IL7RA rs6897932 Polymorphism Is Associated with Better CD4+ T-Cell Recovery in HIV Infected Patients Starting Combination Antiretroviral Therapy
Abstract
1. Background
2. Objective
3. Methods
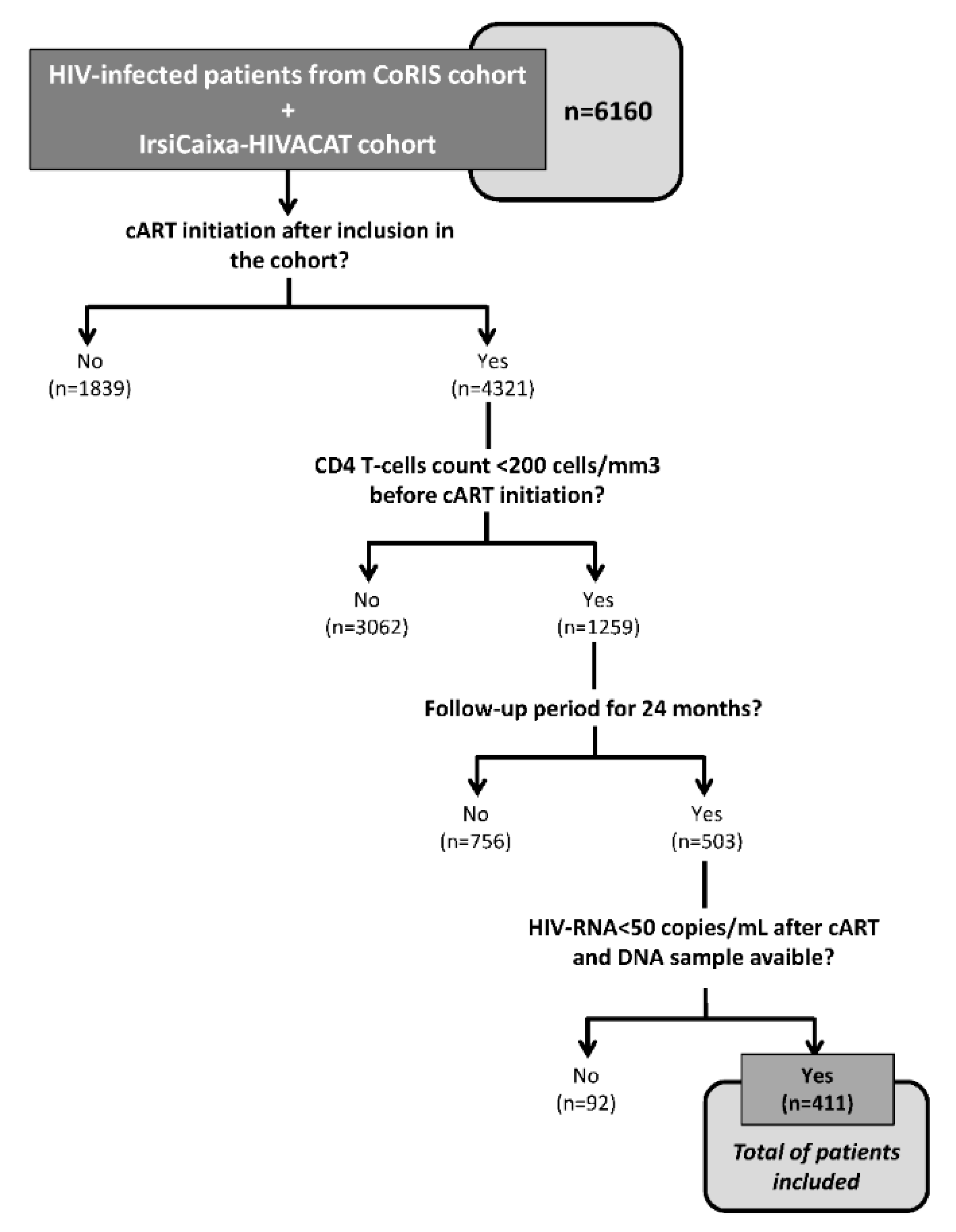
3.1. Study Population
3.2. DNA Genotyping
3.3. Outcome Variables
3.4. Statistical Analysis
4. Results
4.1. Characteristics of the Study Population
4.2. IL7RA rs6897932 Polymorphism and CD4+ T-Cells Recovery
5. Discussion
6. Study Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
Appendix A
References
- Panel de Expertos de Gesida y Plan Nacional sobre el Sida. National consensus document by gesida/national aids plan on antiretroviral treatment in adults infected by the human immunodeficiency virus (january 2011 update). Enferm. Infecc. Microbiol. Clin. 2011, 29, 209.e1-103. [Google Scholar]
- Thompson, M.A.; Aberg, J.A.; Hoy, J.F.; Telenti, A.; Benson, C.; Cahn, P.; Eron, J.J.; Gunthard, H.F.; Hammer, S.M.; Reiss, P.; et al. Antiretroviral treatment of adult hiv infection: 2012 recommendations of the international antiviral society-USA panel. JAMA 2012, 308, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.F.; Kitchen, C.M.; Hunt, P.W.; Rodriguez, B.; Hecht, F.M.; Kitahata, M.; Crane, H.M.; Willig, J.; Mugavero, M.; Saag, M.; et al. Incomplete peripheral CD4+ cell count restoration in hiv-infected patients receiving long-term antiretroviral treatment. Clin. Infect. Dis. 2009, 48, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Helleberg, M.; Kronborg, G.; Larsen, C.S.; Pedersen, G.; Pedersen, C.; Obel, N.; Gerstoft, J. Poor CD4 response despite viral suppression is associated with increased non-aids-related mortality among HIV patients and their parents. AIDS 2013, 27, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.V.; Peng, G.; Rapkin, J.; Krason, D.; Reilly, C.; Cavert, W.P.; Abrams, D.I.; MacArthur, R.D.; Henry, K.; Neaton, J.D. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for aids and non-aids diseases. J. Acquir. Immune Defic. Syndr. 2008, 48, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Fastenackels, S.; Katlama, C.; Ait-Mohand, H.; Schneider, L.; Guihot, A.; Keller, M.; Grubeck-Loebenstein, B.; Simon, A.; Lambotte, O.; et al. Old age and anti-cytomegalovirus immunity are associated with altered t-cell reconstitution in hiv-1-infected patients. AIDS 2011, 25, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.F.; Haley, C.; Koziel, M.J.; Rowley, C.F. Impact of hepatitis c virus on immune restoration in hiv-infected patients who start highly active antiretroviral therapy: A meta-analysis. Clin. Infect. Dis. 2005, 41, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Massanella, M.; Puig, J.; Perez-Alvarez, N.; Gallego-Escuredo, J.M.; Villarroya, J.; Villarroya, F.; Molto, J.; Santos, J.R.; Clotet, B.; et al. Nadir CD4 t cell count as predictor and high