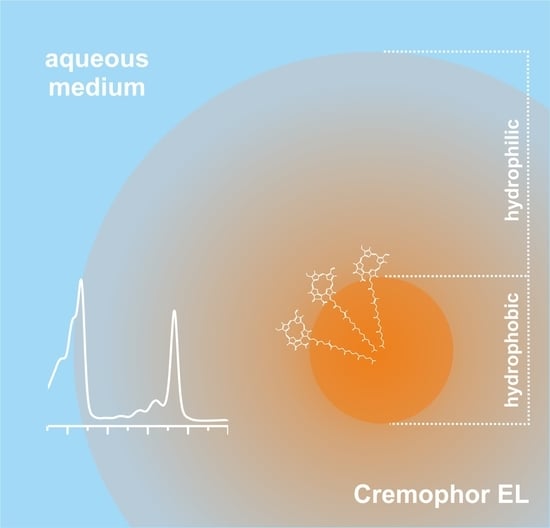
Cremophor EL Nano-Emulsion Monomerizes Chlorophyll a in Water Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chlorophyll a Isolation
2.1.2. Chlorophyll a in Ethanol or PBS Buffer
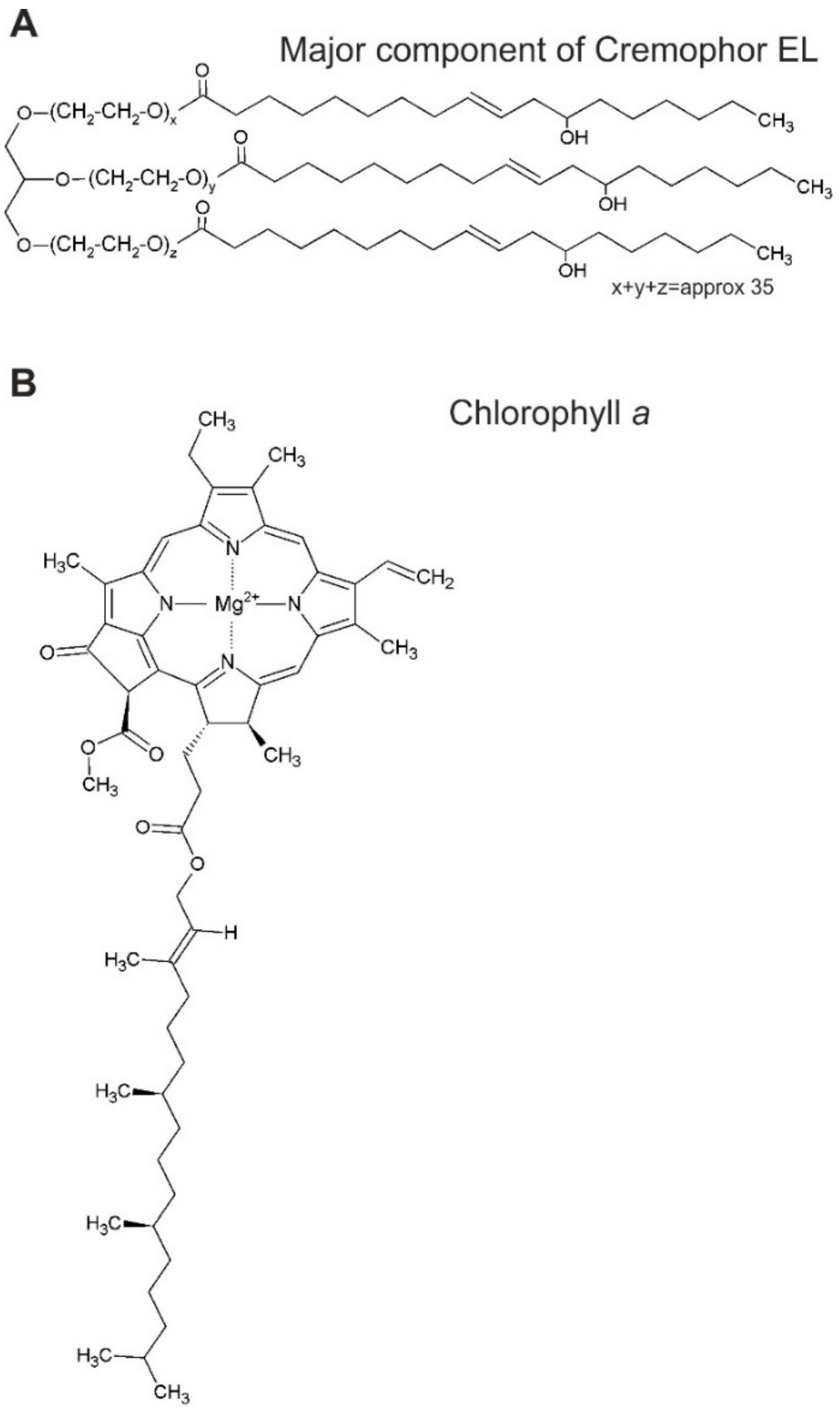
2.1.3. Cremophor Nano-Emulsion Preparation
2.1.4. Complex Formation with Chlorophyll a
2.2. Methods
2.2.1. UV-Vis Absorption Spectroscopy
2.2.2. Circular Dichroism Spectroscopy
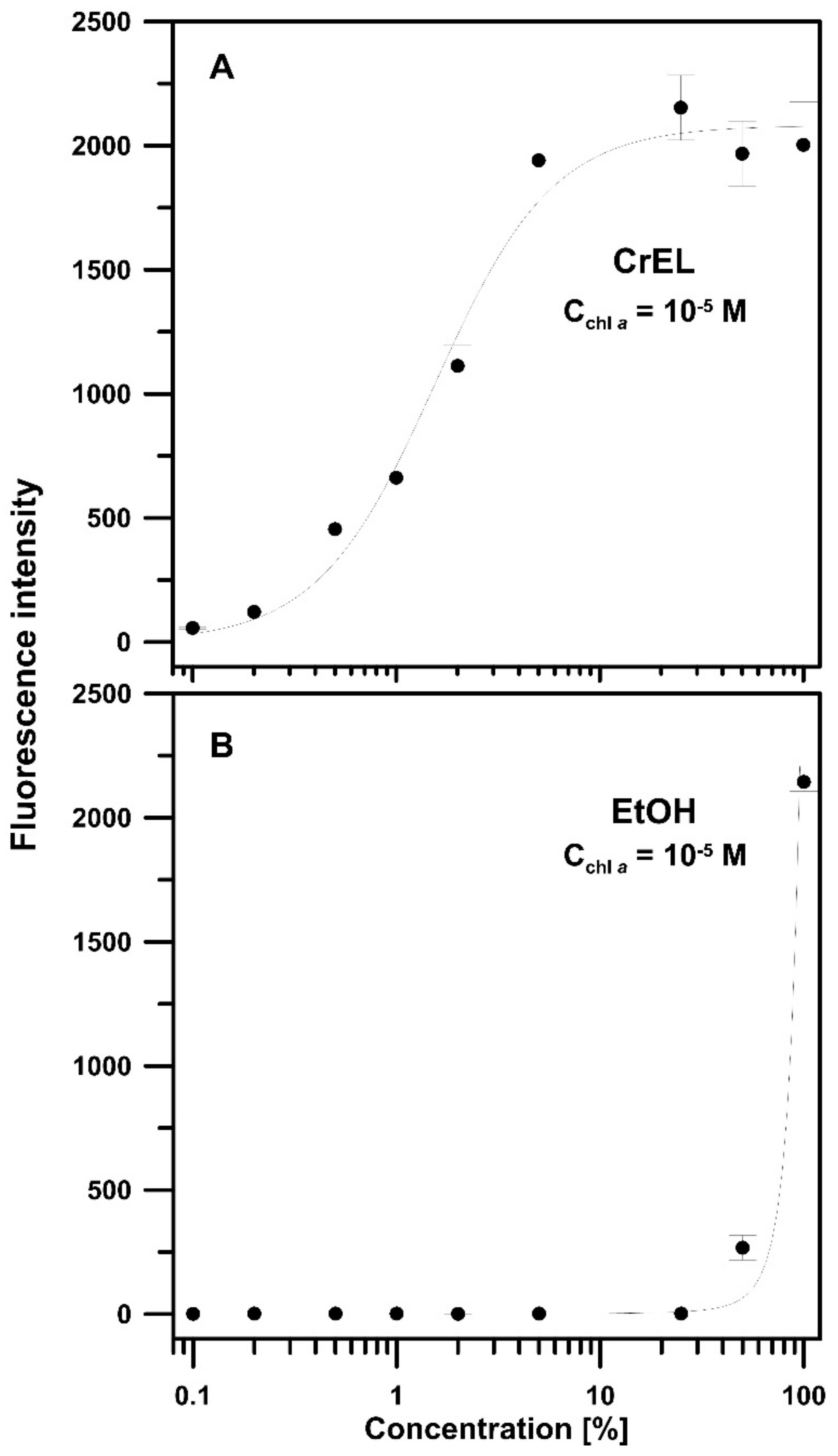
2.2.3. Steady-State Fluorescence Spectroscopy
2.2.4. Time-Resolved Fluorescence Spectroscopy
2.2.5. Raman Spectroscopy
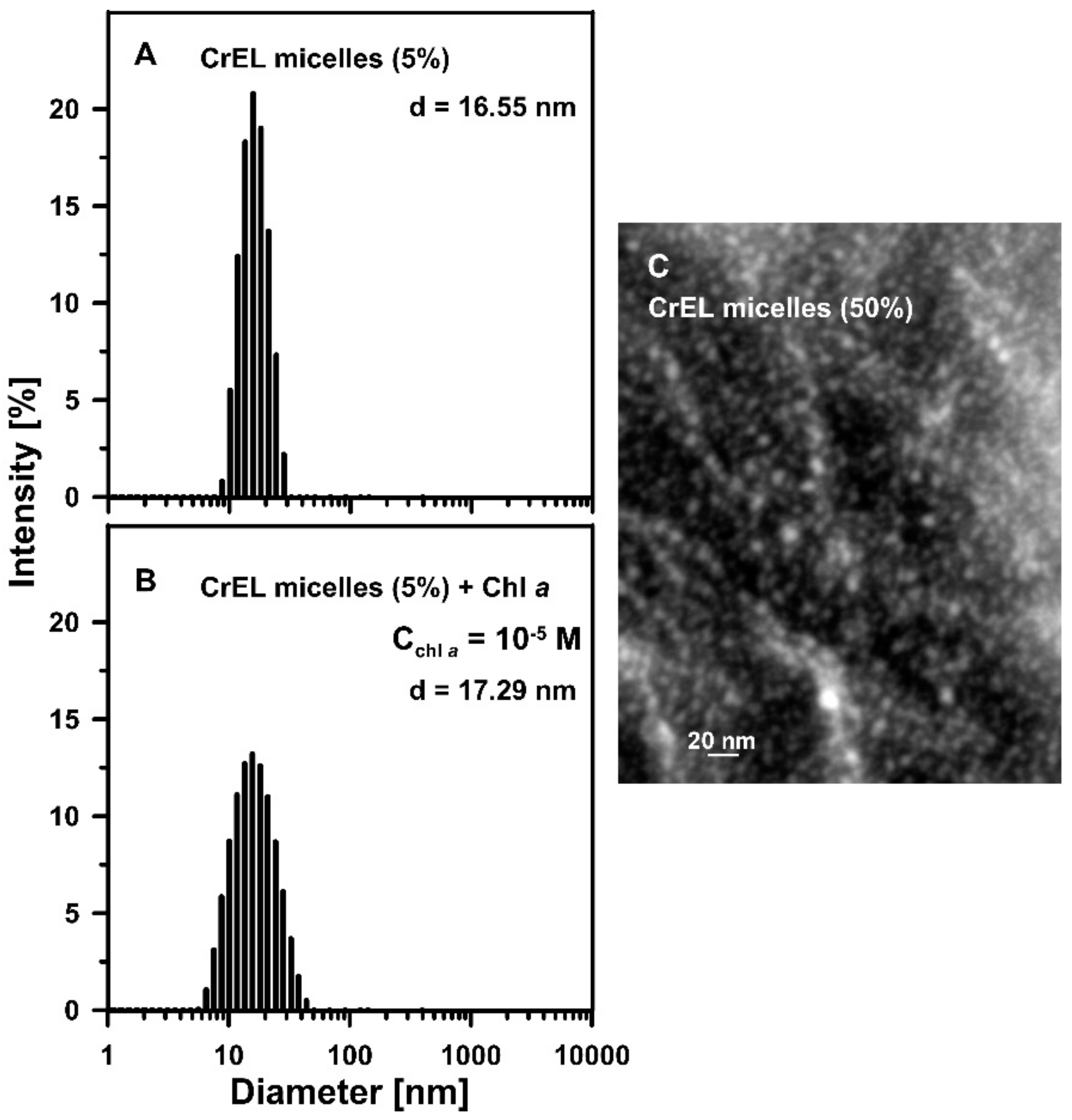
2.2.6. Dynamic Light Scattering
2.2.7. Cryo-Scanning Electron Microscopy
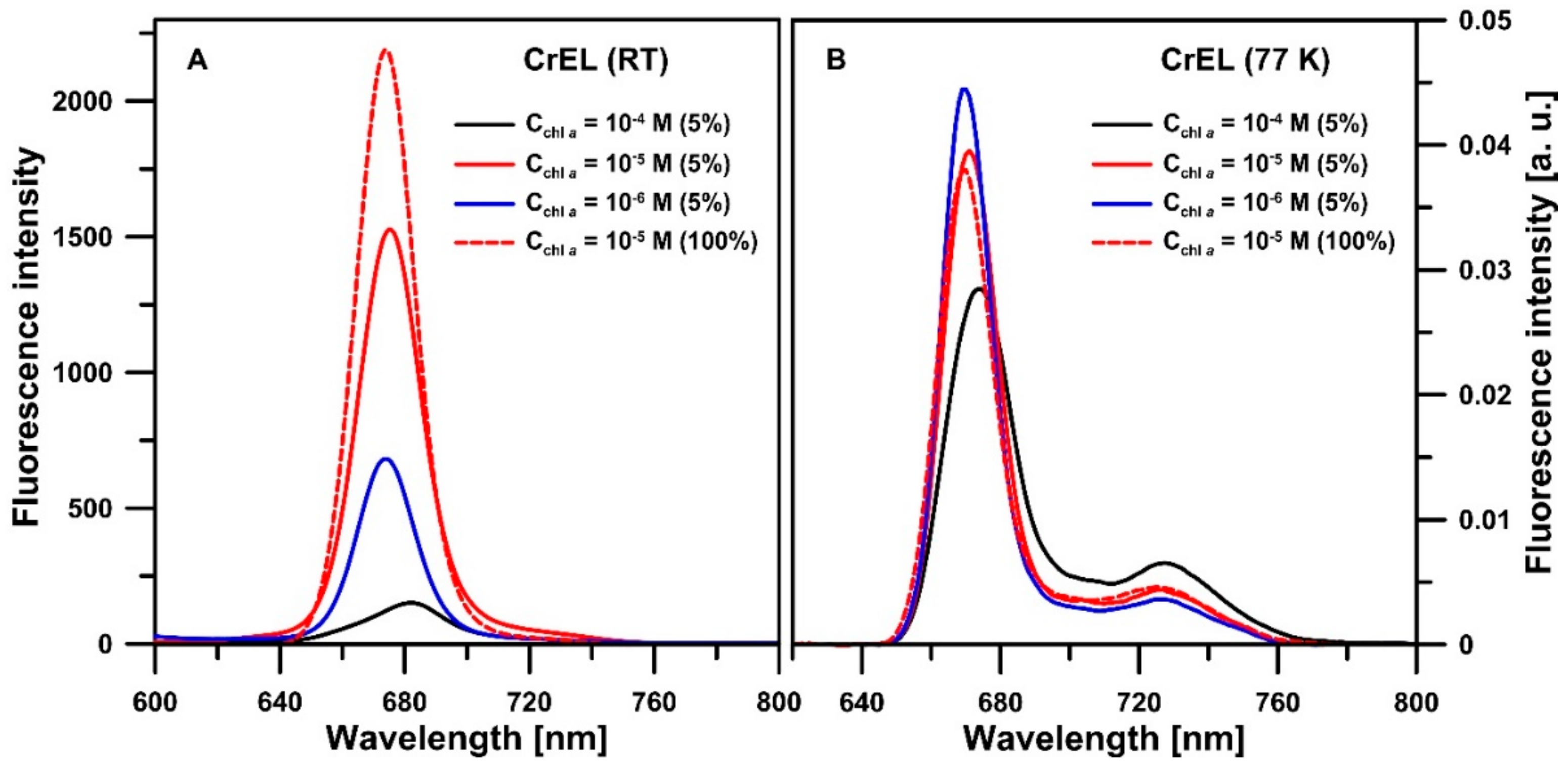
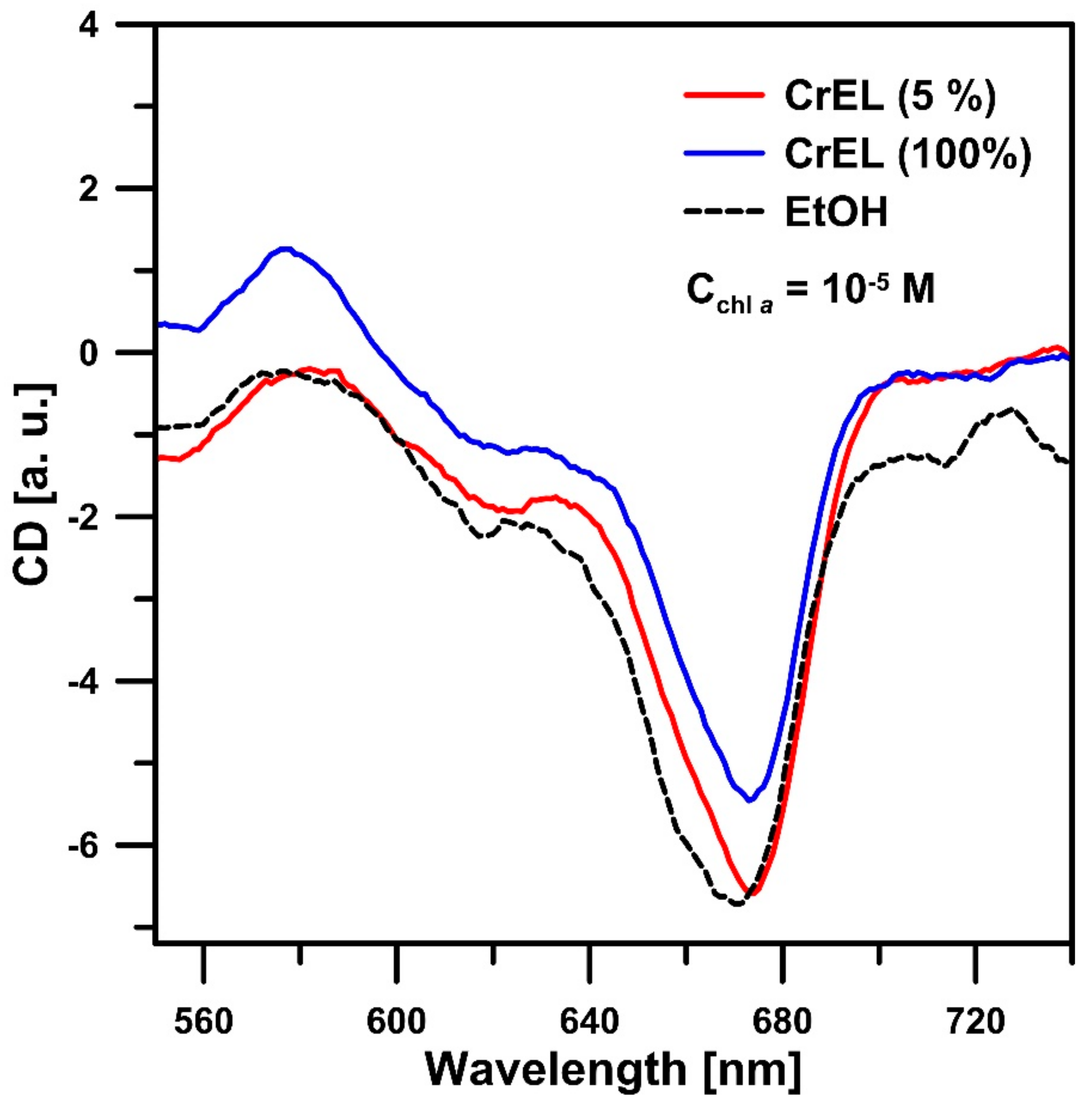
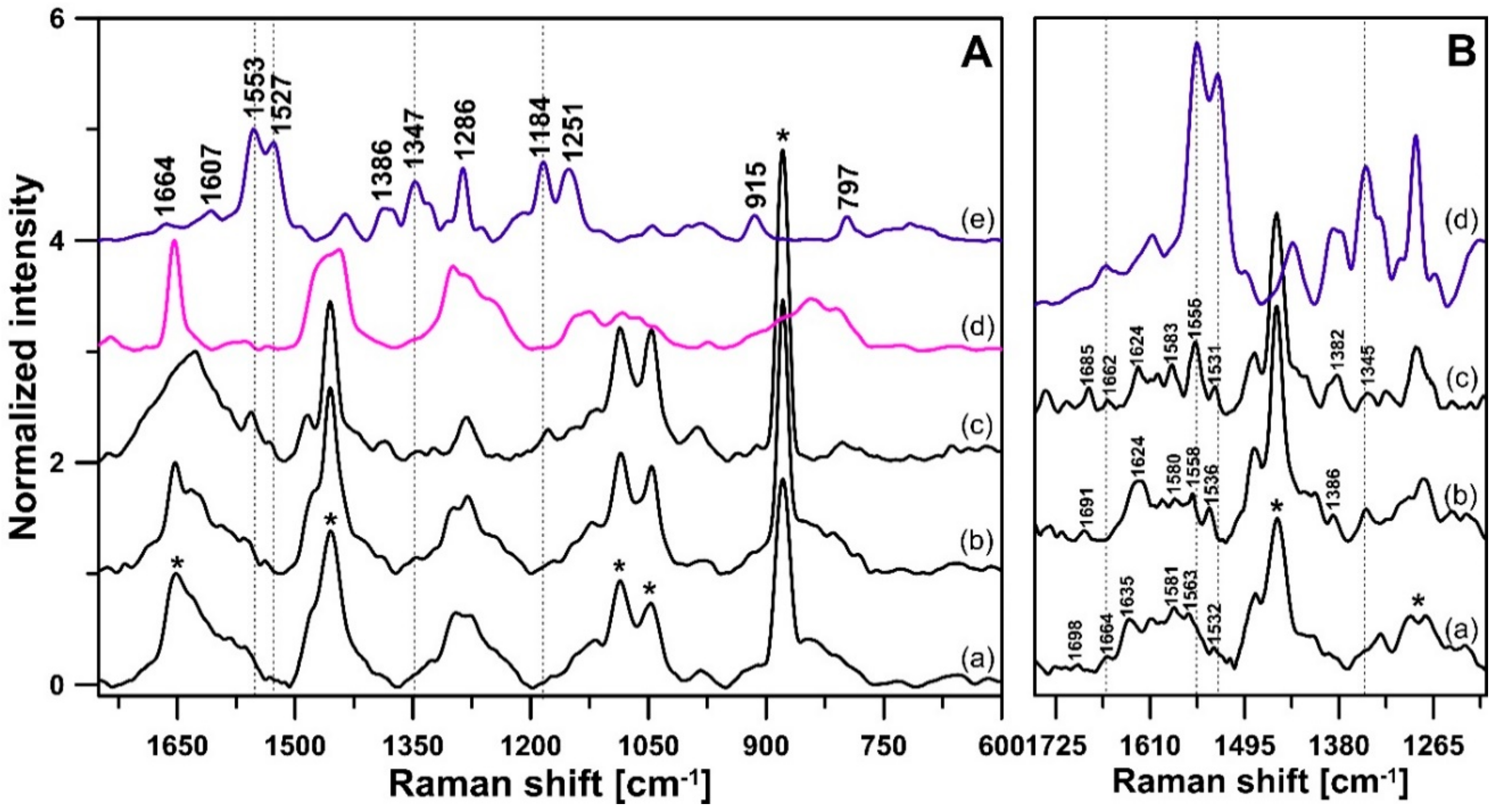
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trauner, D. Richard Willstatter and the 1915 Nobel Prize in chemistry. Angew. Chem. Int. 2015, 54, 11910–11916. [Google Scholar] [CrossRef]
- Willstätter, R.; Stoll, A. Untersuchungen über Chlorophyll: Methoden und Ergebniss; Springer: Berlin, Germany, 1913. [Google Scholar]
- Woodward, R.B.; Ayer, W.A.; Beaton, J.M.; Bickelhaupt, F.; Bonnett, R.; Buchschacher, P.; Closs, G.L.; Dutler, H.; Hannah, J.; Hauck, F.P.; et al. The total synthesis of chlorophyll. J. Am. Chem. Soc. 1960, 82, 3800–3802. [Google Scholar] [CrossRef]
- Battersby, A.R. Tetrapyrroles: The pigments of life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.A.; Senge, M.O. How green is green chemistry? Chlorophylls as a bioresource from biorefineries and their commercial potential in medicine and photovoltaics. Photochem. Photobiol. Sci. 2015, 14, 638–660. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Wang, J.B.; Zhu, Y.R.; Zhang, B.C.; Wu, Y.; Zhang, Q.N.; Qian, G.S.; Kuang, S.Y.; Gange, S.J.; Jacobson, L.P.; et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14601–14606. [Google Scholar] [CrossRef]
