The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the Selected Quality Characteristics, Anthocyanin Stability, and Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Raw Materials
2.3. The Technology of Juice Production
2.4. Analytical Methods
2.4.1. Physicochemical Parameters
2.4.2. HPLC Analysis of Anthocyanins
2.4.3. HPLC Analysis of L-Ascorbic Acid
2.4.4. Analysis of Total Phenolic Content
2.4.5. Antioxidant Activity (ABTS+) Assay
2.4.6. Juice Color Parameters
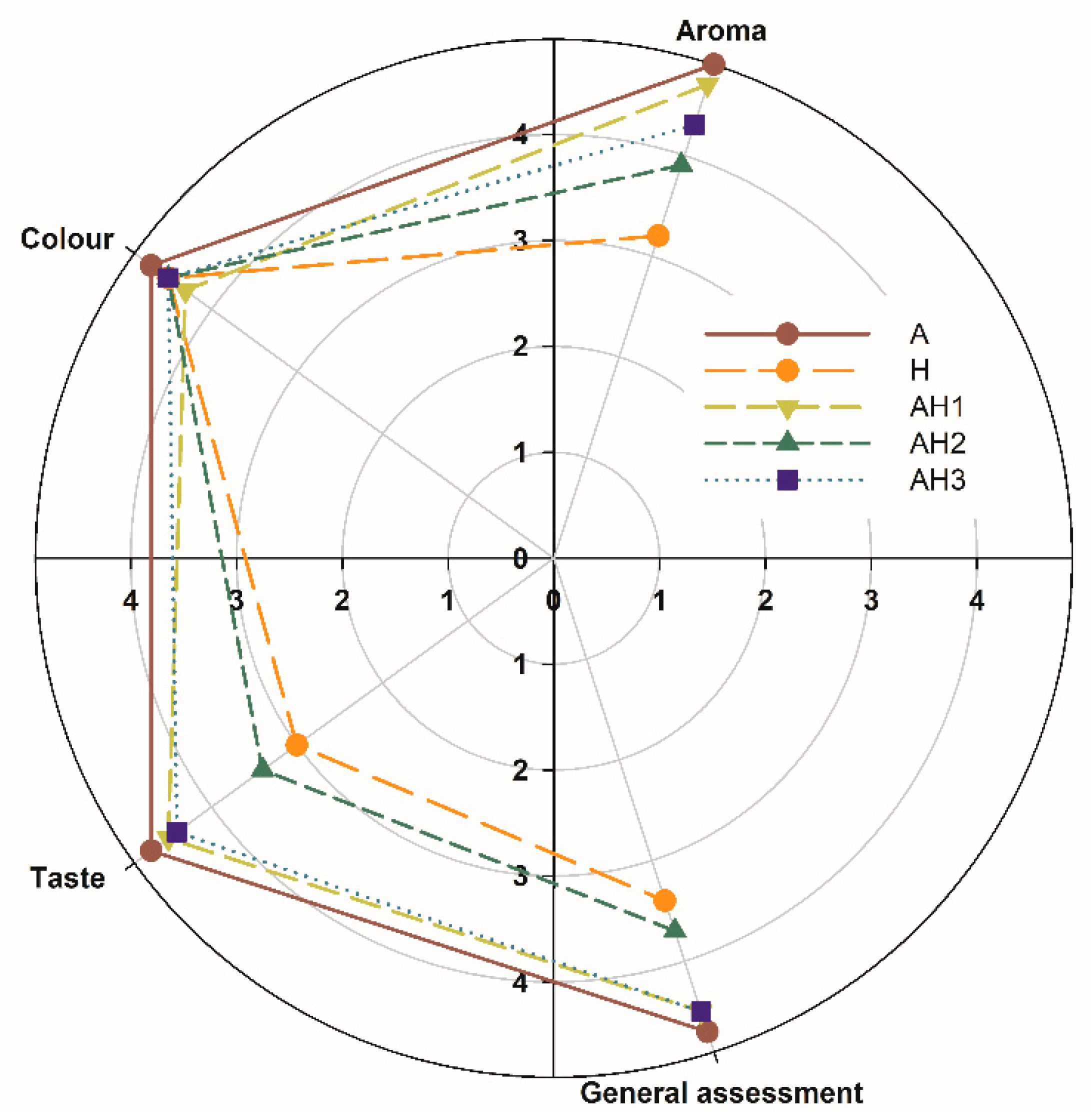
2.5. Sensory Assessment
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters and Sensory Assessment
3.2. L-Ascorbic Acid Content
3.3. Anthocyanin Content
3.4. Total Polyphenols and Antioxidant Activity
3.5. Juice Color Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Snyder, F.; Ni, L. Chinese apples and the emerging world food trade order: Food safety, international trade, and regulatory collaboration between China and the European Union. Chin. J. Comp. Law (CJCL) 2017, 5, 253–307. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Tosato, F.; Folli, G.S.; de Leite, J.A.; Ventura, J.A.; Endringer, D.C.; Filgueiras, P.R.; Romão, W. Controlling the quality of grape juice adulterated by apple juice using ESI (-) FT-ICR mass spectrometry. Microchem. J. 2019, 104033. [Google Scholar] [CrossRef]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Barreira, J.C.; Arraibi, A.A.; Ferreira, I.C. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Cyanogenic glycosides and phenolics in apple seeds and their changes during long term storage. Sci. Hortic. 2019, 255, 30–36. [Google Scholar] [CrossRef]
- Molina, A.K.; Vega, E.N.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.F.; Barreiro, M.F.; Kostić, M.; Soković, M.; et al. Promising antioxidant and antimicrobial food colourants from Lonicera caerulea L. var. Kamtschatica. Antioxidants 2019, 8, 394. [Google Scholar] [CrossRef]
- Auzanneau, N.; Weber, P.; Kosińska-Cagnazzo, A.; Andlauer, W. Bioactive compounds and antioxidant capacity of Lonicera caerulea berries: Comparison of seven cultivars over three harvesting years. J. Food Compos. Anal. 2018, 66, 81–89. [Google Scholar] [CrossRef]
