Bioguided Purification of Active Compounds from Leaves of Anadenanthera colubrina var. cebil (Griseb.) Altschul
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Identification of the Specie Anadenanthera Colubrina Var Cebil
2.2. Preparation of Extracts
2.3. Phytochemical Analysis and Purification
2.3.1. Flash Chromatography
2.3.2. High-Performance Liquid Chromatography (HPLC-DAD)
2.3.3. Preparative High-Performance Liquid Chromatography Coupled To Mass Spectrometry (HPLC-MS)
2.3.4. Ultra-High-Performance Liquid Chromatography Coupled to Mass Spectrometry (UPLC-MS)
2.3.5. Nuclear Magnetic Resonance (NMR)
2.4. Antimicrobial Activity
2.4.1. Microbial Strains
2.4.2. Antimicrobial Assay
2.5. Antioxidant Activity
2.5.1. Free Radical Sequestration
2.5.2. Total Antioxidant Activity (TAA)
2.5.3. Reduction of Ferric ion (FRAP Method)
2.6. Statistical Analysis
3. Results
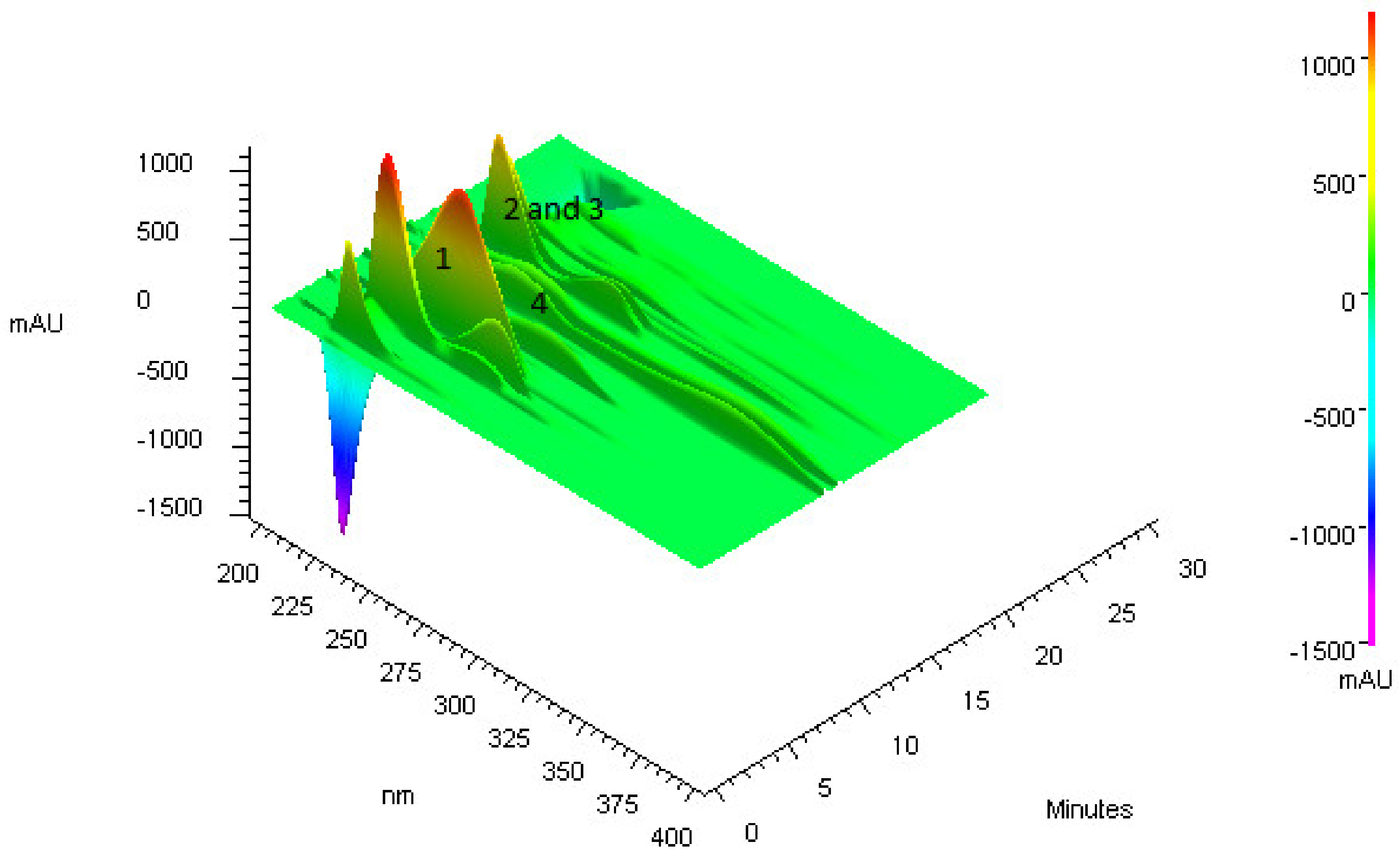
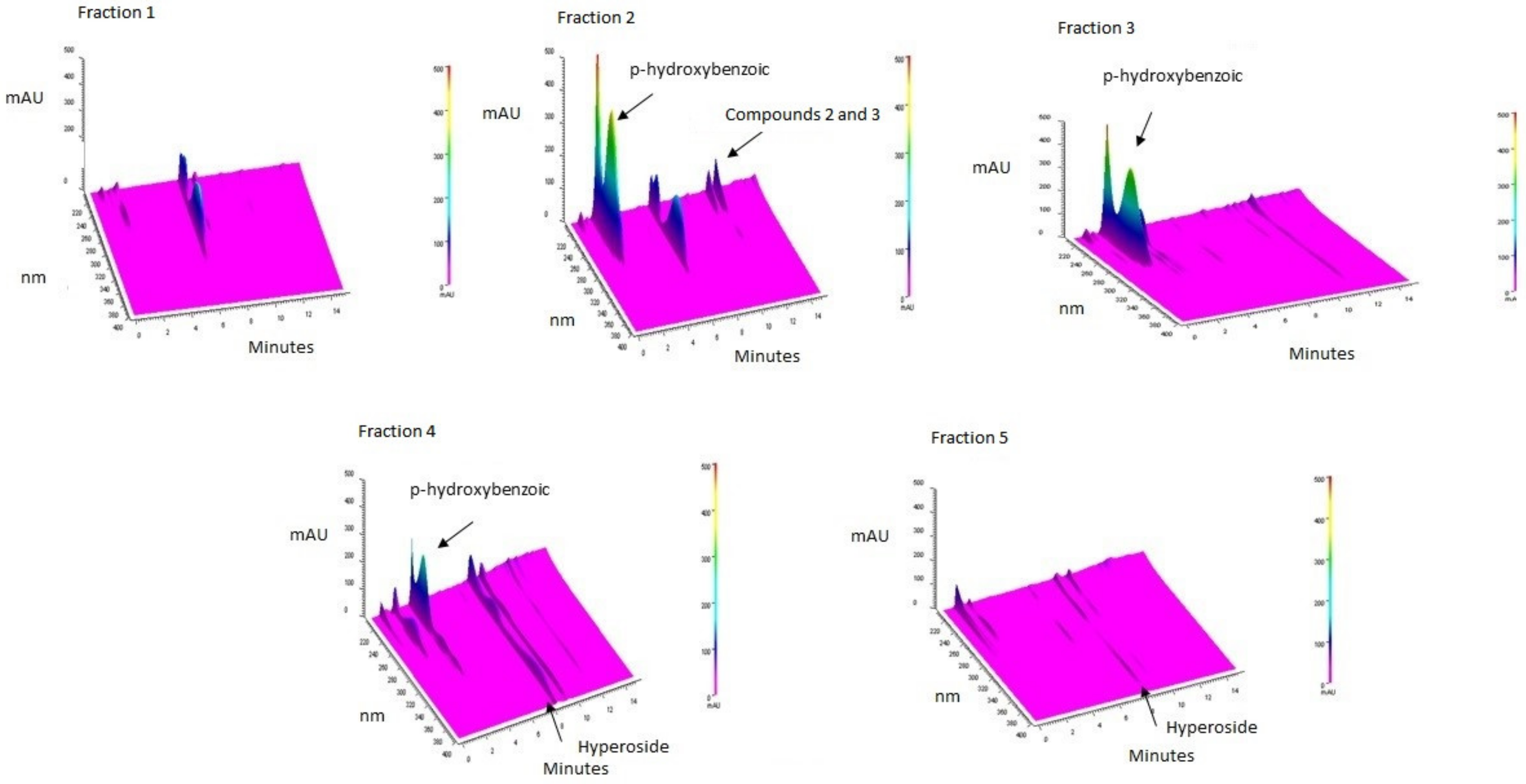
3.1. Purification of Compounds from Anadenanthera Colubrina
3.2. Bioguided Purification of Compounds from Anadenanthera Colubrina
3.3. Nuclear Magnetic Resonance–NMR
3.4. Antimicrobial Activity of Isolated Compounds
3.5. Antioxidant Action of Isolated Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Souza-Moreira, T.M.; Salgado, H.; Pietro, R.C. Brazil in the context of plants and derivates quality control. Rev. Bras. Farmacogn. 2010, 20, 435–440. [Google Scholar] [CrossRef]
- Steinhoff, B. Pyrrolizidine alkaloid contamination in herbal medicinal products: Limits and occurrence. Food Chem. Toxicol. 2019, 130, 262–266. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Heavy metal pollution and potential health risks of commercially available Chinese herbal medicines. Sci. Total Environ. 2019, 653, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Rep. 2017, 50, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, D.P.B.; Florentino, I.F.; da Silva Moreira, L.K.; Brito, A.F.; Carvalho, V.V.; Rodrigues, M.F.; Vasconcelos, G.A.; Vaz, B.G.; Pereira-Junior, M.A.; Fernandes, K.F.; et al. Chemical characterization and pharmacological assessment of polysaccharide free, standardized cashew gum extract (Anacardium occidentale L.). J. Ethnopharmacol. 2018, 213, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, X.; Mi, L.; Jing, J.; Gai, S.; Liu, X.; Wang, Q.; Zhang, S. Simultaneous determinations of four major bioactive components in Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts by LC-MS/MS: Application to its herb-herb interactions based on pharmacokinetic, tissue distribution and excretion studies in rats. Phytomedicine 2019, 56, 64–73. [Google Scholar]
