Photobiomodulation Mitigates Cerebrovascular Leakage Induced by the Parkinsonian Neurotoxin MPTP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. MPTP and Saline Injections
2.3. PBM Treatment
2.4. Animal Perfusion, Tissue Collection and Preparation
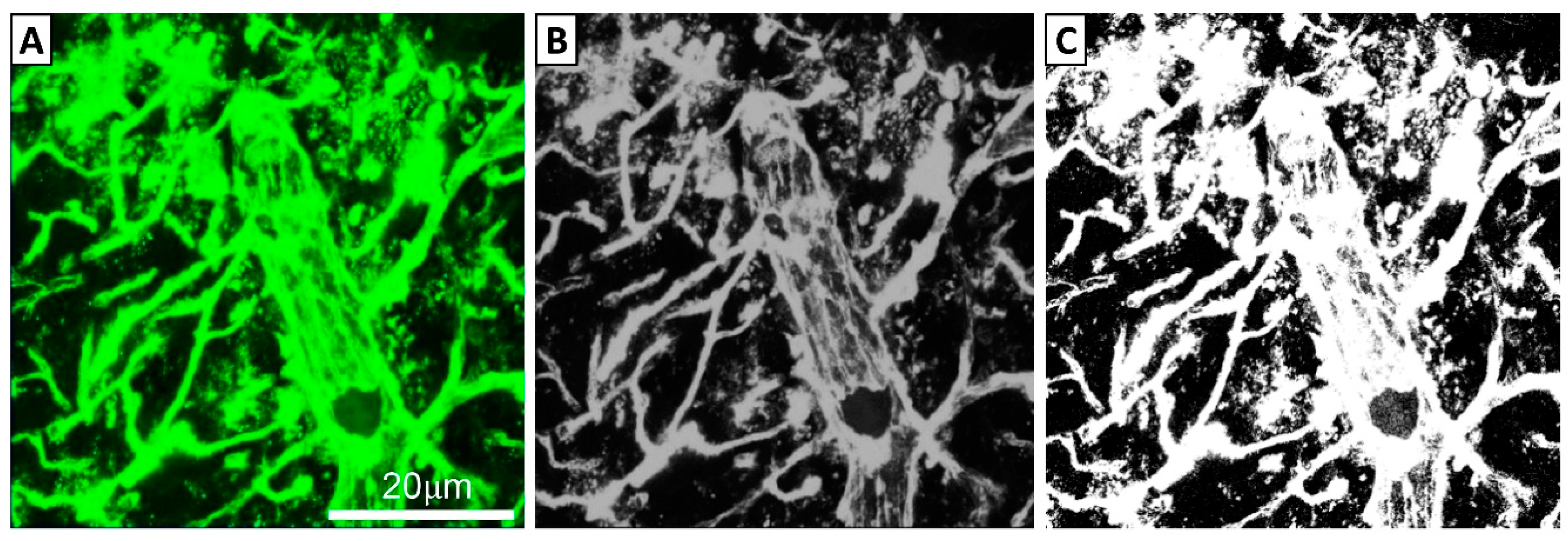
2.5. Imaging and Analysis of FITC-LA Distribution
3. Results
3.1. Time Course of MPTP-Induced Dysfunction of the Cerebrovasculature
3.1.1. Perfusion Time Point Had No Obvious Effect on the Cerebrovasculature of Saline-Injected Controls
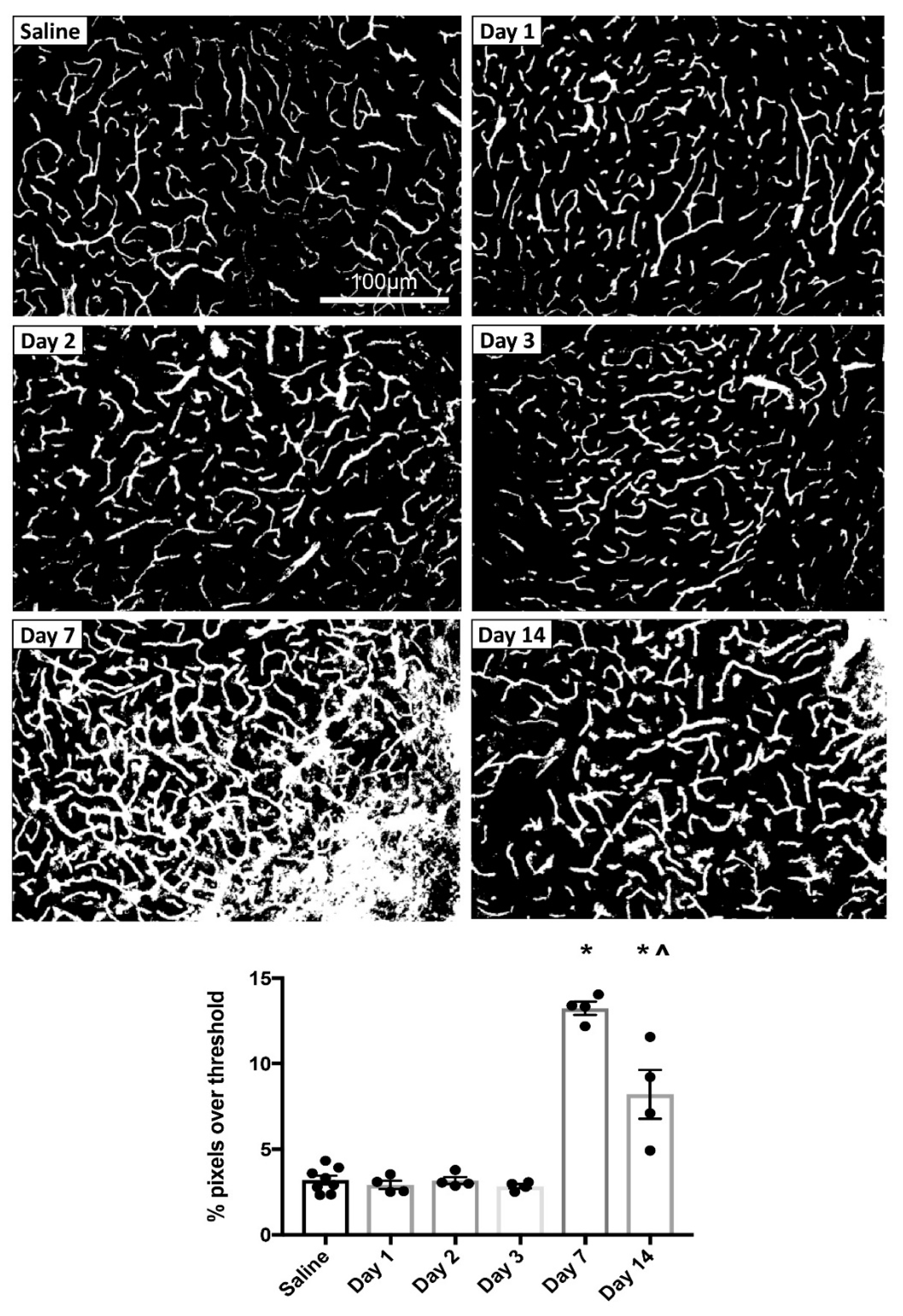
3.1.2. MPTP-Induced Cerebrovascular Leakage in the SNc Peaks at 7 Days Post-Injection
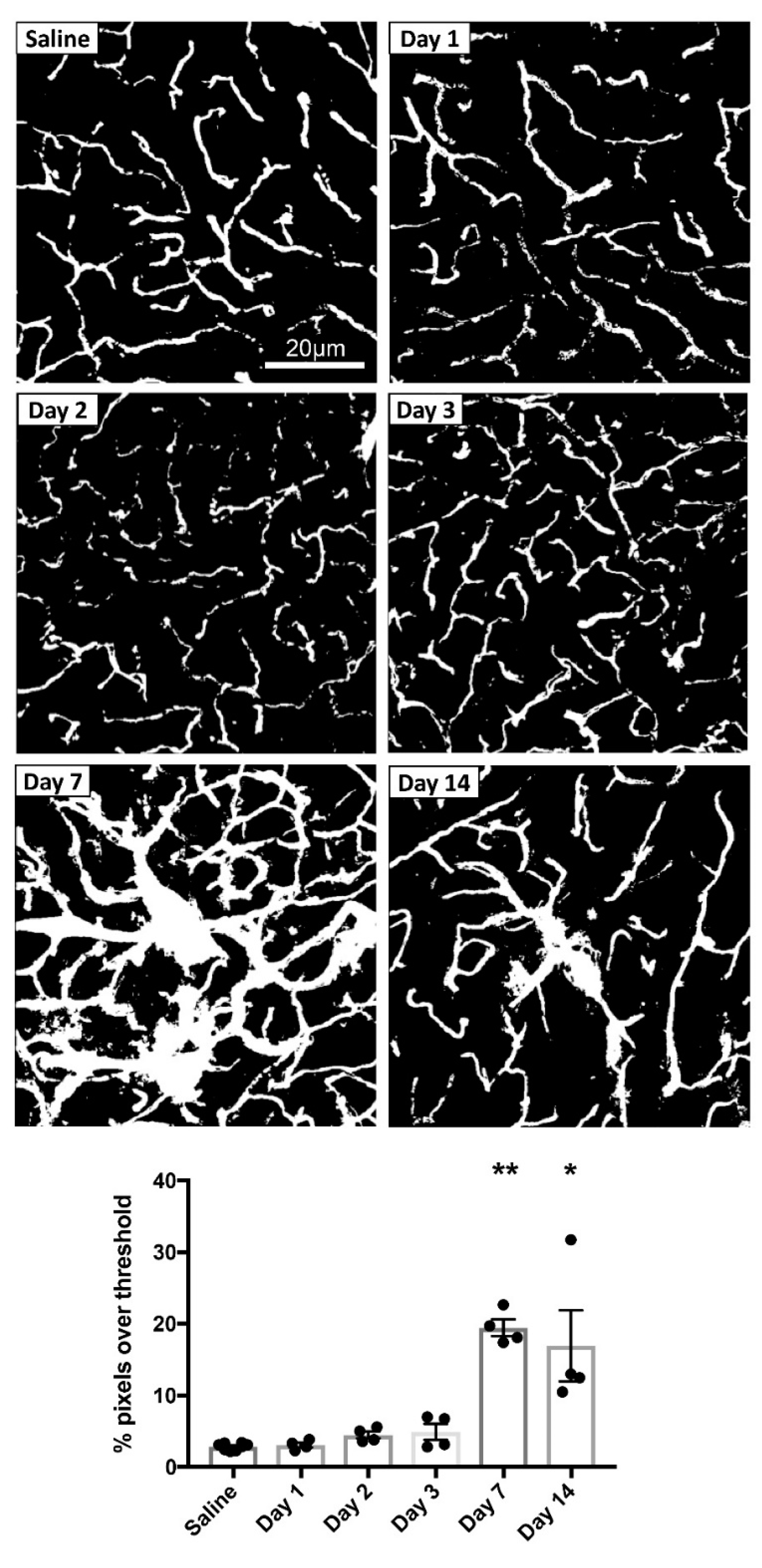
3.1.3. MPTP-Induced Cerebrovascular Leakage in the CPu Peaks at 7 Days Post-Injection
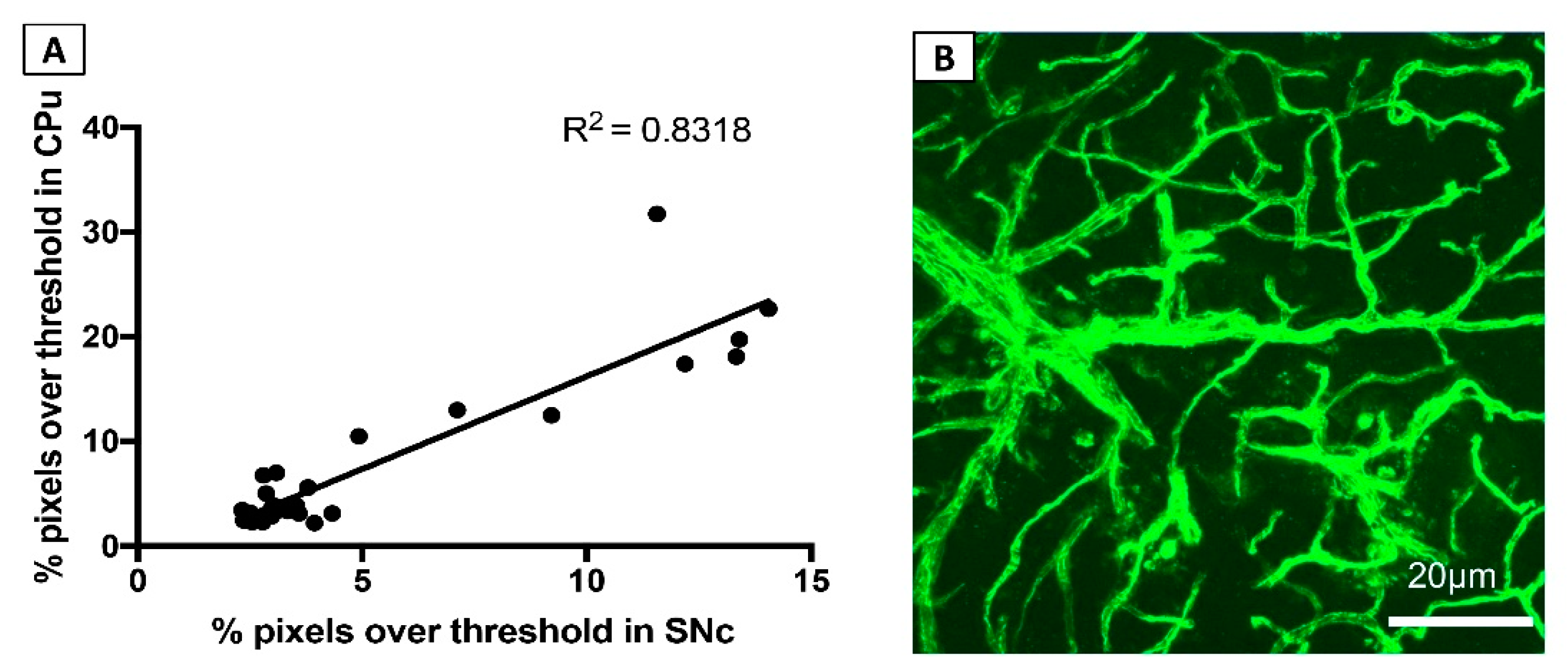
3.1.4. FITC-LA Labelling in the SNc and CPu are Strongly Correlated
3.1.5. MPTP-Induced Cerebrovascular Leakage is Not Confined to the SNc and CPu
3.2. Effects of Transcranial PBM on MPTP-Induced Dysfunction of the Cerebrovasculature
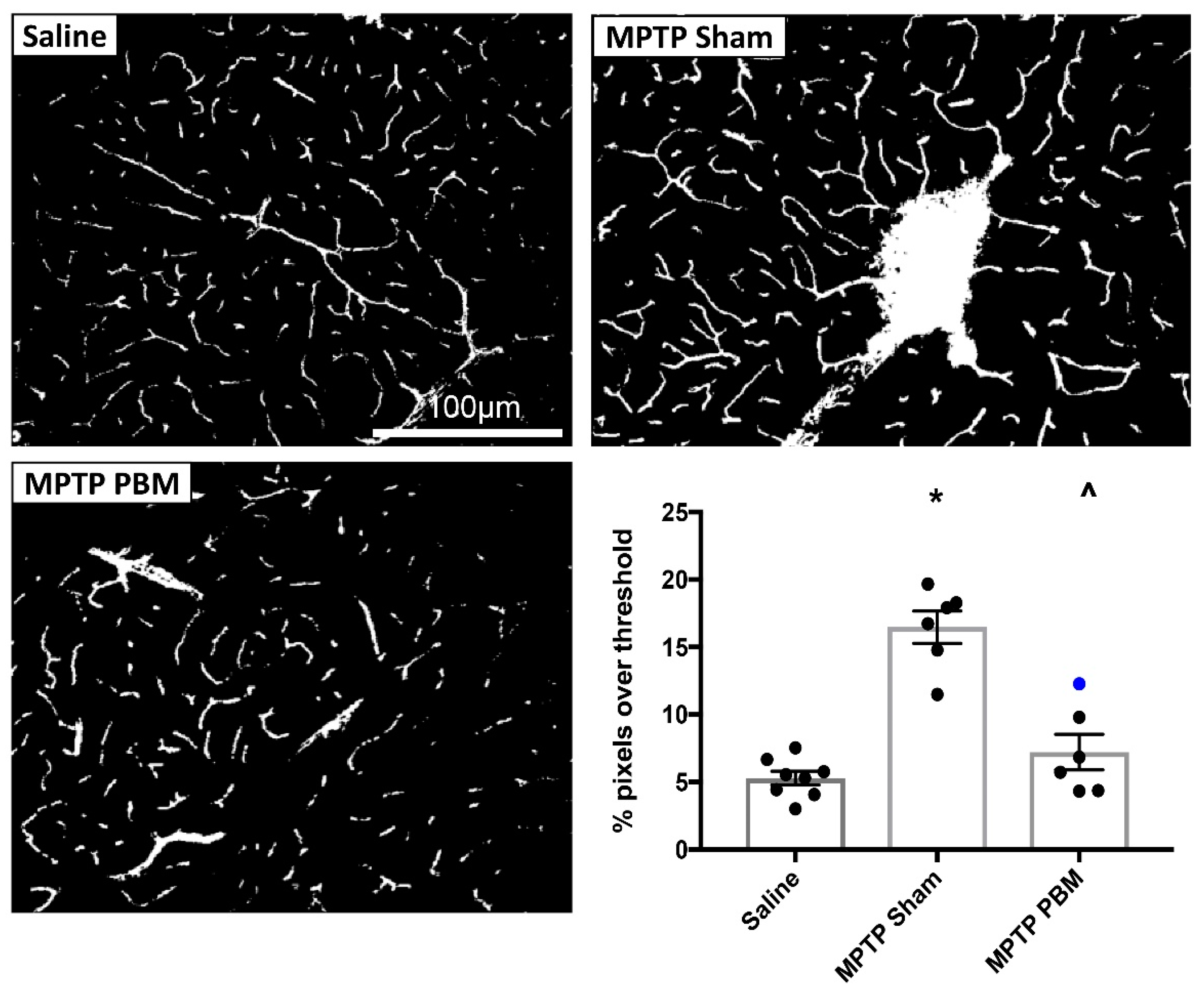
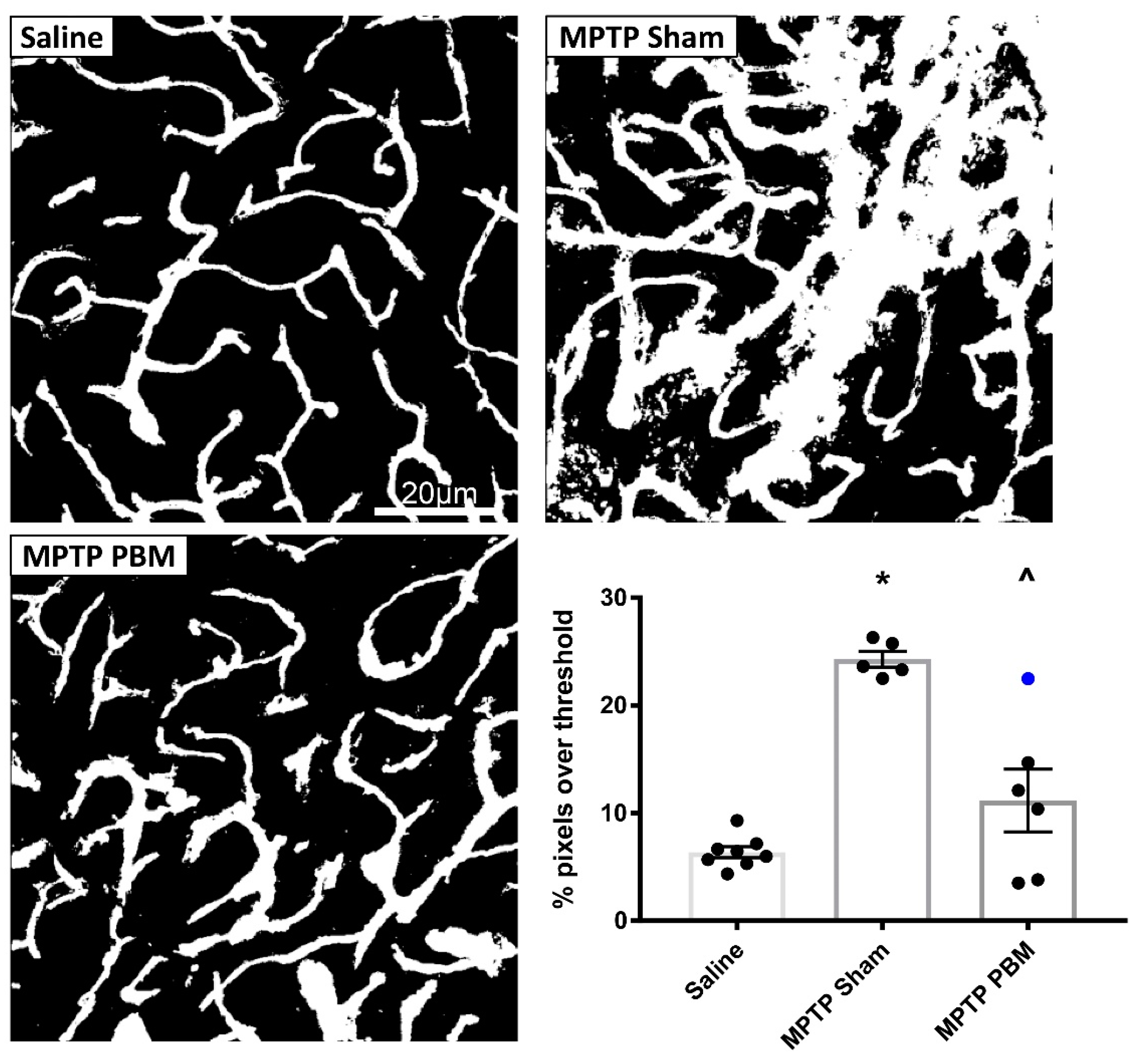
3.2.1. Transcranial PBM Mitigates MPTP-Induced Cerebrovascular Leakage in the SNc
3.2.2. Transcranial PBM Mitigates MPTP-Induced Cerebrovascular Leakage in the CPu
4. Discussion
4.1. Comparison to Previous Studies
4.2. Potential Mechanisms
4.2.1. MPTP-Induced Cerebrovascular Leakage and BBB Dysfunction
4.2.2. PBM-Induced Mitigation of Cerebrovascular Dysfunction
4.3. Strengths and Limitations of the Study
4.4. Implications and Future Directions
4.4.1. Is the MPTP Mouse a Useful Model for Parkinson’s Disease Research?
4.4.2. Might PBM be an Effective Treatment for Other Brain Diseases?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langston, J.W. The MPTP Story. J. Parkinsons Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liss, B.; Haeckel, O.; Wildmann, J.; Miki, T.; Seino, S.; Roeper, J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat. Neurosci. 