The Kiwifruit Allergen Act d 1 Activates NF-κB Signaling and Affects mRNA Expression of TJ Proteins and Innate Pro-Allergenic Cytokines
Abstract
1. Introduction
2. Materials and Methods
2.1. Allergen Preparation
2.2. Animals
2.3. The Dosage Regimen of Act d 1 and Permeability Assay
2.4. Isolation of RNA and qRT-PCR of Mouse Intestinal Samples
2.5. HEK293 Cell Culture, RNA Isolation, and Gene Expression Analysis
2.6. ELISA Detection of TSLP
2.7. NF-ĸB-GFP Reporter Assay
2.8. Statistical Analysis
3. Results
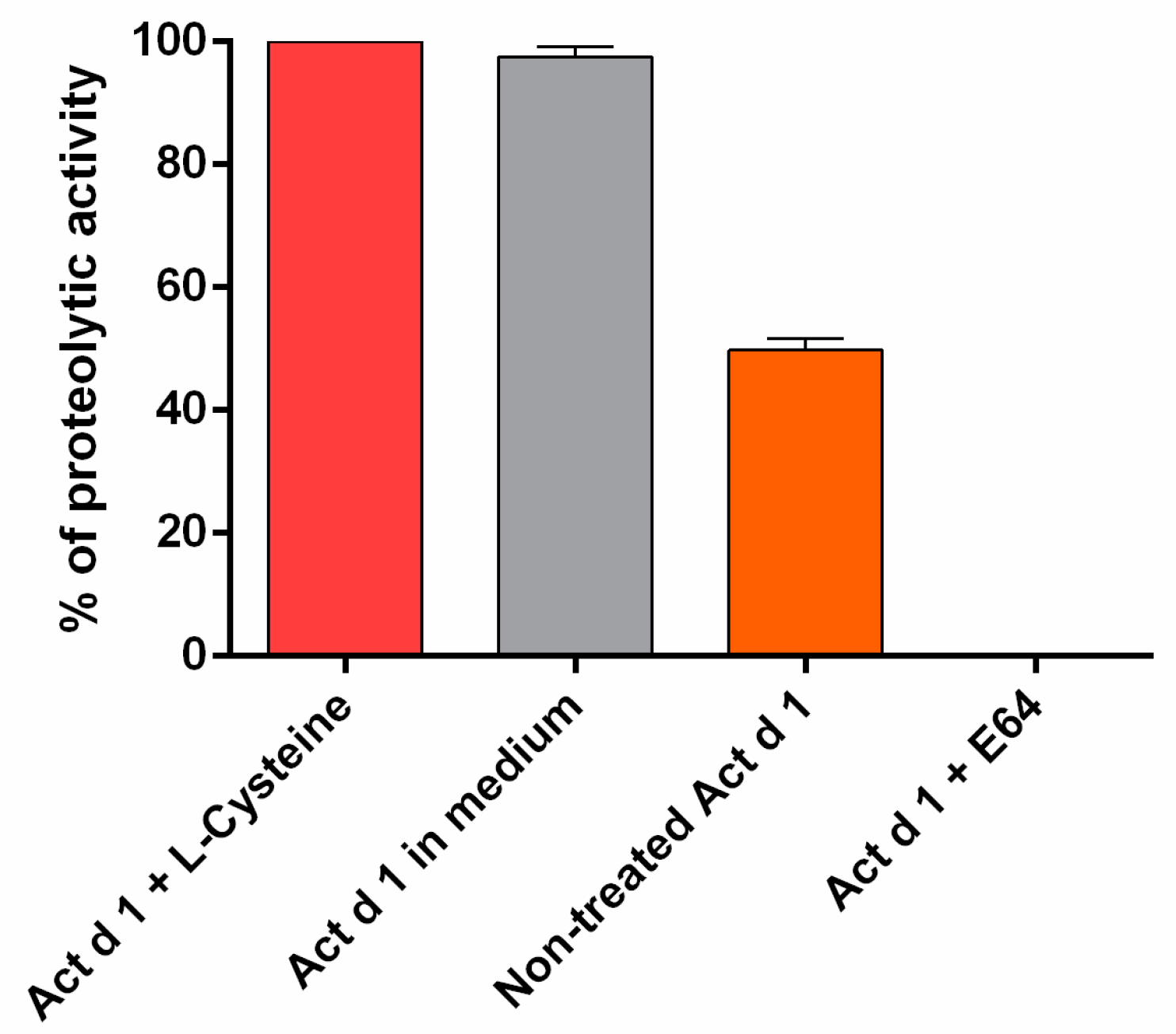
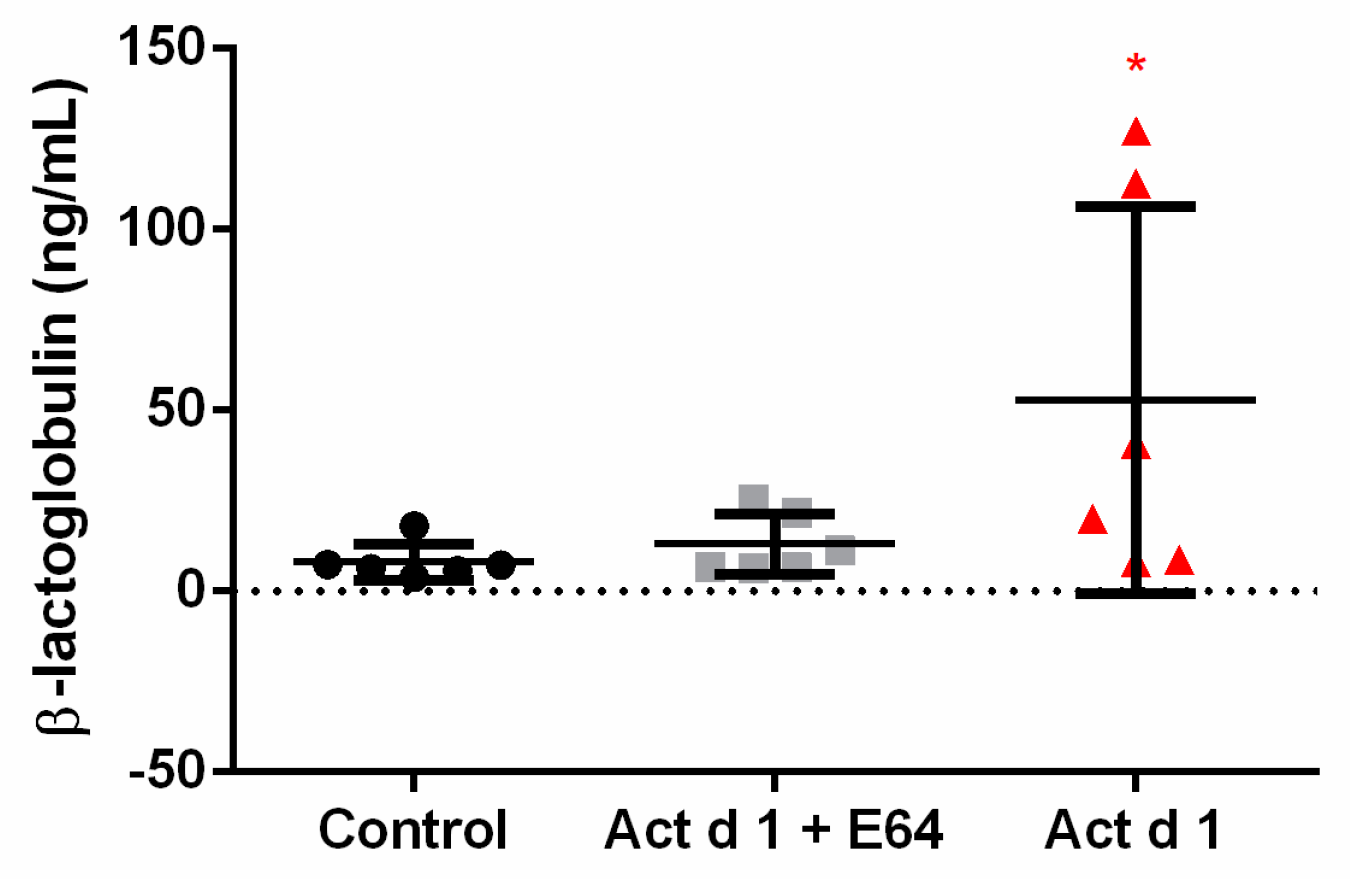
3.1. In Vivo Exposure to Act d 1 Leads to Increased Intestinal Permeability in C57BL/6 Mice
3.2. Act d 1 Differentially Upregulates mRNA Expression of TJ Proteins in Distinct Segments of Mouse Intestine
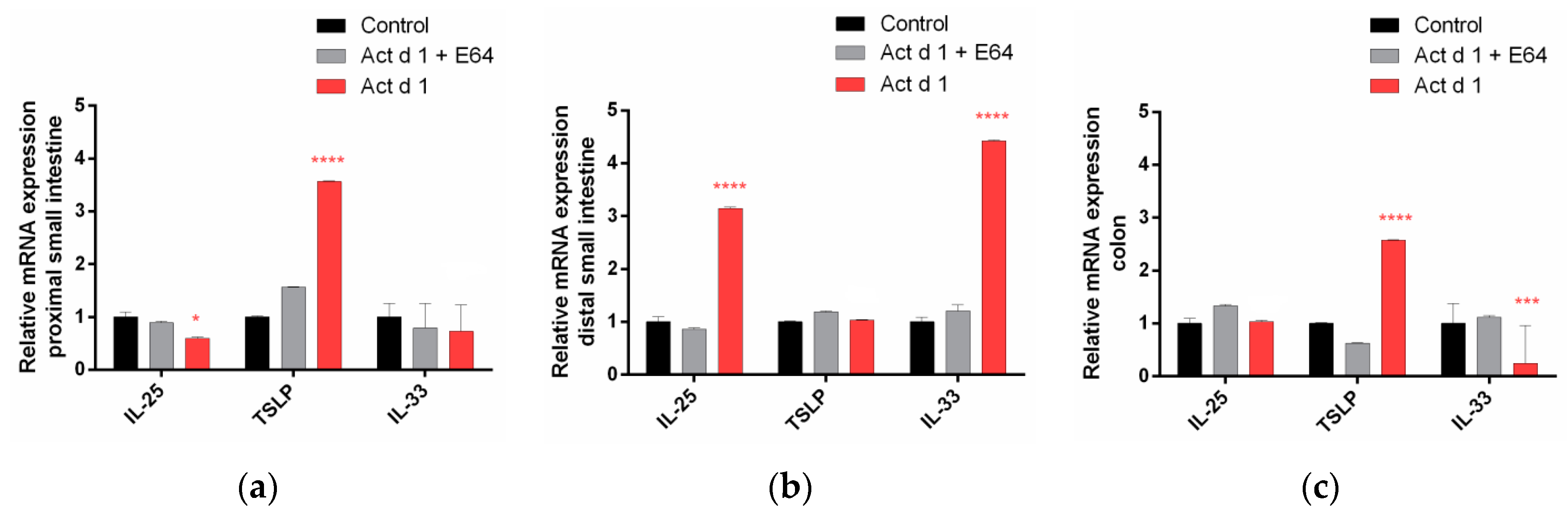
3.3. Act d 1 Induces Upregulation of mRNA for the Innate Pro-Allergenic Cytokines in Vivo
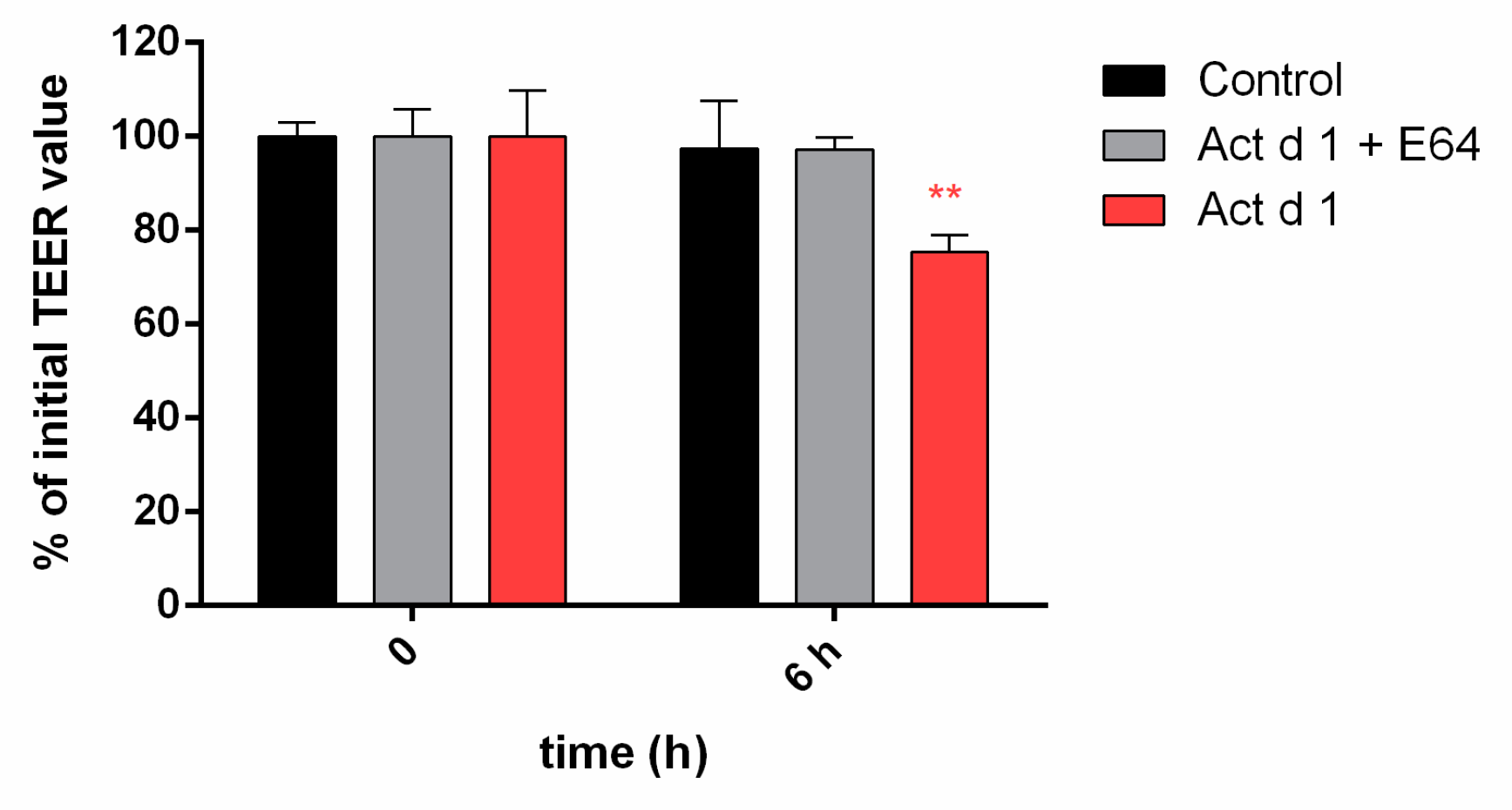
3.4. Act d 1 Affects HEK293 Monolayer Transepithelial Electrical Resistance
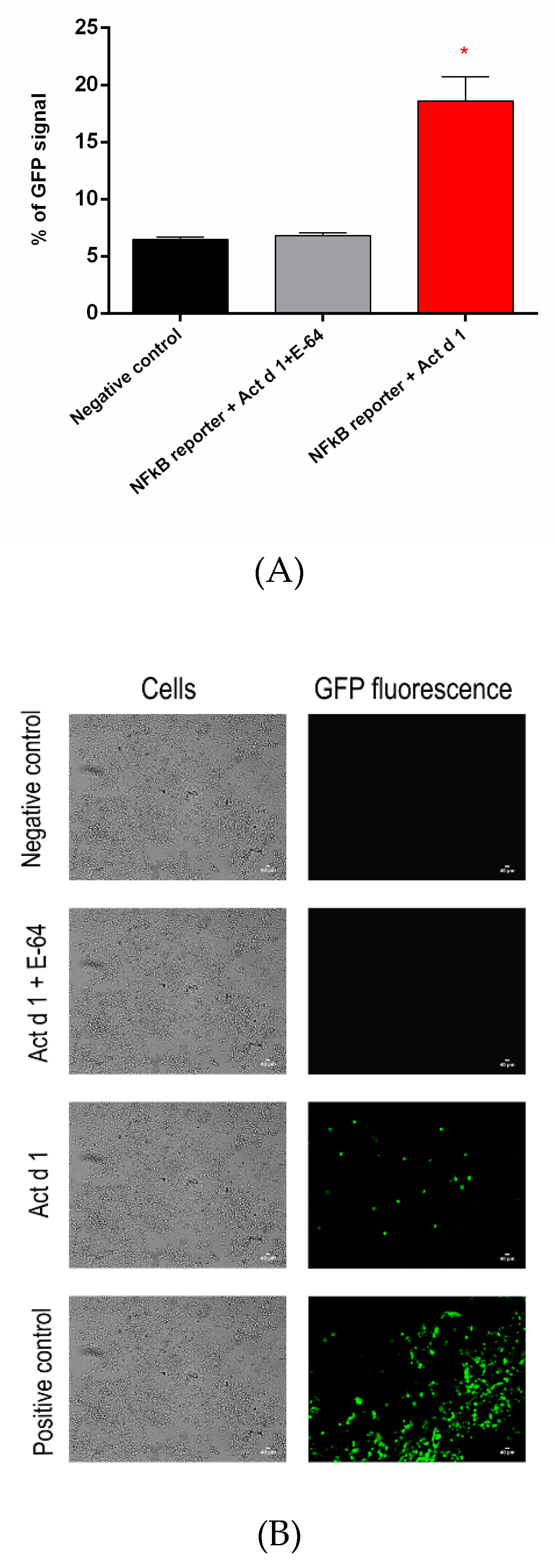
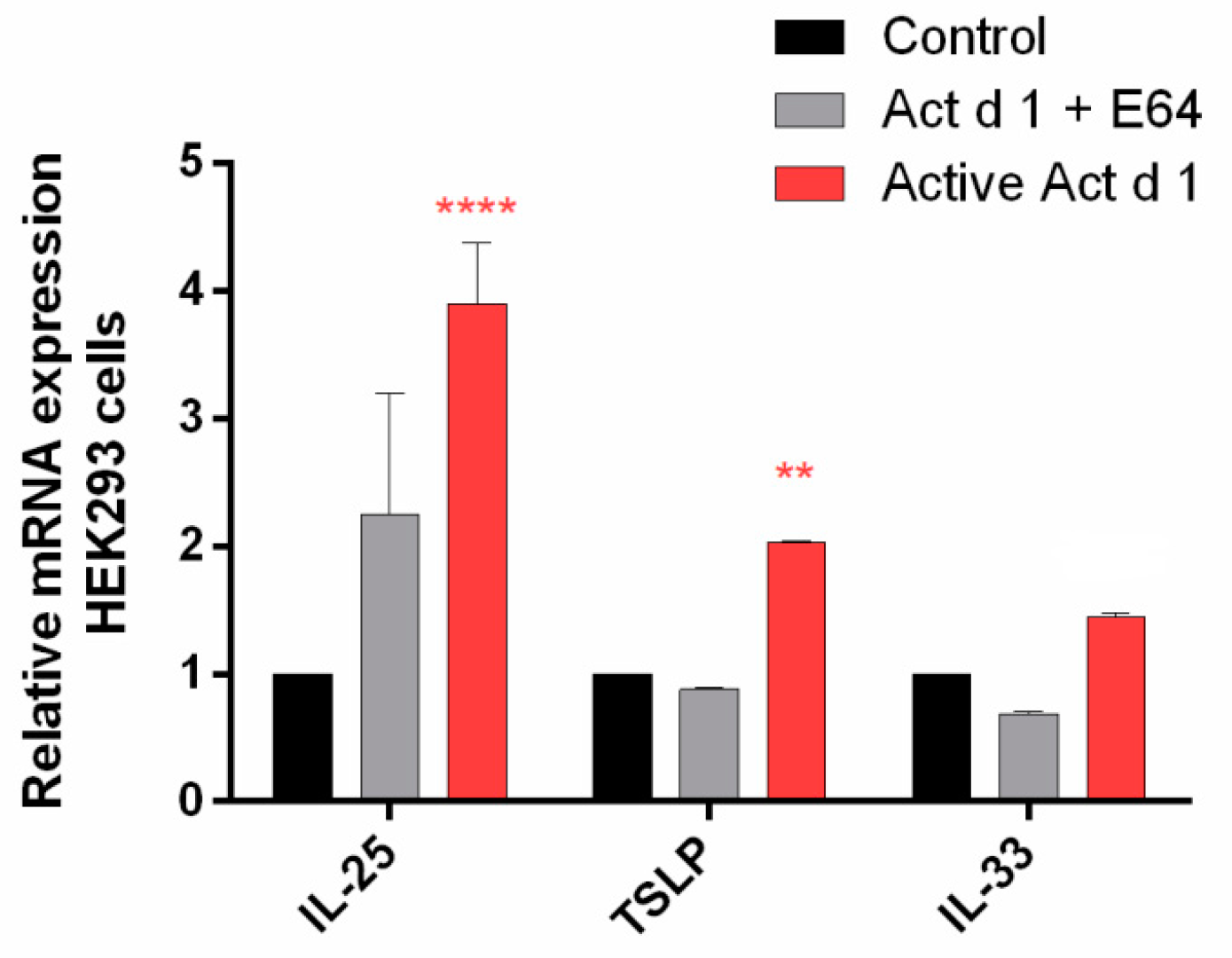
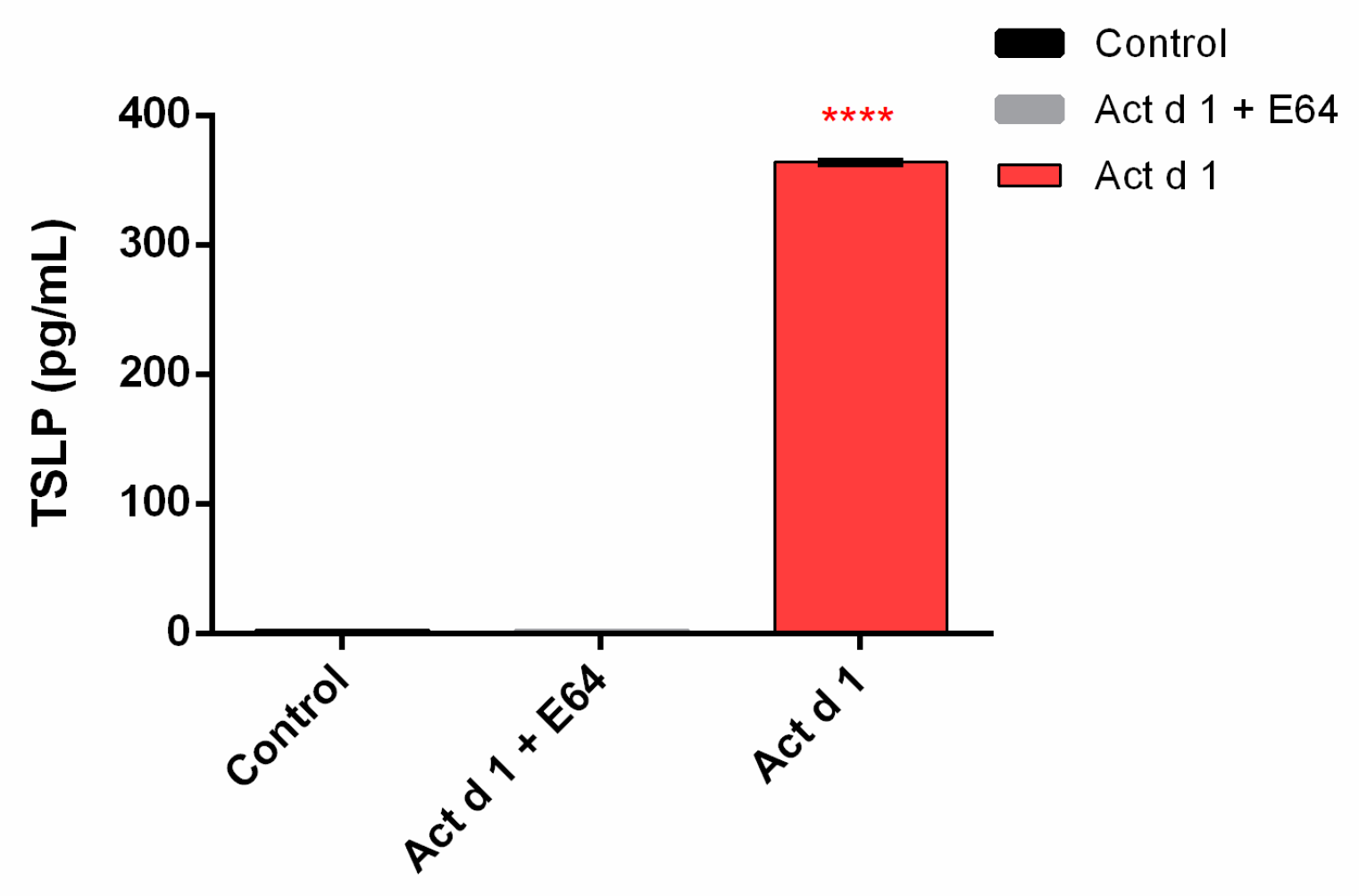
