Amazon Fruits Inhibit Growth and Promote Pro-apoptotic Effects on Human Ovarian Carcinoma Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Extraction of Samples
2.3. Carotenoid Analysis
2.4. Determination of total Phenolics
2.5. Antioxidant Activity Analysis
2.5.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.5.2. Ferric-Reducing Ability of Plasma (FRAP) Assay
2.5.3. Scavenger Radical Activity (DPPH)
2.5.4. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.6. Cell Culture and Treatment Protocol
2.7. Cytotoxic Analysis
2.7.1. MTT Assay
2.7.2. Cell Cycle
2.7.3. Apoptosis Assays
2.8. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Properties of Murici (ME) and Tapereba (TAP) Extracts
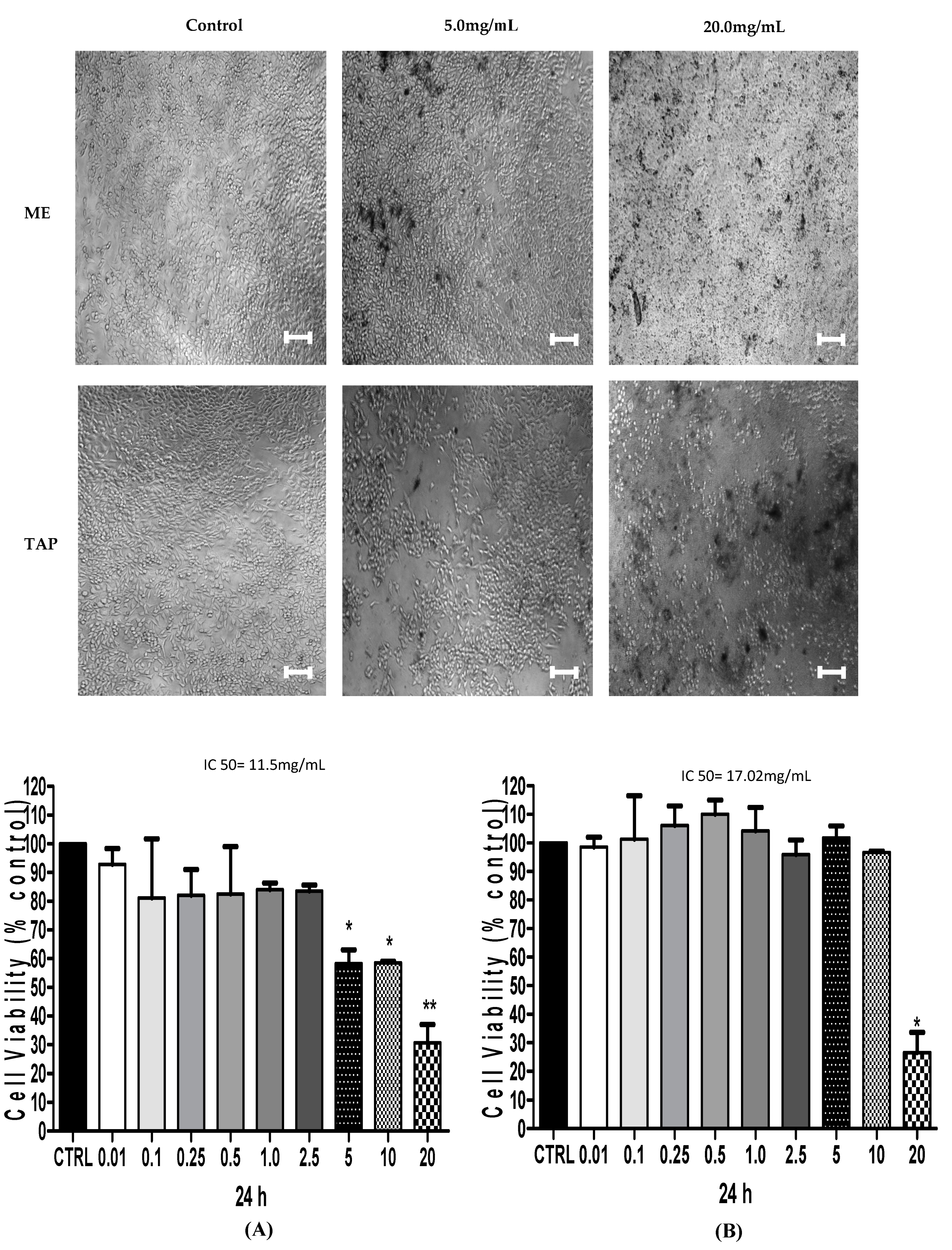
3.2. Cytotoxic Analysis—MTT
3.3. Effect of ME and TAP on Cell Cycle Progression
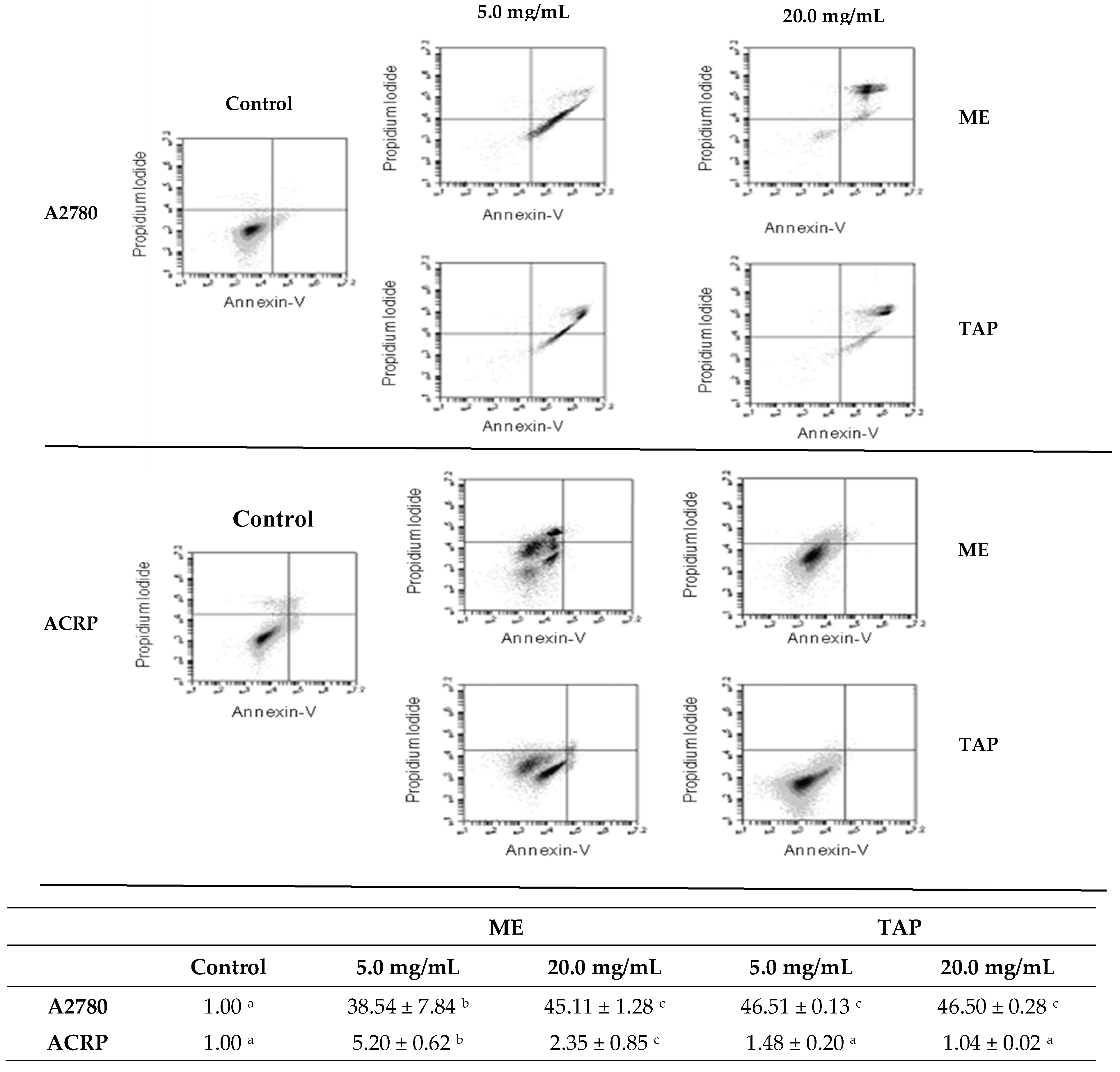
3.4. Effect of Murici and Tapereba Extracts in an Apoptosis Assay
3.5. Cytotoxic Analysis—MTT after Combined Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-azobis(2-methylpropionamidine) dihydrochloride |
| ABTS/TEAC | Trolox equivalent antioxidant capacity |
| A2780 | Human ovarian carcinoma cell line |
| ACRP | Human ovarian carcinoma cell line cisplatin resistant |
| AUC | Area under the curve |
| CDDP | Cisplatin |
| CV | Coefficient of variation |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FBS | Fetal bovine serum |
| FRAP | Ferric reducing ability of plasma |
| GAE | Gallic acid equivalents |
| HPLC | High-performance liquid chromatography |
| INCA | National Cancer Institute |
| LAAF | Laboratory for Analysis of Functional Foods |
| ME | Murici |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) |
| ORAC | Oxygen radical absorbance capacity |
| PBS | Phosphate buffered saline solution |
| PI | Propidium iodide |
| ROS | Reactive oxygen species |
| RPMI 1640 | Cell culture medium |
| TAP | Tapereba |
| TE | Trolox equivalent |
| TPTZ | 2,4,6-Tri(2-pyridyl)-s-triazine |
References