- Chaban, M.F.; Karagianni, C.; Joray, M.B.; Toumpa, D.; Sola, C.; Crespo, M.I.; Palacios, S.M.; Athanassopoulos, C.M.; Carpinella, M.C. Antibacterial effects of extracts obtained from plants of Argentina: Bioguided isolation of compounds from the anti-infectious medicinal plant Lepechinia meyenii. J. Ethnopharmacol. 2019, 239, 111930. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, D.C.; Brandao, G.C.; Nascimento, M.; Oliveira, A.B. Antiplasmodial activity and cytotoxicity, isolation of active alkaloids, and dereplication of Xylopia sericea leaves ethanol extract by UPLC-DAD-ESI-MS/MS. J. Pharm. Pharm. 2019, 71, 260–269. [Google Scholar] [CrossRef] [PubMed]
- De Viana, M.L.; Giamminola, E.; Russo, R.; Ciaccio, M. Morphology and genetics of Anadenanthera colubrina var. cebil (Fabaceae) tree from salta (Northwestern Argentina). Rev. Biol. Trop. 2014, 62, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Barreto, H.M.; Coelho, K.M.; Ferreira, J.H.; Dos Santos, B.H.; de Abreu, A.P.; Coutinho, H.D.; da Silva, R.A.; de Sousa, T.O.; Cito, A.M.; Lopes, J.A. Enhancement of the antibiotic activity of aminoglycosides by extracts from Anadenanthera colubrine (Vell.) Brenan var. cebil against multi-drug resistant bacteria. Nat. Prod. Res. 2016, 30, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.M.; de Almeida Cde, F.; de Albuquerque, U.P.; de Lucena, R.F.; Florentino, A.T.; de Oliveira, R.L. Use and traditional management of Anadenanthera colubrina (Vell.) Brenan in the semi-arid region of northeastern Brazil. J. Ethnobio. Ethnomed. 2006, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, U.P.; de Oliveira, R.F. Is the use-impact on native caatinga species in Brazil reduced by the high species richness of medicinal plants? J. Ethnopharmacol. 2007, 113, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.T.; de Albuquerque, U.P. A new application for the optimal foraging theory: The extraction of medicinal plants. Evid. Based Complement. Altern. Med. 2012, 2012, 364564. [Google Scholar] [CrossRef] [PubMed]
- Lucena, R.F.; Albuquerque, U.P.; Monteiro, J.M.; Almeida Cde, F.; Florentino, A.T.; Ferraz, J.S. Useful plants of the semi-arid northeastern region of Brazil—A look at their conservation and sustainable use. Environ. Monit. Assess. 2007, 125, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Rosalen, P.L.; Freires, I.A.; Sardi, J.C.O.; Lima, R.F.; Lazarini, J.G.; Costa, T.; Pereira, J.V.; Godoy, G.P.; Costa, E. Anadenanthera Colubrina vell Brenan: Anti-Candida and antibiofilm activities, toxicity and therapeutical action. Braz. Oral Res. 2019, 33, e023. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.; Sandes, J.M.; de Paiva, M.M.; de Araujo, J.M.; de Figueiredo, R.C.; da Silva, M.V.; Correia, M.T. Anti-Staphylococcus aureus action of three Caatinga fruits evaluated by electron microscopy. Nat. Prod. Res. 2013, 27, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.; da Silva, C.A., Jr.; de Souza, R.M.; Jose Macedo, A.; da Silva, M.V.; dos Santos Correia, M.T. Comparative analysis of the antioxidant and DNA protection capacities of Anadenanthera colubrina, Libidibia ferrea and Pityrocarpa moniliformis fruits. Food Chem. Toxicol. 