- Becker, R.; Szakiel, A. Phytochemical characteristics and potential therapeutic properties of blue honeysuckle Lonicera caerulea L. (Caprifoliaceae). J. Herb. Med. 2019, 16, 100237. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue honeysuckle (Lonicera cearulea L. subs. edulis) berry; A rich source of some nutrients and their differences among four different cultivars. Sci. Hortic. 2019, 238, 215–221. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kucharska, A.Z. Effect of pre-treatment of blue honeysuckle berries on bioactive iridoid content. Food Chem. 2018, 240, 1087–1091. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Wojdyło, A.; Teleszko, M.; Oszmiański, J. Physicochemical characterisation of quince fruits for industrial use: Yield, turbidity, viscosity and colour properties of juices. Int. J. Food Sci. Technol. 2014, 49, 1818–1824. [Google Scholar] [CrossRef]
- Goiffon, J.-P.; Mouly, P.P.; Gaydou, E.M. Anthocyanic pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A. Effects of blackcurrant and apple mash blending on the phenolics contents, antioxidant capacity, and colour of juices. Czech. J. Food Sci. 2009, 27, 338–351. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Bjork, I.; Trajkowski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cendrowski, A.; Ścibisz, I.; Mitek, M.; Kieliszek, M. Influence of harvest seasons on the chemical composition and antioxidant activity in Rosa rugosa petals. Agrochimica 2018, 62, 157–165. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J. The influence of addition of cranberrybush juice to pear juice on chemical composition and antioxidant properties. J. Food Sci. Technol. 2018, 55, 3399–3407. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Varghese, E.; Kaur, C. Optimization of enzymatic maceration for extraction of carotenoids and total phenolics from sweet pepper using response surface methodology. Indian J. Hortic. 2015, 72, 547–552. [Google Scholar] [CrossRef]
- Islam, M.; Ahmad, I.; Ahmed, S.; Sarker, A. Biochemical Composition and shelf life study of mixed fruit juice from orange & pineapple. J. Environ. Sci. Nat. Resour. 2014, 7, 227–232. [Google Scholar] [CrossRef]
- Jaros, D.; Thamke, I.; Raddatz, H.; Rohm, H. Single-cultivar cloudy juice made from table apples: An attempt to identify the driving force for sensory preference. Eur. Food Res. Technol. 2009, 229, 51–61. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Phenolic compounds in apple (Malus x domestica Borkh.): Compounds characterization and stability during postharvest and after processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [PubMed]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Troszyńska, A.; Narolewska, O.; Robredo, S.; Estrella, I.; Hernández, T.; Lamparski, G.; Amarowicz, R. The effect of polysaccharides on the astringency induced by phenolic compounds. Food Qual. Prefer. 2010, 21, 463–469. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Silva, A.M.; Evtuguin, D.V.; Nunes, F.M.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. The hydrophobic polysaccharides of apple pomace. Carbohydr. Polym. 2019, 223, 115132. [Google Scholar] [CrossRef]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, A.A.; Oszmiański, J.; Golis, T. Variability of phytochemical properties and content of bioactive compounds in Lonicera caerulea L. var. kamtschatica berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Kucharska, A.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, phenolic compounds and antioxidant activity of edible honeysuckle berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Dobler, S.; Petschenka, G.; Pankoke, H. Coping with toxic plant compounds–the insect’s perspective on iridoid glycosides and cardenolides. Phytochemistry 2011, 72, 1593–1604. [Google Scholar] [CrossRef]
- Jurikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (genus Lonicera) and their biological effects. Molecules 2012, 17, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, S.; Yamamoto, N. Functional food products in Japan: A review. Food Sci. Hum. Wellness 2019, 8, 96–101. [Google Scholar] [CrossRef]
- Mark, R.; Lyu, X.; Lee, J.J.L.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Sanaullah Khan, R.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Zhao, C.N.; Li, Y.; Meng, X.; Li, S.; Liu, Q.; Tang, G.Y.; Gan, R.Y.; Li, H. Bin Insight into the roles of vitamins C and D against cancer: Myth or truth? Cancer Lett. 2018, 431, 161–170. [Google Scholar] [CrossRef]
- Zümreoglu-Karan, B. The coordination chemistry of Vitamin C: An overview. Coord. Chem. Rev. 2006, 250, 2295–2307. [Google Scholar] [CrossRef]
- Herbig, A.L.; Maingonnat, J.F.; Renard, C.M.G.C. Oxygen availability in model solutions and purées during heat treatment and the impact on vitamin C degradation. LWT Food Sci. Technol. 2017, 85, 493–499. [Google Scholar] [CrossRef]
- Sapei, L.; Hwa, L. Study on the kinetics of vitamin C degradation in fresh strawberry juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef]
