Retinoic Acid Signaling Is Associated with Cell Proliferation, Muscle Cell Dedifferentiation, and Overall Rudiment Size during Intestinal Regeneration in the Sea Cucumber, Holothuria glaberrima
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection, Maintenance and Evisceration of H. glaberrima Specimens
2.2. Sequence Analyses: Bioinformatics and Phylogeny
2.3. Gene Expression Profile
2.4. Drug Treatments
2.5. Histology: Tissue Fixing, Sectioning, and Labeling
2.6. Statistical Analysis
3. Results
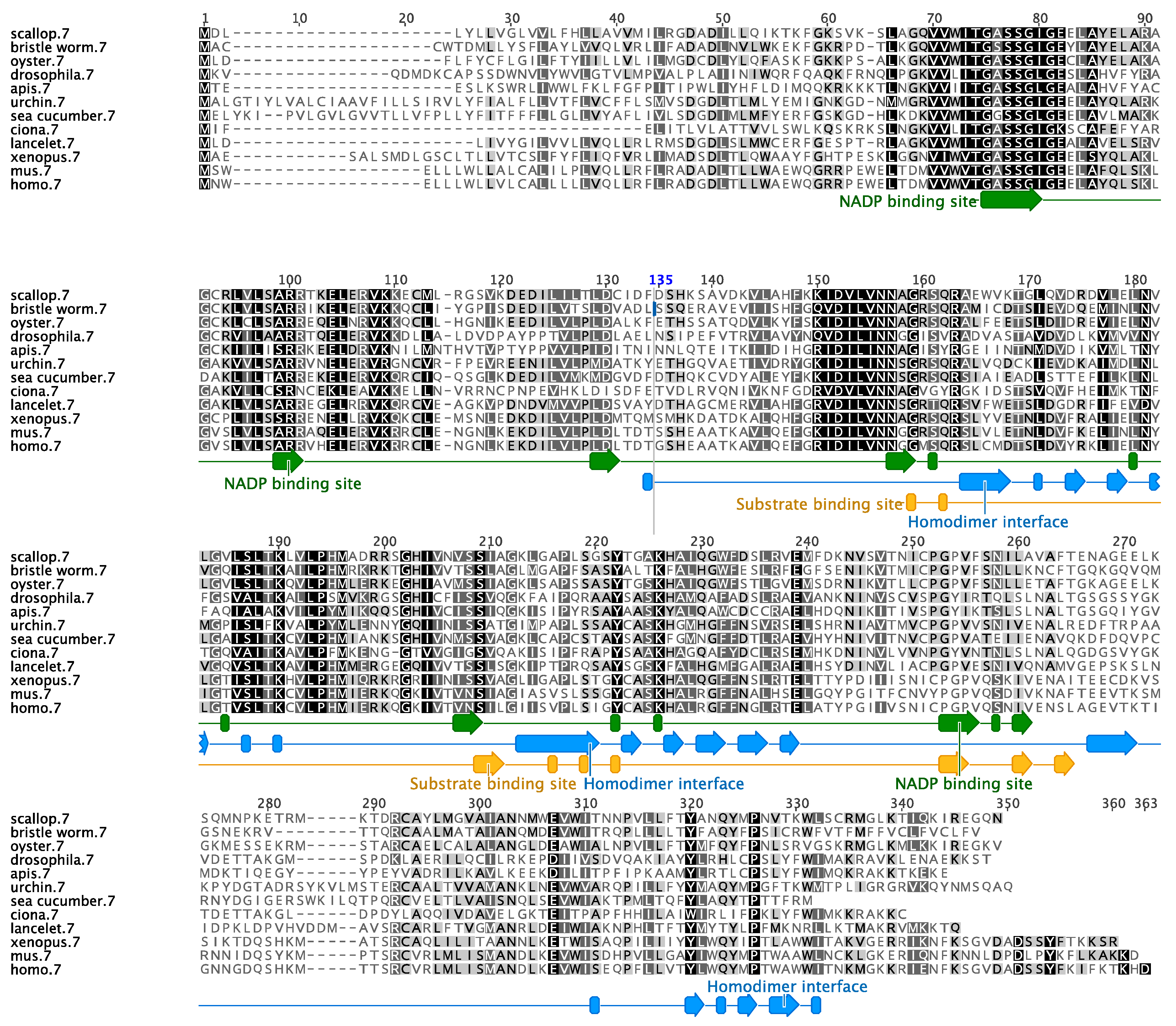
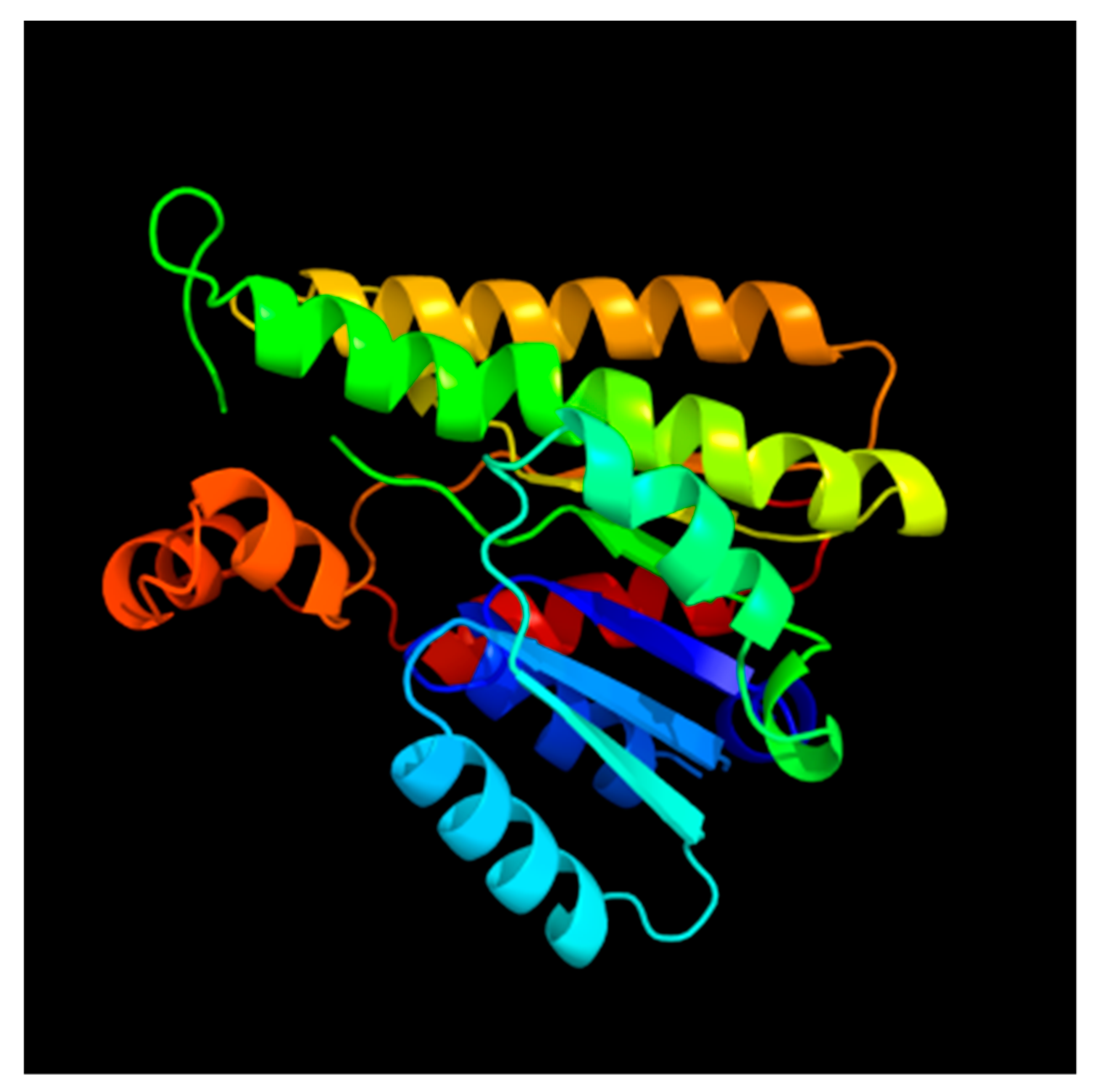
3.1. Sequence Characterization of Two Alcohol Dehydrogenases in the Sea Cucumber
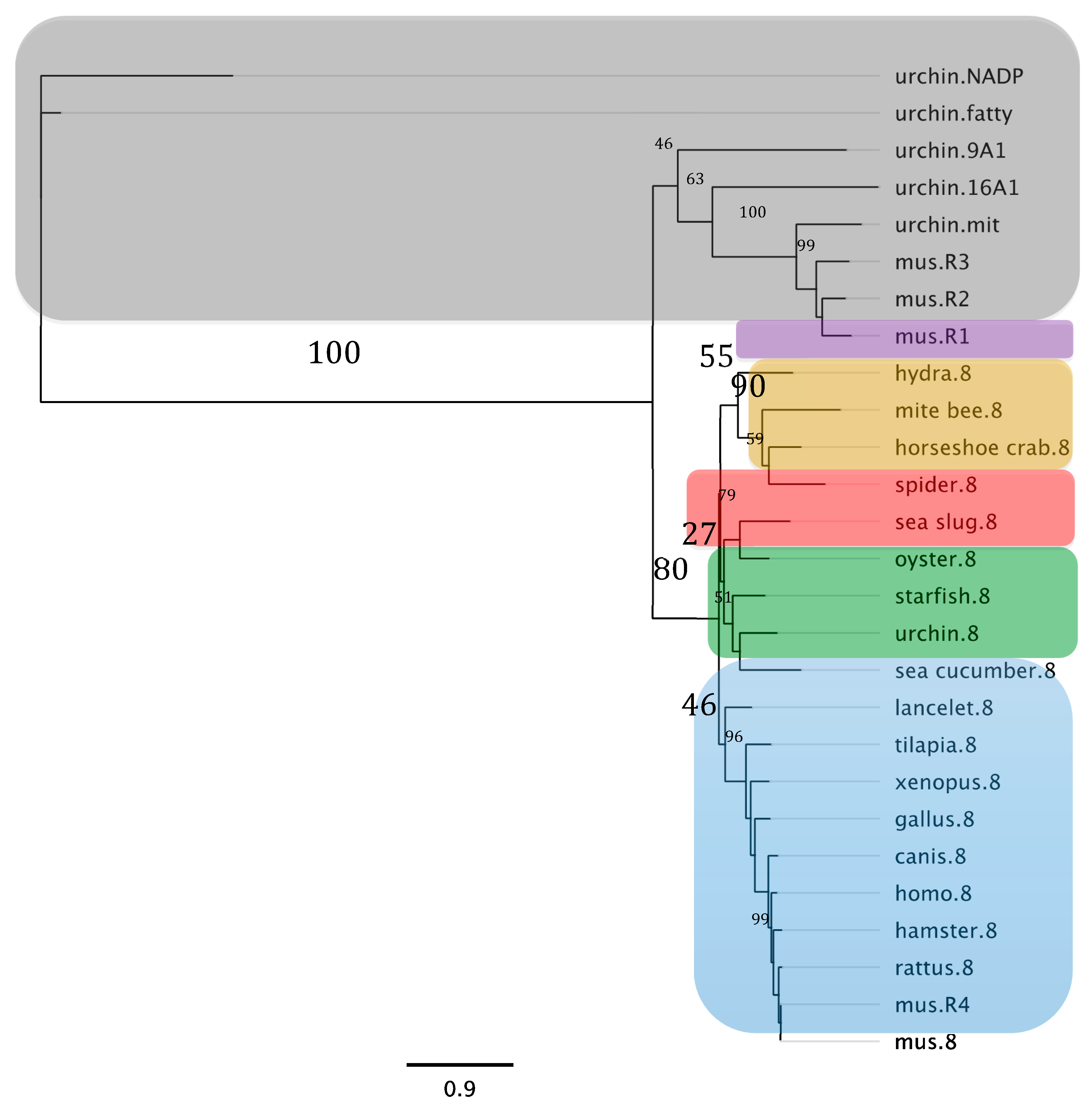
3.2. Phylogenetic Analysis of the Sea Cucumber’s SDR7 and ALDH8A1 Display Ambulacral Ancestry
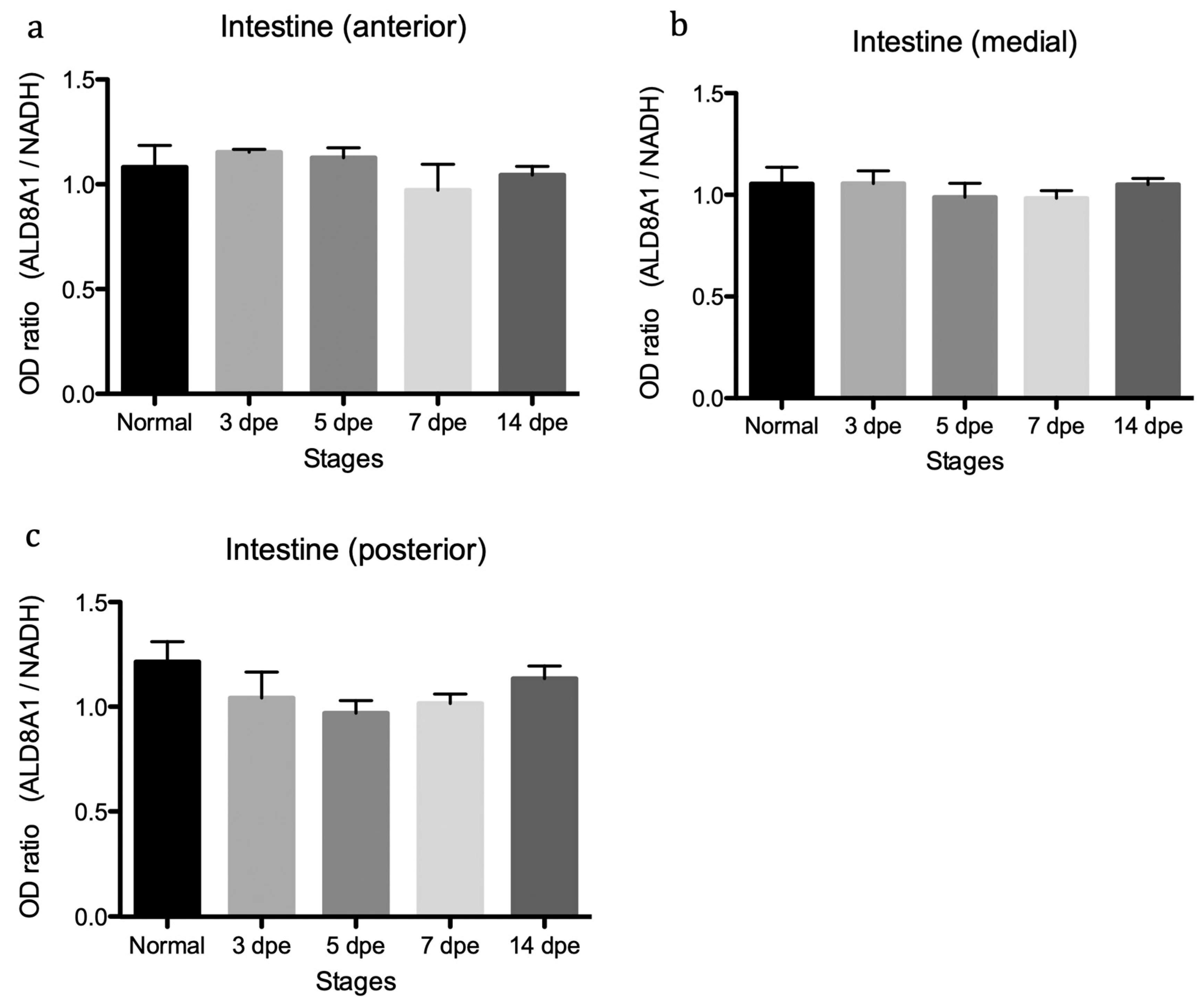
3.3. Quantitative Assessment of Transcript Abundance Showed Regulation of SDR7 during Intestinal Regeneration
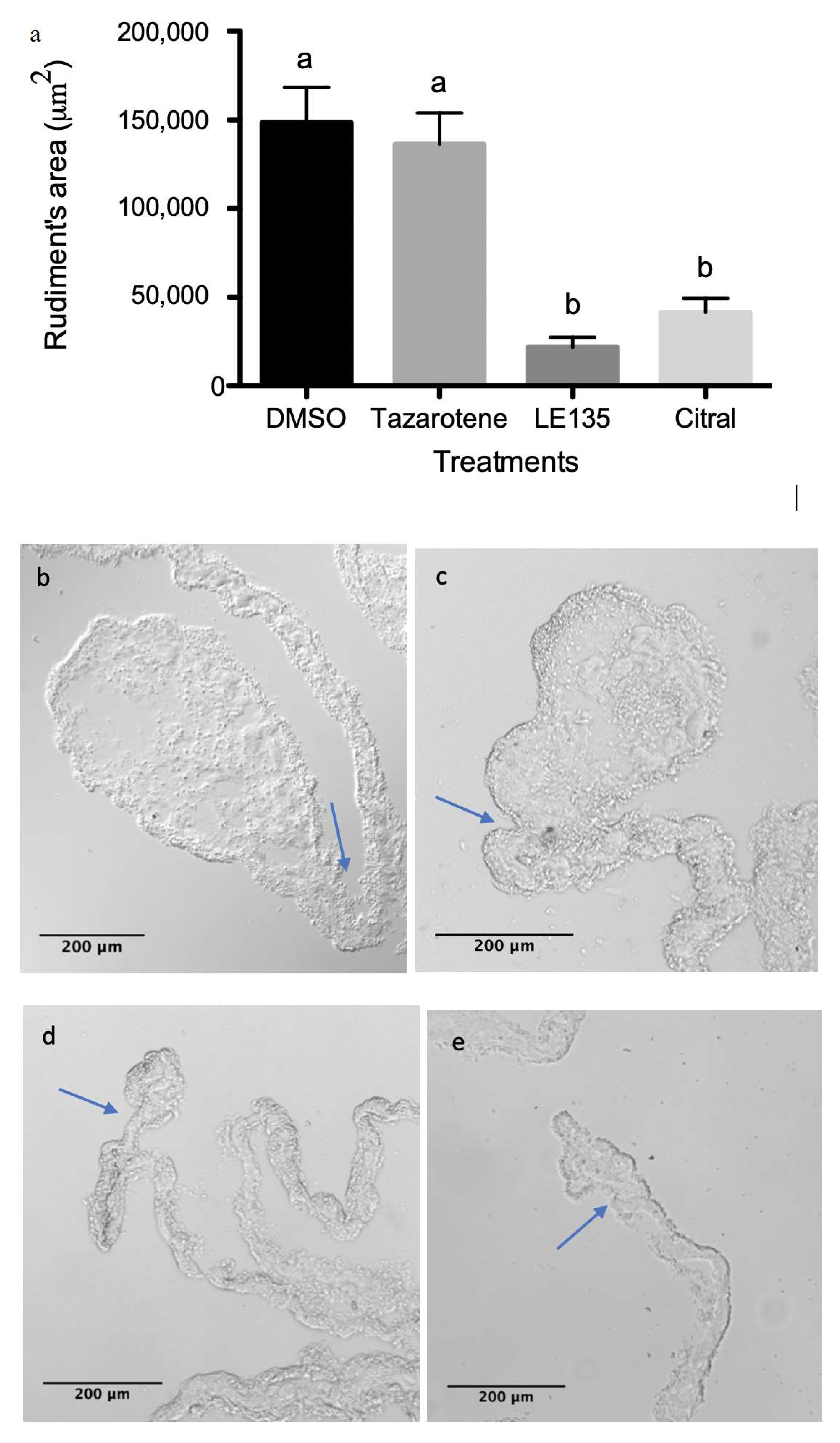
3.4. RA Signaling Modulation Affects Overall Rudiment Size during Regenerative Organogenesis of the Intestine
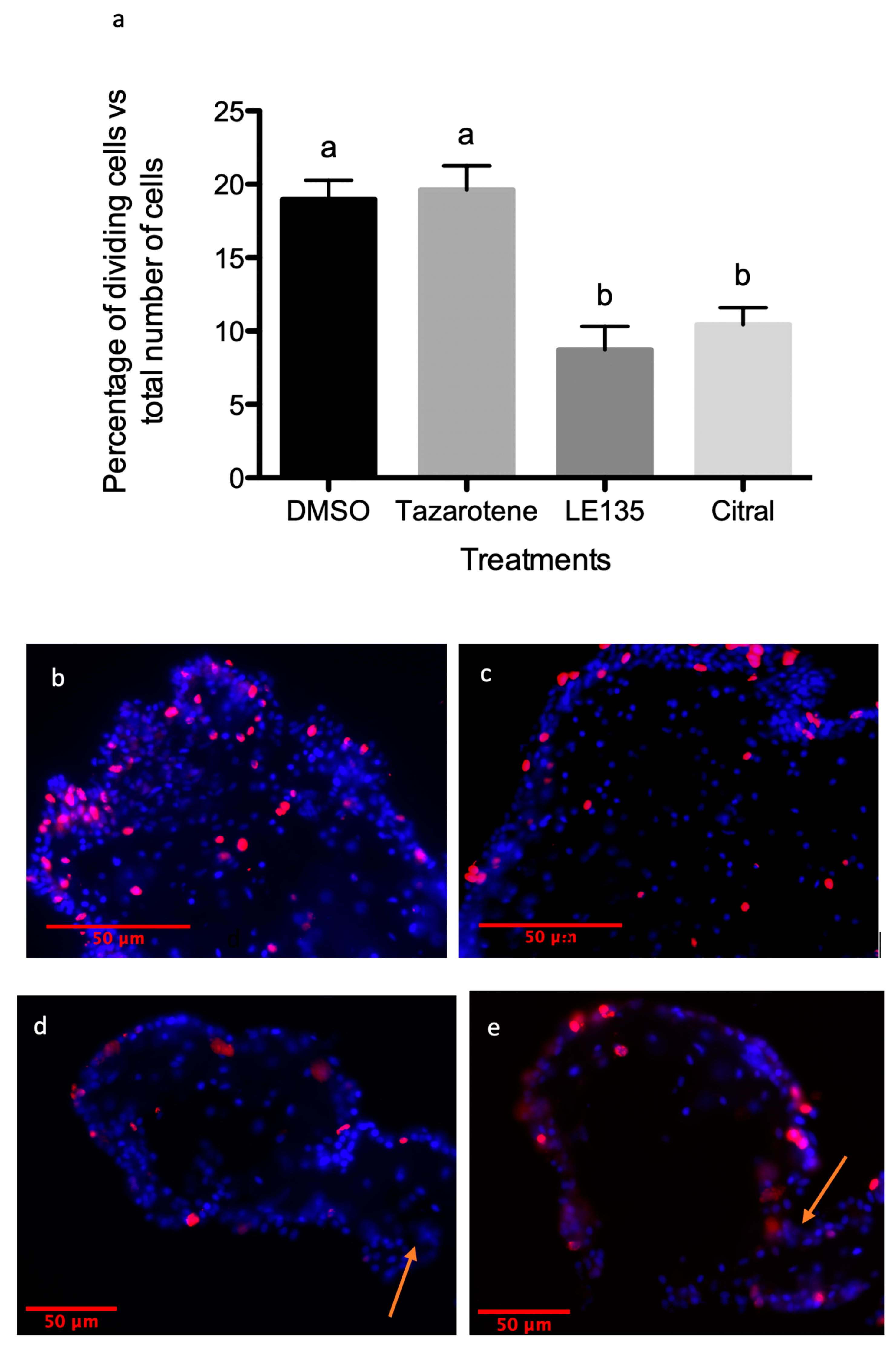
3.5. RA Signaling Modulation Affects Cellular Processes Associated with the Regenerative Organogenesis of the Intestine
4. Discussion
4.1. Holothurian Enzyme Orthologs
4.2. Differential Expression of Genes Linked to RA Signaling during the Regenerative Response
4.3. Functional Depiction of a RA Signaling Pathway during Intestinal Regeneration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Dollé, P. Retinoic acid in development: Towards an integrated view. Genetics 2008, 9, 541–553. [Google Scholar] [CrossRef]
- Bong-Gun, J.; Won-Sun, K. Upregulation of cathepsin D expression in the dedifferentiating salamander limb regenerates and enhancement of its expression by retinoic acid. Wound Rep. Reg. 1998, 6, 349–357. [Google Scholar]
