Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases
Abstract
:1. Introduction
1.1. Epidemiology
1.2. Obesity as a Risk Factor for the Development of IBD
1.3. Effect of Obesity on the Course of IBD
1.4. Skeletal Mass Depletion in IBD
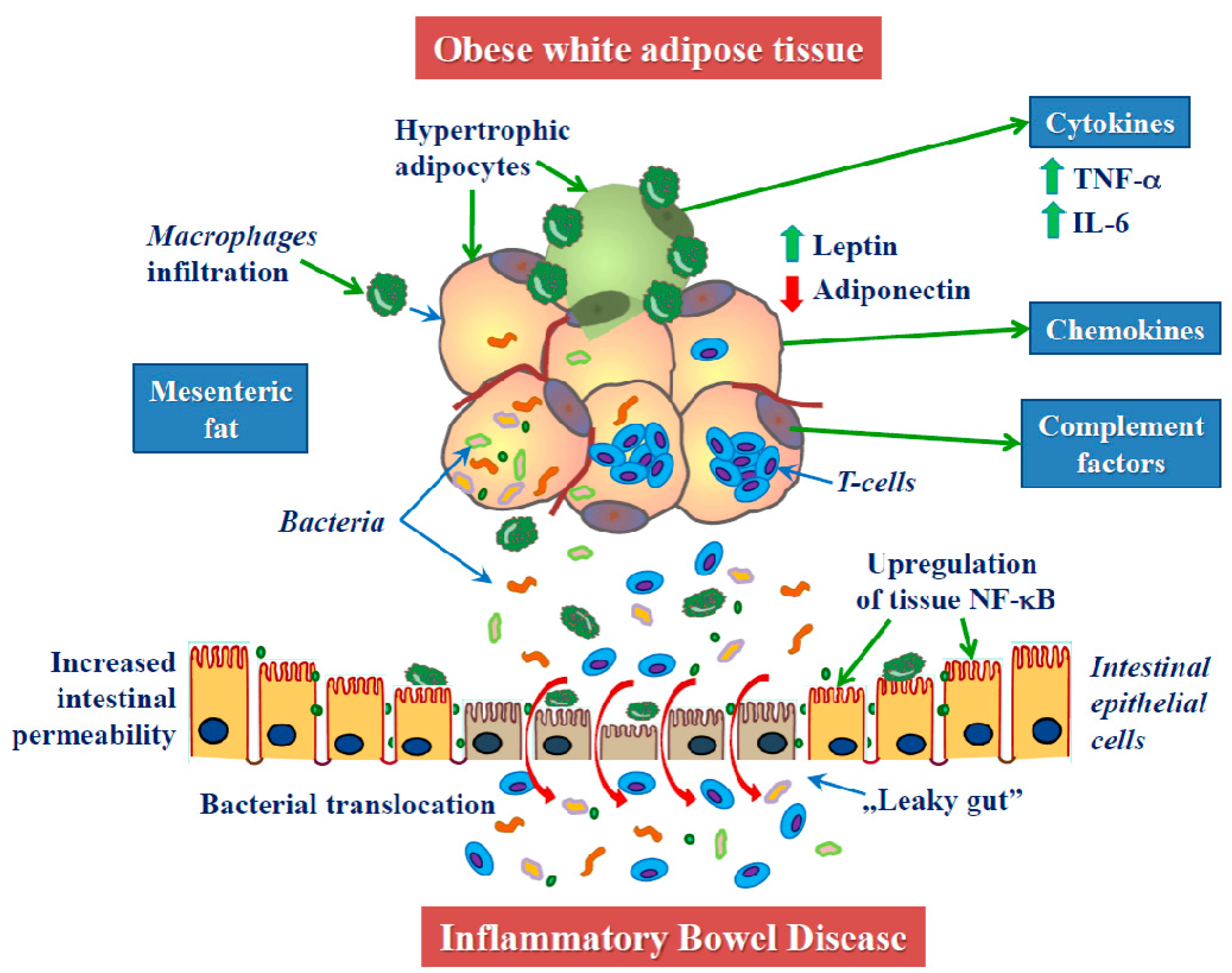
2. Obesity in the Pathogenesis of IBD
2.1. Obesity and Inflammation
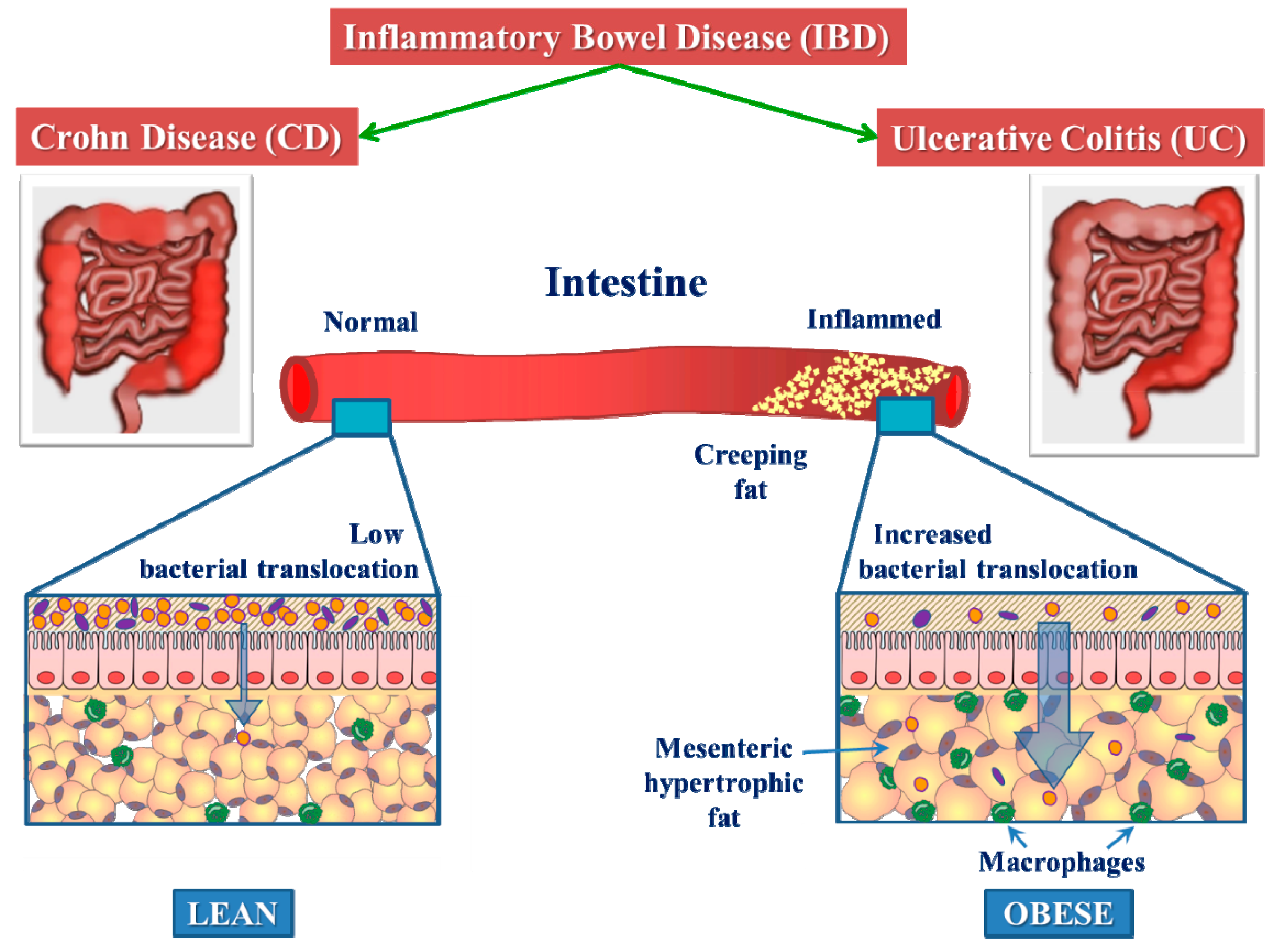
2.2. Mesenteric White Adipose Tissue in CD
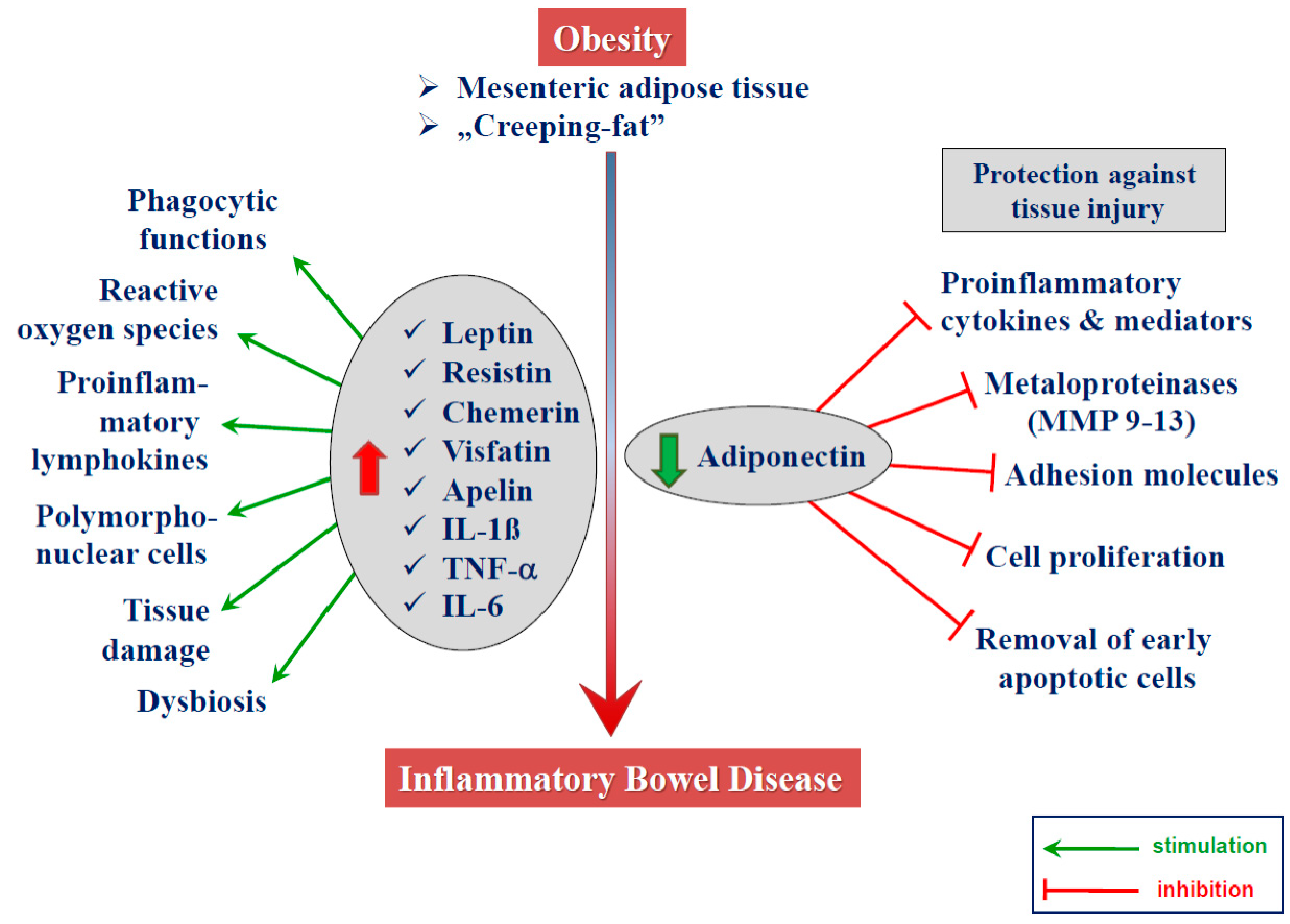
2.3. Adipokines
2.3.1. Leptin
2.3.2. Adiponectin
2.3.3. Other Adipokines
2.4. Dietary Links with IBD
3. Experimental Studies on Role of Adipose Tissue in IBD
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shanahan, F. Crohn’s disease. Lancet 2002, 359, 62–69. [Google Scholar] [CrossRef]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Crohn, B.B.; Ginzburg, L.; Oppenheimer, G.D. Regional ileitis: A pathologic and clinical entity. J. Am. Med Assoc. 1932, 99, 1323–1329. [Google Scholar] [CrossRef]
- Stidham, R.W.; Higgins, P.D.R. Colorectal Cancer in Inflammatory Bowel Disease. Clin. Colon Rectal Surg. 2018, 31, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.W.; Vizuete, J.A.; Martinez, N.; Alvarez, J.J.; Garapati, K.V.; Malakouti, M.; Taboada, C.M. From historical perspectives to modern therapy: A review of current and future biological treatments for Crohn’s disease. Therap. Adv. Gastroenterol. 2015, 8, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, A.; Huang, W.; Liu, X.; Feng, S.; Wu, J.; Yao, Y.; Wang, C.; Li, Q.; Hao, Q.; et al. Increasing rate of inflammatory bowel disease: A 12-year retrospective study in NingXia, China. BMC Gastroenterol. 2016, 16, 2. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef]
- Ho, S.M.; Lewis, J.D.; Mayer, E.A.; Plevy, S.E.; Chuang, E.; Rappaport, S.M.; Croitoru, K.; Korzenik, J.R.; Krischer, J.; Hyams, J.S.; et al. Challenges in IBD Research: Environmental Triggers. Inflamm. Bowel Dis. 2019, 25, S13–S23. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Ng, S.C.; Zeng, Z.; Niewiadomski, O.; Tang, W.; Bell, S.; Kamm, M.A.; Hu, P.; de Silva, H.J.; Niriella, M.A.; Udara, W.S.; et al. Early Course of Inflammatory Bowel Disease in a Population-Based Inception Cohort Study from 8 Countries in Asia and Australia. Gastroenterology 2016, 150, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Environmental risk factors for inflammatory bowel diseases: A review. Dig. Dis. Sci. 2015, 60, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Kujawska-Luczak, M.; Szulinska, M.; Skrypnik, D.; Musialik, K.; Swora-Cwynar, E.; Kregielska-Narozna, M.; Markuszewski, L.; Grzymislawska, M.; Bogdanski, P. The influence of orlistat, metformin and diet on serum levels of insulin-like growth factor-1 in obeses women with and without insulin resistance. J. Physiol. Pharmacol. 2018, 69, 737–745. [Google Scholar] [CrossRef]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. 2018, 69, 663–683. [Google Scholar] [CrossRef]
- Snekvik, I.; Smith, C.H.; Nilsen, T.I.L.; Langan, S.M.; Modalsli, E.H.; Romundstad, P.R.; Saunes, M. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: Prospective Data from the HUNT Study. J. Investig. Dermatol. 2017, 137, 2484–2490. [Google Scholar] [CrossRef]
- Qin, B.; Yang, M.; Fu, H.; Ma, N.; Wei, T.; Tang, Q.; Hu, Z.; Liang, Y.; Yang, Z.; Zhong, R. Body mass index and the risk of rheumatoid arthritis: A systematic review and dose-response meta-analysis. Arthritis Res. Ther. 2015, 17, 86. [Google Scholar] [CrossRef]
- Sterry, W.; Strober, B.E.; Menter, A.; on behalf of the International Psoriasis Council. Obesity in psoriasis: The metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br. J. Dermatol. 2007, 157, 649–655. [Google Scholar] [CrossRef]
- Lu, B.; Hiraki, L.T.; Sparks, J.A.; Malspeis, S.; Chen, C.-Y.; Awosogba, J.A.; Arkema, E.V.; Costenbader, K.H.; Karlson, E.W. Being overweight or obese and risk of developing rheumatoid arthritis among women: A prospective cohort study. Ann. Rheum. Dis. 2014, 73, 1914–1922. [Google Scholar] [CrossRef]
- Maas, F.; Arends, S.; van der Veer, E.; Wink, F.; Efde, M.; Bootsma, H.; Brouwer, E.; Spoorenberg, A. Obesity is common in axial spondyloarthritis and is associated with poor clinical outcome. J. Rheumatol. 2016, 43, 383–387. [Google Scholar] [CrossRef]
- Calkins, B.M.; Mendeloff, A.I. Epidemiology of inflammatory bowel disease. Epidemiol. Rev. 1986, 8, 60–91. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Rawsthorne, P.; Cheang, M.; Blanchard, J.F. A population-based case control study of potential risk factors for IBD. Am. J. Gastroenterol. 2006, 101, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.A.; Goldberg, A.; Levi, Z.; Levy, Y.; Niv, Y.; Bar-Dayan, Y. The prevalence of gastrointestinal diseases in Israeli adolescents and its association with body mass index, gender, and Jewish ethnicity. J. Clin. Gastroenterol. 2008, 42, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.M.; Nguyen, P.; Smith, C.; MacMillan, J.H.; Sherman, P.M. Growth and clinical course of children with Crohn’s disease. Gut 1993, 34, 939–943. [Google Scholar] [CrossRef]
- Azcue, M.; Rashid, M.; Griffiths, A.; Pencharz, P.B. Energy expenditure and body composition in children with Crohn’s disease: Effect of enteral nutrition and treatment with prednisolone. Gut 1997, 41, 203–208. [Google Scholar] [CrossRef]
- Sentongo, T.A.; Semeao, E.J.; Piccoli, D.A.; Stallings, V.A.; Zemel, B.S. Growth, body composition, and nutritional status in children and adolescents with Crohn’s disease. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 33–40. [Google Scholar] [CrossRef]
- Blain, A.; Cattan, S.; Beaugerie, L.; Carbonnel, F.; Gendre, J.P.; Cosnes, J. Crohn’s disease clinical course and severity in obese patients. Clin. Nutr. 2002, 21, 51–57. [Google Scholar] [CrossRef]
- Nic Suibhne, T.; Raftery, T.C.; McMahon, O.; Walsh, C.; O’Morain, C.; O’Sullivan, M. High prevalence of overweight and obesity in adults with Crohn’s disease: Associations with disease and lifestyle factors. J. Crohns Colitis 2013, 7, e241–e248. [Google Scholar] [CrossRef]
- Long, M.D.; Crandall, W.V.; Leibowitz, I.H.; Duffy, L.; del Rosario, F.; Kim, S.C.; Integlia, M.J.; Berman, J.; Grunow, J.; Colletti, R.B.; et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 2162–2168. [Google Scholar] [CrossRef]
- Moran, G.W.; Dubeau, M.F.; Kaplan, G.G.; Panaccione, R.; Ghosh, S. The increasing weight of Crohn’s disease subjects in clinical trials: A hypothesis-generatings time-trend analysis. Inflamm. Bowel Dis. 2013, 19, 2949–2956. [Google Scholar] [CrossRef]
- Steed, H.; Walsh, S.; Reynolds, N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes. Facts 2009, 2, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Kugathasan, S.; Nebel, J.; Skelton, J.A.; Markowitz, J.; Keljo, D.; Rosh, J.; LeLeiko, N.; Mack, D.; Griffiths, A.; Bousvaros, A.; et al. Body mass index in children with newly diagnosed inflammatory bowel disease: Observations from two multicenter North American inception cohorts. J. Pediatr. 2007, 151, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.M.; Harmsen, W.S.; Aniwan, S.; Tremaine, W.J.; Loftus, E.V. Su1855-Prevalence of Obesity and Influence on Phenotype within a Population-Based Cohort of Inflammatory Bowel Disease Patients. Gastroenterology 2018, 154, S-608. [Google Scholar] [CrossRef]
- Lynn, A.M.; Harmsen, W.S.; Tremaine, W.J.; Loftus, E.V. Su1872-Trends in the Prevalence of Overweight and Obesity at the Time of Inflammatory Bowel Disease Diagnosis: A Population-Based Study. Gastroenterology 2018, 154, S-614–S-615. [Google Scholar] [CrossRef]
