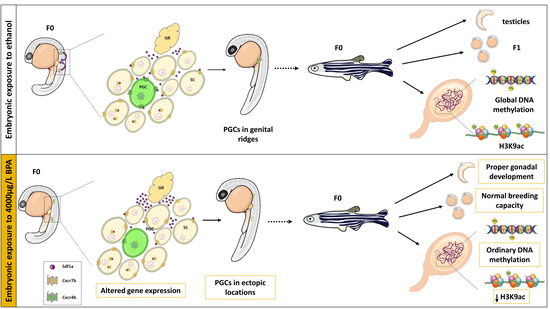
Embryonic Exposure to Bisphenol A Impairs Primordial Germ Cell Migration without Jeopardizing Male Breeding Capacity
Abstract
1. Introduction
2. Results
2.1. Germ Cell Migration
2.2. Epigenetics of Genital Ridges
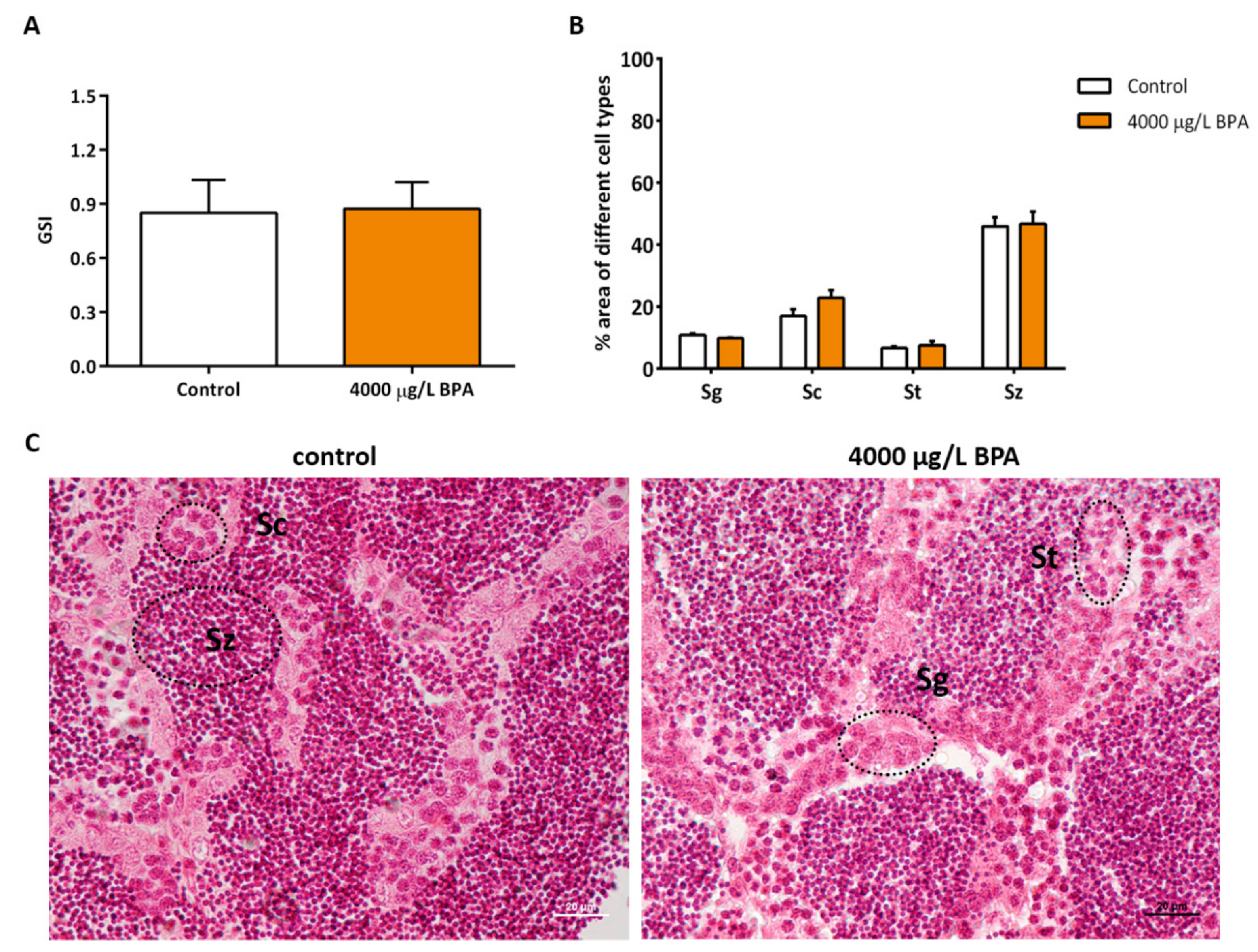
2.3. Gonadosomatic Index and Morphometric Study in Testicles
2.4. Sperm Epigenetics
2.5. Gonadal Differentiation and Male Fertility
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Zebrafish Maintenance and Embryo Collection
4.3. BPA Embryonic Exposure
4.4. Whole Mount Immunostaining
4.5. RNA Extraction and qPCR (Quantitative Polymerase Chain Reaction) Analysis
4.6. Sex Ratio, Fertility, and Early Embryo Development
4.7. Gonadosomatic Index and Morphometric Study of Testicular Cells
4.8. Global DNA Methylation in Genital Ridge and Sperm
4.9. Immunostaining of Epigenetic Marks in Spermatozoa
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Dodds, E.C.; Lawson, W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature 1936, 137, 996. [Google Scholar] [CrossRef]
- MacKay, H.; Abizaid, A. A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA). Horm. Behav. 2018, 101, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhao, Y.; Yang, M.; Farajzadeh, M.; Pan, C.; Wayne, N.L. Actions of bisphenol A and bisphenol S on the reproductive neuroendocrine system during early development in zebrafish. Endocrinology 2016, 157, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Crain, D.A.; Guillette, L.J., Jr. Endocrine-disrupting contaminants and reproduction in vertebrate wildlife. Rev. Toxicol. 1997, 1, 47–70. [Google Scholar]
- Chen, J.; Xiao, Y.; Gai, Z.; Li, R.; Zhu, Z.; Bai, C.; Tanguay, R.L.; Xu, X.; Huang, C.; Dong, Q. Reproductive toxicity of low level bisphenol A exposures in a two-generation zebrafish assay: Evidence of male-specific effects. Aquat. Toxicol. 2015, 169, 204–214. [Google Scholar] [CrossRef]
- Chen, J.; Saili, K.S.; Liu, Y.; Li, L.; Zhao, Y.; Jia, Y.; Bai, C.; Tanguay, R.L.; Dong, Q.; Huang, C. Developmental bisphenol A exposure impairs sperm function and reproduction in zebrafish. Chemosphere 2017, 169, 262–270. [Google Scholar] [CrossRef]
- Laing, L.V.; Viana, J.; Dempster, E.L.; Trznadel, M.; Trunkfield, L.A.; Uren Webster, T.M.; van Aerle, R.; Paull, G.C.; Wilson, R.J.; Mill, J.; et al. Bisphenol A causes reproductive toxicity, decreases dnmt1 transcription, and reduces global DNA methylation in breeding zebrafish (Danio rerio). Epigenetics 2016, 11, 526–538. [Google Scholar] [CrossRef]
