Combining Autoclaving with Mild Alkaline Solution as a Pretreatment Technique to Enhance Glucose Recovery from the Invasive Weed Chloris barbata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Processing of Weed Biomass
2.2. Analysis of Chemical Composition
2.3. Autoclave-Assisted Alkaline Pretreatment
2.4. Enzymatic Hydrolysis
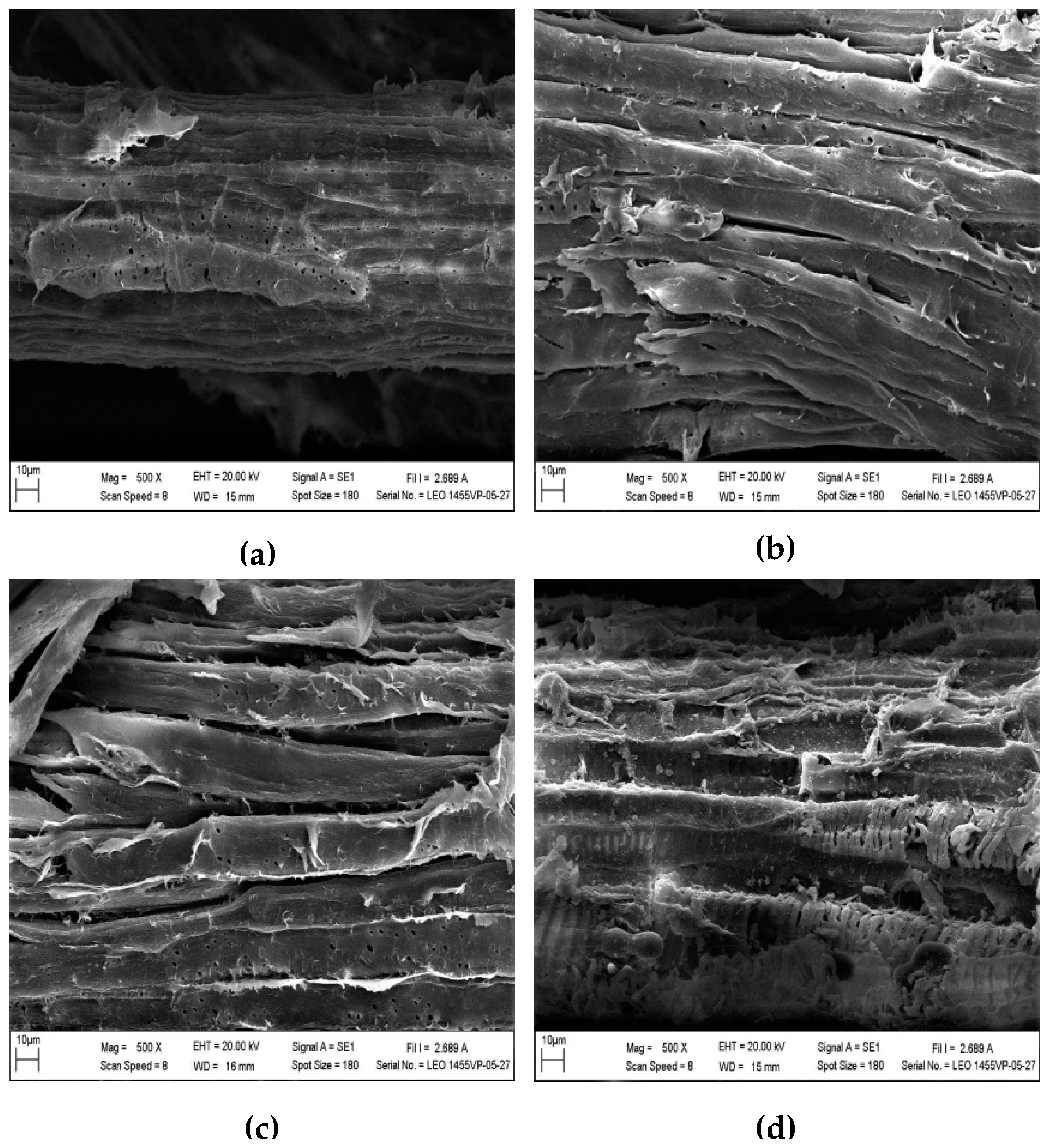
2.5. Surface Morphology of Chloris Barbata
2.6. Cellulose Crystallinity Index
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chloris barbata Characterization
3.2. Optimization of Sodium Hydroxide Concentration for Pretreatment
3.3. Optimization of Pretreatment Temperature
3.4. Characterization of Raw and Pretreated Chloris barbata by Scanning Electron Microscopy and X-ray Diffraction
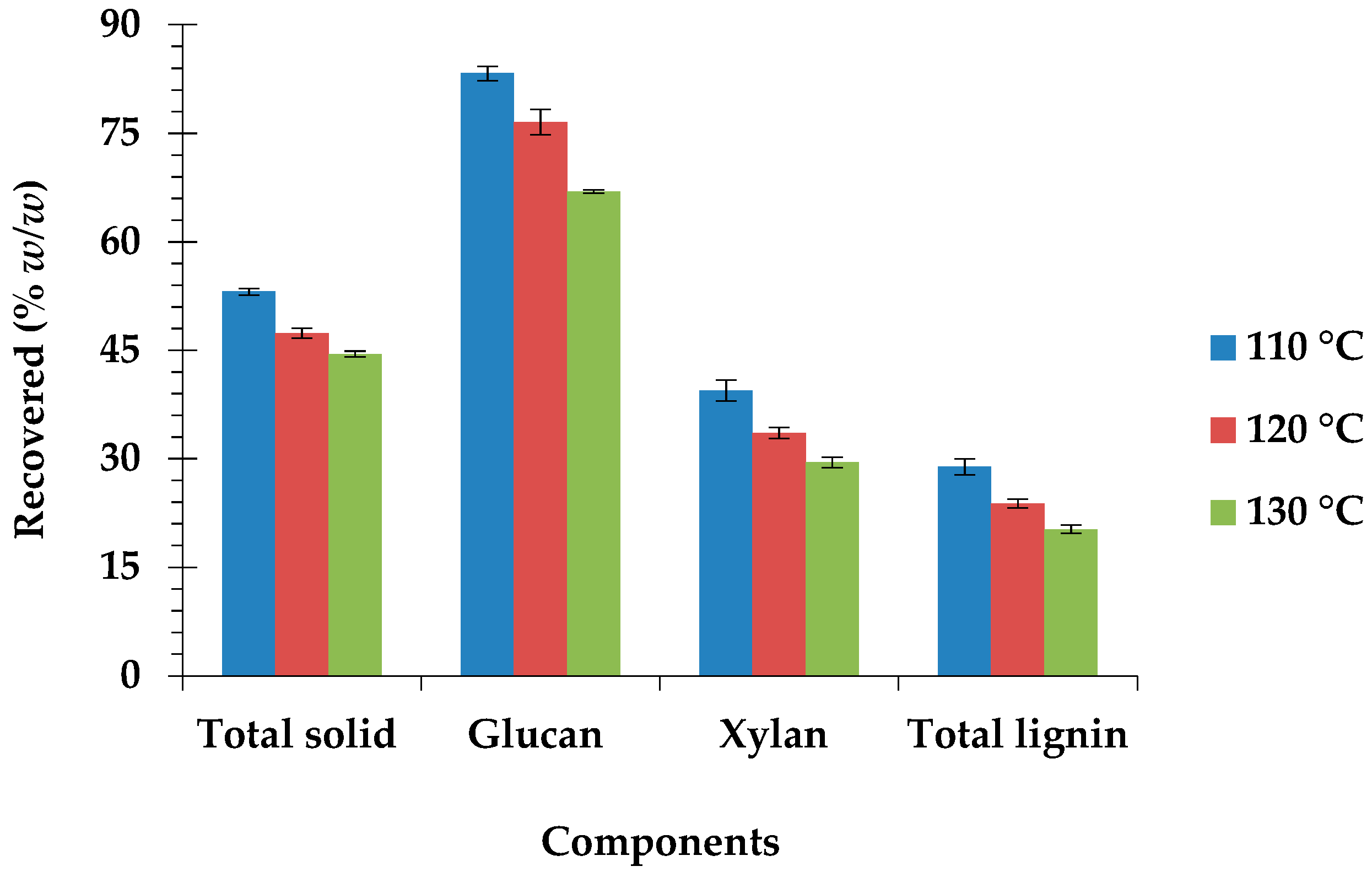
3.5. Material Balance for the Optimum Pretreatment Condition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borah, A.J.; Agarwal, M.; Poudyal, M.; Goyal, A.; Moholkar, V.S. Mechanistic investigation in ultrasound induced enhancement of enzymatic hydrolysis of invasive biomass species. Bioresour. Technol. 2016, 213, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Singh, S.; Goyal, A.; Moholkar, V. An Assessment of invasive weeds as multiple feedstocks for biofuels production. RSC Adv. 2016, 6, 47151–47163. [Google Scholar] [CrossRef]
- Gusain, R.; Suthar, S. Potential of aquatic weeds (Lemna gibba, Lemma minor, Pistia stratiotes and Eichhornia sp.) in biofuel production. Process Saf. Environ. 2017, 109, 233–241. [Google Scholar] [CrossRef]
- Ullah, K.; Sharma, V.K.; Dhingra, S.; Braccio, G.; Ahmad, M.; Sofia, S. Assessing the lignocellulosic biomass resources potential in developing countries: A critical review. Renew. Sustain. Energy Rev. 2015, 51, 682–698. [Google Scholar] [CrossRef]
- Fargione, J.; Hill, J.; Tilman, D.; Polasky, S.; Hawthorne, P. Land clearing and the biofuel carbon debt. Science 2008, 319, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Infante, P.A.; Moore, K.; Hurburgh, C.; Scott, P.; Archontoulis, S.; Lenssen, A.; Fei, S.-Z. Biomass production and composition of temperate and tropical maize in Central Iowa. Agronomy 2018, 8, 88. [Google Scholar] [CrossRef]