CD4 t cell intrinsic apoptosis as final mechanism of poor cd4 t cell recovery in virologically suppressed hiv-infected patients: Clinical implications. Clin. Infect. Dis. 2010, 50, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Mocroft, A.; Lundgren, J.D.; Sabin, M.L.; Monforte, A.; Brockmeyer, N.; Casabona, J.; Castagna, A.; Costagliola, D.; Dabis, F.; De Wit, S.; et al. Risk factors and outcomes for late presentation for hiv-positive persons in europe: Results from the collaboration of observational hiv epidemiological research europe study (cohere). PLoS Med. 2013, 10, e1001510. [Google Scholar] [CrossRef]
- Massanella, M.; Negredo, E.; Perez-Alvarez, N.; Puig, J.; Ruiz-Hernandez, R.; Bofill, M.; Clotet, B.; Blanco, J. CD4 t-cell hyperactivation and susceptibility to cell death determine poor CD4 t-cell recovery during suppressive haart. AIDS 2010, 24, 959–968. [Google Scholar] [CrossRef]
- Li, T.; Wu, N.; Dai, Y.; Qiu, Z.; Han, Y.; Xie, J.; Zhu, T.; Li, Y. Reduced thymic output is a major mechanism of immune reconstitution failure in hiv-infected patients after long-term antiretroviral therapy. Clin. Infect. Dis. 2011, 53, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Medrano, L.M.; Gutierrez-Rivas, M.; Blanco, J.; Garcia, M.; Jimenez-Sousa, M.A.; Pacheco, Y.M.; Montero, M.; Iribarren, J.A.; Bernal, E.; Martinez, O.J.; et al. Mitochondrial haplogroup h is related to CD4+ t cell recovery in hiv infected patients starting combination antiretroviral therapy. J. Transl. Med. 2018, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.K.; Kulkarni, H.; Catano, G.; Agan, B.K.; Camargo, J.F.; He, W.; O’Connell, R.J.; Marconi, V.C.; Delmar, J.; Eron, J.; et al. Ccl3l1-ccr5 genotype influences durability of immune recovery during antiretroviral therapy of hiv-1-infected individuals. Nat. Med. 2008, 14, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Rigato, P.O.; Hong, M.A.; Casseb, J.; Ueda, M.; de Castro, I.; Benard, G.; Duarte, A.J. Better CD4+ t cell recovery in brazilian hiv-infected individuals under haart due to cumulative carriage of sdf-1-3’a, ccr2-v64i, ccr5-d32 and ccr5-promoter 59029a/g polymorphisms. Curr. HIV Res. 2008, 6, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Rosenow, A.A.; James, I.R.; Roberts, S.G.; Nolan, R.C.; French, M.A.; Price, P. Recovery of CD4+ t cells in hiv patients with a stable virologic response to antiretroviral therapy is associated with polymorphisms of interleukin-6 and central major histocompatibility complex genes. J. Acquir. Immune Defic. Syndr. 2006, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Capitini, C.M.; Chisti, A.A.; Mackall, C.L. Modulating t-cell homeostasis with il-7: Preclinical and clinical studies. J. Intern. Med. 2009, 266, 141–153. [Google Scholar] [CrossRef]