- Khalili, H.; Ananthakrishnan, A.N.; Konijeti, G.G.; Higuchi, L.M.; Fuchs, C.S.; Richter, J.M.; Chan, A.T. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2015, 21, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Mendall, M.; Harpsoe, M.C.; Kumar, D.; Andersson, M.; Jess, T. Relation of body mass index to risk of developing inflammatory bowel disease amongst women in the Danish National Birth Cohort. PLoS ONE 2018, 13, e0190600. [Google Scholar] [CrossRef] [Green Version]
- Harpsøe, M.C.; Basit, S.; Andersson, M.; Nielsen, N.M.; Frisch, M.; Wohlfahrt, J.; Nohr, E.A.; Linneberg, A.; Jess, T. Body mass index and risk of autoimmune diseases: A study within the Danish National Birth Cohort. Int. J. Epidemiol. 2014, 43, 843–855. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.S.; Luben, R.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Teucher, B.; Lindgren, S.; Grip, O.; Key, T.; Crowe, F.L.; et al. Body mass index and the risk for Crohn’s disease and ulcerative colitis: Data from a European Prospective Cohort Study (The IBD in EPIC Study). Am. J. Gastroenterol. 2013, 108, 575–582. [Google Scholar] [CrossRef]
- Jensen, C.B.; Angquist, L.H.; Mendall, M.A.; Sorensen, T.I.A.; Baker, J.L.; Jess, T. Childhood body mass index and risk of inflammatory bowel disease in adulthood: A population-based cohort study. Am. J. Gastroenterol. 2018, 113, 694–701. [Google Scholar] [CrossRef]
- Melinder, C.; Hiyoshi, A.; Hussein, O.; Halfvarson, J.; Ekbom, A.; Montgomery, S. Physical Fitness in Adolescence and Subsequent Inflammatory Bowel Disease Risk. Clin. Transl. Gastroenterol. 2015, 6, e121. [Google Scholar] [CrossRef]
- Mendall, M.A.; Gunasekera, A.V.; John, B.J.; Kumar, D. Is obesity a risk factor for Crohn’s disease? Dig. Dis. Sci. 2011, 56, 837–844. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Sundquist, J.; Sundquist, K. Risk of asthma and autoimmune diseases and related conditions in patients hospitalized for obesity. Ann. Med. 2012, 44, 289–295. [Google Scholar] [CrossRef]
- Rahmani, J.; Kord-Varkaneh, H.; Hekmatdoost, A.; Thompson, J.; Clark, C.; Salehisahlabadi, A.; Day, A.S.; Jacobson, K. Body mass index and risk of inflammatory bowel disease: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. Obes. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Uko, V.; Vortia, E.; Achkar, J.P.; Karakas, P.; Fiocchi, C.; Worley, S.; Kay, M.H. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Kredel, L.; Batra, A.; Siegmund, B. Role of fat and adipokines in intestinal inflammation. Curr. Opin. Gastroenterol. 2014, 30, 559–565. [Google Scholar] [CrossRef]
- Kredel, L.I.; Siegmund, B. Adipose-tissue and intestinal inflammation—Visceral obesity and creeping fat. Front. Immunol. 2014, 5, 462. [Google Scholar] [CrossRef] [Green Version]
- Hass, D.J.; Brensinger, C.M.; Lewis, J.D.; Lichtenstein, G.R. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 482–488. [Google Scholar] [CrossRef]
- Malik, T.A.; Manne, A.; Oster, R.A.; Eckhoff, A.; Inusah, S.; Gutierrez, A.M. Obesity is Associated with Poor Surgical Outcome in Crohn’s Disease. Gastroenterol. Res. 2013, 6, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Singla, M.B.; Eickhoff, C.; Betteridge, J. Extraintestinal Manifestations Are Common in Obese Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 1637–1642. [Google Scholar] [CrossRef]
- Pavelock, N.; Masood, U.; Minchenberg, S.; Heisig, D. Effects of obesity on the course of inflammatory bowel disease. Proceedings (Bayl. Univ. Med. Cent.) 2019, 32, 14–17. [Google Scholar] [CrossRef]
- Seminerio, J.L.; Koutroubakis, I.E.; Ramos-Rivers, C.; Hashash, J.G.; Dudekula, A.; Regueiro, M.; Baidoo, L.; Barrie, A.; Swoger, J.; Schwartz, M.; et al. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2857–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, A.; Burstein, E.; Cipher, D.J.; Feagins, L.A. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig. Dis. Sci. 2015, 60, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Pringle, P.L.; Stewart, K.O.; Peloquin, J.M.; Sturgeon, H.C.; Nguyen, D.; Sauk, J.; Garber, J.J.; Yajnik, V.; Ananthakrishnan, A.N.; Chan, A.T.; et al. Body Mass Index, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 2304–2310. [Google Scholar] [CrossRef]
- Stabroth-Akil, D.; Leifeld, L.; Pfutzer, R.; Morgenstern, J.; Kruis, W. The effect of body weight on the severity and clinical course of ulcerative colitis. Int. J. Colorectal Dis. 2015, 30, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Proudfoot, J.; Xu, R.; Sandborn, W.J. Obesity and Response to Infliximab in Patients with Inflammatory Bowel Diseases: Pooled Analysis of Individual Participant Data from Clinical Trials. Am. J. Gastroenterol. 2018, 113, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ren, J.; Li, G.; Wu, X.; Li, J. The Impact of Obesity on the Clinical Course of Inflammatory Bowel Disease: A Meta-Analysis. Med. Sci. Monit. 2017, 23, 2599–2606. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868–7881. [Google Scholar] [CrossRef]
- Erhayiem, B.; Dhingsa, R.; Hawkey, C.J.; Subramanian, V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease. Clin. Gastroenterol. Hepatol. 2011, 9, 684–687. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Gong, J.; Zhang, W.; Gu, L.; Guo, Z.; Cao, L.; Shen, B.; Li, N.; Li, J. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal Dis. 2015, 17, 225–234. [Google Scholar] [CrossRef]
- Bryant, R.V.; Schultz, C.G.; Ooi, S.; Goess, C.; Costello, S.P.; Vincent, A.D.; Schoeman, S.; Lim, A.; Bartholomeusz, F.D.; Travis, S.P.L.; et al. Visceral Adipose Tissue Is Associated with Stricturing Crohn’s Disease Behavior, Fecal Calprotectin, and Quality of Life. Inflamm. Bowel Dis. 2019, 25, 592–600. [Google Scholar] [CrossRef]
- Connelly, T.M.; Juza, R.M.; Sangster, W.; Sehgal, R.; Tappouni, R.F.; Messaris, E. Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn’s disease patients. Dig. Surg. 2014, 31, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.Q.; Moore, G.T.; Strauss, B.J.; Hamilton, A.L.; De Cruz, P.; Kamm, M.A. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment. Pharmacol. Ther. 2017, 45, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sloot, K.W.; Joshi, A.D.; Bellavance, D.R.; Gilpin, K.K.; Stewart, K.O.; Lochhead, P.; Garber, J.J.; Giallourakis, C.; Yajnik, V.; Ananthakrishnan, A.N. Visceral adiposity, genetic susceptibility, and risk of complications among individuals with Crohn’s disease. Inflamm. Bowel Dis. 2016, 23, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desreumaux, P.; Ernst, O.; Geboes, K.; Gambiez, L.; Berrebi, D.; Muller-Alouf, H.; Hafraoui, S.; Emilie, D.; Ectors, N.; Peuchmaur, M.; et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology 1999, 117, 73–81. [Google Scholar] [CrossRef]
- Cooper, C.; Fielding, R.; Visser, M.; van Loon, L.J.; Rolland, Y.; Orwoll, E.; Reid, K.; Boonen, S.; Dere, W.; Epstein, S.; et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 2013, 93, 201–210. [Google Scholar] [CrossRef]
- Adams, D.W.; Gurwara, S.; Silver, H.J.; Horst, S.N.; Beaulieu, D.B.; Schwartz, D.A.; Seidner, D.L. Sarcopenia Is Common in Overweight Patients with Inflammatory Bowel Disease and May Predict Need for Surgery. Inflamm. Bowel Dis. 2017, 23, 1182–1186. [Google Scholar] [CrossRef]
- Bamba, S.; Sasaki, M.; Takaoka, A.; Takahashi, K.; Imaeda, H.; Nishida, A.; Inatomi, O.; Sugimoto, M.; Andoh, A. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS ONE 2017, 12, e0180036. [Google Scholar] [CrossRef]
- Barroso, T.; Conway, F.; Emel, S.; McMillan, D.; Young, D.; Karteszi, H.; Gaya, D.R.; Gerasimidis, K. Patients with inflammatory bowel disease have higher abdominal adiposity and less skeletal mass than healthy controls. Ann. Gastroenterol. 2018, 31, 566. [Google Scholar] [CrossRef]
- Bechtold, S.; Alberer, M.; Arenz, T.; Putzker, S.; Filipiak-Pittroff, B.; Schwarz, H.P.; Koletzko, S. Reduced muscle mass and bone size in pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 216–225. [Google Scholar] [CrossRef]