- Albalat, R. The retinoic acid machinery in invertebrates: Ancestral elements and vertebrate innovations. Mol. Cell. Endocrinol. 2009, 313, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Tata, P.R.; Mou, H.; Pardo-Saganta, A.; Zaho, R.; Prabhu, M.; Law, B.M.; Vinarsky, V.; Cho, J.L.; Breton, S.; Sahay, A.; et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 2013, 503, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Luz-Madrigal, A.; Grajales-Esquivel, E.; McCorkle, A.; DiLorenzo, A.M.; Barbosa-Sabanero, K.; Tsonis, P.A.; Del Rio-Tsonis, K. Reprogramming of the chick retinal pigmented epithelium after retinal injury. BMC Biol. 2014, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Sporn, M.B.; Roberts, A.B. Cervical dysplasia regression induced by all-trans-retinoic acid. J. Natl. Cancer Inst. 1994, 86, 476–477. [Google Scholar]
- Spiegler, E.; Kim, Y.K.; Wassef, L.; Shete, V.; Quadro, L. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim. Biophys. Acta 2012, 1821, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Parés, X.; Farrés, J.; Kedishvili, N.; Duester, G. Medium-chain and short-chain dehydrogenses/reductases in retinoid metabolism. Cell. Mol. Life Sci. 2008, 65, 3936–3949. [Google Scholar] [CrossRef] [Green Version]
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duprey-Díaz, M.V.; Blagburn, J.M.; Blanco, R.E. Exogenous modulation of retinoic acid signaling affects adult RGC survival in the frog visual system after optic nerve injury. PLoS ONE 2016, 11, e0162626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marlétaz, F.; Holland, L.Z.; Laudet, V.; Schubert, M. Retinoic acid signaling and the evolution of chordates. Int. J. Biol. Sci. 2006, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- McEwan, J.; Lynch, J.; Beck, C.W. Expression of key retinoic acid modulating genes suggests active regulation during development and regeneration of the amphibian limb. Dev. Dyn. 2011, 240, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim Biophys. Acta 2012, 1821, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Mashanov, V.S.; García-Arrarás, J.E. Gut regeneration in holoturians: A snapshot of recent developments. Biol. Bull. 2011, 221, 93–109. [Google Scholar] [CrossRef]