3.5. Act d 1 Activates the NF-ĸB Signaling Pathway and Upregulates the Innate Pro-Allergenic Cytokines In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Prim. 2018, 4, 17098. [Google Scholar] [CrossRef]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Pasparakis, M. Role of NF-κB in epithelial biology. Immunol. Rev. 2012, 246, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Food allergy and gastrointestinal disease Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25 and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Berin, M.C.; Sampson, H.A. Immunology of Food Allergy. Immunity 2017, 4747, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Wambre, E.; Bajzik, V.; DeLong, J.H.; O’Brien, K.; Nguyen, Q.A.; Speake, C.; Gersuk, V.H.; DeBerg, H.A.; Whalen, E.; Ni, C.; et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci. Transl. Med. 2017, 9, 9171. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell. Biol. 2016, 17, 564. [Google Scholar] [CrossRef]
- Zihni, C.; Balda, M.S.; Matter, K. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J. Cell. Sci. 2014, 127, 3401–3413. [Google Scholar] [CrossRef]
- Tong, L.; Tergaonkar, V. Rho protein GTPases and their interactions with NFkB: Crossroads of inflammation and matrix biology. Biosci. Rep. 2014, 34, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Wullaert, A. Role of NF-κB activation in intestinal immune homeostasis. Int. J. Med. Microbiol. 