Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Mesenchymal Stem Cells
2.1.1. Rat AD-MSCs
2.1.2. Rat BM-MSCs
2.1.3. Rat DP-MSCs
2.1.4. Pig MSCs
2.2. Lentiviral Transduction of MSCs
2.3. Flow Cytometry of MSCs
2.4. Animals and Investigated Groups
2.4.1. Rats
2.4.2. Pigs
2.5. Surgical Procedures
2.5.1. Rats
2.5.2. Pigs
2.6. Behavioral Assessment
2.6.1. Rats
2.6.2. Pigs
2.7. Electrophysiological Studies
2.8. Histology and Immunohistochemistry
2.9. Real-Time PCR
2.10. Cytokine Assay
2.11. Statistical Analysis
3. Results
3.1. Characterization of Rat Mesenchymal Stem Cells
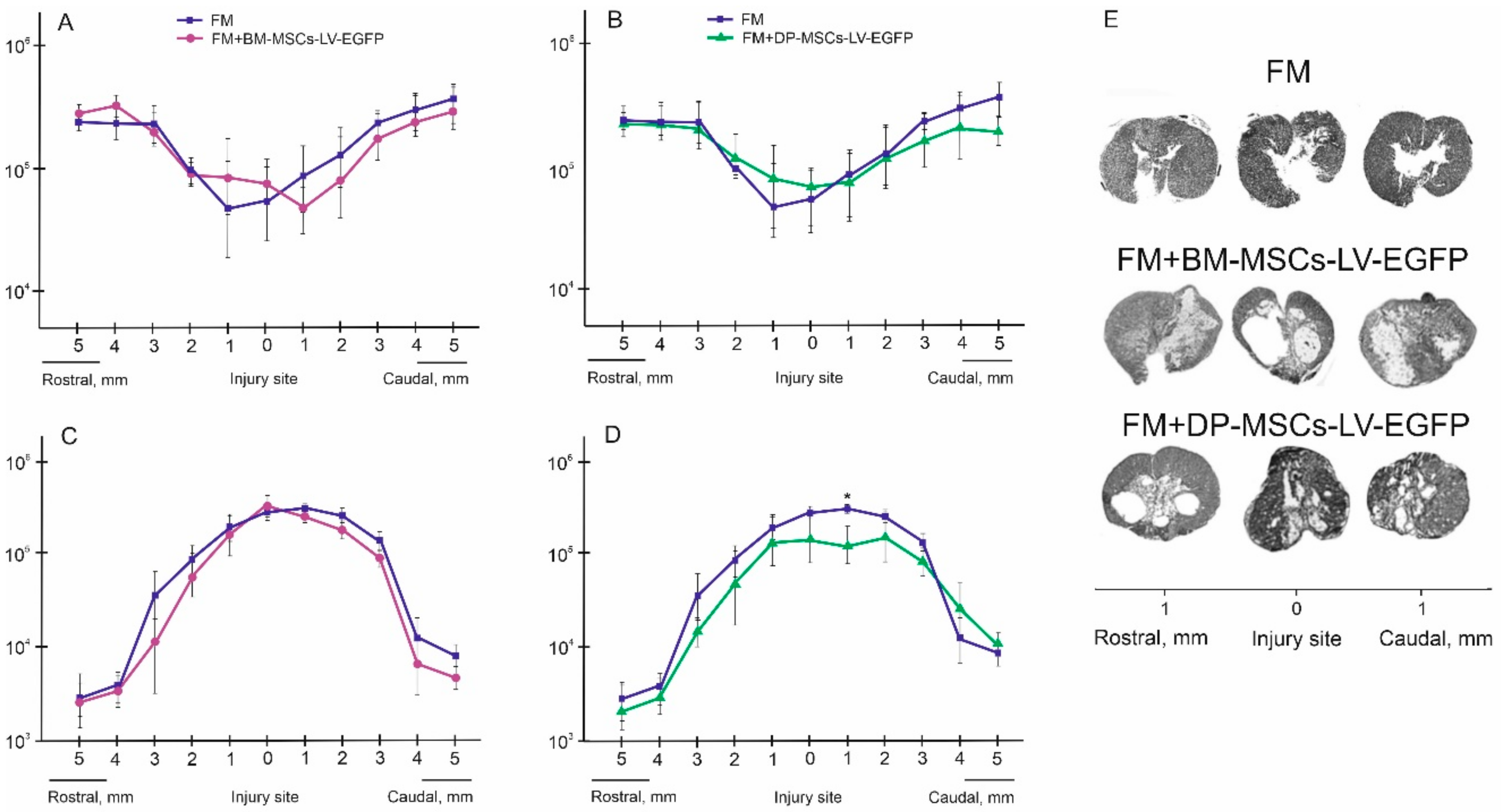
3.2. Distribution and Survival of MSCs in the Area of SCI in Rats
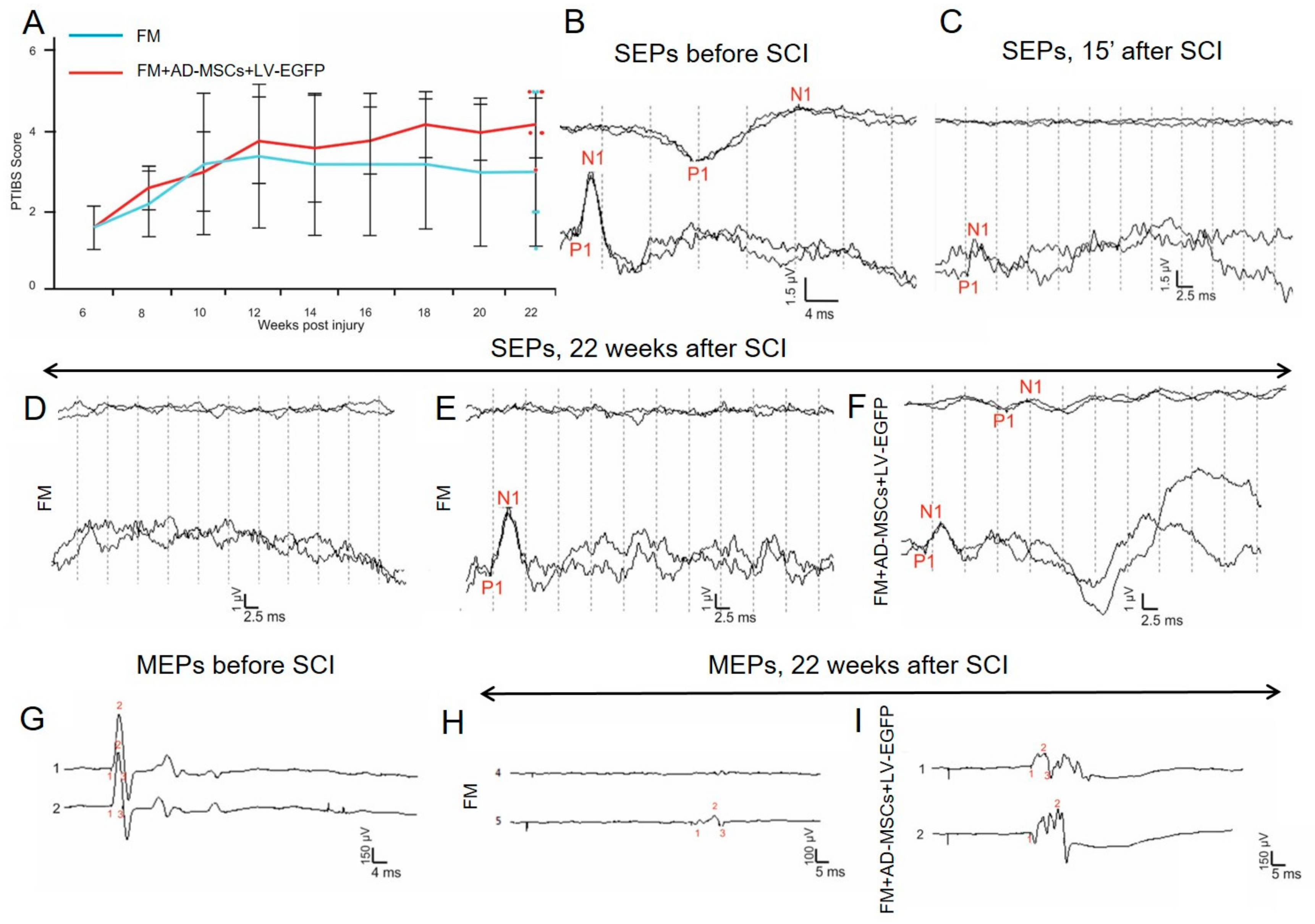
3.3. Behavioral Results in Rats
3.4. Electrophysiological Findings
Rats
3.5. Spinal Cord Morphometry in Rats
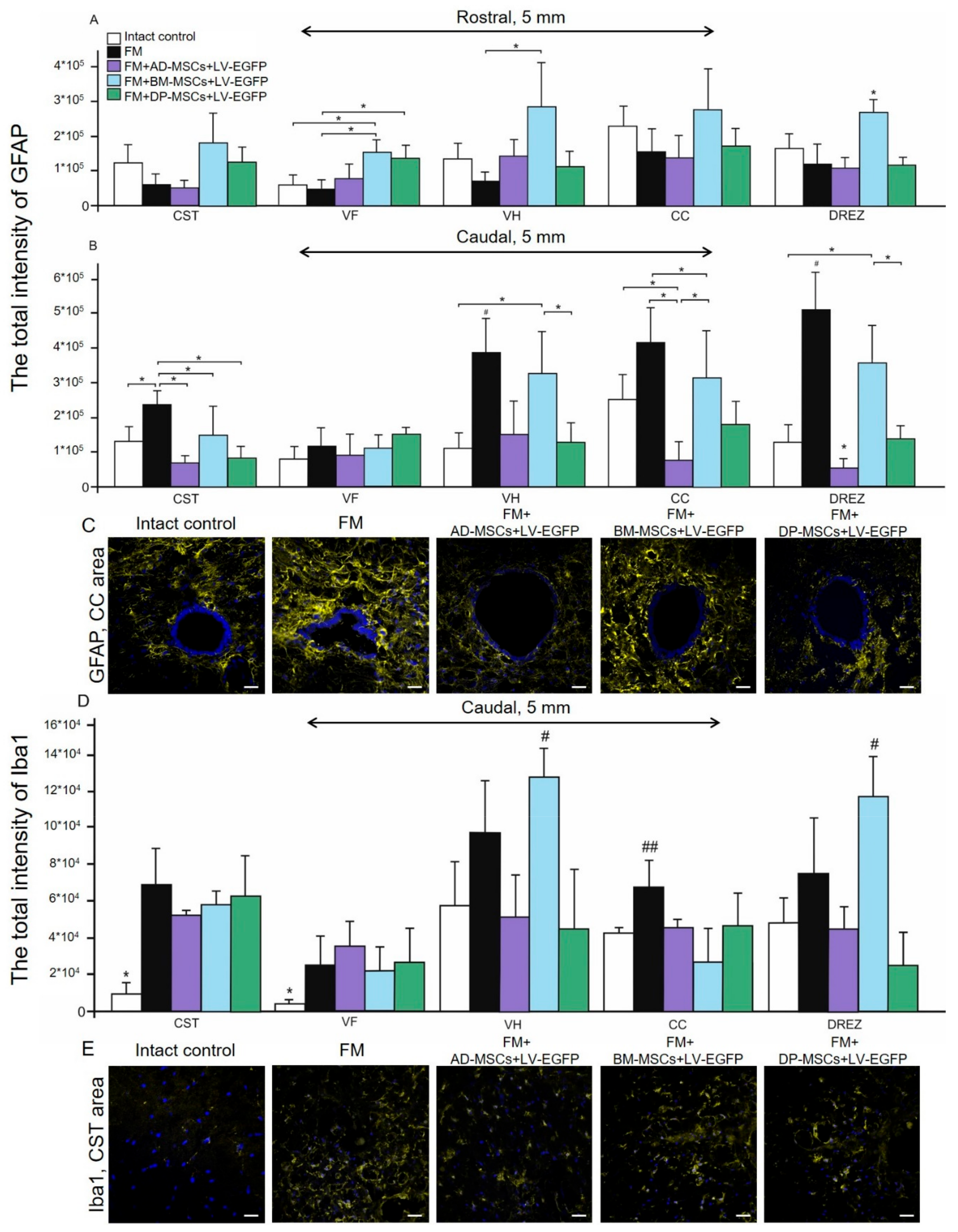
3.6. Assessment of Astroglial and Microglial Cells in the Area of SCI in Rats
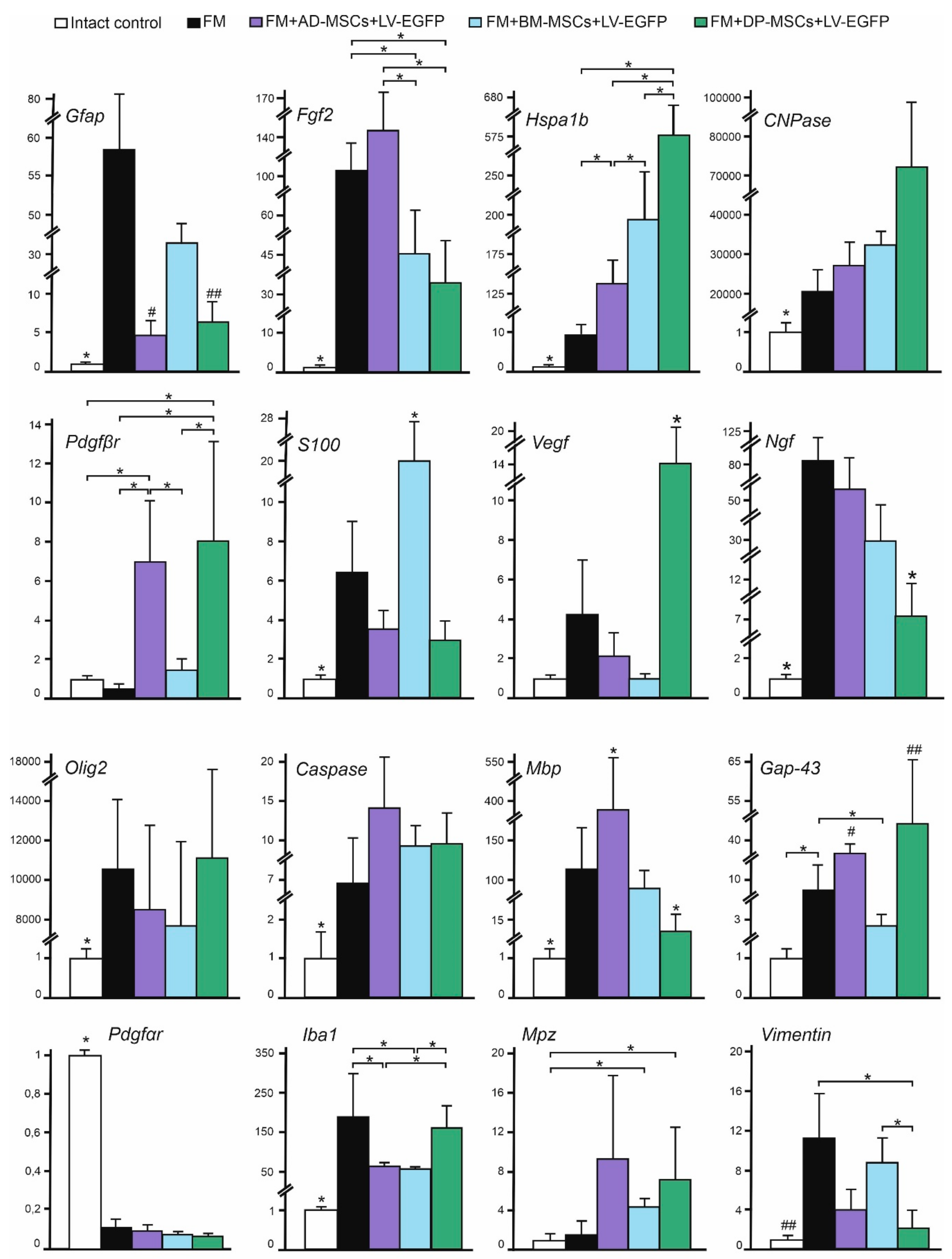
3.7. Analysis of mRNAs Expression in the Area of SCI in Rats
3.8. Summary of Results from Rodent Model
3.9. Characterization of Pig Mesenchymal Stem Cells Obtained from Adipose Tissue
3.10. MSCs Cytokine Profile in Pig
3.11. Distribution and Survival of MSCs Transplanted into the Area of SCI in Pigs
3.12. Behavioral and Electrophysiology Results in Pigs
3.13. Spinal Cord Morphometry in Pigs
3.14. Assessment of Astroglial and Microglial Cells in the Area of SCI in Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise review: MSC adhesion cascade—Insights into homing and transendothelial migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Hassan, Z.M.; Pourfathollah, A.A.; Soudi, S.; Shafiee, A.; Soleimani, M. Comparative immunomodulatory properties of adipose-derived mesenchymal stem cells conditioned media from BALB/c, C57BL/6, and DBA mouse strains. J. Cell. Biochem. 2013, 114, 955–965. [Google Scholar] [CrossRef]
- Lee, D.K.; Song, S.U. Immunomodulatory mechanisms of mesenchymal stem cells and their therapeutic applications. Cell. Immunol. 2018, 326, 68–76. [Google Scholar] [CrossRef]
- Laroni, A.; de Rosbo, N.K.; Uccelli, A. Mesenchymal stem cells for the treatment of neurological diseases: Immunoregulation beyond neuroprotection. Immunol. Lett. 2015, 168, 183–190. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Bianco, J.; De Berdt, P.; Deumens, R.; Des Rieux, A. Taking a bite out of spinal cord injury: Do dental stem cells have the teeth for it? Cell. Mol. Life Sci. 2016, 73, 1413–1437. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.R.; Kostennikov, A.A.; Zakirova, E.Y.; Galieva, L.R.; Garanina, E.E.; Rogozin, A.A.; Kiassov, A.P.; Rizvanov, A.A. Adipose-Derived Mesenchymal Stem Cell Application Combined with Fibrin Matrix Promotes Structural and Functional Recovery Following Spinal Cord Injury in Rats. Front. Pharmacol. 2018, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, V.; Tharion, G.; Kumar, S. Cell therapy augments functional recovery subsequent to spinal cord injury under experimental conditions. Stem Cells Int. 2015, 2015, 132172. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Duarte, A.S.; Longhini, A.L.F.; Pradella, F.; Farias, A.S.; Luzo, A.C.; Oliveira, A.L.; Saad, S.T.O. Neuroprotection and immunomodulation by xenografted human mesenchymal stem cells following spinal cord ventral root avulsion. Sci. Rep. 2015, 5, 16167. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jo, S.H.; Kim, W.H.; Kweon, O.K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef]
- Yalvac, M.E.; Rizvanov, A.A.; Kilic, E.; Sahin, F.; Mukhamedyarov, M.A.; Islamov, R.R.; Palotás, A. Potential role of dental stem cells in the cellular therapy of cerebral ischemia. Curr. Pharm. Des. 2009, 15, 3908–3916. [Google Scholar] [CrossRef] [PubMed]
- Yalvac, M.E.; Ramazanoglu, M.; Rizvanov, A.A.; Sahin, F.; Bayrak, O.F.; Salli, U.; Palotas, A.; Kose, G.T. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: Implications in neo-vascularization, osteo-, adipo-and neurogenesis. Pharm. J. 2010, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Nosrat, I.V.; Smith, C.A.; Mullally, P.; Olson, L.; Nosrat, C.A. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur. J. Neurosci. 2004, 19, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Yalvaç, M.E.; Yarat, A.; Mercan, D.; Rizvanov, A.A.; Palotás, A.; Şahin, F. Characterization of the secretome of human tooth germ stem cells (hTGSCs) reveals neuro-protection by fine-tuning micro-environment. Brainbehav. Immun. 2013, 32, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Minguell, J.J.; Erices, A.; Conget, P. Mesenchymal stem cells. Exp. Biol. Med. 2001, 226, 507–520. [Google Scholar] [CrossRef]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Ryu, H.H.; Kang, B.J.; Park, S.S.; Kim, Y.; Sung, G.J.; Woo, H.M.; Kim, W.H.; Kweon, O.K. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J. Vet. Med. Sci. 2012, 74, 12–65. [Google Scholar] [CrossRef]