- García-Arrarás, J.E.; Greenberg, M. Visceral regeneration in holothurians. Micros. Res. Tech. 2001, 55, 438–451. [Google Scholar] [CrossRef]
- Emson, R.; Wilkie, I. Fission and Autotomy in Echinoderms; Aberdeen University Press: Aberdeen, UK, 1980. [Google Scholar]
- Wilkie, I.C. Autotomy as a prelude to regeneration in echinoderms. Micr. Res. Tech. 2001, 55, 369–396. [Google Scholar] [CrossRef]
- García-Arrarás, J.E.; Estrada-Rodgers, L.; Santiago, R.; Torres, I.I.; Díaz-Miranda, L.; Torres-Avillán, I. Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). J. Exp. Zool. 1998, 281, 288–304. [Google Scholar] [CrossRef]
- García-Arrarás, J.E.; Valentín-Tirado, G.; Flores, J.E.; Rosa, R.J.; Rivera-Cruz, A.; San Miguel-Ruiz, J.E.; Tossas, K. Cell dedifferentiation and epitelial to mesenchymal transitions during intestinal regeneration in H. glaberrima. BMC Dev. Biol. 2011, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- García-Arrarás, J.E.; Dolmatov, I.Y. Echinoderms, potential model systems for studies on muscle regeneration. Curr. Pharm. Des. 2010, 16, 942–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viera-Vera, J.; García-Arrarás, J.E. Molecular characterization and gene expression patterns of retinoid receptors, in normal and regenerating tissues of the sea cucumber, Holothuria glaberrima. Gene 2018, 654, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.; Clark, A.; Spencer, G.; Carlone, R. Cloning and expression of a retinoic acid receptor β2 subtype from the adult newt: Evidence for an early role in tail and caudal spinal cord regeneration. Dev. Dyn. 2011, 240, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Cartagena, C.; Ortiz-Pineda, P.; Ramírez-Gómez, F.; Suárez-Castillo, E.C.; Matos-Cruz, V.; Rodríguez, C.; Ortiz-Zuazaga, H.; García-Arrarás, J.E. Distinct profiles of expressed sequence tags during intestinal regeneration in the sea cucumber Holothuria glaberrima. Physiol. Genom. 