- BenTaieb, A.; Li-Chang, H.; Huntsman, D.; Hamarneh, G. A structured latent model for ovarian carcinoma subtyping from histopathology slides. Med. Image Anal. 2017, 39, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.-L.; Leung, C.S.; Yip, K.-P.; Au Yeung, C.L.; Wong, S.T.C.; Mok, S.C. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol. Cell Physiol. 2015, 309, C444–C456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.J.; Hodeib, M.; Chang, J.; Bristow, R.E. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol Oncol 2013, 130, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Rogez, H.; Eric, B. Chemical composition of the pulp of three typical Amazonian fruits: Araça-boi (Eugenia stipitata), bacuri (Platonia insignis) and cupuaçu (Theobroma grandiflorum). Eur. Food Res. Technol. 2004, 218, 380–384. [Google Scholar] [CrossRef]
- Braga, A.C.C.; Silva, A.E.; Pelais, A.C.A.; Bichara, C.M.G.; Pompeu, D.R. Antioxidant activity and bioactive compounds of the abricó fruits (Mammea americana). Aliment. Nutr. 2010, 21, 31–36. [Google Scholar]
- Sporn, M.B. Approaches to Prevention of Epithelial Cancer during the Preneoplastic Period. Cancer Res. 1976, 36, 2699–2702. [Google Scholar] [PubMed]
- Silva, J.S.; Moura, M.D.; Oliveira, R.A.G.; Diniz, M.F.F.; Barbosa-Filho, J.M. Natural product inhibitors of ovarian neoplasia. Phytomedicine 2003, 10, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O. New Opportunities in Chemoprevention. Cancer Invest. 2002, 20, 237–245. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Mariutti, L.R.B.; Rodrigues, E.; Mercadante, A.Z. Carotenoids from Byrsonima crassifolia: Identification, quantification and in vitro scavenging capacity against peroxyl radicals. J. Food Compos. Anal. 2013, 31, 155–160. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Rodrigues, E.; Chisté, R.C.; Fernandes, E.; Mercadante, A.Z. The Amazonian fruit Byrsonima crassifolia effectively scavenges reactive oxygen and nitrogen species and protects human erythrocytes against oxidative damage. Food Res. Int. 2014, 64, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.; Paulo, E.; Eberlin, M.; Maria, G.; Hai, R. Assessment of