Greensporone A, a Fungal Secondary Metabolite Suppressed Constitutively Activated AKT via ROS Generation and Induced Apoptosis in Leukemic Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Greensporone A from Aquatic Fungi
2.2. Chemicals and Reagents
2.3. Cell Culture
2.4. Cell Proliferation Assay
2.5. Cell Cycle Analysis
2.6. Annexin V/Propidium Iodide Dual Staining
2.7. Cell Lysis and Immunoblotting
2.8. Measurement of Mitochondrial Membrane Potential
2.9. Assay for Release of Cytochrome c
2.10. Measurement of Mitochondrial Superoxide
2.11. Measurement of Reactive Oxygen Species
2.12. Measurement of Reduced Glutathione
2.13. Gene Silencing of AKT Using Small Interference RNA
3. Statistical Analysis
4. Results
4.1. Isolation and Characterization of Greensporone A from an Aquatic Fungus
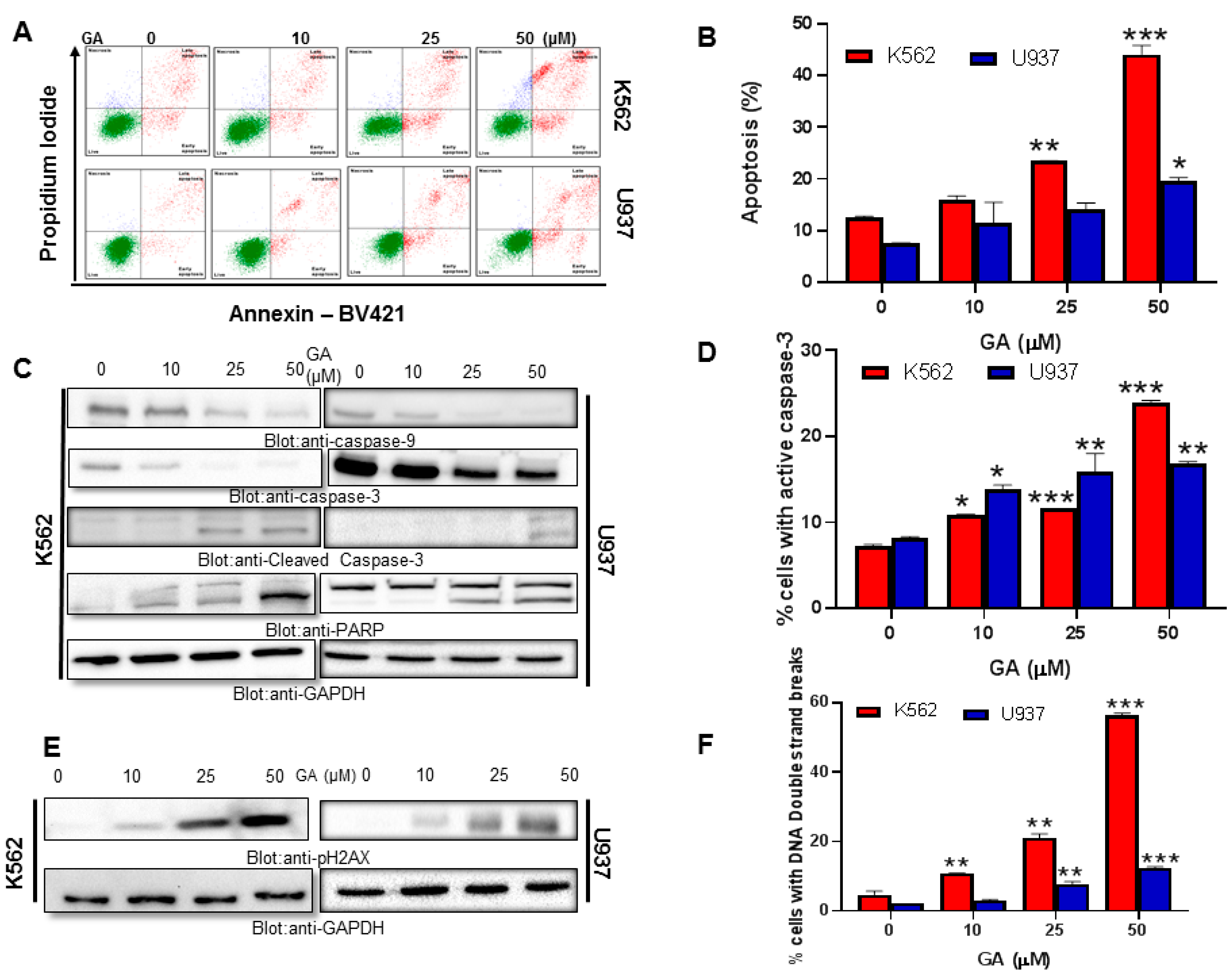
4.2. Effect of Greensporone A Treatment on Cell Proliferation and Apoptosis
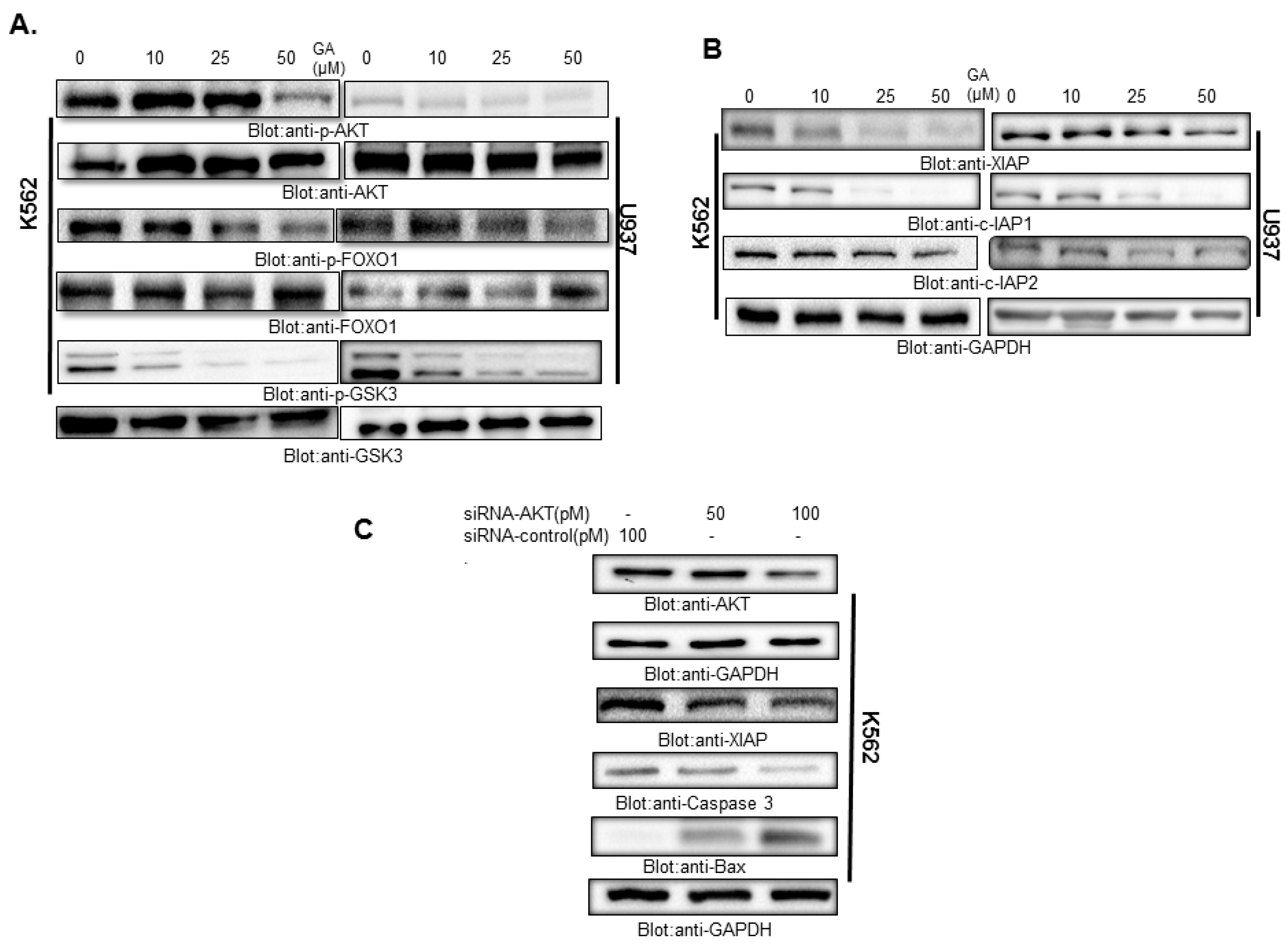
4.3. Treatment of Leukemic Cells with Greensporone A Suppressed the Activation of AKT and Its Associated Proteins
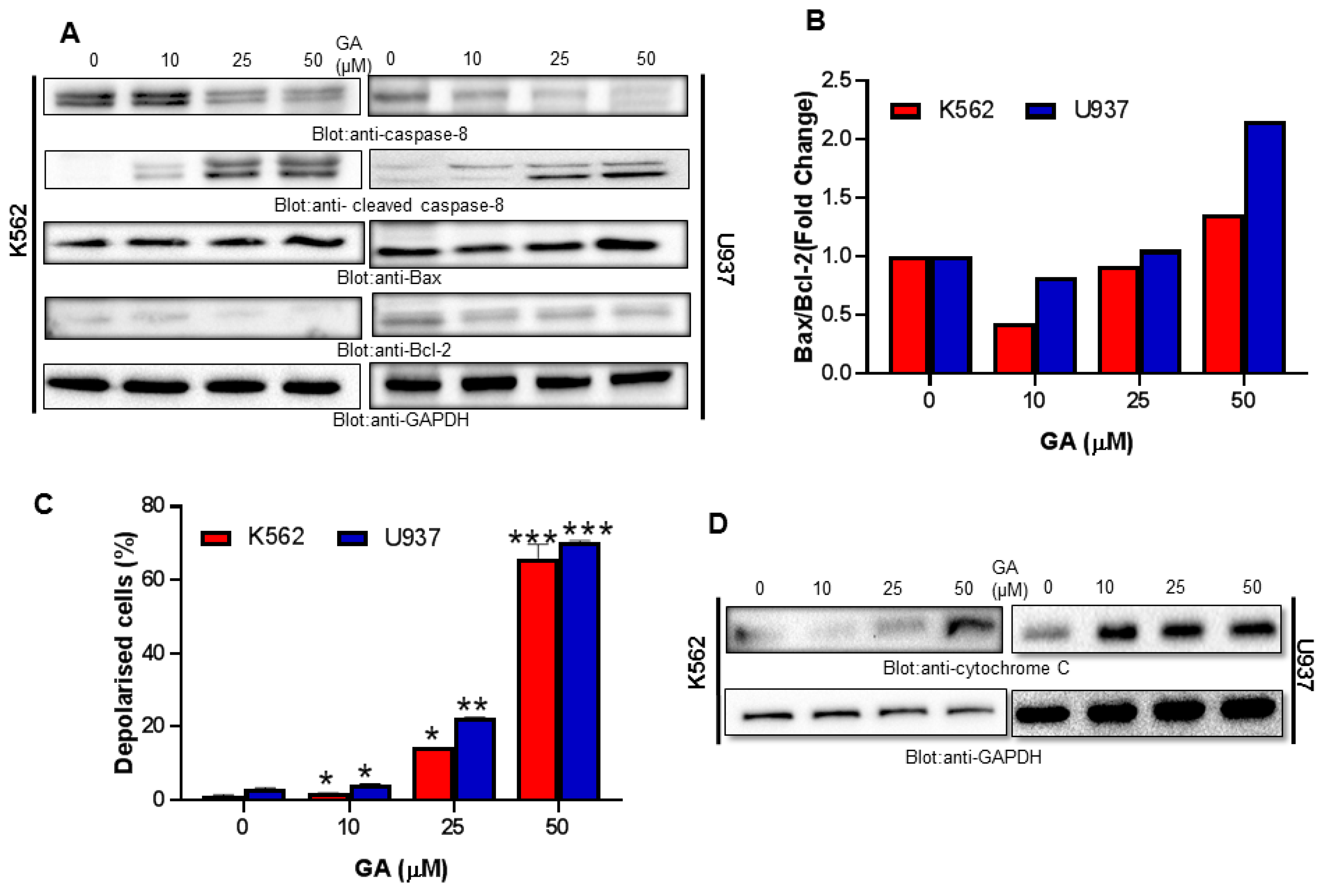
4.4. Greensporone A Treatment Results in Activation of Caspase-8 and Dose-Dependent Decrease in Bcl-2 Protein Causing an Increase in Bax/Bcl-2 Ratio in Leukemic Cells
4.5. Greensporone A-Mediated Activation of Mitochondrial Apoptotic Pathways in Leukemic Cells
4.6. Greensporone A-Mediated Generation of Reactive Oxygen Species (ROS) in Leukemic Cells
4.7. Effect of Greensporone A on Glutathione Leukemic Cell Lines
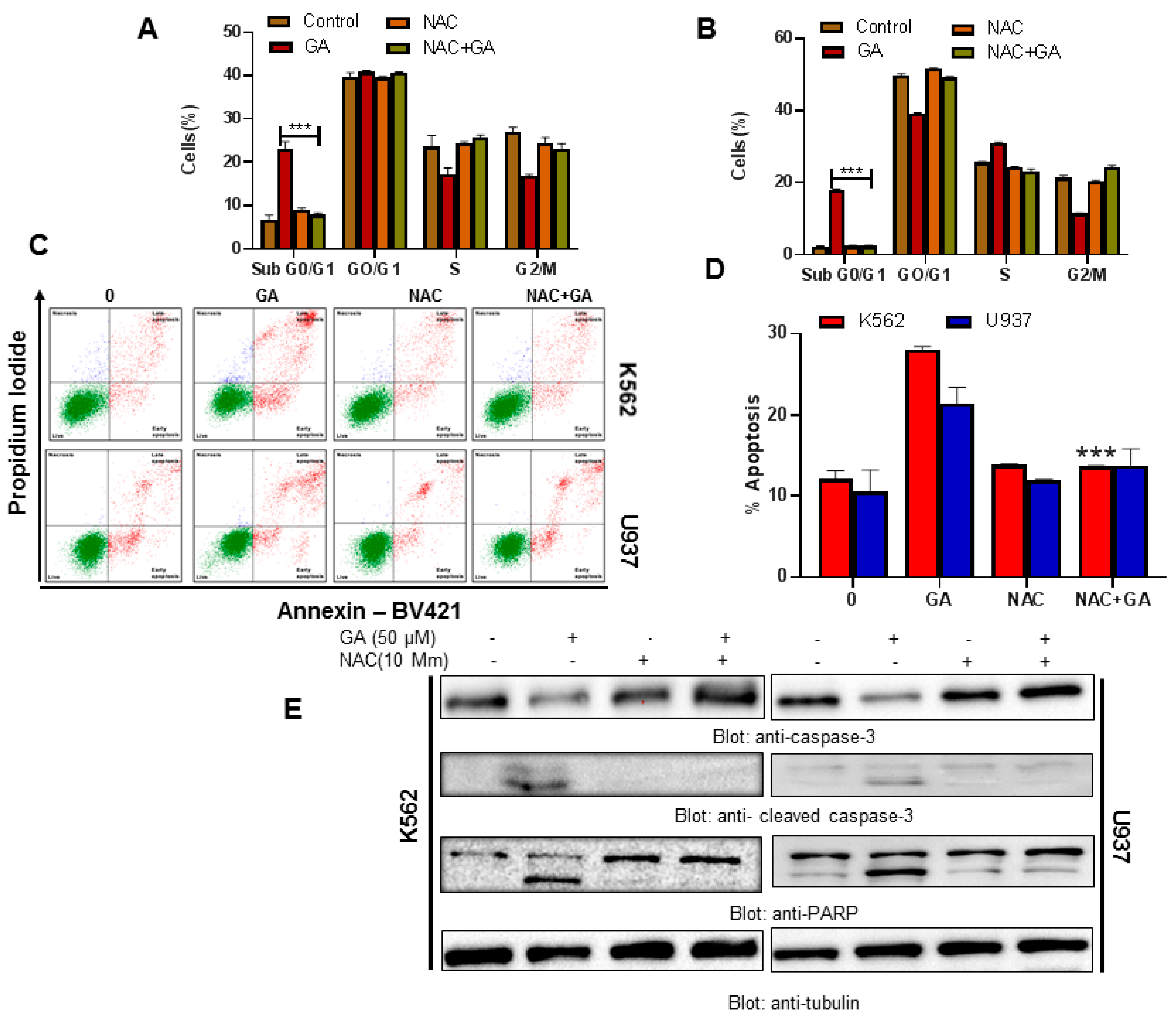
4.8. Greensporone A-Mediated ROS Generation Involved in Apoptotic Cell Death in Leukemic Cells
4.9. Cytotoxic Activity of Imatinib and Greensporone A in Leukemic Cell Lines