2007, 31, 203–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortíz-Pineda, P.; Ramírez-Gómez, F.; Pérez- Ortiz, J.; González-Díaz, S.; Santiago-De Jesús, F.; Hernández-Pasos, J.; Del Valle-Avila, C.; Rojas-Cartagena, C.; Suárez-Castillo, E.C.; Tossas, K.; et al. Gene expression profiling of intestinal regeneration in the sea cucumber. BMC Genom. 2009, 10, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashanov, V.S.; Zueva, O.R.; García-Arrarás, J.E. Transcriptomic changes during regeneration of the central nervous system in an echinoderm. BMC Genom. 2014, 15, 357. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Doerks, T.; Bork, P. SMART 6: Recent updates and new developments. Nucleic Acids Res. 2009, 37, D229–D232. [Google Scholar] [CrossRef]
- Zdobnov, E.M.; Apweiler, R. InterProScan – an integration platform for the signature-recognition methos in InterPro. Bioinformatics 2001, 17, 847–848. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Gascuel, O. Asimple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systsx Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Gómez, F.; Ortiz-Pineda, P.A.; Rivera-Cardona, G.; García-Arrarás, J.E. LPS-inducde genes in intestinal tissue of the sea cucumber Holothuria glaberrima. PLoS ONE 2009, 4, e6178. [Google Scholar] [CrossRef]
- Candelaria, A.G.; Murray, G.; File, S.K.; García-Arrarás, J.E. Contribution of mesenterial muscle dedifferentiation to intestine regeneration in the sea cucumber Holothuria glaberrima. Cell Tissue Res. 2006, 325, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, U.; Filling, C.; Hult, M.; Shafqat, N.; Wu, X.; Lindh, M.; Shafqat, J.; Nordling, E.; Kallberg, Y.; Persson, B.; et al. Short-chain dehydrogenases/reductases (SDR): The 2002 update. Chem-Biol. Interact. 2003, 143–144, 247–253. [Google Scholar] [CrossRef]
