Pyrraline Formation Modulated by Sodium Chloride and Controlled by Encapsulation with Different Coating Materials in the Maillard Reaction
Abstract
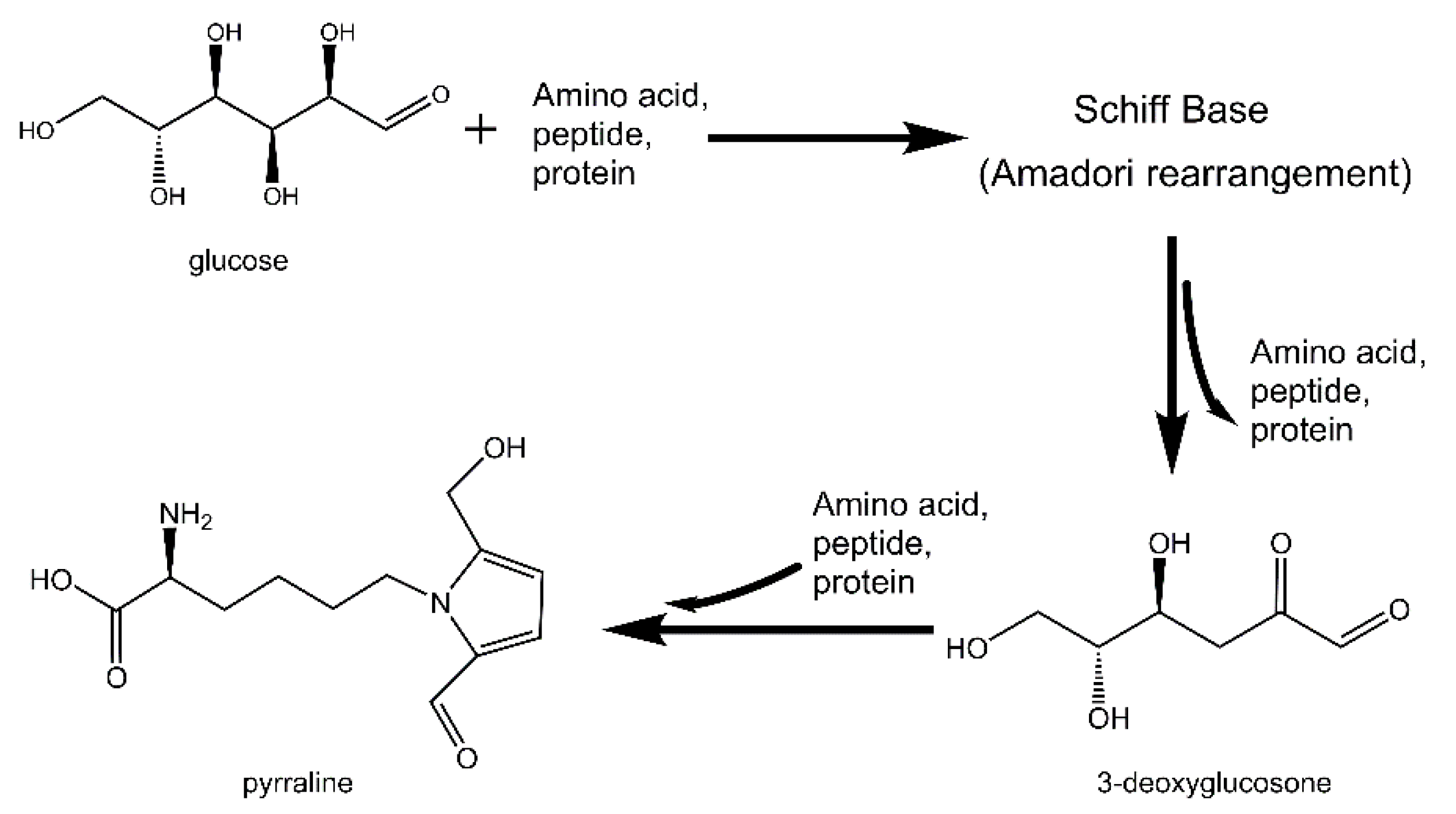
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Glucose–Lysine–NaCl Model Systems
2.3. Encapsulation Process
2.4. Thermal Properties of NaCl Microparticles
2.5. Physical Analysis of the Microparticles
2.5.1. Particle Size Distribution
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. Content of NaCl in Microparticles
2.5.4. Conductimetry
2.6. Preparation of Glucose–Lysine–Microparticle Model Systems
2.7. Extent of Browning
2.8. Pyrraline and 3-DG Analysis
2.8.1. Solid Phase Extraction (SPE) Procedures
2.8.2. LC-MS Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of NaCl in Glucose–Lysine–NaCl Model Systems
3.1.1. Effect of NaCl on the Browning Intensity
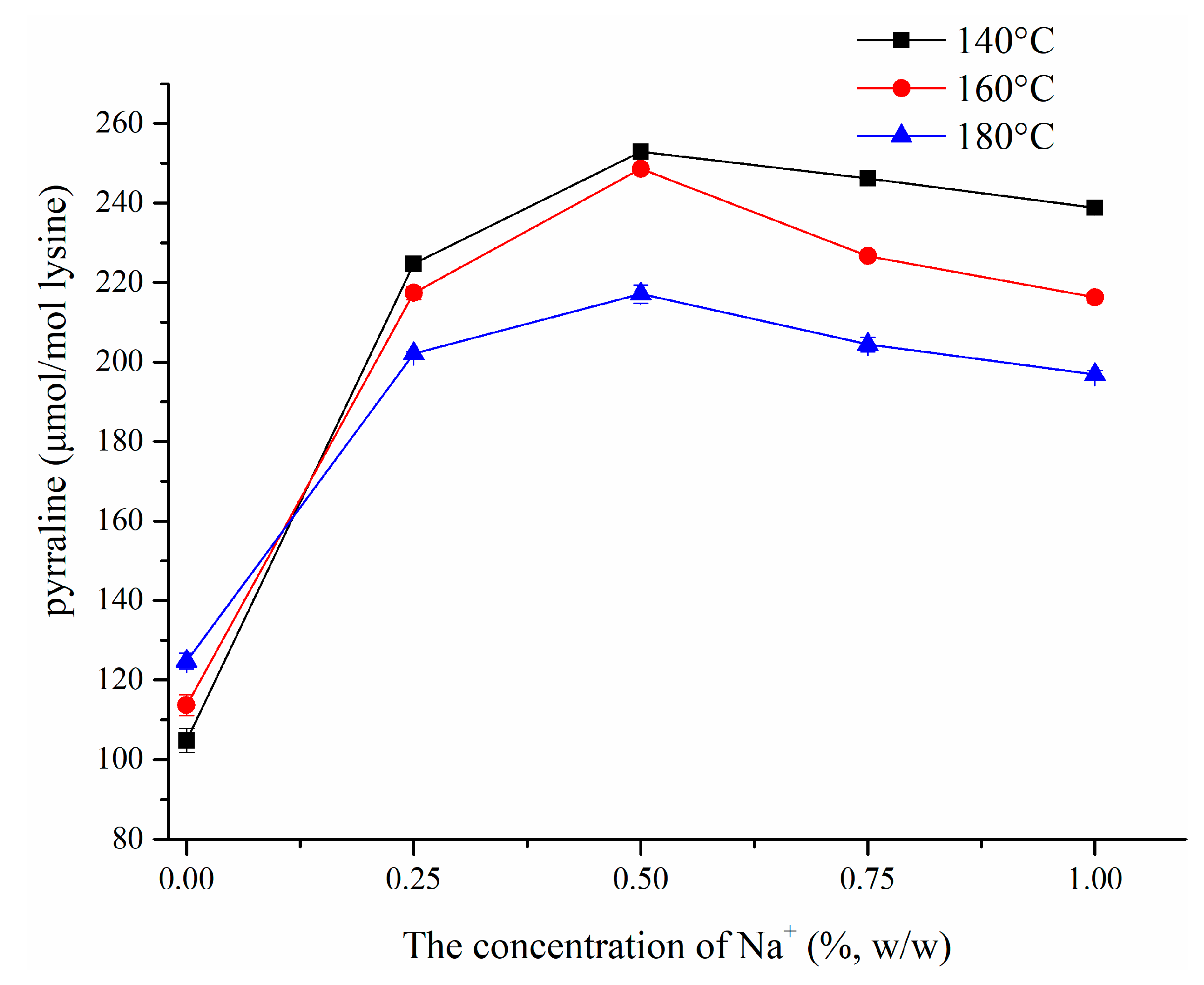
3.1.2. Effect of NaCl on Pyrraline Formation
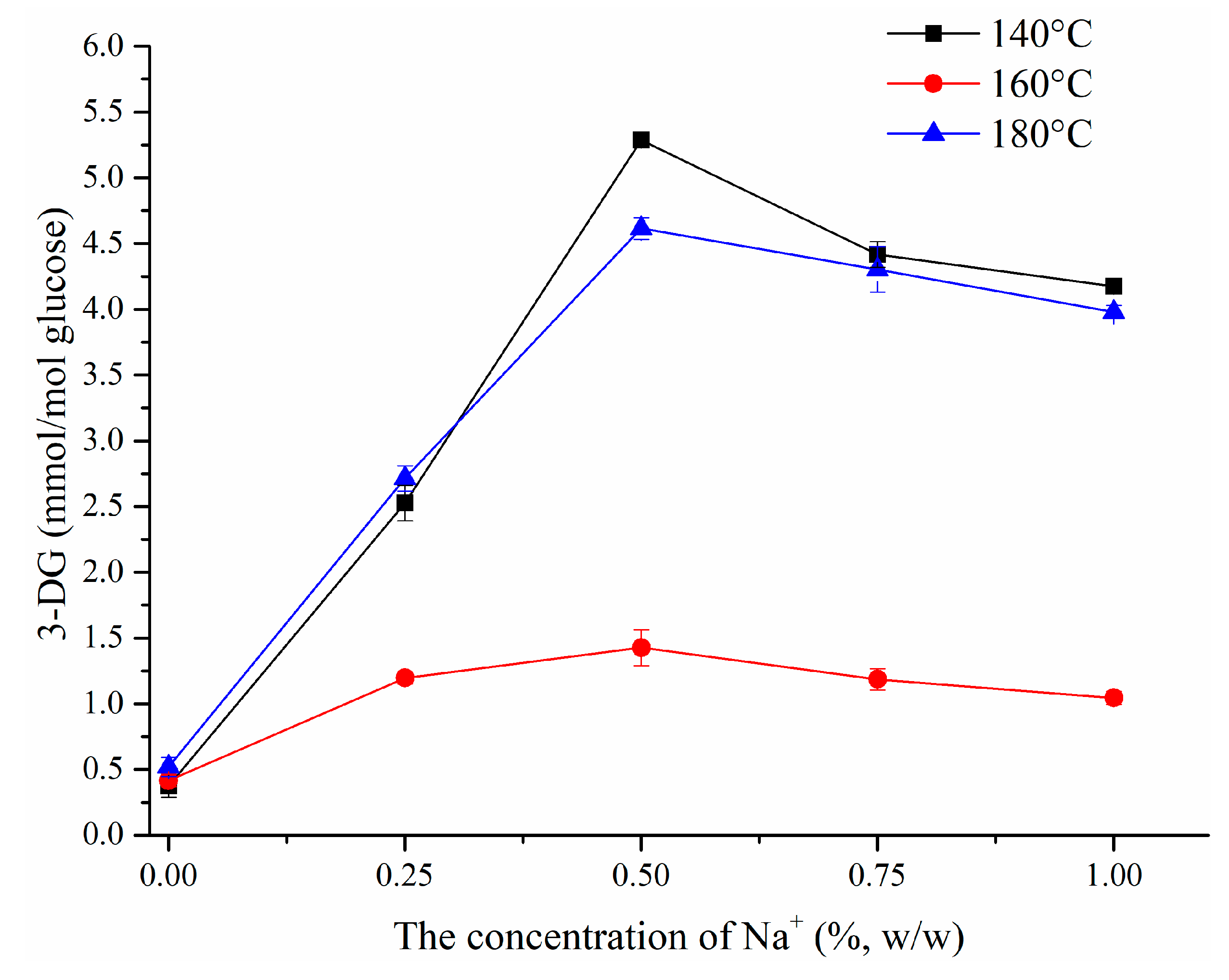
3.1.3. Effect of NaCl on 3-DG Formation
3.2. NaCl Encapsulation Modulates Pyrraline Formation
3.2.1. Thermal (DSC) Properties of NaCl Microparticles
3.2.2. Morphology of NaCl Microparticles
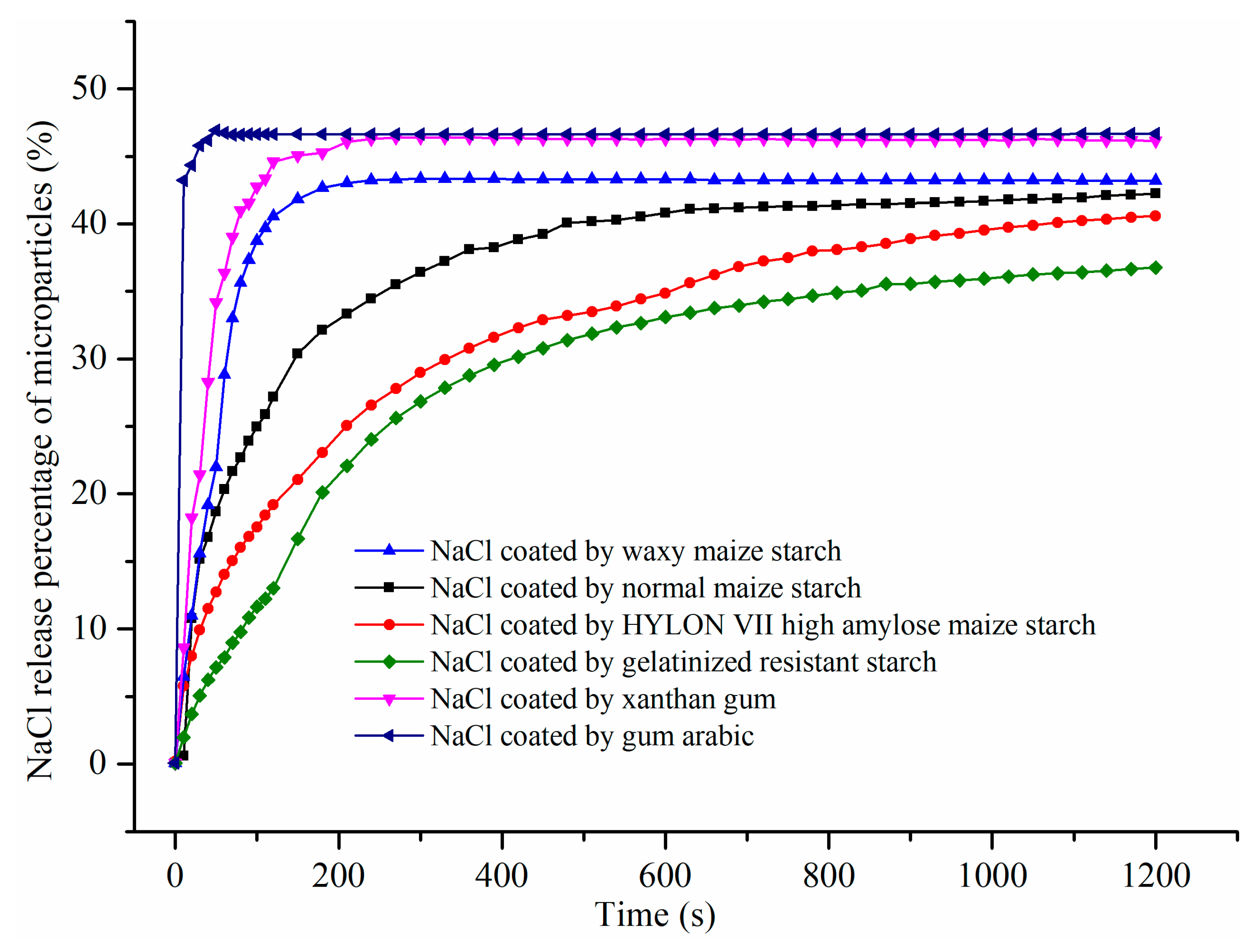
3.2.3. NaCl Release from Microparticles
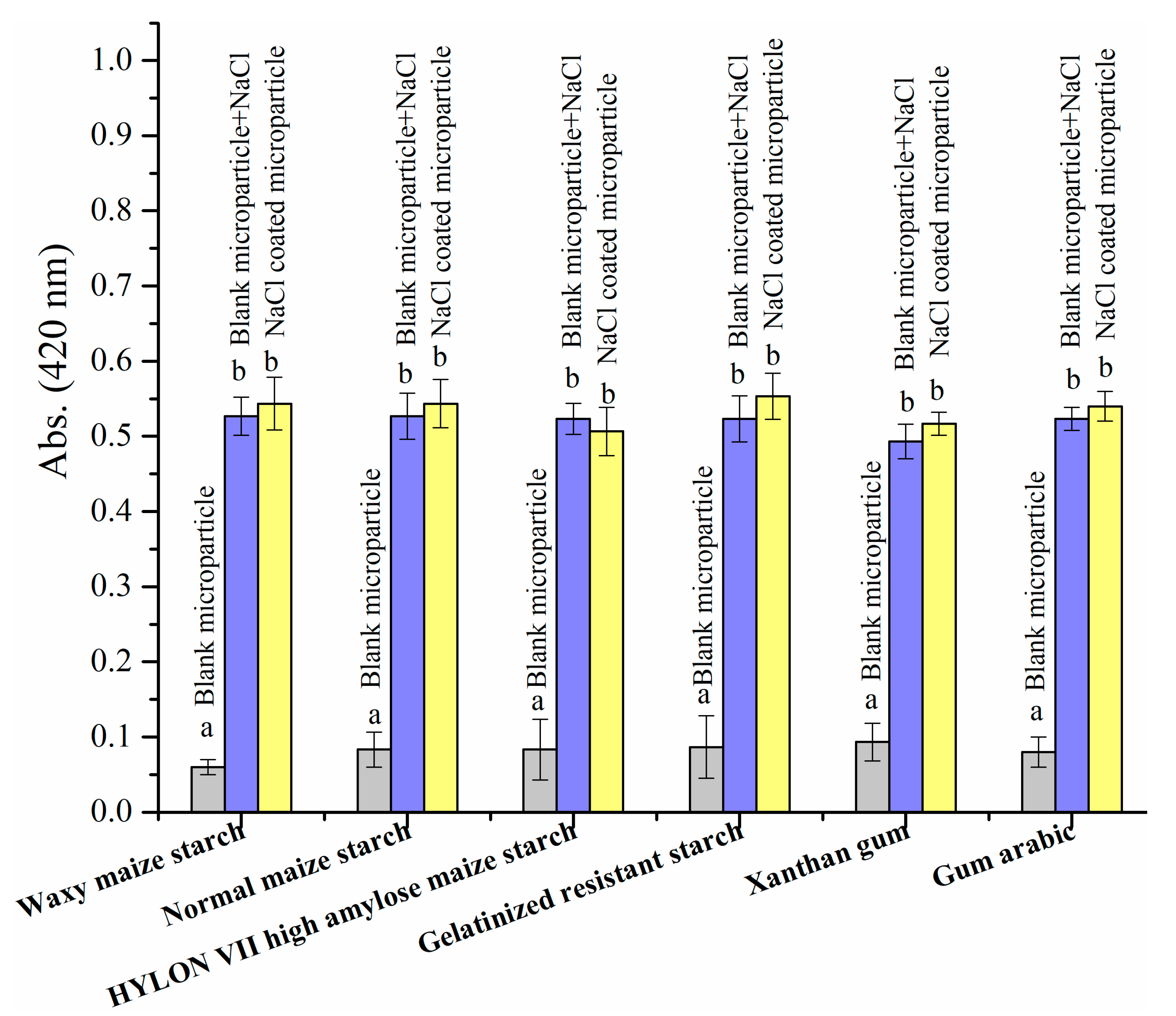
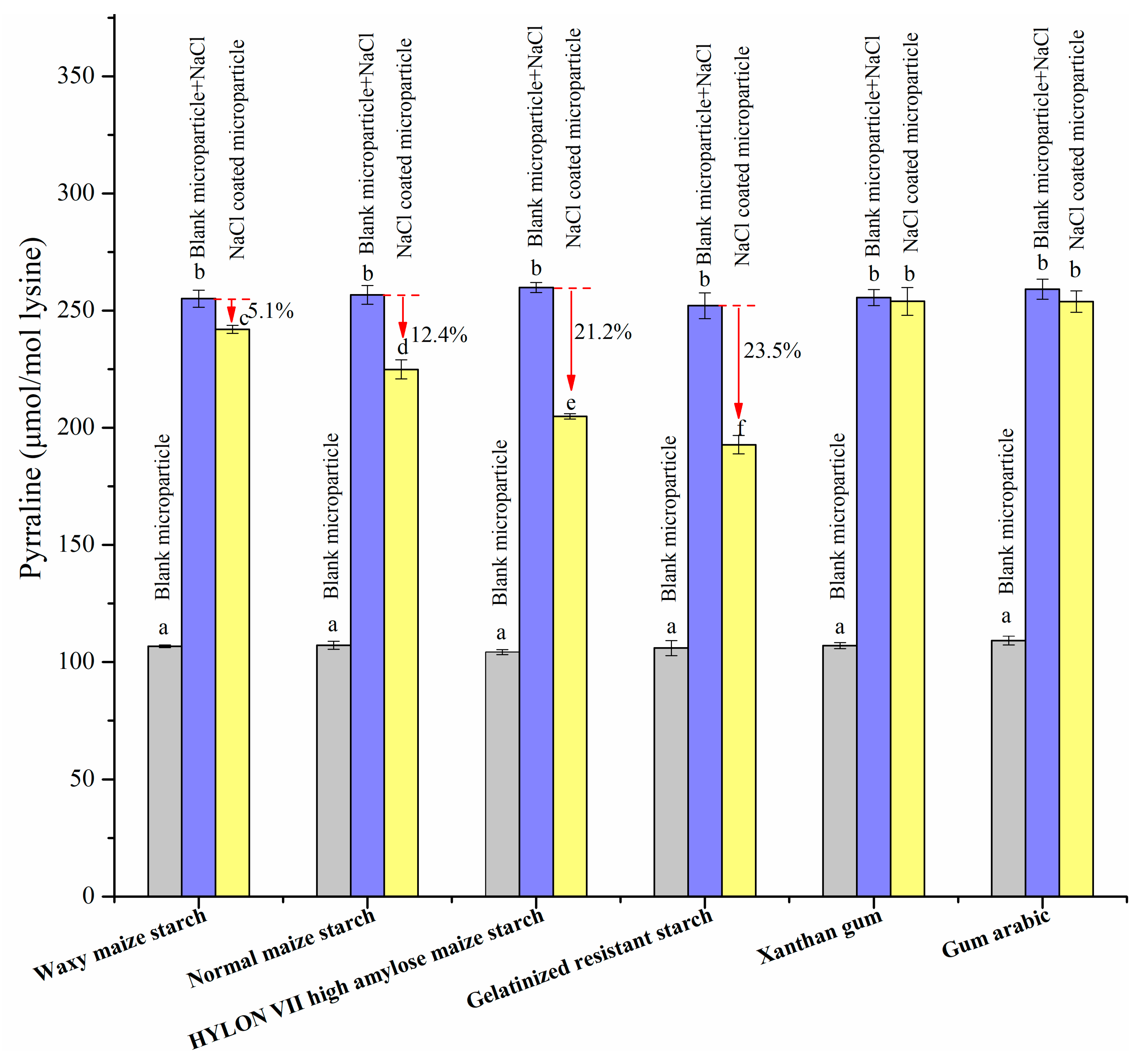
3.2.4. Browning Extent and Pyrraline Formation with the Addition of NaCl Microparticles
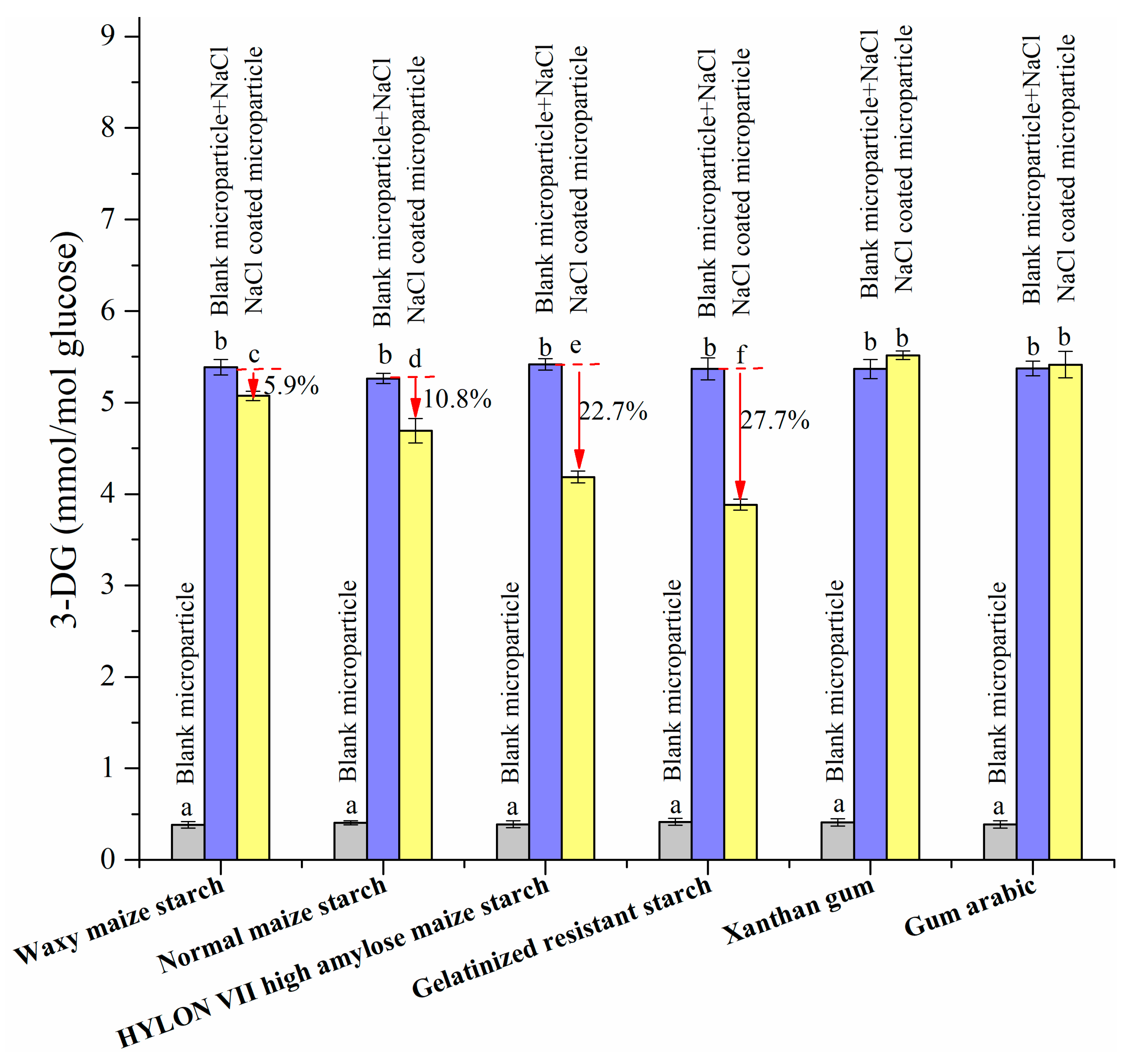
3.2.5. 3-DG Formation with the Addition of NaCl Microparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Nursten, H.E. The Maillard Reaction: Chemistry, Biochemistry, and Implications; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Mechanisms of Disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 285. [Google Scholar] [CrossRef]