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McCormack, V.A.; Boffetta, P. Today’s lifestyles, tomorrow’s cancers: Trends in lifestyle risk factors for cancer in low- and middle-income countries. Ann. Oncol. 2011, 22, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S. Anticancer effect of the extracts from polyalthia evecta against human hepatoma cell line (hepg2). Asian. Pac. J. Trop Biomed. 2012, 2, 368–374. [Google Scholar] [CrossRef]
- Moyo, B.; Mukanganyama, S. Antiproliferative activity of t. Welwitschii extract on jurkat t cells in vitro. Biomed. Res. Int. 2015, 2015, 817624. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; EJ, D.E.B.; Lucas, D.M.; Rakotondraibe, H.L.; Orjala, J.; Soejarto, D.D.; Oberlies, N.H.; Pearce, C.J.; Wani, M.C.; Stockwell, B.R.; et al. Discovery of anticancer agents of diverse natural origin. Anticancer Res. 2016, 36, 5623–5637. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.; Kingston, D.G.I. Newman Anticancer Agents from Natural Products; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Raja, H.A.; Ayers, S.; Kurina, S.J.; Burdette, J.E.; Mattes, Z.; Sabatelle, R.; Bacon, J.W.; Colby, A.H.; Grinstaff, M.W.; et al. Meroterpenoids from neosetophoma sp.: A dioxa[4.3.3]propellane ring system, potent cytotoxicity, and prolific expression. Org. Lett. 2019, 21, 529–534. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Raja, H.A.; Day, C.S.; Chen, W.L.; Swanson, S.M.; Oberlies, N.H. Greensporones: Resorcylic acid lactones from an aquatic halenospora sp. J. Nat. Prod. 2014, 77, 2088–2098. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Xie, L.W.; Ouyang, Y.C.; Zou, K.; Wang, G.H.; Chen, M.J.; Sun, H.M.; Dai, S.K.; Li, X. Isolation and difference in anti-staphylococcus aureus bioactivity of curvularin derivates from fungus eupenicillium sp. Appl. Biochem. Biotechnol. 2009, 159, 284–293. [Google Scholar] [CrossRef]
- Buayairaksa, M.; Kanokmedhakul, S.; Kanokmedhakul, K.; Moosophon, P.; Hahnvajanawong, C.; Soytong, K. Cytotoxic lasiodiplodin derivatives from the fungus syncephalastrum racemosum. Arch. Pharm. Res. 2011, 34, 2037–2041. [Google Scholar] [CrossRef]