2005, 8, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Kang, U.J. Behavioral models of Parkinson’s disease in rodents: A new look at an old problem. Mov. Disord. 2006, 21, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Rademacher, D.J. MPTP Mouse Models of Parkinson’s Disease: An Update. J. Park. Dis. 2011, 1, 19–33. [Google Scholar]
- Sedelis, M.; Hofele, K.; Auburger, G.W.; Morgan, S.; Huston, J.P.; Schwarting, R.K.W. MPTP Susceptibility in the Mouse: Behavioral, Neurochemical, and Histological Analysis of Gender and Strain Differences. Behav. Genet. 2000, 30, 171–182. [Google Scholar] [CrossRef]
- Chao, Y.X.; He, B.P.; Tay, S.S.W. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson’s disease. J. Neuroimmunol. 2009, 216, 39–50. [Google Scholar] [CrossRef]
- Chen, X.; Lan, X.; Roche, I.; Liu, R.; Geiger, J.D. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J. Neurochem. 2008, 107, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Jang, M.; Oh, S.; Nah, S.-Y.; Cho, I.-H. Multi-Target Protective Effects of Gintonin in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Mediated Model of Parkinson’s Disease via Lysophosphatidic Acid Receptors. Front. Pharmacol. 2018, 9, 515. [Google Scholar] [CrossRef]
- Chung, Y.C.; Shin, W.-H.; Baek, J.Y.; Cho, E.J.; Baik, H.H.; Kim, S.R.; Won, S.-Y.; Jin, B.K. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2016, 48, e205. [Google Scholar] [CrossRef]
- Zhao, C.; Ling, Z.; Newman, M.B.; Bhatia, A.; Carvey, P.M. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol. Dis. 2007, 26, 36–46. [Google Scholar] [CrossRef]
- El Massri, N.; Johnstone, D.M.; Peoples, C.L.; Moro, C.; Reinhart, F.; Torres, N.; Stone, J.; Benabid, A.L.; Mitrofanis, J. The effect of different doses of near infrared light on dopaminergic cell survival and gliosis in MPTP-treated mice. Int J. Neurosci 2016, 126, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, D.; El Massri, N.; Moro, C.; Spana, S.; Wang, X.; Torres, N.; Chabrol, C.; De Jaeger, X.; Reinhart, F.; Purushothuman, S.; et al. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism—An abscopal neuroprotective effect. Neuroscience 2014, 274, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Torres, N.; El Massri, N.; Ratel, D.; Johnstone, D.M.; Stone, J.; Mitrofanis, J.; Benabid, A.-L. Photobiomodulation preserves behaviour and midbrain dopaminergic cells from MPTP toxicity: Evidence from two mouse strains. BMC Neurosci. 2013, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Peoples, C.; Spana, S.; Ashkan, K.; Benabid, A.-L.; Stone, J.; Baker, G.E.; Mitrofanis, J. Photobiomodulation enhances nigral dopaminergic cell survival in a chronic MPTP mouse model of Parkinson’s disease. Park. Relat. Disord. 2012, 18, 469–476. [Google Scholar] [CrossRef]
- Reinhart, F.; El Massri, N.; Johnstone, D.M.; Stone, J.; Mitrofanis, J.; Benabid, A.-L.; Moro, C. Near-infrared light (670 nm) reduces MPTP-induced parkinsonism within a broad therapeutic time window. Exp. Brain Res. 2016, 234, 1787–1794. [Google Scholar] [CrossRef]
- Reinhart, F.; El Massri, N.; Darlot, F.; Torres, N.; Johnstone, D.M.; Chabrol, C.; Costecalde, T.; Stone, J.; Mitrofanis, J.; Benabid, A.-L.; et al. 810nm near-infrared light offers neuroprotection and improves locomotor activity in MPTP-treated mice. Neurosci. Res. 2015, 92, 86–90. [Google Scholar] [CrossRef]
- Reinhart, F.; El Massri, N.; Torres, N.; Chabrol, C.; Molet, J.; Johnstone, D.M.; Stone, J.; Benabid, A.-L.; Mitrofanis, J.; Moro, C. The behavioural and neuroprotective outcomes when 670 nm and 810 nm near infrared light are applied together in MPTP-treated mice. Neurosci. Res. 2017, 117, 42–47. [Google Scholar] [CrossRef]
- Shaw, V.E.; Spana, S.; Ashkan, K.; Stone, J.; Baker, G.E.; Mitrofanis, J.; Benabid, A.-L.; Benabid, A. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 2010, 518, 25–40. [Google Scholar] [CrossRef]
- Ganeshan, V.; Skladnev, N.V.; Kim, J.Y.; Mitrofanis, J.; Stone, J.; Johnstone, D.M. Pre-conditioning with Remote Photobiomodulation Modulates the Brain Transcriptome and Protects Against MPTP Insult in Mice. Neuroscience 2019, 400, 85–97. [Google Scholar] [CrossRef]