- Simonich, M.T.; Egner, P.A.; Roebuck, B.D.; Orner, G.A.; Jubert, C.; Pereira, C.; Groopman, J.D.; Kensler, T.W.; Dashwood, R.H.; Williams, D.E.; et al. Natural chlorophyll inhibits aflatoxin B1-induced multi-organ carcinogenesis in the rat. Carcinogenesis 2007, 28, 1294–1302. [Google Scholar] [CrossRef]
- McQuistan, T.J.; Simonich, M.T.; Pratt, M.M.; Pereira, C.B.; Hendricks, J.D.; Dashwood, R.H.; Williams, D.E.; Bailey, G.S. Cancer chemoprevention by dietary chlorophylls: A 12,000-animal dose-dose matrix biomarker and tumor study. Food Chem. Toxicol. 2012, 50, 341–352. [Google Scholar] [CrossRef]
- Diaz, G.D.; Li, Q.; Dashwood, R.H. Caspase-8 and apoptosis-inducing factor mediate a cytochrome c-independent pathway of apoptosis in human colon cancer cells induced by the dietary phytochemical chlorophyllin. Cancer Res. 2003, 63, 1254–1261. [Google Scholar]
- Negishi, T.; Rai, H.; Hayatsu, H. Antigenotoxic activity of natural chlorophylls. Mutat. Res. 1997, 376, 97–100. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 885–891. [Google Scholar] [CrossRef]
- Kim, J.B.; Adler, A.D.; Longo, F.R. Synthesis of porphyrins from monopyrroles. In The Prophyrins V1: Structure and Synthesis Part A; Academic Press Inc.: New York, NY, USA, 1978; Volume 3. [Google Scholar]
- Proll, S.; Wilhelm, B.; Robert, B.; Scheer, H. Myoglobin with modified tetrapyrrole chromophores: Binding specificity and photochemistry. Biochim. Biophys. Acta 2006, 1757, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, L.; Stasiek, M.; Mysliwa-Kurdziel, B.; Strzalka, K. Phytol as one of the determinants of chlorophyll interactions in solution. Photosynth. Res. 2003, 78, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Dashwood, R.; Negishi, T.; Hayatsu, H.; Breinholt, V.; Hendricks, J.; Bailey, G. Chemopreventive properties of chlorophylls toward aflatoxin B1: A review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat. Res. 1998, 399, 245–253. [Google Scholar] [CrossRef]