- Yao, X.S.; Ebizuka, Y.; Noguchi, H.; Kiuchi, F.; Shibuya, M.; Iitaka, Y.; Seto, H.; Sankawa, U. Biologically active constituents of arnebia euchroma: Structure of arnebinol, an ansa-type monoterpenylbenzenoid with inhibitory activity on prostaglandin biosynthesis. Chem. Pharm. Bull. (Tokyo) 1991, 39, 2956–2961. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.; Yuan, J.; Lu, Y.; Zhu, X.; Lin, Y.; Liu, L. Lasiodiplodins from mangrove endophytic fungus lasiodiplodia sp. 318. Nat. Prod. Res. 2016, 30, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qiao, K.; Gao, Z.; Meehan, M.J.; Li, J.W.; Zhao, X.; Dorrestein, P.C.; Vederas, J.C.; Tang, Y. Enzymatic synthesis of resorcylic acid lactones by cooperation of fungal iterative polyketide synthases involved in hypothemycin biosynthesis. J. Am. Chem. Soc. 2010, 132, 4530–4531. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Siveen, K.S.; Kuttikrishnan, S.; Iskandarani, A.N.; Khan, A.Q.; Merhi, M.; Omri, H.E.; Dermime, S.; El-Elimat, T.; Oberlies, N.H.; et al. Greensporone c, a freshwater fungal secondary metabolite induces mitochondrial-mediated apoptotic cell death in leukemic cell lines. Front. Pharmacol. 2018, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Iskandarani, A.; Bhat, A.A.; Siveen, K.S.; Prabhu, K.S.; Kuttikrishnan, S.; Khan, M.A.; Krishnankutty, R.; Kulinski, M.; Nasr, R.R.; Mohammad, R.M.; et al. Bortezomib-mediated downregulation of s-phase kinase protein-2 (skp2) causes apoptotic cell death in chronic myelogenous leukemia cells. Nutr. Metab. 2016, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Qiu, Y.; Shi, Y.; Cai, J.; Wang, B.; Wei, X.; Ke, Q.; Sui, X.; Wang, Y.; et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on t cell acute lymphoblastic leukemia cells. J. Hematol. Oncol. 2018, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of nf-kappab regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648. [Google Scholar] [CrossRef]