2011, 49, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Melo, J.; de Sousa Araujo, T.A.; Thijan Nobre de Almeida e Castro, V.; Lyra de Vasconcelos Cabral, D.; do Desterro Rodrigues, M.; Carneiro do Nascimento, S.; Cavalcanti de Amorim, E.L.; de Albuquerque, U.P. Antiproliferative activity, antioxidant capacity and tannin content in plants of semi-arid northeastern Brazil. Molecules 2010, 15, 8534–8542. [Google Scholar] [CrossRef]
- Damascena, N.P.; Souza, M.T.; Almeida, A.F.; Cunha, R.S.; Damascena, N.P.; Curvello, R.L.; Lima, A.C.; Almeida, E.C.; Santos, C.C.; Dias, A.S.; et al. Antioxidant and orofacial anti-nociceptive activities of the stem bark aqueous extract of Anadenanthera colubrina (Velloso) Brenan (Fabaceae). Nat. Prod. Res. 2014, 28, 753–756. [Google Scholar] [CrossRef]
- Mota, G.S.; Sartori, C.J.; Miranda, I.; Quilho, T.; Mori, F.A.; Pereira, H. Bark anatomy, chemical composition and ethanol-water extract composition of Anadenanthera peregrina and Anadenanthera colubrina. PLoS ONE 2017, 12, e0189263. [Google Scholar] [CrossRef]
- Pessoa, W.S.; Estevao, L.R.; Simoes, R.S.; Barros, M.E.; Mendonca Fde, S.; Baratella-Evencio, L.; Evencio-Neto, J. Effects of angico extract (Anadenanthera colubrina var. cebil) in cutaneous wound healing in rats. Acta Cir. Bras. 2012, 27, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, W.S.; Estevao, L.R.; Simoes, R.S.; Mendonca Fde, S.; Evencio-Luz, L.; Baratella-Evencio, L.; Florencio-Silva, R.; Sa, F.B.; Evencio-Neto, J. Fibrogenesis and epithelial coating of skin wounds in rats treated with angico extract (Anadenanthera colubrina var. cebil). Acta Cir. Bras. 2015, 30, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Guarneire, G.J.; Cardoso Junior, O.; Marques Lima, N.; Aguilar Santos, E.; Aguiar Schulze, C.; Pereira Silva, W.; Pedro Oliveira Batista, J.; de Paula Carli, G.; Castro, S.B.; Alves, C.C.S.; et al. Effect of Anadenanthera colubrina protease inhibitors a Paulinos an inflammatory mediator. Nat. Prod. Res. 2019, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Marinho, R.R.; Ekundi-Valentim, E.; Rodrigues, L.; Yamamoto, M.H.; Teixeira, S.A.; Muscara, M.N.; Costa, S.K.; Thomazzi, S.M. Beneficial effects of Anadenanthera colubrina (Vell.) Brenan extract on the inflammatory and nociceptive responses in rodent models. J. Ethnopharmacol. 2013, 148, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Lima Rde, F.; Alves, E.P.; Rosalen, P.L.; Ruiz, A.L.; Teixeira Duarte, M.C.; Goes, V.F.; de Medeiros, A.C.; Pereira, J.V.; Godoy, G.P.; Melo de Brito Costa, E.M. Antimicrobial and Antiproliferative Potential of Anadenanthera colubrina (Vell.) Brenan. Evid. Based Complement. Altern. Med. 2014, 2014, 802696. [Google Scholar]