- Marchitti, S.A.; Brocker, C.; Stagos, D.; Vasiliou, V. Non-P450 aldehyde oxidizing enzymnes: The aldehyde dehydrogenase superfamily. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 697–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jörnvall, H.; Hedlund, J.; Bergman, T.; Opperman, U.; Persson, B. Superfamilies SDR and MDR: From early ancestry to present forms. Emergenece of three lines, a Zn-metalloenzyme, and distinct variabilities. Biochem. Bioph. Res. Commun. 2010, 396, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.; Bray, J.E.; Bruford, E.; Dellaporta, S.L.; Favia, A.D.; Gonzalez-Duarte, R.; Jörnvall, H.; Kallberg, Y.; Kavanagh, K.L.; Kedishvili, N.; et al. The SDR (shor-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem. Biol. Interact. 2009, 178, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallberg, Y.; Oppermann, U.; Persson, B. Classification of the short-chain dehydrogenase/reductase superfamily using hidden Markov models. FEBS J. 2010, 277, 2375–2386. [Google Scholar] [CrossRef]
- Sophos, N.A.; Vasiliou, V. Aldehyde dehydrogenase gene superfamily: The 2002 update. Chem. Biol. Interact. 2003, 143–144, 5–22. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; DeLuca, H.F. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002, 22, 347–381. [Google Scholar] [CrossRef]
- Duester, G. Retinoid signaling in control of progenitor cell differentiation during mouse development. Semin. Cell Dev. Biol. 2013, 24, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Blum, N.; Begemann, G. Retinoic acid signaling controls the formation, proliferation and survival of the blastema during adult zebrafish fin regeneration. Development 2012, 139, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Napoli, J.L. Retinoic acid: Its biosynthesis and metabolism. Prog. Nucleic Acid Res. Mol. Biol. 1999, 63, 139–188. [Google Scholar] [PubMed]