antioxidant and antiproliferative activities and the identification of phenolic compounds of exotic Brazilian fruits. Food Res. Int. 2011, 53, 417–425. [Google Scholar]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Oliveira Godoy, R.L.; Pacheco, S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef] [Green Version]
- BRASIL IBGE. Statistics-Research on Brazilian production from forestry and plant Extraction (PEVS) 2018. Available online: https://sidra.ibge.gov.br/pesquisa/pevs/quadros/brasil/2018 (accessed on 15 October 2019). (In Portuguese)
- Aromolaran, O.; Badejo, O.K. Efficacy of fresh leaf extracts of Spondias mombin against some clinical bacterial isolates from typhoid patients. Asian Pacific J. Trop. Dis. 2014, 4, 442–446. [Google Scholar] [CrossRef]
- Murillo, E.; Meléndez-Martínez, A.J.; Portugal, F. Screening of vegetables and fruits from Panama for rich sources of lutein and zeaxanthin. Food Chem. 2010, 122, 167–172. [Google Scholar] [CrossRef]
- Matietto, R.D.A.; Lopes, A.S.; Menezes, H.C.D. Physical and physicochemical characterization of caja fruit (Spondias mombin L.) and its pulp, obtained using two types of extractor. Brazilian J. Food Technol. 2010, 13, 156–164. [Google Scholar]
- Vizzotto, M.; Pereira, M.C. Amora-preta (Rubus sp.): Otimização do processo de extração para determinação de compostos fenólicos antioxidantes. Rev. Bras. de Frutic. 2011, 33, 1209–1214. [Google Scholar] [CrossRef]
- dos Santos Guimarães, I.; Ladislau-Magescky, T.; Tessarollo, N.G.; dos Santos, D.Z.; Gimba, E.R.P.; Sternberg, C.; Silva, I.V.; Rangel, L.B.A. Chemosensitizing effects of metformin on cisplatin- and paclitaxel-resistant ovarian cancer cell lines. Pharmacol. Reports 2018, 70, 409–417. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.L.-R.R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Prior, R.L.; Cao, G. Antioxidant Phytochemicals in Fruits and Vegetables: Diet and Health Implications. HortScience 2000, 35, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.D.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP). Comun. Técnico on Line 2006, 1–4. Available online: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/664098/1/cot125.pdf (accessed on 5 November 2019).