- Corbeau, P.; Reynes, J. Immune reconstitution under antiretroviral therapy: The new challenge in hiv-1 infection. Blood 2011, 117, 5582–5590. [Google Scholar] [CrossRef]
- Limou, S.; Melica, G.; Coulonges, C.; Lelievre, J.D.; Do, H.; McGinn, S.; Gut, I.G.; Levy, Y.; Zagury, J.F. Identification of il7ra risk alleles for rapid progression during hiv-1 infection: A comprehensive study in the griv cohort. Curr. HIV Res. 2012, 10, 143–150. [Google Scholar] [CrossRef]
- Guzman-Fulgencio, M.; Berenguer, J.; Jimenez-Sousa, M.A.; Micheloud, D.; Garcia-Alvarez, M.; Bellon, J.M.; Aldamiz-Echevarria, T.; Garcia-Broncano, P.; Catalan, P.; Diez, C.; et al. Il7ra polymorphisms predict the CD4+ recovery in hiv patients on cart. Eur. J. Clin. Investig. 2015, 45, 1192–1199. [Google Scholar] [CrossRef]
- Hartling, H.J.; Thorner, L.W.; Erikstrup, C.; Harritshoj, L.H.; Kronborg, G.; Pedersen, C.; Larsen, C.S.; Helleberg, M.; Gerstoft, J.; Obel, N.; et al. Polymorphism in interleukin-7 receptor alpha gene is associated with faster CD4(+) t-cell recovery after initiation of combination antiretroviral therapy. AIDS 2014, 28, 1739–1748. [Google Scholar] [CrossRef]
- Rajasuriar, R.; Booth, D.R.; Gouillou, M.; Spelman, T.; James, I.; Solomon, A.; Chua, K.; Stewart, G.; Deeks, S.; Bangsberg, D.R.; et al. The role of snps in the alpha-chain of the il-7r gene in CD4+ t-cell recovery in hiv-infected african patients receiving suppressive cart. Genes Immun. 2012, 13, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Rajasuriar, R.; Booth, D.; Solomon, A.; Chua, K.; Spelman, T.; Gouillou, M.; Schlub, T.E.; Davenport, M.; Crowe, S.; Elliott, J.; et al. Biological determinants of immune reconstitution in hiv-infected patients receiving antiretroviral therapy: The role of interleukin 7 and interleukin 7 receptor alpha and microbial translocation. J. Infect. Dis. 2010, 202, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.G.; Schmidt, S.; Seth, P.; Oksenberg, J.R.; Hart, J.; Prokop, A.; Caillier, S.J.; Ban, M.; Goris, A.; Barcellos, L.F.; et al. Interleukin 7 receptor alpha chain (il7r) shows allelic and functional association with multiple sclerosis. Nat. Genet. 2007, 39, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Kreft, K.L.; Verbraak, E.; Wierenga-Wolf, A.F.; van Meurs, M.; Oostra, B.A.; Laman, J.D.; Hintzen, R.Q. Decreased systemic il-7 and soluble il-7ralpha in multiple sclerosis patients. Genes Immun. 2012, 13, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Crawley, A.M.; Faucher, S.; Angel, J.B. Soluble il-7r alpha (scd127) inhibits il-7 activity and is increased in hiv infection. J. Immunol. 2010, 184, 4679–4687. [Google Scholar] [CrossRef] [PubMed]