2010, 300, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.E.; Hoffman, S.M.; Lahue, K.G.; Nolin, J.D.; Anathy, V.; Lundblad, L.K.; Daphtary, N.; Aliyeva, M.; Black, K.E.; Dixon, A.E.; et al. Epithelial NF-κB Orchestrates House Dust Mite–Induced Airway Inflammation, Hyperresponsiveness, and Fibrotic Remodeling. J. Immunol. 2013, 191, 5811–5821. [Google Scholar] [CrossRef] [PubMed]
- Bol-Schoenmakers, M.; Braber, S.; Akbari, P.; De Graaff, P.; Van Roest, M.; Kruijssen, L.; Smit, J.J.; Van Esch, B.C.A.M.; Jeurink, P.V.; Garssen, J.; et al. The mycotoxin deoxynivalenol facilitates allergic sensitization to whey in mice. Mucosal. Immunol. 2016, 9, 1477–1486. [Google Scholar] [CrossRef]
- Fine, A.J. Hypersensitivity reaction to kiwi fruit (Chinese gooseberry, Actinidia chinensis). J. Allergy Clin. Immunol. 1981, 68, 235–237. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Pravettoni, V.; Ispano, M.; Farioli, L.; Ansaloni, R.; Rotondo, F.; Incorvaia, C.; Åsman, I.; Bengtsson, A.; Ortolani, C. Identification of the allergenic components of kiwi fruit and evaluation of their crossreactivity with timothy and birch pollens. J. Allergy Clin. Immunol. 1996, 98, 601–610. [Google Scholar] [CrossRef]
- Moreno Álvarez, A.; Sexto, L.; Bardina, L.; Grishina, G.; Sampson, H. Kiwifruit Allergy in Children: Characterization of Main Allergens and Patterns of Recognition. Children 2015, 2, 424–438. [Google Scholar] [CrossRef]