- Siripong, P.; Duangporn, P.; Takata, E.; Tsutsumi, Y. Phosphoric acid pretreatment of Achyranthes aspera and Sida acuta weed biomass to improve enzymatic hydrolysis. Bioresour. Technol. 2016, 203, 303–308. [Google Scholar] [CrossRef]
- Premjet, S.; Pumira, B.; Premjet, D. Determining the potential of inedible weed biomass for bio-energy and ethanol production. Bioresources 2013, 8, 701–716. [Google Scholar] [CrossRef]
- Choopayak, C.; Woranoot, K.; Naree, P.; Kongbangkerd, A.; Wongkrajang, K.; Buaruaeng, R. Phytotoxic effects of Piper betle L. extracts on germination of Eclipta prostrata L. and Chloris barbata Sw. weeds. NU. Int. J. Sci. 2015, 12, 11–24. [Google Scholar]
- De Menezes, F.F.; Rencoret, J.; Nakanishi, S.C.; Nascimento, V.M.; Silva, V.F.N.; Gutiérrez, A.; del Rio, J.C.; de Moraes Rocha, G.J. Alkaline pretreatment severity leads to different lignin applications in sugar cane biorefineries. ACS Sustain. Chem. Eng. 2017, 5, 5702–5712. [Google Scholar] [CrossRef]
- Chong, G.-G.; He, Y.-C.; Liu, Q.-X.; Kou, X.-Q.; Huang, X.-J.; Di, J.-H.; Ma, C.-L. Effective enzymatic in situ saccharification of bamboo shoot shell pretreated by dilute alkalic salts sodium hypochlorite/sodium sulfide pretreatment under the autoclave system. Bioresour. Technol. 2017, 241, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Obeng, A.K.; Premjet, D.; Premjet, S. A review article of biological pretreatment of agricultural biomass. Pertanika J. Trop. Agric. Sci. 2018, 41, 19–40. [Google Scholar]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Lv, X.; Xiong, C.; Li, S.; Chen, X.; Xiao, W.; Zhang, D.; Li, J.; Gong, Y.; Lin, J.; Liu, Z. Vacuum-assisted alkaline pretreatment as an innovative approach for enhancing fermentable sugar yield and decreasing inhibitor production of sugarcane bagasse. Bioresour. Technol. 2017, 239, 402–411. [Google Scholar] [CrossRef]