- Lundtoft, C.; Seyfarth, J.; Oberstrass, S.; Rosenbauer, J.; Baechle, C.; Roden, M.; Holl, R.W.; Mayatepek, E.; Kummer, S.; Meissner, T.; et al. Autoimmunity risk- and protection-associated il7ra genetic variants differentially affect soluble and membrane il-7ralpha expression. J. Autoimmun. 2019, 97, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.N.; Srinivasula, S.; Hu, Z.; Read, S.W.; Porter, B.O.; Kim, I.; Mican, J.M.; Paik, C.; Degrange, P.; Di Mascio, M.; et al. Decreases in il-7 levels during antiretroviral treatment of hiv infection suggest a primary mechanism of receptor-mediated clearance. Blood 2011, 118, 3244–3253. [Google Scholar] [CrossRef]
- Hartling, H.J.; Ryder, L.P.; Ullum, H.; Odum, N.; Nielsen, S.D. Gene variation in il-7 receptor (il-7r) α affects il-7r response in CD4+ t cells in hiv-infected individuals. Sci. Rep. 2017, 7, 42036. [Google Scholar] [CrossRef]
- Darling, K.E.; Hachfeld, A.; Cavassini, M.; Kirk, O.; Furrer, H.; Wandeler, G. Late presentation to hiv care despite good access to health services: Current epidemiological trends and how to do better. Swiss Med. Wkly. 2016, 146, w14348. [Google Scholar] [CrossRef][Green Version]
- Perez-Molina, J.A.; Diaz-Menendez, M.; Plana, M.N.; Zamora, J.; Lopez-Velez, R.; Moreno, S. Very late initiation of haart impairs treatment response at 48 and 96 weeks: Results from a meta-analysis of randomized clinical trials. J. Antimicrob. Chemother. 2012, 67, 312–321. [Google Scholar] [CrossRef]
| Characteristics | All Patients | IL7RA rs6897932 Genotypes | ||
|---|---|---|---|---|
| CC | CT/TT | p-Value | ||
| No. | 411 | 256 | 155 | |
| Male (n = 411) (%) | 323 (78.6%) | 192 (75%) | 131 (84.5%) | 0.023 |
| Age (n = 411) (years) | 40 (34; 48) | 40 (34; 46) | 50 (33; 49) | 0.488 |
| Caucasian origin (n = 394) (%) | 317 (80.5%) | 187 (75.7%) | 130 (88.4%) | 0.002 |
| Time since HIV diagnosis (n = 411) (years) | 1 (1; 1) | 1 (1; 1) | 1 (1; 1) | 0.517 |
| CD4+ cell count at baseline (n = 411) (cells/μL) | 104 (41; 159) | 92.7 (38; 157) | 115 (47; 162) | 0.198 |
| Hepatitis C infection (n = 411) (%) | 32 (7.8%) | 23 (9%) | 9 (5.8%) | 0.244 |
| Hepatitis B infection (n = 411) (%) | 20 (4.9%) | 14 (5.5%) | 6 (3.9%) | 0.466 |
| cART regimen (n = 411) (%) | 0.054 | |||
| PI-based | 127 (31%) | 73 (28.5%) | 54 (35.1%) | |
| NNRTI-based | 205 (50%) | 134 (52.3%) | 71 (46.1%) | |
| PI+NNRTI-based | 53 (12.9%) | 38 (14.8%) | 15 (9.7%) | |
| Others | 25 (6.1%) | 11 (4.4%) | 14 (9.1%) | |
| HIV transmission route (n = 384) (%) | 0.079 | |||
| Homosexual transmission | 189 (49.2%) | 106 (45.1%) | 83 (55.7%) | |
| Heterosexual transmission | 139 (36.2%) | 95 (40.4%) | 44 (29.5%) | |
| IDU | 56 (14.6%) | 34 (14.5%) | 22 (14.8%) | |
| Outcomes | rs6897932 Genotypes (*) | Unadjusted Analysis (*) | Adjusted Analysis (**) | |||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 256) | CT/TT (n = 155) | Exp(B) (95% CI) | p-Value | q-Value | Exp(B) (95% CI) | p-Value | q-Value | |
| Slope CD4+ recovery | 9.1 (5.9; 12.9) | 10.3 (6.1; 14.8) | 1.12 (0.99; 1.28) | 0.070 | 0.070 | 1.16 (1.03; 1.31) | 0.016 | 0.028 |
