Encapsulation of Antihypertensive Peptides from Whey Proteins and Their Releasing in Gastrointestinal Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Whey Protein Hydrolysis
2.3. Fractionation of Hydrolysate Product
2.4. Determination of ACE Inhibitory Activity of Whey Peptide Fractions
2.5. Encapsulation of the Peptide Fractions
2.6. Simulated Gastrointestinal Digestion of Encapsulated Peptide Fractions
2.7. Analysis and Identification of Amino Acid Sequences from the Released Peptides in Simulated Gastrointestinal Digestion from the Encapsulated f4 Fraction
2.8. Statistical Analysis
3. Results and Discussion
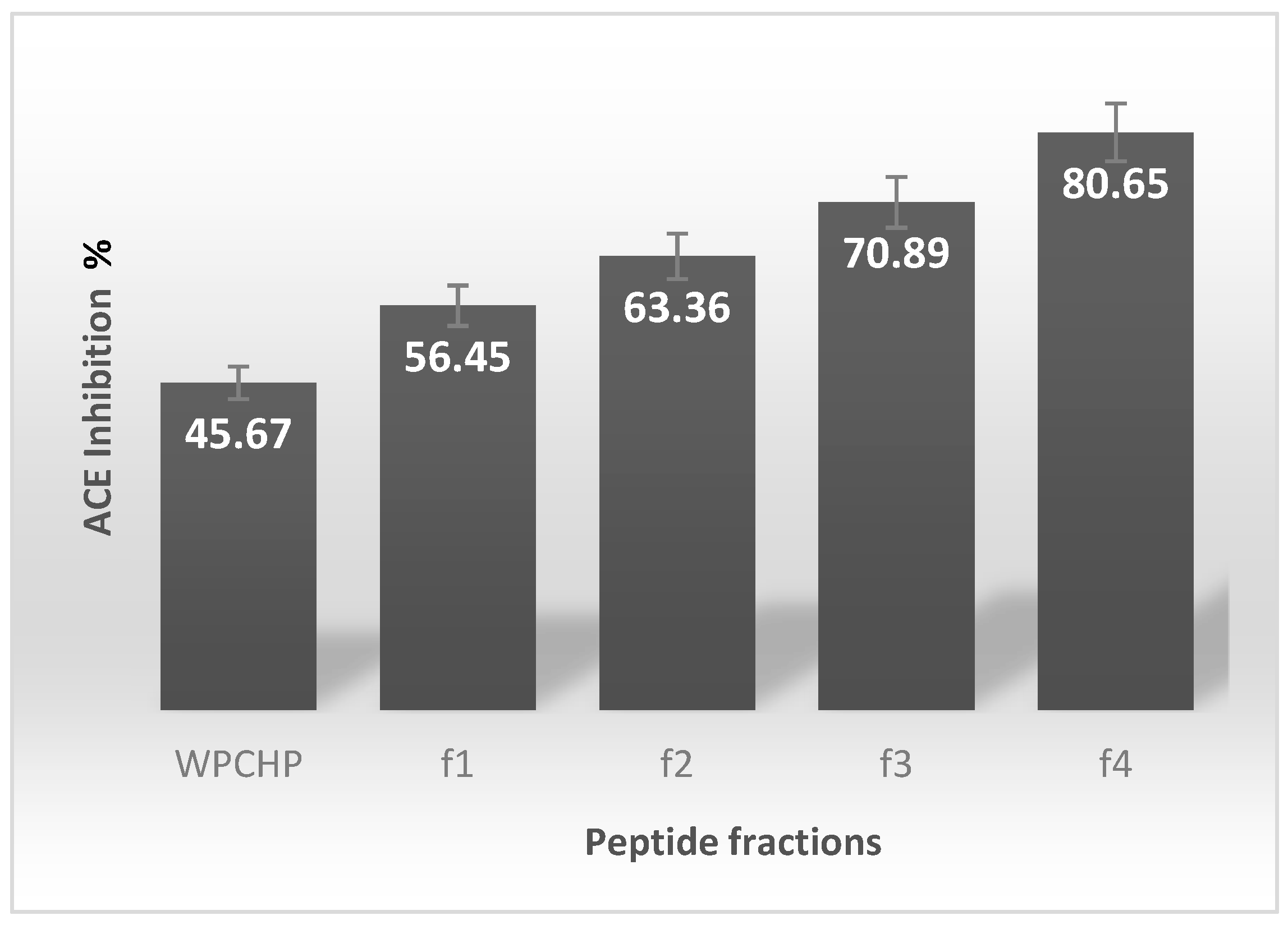
3.1. ACE Inhibitory Activity of Hydrolysate Fractions
3.2. Capsules Characteristics and Encapsulation Efficiency
3.3. Simulated Gastrointestinal Digestion of ACE-Inhibitory Peptide Fractions
3.4. Amino Acid Sequences from Released Peptides in Simulated Gastrointestinal Digestion from Encapsulated f4 Fraction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muro, U.C.; Álvarez, F.R.; Riera, R.F.; Arana, C.A.; Téllez, J.A. Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011, 17, 293–317. [Google Scholar] [CrossRef] [PubMed]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey protein hydrolysates as a source of bioactive peptides for functional foods–Biotechnological facilitation of industrial scale-up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Rocha, G.F.; Kise, F.; Rosso, A.M.; Parisi, M.G. Potential antioxidant peptides produced from whey hydrolysis with an immobilized aspartic protease from Salpichroa origanifolia fruits. Food Chem. 2017, 237, 350–355. [Google Scholar] [CrossRef]
- Muro, U.C.; Riera, R.F.; Alvarado, P.Y. Encapsulation of whey proteins. In Whey Proteins Functional Properties Production and Health Benefits; Wyatt, M., Ed.; Nova Publishers: New York, NY, USA, 2014; pp. 75–116. [Google Scholar]
- Alvarado, P.Y.; Muro, U.C.; Martínez, J.; Nava, M.; Rodríguez, F. Functionalized polymers for enhance oral bioavailability of sensitive molecules. Polymers 2016, 8, 1–22. [Google Scholar]
- Alvarado, P.Y.; Muro, U.C.; Cerda, A.M.; Sánchez, J.A.; Rodríguez, F.R. Antihypertensive and Antioxidant Properties from Whey Protein Hydrolysates Produced by Encapsulated Bacillus subtilis Cells. Int. J. Pep. Res. Ther. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, S.R.; He, Q.S.; Bazinet, L.; Udenigwe, C.C. Encapsulation of food protein hydrolysates and peptides: A review. RSC Adv. 2015, 5, 79270–79278. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Yu, C.Y.; Yin, B.C.; Zhang, W.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Coll. Surf. B. 2009, 68, 245–249. [Google Scholar] [CrossRef]