- Scheer, H. Chlorophylls; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Janik, E.; Bednarska, J.; Zubik, M.; Sowinski, K.; Luchowski, R.; Grudzinski, W.; Gruszecki, W.I. Is it beneficial for the major photosynthetic antenna complex of plants to form trimers? J. Phys. Chem. B 2015, 119, 8501–8508. [Google Scholar] [CrossRef]
- Boussaad, S.; Tazi, A.; Leblanc, R.M. Chlorophyll a dimer: A possible primary electron donor for the photosystem II. Proc. Natl. Acad. Sci. USA 1997, 94, 3504–3506. [Google Scholar] [CrossRef]
- Janik, E.; Bednarska, J.; Sowinski, K.; Luchowski, R.; Zubik, M.; Grudzinski, W.; Gruszecki, W.I. Light-induced formation of dimeric LHCII. Photosynth. Res. 2017, 132, 265–276. [Google Scholar] [CrossRef]
- Katz, J.J.; Shipman, L.L.; Cotton, T.M.; Janson, T.J. Chlorophyll aggregation: Coordination interactions in chlorophyll monomers, dimers, and oligomers. In The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, NY, USA, 1978; Volume 5, pp. 401–458. [Google Scholar]
- Cellamare, B.M.; Fini, P.; Agostiano, A.; Sortino, S.; Cosma, P. Identification of ROS produced by photodynamic activity of chlorophyll/cyclodextrin inclusion complexes. Photochem. Photobiol. 2013, 89, 432–441. [Google Scholar] [CrossRef]
- Semeraro, P.; Chimienti, G.; Altamura, E.; Fini, P.; Rizzi, V.; Cosma, P. Chlorophyll a in cyclodextrin supramolecular complexes as a natural photosensitizer for photodynamic therapy (PDT) applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 85, 47–56. [Google Scholar] [CrossRef]
- Rizzi, V.; Fini, P.; Semeraro, P.; Cosma, P. Detailed investigation of ROS arisen from chlorophyll a/Chitosan based-biofilm. Coll. Surf. B Biointer. 2016, 142, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Vurro, D.; Placido, T.; Fini, P.; Petrella, A.; Semeraro, P.; Cosma, P. Gold-chlorophyll a-hybrid nanoparticles and chlorophyll a/cetyltrimethylammonium chloride self-assembled-suprastructures as novel carriers for chlorophyll a delivery in water medium: Photoactivity and photostability. Coll. Surf. B Biointer. 