- Badmus, J.A.; Ekpo, O.E.; Hussein, A.A.; Meyer, M.; Hiss, D.C. Antiproliferative and apoptosis induction potential of the methanolic leaf extract of holarrhena floribunda (g. Don). Evid. Based Complement Alternat. Med. 2015, 2015, 756482. [Google Scholar] [CrossRef]
- Uddin, S.; Ah-Kang, J.; Ulaszek, J.; Mahmud, D.; Wickrema, A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. Proc. Natl. Acad. Sci. USA 2004, 101, 147–152. [Google Scholar] [CrossRef]
- Uddin, S.; Hussain, A.R.; Manogaran, P.S.; Al-Hussein, K.; Platanias, L.C.; Gutierrez, M.I.; Bhatia, K.G. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene 2005, 24, 7022–7030. [Google Scholar] [CrossRef]
- Syn, G.; Anderson, D.; Blackwell, J.M.; Jamieson, S.E. Toxoplasma gondii infection is associated with mitochondrial dysfunction in-vitro. Front. Cell. Infect. Microbiol. 2017, 7, 512. [Google Scholar] [CrossRef]
- Weidner, C.; Rousseau, M.; Plauth, A.; Wowro, S.J.; Fischer, C.; Abdel-Aziz, H.; Sauer, S. Iberis amara extract induces intracellular formation of reactive oxygen species and inhibits colon cancer. PLoS ONE 2016, 11, e0152398. [Google Scholar] [CrossRef]
- Morzadec, C.; Macoch, M.; Sparfel, L.; Kerdine-Romer, S.; Fardel, O.; Vernhet, L. Nrf2 expression and activity in human t lymphocytes: Stimulation by t cell receptor activation and priming by inorganic arsenic and tert-butylhydroquinone. Free Radic. Biol. Med. 2014, 71, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, B.; Yang, J.; Ma, X.; Deng, S.; Huang, Y.; Wen, Y.; Yuan, J.; Yang, X. Essential oil derived from eupatorium adenophorum spreng. Mediates anticancer effect by inhibiting stat3 and akt activation to induce apoptosis in hepatocellular carcinoma. Front. Pharmacol. 2018, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Uddin, S.; Bu, R.; Khan, O.S.; Ahmed, S.O.; Ahmed, M.; Al-Kuraya, K.S. Resveratrol suppresses constitutive activation of akt via generation of ros and induces apoptosis in diffuse large b cell lymphoma cell lines. PLoS ONE 2011, 6, e24703. [Google Scholar] [CrossRef] [PubMed]