- Heo, J.S.; Choi, Y.; Kim, H.S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Wu, Q.; Xu, C.; Liu, Q. Combination of butylphthalide with umbilical mesenchymal stem cells for the treatment of delayed encephalopathy after carbon monoxide poisoning. Medicine 2016, 95, e5412. [Google Scholar] [CrossRef]
- Panepucci, R.A.; Siufi, J.L.; Silva, W.A., Jr.; Proto-Siquiera, R.; Neder, L.; Orellana, M.; Rocha, V.; Covas, D.T.; Zago, M.A. Comparison of gene expression of umbilical cord vein and bone marrow–Derived mesenchymal stem cells. Stem Cells 2004, 22, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues, superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Jue, S.S.; Cho, Y.A.; Kim, E.C. Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. J. Neurosci. Res. 2015, 93, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Assunção-Silva, R.C.; Gomes, E.D.; Sousa, N.; Silva, N.A.; Salgado, A.J. Hydrogels and cell-based therapies in spinal cord injury regeneration. Stem Cells Int. 2015, 2015, 948040. [Google Scholar] [CrossRef] [PubMed]
- Caron, I.; Rossi, F.; Papa, S.; Aloe, R.; Sculco, M.; Mauri, E.; Sacchetti, A.; Erba, E.; Panini, N.; Parazzi, V.; et al. A new three-dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials 2016, 75, 135–147. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef]
- Sabino, L.; Maria, C.A.; Luca, L.; Valerio, V.; Edda, F.; Giacomo, R.; Gloria, I.; Juan, G.R.; Antonio, C. Engraftment, neuroglial transdifferentiation and behavioral recovery after complete spinal cord transection in rats. Surg. Neurol. Int. 2018, 9. [Google Scholar] [CrossRef]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2. [Google Scholar] [CrossRef]
- Aleksandrov, I.V.; Egorova, E.I.; Vasina, E.Y.; Novikov, V.K.; Matyko, P.G.; Galagudza, M.M. Animal experiments in the area of translational medicine. What would they be? Transl. Med. 2017, 4, 52–70. [Google Scholar]
- Mukhamedshina, Y.O.; Zakirova, E.Y.; Galieva, L.R.; Kostennikov, A.A.; Akhmetzyanova, E.R.; Rizvanov, A.A. Distribution and survival of transplanted adipose-derived mesenchymal stem cells in the spinal cord injury. BioNanoScience 2017, 7, 608–612. [Google Scholar] [CrossRef]
- Masgutova, G.; Mukhamedshina, Y.; Sergeev, M.; Shulman, I.; Ogurtsov, S.; Masgutov, R.; Rizvanov, A. Surgical Procedure for Extracting Pig Teeth for Isolation and Cultivation of Mesenchymal Stem Cells from Dental Pulp for Regenerative Therapy Applications. BioNanoScience 2017, 7, 101–105. [Google Scholar] [CrossRef]
