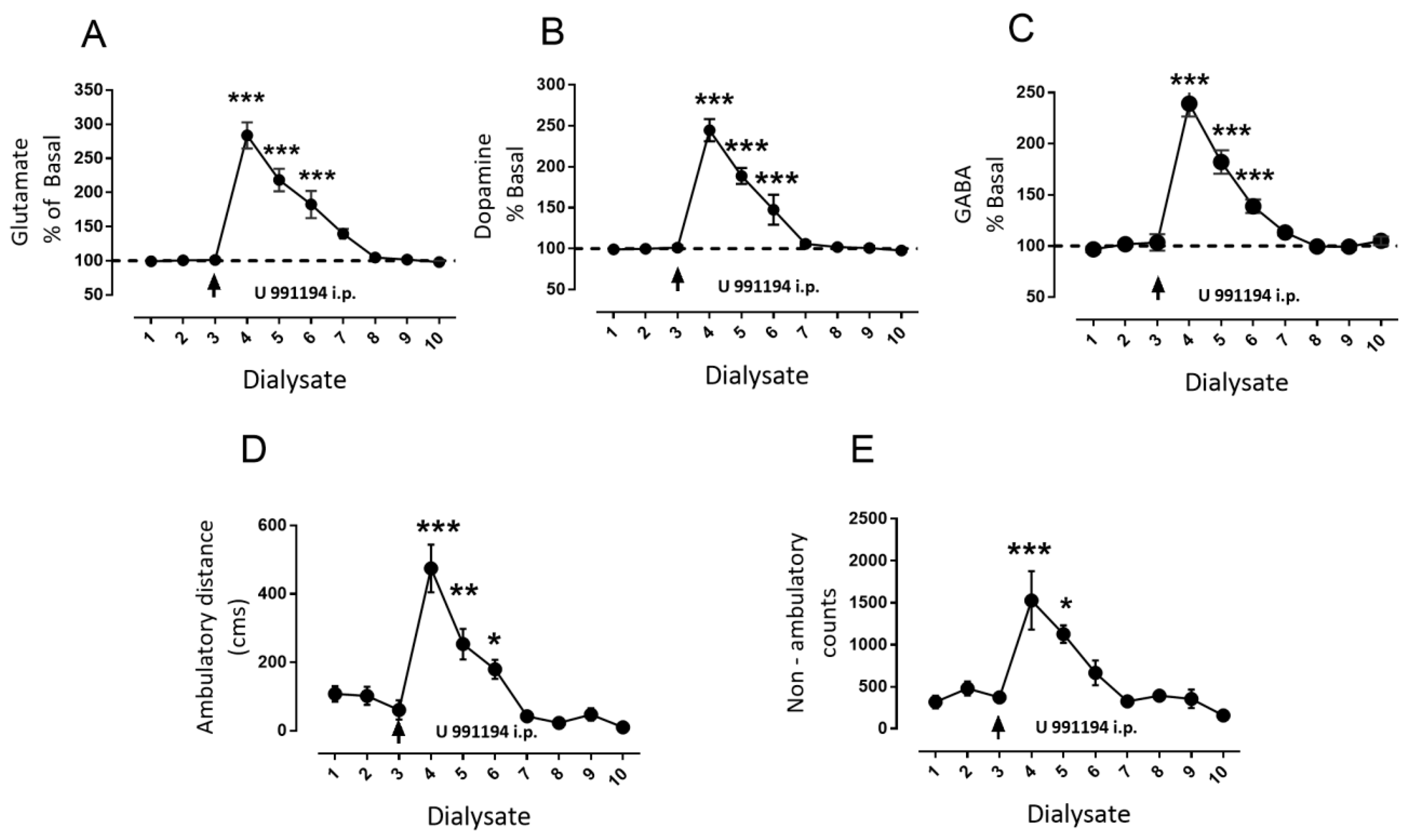
3.4. Systemic Blockade of D3R Mimics the Effect of Intra-Nigral D3R Blockade Role of Nigral D1R
4. Discussion
4.1. Considerations of Drugs Employed
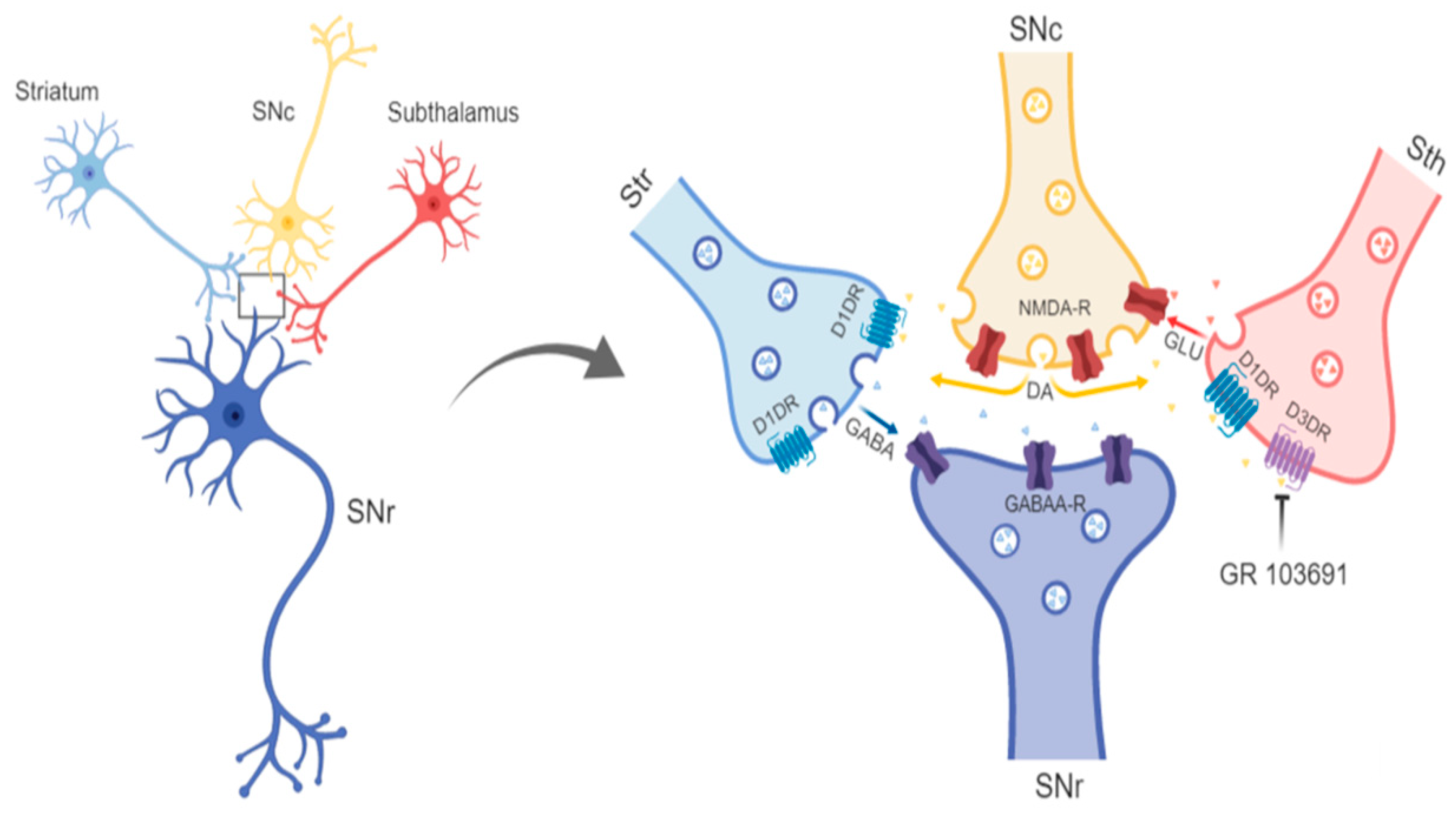
4.2. Role of D3R at Dopamine, GABA, and Glutamate Afferents to SNr in the Neurochemical and Behavioral Effect of Intra-Nigral D3R Blockade
4.3. Disruption of Reciprocal Interaction between Glutamate, Dopamine, and GABA as a Feasible Explanation of the Motor Effects of Intra-Nigral D3R Blockade
- Blockade of D1R abolishes the increase in GABA level and motor behavior [66] (Figure 2). In addition, in our study, the blockade of NMDAR by kynurenic acid also prevented the GABA increment and motor activation (Figure 4 and Figure 5), indicating the lack of activation of striato–nigral D1R. No substantial tonic D1R activation exists under this experimental condition, in that SCH 23,390 did not significantly modify the baseline level of the three neurotransmitters.
- Nigral blockade of GABA A receptors prevents all behavioral effects of the D1R agonist [67]. Thus, postsynaptic nigral glutamate receptors, despite their activation by released glutamate, appear not to participate directly in motor stimulation, but they do promote changes in GABA indirectly through the mobilization of dopamine by NMDAR activation.
- Output neurons appear to possess more sensitivity to GABA than to glutamate with respect to changes in firing rate [51,70]. Additionally, nigral GABA A receptors are more densely expressed in nigra (GABA-sensitive binding: 382 fmol/mg protein [71]) than NMDA receptors (glutamate on NMDA receptors: 112 fmol/mg protein [72]).