2018, 161, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xin, X.; Zhang, Y. Development and characterization of promising Cremophor EL-stabilized o/w nanoemulsions containing short-chain alcohols as a cosurfactant. RSC Adv. 2017, 7, 19815–19827. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Fjallskog, M.L.; Frii, L.; Bergh, J. Is Cremophor EL, solvent for paclitaxel, cytotoxic? Lancet 1993, 342, 873. [Google Scholar] [CrossRef]
- Van Zuylen, L.; Karlsson, M.O.; Verweij, J.; Brouwer, E.; de Bruijn, P.; Nooter, K.; Stoter, G.; Sparreboom, A. Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer. Chemother. Pharmacol. 2001, 47, 309–318. [Google Scholar] [CrossRef]
- Agostiano, A.; Cosma, P.; Trotta, M.; Monsu-Scolaro, L.; Micali, N. Chlorophyll a behavior in aqueous solvents: Formation of nanoscale self-assembled complexes. J. Phys. Chem. B 2002, 106, 12820–12829. [Google Scholar] [CrossRef]
- Vladkova, R. Chlorophyll a self-assembly in polar solvent–water mixtures. Photochem. Photobiol. 2000, 71, 71–83. [Google Scholar] [CrossRef]
- Kaplanova, M.; Parma, L. Effect of excitation and emission wavelength on the fluorescence lifetimes of chlorophyll a. Gen. Physiol. Biophys. 1984, 3, 127–134. [Google Scholar]
- De Paula, J.C.; Robblee, J.H.; Pasternack, R.F. Aggregation of chlorophyll a probed by resonance light scattering spectroscopy. Biophys. J. 1995, 68, 335–341. [Google Scholar] [CrossRef]
- Agostiano, A.; Catucci, L.; Cosma, P.; Fini, P. Aggregation processes and photophysical properties of chlorophyll a in aqueous solutions modulated by the presence of cyclodextrins. Phys. Chem. Chem. Phys. 2003, 5, 2122–2128. [Google Scholar] [CrossRef]
- Van Gurp, M.; van Ginkel, G.; Levine, Y.K. Fluorescence anisotropy of chlorophyll a and chlorophyll b in castor oil. Biochim. Biophys. Acta 1989, 973, 405–413. [Google Scholar] [CrossRef]