- Wu, H.; Dai, X.; Zhou, S.-L.; Gan, Y.-Y.; Xiong, Z.-Y.; Qin, Y.-H.; Ma, J.; Yang, L.; Wu, Z.-K.; Wang, T.-L.; et al. Ultrasound-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Bioresour. Technol. 2017, 241, 70–74. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Monte, L.S.; Escócio, V.A.; de Sousa, A.M.F.; Furtado, C.R.G.; Leite, M.C.A.M.; Visconte, L.L.Y.; Pacheco, E.B.A.V. Study of time reaction on alkaline pretreatment applied to rice husk on biomass component extraction. Biomass Convers. Biorefinery 2018, 8, 189–197. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Berikten, D.; Kıvanç, M.; Bozan, B. Ethanol production from hazelnut shells through enzymatic saccharification and fermentation by low-temperature alkali pretreatment. Fuel 2017, 196, 280–287. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, S.; Zhang, X.; Xu, F. Exploring crystalline-structural variations of cellulose during alkaline pretreatment for enhanced enzymatic hydrolysis. Bioresour. Technol. 2017, 224, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Gabhane, J.; William, S.P.M.P.; Vaidya, A.N.; Mahapatra, K.; Chakrabarti, T. Influence of heating source on the efficacy of lignocellulosic pretreatment—A cellulosic ethanol perspective. Biomass Bioenergy 2011, 35, 96–102. [Google Scholar] [CrossRef]
- Huang, W.; Wang, E.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Zhu, Q.; Lu, F. Effect of Physicochemical pretreatments and enzymatic hydrolysis on corn straw degradation and reducing sugar yield. BioResources 2017, 12, 7002–7015. [Google Scholar] [CrossRef]
- Ahmad Khan, N.; Booker, H.; Yu, P. Effect of heating method on alteration of protein molecular structure in flaxseed: Relationship with changes in protein subfraction profile and digestion in dairy cows. J. Agric. Food Chem. 2015, 63, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2012.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Obeng, A.; Premjet, D.; Premjet, S. Fermentable sugar production from the peels of two durian (Durio zibethinus Murr.) cultivars by phosphoric acid pretreatment. Resources 2018, 7, 60. [Google Scholar] [CrossRef]