- González-Rojo, S.; Lombó, M.; Fernández-Díez, C.; Herráez, M.P. Male exposure to bisphenol a impairs spermatogenesis and triggers histone hyperacetylation in zebrafish testes. Environ. Pollut. 2019, 248, 368–379. [Google Scholar] [CrossRef]
- Hatef, A.; Alavi, S.M.H.; Abdulfatah, A.; Fontaine, P.; Rodina, M.; Linhart, O. Adverse effects of bisphenol A on reproductive physiology in male goldfish at environmentally relevant concentrations. Ecotoxicol. Environ. Saf. 2012, 76, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Haubruge, E.; Petit, F.; Gage, M.J.G. Reduced sperm counts in guppies (Poecilia reticulata) following exposure to low levels of tributyltin and bisphenol A. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F.; Berger, B.; Kletzl, M.; Weismann, T. Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario. Aquat. Toxicol. 2005, 75, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Lombó, M.; Fernández-Díez, C.; González-Rojo, S.; Navarro, C.; Robles, V.; Herráez, M.P. Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure. Environ. Pollut. 2015, 206, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sun, S.; Guo, M.; Song, H. Use of antagonists and morpholinos in loss-of-function analyses: Estrogen receptor ESR2a mediates the effects of 17alpha-ethinylestradiol on primordial germ cell distribution in zebrafish. Reprod. Biol. Endocrinol. 2014, 12, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Doitsidou, M.; Reichman-Fried, M.; Stebler, J.; Köprunner, M.; Dörries, J.; Meyer, D.; Esguerra, C.V.; Leung, T.C.; Raz, E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell 2002, 111, 647–659. [Google Scholar] [CrossRef]
- Raz, E. Primordial germ-cell development: The zebrafish perspective. Nat. Rev. Genet. 2003, 4, 690–700. [Google Scholar] [CrossRef]
- Gamba, L.; Cubedo, N.; Ghysen, A.; Lutfalla, G.; Dambly-Chaudiere, C. Estrogen receptor ESR1 controls cell migration by repressing chemokine receptor CXCR4 in the zebrafish posterior lateral line system. Proc. Natl. Acad. Sci. USA 2010, 107, 6358–6363. [Google Scholar] [CrossRef]
- Yoon, C.; Kawakami, K.; Hopkins, N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 1997, 124, 3157–3165. [Google Scholar]
- Bussmann, J.; Raz, E. Chemokine-guided cell migration and motility in zebrafish development. EMBO J. 2015, 34, 1309–1318. [Google Scholar] [CrossRef]
- Eddy, E.M. Fine structural observations on the form and distribution of nuage in germ cells of the rat. Anat. Rec. 1974, 178, 731–757. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.C.; Aasland, R.; Fjose, A. A vasa-like gene in zebrafish identifies putative primordial germ cells. Mech. Dev. 1997, 66, 95–105. [Google Scholar] [CrossRef]