- Cavic, M.; Grozdanovic, M.M.; Bajic, A.; Jankovic, R.; Andjus, P.R.; Gavrovic-Jankulovic, M. The effect of kiwifruit (Actinidia deliciosa) cysteine protease actinidin on the occludin tight junction network in T84 intestinal epithelial cells. Food Chem. Toxicol. 2014, 72, 61–68. [Google Scholar] [CrossRef]
- Grozdanovic, M.M.; Čavić, M.; Nešić, A.; Andjelković, U.; Akbari, P.; Smit, J.J.; Gavrović-Jankulović, M. Kiwifruit cysteine protease actinidin compromises the intestinal barrier by disrupting tight junctions. Biochim. Biophys. Acta(BBA)-Gen. Subj. 2016, 1860, 516–526. [Google Scholar] [CrossRef]
- Drapeau, G.R. Protease from Staphyloccus aureus. Methods Enzymol. 1976, 45, 469–475. [Google Scholar] [CrossRef]
- Nešić, A.; Stam, A.; Čavić, M.; Ten Klooster, J.P.; Pieters, R.; Smit, J.; Gavrović-Jankulović, M. Activation of epithelial cells by the major kiwifruit allergen Act d 1 in human and mouse-derived intestinal model. J. Funct. Foods 2019, 62, 103556. [Google Scholar] [CrossRef]
- Nikolić, J.; Nešić, A.; Čavić, M.; Đorđević, N.; Anđelković, U.; Atanasković-Marković, M.; Drakulić, B.; Gavrović-Jankulović, M. Effect of malondialdehyde on the ovalbumin structure and its interactions with T84 epithelial cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Klems, M.; Untersmayr, E. The role of gastrointestinal permeability in food allergy. Ann. Allergy Asthma. Immunol. 