- Souza Dos Santos, B.; Bezerra Filho, C.M.; Alves do Nascimento Junior, J.A.; Brust, F.R.; Bezerra-Silva, P.C.; Lino da Rocha, S.K.; Krogfelt, K.A.; Maria do Amaral Ferraz Navarro, D.; Tereza Dos Santos Correia, M.; Napoleao, T.H.; et al. Anti-staphylococcal activity of Syagrus coronata essential oil: Biofilm eradication and in vivo action on Galleria mellonela infection model. Microb. Pathog. 2019, 131, 150–157. [Google Scholar] [PubMed]
- Quave, C.L.; Plano, L.R.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Silva, T.; Carvalho, M.; Braz-Filho, R. Estudo espectroscópico em elucidação estrutural de flavonoides de Solanum jabrense Agra & Nee e S. paludosum Moric. Quim. NOVA 2009, 32, 1119–1128. [Google Scholar]
- Cho, J.-Y.; Moon, J.-H.; Seong, K.-Y.; PARK, K.-H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Júnior, B.; Bomfim, C.; Kuster, R.M.; Simas, N.K.; Sakuragui, C.M.; Porzel, A.; Wessjohann, L. Flavonoids and a neolignan glucoside from Guarea macrophylla (Meliaceae). Química NOVA 2012, 35, 1123–1126. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, D.R.C.; da Silva, L.C.N.; Harand, W.; Fernandes, J.M.; da Cunha Soares, T.; Langassner, S.M.Z.; Giordani, R.B.; Ximenes, R.M.; da Silva, A.G.; da Silva, M.V. Effects of Rainfall on the Antimicrobial Activity and Secondary Metabolites Contents of Leaves and Fruits of Anadenanthera colubrina from Caatinga Area. Pharmacogn. J. 2017, 9, 435–440. [Google Scholar] [CrossRef]
- Araújo, D.; Da Silva, L.C.N.; Da Silva, A.G.; Macedo, A.; Correia, M.; Da Silva, M. Comparative analysis of anti-Staphylococcus aureus action of leaves and fruits of Anadenanthera colubrina var. cebil (Griseb.) Altschul. Afr. J. Microbiol. Res 2014, 8, 2690–2696. [Google Scholar]
- Bar-Or, D.; Carrick, M.M.; Mains, C.W.; Rael, L.T.; Slone, D.; Brody, E.N. Sepsis, oxidative stress, and hypoxia: Are there clues to better treatment? Redox Rep. 2015, 20, 193–197. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In Vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Lin, Y.H. MicroRNA Networks Modulate Oxidative Stress in Cancer. Int. J. Mol. Sci. 2019, 20, 4497. [Google Scholar] [CrossRef]
- Villanueva, M.; Garcia, B.; Valle, J.; Rapun, B.; Ruiz de Los Mozos, I.; Solano, C.; Marti, M.; Penades, J.R.; Toledo-Arana, A.; Lasa, I. Sensory deprivation in Staphylococcus aureus. Nat. Commun. 2018, 9, 523. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kalemaki, D. Evaluation and management of Staphylococcus aureus bacteriuria: An updated review. Infection 2018, 46, 293–301. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Prabhoo, R.; Chaddha, R.; Iyer, R.; Mehra, A.; Ahdal, J.; Jain, R. Overview of methicillin resistant Staphylococcus aureus mediated bone and joint infections in India. Orthop. Rev. 2019, 11, 8070. [Google Scholar] [CrossRef] [PubMed]