- Milne, R.; Brownstein, S. Advanced glycation end products and diabetic retinopathy. Amino Acids 2013, 44, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Franke, S.; Rüster, C.; Wolf, G. Advanced glycation end-products and the kidney. Eur. J. Clin. Investig. 2010, 40, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Mallipattu, S.K.; Uribarri, J. Advanced glycation end product accumulation: A new enemy to target in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2014, 23, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Matsui, T. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. Nutrition 2016, 32, 157–165. [Google Scholar] [CrossRef]
- Luévano-Contreras, C.; Gómez-Ojeda, A.; Macías-Cervantes, M.H.; Garay-Sevilla, M.E. Dietary Advanced Glycation End Products and Cardiometabolic Risk. Curr. Diabetes Rep. 2017, 17, 63. [Google Scholar] [CrossRef]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary Advanced Glycation End Products and Aging. Nutrients 2010, 2, 1247–1265. [Google Scholar] [CrossRef]
- Clarke, R.E.; Dordevic, A.L.; Tan, S.M.; Ryan, L.; Coughlan, M.T. Dietary Advanced Glycation End Products and Risk Factors for Chronic Disease: A Systematic Review of Randomised Controlled Trials. Nutrients 2016, 8, 125. [Google Scholar] [CrossRef]
- Gugliucci, A.; Kotani, K.; Taing, J.; Matsuoka, Y.; Sano, Y.; Yoshimura, M.; Egawa, K.; Horikawa, C.; Kitagawa, Y.; Kiso, Y.; et al. Short-Term Low Calorie Diet Intervention Reduces Serum Advanced Glycation End Products in Healthy Overweight or Obese Adults. Ann. Nutr. Metab. 2009, 54, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Clarke, R.E.; Coughlan, M.T. Stirring the Pot: Can Dietary Modification Alleviate the Burden of CKD? Nutrients 2017, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Witte, S.; Henle, T. Free and Protein-Bound Maillard Reaction Products in Beer: Method Development and a Survey