- Bryant, R.V.; Schultz, C.G.; Ooi, S.; Goess, C.; Costello, S.P.; Vincent, A.D.; Schoeman, S.N.; Lim, A.; Bartholomeusz, F.D.; Travis, S.P.L.; et al. Obesity in Inflammatory Bowel Disease: Gains in Adiposity despite High Prevalence of Myopenia and Osteopenia. Nutrients 2018, 10, 1192. [Google Scholar] [CrossRef] [Green Version]
- Bryant, R.V.; Ooi, S.; Schultz, C.G.; Goess, C.; Grafton, R.; Hughes, J.; Lim, A.; Bartholomeusz, F.D.; Andrews, J.M. Low muscle mass and sarcopenia: Common and predictive of osteopenia in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 41, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Trott, M.J.; Bartholomeusz, F.D.; Andrews, J.M. Systematic review: Body composition in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Cabalzar, A.L.; Oliveira, D.J.F.; Reboredo, M.d.M.; Lucca, F.A.; Chebli, J.M.F.; Malaguti, C. Muscle function and quality of life in the Crohn’s disease. Fisioter. em Mov. 2017, 30, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, D.; Viana, C.; Marques, I.; Costa, C.; Martins, S.F. Sarcopenia is associated with Postoperative Outcome in Patients with Crohn’s Disease Undergoing Bowel Resection. Gastrointest. Disord. 2019, 1, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Cuoco, L.; Vescovo, G.; Castaman, R.; Ravara, B.; Cammarota, G.; Angelini, A.; Salvagnini, M.; Dalla Libera, L. Skeletal muscle wastage in Crohn’s disease: A pathway shared with heart failure? Int. J. Cardiol. 2008, 127, 219–227. [Google Scholar] [CrossRef]
- Pedersen, M.; Cromwell, J.; Nau, P. Sarcopenia is a Predictor of Surgical Morbidity in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1867–1872. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Schneider, S.M.; Al-Jaouni, R.; Filippi, J.; Wiroth, J.B.; Zeanandin, G.; Arab, K.; Hebuterne, X. Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission. Inflamm. Bowel Dis. 2008, 14, 1562–1568. [Google Scholar] [CrossRef]
- Subramaniam, K.; Fallon, K.; Ruut, T.; Lane, D.; McKay, R.; Shadbolt, B.; Ang, S.; Cook, M.; Platten, J.; Pavli, P.; et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 41, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Thangarajah, D.; Hyde, M.J.; Konteti, V.K.; Santhakumaran, S.; Frost, G.; Fell, J.M. Systematic review: Body composition in children with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 42, 142–157. [Google Scholar] [CrossRef] [Green Version]
- Vadan, R.; Gheorghe, L.S.; Constantinescu, A.; Gheorghe, C. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn’s disease treated with Infliximab. Clin. Nutr. 2011, 30, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Van Langenberg, D.R.; Gatta, P.D.; Hill, B.; Zacharewicz, E.; Gibson, P.R.; Russell, A.P. Delving into disability in Crohn’s disease: Dysregulation of molecular pathways may explain skeletal muscle loss in Crohn’s disease. J. Crohns. Colitis 2013. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Brzozowski, B.; Magierowski, M.; Zahradnik-Bilska, J.; Wojcik, D.; Magierowska, K.; Kwiecien, S.; Mach, T.; Brzozowski, T. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacol. Rep. 2016, 68, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Brzozowski, B.; Mazur-Bialy, A.; Sliwowski, Z.; Brzozowski, T. The role of physical exercise in inflammatory bowel disease. BioMed Res. Int. 2014, 2014, 429031. [Google Scholar] [CrossRef]
- Bilski, J.; Mazur-Bialy, A.I.; Wierdak, M.; Brzozowski, T. The impact of physical activity and nutrition on inflammatory bowel disease: The potential role of cross talk between adipose tissue and skeletal muscle. J. Physiol. Pharmacol. 2013, 64, 143–155. [Google Scholar]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and Their Role in Intestinal Inflammation. Front. Immunol. 2018, 9, 1974. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Proudfoot, J.; Xu, R.; Sandborn, W.J. Impact of Obesity on Short- and Intermediate-Term Outcomes in Inflammatory Bowel Diseases: Pooled Analysis of Placebo Arms of Infliximab Clinical Trials. Inflamm. Bowel Dis. 2018, 24, 2278–2284. [Google Scholar] [CrossRef]
- Lanthier, N.; Leclercq, I.A. Adipose tissues as endocrine target organs. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, M.; Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014, 3, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and Metabolic Implications of Regional Differences among Fat Depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagiannides, I.; Pothoulakis, C. Obesity, innate immunity and gut inflammation. Curr. Opin. Gastroenterol. 2007, 23, 661–666. [Google Scholar] [CrossRef]
- Nam, S.Y. Obesity-Related Digestive Diseases and Their Pathophysiology. Gut Liver 2017, 11, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Clement, K.; Langin, D. Regulation of inflammation-related genes in human adipose tissue. J. Intern. Med. 2007, 262, 422–430. [Google Scholar] [CrossRef]
- Karagiannides, I.; Pothoulakis, C. Neuropeptides, mesenteric fat, and intestinal inflammation. Ann. N. Y. Acad. Sci. 2008, 1144, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Chamaillard, M.; Gonzalez, F.; Beclin, E.; Decourcelle, C.; Antunes, L.; Gay, J.; Neut, C.; Colombel, J.F.; Desreumaux, P. Mesenteric fat in Crohn’s disease: A pathogenetic hallmark or an innocent bystander? Gut 2007, 56, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.; Trystram, L.; Assmann, K.; Salem, J.E.; Vaillant, J.C.; Oppert, J.M.; Laugerette, F.; et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef]
- Fink, C.; Karagiannides, I.; Bakirtzi, K.; Pothoulakis, C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2012, 18, 1550–1557. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, A.L.; Warren, B.F.; Gear, M.W.; Shepherd, N.A. Fat-wrapping in Crohn’s disease: Pathological basis and relevance to surgical practice. Br. J. Surg. 1992, 79, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Gonzalez, F.; Dubuquoy, L.; Rousseaux, C.; Dubuquoy, C.; Decourcelle, C.; Saudemont, A.; Tachon, M.; Beclin, E.; Odou, M.F.; et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 2012, 61, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Tilg, H. “Metabolic aspects” in inflammatory bowel diseases. Curr. Drug. Deliv. 2012, 9, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Kurada, S.; Gordon, I.O.; Baker, M.E.; Gandhi, N.; McDonald, C.; Coffey, J.C.; Rieder, F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Westcott, E.D.; Mattacks, C.A.; Windsor, A.C.; Knight, S.C.; Pond, C.M. Perinodal adipose tissue and fatty acid composition of lymphoid tissues in patients with and without Crohn’s disease and their implications for the etiology and treatment of CD. Ann. N. Y. Acad. Sci. 2006, 1072, 395–400. [Google Scholar] [CrossRef]
- Kredel, L.I.; Batra, A.; Stroh, T.; Kuhl, A.A.; Zeitz, M.; Erben, U.; Siegmund, B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut 2013, 62, 852–862. [Google Scholar] [CrossRef]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef]
- Batra, A.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Glauben, R.; Kunkel, D.; Scheffold, A.; Erben, U.; Kuhl, A.; Loddenkemper, C.; et al. Mesenteric fat—Control site for bacterial translocation in colitis? Mucosal Immunol. 2012, 5, 580–591. [Google Scholar] [CrossRef] [Green Version]
- Batra, A.; Zeitz, M.; Siegmund, B. Adipokine signaling in inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 1897–1905. [Google Scholar] [CrossRef]
- Kruis, T.; Batra, A.; Siegmund, B. Bacterial translocation—Impact on the adipocyte compartment. Front. Immunol. 2014, 4, 510. [Google Scholar] [CrossRef] [Green Version]