4.4. Behavioral Effects of the Blockade of Nigral versus Systemic D3R
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Damsma, G.; Bottema, T.; Westerink, B.H.; Tepper, P.G.; Dijkstra, D.; Pugsley, T.A.; Wikström, H. Pharmacological aspects of R-(+)-7-OH-DPAT, a putative dopamine D3 receptor ligand. Eur. J. Pharmacol. 1993, 249, R9–R10. [Google Scholar] [CrossRef]
- Waters, N.; Svensson, K.; Haadsma-Svensson, S.R.; Smith, M.W.; Carlsson, A. The dopamine D3-receptor: A postsynaptic receptor inhibitory on rat locomotor activity. J. Neural Transm. 1993, 94, 11–19. [Google Scholar] [CrossRef]
- Svensson, K.; Carlsson, A.; Waters, N. Locomotor inhibition by the D3 ligand R-(+)-7-OH-DPAT is independent of changes in dopamine release. J. Neural Transm. Gen. Sect. 1994, 95, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Pugsley, T.A.; Davis, M.D.; Akunne, H.C.; MacKenzie, R.G.; Shih, Y.H.; Damsma, G.; Wikstrom, H.; Whetzel, S.Z.; Georgic, L.M.; Cooke, L.W. Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J. Pharmacol. Exp. Ther. 1995, 275, 1355–1366. [Google Scholar] [PubMed]
- Sautel, F.; Griffon, N.; Sokoloff, P.; Schwartz, J.C.; Launay, C.; Simon, P.; Costentin, J.; Schoenfelder, A.; Garrido, F.; Mann, A. Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. J. Pharmacol. Exp. Ther. 1995, 275, 1239–1246. [Google Scholar] [PubMed]
- Bristow, L.J.; Cook, G.P.; Gay, J.C.; Kulagowski, J.J.; Landon, L.; Murray, F.; Saywell, L.; Young, L.; Hutson, P.H. The behavioural and neurochemical profile of the putative dopamine D3 receptor agonist, (+)-PD 128907, in the rat. Neuropharmacology 1996, 35, 285–294. [Google Scholar] [CrossRef]
- Audinot, V.; Gluck, L.; Newman-Tancredi, A.; Bervoets, K.; Deposte, I.; Brocco, M.; Millan, M.J. A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194. Eur. Neuropsychopharmacol. 1996, 287, 187–197. [Google Scholar]
- Clifford, J.J.; Waddington, J.L. Heterogeneity of behavioural profile between three new putative selective D 3 dopamine receptor antagonists using an ethologically based approach. Psychopharmacology 1998, 136, 284–290. [Google Scholar] [CrossRef]
- Gendreau, P.L.; Petitto, J.M.; Schnauss, R.; Frantz, K.J.; Van Hartesveldt, C.; Gariépy, J.-L.; Lewis, M.H. Effects of the putative dopamine D3 receptor antagonist PNU 99194A on motor behavior and emotional reactivity in C57BL/6J mice. Eur. J. Pharmacol. 1997, 337, 147–155. [Google Scholar] [CrossRef]
- Millan, M.J.; Gobert, A.; Newman-Tancredi, A.; Lejeune, F.; Cussac, D.; Rivet, J.M.; Audinot, V.; Dubuffet, T.; Lavielle, G. S33084, a novel, potent, selective, and competitive antagonist at dopamine D(3)-receptors: I. Receptorial, electrophysiological and neurochemical profile compared with GR218,231 and L741,626. J. Pharmacol. Exp. Ther. 2000, 293, 1048–1062. [Google Scholar]
- Carr, K.D.; Yamamoto, N.; Omura, M.; de Vaca, S.C.; Krahne, L. Effects of the D(3) dopamine receptor antagonist, U99194A, on brain stimulation and d-amphetamine reward, motor activity, and c-fos expression in ad libitum fed and food-restricted rats. Psychopharmacology 2002, 163, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Gyertyán, I.; Sághy, K. Effects of dopamine D3 receptor antagonists on spontaneous and agonist-reduced motor activity in NMRI mice and Wistar rats: Comparative study with nafadotride, U 99194A and SB 277011. Behav. Pharmacol. 2004, 15, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, L.M.; Newman, A.H.; McNamara, R.K.; Logue, A.D.; Taylor, B.; Welge, J.A.; Xu, M.; Zhang, J.; Richtand, N.M. The dopamine D3 receptor antagonist NGB 2904 increases spontaneous and amphetamine-stimulated locomotion. Pharmacol. Biochem. Behav. 2007, 86, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Guitart-Masip, M.; Johansson, B.; Fernández-Teruel, A.; Tobeña, A.; Giménez-Llort, L. Divergent effect of the selective D3 receptor agonist pd-128,907 on locomotor activity in Roman high- and low-avoidance rats: Relationship to NGFI-A gene expression in the Calleja islands. Psychopharmacology 2008, 196, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Cote, S.R.; Kuzhikandathil, E.V. In vitro and in vivo characterization of the agonist-dependent D3 dopamine receptor tolerance property. Neuropharmacology 2014, 79, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Eberwine, J.; Wilson, C.J.; Cao, Y.; Stefani, A.; Kitai, S.T. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc. Natl. Acad. Sci. USA 1992, 89, 10178–10182. [Google Scholar] [CrossRef]