- Kim, B.; Mitrofanis, J.; Stone, J.; Johnstone, D.M. Remote tissue conditioning is neuroprotective against MPTP insult in mice. IBRO Rep. 2018, 4, 14–17. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Kim, Y.-S.; Bok, E.; Yune, T.Y.; Maeng, S.; Jin, B.K. MMP-3 Contributes to Nigrostriatal Dopaminergic Neuronal Loss, BBB Damage, and Neuroinflammation in an MPTP Mouse Model of Parkinson’s Disease. Mediat. Inflamm. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.O.; Rosa, A.I.; Carvalho, A.N.; Nunes, M.J.; Dionísio, P.; Rodrigues, E.; Costa, D.; Duarte-Silva, S.; Maciel, P.; Rodrigues, C.M.P.; et al. Neurotoxic effects of MPTP on mouse cerebral cortex: Modulation of neuroinflammation as a neuroprotective strategy. Mol. Cell. Neurosci. 2019, 96, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beal, M.F. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2003, 991, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Harry, G.J. Features of Microglia and Neuroinflammation Relevant to Environmental Exposure and Neurotoxicity. Int. J. Environ. Res. Public Heal. 2011, 8, 2980–3018. [Google Scholar] [CrossRef] [Green Version]
- Vroon, A.; Drukarch, B.; Bol, J.G.; Cras, P.; Brevé, J.J.; Allan, S.M.; Relton, J.K.; Hoogland, P.V.; Van Dam, A.-M. Neuroinflammation in Parkinson’s patients and MPTP-treated mice is not restricted to the nigrostriatal system: Microgliosis and differential expression of interleukin-1 receptors in the olfactory bulb. Exp. Gerontol. 2007, 42, 762–771. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [Green Version]
- A O’Neill, L.; Kaltschmidt, C. NF-kappa B: A crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997, 20, 252–258. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Peterson, L.J.; Flood, P.M. Oxidative Stress and Microglial Cells in Parkinson’s Disease. Mediat. Inflamm. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E.; R, L. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, R.; Avila, M.; Gonzalez, J.; El-Bacha, R.S.; Baez, E.; Garcia-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson’s disease. Front. Cell Neurosci 2014, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-L.; Indyk, J.A.; Bugge, T.H.; Kombrinck, K.W.; Degen, J.L.; Strickland, S. Neuronal Death and Blood–Brain Barrier Breakdown after Excitotoxic Injury Are Independent Processes. J. Neurosci. 1999, 19, 9813–9820. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Farkas, A.E.; Hilgarth, R.S.; Krug, S.M.; Wolf, M.F.; Benedik, J.K.; Fromm, M.; Koval, M.; Parkos, C.; Nusrat, A. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol. Boil. Cell 2014, 25, 2710–2719. [Google Scholar] [CrossRef]
- Greene, C.; Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 2016, 4, e1138017. [Google Scholar] [CrossRef]
- Huang, D.; Xu, J.; Wang, J.; Tong, J.; Bai, X.; Li, H.; Wang, Z.; Huang, Y.; Wu, Y.; Yu, M.; et al. Dynamic Changes in the Nigrostriatal Pathway in the MPTP Mouse Model of Parkinson’s Disease. Park. Dis. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, Y.; Liu, H.; Tang, J.; Veenstra, A.; Kern, T.S. Photobiomodulation Inhibits Long-term Structural and Functional Lesions of Diabetic Retinopathy. Diabetes 2018, 67, 291–298. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- De Marchi, T.; Leal Junior, E.C.; Bortoli, C.; Tomazoni, S.S.; Lopes-Martins, R.A.; Salvador, M. Low-level laser therapy (LLLT) in human progressive-intensity running: Effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med. Sci. 2012, 27, 231–236. [Google Scholar] [CrossRef]
- Tatmatsu-Rocha, J.C.; Ferraresi, C.; Hamblin, M.R.; Damasceno, F.M.; Nascimento, N.R.F.D.; Driusso, P.; Parizotto, N.A. Low-Level Laser Therapy (904nm) Can Increase Collagen and Reduce Oxidative and Nitrosative Stress in Diabetic Wounded Mouse Skin. J. Photochem. Photobiol. B: Boil. 2016, 164, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.-H.; Huang, Y.-Y.; Sharma, S.K.; Hamblin, M.R. Effects of 810-nm Laser on Murine Bone-Marrow-Derived Dendritic Cells. Photomed. Laser Surg. 2011, 29, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, M.H.; Shin, J.H.; Kim, K.S.; Yoo, C.M.; Jo, G.E.; Kim, J.H.; Choi, H. Low Level Light Therapy Modulates Inflammatory Mediators Secreted by Human Annulus Fibrosus Cells during Intervertebral Disc Degeneration In Vitro. Photochem. Photobiol. 2015, 91, 403–410. [Google Scholar] [CrossRef]