- Mercali, G.D.; Jaeschke, D.P.; Tessaro, I.C.; Marczak, L.D.F. Study of vitamin C degradation in acerola pulp during ohmic and conventional heat treatment. LWT Food Sci. Technol. 2012, 47, 91–95. [Google Scholar] [CrossRef]
- Gu, K.D.; Wang, C.K.; Hu, D.G.; Hao, Y.J. How do anthocyanins paint our horticultural products? Sci. Hortic. 2019, 249, 257–262. [Google Scholar] [CrossRef]
- Sinopoli, A.; Calogero, G.; Bartolotta, A. Computational aspects of anthocyanidins and anthocyanins: A review. Food Chem. 2019, 297, 124898. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante Braga, A.R.; Murador, D.C.; Mendes De Souza Mesquita, L.; Vera De Rosso, V. Critical review Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Aspects Med. 2018, 61, 76–82. [Google Scholar] [CrossRef]
- Medina dos Santos, N.; Berilli Batista, P.; Batista, Â.G.; Maróstica Júnior, M.R. Current evidence on cognitive improvement and neuroprotection promoted by anthocyanins. Curr. Opin. Food Sci. 2019, 26, 71–78. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Hładyszowski, J.; Mitek, M. Stabilization of anthocyanin and skullcap flavone complexes–Investigations with computer simulation and experimental methods. Food Chem. 2013, 138, 491–500. [Google Scholar] [CrossRef]
- Muche, B.M.; Speers, R.A.; Rupasinghe, H.P.V. Storage temperature impacts on anthocyanins degradation, color changes and haze development in juice of “Merlot” and “Ruby” grapes (Vitis vinifera). Front. Nutr. 2018, 5, 100. [Google Scholar] [CrossRef]
- Roidoung, S.; Dolan, K.D.; Siddiq, M. Estimation of kinetic parameters of anthocyanins and color degradation in vitamin C fortified cranberry juice during storage. Food Res. Int. 2017, 94, 29–35. [Google Scholar] [CrossRef]
- Buvé, C.; Kebede, B.T.; De Batselier, C.; Carrillo, C.; Pham, H.T.T.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Kinetics of colour changes in pasteurised strawberry juice during storage. J. Food Eng. 2018, 216, 42–51. [Google Scholar] [CrossRef]

| Symbol of the Juice Type | The Percentage Share of the Individual Juice [%] |
|---|---|
| A | 100% A |
| H | 100% H |
| AH1 | 90% A-10% H |
| AH2 | 80% A-20% H |
| AH3 | 70% A-30% H |
| Juice | Parameters | Time of Storage | ||||
|---|---|---|---|---|---|---|
| After Production | 1 Month | 2 Months | 3 Months | 4 Months | ||