| ≥10 CD4+ cells/mm3 per month | 105 (41%) | 82 (52.9%) | 1.61 (1.08; 2.41) | 0.019 | 0.044 | 1.75 (1.14; 2.69) | 0.010 | 0.028 |
| ≥15 CD4+ cells/mm3 per month | 38 (14.8%) | 37 (23.9%) | 1.79 (1.08; 2.88) | 0.023 | 0.044 | 1.94 (1.14; 3.30) | 0.015 | 0.028 |
| CD4+ increase (ΔCD4+) | 258 (167; 381) | 295 (189; 442) | 1.15 (1.02; 1.31) | 0.020 | 0.044 | 1.19 (1.06; 1.34) | 0.004 | 0.022 |
| ≥200 CD4+ cells/mm3 | 164 (64.1%) | 114 (73.5%) | 1.56 (1.01; 2.42) | 0.047 | 0.054 | 1.63 (1.03; 2.57) | 0.036 | 0.044 |
| ≥300 CD4+ cells/mm3 | 97 (37.9%) | 75 (44.8%) | 1.53 (1.03; 2.30) | 0.037 | 0.051 | 1.63 (1.06; 2.49) | 0.025 | 0.034 |
| ≥400 CD4+ cells/mm3 | 54 (21.1%) | 48 (31%) | 1.67 (1.06; 2.64) | 0.025 | 0.044 | 1.63 (1.01; 2.63) | 0.047 | 0.052 |
| ≥500 CD4+ cells/mm3 | 24 (9.4%) | 26 (16.8%) | 1.94 (1.06; 3.53) | 0.028 | 0.044 | 2.16 (1.14; 4.11) | 0.018 | 0.028 |
| CD4+ at the end of follow-up | 362 (260; 463) | 425 (274; 558) | 1.14 (1.03; 1.25) | 0.009 | 0.044 | 1.13 (1.03; 1.24) | 0.006 | 0.022 |
| ≥350 CD4+ cells/mm3 | 133 (52%) | 96 (61%) | 1.51 (1.01; 2.26) | 0.049 | 0.054 | 1.51 (0.96; 2.37) | 0.077 | 0.077 |
| ≥500 CD4+ cells/mm3 | 52 (20.3%) | 59 (38.1%) | 2.41 (1.54; 3.76) | <0.001 | 0.001 | 2.44 (1.49; 3.99) | 0.006 | 0.022 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resino, S.; Navarrete-Muñoz, M.A.; Blanco, J.; Pacheco, Y.M.; Castro, I.; Berenguer, J.; Santos, J.; Vera-Méndez, F.J.; Górgolas, M.; Jiménez-Sousa, M.A.Á.; et al. IL7RA rs6897932 Polymorphism Is Associated with Better CD4+ T-Cell Recovery in HIV Infected Patients Starting Combination Antiretroviral Therapy. Biomolecules 2019, 9, 233. https://doi.org/10.3390/biom9060233
Resino S, Navarrete-Muñoz MA, Blanco J, Pacheco YM, Castro I, Berenguer J, Santos J, Vera-Méndez FJ, Górgolas M, Jiménez-Sousa MAÁ, et al. IL7RA rs6897932 Polymorphism Is Associated with Better CD4+ T-Cell Recovery in HIV Infected Patients Starting Combination Antiretroviral Therapy. Biomolecules. 2019; 9(6):233. https://doi.org/10.3390/biom9060233
Chicago/Turabian StyleResino, Salvador, María A. Navarrete-Muñoz, Julià Blanco, Yolanda M. Pacheco, Iván Castro, Juan Berenguer, Jesús Santos, Francisco J. Vera-Méndez, Miguel Górgolas, M. A. Ángeles Jiménez-Sousa, and et al. 2019. "IL7RA rs6897932 Polymorphism Is Associated with Better CD4+ T-Cell Recovery in HIV Infected Patients Starting Combination Antiretroviral Therapy" Biomolecules 9, no. 6: 233. https://doi.org/10.3390/biom9060233
APA StyleResino, S., Navarrete-Muñoz, M. A., Blanco, J., Pacheco, Y. M., Castro, I., Berenguer, J., Santos, J., Vera-Méndez, F. J., Górgolas, M., Jiménez-Sousa, M. A. Á., Benito, J. M., & Rallón, N. (2019). IL7RA rs6897932 Polymorphism Is Associated with Better CD4+ T-Cell Recovery in HIV Infected Patients Starting Combination Antiretroviral Therapy. Biomolecules, 9(6), 233. https://doi.org/10.3390/biom9060233