- Yamaura, M.; Yao, M.; Yaroslavsky, I.; Cohen, R.; Smotrich, M.; Kochevar, I.E. Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg. Med. 2009, 41, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Khuman, J.; Zhang, J.; Park, J.; Carroll, J.D.; Donahue, C.; Whalen, M.J. Low-Level Laser Light Therapy Improves Cognitive Deficits and Inhibits Microglial Activation after Controlled Cortical Impact in Mice. J. Neurotrauma 2012, 29, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Whelan, H. Harnessing the cell’s own ability to repair and prevent neurodegenerative disease. SPIE Newsroom 2008, 1–3. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Mollgard, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef]
- Natarajan, R.; Northrop, N.; Yamamoto, B. Fluorescein Isothiocyanate (FITC)-Dextran Extravasation as a Measure of Blood-Brain Barrier Permeability; Wiley: Hoboken, NJ, USA, 2017; Volume 79. [Google Scholar]
- Blinder, P.; Tsai, P.S.; Kaufhold, J.P.; Knutsen, P.M.; Suhl, H.; Kleinfeld, D. The cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 2013, 16, 889–897. [Google Scholar] [CrossRef]
- Bartels, A.L.; Willemsen, A.T.M.; Kortekaas, R.; De Jong, B.M.; De Vries, R.; De Klerk, O.; Van Oostrom, J.C.H.; Portman, A.; Leenders, K.L. Decreased blood–brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J. Neural Transm. 2008, 115, 1001–1009. [Google Scholar] [CrossRef]
- Desai Bradaric, B.; Patel, A.; Schneider, J.A.; Carvey, P.M.; Hendey, B. Evidence for angiogenesis in Parkinson’s disease, incidental Lewy body disease, and progressive supranuclear palsy. J. Neural Transm. (Vienna) 2012, 119, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.T.; Woulfe, J.M. Striatal blood–brain barrier permeability in Parkinson’s disease. Br. J. Pharmacol. 2015, 35, 747–750. [Google Scholar] [CrossRef]