of Different Beer Types. J. Agric. Food Chem. 2016, 64, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Gensberger-Reigl, S.; Henle, T.; Pischetsrieder, M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin. Cancer Biol. 2018, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Snooks, H.; Sang, S. Complexity of Advanced Glycation End Products in Foods: Where Are We Now? J. Agric. Food Chem. 2018, 66, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Wellner, A.; Huettl, C.; Henle, T. Formation of Maillard Reaction Products during Heat Treatment of Carrots. J. Agric. Food Chem. 2011, 59, 7992–7998. [Google Scholar] [CrossRef] [PubMed]
- Resmini, P.; Pellegrino, L. Occurrence of protein-bound lysylpyrrolaldehyde in dried pasta. Cereal Chem. 1994, 71, 254–262. [Google Scholar]
- Li, H.; Yu, S.-J. Review of pentosidine and pyrraline in food and chemical models: Formation, potential risks and determination. J. Sci. Food Agric. 2018, 98, 3225–3233. [Google Scholar] [CrossRef]
- Gökmen, V.; Şenyuva, H.Z. Effects of some cations on the formation of acrylamide and furfurals in glucose–asparagine model system. Eur. Food Res. Technol. 2007, 225, 815–820. [Google Scholar] [CrossRef]
- Levine, R.A.; Ryan, S.M. Determining the Effect of Calcium Cations on Acrylamide Formation in Cooked Wheat Products Using a Model System. J. Agric. Food Chem. 2009, 57, 6823–6829. [Google Scholar] [CrossRef]
- Claus, A.; Mongili, M.; Weisz, G.; Schieber, A.; Carle, R. Impact of formulation and technological factors on the acrylamide content of wheat bread and bread rolls. J. Cereal Sci. 2008, 47, 546–554. [Google Scholar] [CrossRef]
- Fiore, A.; Troise, A.D.; Ataç Mogol, B.; Roullier, V.; Gourdon, A.; El Mafadi Jian, S.; Hamzalıoğlu, B.A.; Gökmen, V.; Fogliano, V. Controlling the Maillard Reaction by Reactant Encapsulation: Sodium Chloride in Cookies. J. Agric. Food Chem. 2012, 60, 10808–10814. [Google Scholar] [CrossRef] [PubMed]