- Lee, J.H.; Jones, C.F.; Okon, E.B.; Anderson, L.; Tigchelaar, S.; Kooner, P.; Godbey, T.; Chua, B.; Gray, G.; Hildebrandt, R.; et al. A novel porcine model of traumatic thoracic spinal cord injury. J. Neurotrauma 2013, 30, 142–159. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Gilazieva, Z.E.; Arkhipova, S.S.; Galieva, L.R.; Garanina, E.E.; Shulman, A.A.; Rizvanov, A.A. Electrophysiological, morphological, and ultrastructural features of the injured spinal cord tissue after transplantation of human umbilical cord blood mononuclear cells genetically modified with the VEGF and GDNF genes. Neural Plast. 2017, 2017, 9857918. [Google Scholar] [CrossRef]
- Benavides, F.D.; Santamaria, A.J.; Bodoukhin, N.; Guada, L.G.; Solano, J.P.; Guest, J.D. Characterization of motor and somatosensory evoked potentials in the Yucatan micropig using transcranial and epidural stimulation. J. Neurotrauma 2017, 34, 2595–2608. [Google Scholar] [CrossRef]
- Cuellar, C.A.; Mendez, A.A.; Islam, R.; Calvert, J.S.; Grahn, P.J.; Knudsen, B.; Pham, T.; Lee, K.H.; Lavrov, I.A. The role of functional neuroanatomy of the lumbar spinal cord in effect of epidural stimulation. Front. Neuroanat. 2017, 11, 82. [Google Scholar] [CrossRef]
- Navarro, X.; Verdu, E.; Butı, M. Comparison of regenerative and reinnervating capabilities of different functional types of nerve fibers. Exp. Neurol. 1994, 129, 217–224. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.O.; Garanina, E.E.; Masgutova, G.A.; Galieva, L.R.; Sanatova, E.R.; Chelyshev, Y.A.; Rizvanov, A.A. Assessment of glial scar, tissue sparing, behavioral recovery and axonal regeneration following acute transplantation of genetically modified human umbilical cord blood cells in a rat model of spinal cord contusion. PLoS ONE 2016, 11, e0151745. [Google Scholar] [CrossRef]
- Meinck, H.M. Occurrence of the H reflex and the F wave in the rat. Electroencephalogr. Clin. Neurophysiol. 1976, 41, 530–533. [Google Scholar] [CrossRef]
- Skinner, S.A.; Transfeldt, E.E. Electromyography in the detection of mechanically induced spinal motor tract injury: Observations in diverse porcine models. J. Neurosurg. Spine 2009, 11, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Shaymardanova, G.F.; Garanina, E.E.; Salafutdinov, I.I.; Rizvanov, A.A.; Islamov, R.R.; Chelyshev, Y.A. Adenoviral vector carrying glial cell-derived neurotrophic factor for direct gene therapy in comparison with human umbilical cord blood cell-mediated therapy of spinal cord injury in rat. Spinal Cord 2016, 54, 347. [Google Scholar] [CrossRef] [PubMed]