- Sibley, D.R.; Ariano, M.A. Dopamine receptor distribution in the rat CNS: Elucidation using anti-peptide antisera directed against D1A and D3 subtypes. Brain Res. 1994, 649, 95–110. [Google Scholar]
- Surmeier, D.J.; Song, W.-J.; Yan, Z. Coordinated Expression of Dopamine Receptors in Neostriatal Medium Spiny Neurons. J. Neurosci. 1996, 16, 6579–6591. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Trujillo, R.; Avalos-Fuentes, A.; Rangel-Barajas, C.; Paz-Bermúdez, F.; Sierra, A.; Escartín-Perez, E.; Aceves, J.; Erlij, D.; Florán, B. D3 dopamine receptors interact with dopamine D1 but not D4 receptors in the GABAergic terminals of the SNr of the rat. Neuropharmacology 2013, 67, 370–378. [Google Scholar] [CrossRef]
- Avalos-Fuentes, A.; Loya-López, S.; Flores-Pérez, A.; Recillas-Morales, S.; Cortés, H.; Paz-Bermúdez, F.; Florán, B. Presynaptic CaMKII alpha modulates dopamine D3 receptor activation in striatonigral terminals of the rat brain in a Ca2+ dependent manner. Neuropharmacology 2013, 71, 273–281. [Google Scholar] [CrossRef]
- Bouthenet, M.-L.; Souil, E.; Martres, M.-P.; Sokoloff, P.; Giros, B.; Schwartz, J.-C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: Comparison with dopamine D2 receptor mRNA. Brain Res. 1991, 564, 203–219. [Google Scholar] [CrossRef]
- Flores, G.; Liang, J.; Sierra, A.; Martínez-Fong, D.; Quirion, R.; Aceves, J.; Srivastava, L. Expression of dopamine receptors in the subthalamic nucleus of the rat: Characterization using reverse transcriptase–polymerase chain reaction and autoradiography. Neuroscience 1999, 91, 549–556. [Google Scholar] [CrossRef]
- Ibanez-Sandoval, O.; Hernández, A.; Florán, B.; Galarraga, E.; Tapia, D.; Valdiosera, R.; Erlij, D.; Aceves, J.; Bargas, J.; Hernandez-Cortes, A. Control of the Subthalamic Innervation of Substantia Nigra Pars Reticulata by D1 and D2 Dopamine Receptors. J. Neurophysiol. 2006, 95, 1800–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, S.; Tkatch, T.; Atherton, J.F.; Wilson, C.J.; Bevan, M.D. D2-Like Dopamine Receptors Modulate SKCa Channel Function in Subthalamic Nucleus Neurons Through Inhibition of Cav2.2 Channels. J. Neurophysiol. 2008, 99, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-Z.; Johnson, S.W. Regulation of polysynaptic subthalamonigral transmission by D2, D3 and D4 dopamine receptors in rat brain slices. J. Physiol. 2012, 590, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Briones-Lizardi, L.J.; Cortés, H.; Avalos-Fuentes, J.A.; Paz-Bermúdez, F.J.; Aceves, J.; Erlij, D.; Florán, B. Presynaptic control of [3H]-glutamate release by dopamine receptor subtypes in the rat substantia nigra. Central role of D1 and D3 receptors. Neuroscience 2019, 406, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Todd, R.D.; O’Malley, K.L. Dopamine D2 and D3 receptors inhibit dopamine release. J. Pharmacol. Exp. Ther. 1994, 270, 475–479. [Google Scholar] [PubMed]
- Diaz, J.; Levesque, D.; Lammers, C.; Griffon, N.; Martres, M.-P.; Schwartz, J.-C.; Sokoloff, P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 1995, 65, 731–745. [Google Scholar] [CrossRef]
- Díaz, J.; Pilon, C.; Le Foll, B.; Gros, C.; Triller, A.; Schwartz, J.-C.; Sokoloff, P. Dopamine D3Receptors Expressed by All Mesencephalic Dopamine Neurons. J. Neurosci. 2000, 20, 8677–8684. [Google Scholar] [CrossRef]
- Stanwood, G.D.; Artymyshyn, R.P.; Kung, M.P.; Kung, H.F.; Lucki, I.; Mcgonigle, P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: Evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J. Pharmacol. Exp. Ther. 2000, 295, 1223–1231. [Google Scholar]