- Bensah, E.C.; Kadar, Z.; Mensah, M.Y. Ethanol production from hydrothermally-treated biomass from West Africa. Bioresources 2015, 10, 6522–6537. [Google Scholar] [CrossRef]
- Udeh, B.A.; Erkurt, E.A. Compositional and structural changes in Phoenix canariensis and Opuntia ficus-indica with pretreatment: Effects on enzymatic hydrolysis and second generation ethanol production. Bioresour. Technol. 2017, 224, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.Y.; Lee, J.H.; Kim, D.S.; Lee, J.H.; Lee, S.K.; Lee, S.J.; Park, C.; Kim, S.W. Enhancement of glucose yield from canola agricultural residue by alkali pretreatment based on multi-regression models. J. Ind. Eng. Chem. 2017, 51, 303–311. [Google Scholar] [CrossRef]
- Cai, D.; Li, P.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Wang, Z.; Tan, T. Effect of dilute alkaline pretreatment on the conversion of different parts of corn stalk to fermentable sugars and its application in acetone–butanol–ethanol fermentation. Bioresour. Technol. 2016, 211, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bolado-Rodríguez, S.; Toquero, C.; Martín-Juárez, J.; Travaini, R.; García-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Sun, Y.; Li, L.; Kong, X.; Yuan, Z. Improving methane production from anaerobic digestion of Pennisetum Hybrid by alkaline pretreatment. Bioresour. Technol. 2018, 255, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Phitsuwan, P.; Sakka, K.; Ratanakhanokchai, K. Structural changes and enzymatic response of Napier grass (Pennisetum purpureum) stem induced by alkaline pretreatment. Bioresour. Technol. 2016, 218, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cheng, J.J. Pretreatment of switchgrass for sugar production with the combination of sodium hydroxide and lime. Bioresour. Technol. 2011, 102, 3861–3868. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cheng, J.J.; Sharma-Shivappa, R.R.; Burns, J.C. Sodium hydroxide pretreatment of switchgrass for ethanol production. Energy Fuels 2010, 24, 2113–2119. [Google Scholar] [CrossRef]