- Shaymardanova, G.F.; Mukhamedshina, Y.O.; Salafutdinov, I.I.; Rizvanov, A.A.; Chelyshev, Y.A. Usage of plasmid vector carrying Vegf and Fgf2 genes after spinal cord injury in rats. Bull. Exp. Biol. Med. 2013, 154, 544–547. [Google Scholar] [CrossRef]
- Uchida, K.; Nakajima, H.; Guerrero, A.R.; Johnson, W.E.; Masri, W.E.; Baba, H. Gene therapy strategies for the treatment of spinal cord injury. Ther. Deliv. 2014, 5, 591–607. [Google Scholar] [CrossRef]
- Galieva, L.R.; Mukhamedshina, Y.O.; Arkhipova, S.S.; Rizvanov, A.A. Human umbilical cord blood cell transplantation in neuroregenerative strategies. Front. Pharmacol. 2017, 8, 628. [Google Scholar] [CrossRef]
- Manley, N.C.; Priest, C.A.; Denham, J.; Wirth, E.D.; Lebkowski, J.S. Human embryonic stem cell-derived oligodendrocyte progenitor cells: Preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl. Med. 2017, 6, 1917–1929. [Google Scholar] [CrossRef]
- Nagoshi, N.; Okano, H. iPSC-derived neural precursor cells: Potential for cell transplantation therapy in spinal cord injury. Cell. Mol. Life Sci. 2018, 75, 989–1000. [Google Scholar] [CrossRef]
- Galieva, L.R.; James, V.; Mukhamedshina, Y.O.; Rizvanov, A.A. Therapeutic Potential of Extracellular Vesicles for the Treatment of Nerve Disorders. Front. Neurosci. 2019, 13, 163. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.O.; Gracheva, O.A.; Mukhutdinova, D.M.; Chelyshev, Y.A.; Rizvanov, A.A. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen. Res. 2019, 14, 227–237. [Google Scholar] [CrossRef]
- Xu, P.; Yang, X. The efficacy and safety of mesenchymal stem cell transplantation for spinal cord injury patients: A meta-analysis and systematic review. Cell Transpl. 2019, 28, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J. Bone Miner. Metab. 2015, 33, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Rosochacki, S.J.; Matejczyk, M. Green fluorescent protein as a molecular marker in microbiology. Acta Microbiol. Pol. 2002, 51, 205–216. [Google Scholar] [PubMed]
- Yang, J.; Wang, N.; Chen, D.; Yu, J.; Pan, Q.; Wang, D.; Liu, J.; Shi, X.; Dong, X.; Cao, H.; et al. The Impact of GFP Reporter Gene Transduction and Expression on Metabolomics of Placental Mesenchymal Stem Cells Determined by UHPLC-Q/TOF-MS. Stem Cells Int. 2017, 2017, 3167985. [Google Scholar] [CrossRef]