- Weerapol, Y.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P. Self-nanoemulsifying drug delivery system of nifedipine: Impact of hydrophilic-lipophilic balance and molecular structure of mixed surfactants. AAPS Pharm. Sci. Tech. 2014, 15, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.T.; Naguib, M. Propofol: The challenges of formulation. Anesthesiology 2005, 103, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, M.; Kalkowski, J.; Luo, H.; Donovan, A.J.; Zhang, P.; Liu, C.; Shang, W.; Irving, T.; Herrera-Alonso, M.; Liu, Y. Core-shell structure and aggregation number of micelles composed of amphiphilic block copolymers and amphiphilic heterografted polymer brushes determined by small-angle X-ray scattering. ACS Macro. Lett. 2017, 6, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Croy, S.R.; Kwon, G.S. Polysorbate 80 and Cremophor EL micelles deaggregate and solubilize nystatin at the core-corona interface. J. Pharm. Sci. 2005, 94, 2345–2354. [Google Scholar] [CrossRef]
- Cinar, K. A review on nanoemulsions: Preparation methods and stability. Trakaya Univ. J. Eng. Sci. 2017, 18, 79–83. [Google Scholar]
- Nazzal, S.; Smalyukh, I.; Lavrentovich, O.D.; Khan, M.A. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: Mechanism and progress of emulsion formation. Int. J. Pharm. 2002, 235, 247–265. [Google Scholar] [CrossRef]
- Mohsin, K.; Alamri, R.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Hussain, M.D. Development of self-nanoemulsifying drug delivery systems for the enhancement of solubility and oral bioavailability of fenofibrate, a poorly water-soluble drug. Int. J. Nanomed. 2016, 11, 2829–2838. [Google Scholar]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomedicine (Lond) 2010, 5, 1595–1616. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.-G.; Raval, K.; Khan, R.-A.; Chaurasia, M.; Jain, N.-K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Controlled Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Nikam, T.-H.; Patil, M.-P.; Patil, S.-S.; Vadnere, G.-P.; Lodhi, S. Nanoemulsion: A brief review on development and application in parenteral drug delivery. Adv. Pharm. J. 2018, 3, 43–54. [Google Scholar] [CrossRef]