- Astley, R.; Miller, F.C.; Mursalin, M.H.; Coburn, P.S.; Callegan, M.C. An Eye on Staphylococcus aureus Toxins: Roles in Ocular Damage and Inflammation. Toxins 2019, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Troeman, D.P.R.; Van Hout, D.; Kluytmans, J. Antimicrobial approaches in the prevention of Staphylococcus aureus infections: A review. J. Antimicrob. Chemother. 2019, 74, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.M.; Nedeljkovic, M.; Dessen, A. New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus. Drug Resist. Updates 2017, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.S.; Pinto, B.L.S.; Monteiro, J.M.; Ferreira, R.M.; Ribeiro, P.C.S.; Bando, S.Y.; Marques, S.G.; Silva, L.C.N.; Neto, W.R.N.; Ferreira, G.F.; et al. Phylogenetic and Molecular Profile of Staphylococcus aureus Isolated from Bloodstream Infections in Northeast Brazil. Microorganisms 2019, 7, 210. [Google Scholar] [CrossRef]
- Rello, J.; Parisella, F.R.; Perez, A. Alternatives to antibiotics in an era of difficult-to-treat resistance: New insights. Expert Rev. Clin. Pharm. 2019, 12, 635–642. [Google Scholar] [CrossRef]
- Diniz, R.C.; Soares, L.W.; Nascimento da Silva, L.C. Virtual Screening for the Development of New Effective Compounds Against Staphylococcus aureus. Curr. Med. Chem. 2018, 25, 5975–5985. [Google Scholar] [CrossRef]
- Ren, G.; Xue, P.; Sun, X.; Zhao, G. Determination of the volatile and polyphenol constituents and the antimicrobial, antioxidant, and tyrosinase inhibitory activities of the bioactive compounds from the by-product of Rosa rugosa Thunb. var. plena Regal tea. BMC Complement. Altern. Med. 2018, 18, 307. [Google Scholar] [CrossRef]
- Da Costa Cordeiro, B.M.P.; de Lima Santos, N.D.; Ferreira, M.R.A.; de Araujo, L.C.C.; Junior, A.R.C.; da Conceicao Santos, A.D.; de Oliveira, A.P.; da Silva, A.G.; da Silva Falcao, E.P.; Dos Santos Correia, M.T.; et al. Hexane extract from Spondias tuberosa (Anacardiaceae) leaves has antioxidant activity and is an anti-Candida agent by causing mitochondrial and lysosomal damages. BMC Complement. Altern. Med. 2018, 18, 284. [Google Scholar] [CrossRef]
- Andriamadio, J.H.; Rasoanaivo, L.H.; Benedec, D.; Vlase, L.; Gheldiu, A.M.; Duma, M.; Toiu, A.; Raharisololalao, A.; Oniga, I. HPLC/MS analysis of polyphenols, antioxidant and antimicrobial activities of Artabotrys hildebrandtii O. Hffm. extracts. Nat. Prod. Res. 2015, 29, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Tadic, V.; Oliva, A.; Bozovic, M.; Cipolla, A.; De Angelis, M.; Vullo, V.; Garzoli, S.; Ragno, R. Chemical and Antimicrobial Analyses of Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, an Endemic of the Western Balkan. Molecules 2017, 22, 1395. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.S.; Bekbolatova, E.; Cotrim, M.D.; Sakipova, Z.; Ibragimova, L.; Kukula-Koch, W.; Giorno, T.B.S.; Fernandes, P.D.; Fonseca, D.A.; Boylan, F. Chemistry and Pharmacology of the Kazakh Crataegus Almaatensis Pojark: An Asian Herbal Medicine. Antioxidants 2019, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Qiu, X.; Dai, M.; Zhang, X.; Jin, G. Hyperoside Attenuates Hepatic Ischemia-Reperfusion Injury by Suppressing Oxidative Stress and Inhibiting Apoptosis in Rats. Transpl. Proc. 2019, 51, 2051–2059. [Google Scholar]