- Dötsch, M.; Busch, J.; Batenburg, M.; Liem, G.; Tareilus, E.; Mueller, R.; Meijer, G. Strategies to Reduce Sodium Consumption: A Food Industry Perspective. Crit. Rev. Food Sci. Nutr. 2009, 49, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Koszucka, A.; Nowak, A. Thermal processing food-related toxicants: A review. Crit. Rev. Food Sci. Nutr. 2018, 1–18. [Google Scholar] [CrossRef]
- Li, L.; Han, L.; Fu, Q.; Li, Y.; Liang, Z.; Su, J.; Li, B. Formation and Inhibition of Nε-(Carboxymethyl)lysine in Saccharide-Lysine Model Systems during Microwave Heating. Molecules 2012, 17, 12758–12770. [Google Scholar] [CrossRef]
- Liang, Z.; Li, L.; Fu, Q.; Zhang, X.; Xu, Z.; Li, B. Formation and elimination of pyrraline in the Maillard reaction in a saccharide–lysine model system. J. Sci. Food Agric. 2016, 96, 2555–2564. [Google Scholar] [CrossRef]
- Liang, Z.; Li, L.; Qi, H.; Zhang, Z.X.X.; Li, B. Kinetic Study on Peptide-Bound Pyrraline Formation and Elimination in the Maillard Reaction Using Single- and Multiple-Response Models. J. Food Sci. 2016, 81, C2405–C2424. [Google Scholar] [CrossRef]
- Lee, A.K.; Schieb, L.J.; Yuan, K.; Maalouf, J.; Gillespie, C.; Cogswell, M.E. Sodium content in packaged foods by census division in the United States, 2009. Prev. Chronic Dis. 2015, 12, E43. [Google Scholar] [CrossRef]
- Eyles, H.; Shields, E.; Webster, J.; Ni Mhurchu, C. Achieving the WHO sodium target: Estimation of reductions required in the sodium content of packaged foods and other sources of dietary sodium. Am. J. Clin. Nutr. 2016, 104, 470–479. [Google Scholar] [CrossRef]
- Wolfson, J.A.; Moran, A.J.; Jarlenski, M.P.; Bleich, S.N. Trends in Sodium Content of Menu Items in Large Chain Restaurants in the U.S. Am. J. Prev. Med. 2018, 54, 28–36. [Google Scholar] [CrossRef]