2018, 121, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Stremnitzer, C.; Manzano-Szalai, K.; Willensdorfer, A.; Starkl, P.; Pieper, M.; König, P.; Mildner, M.; Tschachler, E.; Reichart, U.; Jensen-Jarolim, E. Papain degrades tight junction proteins of human keratinocytes in vitro and sensitizes C57BL/6 mice via the skin independent of its enzymatic activity or TLR4 activation. J. Investig. Dermatol. 2015, 135, 1790–1800. [Google Scholar] [CrossRef]
- Grozdanovic, M.; Ostojic, S.; Aleksic, I.; Andjelkovic, U.; Petersen, A.; Gavrovic-Jankulovic, M. Active actinidin retains function upon gastro-intestinal digestion and is more thermostable than the E-64-inhibited counterpart. J. Sci. Food Agric. 2014, 94, 3046–3052. [Google Scholar] [CrossRef]
- Salazar, F.; Ghaemmaghami, A.M. Allergen recognition by innate immune cells: Critical role of dendritic and epithelial cells. Front. Immunol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Yang, G.; Geng, X.R.; Cao, Y.; Li, N.; Ma, L.; Chen, S.; Yang, P.C.; Liu, Z. Microbial Products Induce Claudin-2 to Compromise Gut Epithelial Barrier Function. PLoS ONE 2013, 8, e68547. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Y.-H. Claudin Distributions and Functions in Intestines. Tissue Barriers 2013, 13, 1–14. [Google Scholar] [CrossRef]