- Brand-Wiliams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.D.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre DPPH. Comun. Técnico on-line. 2007, 127, 1–4. [Google Scholar]
- Sherman-Baust, C.A.; Weeraratna, A.T.; Rangel, L.B.A.; Pizer, E.S.; Cho, K.R.; Schwartz, D.R.; Shock, T.; Morin, P.J. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell 2003, 3, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Archiv, V. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Cell Pathol. 1977, 24, 227–242. [Google Scholar]
- Mozarina, M.; Almeida, B.; Henrique, P.; De Sousa, P.H.M.; Martha, Â.; Arriaga, C.; Matias, G.; Emanuel, C.; Magalhães, D.C.; Arraes, G.; et al. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [Green Version]
- Rodriguez-amaya, D.B.; Kimura, M.; Amaya-Farfan, J. Fontes de carotenóides: Tabela brasileira de composição de carotenóides em alimentos. Bras. Ministério Meio Ambient. Biodiversidade e Florestas; 2008; p. 99. Available online: https://www.mma.gov.br/estruturas/sbf_agrobio/_publicacao/89_publicacao09032009113306.pdf (accessed on 24 June 2019).
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, VItamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222318/ (accessed on 2 June 2019).
- Hamacek, F.R.; Martino, H.S.; Pinheiro-Sant’Ana, H.M. Murici, fruit from the Cerrado of Minas Gerais, Brazil: Physical and physicochemical characteristics, and occurrence and concentration of carotenoids and vitamins. Fruits 2014, 6, 459–472. [Google Scholar] [CrossRef]
- Otles, S.; Çagindi, O. Carotenoids as Natural Colorants in Carmen Socaciu. Food Color. Chem. Funct. Prop. 2007, 51–70. [Google Scholar]
- Rodríguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; International Life Sciences Institute: Washington, DC, USA, 2001; p. 64. Available online: http://beauty-review.nl/wp-content/uploads/2014/11/A-guide-to-carotenoid-analysis-in-foods.pdf (accessed on 2 June 2019).