- Alzeer, J.; Hadeed, K.H. Ethanol and its Halal status in food industries. Trends Food Sci. Tech. 2016, 58, 14–20. [Google Scholar] [CrossRef]
- Agostiano, A.; Cosma, P.; Della Monica, M. Spectroscopic and electrochemical characterization of chlorophyll a in different water + organic solvent mixtures. Bioelectrochem. Bioenerg. 1990, 23, 311–324. [Google Scholar] [CrossRef]
- Correia, R.F.; Viseu, M.I.; Andrade, S.M. Aggregation/disaggregation of chlorophyll a in model phospholipid-detergent vesicles and micelles. Photochem. Photobiol. Sci. 2014, 13, 907–916. [Google Scholar]
- Broyde, S.B.; Brody, S.S. Emission spectra of chlorophyll-a in polar and nonpolar solvents. J. Chem. Phys. 1967, 46, 3334–3340. [Google Scholar] [CrossRef]
- Oksanen, J.A.I.; Zenkevich, E.I.; Knyukshto, V.N.; Pakalnis, S.; Hynninen, P.H.; Korppi-Tommola, J.E.I. Investigations of Chl a aggregates cross-linked by dioxane in 3-methylpentane. Biochim. Biophys. Acta 1997, 1321, 165–178. [Google Scholar] [CrossRef]
- Dentuto, P.L.; Catucci, L.; Cosma, P.; Fini, P.; Agostiano, A.; Hackbarth, S.; Rancan, F.; Roeder, B. Cyclodextrin/chlorophyll a complexes as supramolecular photosensitizers. Bioelectrochemistry 2007, 70, 39–43. [Google Scholar]
- Belgio, E.; Johnson, M.P.; Juric, S.; Ruban, A.V. Higher plant photosystem II light-harvesting antenna, not the reaction center, determines the excited-state lifetime-both the maximum and the nonphotochemically quenched. Biophys. J. 2012, 102, 2761–2771. [Google Scholar] [CrossRef]
- Helenius, V.M.; Hynninen, P.H.; Korppi-Tommola, J.E.I. Chlorophyll a aggregates in hydrocarbon solution, a picosecond spectroscopy and molecular modeling study. Photochem. Photobiol. 1993, 58, 867–873. [Google Scholar] [CrossRef]
- Wrobel, D.; Dudkowiak, A.; Goc, J. Fluorescence spectroscopy in optoelectronics, photomedicine, and investigetion of biomolecular systems. In Reviews in Fluorescence; Geddes, D.M., Ed.; Springer: New York, NY, USA, 2008; pp. 237–276. [Google Scholar]