- Joseph, J.; Wang, Y.-M.; Miles, P.; Budygin, E.; Picetti, R.; Gainetdinov, R.; Caron, M.; Wightman, R.; Gainetdinov, R. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D3 receptors. Neuroscience 2002, 112, 39–49. [Google Scholar] [CrossRef]
- Davila, V.; Yan, Z.; Craciun, L.C.; Logothetis, D.; Sulzer, D. D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J. Neurosci. 2003, 23, 5693–5697. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, I.; Starr, M.; Fletcher, A.; James, T.; MacLeod, N. Evidence for a GABAergic nigrothalamic pathway in the rat. Exp. Brain Res. 1980, 40, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Loya, S.; Escartín, E.; Ayala, V.; Paz, F.; Erlij, D.; Floran, B. D3 receptor blockade increases dopamine, glutamate and GABA levels in the substantia nigra reticulata and increases locomotor activivity. Presented at the SFN Congress, Washington, DC, USA, 11–15 November 2017. [Google Scholar]
- Rosales, M.G.; Flores, G.; Hernández, S.; Martinez-Fong, D.; Aceves, J. Activation of subthalamic neurons produces NMDA receptor-mediated dendritic dopamine release in substantia nigra pars reticulata: A microdialysis study in the rat. Brain Res. 1994, 645, 335–337. [Google Scholar] [CrossRef]
- Zapata, A.; Chefer, V.I.; Shippenberg, T.S.; Denoroy, L. Detection and quantification of neurotransmitters in dialysates. Curr. Protoc. Neurosci. 2009, 48, 7.4.1–7.4.30. [Google Scholar]
- Quiroz-Gonzalez, S.; Escartín-Pérez, R.E.; Paz-Bermudez, F.; Segura-Alegría, B.; Reyes-Legorreta, C.; Guadarrama-Olmos, J.C.; Jiménez-Estrada, I. Endogenous content and release of [(3)H]-GABA and [(3)H]-glutamate in the spinal cord of chronically undernourished rat. Neurochem. Res. 2013, 38, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G. The Rat Brain in Stereotaxic Coordinates. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1467687/ (accessed on 1 January 2019).
- Rosales, M.; Martínez-Fong, D.; Morales, R.; Nunez, A.; Flores, G.; Góngora-Alfaro, J.L.; Floran, B.; Aceves, J. Reciprocal interaction between glutamate and dopamine in the pars reticulata of the rat substantia nigra: A microdialysis study. Neuroscience 1997, 80, 803–810. [Google Scholar] [CrossRef]
- Neumeyer, J. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur. J. Pharmacol. 2003, 474, 137–140. [Google Scholar] [CrossRef]
- Mintz, I.; Hammond, C.; Guibert, B.; Leviel, V. Stimulation of the subthalamic nucleus enhances the release of dopamine in the rat substantia nigra. Brain Res. 1986, 376, 406–408. [Google Scholar] [CrossRef]
- Araneda, R.; Bustos, G. Modulation of Dendritic Release of Dopamine by N-Methyl-D-Aspartate Receptors in Rat Substantia Nigra. J. Neurochem. 1989, 52, 962–970. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Rassoulpour, A.; Wu, H.-Q.; Ferre, S.; Schwarcz, R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J. Neurochem. 2005, 93, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Kho, C.M.; Ab Rahim, S.K.E.; Ahmad, Z.A.; Abdullah, N.S. A Review on Microdialysis Calibration Methods: The Theory and Current Related Efforts. Mol. Neurobiol. 2017, 54, 3506–3527. [Google Scholar] [CrossRef] [PubMed]
- Starke, K.; Göthert, M.; Kilbinger, H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 1989, 69, 864–989. [Google Scholar] [CrossRef] [PubMed]
- L’Hirondel, M.; Cheramy, A.; Godeheu, G.; Artaud, F.; Saiardi, A.; Borrelli, E.; Glowinski, J. Lack of autoreceptor-mediated inhibitory control of dopamine release in striatal synaptosomes of D2 receptor-deficient mice. Brain Res. 1998, 792, 253–262. [Google Scholar] [CrossRef]
- Féger, J.; Robledo, P. Excitatory influence of rat subthalamic nucleus to substantia nigra pars reticulata and the pallidal complex: Electrophysiological data. Brain Res. 1990, 518, 47–54. [Google Scholar]
- Ammari, R.; Lopez, C.; Bioulac, B.; Garcia, L.; Hammond, C. Subthalamic nucleus evokes similar long lasting glutamatergic excitations in pallidal, entopeduncular and nigral neurons in the basal ganglia slice. Neuroscience 2010, 166, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Maina, F.K.; Mathews, T.A. A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem. Neurosci. 2010, 1, 450–462. [Google Scholar] [CrossRef]