- Rivera Rivera, A.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-breast cancer potential of quercetin via the akt/ampk/mammalian target of rapamycin (mtor) signaling cascade. PLoS ONE 2016, 11, e0157251. [Google Scholar] [CrossRef]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.J.; Xu, J.; Zheng, W. Foxo signaling pathways as therapeutic targets in cancer. Int. J. Biol. Sci. 2017, 13, 815–827. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chan, C.Y.; Chou, I.T.; Lien, C.H.; Hung, H.C.; Lee, M.F. Quercetin induces growth arrest through activation of foxo1 transcription factor in egfr-overexpressing oral cancer cells. J. Nutr. Biochem. 2013, 24, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Guisado, E.; Lorenzo-Benayas, M.J.; Fernandez-Salguero, P.M. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: Relevance in cell proliferation. Int. J. Cancer 2004, 109, 167–173. [Google Scholar] [CrossRef]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Busselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (iaps). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef]
- Dan, H.C.; Sun, M.; Kaneko, S.; Feldman, R.I.; Nicosia, S.V.; Wang, H.G.; Tsang, B.K.; Cheng, J.Q. Akt phosphorylation and stabilization of x-linked inhibitor of apoptosis protein (xiap). J. Biol. Chem. 2004, 279, 5405–5412. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.J.; Park, P.G.; Cha, H.R.; Ahn, S.G.; Kim, M.J.; Kim, H.; Koo, J.S.; Jeong, J.; Park, J.H.; Dong, S.M.; et al. Cellular inhibitor of apoptosis protein 2 promotes the epithelial-mesenchymal transition in triple-negative breast cancer cells through activation of the akt signaling pathway. Oncotarget 2017, 8, 78781–78795. [Google Scholar] [CrossRef]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The bax/bcl-2 ratio determines the susceptibility of human melanoma cells to cd95/fas-mediated apoptosis. J. Invest. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; El Rouby, S.; Reed, J.C.; Krajewski, S.; Silber, R.; Potmesil, M.; Newcomb, E.W. Drug-induced apoptosis in b-cell chronic lymphocytic leukemia: Relationship between p53 gene mutation and bcl-2/bax proteins in drug resistance. Oncogene 1996, 12, 1055–1062. [Google Scholar] [PubMed]
- Pepper, C.; Hoy, T.; Bentley, D.P. Bcl-2/bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br. J. Cancer 1997, 76, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef]
- Thamizhiniyan, V.; Young-Woong, C.; Young-Kyoon, K. The cytotoxic nature of acanthopanax sessiliflorus stem bark extracts in human breast cancer cells. Saudi. J. Biol. Sci. 2015, 22, 752–759. [Google Scholar] [CrossRef]
- Cai, Z.; Lin, M.; Wuchter, C.; Ruppert, V.; Dorken, B.; Ludwig, W.D.; Karawajew, L. Apoptotic response to homoharringtonine in human wt p53 leukemic cells is independent of reactive oxygen species generation and implicates bax translocation, mitochondrial cytochrome c release and caspase activation. Leukemia 2001, 15, 567–574. [Google Scholar] [CrossRef]
- Looi, C.Y.; Arya, A.; Cheah, F.K.; Muharram, B.; Leong, K.H.; Mohamad, K.; Wong, W.F.; Rai, N.; Mustafa, M.R. Induction of apoptosis in human breast cancer cells via caspase pathway by vernodalin isolated from centratherum anthelminticum (l.) seeds. PloS ONE 2013, 8, e56643. [Google Scholar] [CrossRef]
- Chandra, D.; Liu, J.W.; Tang, D.G. Early mitochondrial activation and cytochrome c up-regulation during apoptosis. J. Biol. Chem. 2002, 277, 50842–50854. [Google Scholar] [CrossRef]