- Kortekaas, R.; Leenders, K.L.; Van Oostrom, J.C.H.; Vaalburg, W.; Bart, J.; Willemsen, A.T.M.; Hendrikse, N.H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005, 57, 176–179. [Google Scholar] [CrossRef] [PubMed]
- A Banks, W. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol 2009, 9, S3. [Google Scholar] [CrossRef]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garçon, G.; Rouaix, N.; et al. Targeting Chelatable Iron as a Therapeutic Modality in Parkinson’s Disease. Antioxidants Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Ward, R.J.; Newbould, R.; Piccini, P.; Sharp, D.; Kabba, C.; Patel, M.C.; Spino, M.; Connelly, J.; Tricta, F.; et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 2017, 7, 1398. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Statton, S.A.; Whitmore, C.; Freinbichler, W.; Weinberger, P.; Tipton, K.F.; Della Corte, L.; Ward, R.J.; Crichton, R.R. Clinically available iron chelators induce neuroprotection in the 6-OHDA model of Parkinson’s disease after peripheral administration. J. Neural Transm. (Vienna) 2011, 118, 223–231. [Google Scholar] [CrossRef]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, D.M.; Moro, C.; Stone, J.; Benabid, A.-L.; Mitrofanis, J. Turning On Lights to Stop Neurodegeneration: The Potential of Near Infrared Light Therapy in Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2016, 9, 500. [Google Scholar] [CrossRef]
- Minagar, A.; Alexander, J.S. Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. J. 2003, 9, 540–549. [Google Scholar] [CrossRef]
- Rosenberg, G. Blood-Brain Barrier Permeability in Aging and Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2014, 1, 138–139. [Google Scholar]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.M.; Kócsi, Z.; Stone, J. Pericapillary Haem-Rich Deposits: Evidence for Microhaemorrhages in Aging Human Cerebral Cortex. Br. J. Pharmacol. 