- Dunford, E.K.; Ni Mhurchu, C.; Huang, L.; Vandevijvere, S.; Swinburn, B.; Pravst, I.; Tolentino-Mayo, L.; Reyes, M.; L’Abbe, M.; Neal, B.C. A comparison of the healthiness of packaged foods and beverages from 12 countries using the Health Star Rating nutrient profiling system, 2013–2018. Obes. Rev. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, J.K.C.; Li, Y.; Haytowitz, D.B.; Bahadur, R.; Pehrsson, P.R.; Cogswell, M.E. Assessing Changes in Sodium Content of Selected Popular Commercially Processed and Restaurant Foods: Results from the USDA: CDC Sentinel Foods Surveillance Program. Nutrients 2019, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Saltmarch, M.; Labuza, T.P. Nonenzymatic Browning via the Maillard Reaction in Foods. Diabetes 1982, 31, 29–36. [Google Scholar] [CrossRef]
- Kwak, E.-J.; Lim, S.-I. The effect of sugar, amino acid, metal ion, and NaCl on model Maillard reaction under pH control. Amino Acids 2004, 27, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Moreau, L.; Bindzus, W.; Hill, S. Influence of Sodium Chloride on Color Development of Cereal Model Systems Through Changes in Glass Transition Temperature and Water Retention. Cereal Chem. 2009, 86, 232–238. [Google Scholar] [CrossRef]
- Moreau, L.; Lagrange, J.; Bindzus, W.; Hill, S. Influence of sodium chloride on colour, residual volatiles and acrylamide formation in model systems and breakfast cereals. Int. J. Food Sci. Technol. 2009, 44, 2407–2416. [Google Scholar] [CrossRef]
- Rizzi, G.P. Effects of Cationic Species on Visual Color Formation in Model Maillard Reactions of Pentose Sugars and Amino Acids. J. Agric. Food Chem. 2008, 56, 7160–7164. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Noumi, Y.; Nakajima, K.; Nagatsuka, C.; Aizawa, H.; Nakawaki, R.; Mizude, E.; Otsuka, Y.; Homma, T.; Chuyen, N.V. Effects of Salt Concentration on the Reaction Rate of Glc with Amino Acids, Peptides, and Proteins. Biosci. Biotechnol. Biochem. 2009, 73, 2379. [Google Scholar] [CrossRef][Green Version]