- Yang, S.; Mao, X.Y.; Li, F.F.; Zhang, D.; Leng, X.J.; Ren, F.Z.; Teng, G.X. The improving effect of spray-drying encapsulation process on the bitter taste and stability of whey protein hydrolysate. Eur. Food Res. Technol. 2012, 235, 91–97. [Google Scholar] [CrossRef]
- Kavianinia, I.; Plieger, P.G.; Kandile, N.G.; Harding, D.R. In vitro evaluation of spray-dried chitosan microspheres crosslinked with pyromellitic dianhydride for oral colon-specific delivery of protein drugs. J. Appl. Polym. Sci. 2014, 131, 1–13. [Google Scholar] [CrossRef]
- Ma, J.J.; Mao, X.Y.; Wang, Q.; Yang, S.; Zhang, D.; Chen, S.W.; Li, Y.H. Effect of spray drying and freeze drying on the immunomodulatory activity, bitter taste and hygroscopicity of hydrolysate derived from whey protein concentrate. LWT Food Sci. Technol. 2014, 56, 296–302. [Google Scholar] [CrossRef]
- Mohan, A.; McClements, D.J.; Udenigwe, C.C. Encapsulation of bioactive whey peptides in soy lecithin-derived nanoliposomes: Influence of peptide molecular weight. Food Chem. 2016, 213, 143–148. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Tu, Z.C.; Xiao, H.; Wang, H.; Huang, X.Q.; Liu, G.X.; Lin, D.R. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod. Process. 2014, 92, 30–37. [Google Scholar] [CrossRef]
- Kostadinova, A.I.; Middelburg, J.; Ciulla, M.; Garssen, J.; Hennink, W.E.; Knippels, L.M.; Willemsen, L.E. PLGA nanoparticles loaded with beta-lactoglobulin-derived peptides modulate mucosal immunity and may facilitate cow’s milk allergy prevention. Eur. J. Pharmacol. 2018, 818, 211–220. [Google Scholar] [CrossRef]
- Gomez-Mascaraque, L.G.; Morfin, R.C.; Pérez-Masiá, R.; Sanchez, G.; Lopez-Rubio, A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT Food Sci. Technol. 2016, 69, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. In-vitro digestion of different forms of bovine lactoferrin encapsulated in alginate micro-gel particles. Food Hydrocoll. 2016, 52, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Giroux, H.J.; Robitaille, G.; Britten, M. Controlled release of casein-derived peptides in the gastrointestinal environment by encapsulation in water-in-oil-in-water double emulsions. LWT Food Sci. Technol. 2016, 69, 225–232. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Dufour, C. A standardized static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- UniProt database. The UniProt Consortium UniProt: The Universal Protein Knowledgebase Nucleic Acids Res. 45: D158-D169, 2019. Available online: http://www.uniprot.org/peptidesearch/ (accessed on 19 September 2018).