- Zou, L.; Chen, S.; Li, L.; Wu, T. The protective effect of hyperoside on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. Exp. Toxicol. Pathol. 2017, 69, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Q.; Liu, S.; An, X.; Huang, Z.; Zhang, B.; Yuan, Y.; Xing, C. Protective effect of hyperoside against renal ischemia-reperfusion injury via modulating mitochondrial fission, oxidative stress, and apoptosis. Free Radic. Res. 2019, 53, 727–736. [Google Scholar] [PubMed]
- Wang, X.; Fan, G.; Wei, F.; Bu, Y.; Huang, W. Hyperoside protects rat ovarian granulosa cells against hydrogen peroxide-induced injury by sonic hedgehog signaling pathway. Chem. Biol. Interact. 2019, 310, 108759. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, L.; Wang, X.; Lan, R.; Wang, M.; Du, G.; Guan, W.; Liu, J.; Brennan, M.; Guo, H.; et al. Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces Cerevisiae as a Model. Molecules 2019, 24, 788. [Google Scholar] [CrossRef]
- Yang, L.; Shen, L.; Li, Y.; Li, Y.; Yu, S.; Wang, S. Hyperoside attenuates dextran sulfate sodium-induced colitis in mice possibly via activation of the Nrf2 signalling pathway. J. Inflamm. 2017, 14, 25. [Google Scholar] [CrossRef]
- Jin, X.N.; Yan, E.Z.; Wang, H.M.; Sui, H.J.; Liu, Z.; Gao, W.; Jin, Y. Hyperoside exerts anti-inflammatory and anti-arthritic effects in LPS-stimulated human fibroblast-like synoviocytes in vitro and in mice with collagen-induced arthritis. Acta Pharm. Sin. 2016, 37, 674–686. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.H.; Zhu, L.B.; Li, T.; Zhu, H.; Wang, Y.N.; Ren, X.L.; Hu, B.L.; Huang, C.P.; Zhu, J.H.; Zhang, X. Hyperoside inhibits lipopolysaccharide-induced inflammatory responses in microglial cells via p38 and NFkappaB pathways. Int. Immunopharmacol. 2017, 50, 14–21. [Google Scholar]
- Guo, W.; Yu, H.; Zhang, L.; Chen, X.; Liu, Y.; Wang, Y.; Zhang, Y. Effect of hyperoside on cervical cancer cells and transcriptome analysis of differentially expressed genes. Cancer Cell Int. 2019, 19, 235. [Google Scholar] [PubMed]
- Li, Y.; Wang, Y.; Li, L.; Kong, R.; Pan, S.; Ji, L.; Liu, H.; Chen, H.; Sun, B. Hyperoside induces apoptosis and inhibits growth in pancreatic cancer via Bcl-2 family and NF-kappaB signaling pathway both in vitro and in vivo. Tumour Biol. 2016, 37, 7345–7355. [Google Scholar] [CrossRef] [PubMed]
- Berkoz, M. Effect of Hyperoside on the Inhibition of Adipogenesis in 3t3-L1 Adipocytes. Acta Endocrinol. 2019, 15, 165–172. [Google Scholar]
- Zhang, Y.; Wang, M.; Dong, H.; Yu, X.; Zhang, J. Anti-hypoglycemic and hepatocyte-protective effects of hyperoside from Zanthoxylum bungeanum leaves in mice with high-carbohydrate/high-fat diet and alloxan-induced diabetes. Int. J. Mol. Med. 2018, 41, 77–86. [Google Scholar] [CrossRef]