- Bell, L.N. Maillard reaction as influenced by buffer type and concentration. Food Chem. 1997, 59, 143–147. [Google Scholar] [CrossRef]
- Rizzi, G.P. Role of Phosphate and Carboxylate Ions in Maillard Browning. J. Agric. Food Chem. 2004, 52, 953–957. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Gökmen, V. Effects of Sodium Chloride, Potassium Chloride, and Calcium Chloride on the Formation of α-Dicarbonyl Compounds and Furfurals and the Development of Browning in Cookies during Baking. J. Agric. Food Chem. 2016, 64, 7838–7848. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Smith, R.E. Sources of Variability of Acrylamide Levels in a Cracker Model. J. Agric. Food Chem. 2005, 53, 4410–4416. [Google Scholar] [CrossRef] [PubMed]
- Portero-Otin, M.; Nagaraj, R.H.; Monnier, V.M. Chromatographic evidence for pyrraline formation during protein glycation in vitro and in vivo. Biochim. Et Biophys. Acta (Bba)-Protein Struct. Mol. Enzymol. 1995, 1247, 74–80. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated Foods, Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Gökmen, V. Effect of Sodium Chloride on α-Dicarbonyl Compound and 5-Hydroxymethyl-2-furfural Formations from Glucose under Caramelization Conditions: A Multiresponse Kinetic Modeling Approach. J. Agric. Food Chem. 2016, 64, 6333–6342. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Mayes, H.B.; Nolte, M.W.; Beckham, G.T.; Shanks, B.H.; Broadbelt, L.J. The Alpha–Bet(a) of Salty Glucose Pyrolysis: Computational Investigations Reveal Carbohydrate Pyrolysis Catalytic Action by Sodium Ions. ACS Catal. 2015, 5, 192–202. [Google Scholar] [CrossRef]