- Yu, H.; Fischer, G.; Ebert, A.D.; Wu, H.E.; Bai, X.; Hogan, Q.H. Analgesia for neuropathic pain by dorsal root ganglion transplantation of genetically engineered mesenchymal stem cells: Initial results. Mol. Pain 2015, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, R.S.; Bello, S.; Biering-Sørensen, F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef]
- Nakano, N.; Nakai, Y.; Seo, T.B.; Homma, T.; Yamada, Y.; Ohta, M.; Suzuki, Y.; Nakatani, T.; Fukushima, M.; Hayashibe, M.; et al. Effects of bone marrow stromal cell transplantation through CSF on the subacute and chronic spinal cord injury in rats. PLoS ONE 2013, 8, e73494. [Google Scholar] [CrossRef]
- Neirinckx, V.; Agirman, G.; Coste, C.; Marquet, A.; Dion, V.; Rogister, B.; Franzen, R.; Wislet, S. Adult bone marrow mesenchymal and neural crest stem cells are chemo attractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res. Ther. 2015, 6, 211. [Google Scholar] [CrossRef]
- Sun, G.; Li, G.; Li, D.; Huang, W.; Zhang, R.; Zhang, H.; Duan, Y.; Wang, B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 194–204. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, H. Roles of Mesenchymal Stem Cells in Spinal Cord Injury. Stem Cells Int. 2017, 2017, 5251313. [Google Scholar] [CrossRef]
- Ruppert, K.A.; Nguyen, T.T.; Prabhakara, K.S.; Toledano Furman, N.E.; Srivastava, A.K.; Harting, M.T.; Cox, C.S., Jr.; Olson, S.D. Human mesenchymal stromal cell-derived extracellular vesicles modify microglial response and improve clinical outcomes in experimental spinal cord injury. Sci. Rep. 2018, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Krupa, P.; Vackova, I.; Ruzicka, J.; Zaviskova, K.; Dubisova, J.; Koci, Z.; Turnovcova, K.; Urdzikova, L.M.; Kubinova, S.; Rehak, S.; et al. The effect of human mesenchymal stem cells derived from Wharton’s jelly in spinal cord injury treatment is dose-dependent and can be facilitated by repeated application. Int. J. Mol. Sci. 2018, 19, 1503. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, G.; Ma, F.; Yu, B.; Chen, F.; Yang, J.; Feng, J.; Wang, Q. Repeated injections of human umbilical cord blood-derived mesenchymal stem cells significantly promotes functional recovery in rabbits with spinal cord injury of two noncontinuous segments. Stem Cell Res. Ther. 2018, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Zhuravleva, M.N.; Sergeev, M.A.; Zakirova, E.Y.; Gracheva, O.A.; Mukhutdinova, D.M.; Rizvanov, A.A. Improving culture conditions, proliferation and migration of porcine mesenchymal stem cells on spinal cord concussion injury model in vitro. Unpublished work.