- Sideri, A.; Bakirtzi, K.; Shih, D.Q.; Koon, H.W.; Fleshner, P.; Arsenescu, R.; Arsenescu, V.; Turner, J.R.; Karagiannides, I.; Pothoulakis, C. Substance P mediates proinflammatory cytokine release form mesenteric adipocytes in Inflammatory Bowel Disease patients. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 420–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drouet, M.; Dubuquoy, L.; Desreumaux, P.; Bertin, B. Visceral fat and gut inflammation. Nutrition 2012, 28, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Acedo, S.C.; Gotardo, E.M.; Lacerda, J.M.; de Oliveira, C.C.; de Oliveira Carvalho, P.; Gambero, A. Perinodal adipose tissue and mesenteric lymph node activation during reactivated TNBS-colitis in rats. Dig. Dis. Sci. 2011, 56, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, A.T. Deciphering the Role of Mesenteric Fat in Inflammatory Bowel Disease. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 352–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, L.; Li, Y.; Zhu, W.; Shen, B.; Gong, J.; Guo, Z.; Zhang, W.; Wu, R.; Gu, L.; Li, N. Mesenteric Adipocyte Dysfunction in Crohn’s Disease is Associated with Hypoxia. Inflamm. Bowel Dis. 2015, 22, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Van, A.G.; Rutgeerts, P. The role of C-reactive protein as an inflammatory marker in gastrointestinal diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 580–586. [Google Scholar] [CrossRef]
- Zulian, A.; Cancello, R.; Micheletto, G.; Gentilini, D.; Gilardini, L.; Danelli, P.; Invitti, C. Visceral adipocytes: Old actors in obesity and new protagonists in Crohn’s disease? Gut 2012, 61, 86–94. [Google Scholar] [CrossRef]
- Zulian, A.; Cancello, R.; Ruocco, C.; Gentilini, D.; Di Blasio, A.M.; Danelli, P.; Micheletto, G.; Cesana, E.; Invitti, C. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn’s disease. An in vivo and in vitro study. PLoS ONE 2013, 8, e78495. [Google Scholar] [CrossRef]
- Charriere, G.; Cousin, B.; Arnaud, E.; Andre, M.; Bacou, F.; Penicaud, L.; Casteilla, L. Preadipocyte conversion to macrophage. Evidence of plasticity. J. Biol. Chem. 2003, 278, 9850–9855. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Shelley-Fraser, G.; Borley, N.R.; Warren, B.F.; Shepherd, N.A. The connective tissue changes of Crohn’s disease. Histopathology 2012, 60, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Karagiannides, I.; Bakirtzi, K.; Pothoulakis, C. Neuropeptide—Adipose tissue communication and intestinal pathophysiology. Curr. Pharm. Des. 2011, 17, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Sideri, A.; Bakirtzi, K.; Arsenescu, R.; Fleshner, P.; Shih, D.Q.; Karagiannidis, I.; Pothoulakis, C. Effects of Substance P on Pro and Anti-Inflammatory Responses of Human Mesenteric Preadipocytes Isolated from IBD Patients. Gastroenterology 2013, 144, S-100. [Google Scholar] [CrossRef]
- Sideri, A.; Bakirtzi, K.; Arsenescu, R.; Fleshner, P.; Shih, D.Q.; Pothoulakis, C.; Karagiannidis, I. Preadipocyte-Specific Effects on Human Colonocyte Proinflammatory Responses Are Obesity and IBD-Dependent. Gastroenterology 2013, 144, S-820. [Google Scholar] [CrossRef]
- Karagiannides, I.; Kokkotou, E.; Tansky, M.; Tchkonia, T.; Giorgadze, N.; O’Brien, M.; Leeman, S.E.; Kirkland, J.L.; Pothoulakis, C. Induction of colitis causes inflammatory responses in fat depots: Evidence for substance P pathways in human mesenteric preadipocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 5207–5212. [Google Scholar] [CrossRef] [Green Version]
- Karmiris, K.; Koutroubakis, I.E.; Kouroumalis, E.A. Leptin, adiponectin, resistin, and ghrelin–implications for inflammatory bowel disease. Mol. Nutr. Food Res. 2008, 52, 855–866. [Google Scholar] [CrossRef]
- Sideri, A.; Stavrakis, D.; Bowe, C.; Shih, D.Q.; Fleshner, P.; Arsenescu, V.; Arsenescu, R.; Turner, J.R.; Pothoulakis, C.; Karagiannides, I. Effects of obesity on severity of colitis and cytokine expression in mouse mesenteric fat. Potential role of adiponectin receptor 1. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G591–G604. [Google Scholar] [CrossRef] [Green Version]
- Lam, Y.Y.; Ha, C.W.; Hoffmann, J.; Oscarsson, J.; Dinudom, A.; Mather, T.J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity 2015, 23, 1429–1439. [Google Scholar] [CrossRef]
- Morshedzadeh, N.; Rahimlou, M.; Asadzadeh Aghdaei, H.; Shahrokh, S.; Reza Zali, M.; Mirmiran, P. Association Between Adipokines Levels with Inflammatory Bowel Disease (IBD): Systematic Reviews. Dig. Dis. Sci. 2017, 62, 3280–3286. [Google Scholar] [CrossRef]
- Azamar-Llamas, D.; Hernandez-Molina, G.; Ramos-Avalos, B.; Furuzawa-Carballeda, J. Adipokine Contribution to the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2017, 2017, 5468023. [Google Scholar] [CrossRef] [Green Version]
- Graßmann, S.; Wirsching, J.; Eichelmann, F.; Aleksandrova, K. Association Between Peripheral Adipokines and Inflammation Markers: A Systematic Review and Meta-Analysis. Obesity 2017, 25, 1176–1785. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Singh, N.P.; Guan, H.; Busbee, B.; Price, R.L.; Taub, D.D.; Mishra, M.K.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. The emerging role of leptin antagonist as potential therapeutic option for inflammatory bowel disease. Int. Rev. Immunol. 2014, 33, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, U.P.; Singh, N.P.; Guan, H.; Busbee, B.; Price, R.L.; Taub, D.D.; Mishra, M.K.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Leptin antagonist ameliorates chronic colitis in IL-10-/- mice. Immunobiology 2013, 218, 1439–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesiada, G.; Czepiel, J.; Ptak-Belowska, A.; Targosz, A.; Krzysiek-Maczka, G.; Strzalka, M.; Konturek, S.J.; Brzozowski, T.; Mach, T. Expression and release of leptin and proinflammatory cytokines in patients with ulcerative colitis and infectious diarrhea. J. Physiol. Pharmacol. 2012, 63, 471–481. [Google Scholar] [PubMed]
- Tuzun, A.; Uygun, A.; Yesilova, Z.; Ozel, A.M.; Erdil, A.; Yaman, H.; Bagci, S.; Gulsen, M.; Karaeren, N.; Dagalp, K. Leptin levels in the acute stage of ulcerative colitis. J. Gastroenterol. Hepatol. 2004, 19, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, R.; Calhan, T.; Sahin, A.; Ozdil, K.; Caliskan, Z.; Bireller, E.S.; Cakmakoglu, B. Are adipocytokines inflammatory or metabolic mediators in patients with inflammatory bowel disease? Ther. Clin. Risk Manag. 2017, 13, 1295–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Voudouri, T.; Kouroumalis, E.A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 100–105. [Google Scholar] [CrossRef]
- Chouliaras, G.; Panayotou, I.; Margoni, D.; Mantzou, E.; Pervanidou, P.; Manios, Y.; Chrousos, G.P.; Roma, E. Circulating leptin and adiponectin and their relation to glucose metabolism in children with Crohn’s disease and ulcerative colitis. Pediatr. Res. 2013, 74, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, V.S.; Milanski, M.; Fagundes, J.J.; Torsoni, A.S.; Ayrizono, M.L.; Nunez, C.E.; Dias, C.B.; Meirelles, L.R.; Dalal, S.; Coy, C.S.; et al. Serum levels and mesenteric fat tissue expression of adiponectin and leptin in patients with Crohn’s disease. Clin. Exp. Immunol. 2012, 170, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Valentini, L.; Wirth, E.K.; Schweizer, U.; Hengstermann, S.; Schaper, L.; Koernicke, T.; Dietz, E.; Norman, K.; Buning, C.; Winklhofer-Roob, B.M.; et al. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition 2009, 25, 172–181. [Google Scholar] [CrossRef]
- Nishi, Y.; Isomoto, H.; Ueno, H.; Ohnita, K.; Wen, C.Y.; Takeshima, F.; Mishima, R.; Nakazato, M.; Kohno, S. Plasma leptin and ghrelin concentrations in patients with Crohn’s disease. World J. Gastroenterol. 2005, 11, 7314–7317. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.; Vidal, H.; Desreumaux, P.; Dubuquoy, L.; Bourreille, A.; Colombel, J.F.; Cherbut, C.; Galmiche, J.P. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol. Clin. Biol. 2003, 27, 987–991. [Google Scholar] [CrossRef]