- Windels, F.; Kiyatkin, E. GABAergic mechanisms in regulating the activity state of substantia nigra pars reticulata neurons. Neuroscience 2006, 140, 1289–1299. [Google Scholar] [CrossRef]
- Grace, A.; Bunney, B. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience 1983, 10, 301–315. [Google Scholar] [CrossRef]
- Kiyatkin, E.A.; Rebec, G.V. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J. Neurophysiol. 1996, 75, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Hatzipetros, T.; Yamamoto, B.K. Dopaminergic and GABAergic modulation of glutamate release from rat subthalamic nucleus efferents to the substantia nigra. Brain Res. 2006, 1076, 60–67. [Google Scholar] [CrossRef]
- Desce, J.; Godeheu, G.; Galli, T.; Artaud, F.; Cheramy, A.; Glowinski, J. l-Glutamate-evoked release of dopamine from synaptosomes of the rat striatum: Involvement of AMPA and N-methyl-d-aspartate receptors. Neuroscience 1992, 47, 333–339. [Google Scholar] [CrossRef]
- Christoffersen, C.; Meltzer, L. Evidence for N-methyl-d-aspartate and AMPA subtypes of the glutamate receptor on substantia nigra dopamine neurons: Possible preferential role for N-methyl-d-aspartate receptors. Neuroscience 1995, 67, 373–381. [Google Scholar] [CrossRef]
- Biggs, C.S.; Fowler, L.J.; Whitton, P.S.; Starr, M.S. NMDA receptor antagonists increase the release of dopamine in the substantia nigra of reserpine-treated rats. Eur. J. Pharmacol. 1996, 299, 83–91. [Google Scholar] [CrossRef]
- Cobb, W.S.; Abercrombie, E.D. Distinct Roles for Nigral GABA and Glutamate Receptors in the Regulation of Dendritic Dopamine Release under Normal Conditions and in Response to Systemic Haloperidol. J. Neurosci. 2002, 22, 1407–1413. [Google Scholar] [CrossRef] [Green Version]
- Smith, I.D.; Grace, A.A. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse 1992, 12, 287–303. [Google Scholar] [CrossRef]
- Chergui, K.; Akaoka, H.; Charlety, P.J.; Saunier, C.F.; Buda, M.; Chouvet, G. Subthalamic nucleus modulates burst firing of nigral dopamine neurones via NMDA receptors. NeuroReport 1994, 5, 1185–1188. [Google Scholar] [CrossRef]
- Blythe, S.N.; Atherton, J.F.; Bevan, M.D. Synaptic Activation of Dendritic AMPA and NMDA Receptors Generates Transient High-Frequency Firing in Substantia Nigra Dopamine Neurons In Vitro. J. Neurophysiol. 2007, 97, 2837–2850. [Google Scholar] [CrossRef] [Green Version]
- Porceddu, M.; Giorgi, O.; Ongini, E.; Mele, S.; Biggio, G. 3H-SCH 23390 binding sites in the rat substantia nigra: Evidence for a presynaptic localization and innervation by dopamine. Life Sci. 1986, 39, 321–328. [Google Scholar] [CrossRef]
- Jackson, E.; Kelly, P.H. Nigral dopaminergic mechanisms in drug-induced circling. Brain Res. Bull. 1983, 11, 605–611. [Google Scholar] [CrossRef]
- Asin, K.; Montana, W. Rotation following intranigral injections of a selective D1 or a selective D2 dopamine receptor agonist in rats. Pharmacol. Biochem. Behav. 1988, 29, 89–92. [Google Scholar] [CrossRef]
- Starr, M.S.; Starr, B.S. Circling evoked by intranigral SKF 38393: A GABA-mediated D-1 response? Pharmacol. Biochem. Behav. 1989, 32, 849–851. [Google Scholar] [CrossRef]
- Trevitt, J.T.; Carlson, B.B.; Nowend, K.; Salamone, J.D. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology 2001, 156, 32–41. [Google Scholar] [PubMed]
- Trevitt, T.; Carlson, B.; Correa, M.; Keene, A.; Morales, M.; Salamone, J. Interactions between dopamine D1 receptors and gamma-aminobutyric acid mechanisms in substantia nigra pars reticulata of the rat: Neurochemical and behavioral studies. Psychopharmacology 2002, 159, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Papp, N.; Bacino, C. Contralateral turning evoked by the intranigral microinjection of muscimol and other GABA agonists. Brain Res. 1978, 155, 297–312. [Google Scholar] [CrossRef]
- Arnt, J. Turning behaviour and catalepsy after injection of excitatory amino acids into rat substantia nigra. Neurosci. Lett. 1981, 23, 337–342. [Google Scholar] [CrossRef]
- Windels, F.; Kiyatkin, E.A. GABA, Not Glutamate, Controls the Activity of Substantia Nigra Reticulata Neurons in Awake, Unrestrained Rats. J. Neurosci. 2004, 24, 6751–6754. [Google Scholar] [CrossRef]