- Pawlowski, J.; Kraft, A.S. Bax-induced apoptotic cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 529–531. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; G, M.M.; G, S.; Malla, R. Reactive oxygen species: A key constituent in cancer survival. Biomark Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Lee, C.W.; Yen, F.L.; Ko, H.H.; Li, S.Y.; Chiang, Y.C.; Lee, M.H.; Tsai, M.H.; Hsu, L.F. Cudraflavone c induces apoptosis of a375.S2 melanoma cells through mitochondrial ros production and mapk activation. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. Ros inhibitor n-acetyl-l-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase akt pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hall, T.; Eathiraj, S.; Wick, M.J.; Schwartz, B.; Abbadessa, G. In-vitro and in-vivo combined effect of arq 092, an akt inhibitor, with arq 087, a fgfr inhibitor. Anticancer Drugs 2017, 28, 503–513. [Google Scholar] [CrossRef]
- Uddin, S.; Hussain, A.R.; Siraj, A.K.; Manogaran, P.S.; Al-Jomah, N.A.; Moorji, A.; Atizado, V.; Al-Dayel, F.; Belgaumi, A.; El-Solh, H.; et al. Role of phosphatidylinositol 3’-kinase/akt pathway in diffuse large b-cell lymphoma survival. Blood 2006, 108, 4178–4186. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Yunes, J.A.; Cardoso, B.A.; Martins, L.R.; Jotta, P.Y.; Abecasis, M.; Nowill, A.E.; Leslie, N.R.; Cardoso, A.A.; Barata, J.T. Pten posttranslational inactivation and hyperactivation of the pi3k/akt pathway sustain primary t cell leukemia viability. J. Clin. Invest. 2008, 118, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, V.; St-Germain, M.E.; Parent, S.; Asselin, E. Akt activity in endometrial cancer cells: Regulation of cell survival through ciap-1. Int. J. Oncol. 2003, 23, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Seol, D.W. Up-regulation of iaps by pi-3k: A cell survival signal-mediated anti-apoptotic mechanism. Biochem. Biophys. Res. Commun. 