- Paul, G.; Schaffler, A.; Neumeier, M.; Furst, A.; Bataillle, F.; Buechler, C.; Muller-Ladner, U.; Scholmerich, J.; Rogler, G.; Herfarth, H. Profiling adipocytokine secretion from creeping fat in Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Weigert, J.; Obermeier, F.; Neumeier, M.; Wanninger, J.; Filarsky, M.; Bauer, S.; Aslanidis, C.; Rogler, G.; Ott, C.; Schaffler, A.; et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 630–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waluga, M.; Hartleb, M.; Boryczka, G.; Kukla, M.; Zwirska-Korczala, K. Serum adipokines in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 6912–6917. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kiyohara, T.; Murayama, Y.; Kihara, S.; Okamoto, Y.; Funahashi, T.; Ito, T.; Nezu, R.; Tsutsui, S.; Miyagawa, J.I.; et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut 2005, 54, 789–796. [Google Scholar] [CrossRef]
- Han, S.; Wang, G.; Qiu, S.; de la Motte, C.; Wang, H.Q.; Gomez, G.; Englander, E.W.; Greeley, G.H., Jr. Increased colonic apelin production in rodents with experimental colitis and in humans with IBD. Regul. Pept. 2007, 142, 131–137. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Chen, Q.; Zhu, W.; Zuo, L.; Guo, Z.; Gong, J.; Cao, L.; Gu, L.; Li, J. Adipokine apelin ameliorates chronic colitis in Il-10−/− mice by promoting intestinal lymphatic functions. Biochem. Pharmacol. 2018, 148, 202–212. [Google Scholar] [CrossRef]
- Terzoudis, S.; Malliaraki, N.; Damilakis, J.; Dimitriadou, D.A.; Zavos, C.; Koutroubakis, I.E. Chemerin, visfatin, and vaspin serum levels in relation to bone mineral density in patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2016, 28, 814–819. [Google Scholar] [CrossRef]
- Al-Suhaimi, E.A.; Shehzad, A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. Prostaglandins 2013, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Konrad, A.; Lehrke, M.; Schachinger, V.; Seibold, F.; Stark, R.; Ochsenkuhn, T.; Parhofer, K.G.; Goke, B.; Broedl, U.C. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur. J. Gastroenterol. Hepatol. 2007, 19, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Kouroumalis, E.A. The effect of infliximab on circulating levels of leptin, adiponectin and resistin in patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2007, 19, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Guven, K.; Celikbilek, M.; Deniz, K.; Saraymen, B.; Gursoy, S. Serum Visfatin Levels in Ulcerative Colitis. J. Clin. Lab. Anal. 2016, 30, 552–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [Green Version]
- Starr, A.E.; Deeke, S.A.; Ning, Z.; Chiang, C.K.; Zhang, X.; Mottawea, W.; Singleton, R.; Benchimol, E.I.; Wen, M.; Mack, D.R.; et al. Proteomic analysis of ascending colon biopsies from a paediatric inflammatory bowel disease inception cohort identifies protein biomarkers that differentiate Crohn’s disease from UC. Gut 2017, 66, 1573–1583. [Google Scholar] [CrossRef]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Morisaki, T.; Takeshima, F.; Fukuda, H.; Matsushima, K.; Akazawa, Y.; Yamaguchi, N.; Ohnita, K.; Isomoto, H.; Takeshita, H.; Sawai, T.; et al. High serum vaspin concentrations in patients with ulcerative colitis. Dig. Dis. Sci. 2014, 59, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014, 25, 348–355. [Google Scholar] [CrossRef]
- Yin, J.; Hou, P.; Wu, Z.; Nie, Y. Decreased levels of serum omentin-1 in patients with inflammatory bowel disease. Med. Sci. Monit. 2015, 21, 118–122. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, L.; Liu, L.; Feng, Y.; Lu, L.; Ren, X.; Dong, X.; Sang, W. Serum omentin-1 as a disease activity marker for Crohn’s disease. Dis. Markers 2014, 2014, 162517. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.L.; Li, Z.Y.; Song, J.; Liu, J.M.; Miao, C.Y. Metrnl: A secreted protein with new emerging functions. Acta Pharmacol. Sin. 2016, 37, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, L.; Ge, S.; Ge, Y.; Li, J.; Zhu, B.; Zhang, Z.; Jiang, C.; Li, J.; Wang, S.; Liu, M. The adipokine metrnl ameliorates chronic colitis in Il-10–/–mice by attenuating mesenteric adipose tissue lesions during spontaneous colitis. J. Crohn’s Colitis 2019, 13, 931–941. [Google Scholar] [CrossRef] [PubMed]
- DeClercq, V.; Langille, M.G.I.; Van Limbergen, J. Differences in adiposity and diet quality among individuals with inflammatory bowel disease in Eastern Canada. PLoS ONE 2018, 13, e0200580. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Muniesa, P.; Martinez-Gonzalez, M.A.; Hu, F.B.; Despres, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Shoda, R.; Matsueda, K.; Yamato, S.; Umeda, N. Epidemiologic analysis of Crohn disease in Japan: Increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am. J. Clin. Nutr. 1996, 63, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Wright, E.K.; Kamm, M.A.; Teo, S.M.; Inouye, M.; Wagner, J.; Kirkwood, C.D. Recent advances in characterizing the gastrointestinal microbiome in Crohn’s disease: A systematic review. Inflamm. Bowel Dis. 2015, 21, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Schaubeck, M.; Haller, D. Reciprocal interaction of diet and microbiome in inflammatory bowel diseases. Curr. Opin. Gastroenterol. 2015, 31, 464–470. [Google Scholar] [CrossRef]
- De, F.C.; Cavalieri, D.; Di, P.M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Albenberg, L.G.; Lewis, J.D.; Wu, G.D. Food and the gut microbiota in inflammatory bowel diseases: A critical connection. Curr. Opin. Gastroenterol. 2012, 28, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2015, 49 (Suppl. 1), S20–S24. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [Green Version]
- Schink, M.; Konturek, P.C.; Tietz, E.; Dieterich, W.; Pinzer, T.C.; Wirtz, S.; Neurath, M.F.; Zopf, Y. Microbial patterns in patients with histamine intolerance. J. Physiol. Pharmacol. 2018, 69, 579–593. [Google Scholar] [CrossRef]
- Pendyala, S.; Walker, J.M.; Holt, P.R. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 2012, 142, 1100–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, D.; Skonieczna-Zydecka, K.; Lukomska, A.; Gutowska, I.; Dec, K.; Kupnicka, P.; Palma, J.; Pilutin, A.; Marlicz, W.; Stachowska, E. The short chain fatty acids and lipopolysaccharides status in Sprague-Dawley rats fed with high-fat and high-cholesterol diet. J. Physiol. Pharmacol. 2018, 69, 6. [Google Scholar] [CrossRef]
- Shi, C.; Li, H.; Qu, X.; Huang, L.; Kong, C.; Qin, H.; Sun, Z.; Yan, X. High fat diet exacerbates intestinal barrier dysfunction and changes gut microbiota in intestinal-specific ACF7 knockout mice. Biomed Pharm. 2019, 110, 537–545. [Google Scholar] [CrossRef]
- Ma, X.; Torbenson, M.; Hamad, A.R.; Soloski, M.J.; Li, Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin. Exp. Immunol. 2008, 151, 130–138. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, H.; Qiang, Y.; Wu, S.; Yan, C.; Han, M.; Xiao, T.; Yan, N.; An, H.; Zhou, X. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int. Immunopharmacol. 2016, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.G.; Leonel, A.J.; Aguilar, E.C.; Batista, N.V.; Alves, A.C.; Coimbra, C.C.; Ferreira, A.V.; de Faria, A.M.; Cara, D.C.; Alvarez Leite, J.I. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011, 10, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Magierowski, M.; Surmiak, M.; Kwiecien, S.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Brzozowski, T. Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers. Nutrients 2019, 11, 1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur-Bialy, A.I.; Bilski, J.; Wojcik, D.; Brzozowski, B.; Surmiak, M.; Hubalewska-Mazgaj, M.; Chmura, A.; Magierowski, M.; Magierowska, K.; Mach, T.; et al. Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients 2017, 9, 410. [Google Scholar] [CrossRef] [Green Version]