- Gale, K. Chronic blockade of dopamine receptors by antischizophrenic drugs enhances GABA binding in substantia nigra. Nature 1980, 283, 569–570. [Google Scholar] [CrossRef]
- Albin, R.; Makowiec, R.; Hollingsworth, Z.; Dure, L.; Penney, J.; Young, A. Excitatory amino acid binding sites in the basal ganglia of the rat: A quantitative autoradiographic study. Neuroscience 1992, 46, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Westerink, B.H.C.; Santiago, M.; De Vries, J.B.; Vries, J. In vivo evidence for a concordant response of terminal and dendritic dopamine release during intranigral infusion of drugs. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1992, 346, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef]
- Wittmann, M.; Marino, M.J.; Bradley, S.R.; Conn, P.J. Activation of Group III mGluRs Inhibits GABAergic and Glutamatergic Transmission in the Substantia Nigra Pars Reticulata. J. Neurophysiol. 2001, 85, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Nava-Asbell, C.; Paz-Bermudez, F.; Erlij, D.; Aceves, J.; Florán, B. GABA(B) receptor activation inhibits dopamine D1 receptor-mediated facilitation of H-3 GABA release in substantia nigra pars reticulata. Neuropharmacology 2007, 53, 631–637. [Google Scholar] [CrossRef]
- Cortes, H.; Paz, F.; Erlij, D.; Aceves, J.; Florán, B. GABA(B) receptors modulate depolarization-stimulated H-3 glutamate release in slices of the pars reticulata of the rat substantia nigra. Eur. J. Pharmacol. 2010, 649, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Floran, B.; Arias-Montano, J.A.; Young, J.M.; Aceves, J. Histamine H-3 receptor activation selectively inhibits dopamine D-1 receptor-dependent H-3 GABA release from depolarization-stimulated slices of rat substantia nigra pars reticulata. Neuroscience 1997, 80, 241–249. [Google Scholar] [CrossRef]
- Bevan, M.D.; Bolam, J.P.; Crossman, A.R. Convergent Synaptic Input From the Neostriatum and the Subthalamus Onto Identified Nigrothalamic Neurons in the Rat. Eur. J. Neurosci. 1994, 6, 320–334. [Google Scholar] [CrossRef]
- Henny, P.; Brown, M.T.C.; Northrop, A.; Faunes, M.A.; Ungless, M.; Magill, P.J.; Bolam, J.P. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat. Neurosci. 2012, 15, 613–619. [Google Scholar] [CrossRef]
- Yung, K.; Bolam, J.P.; Smith, A.; Hersch, S.; Ciliax, B.; Levey, A.; Smith, D. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: Light and electron microscopy. Neuroscience 1995, 65, 709–730. [Google Scholar] [CrossRef]
- Caillé, I.; Dumartin, B.; Bloch, B. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 1996, 730, 17–31. [Google Scholar] [CrossRef]
- Chatha, B.; Bernard, V.; Streit, P.; Bolam, J.P. Synaptic localization of ionotropic glutamate receptors in the rat substantia nigra. Neuroscience 2000, 101, 1037–1051. [Google Scholar] [CrossRef]
- Rice, M.E.; Cragg, S.J. Dopamine Spillover after Quantal Release: Rethinking Dopamine Transmission in the Nigrostriatal Pathway. Brain Res. Rev. 2008, 58, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Kitai, S.; Kita, H. The ultrastructural morphology of the subthalamic-nigral axon terminals intracellularly labeled with horseradish peroxidase. Brain Res. 1984, 299, 182–185. [Google Scholar] [CrossRef]
- Fujiyama, F.; Stephenson, F.A.; Bolam, J.P. Synaptic localization of GABA(A) receptor subunits in the substantia nigra of the rat: Effects of quinolinic acid lesions of the striatum. Eur. J. Neurosci. 2002, 15, 1961–1975. [Google Scholar] [CrossRef]
- Boyes, J.; Bolam, J.P. Localization of GABA receptors in the basal ganglia. Prog. Brain Res. 2007, 160, 229–243. [Google Scholar]
- Ahlenius, S.; Salmi, P. Behavioral and biochemical effects of the dopamine D3 receptor-selective ligand, 7-OH-DPAT, in the normal and the reserpine-treated rat. Eur. J. Pharmacol. 1994, 260, 177–181. [Google Scholar] [CrossRef]
- Haadsma-Svensson, S.; Svensson, K. PNU-99194A: A preferentail dopamine D3 receptor antagonist. CNS Drug Rev. 1998, 4, 42–53. [Google Scholar] [CrossRef]