- Sowa, A.; Zgorka, G.; Szykula, A.; Franiczek, R.; Zbikowska, B.; Gamian, A.; Sroka, Z. Analysis of Polyphenolic Compounds in Extracts from Leaves of Some Malus domestica Cultivars: Antiradical and Antimicrobial Analysis of These Extracts. BioMed Res. Int. 2016, 2016, 6705431. [Google Scholar] [CrossRef]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic Compounds in Antimicrobial Therapy. J. Med. Food 2017, 20, 1031–1038. [Google Scholar]
- Heleno, S.A.; Ferreira, I.C.; Esteves, A.P.; Ciric, A.; Glamoclija, J.; Martins, A.; Sokovic, M.; Queiroz, M.J. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Peungvicha, P.; Thirawarapan, S.S.; Watanabe, H. Possible mechanism of hypoglycemic effect of 4-hydroxybenzoic acid, a constituent of Pandanus odorus root. Jpn. J. Pharm. 1998, 78, 395–398. [Google Scholar]
- Franklin, Z.J.; McDonnell, B.; Montgomery, I.A.; Flatt, P.R.; Irwin, N. Dual modulation of GIP and glucagon action by the low molecular weight compound 4-hydroxybenzoic acid 2-bromobenzylidene hydrazide. Diabetes Obes. Metab. 2011, 13, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.R.; Jeong, S.J.; Lee, N.R.; Shin, H.K.; Seo, C.S. Simultaneous determination and anti-inflammatory effects of four phenolic compounds in Dendrobii Herba. Nat. Prod. Res. 2017, 31, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Chatterjee, S.S.; Kumar, V. Low dose aspirin like analgesic and anti-inflammatory activities of mono-hydroxybenzoic acids in stressed rodents. Life Sci. 2016, 148, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Eerdunbayaer, A.D.; Kuroda, T.; Zhang, G.; Hatano, T.; Chen, G. Polyphenolic constituents of Cynomorium songaricum Rupr. and antibacterial effect of polymeric proanthocyanidin on methicillin-resistant Staphylococcus aureus. J. Agric. Food Chem. 2012, 60, 7297–7305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, C.; Song, L.; Li, T.; Cui, S.; Zhang, L.; Jia, Y. Antimicrobial activity and mechanism of Larch bark procyanidins against Staphylococcus aureus. Acta Biochim. Biophys. Sin. 2017, 49, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. Biomed. Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, S.; Xu, C.; Li, L.; Zhang, Y.; Guo, Y.; Zhang, C.; Li, P.; Long, M.; He, J. Proanthocyanidins Protect against beta-Hydroxybutyrate-Induced Oxidative Damage in Bovine Endometrial Cells. Molecules 2019, 24, 400. [Google Scholar] [CrossRef]
- Gonzalez-Quilen, C.; Gil-Cardoso, K.; Gines, I.; Beltran-Debon, R.; Pinent, M.; Ardevol, A.; Terra, X.; Blay, M.T. Grape-Seed Proanthocyanidins are Able to Reverse Intestinal Dysfunction and Metabolic Endotoxemia Induced by a Cafeteria Diet in Wistar Rats. Nutrients 2019, 11, 979. [Google Scholar] [CrossRef]
| Samples | Antimicrobial Activity | Antioxidant Activity | |||