- Bilski, J.; Mazur-Bialy, A.I.; Brzozowski, B.; Magierowski, M.; Jasnos, K.; Krzysiek-Maczka, G.; Urbanczyk, K.; Ptak-Belowska, A.; Zwolinska-Wcislo, M.; Mach, T.; et al. Moderate Exercise Training Attenuates the Severity of Experimental Rodent Colitis: The Importance of Crosstalk between Adipose Tissue and Skeletal Muscles. Mediat. Inflamm. 2015, 2015, 605071. [Google Scholar] [CrossRef]
- Bibi, S.; de Sousa Moraes, L.F.; Lebow, N.; Zhu, M.J. Dietary Green Pea Protects against DSS-Induced Colitis in Mice Challenged with High-Fat Diet. Nutrients 2017, 9, 509. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.X.; Wang, T.; Zhou, F.; Wang, Y.; Xing, J.W.; Zhang, S.; Gu, S.Z.; Sang, L.X.; Dai, C.; Wang, H.L. Voluntary exercise prevents colonic inflammation in high-fat diet-induced obese mice by up-regulating PPAR-gamma activity. Biochem. Biophys. Res. Commun. 2015, 459, 475–480. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.; Magness, S.; Jobin, C.; Lund, P.K. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef] [Green Version]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermudez-Humaran, L.G.; Smirnova, N.; Berge, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ha, C.W.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maillard, F.; Vazeille, E.; Sauvanet, P.; Sirvent, P.; Bonnet, R.; Combaret, L.; Chausse, P.; Chevarin, C.; Otero, Y.F.; Delcros, G.; et al. Preventive Effect of Spontaneous Physical Activity on the Gut-Adipose Tissue in a Mouse Model That Mimics Crohn’s Disease Susceptibility. Cells 2019, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thevenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassaganya-Riera, J.; Ferrer, G.; Casagran, O.; Sanchez, S.; de Horna, A.; Duran, E.; Orpi, M.; Guri, A.J.; Hontecillas, R. F4/80hiCCR2hi macrophage infiltration into the intra-abdominal fat worsens the severity of experimental IBD in obese mice with DSS colitis. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2009, 4, e90–e97. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR-4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, H.Y.; Kim, T.K.; Kim, M.S.; Park, Y.M.; Kim, J.; Park, K.; Kweon, M.N.; Kim, S.H.; Bae, J.W.; et al. Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis. PLoS ONE 2017, 12, e0187515. [Google Scholar] [CrossRef] [Green Version]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef]
- Stenman, L.K.; Holma, R.; Gylling, H.; Korpela, R. Genetically obese mice do not show increased gut permeability or faecal bile acid hydrophobicity. Br. J. Nutr. 2013, 110, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Brun, P.; Castagliuolo, I.; Di Leo, V.; Buda, A.; Pinzani, M.; Palu, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Hara, H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr. Metab. 2010, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, L.; Kisling, S.; Lichti, P.; Martin, F.P.; May, S.; Klingenspor, M.; Lichtenegger, M.; Rychlik, M.; Haller, D. High fat diet accelerates pathogenesis of murine Crohn’s disease-like ileitis independently of obesity. PLoS ONE 2013, 8, e71661. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Kang, Y.; Du, M.; Zhu, M.J. Maternal high-fat diet consumption enhances offspring susceptibility to DSS-induced colitis in mice. Obesity 2017, 25, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, B.; Lehr, H.A.; Fantuzzi, G. Leptin: A pivotal mediator of intestinal inflammation in mice. Gastroenterology 2002, 122, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Rennick, D.M.; Fort, M.M. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(-/-) mice and intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G829–G833. [Google Scholar] [CrossRef]
- Siegmund, B.; Sennello, J.A.; Lehr, H.A.; Batra, A.; Fedke, I.; Zeitz, M.; Fantuzzi, G. Development of intestinal inflammation in double IL-10- and leptin-deficient mice. J. Leukoc. Biol. 2004, 76, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, T.; Matsuda, M.; Araki, H.; Oshima, K.; Kihara, S.; Funahashi, T.; Shimomura, I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology 2006, 131, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Fletcher, E.; Larsen, B.; Baliga, M.S.; Durstine, J.L.; Fayad, R. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. J. Inflamm. 2012, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Obeid, S.; Wankell, M.; Charrez, B.; Sternberg, J.; Kreuter, R.; Esmaili, S.; Ramezani-Moghadam, M.; Devine, C.; Read, S.; Bhathal, P.; et al. Adiponectin confers protection from acute colitis and restricts a B cell immune response. J. Biol. Chem. 2017, 292, 6569–6582. [Google Scholar] [CrossRef] [Green Version]
- Arsenescu, V.; Narasimhan, M.L.; Halide, T.; Bressan, R.A.; Barisione, C.; Cohen, D.A.; de Villiers, W.J.; Arsenescu, R. Adiponectin and plant-derived mammalian adiponectin homolog exert a protective effect in murine colitis. Dig. Dis. Sci. 2011, 56, 2818–2832. [Google Scholar] [CrossRef]
- Fayad, R.; Pini, M.; Sennello, J.A.; Cabay, R.J.; Chan, L.; Xu, A.; Fantuzzi, G. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology 2007, 132, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Saxena, A.; Larsen, B.; Truman, S.; Biyani, N.; Fletcher, E.; Baliga, M.S.; Ponemone, V.; Hegde, S.; Chanda, A.; et al. Mucus mediated protection against acute colitis in adiponectin deficient mice. J. Inflamm. 2015, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pini, M.; Gove, M.E.; Fayad, R.; Cabay, R.J.; Fantuzzi, G. Adiponectin deficiency does not affect development and progression of spontaneous colitis in IL-10 knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G382–G387. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Sideri, A.; Ruiz, J.J.; Stavrakis, D.; Shih, D.Q.; Turner, J.R.; Pothoulakis, C.; Karagiannides, I. Mesenteric Adipose-derived Stromal Cells from Crohn’s Disease Patients Induce Protective Effects in Colonic Epithelial Cells and Mice with Colitis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zuo, L.; Zhu, W.; Gong, J.; Zhang, W.; Guo, Z.; Gu, L.; Li, N.; Li, J. Telmisartan attenuates the inflamed mesenteric adipose tissue in spontaneous colitis by mechanisms involving regulation of neurotensin/microRNA-155 pathway. Biochem. Pharmacol. 2015, 93, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.C.; Pershadsingh, H.A.; Ho, C.I.; Chittiboyina, A.; Desai, P.; Pravenec, M.; Qi, N.; Wang, J.; Avery, M.A.; Kurtz, T.W. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 2004, 43, 993–1002. [Google Scholar] [CrossRef] [Green Version]
- Desreumaux, P.; Dubuquoy, L.; Nutten, S.; Peuchmaur, M.; Englaro, W.; Schoonjans, K.; Derijard, B.; Desvergne, B.; Wahli, W.; Chambon, P.; et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J. Exp. Med. 2001, 193, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Katayama, K.; Wada, K.; Nakajima, A.; Mizuguchi, H.; Hayakawa, T.; Nakagawa, S.; Kadowaki, T.; Nagai, R.; Kamisaki, Y.; Blumberg, R.S.; et al. A novel PPARγ gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterology 2003, 124, 1315–1324. [Google Scholar] [CrossRef]
- Lytle, C.; Tod, T.J.; Vo, K.T.; Lee, J.W.; Atkinson, R.D.; Straus, D.S. The peroxisome proliferator-activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm. Bowel Dis. 2005, 11, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Chujo, D.; Yagi, K.; Asano, A.; Muramoto, H.; Sakai, S.; Ohnishi, A.; Shintaku-Kubota, M.; Mabuchi, H.; Yamagishi, M.; Kobayashi, J. Telmisartan treatment decreases visceral fat accumulation and improves serum levels of adiponectin and vascular inflammation markers in Japanese hypertensive patients. Hypertens. Res. 2007, 30, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Aubert, G.; Burnier, M.; Dulloo, A.; Perregaux, C.; Mazzolai, L.; Pralong, F.; Zanchi, A. Neuroendocrine characterization and anorexigenic effects of telmisartan in diet- and glitazone-induced weight gain. Metabolism 2010, 59, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Vinci, A.; Behnsen, J.; Cheng, C.; Jellbauer, S.; Raffatellu, M.; Sousa, K.M.; Edwards, R.; Nguyen, N.T.; Stamos, M.J.; et al. Bariatric surgery attenuates colitis in an obese murine model. Surg. Obes. Relat. Dis. 2017, 13, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. J. Physiol. Pharmacol. 2018, 69, 117–125. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Bilski, J.; Pochec, E.; Brzozowski, T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J. Physiol. Pharmacol. 2017, 68, 243–251. [Google Scholar] [PubMed]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef] [PubMed]