- Liang, Z.; Li, L.; Qi, H.; Wan, L.; Cai, P.; Xu, Z.; Li, B. Formation of Peptide Bound Pyrraline in the Maillard Model Systems with Different Lys-Containing Dipeptides and Tripeptides. Molecules 2016, 21, 463. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A. Influence of alkali metals on the kinetics of the thermal decomposition of biomass. Fuel Process. Technol. 2012, 104, 189–197. [Google Scholar] [CrossRef]
- Mayes, H.B.; Tian, J.; Nolte, M.W.; Shanks, B.H.; Beckham, G.T.; Gnanakaran, S.; Broadbelt, L.J. Sodium Ion Interactions with Aqueous Glucose: Insights from Quantum Mechanics, Molecular Dynamics, and Experiment. J. Phys. Chem. B 2014, 118, 1990–2000. [Google Scholar] [CrossRef][Green Version]
- Lutz, R.; Aserin, A.; Wicker, L.; Garti, N. Release of electrolytes from W/O/W double emulsions stabilized by a soluble complex of modified pectin and whey protein isolate. Colloids Surf. B: Biointerfaces 2009, 74, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Sapei, L.; Naqvi, M.A.; Rousseau, D. Stability and release properties of double emulsions for food applications. Food Hydrocoll. 2012, 27, 316–323. [Google Scholar] [CrossRef]
- Chen, X.; He, X.-W.; Zhang, B.; Fu, X.; Jane, J.-L.; Huang, Q. Effects of adding corn oil and soy protein to corn starch on the physicochemical and digestive properties of the starch. Int. J. Biol. Macromol. 2017, 104, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Sworn, G. 8-Xanthan gum. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Boca Raton, FL, USA, 2009; pp. 186–203. [Google Scholar]
- Williams, P.A.; Phillips, G.O. 11-Gum arabic. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Boca Raton, FL, USA, 2009; pp. 252–273. [Google Scholar]
- Venugopal, V. Edible Films and Carrier Matrices from Marine Polysaccharides. In Marine Polysaccharides: Food Applications; Venugopal, V., Ed.; CRC press: Boca Raton, FL, USA; pp. 291–293.