| A | TSS A | 13.54 ± 0.08 a | 13.44 ± 0.00 a | 13.50 ± 0.00 a | 13.44 ±0.08 a | 13.54 ± 0.08 a |
| TTA B | 0.56 ± 0.00 a | 0.54 ± 0.00 b | 0.50 ± 0.00 c | 0.49 ± 0.00 c | 0.50 ± 0.00 c | |
| TSS/TTA | 24.17 | 24.88 | 27.00 | 27.42 | 27.08 | |
| pH | 3.13 ± 0.01 c | 3.19 ± 0.01 b | 3.24 ± 0.02 b | 3.19 ± 0.02 b | 3.51 ± 0.02 a | |
| L-ascorbic acid C | 0.52 ± 0.00 a | 0.44 ± 0.00 b | 0.31 ± 0.00 c | 0.24 ± 0.00 d | 0.17 ± 0.01 e | |
| H | TSS A | 14.15 ± 0.08 a,b | 14.05 ± 0.08 b | 14.25 ± 0.08 a | 14.15 ±0.08 a,b | 14.12 ± 0.02 a,b |
| TTA B | 3.64 ± 0.02 a | 3.63 ± 0.02 a | 3.59 ± 0.02 a,b | 3.56 ± 0.02 b | 3.56 ± 0.02 b | |
| TSS/TTA | 3.88 | 3.87 | 3.96 | 3.97 | 3.96 | |
| pH | 2.63 ± 0.00 c | 2.67 ± 0.00 b | 2.68 ± 0.00 b | 2.68 ± 0.01 b | 2.72 ± 0.00 a | |
| L-ascorbic acid C | 32.59 ± 0.19 a | 11.98 ± 0.07 b | 11.62 ± 0.07 c | 10.44 ± 0.06 d | 9.36 ± 0.05 e | |
| AH1 | TSS A | 13.54 ± 0.08 a | 13.53 ± 0.08 a | 13.54 ± 0.08 a | 13.54 ± 0.08 a | 13.64 ± 0.08 a |
| TTA | 0.78 ± 0.01 a | 0.76 ± 0.00 b | 0.74 ± 0.01 c | 0.76 ± 0.00 b | 0.69 ± 0.00 d | |
| TSS/TTA | 17.35 | 17.80 | 18.29 | 17.81 | 19.76 | |
| pH | 3.02 ± 0.01 c | 3.08 ± 0.00 b | 3.08 ± 0.00 b | 3.08 ± 0.02 b | 3.40 ± 0.01 a | |
| L-ascorbic acid C | 1.14 ± 0.01 a | 0.86 ± 0.00 b | 0.52 ± 0.00 c | 0.40 ± 0.00 d | 0.20 ± 0.04 e | |
| AH2 | TSS A | 13.64 ± 0.08 a | 13.58 ± 0.00 a | 13.54 ± 0.08 a | 13.64 ± 0.08 a | 13.64 ± 0.08 a |
| TTA B | 1.08 ± 0.01 a | 1.06 ± 0.01 b | 1.03 ± 0.01 c | 1.08 ± 0.01 a | 0.97 ± 0.01 d | |
| TSS/TTA | 12.62 | 12.81 | 13.14 | 12.62 | 14.06 | |
| pH | 2.91 ± 0.01 d | 2.97 ± 0.02 c | 2.98 ± 0.00 c | 3.19 ± 0.02 b | 3.31 ± 0.01 a | |
| L-ascorbic acid C | 4.23 ± 0.02 a | 2.58 ± 0.01 b | 1.45 ± 0.01 c | 1.14 ± 0.01 d | 0.96 ± 0.05 e | |
| AH3 | TSS A | 13.81 ± 0.08 a | 13.95 ± 0.08 a | 13.95 ± 0.07 a | 13.75 ± 0.08 a | 13.95 ± 0.08 a |
| TTA B | 1.34 ± 0.01 a | 1.33 ± 0.01 a | 1.21 ± 0.01 d | 1.27 ± 0.01 b | 1.24 ± 0.01 c | |
| TSS/TTA | 10.30 | 10.48 | 11.52 | 10.82 | 11.25 | |
| pH | 2.86 ± 0.01 c | 2.90 ± 0.00 b c | 2.94 ± 0.01 b | 2.90 ± 0.01 b c | 3.25 ± 0.01 a | |
| L-ascorbic acid C | 8.07 ± 0.05 a | 6.10 ± 0.04 b | 3.47 ± 0.02 c | 2.89 ± 0.57 c | 2.14 ± 0.01 d | |
| Juice | Parameters | Time of Storage | ||||
|---|---|---|---|---|---|---|
| After Production | 1 Month | 2 Months | 3 Months | 4 Months | ||