| Reference | Year | Study Design | Sample | Marker of Obesity/Overweight | Conclusion |
|---|---|---|---|---|---|
| Blain et al. [27] | 2002 | Retrospective | 2065 CD patients | BMI ≥ 25.0 kg/m2 at disease onset and BMI > 30.0 kg/m2 anytime during the course of the disease | Obesity was associated with more frequent anoperineal. complications and more marked year-by-year disease activity, but does not alter significantly the long-term course of the disease. |
| Hass et al. [47] | 2006 | Cross-sectional | 148 CD patients | BMI ≥ 25.0 kg/m2 | Patients with a BMI > 25 kg/m2 had a shorter time to first surgery than those with a BMI of less than 18.5 kg/m2. |
| Long et al. [29] | 2011 | Cross-sectional | 1598 children with IBD | BMI | Obese IBD patients have an increased need for surgery. |
| Erhayiem et al. [58] | 2011 | Retrospective | 50 CD patients | CT scans, MFI defined as the ratio of areas of VAT to SAT | MFI was significantly higher in patients with complicated (strictures and fistulas) disease. |
| Malik et al. [48] | 2013 | Retrospective | 90 CD patients | BMI ≥ 30.0 kg/m2 | Obese CD patients had a poor surgical outcome when compared to not obese CD patients. |
| Connelly et al. [61] | 2014 | Retrospective | 143 CD patients after elective ileocolectomy | CT scans BMI | The VAT/SAT ratio was a predictor of increased risk for postoperative complications in patients after elective ileocolectomy. |
| Seminerio et al. [51] | 2015 | Retrospective | 1494 IBD patients | BMI ≥ 30 kg/m2 | Obesity was not associated with increased health-care utilization and IBD-related surgeries. |
| Flores et al. [52] | 2015 | Retrospective | 581 IBD patients (297 CD and 284 UC). | BMI ≥ 30 kg/m2 | Obese IBD patients were less likely to have need for anti-TNF therapy, surgery or hospitalization than normal or underweight patients. |
| Pringle et al. [53] | 2015 | Cross-sectional | 846 patients with CD | BMI ≥ 30 kg/m2 | There were no associations between obesity and risk of perianal disease, structuring disease, or surgery. Compared with normal-weight individuals, obesity was associated with lower risk of penetrating disease. |
| Stabroth-Akil et al. [54] | 2015 | Retrospective | 202 UC patients | High BMI had a favourable effect on the prognosis; low BMI pointed to a more severe course of the disease. | |
| Li et al. [59] | 2015 | Retrospective | 117 CD patients after ileocolic resection | CT scans | High visceral fat area value was associated with higher postoperative recurrence, defined as the reappearance of the clinical manifestations of Crohn’s disease. |
| Van Der Sloot et al. [63] | 2016 | Prospective | 482 patients | CT scans | VAT volume was associated with an increased risk of surgery and penetrating disease but not structuring or perianal disease among CD patients. |
| Singla et al. [49] | 2017 | Retrospective | 209 CD patients | BMI | Patients with higher BMI were more likely to have extraintestinal manifestations. |
| Holt et al. [62] | 2017 | Prospective | 44 post-operative Crohn’s disease patients | CT or MRI scans. Waist circumference BMI | Excessive visceral adiposity was an independent risk factor for endoscopic recurrence of Crohn’s disease after surgery. Lower skeletal muscle area correlated with increased fecal inflammatory markers. |
| Singh et al. [90] | 2018 | Post hoc analysis | 575 IBD placebo-treated patients (pooled analysis of placebo arms, using data from clinical trials of infliximab in IBD) | BMI ≥ 30 kg/m2 | Obesity does not significantly impact short- and intermediate-term clinical outcomes in patients with IBD. |
| Pavelock et al. [50] | 2019 | Retrospective | 55 IBD patients (27 CD, 18 UC) | overweight BMI ≥ 25.0 kg/m2 obese BMI > 30.0 kg/m2 | An increasing trend in mean number of clinic visits, hospitalizations/flares, and mean escalations in therapy with an increase in BMI. |
| Bryant et al. [60] | 2019 | Prospective | 97 CD patients | DXA, BMI, WHR | VAT was associated with structuring CD behavior and prospective disease activity and QoL in a disease-distribution-dependent manner. |
| Reference | Year | Sample | Conclusion |
|---|---|---|---|
| Barbier et al. [142] | 2003 | 19 IBD patients | Leptin mRNA levels are significantly higher in mWAT of CD and UC patients than in controls. |
| Tuzun et al. [135] | 2004 | 29 patients with active UC | Serum leptin levels are significantly higher in patients with acute UC in comparison to controls. |
| Nishi et al. [141] | 2005 | 28 CD patients | There are no differences in the plasma leptin levels between CD patients and healthy controls. |
| Yamamoto et al. [146] | 2005 | 30 IBD patients | Tissue concentrations and release of APN are significantly increased in pathologically altered mWAT in CD patients in comparison to paired normal mWAT from the same subjects. APN mRNA levels are significantly higher in pathologically altered mWAT of CD patients than with normal mWAT of the same CD patients. |
| Paul et al. [143] | 2006 | 10 CD patients | The secretion of APN and leptin is significantly upregulated in mWAT specimen. |
| Karmiris et al. [137] | 2006 | 100 IBD patients | Serum levels of adiponectin, resistin, and active ghrelin are higher and serum levels of leptin are lower in patients with IBD than in healthy controls. |
| Han et al. [147] | 2007 | IBD patients | In IBD patients, apelin immunostaining demonstrates elevated intestinal apelin content. |
| Moschen et al. [154] | 2007 | 74 IBD patients | In IBD patients, the plasma visfatin levels are significantly higher and visfatin mRNA expression is significantly elevated in colonic tissue in comparison to healthy controls. |
| Valentini et al. [140] | 2009 | 128 IBD patients | There are no differences in serum leptin levels between IBD patients and healthy controls. Serum resistin and visfatin concentrations are elevated in patients with active disease, but not in in those in remission. APN serum concentrations are lower in IBD patients and retinol-binding protein-4 is higher in comparison to healthy controls. |
| Weigert et al. [144] | 2010 | 310 IBD patients | Chemerin serum levels are elevated in IBD patients in comparison to healthy controls, whereas APN serum levels are higher in UC patients in comparison to healthy controls. CD patients have lower APN serum levels than UC patients, and APN serum level are lower in female CD patients in comparison to female healthy controls. |
| Biesiada et al. [134] | 2012 | 50 patients with active UC | Serum concentrations of leptin are significantly higher in UC patients with exacerbation of the disease than in patients in remission. The expression of leptin mRNA in colonic mucosa of patients with exacerbation of UC is higher in comparison to those in patients with UC in remission. |
| Rodrigues et al. [139]. | 2012 | 16 patients with ileocecal CD | Serum APN is lower in the active CD patients in comparison to the control, but no differences are seen when comparing the active CD patients to those in remission. APM expression in mWAT is lower in the active CD group in comparison to the control. Serum leptin is similar in all groups. |