- Park, S.S.; Byeon, Y.E.; Ryu, H.H.; Kang, B.J.; Kim, Y.; Kim, W.H.; Kang, K.S.; Han, H.J.; Kweon, O.K. Comparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: Involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotrophin-3. Cell Transpl. 2011, 20, 1867–1880. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lee, Y.J.; Lee, S.H.; Lee, D.; Choi, K.; Kim, W.H.; Kweon, O.K.; Han, H.J. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy 2012, 14, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Byeon, Y.E.; Ryu, H.H.; Jeong, Y.H.; Lee, Y.W.; Kim, W.H.; Kang, K.S.; Kweon, O.K. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J. Vet. Sci. 2007, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Zurita, M.; Vaquero, J.; Bonilla, C.; Santos, M.; De Haro, J.; Oya, S.; Aguayo, C. Functional Recovery of Chronic Paraplegic Pigs After Autologous Transplantation of Bone Marrow Stromal Cells. Transplantation 2008, 86, 845–853. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q.; Zhang, L.; Lin, H.; Hu, J.; Li, D.; Shi, S.; Cui, S.; Zhou, J.; Ji, J.; et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS ONE 2012, 7, e43768. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef]
- Song, W.J.; Li, Q.; Ryu, M.O.; Ahn, J.O.; Ha Bhang, D.; Chan Jung, Y.; Youn, H.Y. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in mice. Sci. Rep. 2017, 7, 5187. [Google Scholar] [CrossRef]
- Chaubey, S.; Thueson, S.; Ponnalagu, D.; Alam, M.A.; Gheorghe, C.P.; Aghai, Z.; Singh, H.; Bhandari, V. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res. Ther. 2018, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Yang, L.; Cole, A.; Sun, L.; Chiang, A.C.; Fowler, S.W.; Shim, D.J.; Rodriguez-Rivera, J.; Taglialatela, G.; Jankowsky, J.L.; et al. NF kappa B-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 2015, 85, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; Singh, S.; van der Pol, S.; Kipp, M.; Lim, J.L.; Peferoen, L.; Gerritsen, W.; Kooi, E.J.; Witte, M.E.; Geurts, J.J.; et al. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J. Neuroinflamm. 2012, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Nakajima, H.; Uchida, K.; Takeura, N.; Honjoh, K.; Watanabe, S.; Kitade, M.; Kokubo, Y.; Johnson, W.E.B.; Matsumine, A. Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury. Cell Transpl. 2018, 27, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Montzka, K.; Lassonczyk, N.; Tschöke, B.; Neuss, S.; Führmann, T.; Franzen, R.; Smeets, R.; Brook, G.A.; Wöltje, M. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: Misleading marker gene expression. BMC Neurosci. 2009, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, S.V.; Krishnappa, V.; Haga, C.L.; Ortiz, L.A.; Phinney, D.G. A clinical indications prediction scale based on TWIST1 for human mesenchymal stem cells. EBioMedicine 2016, 4, 62–73. [Google Scholar] [CrossRef] [PubMed]




| Antibody | Host | Dilution | Source |
|---|---|---|---|
| Thy-1 (CD90) conjugated with РЕ/Cу5 | Mouse | 1:100 | Вiolegend |
| CD 73 | Rat | 1:100 | Вiolegend |
| CD 44 conjugated with APC/Cy7 | Rat | 1:100 | Вiolegend |
| CD 29 conjugated with РЕ | Hamster | 1:100 | Вiolegend |
| CD34 | Mouse | 1:100 | Santa Cruz |
| CD45 | Rat | 1:100 | Sony |
| GFAP | Mouse | 1:200 | Santa Cruz |
| Iba1 | Goat | 1:300 | Abcam |
| Anti-goat IgG conjugated with Alexa 555 | Donkey | 1:200 | Invitrogen |
| Anti-mouse IgG conjugated with Alexa 546 | Donkey | 1:200 | Invitrogen |
| Source of MSC | Thy-1 (CD90) | CD73 | CD44 | CD29 |
|---|---|---|---|---|