| A | Total anthocyanins | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| H | Cyanidin 3,5-O-diglucoside | 60.27 ± 0.47 a | 47.93 ± 0.51 b | 45.75 ± 0.25 c | 35.02 ± 1.44 d | 30.88 ± 0.15 e |
| Cyanidin 3-O-glucoside | 460.26 ± 2.60 a | 359.38 ± 2.98 b | 340.18 ± 3.40 c | 337.68 ± 12.63 c | 305.54 ± 1.62 d | |
| Cyanidin 3-O-rutinoside | 35.19 ± 0.31 a | 29.34 ± 0.35 b | 26.36 ± 0.36 c | 25.43 ± 1.09 c | 23.06 ± 0.15 d | |
| Pelargonidin 3-O-glucoside | 9.32 ± 0.13 a | 9.14 ± 0.06 a,b | 8.80 ± 0.06 b c | 8.40 ± 0.38 c | 5.30 ± 0.03 d | |
| Peonidin 3-O-glucoside | 23.71 ± 0.22 a | 20.29 ± 0.22 b | 18.70 ± 0.49 c | 17.28 ± 0.72 d | 15.38 ± 0.10 e | |
| Peonidin 3-O-rutinoside | 6.63 ± 0.06 a | 5.31 ± 0.33 b | 4.29 ± 0.11 c | 3.51 ± 0.04 d | 3.38 ± 0.05 d | |
| Total anthocyanins | 595.39 ± 3.77 a | 471.39 ± 4.44 b | 444.09 ± 3.74 c | 427.32 ± 5.28 d | 383.56 ± 2.09 e | |
| AH1 | Cyanidin 3,5-O-diglucoside | 6.48 ± 0.10 a | 5.07 ± 0.03 b | 3.38 ± 0.05 c | 2.95 ± 0.08 d | 2.62 ± 0.02 e |
| Cyanidin 3-O-glucoside | 34.33 ± 0.84 a | 17.12 ± 0.61 b | 15.90 ± 0.16 b | 14.15 ± 0.89 c | 12.49 ± 0.17 c | |
| Cyanidin 3-O-rutinoside | 4.56 ± 0.36 a | 2.16 ± 0.01 b | 1.15 ± 0.02 c | 0.95 ± 0.02 c | 0.81 ± 0.01 c | |
| Pelargonidin 3-O-glucoside | 1.13 ± 0.02 a | 1.15 ± 0.01 a | 1.09 ± 0.01 b | 0.96 ± 0.01 c | 0.58 ± 0.01 d | |
| Peonidin 3-O-glucoside | 3.17 ± 0.07 a | 1.34 ± 0.04 b | 1.29 ± 0.01 b | 0.62 ± 0.01 c | 0.34 ± 0.01 d | |
| Peonidin 3-O-rutinoside | 0.18 ± 0.01 a | 0.16 ± 0.00 b | 0.15 ± 0.00 b c | 0.15 ± 0.01 c | 0.10 ± 0.00 d | |
| Total anthocyanins | 49.86 ± 1.36 a | 27.00 ± 0.72 b | 22.97 ± 1.92 c | 19.77 ± 1.03 d | 16.94 ± 0.33 e | |
| AH2 | Cyanidin 3,5-O-diglucoside | 9.37 ± 0.08 b | 10.10 ± 0.10 a | 5.95 ± 0.04 c | 5.90 ± 0.05 c | 5.59 ± 0.10 d |
| Cyanidin 3-O-glucoside | 68.76 ± 0.41 a | 31.83 ± 0.16 b | 30.63 ± 0.31 c | 27.62 ± 0.18 d | 25.55 ± 0.13 e | |
| Cyanidin 3-O-rutinoside | 5.04 ± 0.03 a | 4.82 ± 0.03 b | 2.86 ± 0.02 c | 2.65 ± 0.02 d | 2.62 ± 0.01 d | |
| Pelargonidin 3-O-glucoside | 1.20 ± 0.01 a | 1.30 ± 0.07 a | 0.75 ± 0.01 b | 0.79 ± 0.05 b | 0.80 ± 0.05 b | |
| Peonidin 3-O-glucoside | 3.28 ± 0.02 a | 2.85 ± 0.03 b | 1.55 ± 0.01 c | 1.41 ± 0.07 d | 1.29 ± 0.07 e | |
| Peonidin 3-O-rutinoside | 0.58 ± 0.00 a | 0.54 ± 0.00 b | 0.39 ± 0.00 c | 0.36 ± 0.00 d | 0.27 ± 0.00 e | |
| Total anthocyanins | 88.22 ± 0.52 a | 51.45 ± 0.38 b | 42.12 ± 0.22 c | 38.73 ± 0.34 d | 36.11 ± 0.35 e | |