|---|---|---|---|---|---|
| IN50 (μg/mL) | DPPH | TAA | FRAP | ||
| S. aureus UFPEDA 02 | S. aureus UFPEDA 705 | IC50 (μg/mL) | % | μg FeSO4/mg | |
| ASE-AcOEt | 312.5 | 2500 | 142 ± 10 | N/E | N/E |
| Fraction 1 (F1) | 2500 | 2500 | N/A | N/E | N/E |
| Fraction 2 (F2) | 19.53 | 2500 | 202 ± 6 a | N/E | N/E |
| Fraction 3 (F3) | 78.12 | >2500 | 263 ± 17 b | N/E | N/E |
| Fraction 4 (F4) | 19.53 | >2500 | 133 ± 9 c | N/E | N/E |
| Fraction 5 (F5) | >2500 | >2500 | 218 ±7.6 a | N/E | N/E |
| p-hydroxybenzoic acid (1) | 500 | >500 | N/A | N/E | N/E |
| Proanthocyanidin (2) | 500 | >500 | 124.8 ± 7 c | 60 ± 3.3 a | 550.22 ± 10.43 |
| Proanthocyanidin (3) | 62.5 | >500 | 178.4 ± 5 a | 35 ± 0.67 b | 552.62 ± 5.23 |
| Hyperoside (4) | 250 | 62.5 | 258 ± 20 b | 4.31 ± 0.81 c | 529.91 ± 21.55 |
| Ampicillin | <1.9 | 3.9 | N/E | N/E | N/E |
| Tetracycline | <1.9 | 15.6 | N/E | N/E | N/E |
| Trolox | N/E | N/E | N/E | N/E | 566.08 ± 13.81 |
| Gallic acid | N/E | N/E | <312 | N/E | N/E |
| Ascorbic acid | N/E | N/E | N/E | 100 | N/E |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo Cavalcante de Araújo, D.; Diego da Silva, T.; Harand, W.; Sampaio de Andrade Lima, C.; Paulo Ferreira Neto, J.; de Azevedo Ramos, B.; Alves Rocha, T.; da Silva Alves, H.; Sobrinho de Sousa, R.; Paula de Oliveira, A.; et al. Bioguided Purification of Active Compounds from Leaves of Anadenanthera colubrina var. cebil (Griseb.) Altschul. Biomolecules 2019, 9, 590. https://doi.org/10.3390/biom9100590
Rodrigo Cavalcante de Araújo D, Diego da Silva T, Harand W, Sampaio de Andrade Lima C, Paulo Ferreira Neto J, de Azevedo Ramos B, Alves Rocha T, da Silva Alves H, Sobrinho de Sousa R, Paula de Oliveira A, et al. Bioguided Purification of Active Compounds from Leaves of Anadenanthera colubrina var. cebil (Griseb.) Altschul. Biomolecules. 2019; 9(10):590. https://doi.org/10.3390/biom9100590
Chicago/Turabian StyleRodrigo Cavalcante de Araújo, Daniel, Túlio Diego da Silva, Wolfgang Harand, Claudia Sampaio de Andrade Lima, João Paulo Ferreira Neto, Bárbara de Azevedo Ramos, Tamiris Alves Rocha, Harley da Silva Alves, Rayane Sobrinho de Sousa, Ana Paula de Oliveira, and et al. 2019. "Bioguided Purification of Active Compounds from Leaves of Anadenanthera colubrina var. cebil (Griseb.) Altschul" Biomolecules 9, no. 10: 590. https://doi.org/10.3390/biom9100590
APA StyleRodrigo Cavalcante de Araújo, D., Diego da Silva, T., Harand, W., Sampaio de Andrade Lima, C., Paulo Ferreira Neto, J., de Azevedo Ramos, B., Alves Rocha, T., da Silva Alves, H., Sobrinho de Sousa, R., Paula de Oliveira, A., Cláudio Nascimento da Silva, L., Roberto Guedes da Silva Almeida, J., Vanusa da Silva, M., & Tereza dos Santos Correia, M. (2019). Bioguided Purification of Active Compounds from Leaves of Anadenanthera colubrina var. cebil (Griseb.) Altschul. Biomolecules, 9(10), 590. https://doi.org/10.3390/biom9100590