- Le Maux, S.; Nongonierma, A.B.; FitzGerald, R.J. Improved short peptide identification using HILIC–MS/MS: Retention time prediction model based on the impact of amino acid position in the peptide sequence. Food Chem. 2015, 173, 847–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemil, I.; Abdelhedi, O.; Nasri, R.; Mora, L.; Jridi, M.; Aristoy, M.C.; Nasri, M. Novel bioactive peptides from enzymatic hydrolysate of Sardinelle (Sardinella aurita) muscle proteins hydrolysed by Bacillus subtilis A26 proteases. Food Res. Int. 2017, 100, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Yamauchi, R.; Iwamoto, S. Stability of bioactive compounds from Thai Riceberry bran extract encapsulated within gelatin matrix during in vitro gastrointestinal digestion. Coll. Surf. A Physicochem. Eng. Aspects 2018, 546, 136–142. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure−Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 4, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Paulson, T.; Rousseau, D. Biopolymers in controlled-release delivery systems. In Modern Biopolymer Science; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Academic Press: Philadelphia, PA, USA, 2009; pp. 519–557. [Google Scholar]
- Groening, R.; Bensmann, H. High frequency controlled capsules with integrated gas producing cells. Eur. J. Pharm. Biophar. 2009, 72, 282–284. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; McClements, D.J. Protein encapsulation in alginate hydrogel beads: Effect of pH on microgel stability, protein retention and protein release. Food Hydrocoll. 2016, 58, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Yamauchi, T.; Katsuda, T.; Yamaji, H.; Katoh, S. Angiotensin-I converting enzyme (ACE) inhibitory mechanism of tripeptides containing aromatic residues. J. Biosci. Bioeng. 2008, 106, 310–312. [Google Scholar] [CrossRef]
- Cicero, A.F.; Borghi, C. Evidence of clinically relevant efficacy for dietary supplements and nutraceuticals. Curr. Hypertens. Rep. 2013, 15, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.G.; Monteiro, K.M.; Possenti, A.; Pintado, M.E.; Carvalho, J.E.; Malcata, F. X Antiulcerogenic activity of peptide concentrates obtained from hydrolysis of whey proteins by proteases from Cynara cardunculus. Int. Dairy J. 2011, 21, 934–939. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Ahmed, A.S.; Miyata, T. Novel angiotensin-converting enzyme inhibitory peptides from caseins and whey proteins of goat milk. J. Adv. Res. 2017, 8, 63–71. [Google Scholar] [CrossRef] [PubMed]