- Shigetomi, K.; Ikenouchi, J. Regulation of the epithelial barrier by post-translational modifications of tight junction membrane proteins. J. Biochem. 2018, 163, 265–272. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. Pores in the wall: Claudins constitute tight junction strands containing aqueous pores. J. Cell. Biol. 2000, 149, 13–16. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Claudins and Epithelial Paracellular Transport. Annu. Rev. Physiol. 2005, 68, 403–429. [Google Scholar] [CrossRef] [PubMed]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Gremmels, H.; Koelink, P.J.; Verheijden, K.A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014, 28, 2414–2429. [Google Scholar] [CrossRef] [PubMed]
- Pizzuti, D.; Senzolo, M.; Buda, A.; Chiarelli, S.; Giacomelli, L.; Mazzon, E.; Curioni, A.; Faggian, D.; De Lazzari, F. In vitro model for IgE mediated food allergy. Scand. J. Gastroenterol. 2011, 46, 177–187. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. Allergens and the airway epithelium response: Gateway to allergic sensitization. J. Allergy Clin. Immunol. 2014, 134, 499–507. [Google Scholar] [CrossRef]
- Yu, H.S.; Angkasekwinai, P.; Chang, S.H.; Chung, Y.; Dong, C. Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J. Korean Med. Sci. 2010, 25, 829–834. [Google Scholar] [CrossRef] [PubMed]

| Target Gene (Reference) | Murine Primer Sequence (5′–3′) | Tm (°C) |