- Duester, G. Families of retinoid dehydrogenases regulating vitamin A function: Production of visual pigment and retinoic acid. Eur. J. Biochem. 2000, 267, 4315–4324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belyaeva, O.V.; Johnson, M.P.; Kedishvili, N.Y. Kinetic analysis of human enzyme RDH10 defines the characteristics of a physiologically relevant retinol dehydrogenase. J. Biol. Chem. 2008, 283, 20299–20308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parés, X.; Farrés, J.; Kedishvili, N.; Duester, G. Medium- and short-chain dehydrogenase/reductase gene and protein families: Medium-chain and short- chain dehydrogenases/reductases in retinoid metabolism. Cell. Mol. Life Sci. 2008, 65, 3936–3949. [Google Scholar] [CrossRef] [Green Version]
- Scadding, S.R. Citral, an inhibitor of retinoic acid synthesis, modifies pattern formation during limb regeneration in the axolotl Ambystoma mexicanum. Can. J. Zool. 1999, 77, 1835–1837. [Google Scholar] [CrossRef]
- Kaneko, N.; Katsuyama, Y.; Kawamura, K.; Fujiwara, S. Regeneration of the gut requires retinoic acid in the budding ascidian Polyandrocarpa misakiensis. Develp. Growth Differ. 2010, 52, 457–468. [Google Scholar] [CrossRef]
- Nguyen, M.; Singhal, P.; Piet, J.W.; Shefelbine, S.J.; Maden, M.; Randal-Voss, S.; Monaghan, J.R. Retinoic acid receptor regulation of epimorphic and homeostatic regeneration in the axolotl. Develpoment 2017, 144, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Maden, M. Retinoids as endogenous components of the regenerating limb and tail. Wound Repair Regen. 1998, 6, 358–365. [Google Scholar] [CrossRef]
- Cuervo, R.; Chimal-Monroy, J. Chemical activation of RARβ induces post-embryonically bilateral limb duplication during Xenopus limb regeneration. Sci. Rep. 2013, 3, 1886. [Google Scholar] [CrossRef]