- Cai, D.; Li, P.; Chen, C.; Wang, Y.; Hu, S.; Cui, C.; Qin, P.; Tan, T. Effect of chemical pretreatments on corn stalk bagasse as immobilizing carrier of Clostridium acetobutylicum in the performance of a fermentation-pervaporation coupled system. Bioresour. Technol. 2016, 220, 68–75. [Google Scholar] [CrossRef]
- Pocan, P.; Bahcegul, E.; Oztop, M.H.; Hamamci, H. Enzymatic hydrolysis of fruit peels and other lignocellulosic biomass as a source of sugar. Waste Biomass Valoriz. 2018, 9, 929–937. [Google Scholar] [CrossRef]
- Kim, I.; Han, J.-I. Optimization of alkaline pretreatment conditions for enhancing glucose yield of rice straw by response surface methodology. Biomass Bioenergy 2012, 46, 210–217. [Google Scholar] [CrossRef]
- Premjet, S.; Premjet, D.; Yoo, H.Y.; Kim, S.W. Improvement of sugar recovery from Sida acuta (Thailand Weed) by NaOH pretreatment and application to bioethanol production. Korean J. Chem. Eng. 2018, 35, 2413–2420. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Zhang, P.; Jin, S.; Tao, X.; Tang, X.; Ye, J. Ultrasound assisted alkaline pretreatment to enhance enzymatic saccharification of grass clipping. Energy Convers. Manag. 2017, 149, 409–415. [Google Scholar] [CrossRef]
- Kacem, I.; Koubaa, M.; Maktouf, S.; Chaari, F.; Najar, T.; Chaabouni, M.; Ettis, N.; Chaabouni, S.E. Multistage process for the production of bioethanol from almond shell. Bioresour. Technol. 2016, 211, 154–163. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]



| Component | % dw (w/w) |
|---|---|
| Glucan | 36.88 ± 0.32 |
| Xylan | 17.23 ± 0.27 |
| Galactan | 1.35 ± 0.08 |
| Ethanol extractive | 7.45 ± 0.35 |
| Ash | 7.67 ± 0.25 |
| AIL | 14.78 ± 0.40 |
| ASL | 4.89 ± 0.18 |
| Total lignin | 19.67 ± 0.58 |
| Composition % dw (w/w) | Raw Biomass (% w/w) | 1 (% w/w) | 2 (% w/w) | 3 (% w/w) | 4 (% w/w) |
|---|---|---|---|---|---|
| Glucan | 36.88 ± 0.32 d | 50.30 ± 0.43 c | 59.62 ± 0.52 b | 61.06 ± 0.41 a | 62.03 ± 0.38 a |
| Xylan | 17.23 ± 0.27 a | 13.80 ± 0.32 b | 12.21 ± 0.24 c | 11.19 ± 0.31 d | 10.12 ± 0.45 e |
| ASL | 4.89 ± 0.18 a | 3.12 ± 0.10 b | 2.58 ± 0.13 c | 2.17 ± 0.11 d | 1.79 ± 0.11 e |
| AIL | 14.78 ± 0.40 a | 8.42 ± 0.22 b | 7.31 ± 0.37 c | 6.36 ± 0.34 d | 4.90 ± 0.24 e |