| AH3 | Cyanidin 3,5-O-diglucoside | 20.17 ± 0.19 a | 18.59 ± 0.11 b | 12.31 ± 0.06 c | 11.55 ± 0.07 d | 6.74 ± 0.08 e |
| Cyanidin 3-O-glucoside | 143.96 ± 0.96 a | 91.77 ± 0.58 b | 87.87 ± 0.87 c | 84.79 ± 0.51 c | 68.89 ± 2.09 d | |
| Cyanidin 3-O-rutinoside | 11.12 ± 0.06 a | 10.76 ± 0.07 b | 7.65 ± 0.05 c | 7.38 ± 0.15 d | 4.43 ± 0.08 e | |
| Pelargonidin 3-O-glucoside | 2.71 ± 0.03 a | 2.76 ± 0.11 a | 2.03 ± 0.06 b | 1.78 ± 0.10 c | 1.15 ± 0.01 d | |
| Peonidin 3-O-glucoside | 7.51 ± 0.04 a | 6.73 ± 0.09 a | 4.51 ± 0.09 b | 3.94 ± 0.16 b | 2.78 ± 0.82 c | |
| Peonidin 3-O-rutinoside | 0.90 ± 0.03 a | 0.88 ± 0.01 a | 0.50 ± 0.06 b | 0.43 ± 0.01 b c | 0.39 ± 0.00 c | |
| Total anthocyanins | 186.37 ± 1.3 a | 131.49 ± 0.95 b | 114.87 ± 1.11 c | 109.87 ± 0.99 d | 84.38 ± 3.08 e | |
| Juice | Parameters | Time of Storage | ||||
|---|---|---|---|---|---|---|
| After Production | 1 Month | 2 Months | 3 Months | 4 Months | ||
| A | TP | 47.39 ± 0.47 a | 42.80 ± 0.43 b | 41.28 ± 0.41 c | 32.21 ± 0.32 d | 28.65 ± 0.29 e |
| ABTS | 3.94 ± 0.00 a | 3.54 ± 0.02 b | 3.17 ± 0.03 c | 2.61 ± 0.18 d | 2.26 ± 0.04 e | |
| H | TP | 767.88 ± 7.68 a | 762.80 ± 7.63 a | 750.10 ± 7.50 a | 706.92 ± 7.07 b | 635.80 ± 6.36 c |
| ABTS | 96.44 ± 0.48 a | 95.24 ± 0.47 a,b | 92.68 ± 1.11 b | 88.15 ± 1.87 c | 61.90 ± 0.57 d | |
| AH1 | TP | 128.07 ± 0.76 a | 115.98 ± 1.16 b | 108.98 ± 1.09 c | 94.07 ± 0.94 d | 85.61 ± 0.86 e |
| ABTS | 20.79 ± 0.56 a | 17.31 ± 0.23 b | 10.87 ± 0.88 c | 10.22 ± 0.12 c,d | 8.95 ± 0.19 d | |
| AH2 | TP | 165.50 ± 4.47 a | 143.77 ± 1.44 b | 135.41 ± 1.35 c | 129.45 ± 1.29 c,d | 126.00 ± 1.26 d |
| ABTS | 24.8 ± 0.76 a | 19.25 ± 1.24 b | 15.41 ± 0.50 c | 13.75 ± 0.62 c | 10.68 ± 0.08 d | |
| AH3 | TP | 287.54 ± 2.37 a | 258.71 ± 1.98 b | 193.70 ± 1.93 c | 186.07 ± 1.86 d | 174.60 ± 1.76 e |
| ABTS | 30.09 ± 0.15 a | 27.52 ± 0.31 b | 26.46 ± 0.13 c | 24.49 ± 0.30 d | 22.09 ± 0.33 e | |
| Juice | Parameters | Time of Storage | ||||
|---|---|---|---|---|---|---|
| After Production | 1 Month | 2 Months | 3 Months | 4 Months | ||
| A | L* | 98.28 ± 0.98 a | 98.28 ± 0.98 a | 98.35 ± 0.98 a | 98.61 ± 0.99 a | 98.87 ± 0.99 a |
| a* | 0.21 ± 0.00 a | 0.17 ± 0.00 b | 0.21 ± 0.00 a | 0.17 ± 0.00 b | 0.15 ± 0.00 c | |
| b* | 1.63 ± 0.02 d | 2.06 ± 0.02 c | 2.86 ± 0.03 b | 3.52 ± 0.04 a | 3.45 ± 0.03 a | |
| ΔE | - | 0.43 | 1.23 | 1.92 | 1.91 | |