- Zobel, H.F. CHAPTER IX-Gelatinization of starch and mechanical properties of starch pastes. In Starch: Chemistry and Technology, 2nd ed.; Whistler, R.L., Bemiller, J.N., Paschall, E.F., Eds.; Academic Press: San Diego, CA, USA, 1984; pp. 285–309. [Google Scholar]


| Setup | Amount in the PTFE Lined Tube (mg) | ||
|---|---|---|---|
| NaCl | Coated NaCl Microparticles | Coating Materials Microparticles | |
| NaCl 0.000% (Na+ 0.00%) | 0 | ||
| NaCl 0.625% (Na+ 0.25%) | 94 | ||
| NaCl 1.250% (Na+ 0.50%) | 188 | ||
| NaCl 1.875% (Na+ 0.75%) | 281 | ||
| NaCl 2.500% (Na+ 1.00%) | 375 | ||
| encapsulated NaCl (WMS) | 662 | ||
| blank microparticle (WMS) + NaCl | 188 | 474 | |
| blank microparticle (WMS) | 474 | ||
| encapsulated NaCl (NMS) | 699 | ||
| blank microparticle (NMS) + NaCl | 188 | 511 | |
| blank microparticle (NMS) | 511 | ||
| encapsulated NaCl (HAMS) | 729 | ||
| blank microparticle (HAMS) + NaCl | 188 | 541 | |
| blank microparticle (HAMS) | 541 | ||
| encapsulated NaCl (GRS) | 761 | ||
| blank microparticle (GRS) + NaCl | 188 | 573 | |
| blank microparticle (GRS) | 573 | ||
| encapsulated NaCl (XG) | 689 | ||
| blank microparticle (XG) + NaCl | 188 | 501 | |
| blank microparticle (XG) | 501 | ||
| encapsulated NaCl (GA) | 653 | ||
| blank microparticle (GA) + NaCl | 188 | 465 | |
| blank microparticle (GA) | 465 | ||
| Sample | D3,2 (μm) |
|---|---|
| NaCl coated by WMS | 789 |
| NaCl coated by NMS | 866 |
| NaCl coated by HAMS | 773 |
| NaCl coated by GRS | 766 |
| NaCl coated by XG | 780 |
| NaCl coated by GA | 795 |
| Samples | Gelatinization of Coating Materials | ||||
|---|---|---|---|---|---|
| To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | Tc -To (°C) | |
| NaCl coated by WMS | 55.79 | 58.26 | 63.08 | 2.770 | 7.29 |
| NaCl coated by NMS | 93.42 | 102.37 | 113.28 | 2.133 | 19.86 |
| NaCl coated by HAMS | 130.12 | 130.71 | 132.70 | 0.212 | 2.58 |
| NaCl coated by GRS | 155.55 | 158.65 | 161.53 | 0.539 | 5.98 |
| NaCl coated by XG | 150.31 | 157.55 | 163.61 | 3.305 | 13.30 |
| NaCl coated by GA | 57.20 | 57.90 | 60.97 | 1.301 | 3.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Chen, X.; Yang, Z.; Lai, Y.; Yang, Y.; Lei, C.; Zeng, Y. Pyrraline Formation Modulated by Sodium Chloride and Controlled by Encapsulation with Different Coating Materials in the Maillard Reaction. Biomolecules 2019, 9, 721. https://doi.org/10.3390/biom9110721
Liang Z, Chen X, Yang Z, Lai Y, Yang Y, Lei C, Zeng Y. Pyrraline Formation Modulated by Sodium Chloride and Controlled by Encapsulation with Different Coating Materials in the Maillard Reaction. Biomolecules. 2019; 9(11):721. https://doi.org/10.3390/biom9110721
Chicago/Turabian StyleLiang, Zhili, Xu Chen, Zhao Yang, Yuzhu Lai, Yinling Yang, Chuying Lei, and Ya Zeng. 2019. "Pyrraline Formation Modulated by Sodium Chloride and Controlled by Encapsulation with Different Coating Materials in the Maillard Reaction" Biomolecules 9, no. 11: 721. https://doi.org/10.3390/biom9110721
APA StyleLiang, Z., Chen, X., Yang, Z., Lai, Y., Yang, Y., Lei, C., & Zeng, Y. (2019). Pyrraline Formation Modulated by Sodium Chloride and Controlled by Encapsulation with Different Coating Materials in the Maillard Reaction. Biomolecules, 9(11), 721. https://doi.org/10.3390/biom9110721