2008, 377, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Achkar, I.W.; Kuttikrishnan, S.; Akhtar, S.; Khan, A.Q.; Siveen, K.S.; Uddin, S. Embelin: A benzoquinone possesses therapeutic potential for the treatment of human cancer. Future Med. Chem. 2018, 10, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Kabeer, F.A.; Rajalekshmi, D.S.; Nair, M.S.; Prathapan, R. Molecular mechanisms of anticancer activity of deoxyelephantopin in cancer cells. Integr. Med. Res. 2017, 6, 190–206. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhu, K.S.; Siveen, K.S.; Kuttikrishnan, S.; Jochebeth, A.; Ali, T.A.; Elareer, N.R.; Iskandarani, A.; Quaiyoom Khan, A.; Merhi, M.; Dermime, S.; et al. Greensporone A, a Fungal Secondary Metabolite Suppressed Constitutively Activated AKT via ROS Generation and Induced Apoptosis in Leukemic Cell Lines. Biomolecules 2019, 9, 126. https://doi.org/10.3390/biom9040126
Prabhu KS, Siveen KS, Kuttikrishnan S, Jochebeth A, Ali TA, Elareer NR, Iskandarani A, Quaiyoom Khan A, Merhi M, Dermime S, et al. Greensporone A, a Fungal Secondary Metabolite Suppressed Constitutively Activated AKT via ROS Generation and Induced Apoptosis in Leukemic Cell Lines. Biomolecules. 2019; 9(4):126. https://doi.org/10.3390/biom9040126
Chicago/Turabian StylePrabhu, Kirti S., Kodappully S. Siveen, Shilpa Kuttikrishnan, Anh Jochebeth, Tayyiba A. Ali, Noor R. Elareer, Ahmad Iskandarani, Abdul Quaiyoom Khan, Maysaloun Merhi, Said Dermime, and et al. 2019. "Greensporone A, a Fungal Secondary Metabolite Suppressed Constitutively Activated AKT via ROS Generation and Induced Apoptosis in Leukemic Cell Lines" Biomolecules 9, no. 4: 126. https://doi.org/10.3390/biom9040126
APA StylePrabhu, K. S., Siveen, K. S., Kuttikrishnan, S., Jochebeth, A., Ali, T. A., Elareer, N. R., Iskandarani, A., Quaiyoom Khan, A., Merhi, M., Dermime, S., El-Elimat, T., Oberlies, N. H., Alali, F. Q., Steinhoff, M., & Uddin, S. (2019). Greensporone A, a Fungal Secondary Metabolite Suppressed Constitutively Activated AKT via ROS Generation and Induced Apoptosis in Leukemic Cell Lines. Biomolecules, 9(4), 126. https://doi.org/10.3390/biom9040126