| Adipose tissue | 99.9 ± 0.17/98.6 ± 2.3 | 94 ± 0.5/87.5 ± 4.4 | 98.4 ± 2.9/98.7 ± 2.6 | 88 ± 4/80 ± 8.5 |
| Bone marrow | 98.9 ± 1.6/96.8 ± 5.8 | 91 ± 0.5/88 ± 4 | 90.6 ± 5.8/93 ± 11.3 | 59 ± 7.3/51 ± 9 * |
| Dental pulp | 91 ± 0.5/88.5 ± 1.2 *# | 87.7 ± 1.7/87 ± 8 | 74 ± 0.5/69 ± 9 *# | 49 ± 5.3/44 ± 0.5 * |
| Groups of Animals | Amplitude of MEPs (mV) | Latency of MEPs (ms) | Lack of MEPs in Both Legs (in % of Rats) | Registration of MEPs from One Leg (in No. of Rats) |
|---|---|---|---|---|
| Intact control | 17.66 ± 5.30 | 5.81 ± 0.63 | - | - |
| FM | 0.5 ± 0.36 | 15.83 ± 1.14 | 44% | 1 |
| FM + AD-MSCs + LV-EGFP | 1.57 ± 0.88 | 14.08 ± 3.83 | 20% | 1 |
| FM + BM-MSCs + LV-EGFP | 1.68 ± 0.7 | 14.77 ± 3.2 | 32% | - |
| FM + DP-MSCs + LV-EGFP | 1.01 ± 0.75 | 12.85 ± 3.10 | 32% | - |
| Cytokine/ Chemokine | Pig 1 | Pig 2 | Pig 3 | Pig 4 | Pig 5 |
|---|---|---|---|---|---|
| GM-CSF | <0.009/<0.009 | <0.009/<0.009 | <0.009/0.01 ± 0.0005 | <0.009/<0.009 | <0.009/<0.009 |
| IFN-g | <0.12/<0.12 | <0.12/<0.12 | <0.12/<0.12 | 1.4 ± 0.1 */1.33 ± 0.15 * | 1.2 ± 0.05 */<0.12 |
| IL-1a | 0.008 ± 0/0.009 ± 0.0005 | 0.008 ± 0/0.008 ± 0 | 0.009 ± 0/0.008 ± 0 | 0.009 ± 0.0005/0.008 ± 0 | 0.008 ± 0/0.008 ± 0 |
| IL-1b | 0.01 ± 0/0.01 ± 0 | 0.01 ± 0.0005/0.01 ± 0 | 0.01 ± 0/0.009 ± 0.001 | 0.009 ± 0/0.01 ± 0 | 0.009 ± 0.0005/0.01 ± 0 |
| IL-1Ra | <0.03/<0.03 | <0.03/<0.03 | <0.03/<0.03 | <0.03/<0.03 | <0.03/<0.03 |
| IL-2 | <0.01/<0.01 | <0.01/<0.01 | <0.01/<0.01 | <0.01/<0.01 | <0.01/<0.01 |
| IL-4 | <0.05/<0.05 | <0.05/<0.05 | <0.05/0.05 ± 0.005 | <0.05/0.03 ± 0.005 | <0.05/<0.05 |
| IL-6 | 2.77 ± 0.1 */2.5 ± 0.05 * | 0.15 ± 0 */0.15 ± 0.01 * | 9.21 ± 0.3 */9.4 ± 0.2 * | 0.48 ± 0.01 */0.49 ± 0 * | 0.57 ± 0.05 */0.62 ± 0 * |
| IL-8 | 21.05 ± 0.5 */20.5 ± 0.8 * | 0.21 ± 0.01 */0.22 ± 0 * | 14.55 ± 0.3 */15 ± 0.1 * | 4.56 ± 0.2 */4.6 ± 0.05 * | 3.02 ± 0.05 */3.2 ± 0.1 * |
| IL-10 | <0.009/<0.009 | <0.009/<0.009 | <0.009/<0.009 | 0.01 ± 0.0005/<0.009 | 0.01 ± 0.0005/<0.009 |
| IL-12 | <0.03/<0.03 | <0.03/<0.03 | <0.03/0.03 ± 0.005 | <0.03/0.03 ± 0.005 | <0.03/<0.03 |
| IL-18 | 0.1 ± 0 */0.1 ± 0 * | 0.08 ± 0.001/0.09 ± 0 | 0.09 ± 0.001/0.09 ± 0 | 0.17 ± 0.02 */0.2 ± 0 * | 0.35 ± 0 */0.4 ± 0.05 * |
| TNF-a | 0.01 ± 0/0.01 ± 0 | 0.01 ± 0.0005/0.01 ± 0 | 0.01 ± 0/0.01 ± 0.005 | 0.01 ± 0/0.01 ± 0.005 | 0.01 ± 0.0005/0.01 ± 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhamedshina, Y.; Shulman, I.; Ogurcov, S.; Kostennikov, A.; Zakirova, E.; Akhmetzyanova, E.; Rogozhin, A.; Masgutova, G.; James, V.; Masgutov, R.; et al. Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models. Biomolecules 2019, 9, 811. https://doi.org/10.3390/biom9120811
Mukhamedshina Y, Shulman I, Ogurcov S, Kostennikov A, Zakirova E, Akhmetzyanova E, Rogozhin A, Masgutova G, James V, Masgutov R, et al. Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models. Biomolecules. 2019; 9(12):811. https://doi.org/10.3390/biom9120811
Chicago/Turabian StyleMukhamedshina, Yana, Iliya Shulman, Sergei Ogurcov, Alexander Kostennikov, Elena Zakirova, Elvira Akhmetzyanova, Alexander Rogozhin, Galina Masgutova, Victoria James, Ruslan Masgutov, and et al. 2019. "Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models" Biomolecules 9, no. 12: 811. https://doi.org/10.3390/biom9120811
APA StyleMukhamedshina, Y., Shulman, I., Ogurcov, S., Kostennikov, A., Zakirova, E., Akhmetzyanova, E., Rogozhin, A., Masgutova, G., James, V., Masgutov, R., Lavrov, I., & Rizvanov, A. (2019). Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models. Biomolecules, 9(12), 811. https://doi.org/10.3390/biom9120811