- Hindman, J.C.; Kugel, R.; Wasielewski, M.R.; Katz, J.J. Coherent stimulated light emission (lasing) in covalently linked chlorophyll dimers. Proc. Natl. Acad. Sci. USA 1978, 75, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Garab, G.; van Amerongen, H. Linear dichroism and circular dichroism in photosynthesis research. Photosynth. Res. 2009, 101, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M.; Cox, R.P. Monomers, dimers, and tetramers of 4-n-propyl-5-ethyl farnesyl bacteriochlorophyll c in dichloromethane and carbon tetrachloride. Photosynth. Res. 1991, 30, 35–43. [Google Scholar] [PubMed]
- Lutz, M. Antenna chlorophyll in photosynthetic membranes. A study by resonance Raman spectroscopy. Biochim Biophys Acta 1977, 460, 408–430. [Google Scholar] [CrossRef]
- Krawczyk, S. The effects of hydrogen bonding and coordination interaction in visible absorption and vibrational spectra of chlorophyll a. Biochim. Biophys. Acta 1989, 976, 140–149. [Google Scholar] [CrossRef]
- Koyama, Y.; Umemoto, Y.; Akamatsu, A.; Uehara, K.; Tanaka, M. Raman-spectra of chlorophyll forms. J. Mol. Struct. 1986, 146, 273–287. [Google Scholar] [CrossRef]
- Fujiwara, M.; Tasumi, M. Resonance Raman and infrared studies on axial coordination to chlorophylls-a and chlorophylls-b invitro. J. Phys. Chem-Us 1986, 90, 250–255. [Google Scholar] [CrossRef]
- Wrobel, D. Resonance Raman-spectra of chlorophylls dissolved in liquid-crystal matrices.1. The interaction between chlorophylls and a liquid-crystalline Mbba + Ebba mixture. Biophys Chem. 1987, 26, 91–99. [Google Scholar] [CrossRef]
- Mattioli, T.A.; Williams, J.C.; Allen, J.P.; Robert, B. Changes in primary donor hydrogen-bonding interactions in mutant reaction centers from Rhodobacter sphaeroides: Identification of the vibrational frequencies of all the conjugated carbonyl groups. Biochemistry 1994, 33, 1636–1643. [Google Scholar] [CrossRef]
- Spiedel, D.; Roszak, A.W.; McKendrick, K.; McAuley, K.E.; Fyfe, P.K.; Nabedryk, E.; Breton, J.; Robert, B.; Cogdell, R.J.; Isaacs, N.W.; et al. Tuning of the optical and electrochemical properties of the primary donor bacteriochlorophylls in the reaction centre from Rhodobacter sphaeroides: Spectroscopy and structure. Biochim. Biophys. Acta 2002, 1554, 75–93. [Google Scholar] [CrossRef]