2005, 25, 1656–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.; Johnstone, D.M.; Mitrofanis, J.; O’Rourke, M. The mechanical cause of age-related dementia (Alzheimer’s disease): The brain is destroyed by the pulse. J. Alzheimer’s Dis. 2015, 44, 355–373. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.-F.; Wan, C.-Q.; Cai, M.; Zhou, N.-K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- De Taboada, L.; Yu, J.; El-Amouri, S.; Gattoni-Celli, S.; Richieri, S.; McCarthy, T.; Streeter, J.; Kindy, M.S. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J. Alzheimers Dis. 2011, 23, 521–535. [Google Scholar] [CrossRef]
- Grillo, S.L.; Duggett, N.A.; Ennaceur, A.; Chazot, P.L. Non-invasive infra-red therapy (1072 nm) reduces beta-amyloid protein levels in the brain of an Alzheimer’s disease mouse model, TASTPM. J. Photochem. Photobiol. B 2013, 123, 13–22. [Google Scholar] [CrossRef]
- Purushothuman, S.; Johnstone, D.M.; Nandasena, C.; Mitrofanis, J.; Stone, J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex—Evidence from two transgenic mouse models. Alzheimer’s Res. Ther. 2014, 6, 2. [Google Scholar] [CrossRef]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Miguel, M.; Martin, K.L.; Stone, J.; Johnstone, D.M. Photobiomodulation Mitigates Cerebrovascular Leakage Induced by the Parkinsonian Neurotoxin MPTP. Biomolecules 2019, 9, 564. https://doi.org/10.3390/biom9100564
San Miguel M, Martin KL, Stone J, Johnstone DM. Photobiomodulation Mitigates Cerebrovascular Leakage Induced by the Parkinsonian Neurotoxin MPTP. Biomolecules. 2019; 9(10):564. https://doi.org/10.3390/biom9100564
Chicago/Turabian StyleSan Miguel, Mia, Kristy L. Martin, Jonathan Stone, and Daniel M. Johnstone. 2019. "Photobiomodulation Mitigates Cerebrovascular Leakage Induced by the Parkinsonian Neurotoxin MPTP" Biomolecules 9, no. 10: 564. https://doi.org/10.3390/biom9100564
APA StyleSan Miguel, M., Martin, K. L., Stone, J., & Johnstone, D. M. (2019). Photobiomodulation Mitigates Cerebrovascular Leakage Induced by the Parkinsonian Neurotoxin MPTP. Biomolecules, 9(10), 564. https://doi.org/10.3390/biom9100564