| Total lignin | 19.67 ± 0.58 a | 11.54 ± 0.25 b | 9.89 ± 0.40 c | 8.53 ± 0.44 d | 6.68 ± 0.14 e |
| Hydrolysis efficiency | 15.15 ± 0.56 d | 61.53 ± 0.86 c | 79.64 ± 0.72 b | 80.63 ± 0.53 ab | 82.26 ± 0.61 a |
| Glucose recovery | 6.20 ± 0.22 e | 19.77 ± 0.29 d | 24.96 ± 0.33 a | 22.43 ± 0.29 b | 21.23 ± 0.35 c |
| Composition % dw (w/w) | Raw Biomass (% w/w) | 110 °C | 120 °C | 130 °C |
|---|---|---|---|---|
| Glucan | 36.88 ± 0.32 d | 57.84 ± 0.36 b | 59.62 ± 0.52 a | 55.53 ± 0.44 c |
| Xylan | 17.23 ± 0.27 a | 12.78 ± 0.38 b | 12.21 ± 0.24 bc | 11.42 ± 0.40 c |
| ASL | 4.89 ± 0.18 a | 2.76 ± 0.11 b | 2.58 ± 0.13 bc | 2.28 ± 0.10 c |
| AIL | 14.78 ± 0.40 a | 7.92 ± 0.21 b | 7.31 ± 0.37 bc | 6.67 ± 0.25 c |
| Total lignin | 19.67 ± 0.58 a | 10.69 ± 0.32 b | 9.89 ± 0.40 bc | 8.95 ± 0.31 c |
| Hydrolysis efficiency | 15.15 ± 0.56 e | 90.03 ± 0.94 a | 79.64 ± 0.72 b | 70.29 ± 1.14 c |
| Glucose recovery | 6.20 ± 0.22 d | 30.70 ± 0.30 a | 24.96 ± 0.33 b | 19.27 ± 0.20 c |
| NaOH Concentration (%) | Temperature (°C) | CrI (%) |
|---|---|---|
| Raw biomass | 32.43 | |
| 1 | 120 | 46.68 |
| 2 | 120 | 52.27 |
| 3 | 120 | 56.38 |
| 4 | 120 | 61.24 |
| 2 | 110 | 61.53 |
| 2 | 120 | 52.27 |
| 2 | 130 | 47.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obeng, A.K.; Premjet, D.; Premjet, S. Combining Autoclaving with Mild Alkaline Solution as a Pretreatment Technique to Enhance Glucose Recovery from the Invasive Weed Chloris barbata. Biomolecules 2019, 9, 120. https://doi.org/10.3390/biom9040120
Obeng AK, Premjet D, Premjet S. Combining Autoclaving with Mild Alkaline Solution as a Pretreatment Technique to Enhance Glucose Recovery from the Invasive Weed Chloris barbata. Biomolecules. 2019; 9(4):120. https://doi.org/10.3390/biom9040120
Chicago/Turabian StyleObeng, Abraham Kusi, Duangporn Premjet, and Siripong Premjet. 2019. "Combining Autoclaving with Mild Alkaline Solution as a Pretreatment Technique to Enhance Glucose Recovery from the Invasive Weed Chloris barbata" Biomolecules 9, no. 4: 120. https://doi.org/10.3390/biom9040120