|---|---|---|
| CLDN1 (NM_016674.4) | F: TCTACGAGGGACTGTGGATG | 57 |
| R: TCAGATTCAGCTAGGAGTCG | ||
| CLDN2 (NM_016675.4) | F: GGCTGTTAGGCTCATCCAT | 55 |
| R: TGGCACCAACATAGGAACTC | ||
| CLDN3 (NM_009902.4) | F: AAGCCGAATGGACAAAGAA | 58.7 |
| R: CTGGCAAGTAGCTGCAGTG | ||
| CLDN4 (NM_009903.2) | F: CGCTACTCTTGCCATTACG | 55 |
| R: CTGGCAAGTAGCTGCAGTG | ||
| OCLN (NM_008756.2) | F: ATGTCCGGCCGATGCTCTC | 61.2 |
| R: TTTGGCTGCTCTTGGGTCTGTAT | ||
| ZO1 (NM_009386.2) | F: CGAGGCATCATCCCAAATAAGAAC | 58.7 |
| R: TCCAGAAGTCTGCCCGATCAC | ||
| E-cadherin (NM_009864.2) | F: ACTGTGAAGGGACGGTCAAC | 64.3 |
| R: GGAGCAGCAGGATCAGAATC | ||
| IL-33 (NM_001164724.1) | F: GGTGTGGATGGGAAGAAGCTG | 61 |
| R: GAGGACTTTTTGTGAAGGACG | ||
| TSLP (NM_021367.2) | F: CGGATGGGGCTAACTTACA | 61 |
| R: TCCTCGATTTGCTCGAACTT | ||
| IL-25 (NM_080729.3) | F: CAGCAAAGAGCAAGAACC | 61 |
| R: CCCTGTCCAACTCATAGC | ||
| ACTB (NM_007393.3) | F: ATGCTCCCCGGGCTGTAT | 61 |
| R: CATAGGAGTCCTTCTGACCCATTC |
| Target Gene (Reference) | Human Primer Sequence (5′–3′) | Tm (°C) |
|---|---|---|
| TSLP (NM_009903.2) | F: CGCTACTCTTGCCATTACG | 55 |
| R: ACTCAGCACACCATGACTTG | ||
| IL-25 (NM_022789.3) | F: CCAGGTGGTTGCATTCTTGG | 49 |
| R: TGGCTGTAGGTGTGGGTTCC | ||
| IL-33 (NM_033439.3) | F: CACCCCTCAAATGAATCAGG | 51 |
| R: GGAGCTCCACAGAGTGTTCC | ||
| GAPDH (NM_009386.2) | F: CGAGGCATCATCCCAAATAAGAAC | 58.7 |