- Feiler, U.; Mattioli, T.A.; Katheder, I.; Scheer, H.; Lutz, M. Effects of vinyl substitutions on Resonance Raman spectra of (Bacterio)chlorophylls. J. Raman Spectrosc. 1994, 25. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green natural colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef] [PubMed]
- Özkan, G.; Bilek, S.-E. Microencapsulation of natural food colourants. Int. J. Food Sci. Nutr. 2014, 3, 145–156. [Google Scholar]
- Shen, S.-C.; Hsu, H.-Y.; Huang, C.-N.; Wu, J.S.-B. Color loss in ethanolic solutions of chlorophyll a. J. Agric. Food Chem. 2010, 58, 8056–8060. [Google Scholar] [CrossRef]
- Yujie, Y.; Biru, H.; Zhiming, L.; Wenjian, W. Preparation of photostable chlorophyll/PVA film. Advan. Mater. Res. 2011, 239-242, 2707–2710. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik-Zabrotowicz, E.; Arczewska, M.; Zubik, M.; Terpilowski, K.; Skrzypek, T.H.; Swietlicka, I.; Gagos, M. Cremophor EL Nano-Emulsion Monomerizes Chlorophyll a in Water Medium. Biomolecules 2019, 9, 881. https://doi.org/10.3390/biom9120881
Janik-Zabrotowicz E, Arczewska M, Zubik M, Terpilowski K, Skrzypek TH, Swietlicka I, Gagos M. Cremophor EL Nano-Emulsion Monomerizes Chlorophyll a in Water Medium. Biomolecules. 2019; 9(12):881. https://doi.org/10.3390/biom9120881
Chicago/Turabian StyleJanik-Zabrotowicz, Ewa, Marta Arczewska, Monika Zubik, Konrad Terpilowski, Tomasz H. Skrzypek, Izabela Swietlicka, and Mariusz Gagos. 2019. "Cremophor EL Nano-Emulsion Monomerizes Chlorophyll a in Water Medium" Biomolecules 9, no. 12: 881. https://doi.org/10.3390/biom9120881
APA StyleJanik-Zabrotowicz, E., Arczewska, M., Zubik, M., Terpilowski, K., Skrzypek, T. H., Swietlicka, I., & Gagos, M. (2019). Cremophor EL Nano-Emulsion Monomerizes Chlorophyll a in Water Medium. Biomolecules, 9(12), 881. https://doi.org/10.3390/biom9120881