| Chouliaras et al. [138] | 2013 | 50 pediatric IBD patients | In pediatric CD, there is no difference between those in remission and active disease. UC patients in remission have significantly elevated leptin in comparison to those with active disease. |
| Waluga et al. [145] | 2014 | 40 IBD patients | Serum leptin levels are significantly lower in IBD patients in comparison to healthy controls, and are significantly increased in CD but not UC patients after three months of therapy with corticosteroids and/or azathioprine. Serum resistin and visfatin levels are significantly elevated in IBD patients in comparison to healthy controls. Treatment induces a decrease in the serum resistin concentration only in UC patients and in the serum visfatin concentrations only in CD patients. There are no significant changes in the serum concentrations of adiponectin, chemerin and tissue growth factor-β1 between IBD patients in comparison to healthy controls, and these serum concentrations are not altered by therapy. |
| Morisaki et al. [157] | 2014 | 63 IBD patients | Serum vaspin concentrations are significantly higher in patients with UC than in patients with CD and healthy controls. |
| Lu et al. [160] | 2014 | 240 CD patients | Serum omentin-1 levels and colonic omentin-1 expressions are decreased in active CD patients. |
| Yin et al. [159] | 2015 | 192 IBD patients | Serum omentin-1 levels are significantly lower in both CD and UC patients than in healthy controls. |
| Terzoudis et al. [149] | 2016 | 120 IBD patients | The chemerin serum is significantly elevated in IBD patients than in healthy controls. Serum visfatin levels in CD patients are significantly higher than in UC patients. |
| Dogan et al. [153] | 2016 | 31 UC patients | The visfatin serum level is increased in the active UC patients in comparison to post-treatment remission patients and the healthy controls. |
| Starr et al. [155] | 2017 | 99 pediatric IBD patients | In colonic biopsies from IBD patients, the higher expression of visfatin was observed comparing to controls and there was a correlation between visfatin levels in the colonic biopsies and disease activity. |
| Kahraman et al. [136] | 2017 | 105 IBD patients | Serum adiponectin levels are significantly lower and leptin is significantly higher in patients with CD and UC. |
| Ge et al. [148] | 2018 | 24 CD patients | mWAT from CD patients express a higher level of apelin in comparison to controls. |
| Zuo et al. [163] | 2019 | 24 CD patients | mWAT from CD patients expressed a higher level of Metrnl in comparison to controls. |
| Reference | Year | Study Type | Conclusion |
|---|---|---|---|
| Siegmund et al. [204] | 2002 | Acute and chronic colitis induced in leptin-deficient ob/ob or WT mice, using DSS or TNBS | In the DSS acute model, ob/ob mice exhibit a 72% reduction of colitis severity and spontaneous release of proinflammatory cytokines from the colon in comparison to WT mice. Replacement of leptin in ob/ob mice converts the disease resistance to susceptibility, indicating that leptin deficiency, not obesity, accounts for the resistance to acute DSS-induced colitis. |
| Siegmund et al. [206] | 2004 | Spontaneously developing colitis in leptin-deficient IL-10−/− mice (IL-10−/− ob/ob) | Both IL-10−/− ob/ob and in IL-10−/− mice have a similar degree of intestinal inflammation. |
| Nishihara et al. [207] | 2006 | DSS- and TNBS-induced colitis in APN-KO mice | APN-KO mice develop a larger degree of severe colitis in comparison to WT mice. Adenovirus-mediated administration of APN significantly ameliorates the severity of colitis. APN receptors are expressed in intestinal epithelial cells, and APN inhibits LPS-induced IL-8 production in intestinal epithelial cells. |
| Fayad et al. [211] | 2007 | DSS- and TNBS-induced colitis in APN-KO mice | APN KO mice are protected from chemically induced colitis; the administration of exogenous APN completely restores the intestinal inflammatory response to DSS. |
| Han et al. [147] | 2007 | DSS-induced colitis in C57/BL6 mice and Sprague–Dawley rats | In both mice and rats with experimental colitis, colonic apelin mRNA levels are elevated during DSS-induced colitis. |
| Teixeira et al. [182] | 2011 | DSS-induced colitis in C57/BL6 mice | Leptin serum levels are increased in HFD-fed mice in comparison to control and colitis groups. Leptin expression in adipose tissue is elevated in both HFD groups in comparison to the colitis (normal-diet) group. |
| Arsenescu et al. [210] | 2011 | DSS-induced colitis in C57/BL6 mice | Adenovirus-mediated administration of APN ameliorates the severity of DSS-induced colitis. The APP homolog osmotin similarly reduces colitis severity. |
| Saxena et al. [208] | 2012 | DSS-induced colitis in APN-KO mice | APN deficiency exacerbates the severity of DSS-induced colitis and increases the production of proinflammatory cytokines. In WT mice in DSS-induced colitis. There is a decrease in the serum adiponectin level in comparison to the control. |
| Singh et al. [133] | 2013 | Spontaneously developing chronic colitis in IL-10−/− mice | Pegylated leptin antagonist ameliorates the development of chronic experimental colitis. |
| Sideri et al. [128] | 2015 | TNBS-induced colitis in C57/BL6 mice | Silencing adiponectin receptor 1 exacerbates TNBS-induced colitis in mice. |
| Kaur et al. [212] | 2015 | DSS-induced colitis in C57/BL6 mice | APN KO mice are less susceptible to DSS-induced colitis than WT mice and have a reduced release of proinflammatory cytokines. |
| Bilski et al. [185] | 2015 | TNBS-induced colitis Sprague–Dawley rats | The impaired healing of colitis observed in rats fed the HFD is accompanied by an increase in in leptin but also the reduction in adiponectin plasma levels. |
| Mazur-Bialy et al. [184] | 2017 | TNBS-induced colitis in C57/BL6 mice | There is increased leptin and decreased adiponectin plasma levels and elevated leptin and decreased adiponectin expression in adipose tissue, which correspond to disease exacerbation in HFD animals. |
| Obeid et al. [209] | 2017 | DSS-induced colitis in APN-KO mice | APN-KO mice which have shown an aggravation of DSS-induced colitis have a greater inflammatory cell infiltration and higher presence of activated B cells in comparison to controls, accompanied by an elevated proinflammatory cytokine profile production. |
| Ge et al. [148] | 2018 | Spontaneously developing chronic colitis in IL-10−/− mice | Apelin significantly ameliorates chronic colitis in Il-10−/− mice, demonstrated by the decreased disease activity index, inflammatory scores, and decreased levels of proinflammatory cytokines. |
| Zuo et al. [163] | 2019 | Spontaneously developing chronic colitis in IL-10−/− mice | In IL-10−/− mice with spontaneous colitis, administration of metrnl decreases pathological alterations in mWAT, increases adipocyte size, and ameliorates inflammation. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Surmiak, M.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Danielak, A.; Ptak-Belowska, A.; et al. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules 2019, 9, 780. https://doi.org/10.3390/biom9120780
Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, et al. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules. 2019; 9(12):780. https://doi.org/10.3390/biom9120780
Chicago/Turabian StyleBilski, Jan, Agnieszka Mazur-Bialy, Dagmara Wojcik, Marcin Surmiak, Marcin Magierowski, Zbigniew Sliwowski, Robert Pajdo, Slawomir Kwiecien, Aleksandra Danielak, Agata Ptak-Belowska, and et al. 2019. "Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases" Biomolecules 9, no. 12: 780. https://doi.org/10.3390/biom9120780
APA StyleBilski, J., Mazur-Bialy, A., Wojcik, D., Surmiak, M., Magierowski, M., Sliwowski, Z., Pajdo, R., Kwiecien, S., Danielak, A., Ptak-Belowska, A., & Brzozowski, T. (2019). Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules, 9(12), 780. https://doi.org/10.3390/biom9120780