- Reavill, C.; Taylor, S.G.; Wood, M.D.; Ashmeade, T.E.; Austin, N.; Avenell, K.Y.; Boyfield, I.; Branch, C.L.; Cilia, J.; Coldwell, M.C.; et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J. Pharmacol. Exp. Ther. 2000, 294, 1154–1165. [Google Scholar]
- Sokoloff, P.; Giros, B.; Martres, M.-P.; Bouthenet, M.-L.; Schwartz, J.-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 1990, 347, 146–151. [Google Scholar] [CrossRef]
- Kling-Petersen, T.; Ljung, E.; Svensson, K. Effects on locomotor activity after local application of D3 preferring compounds in discrete areas of the rat brain. J. Neural Transm. 1995, 102, 209–220. [Google Scholar] [CrossRef]
- Ouagazzal, A.-M.; Creese, I. Intra-accumbens infusion of D3 receptor agonists reduces spontaneous and dopamine-induced locomotion. Pharmacol. Biochem. Behav. 2000, 67, 637–645. [Google Scholar] [CrossRef]
- Stewart, J.; Vezina, P. Microinjections of Sch-23390 into the ventral tegmental area and substantia nigra pars reticulata attenuate the development of sensitization to the locomotor activating effects of systemic amphetamine. Brain Res. 1989, 495, 401–406. [Google Scholar] [CrossRef]
- Robertson, G.; Robertson, H. Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J. Neurosci. 1989, 9, 3326–3331. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, A.J.; Trevitt, J.T.; Conlan, A.; Gianutsos, G.; Salamone, J.D. Striatal and nigral D 1 mechanisms involved in the antiparkinsonian effects of SKF 82958 (APB): Studies of tremulous jaw movements in rats. Psychopharmacology 1999, 143, 72–81. [Google Scholar] [CrossRef] [PubMed]
- David, H.N. Towards a Reconceptualization of Striatal Interactions Between Glutamatergic and Dopaminergic Neurotransmission and Their Contribution to the Production of Movements. Curr. Neuropharmacol. 2009, 7, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dray, A.; Oakley, N.R. Methiothepin and a 5-HT pathway to rat substantia nigra. Cell. Mol. Life Sci. 1977, 33, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Walaas, I.; Fonnum, F. Biochemical evidence for gamma-aminobutyrate containing fibres from the nucleus accumbens to the substantia nigra and ventral tegmental area in the rat. Neuroscience 1980, 5, 63–72. [Google Scholar] [CrossRef]
- Strahlendorf, H.K.; Barnes, C.D. Control of substantia nigra pars reticulata neurons by the nucleus accumbens. Brain Res. Bull. 1983, 11, 259–263. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sánchez, M.; Escartín-Pérez, R.E.; Leyva-Gómez, G.; Avalos-Fuentes, J.A.; Paz-Bermúdez, F.J.; Loya-López, S.I.; Aceves, J.; Erlij, D.; Cortés, H.; Florán, B. Blockade of Intranigral and Systemic D3 Receptors Stimulates Motor Activity in the Rat Promoting a Reciprocal Interaction among Glutamate, Dopamine, and GABA. Biomolecules 2019, 9, 511. https://doi.org/10.3390/biom9100511
Rodríguez-Sánchez M, Escartín-Pérez RE, Leyva-Gómez G, Avalos-Fuentes JA, Paz-Bermúdez FJ, Loya-López SI, Aceves J, Erlij D, Cortés H, Florán B. Blockade of Intranigral and Systemic D3 Receptors Stimulates Motor Activity in the Rat Promoting a Reciprocal Interaction among Glutamate, Dopamine, and GABA. Biomolecules. 2019; 9(10):511. https://doi.org/10.3390/biom9100511
Chicago/Turabian StyleRodríguez-Sánchez, Marina, Rodrigo Erick Escartín-Pérez, Gerardo Leyva-Gómez, José Arturo Avalos-Fuentes, Francisco Javier Paz-Bermúdez, Santiago Iván Loya-López, Jorge Aceves, David Erlij, Hernán Cortés, and Benjamín Florán. 2019. "Blockade of Intranigral and Systemic D3 Receptors Stimulates Motor Activity in the Rat Promoting a Reciprocal Interaction among Glutamate, Dopamine, and GABA" Biomolecules 9, no. 10: 511. https://doi.org/10.3390/biom9100511
APA StyleRodríguez-Sánchez, M., Escartín-Pérez, R. E., Leyva-Gómez, G., Avalos-Fuentes, J. A., Paz-Bermúdez, F. J., Loya-López, S. I., Aceves, J., Erlij, D., Cortés, H., & Florán, B. (2019). Blockade of Intranigral and Systemic D3 Receptors Stimulates Motor Activity in the Rat Promoting a Reciprocal Interaction among Glutamate, Dopamine, and GABA. Biomolecules, 9(10), 511. https://doi.org/10.3390/biom9100511