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- de Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Pessoa, C.; Costa-Lotufo, L.V.; Leyva, A.; de Moraes, M.E.A.; de Moraes, M.O. Anticancer potential of Northeast Brazilian plants. Adv. Phytomedicine 2006, 2, 197–211. [Google Scholar]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Looi, C.Y.; Arya, A.; Cheah, F.K.; Muharram, B.; Leong, K.H. Induction of Apoptosis in Human Breast Cancer Cells via Caspase Pathway by Vernodalin Isolated from Centratherum anthelminticum (L.) Seeds. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.J.; Baumann, W.T.; Chen, C.; Verdugo, A.; Tavassoly, I.; Wang, Y.; Clarke, R.; Weiner, L.M. Dynamic modeling of estrogen signaling and cell fate in breast cancer cells. Nature 2012, 11, 523–532. [Google Scholar]
- Niranjana, R.; Gayathri, R.; Nimish Mol, S.; Sugawara, T.; Hirata, T.; Miyashita, K.; Ganesan, P. Carotenoids modulate the hallmarks of cancer cells. J. Funct. Foods 2015, 18, 968–985. [Google Scholar] [CrossRef]
- Livny, O.; Kaplan, I.; Reifen, R.; Polak-Charcon, S.; Madar, Z.; Schwartz, B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumour cells. J. Nutr. 2002, 132, 3754–3759. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lu, Z.; Bai, L.; Shi, Z.; Zhao, W.E.; Zhao, B. β-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur. J. Cancer 2007, 43, 2590–2601. [Google Scholar] [CrossRef] [PubMed]



| Parameter | ME | TAP |
|---|---|---|
| Total phenolics (mg gallic acid (GAE)/mL) | 1634.05 ± 278.18 a | 1049.09 ± 95.68 b |
| ORAC assay (μM TE/g) | 1020.39 ± 88.43 a | 623.72 ± 38.75 b |
| FRAP assay (μmol ferrous sulphate/g) | 1014.71 ± 2.08 a | 644.55 ± 10.89 b |
| DPPH assay (% reduction) | 70.17 ± 4.61 a | 78.70 ± 0.28 b |
| TEAC assay (μmol TE/g) | 1620.95 ± 114.65 a | 1090.90 ± 296.04 b |
| Parameter | ME | TAP |
|---|---|---|
| Total carotenoids | 86.30 ± 8.82 b | 185.92 ± 12.86 a |
| Lutein | 23.39 ± 1.41 a | 11.96 ± 0.07 b |
| Zeaxanthin | 5.20 ± 1.02 a | 1.25 ± 0.10 b |
| Zeinoxanthin | 1.92 ± 0.21 b | 45.72 ± 2.92 a |
| β-cryptoxanthin | 1.32 ± 0.34 b | 89.81 ± 4.58 a |
| α-carotene | 0.48 ± 0.11 b | 18.25 ± 2.99 a |
| β-carotene | 4.61 ± 1.62 b | 17.45 ± 3.57 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, V.R.; Brum, M.C.M.; Guimarães, I.d.S.; dos Santos, P.d.F.; do Amaral, T.O.; Abreu, J.P.; Passos, T.; Freitas-Silva, O.; Gimba, E.R.P.; Teodoro, A.J. Amazon Fruits Inhibit Growth and Promote Pro-apoptotic Effects on Human Ovarian Carcinoma Cell Lines. Biomolecules 2019, 9, 707. https://doi.org/10.3390/biom9110707
de Souza VR, Brum MCM, Guimarães IdS, dos Santos PdF, do Amaral TO, Abreu JP, Passos T, Freitas-Silva O, Gimba ERP, Teodoro AJ. Amazon Fruits Inhibit Growth and Promote Pro-apoptotic Effects on Human Ovarian Carcinoma Cell Lines. Biomolecules. 2019; 9(11):707. https://doi.org/10.3390/biom9110707
Chicago/Turabian Stylede Souza, Vanessa Rosse, Mariana Concentino Menezes Brum, Isabella dos Santos Guimarães, Paula de Freitas dos Santos, Thuane Oliveira do Amaral, Joel Pimentel Abreu, Thuane Passos, Otniel Freitas-Silva, Etel Rodrigues Pereira Gimba, and Anderson Junger Teodoro. 2019. "Amazon Fruits Inhibit Growth and Promote Pro-apoptotic Effects on Human Ovarian Carcinoma Cell Lines" Biomolecules 9, no. 11: 707. https://doi.org/10.3390/biom9110707