| R: TCCAGAAGTCTGCCCGATCAC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nešić, A.; Čavić, M.; Popović, M.; Zlatanova, M.; Pieters, R.; Smit, J.; Gavrović-Jankulović, M. The Kiwifruit Allergen Act d 1 Activates NF-κB Signaling and Affects mRNA Expression of TJ Proteins and Innate Pro-Allergenic Cytokines. Biomolecules 2019, 9, 816. https://doi.org/10.3390/biom9120816
Nešić A, Čavić M, Popović M, Zlatanova M, Pieters R, Smit J, Gavrović-Jankulović M. The Kiwifruit Allergen Act d 1 Activates NF-κB Signaling and Affects mRNA Expression of TJ Proteins and Innate Pro-Allergenic Cytokines. Biomolecules. 2019; 9(12):816. https://doi.org/10.3390/biom9120816
Chicago/Turabian StyleNešić, Andrijana, Milena Čavić, Milica Popović, Milena Zlatanova, Raymond Pieters, Joost Smit, and Marija Gavrović-Jankulović. 2019. "The Kiwifruit Allergen Act d 1 Activates NF-κB Signaling and Affects mRNA Expression of TJ Proteins and Innate Pro-Allergenic Cytokines" Biomolecules 9, no. 12: 816. https://doi.org/10.3390/biom9120816
APA StyleNešić, A., Čavić, M., Popović, M., Zlatanova, M., Pieters, R., Smit, J., & Gavrović-Jankulović, M. (2019). The Kiwifruit Allergen Act d 1 Activates NF-κB Signaling and Affects mRNA Expression of TJ Proteins and Innate Pro-Allergenic Cytokines. Biomolecules, 9(12), 816. https://doi.org/10.3390/biom9120816