| Capsules | Pore Volume (cm3/g) | Surface Area (m2/g) | Pore Diameter (nm) | Encapsulation Efficiency % |
|---|---|---|---|---|
| SA | 0.0252 | 1.003 | 6.9 | 70 |
| SA-CO | 0.0019 | 1.405 | 5.5 | 90 |
| SA-AG | 0.0016 | 1.082 | 6.1 | 95 |
| SA-GE | 0.0018 | 1.95 | 3.8 | 80 |
| Gastric Phase | Time (min) | Ratio of Released Peptides (mg/L) | ACE Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Loaded Capsules of f4 | Non-encapsulated f4 | Loaded Capsules of f4 | Non-encapsulated f4 | ||||||
| SA-CO | SA-AG | SA-GE | SA-CO | SA-AG | SA-GE | ||||
| Oral | 10 | 1.5 | 0.5 | 1.0 | 2.3 | 25.3 | 9.6 | 18.6 | 15.2 |
| Gastric | 120 | 2.1 | 2.7 | 1.6 | 0.8 | 39.5 | 57.4 | 30.3 | 2.7 |
| Duodenal | 120 | 0.3 | 0.6 | 1.4 | 0.1 | 9.5 | 20.4 | 5.0 | 0.0 |
| Total | 250 | 3.9 | 3.8 | 4.0 | 3.8 | 74.3 | 86.4 | 53.9 | 17.9 |
| Sample Fraction f4 | Digest Phase | Released Fragments/Suggested Sequence | Identified Sequence | Experimental Mass (Da) | Theoretical Mass (Da) | Protein Origin |
|---|---|---|---|---|---|---|
| Non-encapsulated | Undigested | 1. VNLSMYNGIAL 2. ITPAVQMN 3.TVVSAPNYTLR 4. VAGTWY 5.LMTGYPVILYP | VAGTWY | 695.4 | 695.2 | β-Lg |
| Non-encapsulated | Gastric | 1.SAPLR 2.GTW 3. TYV | SAPLR | 543.6 | 543.2 | β-Lg |
| Capsules SA-CO | Gastric | 1. VLDTDYK 2. PAVQM 3. TSGYPV | VLDTDYK | 852.6 | 852.4 | β-Lg |
| Duodenal | 1.VDY 2.KIDAL | KIDAL | 558.1 | 558.3 | β-Lg | |
| Capsules SA-AG | Gastric | 1. ENSAEP 2. IPAVFK 3. VAGTWY 4. VSYT | ENSAEP IPAVFK VAGTWY | 645.5 673.7 695.4 | 645.3 673.4 695.2 | β-Lg |
| Duodenal | 1. VYT 2. AMAASDE 3. IPAVF 4. MDSF | IPAVF | 545.5 | 545.3 | β-Lg | |
| Capsules SA-GE | Gastric | 1.DGFYELYAME 2.VKTYMNATIK 3. KIPAVF | KIPAVF | 673.1 | 673.4 | β-Lg |
| Duodenal | 1. SAAGYT 2. DFELY 3. HTSGY | HTSGY | 563.2 | 563.2 | α-La |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado, Y.; Muro, C.; Illescas, J.; Díaz, M.d.C.; Riera, F. Encapsulation of Antihypertensive Peptides from Whey Proteins and Their Releasing in Gastrointestinal Conditions. Biomolecules 2019, 9, 164. https://doi.org/10.3390/biom9050164
Alvarado Y, Muro C, Illescas J, Díaz MdC, Riera F. Encapsulation of Antihypertensive Peptides from Whey Proteins and Their Releasing in Gastrointestinal Conditions. Biomolecules. 2019; 9(5):164. https://doi.org/10.3390/biom9050164
Chicago/Turabian StyleAlvarado, Yolanda, Claudia Muro, Javier Illescas, María del Carmen Díaz, and Francisco Riera. 2019. "Encapsulation of Antihypertensive Peptides from Whey Proteins and Their Releasing in Gastrointestinal Conditions" Biomolecules 9, no. 5: 164. https://doi.org/10.3390/biom9050164