- Al Haj Zen, A.; Nawrot, D.A.; Howarth, A.; Caporali, A.; Ebner, D.; Vernet, A.; Schneider, J.E.; Bhattacharya, S. The retinoid agonist tazarotene promotes angiogenesis and wound healing. Mol. Ther. 2016, 24, 1745–1759. [Google Scholar] [CrossRef] [Green Version]
- Maden, M. Who needs stem cells if you can dedifferentiate? Cell Stem Cell. 2013, 13, 640–641. [Google Scholar] [CrossRef] [Green Version]
- San Miguel-Ruiz, J.E.; García-Arrarás, J.E. Common cellular events occurring during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Dev. Biol. 2007, 17, 115. [Google Scholar]
- Liu, H.; Ly, I.; Hu, Y.; Wan, Y.Y. Retinoic acid regulates cell cycle genes and accelerates normal mouse liver regeneration. Biochem. Pharmacol. 2014, 91, 256–265. [Google Scholar] [CrossRef] [Green Version]






| Short-Chain Dehydrogenase Reductase 7 | ||
| Primer Name | Sequence (5′-3′) | Amplicon Product Region |
| SDR 5′F | CTCTAGTCAGTAGGCAGAAGGA | 5′UTR/ORF |
| SDR 5′R | TCAGAGAGCACTATCAGGAAGG | 5′UTR/ORF |
| SDR F* | TGACTTTGCTGGTGTTTCCACTGC | ORF |
| SDR R | CACTGCTCATGTTAGCTATGTGGC | ORF |
| SDR F2 | GCCACATAGTCAACATGAGCAG | ORF |
| SDR R2* | GCAAGATAGAACTGGGTGAGCA | ORF |
| SDR 3′R | GCCTATGGATATGCCACTCT | ORF/3′UTR |
| Aldehyde Dehydrogenase Family 8A1 | ||
| Primer Name | Sequence (5′-3′) | Amplicon Product Region |
| ALDH81 5′F | CACTGTAATCTAGAGATTGGTATGGAC | 5′UTR/ORF |
| ALDH81 5′R | GTTTGTGCTGGATGGCAGTTGC | 5′UTR/ORF |
| ALDH81 FA | GCAACTGCCATCCAGCACAAACTA | ORF |
| ALDH81 RA | AACTTGATCGTACCGTGGTTGGGA | ORF |
| ALDH81 FB | CGGTACGATCAAGTTTCTCAAATCAGGGG | ORF |
| ALDH81 RB | GTTTGTTGTGCTCTCTGTTGGAGGTC | ORF |
| ALDH81 FC* | GACCTCCAACAGAGAGCACAACAAAC | ORF |
| ALDH81 RC* | GCTGCCCTCTCCACACTCTATTTTC | ORF |
| ALDH81 FRT2 | AGCTGCCTACGAGAATGTGAGCAT | ORF |
| ALDH81 RRT2 | TCTTCCCTTACTGCACCTCCACAA | ORF |
| ALDH81 FF | GTGGAGGTGCAGTAAGGGAAGA | ORF |
| ALDH81 3′F | GCATCCTCTAAACACTACCAGTC | ORF/3′UTR |