| H | L* | 4.47 ± 0.04 d | 4.71 ± 0.05 c | 5.44 ± 0.05 b | 6.30 ± 0.06 a | 6.40 ± 0.06 a |
| a* | 35.34 ± 0.35 a | 35.11 ± 0.35 a | 32.97 ± 0.33 b | 30.29 ± 0.30 c | 29.22 ± 0.29 d | |
| b* | 7.66 ± 0.08 d | 8.03 ± 0.08 c | 9.35 ± 0.09 b | 10.81 ± 0.11 a | 10.91 ± 0.11 a | |
| ΔE | - | 0.50 | 3.07 | 6.23 | 7.19 | |
| AH1 | L* | 49.59 ± 0.50 c | 50.76 ± 0.51 b c | 51.81 ± 0.52 b | 51.96 ± 0.52 b | 53.48 ± 0.53 a |
| a* | 57.98 ± 0.70 a | 58.28 ± 0.58 a | 56.95 ± 0.57 a | 57.29 ± 0.57 a | 55.16 ± 0.55 b | |
| b* | 10.04 ± 0.10 e | 11.78 ± 0.12 d | 14.48 ± 0.14 c | 20.22 ± 0.20 b | 33.77 ± 0.34 a | |
| ΔE | - | 2.12 | 5.07 | 10.48 | 24.21 | |
| AH2 | L* | 33.35 ± 0.33 d | 34.83 ± 0.35 c | 34.84 ± 0.35 c | 36.39 ± 0.36 b | 38.55 ± 0.39 a |
| a* | 60.05 ± 3.44 a | 60.38 ± 0.60 a | 55.27 ± 0.55 b | 52.97 ± 0.53 b c | 49.59 ± 0.50 c | |
| b* | 29.64 ± 0.30 e | 32.33 ± 0.32 d | 37.92 ± 0.38 c | 46.04 ± 0.46 b | 59.34 ± 0.59 a | |
| ΔE | - | 3.09 | 9.68 | 18.12 | 31.92 | |
| AH3 | L* | 24.10 ± 0.24 c | 24.29 ± 0.38 c | 25.39 ± 0.25 b | 26.39 ± 0.26 a | 27.26 ± 0.27 a |
| a* | 65.79 ± 0.66 a | 65.28 ± 1.77 a | 61.57 ± 0.62 b | 62.30 ± 0.70 b | 60.95 ± 0.71 b | |
| b* | 39.22 ± 0.39 d | 39.31 ± 0.42 d | 42.69 ± 0.42 c | 44.91 ± 0.44 b | 46.88 ± 0.46 a | |
| ΔE | - | 0.55 | 5.61 | 7.06 | 9.60 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grobelna, A.; Kalisz, S.; Kieliszek, M. The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the Selected Quality Characteristics, Anthocyanin Stability, and Antioxidant Properties. Biomolecules 2019, 9, 744. https://doi.org/10.3390/biom9110744
Grobelna A, Kalisz S, Kieliszek M. The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the Selected Quality Characteristics, Anthocyanin Stability, and Antioxidant Properties. Biomolecules. 2019; 9(11):744. https://doi.org/10.3390/biom9110744
Chicago/Turabian StyleGrobelna, Anna, Stanisław Kalisz, and Marek Kieliszek. 2019. "The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the Selected Quality Characteristics, Anthocyanin Stability, and Antioxidant Properties" Biomolecules 9, no. 11: 744. https://doi.org/10.3390/biom9110744
APA StyleGrobelna, A., Kalisz, S., & Kieliszek, M. (2019). The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the Selected Quality Characteristics, Anthocyanin Stability, and Antioxidant Properties. Biomolecules, 9(11), 744. https://doi.org/10.3390/biom9110744