| ALDH81 3′R | GATCAAGGGTTCTGTTGATTACTT | ORF/3′UTR |
| NADH | ||
| Primer Name | Sequence (5′-3′) | Amplicon Product Region |
| NADH F* | CGCAGAAGTAGCCGCGAATAT | ORF |
| NADH R* | CAATGGTTGTTGCTGGAGTCTTT | ORF |
| Short-Chain Dehydrogenase Reductase 7 | Aldehyde Dehydrogenase Family 8 A1 | |||
|---|---|---|---|---|
| Organism’s Nickname | Identity (Similarity ) | Organism’s Nickname | Identity (Similarity) | |
| scallop | 41 (59) | hydra | 55 (66) | |
| bristle worm | 39 (59) | sea slug | 54 (66) | |
| oyster | 41 (57) | oyster | 57 (69) | |
| drosophila | 27 (45) | horseshoe crab | 50 (65) | |
| apis | 26 (42) | spider | 45 (62) | |
| urchin | 46 (70) | starfish | 55 (69) | |
| lancelet | 40 (58) | urchin | 44 (60) | |
| ciona | 22 (36) | urochord | 45 (59) | |
| frog | 37 (54) | sea squirt | 52 (62) | |
| mus | 36 (53) | lancelet | 57 (69) | |
| homo | 37 (53) | frog | 56 (70) | |
| mus | 57 (72) | |||
| homo | 57 (71) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viera-Vera, J.; García-Arrarás, J.E. Retinoic Acid Signaling Is Associated with Cell Proliferation, Muscle Cell Dedifferentiation, and Overall Rudiment Size during Intestinal Regeneration in the Sea Cucumber, Holothuria glaberrima. Biomolecules 2019, 9, 873. https://doi.org/10.3390/biom9120873
Viera-Vera J, García-Arrarás JE. Retinoic Acid Signaling Is Associated with Cell Proliferation, Muscle Cell Dedifferentiation, and Overall Rudiment Size during Intestinal Regeneration in the Sea Cucumber, Holothuria glaberrima. Biomolecules. 2019; 9(12):873. https://doi.org/10.3390/biom9120873
Chicago/Turabian StyleViera-Vera, Jorge, and José E. García-Arrarás. 2019. "Retinoic Acid Signaling Is Associated with Cell Proliferation, Muscle Cell Dedifferentiation, and Overall Rudiment Size during Intestinal Regeneration in the Sea Cucumber, Holothuria glaberrima" Biomolecules 9, no. 12: 873. https://doi.org/10.3390/biom9120873