- Knaut, H.; Steinbeisser, H.; Schwarz, H.; Nüsslein-Volhard, C. An evolutionary conserved region in the vasa 3′UTR targets RNA translation to the germ cells in the zebrafish. Curr. Biol. 2002, 12, 454–466. [Google Scholar] [CrossRef]
- Saito, T.; Goto-Kazeto, R.; Arai, K.; Yamaha, E. Xenogenesis in Teleost Fish through Generation of Germ-Line Chimeras by Single Primordial Germ Cell Transplantation1. Biol. Reprod. 2007, 78, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Valcarce, D.G.; Alfonso, J.; Herráez, M.P.; Robles, V. In Vitro Generation of Zebrafish PGC-Like Cells1. Biol. Reprod. 2014, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [PubMed]
- Hales, B.F.; Grenier, L.; Lalancette, C.; Robaire, B. Epigenetic programming: From gametes to blastocyst. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 652–665. [Google Scholar] [CrossRef]
- Jammes, H.; Junien, C.; Chavatte-Palmer, P. Epigenetic control of development and expression of quantitative traits. Reprod. Fertil. Dev. 2011, 23, 64–74. [Google Scholar] [CrossRef]
- Hill, R.L.; Janz, D.M. Developmental estrogenic exposure in zebrafish (Danio rerio): I. Effects on sex ratio and breeding success. Aquat. Toxicol. 2003, 63, 417–429. [Google Scholar] [CrossRef]
- Willey, J.B.; Krone, P.H. Effects of endosulfan and nonylphenol on the primordial germ cell population in pre-larval zebrafish embryos. Aquat. Toxicol. 2001, 54, 113–123. [Google Scholar] [CrossRef]
- Weber, L.P.; Hill, R.L.; Janz, D.M. Developmental estrogenic exposure in zebrafish (Danio rerio): II. Histological evaluation of gametogenesis and organ toxicity. Aquat. Toxicol. 2003, 63, 431–446. [Google Scholar] [CrossRef]
- Lam, S.H.; Hlaing, M.M.; Zhang, X.; Yan, C.; Duan, Z.; Zhu, L.; Ung, C.Y.; Mathavan, S.; Ong, C.N.; Gong, Z. Toxicogenomic and phenotypic analyses of bisphenol-a early-life exposure toxicity in zebrafish. PLoS ONE 2011, 6, e28273. [Google Scholar] [CrossRef] [PubMed]
- Lombó, M.; González-Rojo, S.; Fernández-Díez, C.; Herráez, M.P. Cardiogenesis impairment promoted by bisphenol A exposure is successfully counteracted by epigallocatechin gallate. Environ. Pollut. 2019, 246, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, S.; Maradonna, F.; Gioacchini, G.; Cobellis, G.; Piccinetti, C.C.; Valle, L.D.; Carnevali, O. BPA-Induced Deregulation of Epigenetic Patterns: Effects on Female Zebrafish Reproduction. Sci. Rep. 2016, 6, 21982. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Metcalfe, T.L.; Kiparissis, Y.; Koenig, B.G.; Khan, C.; Hughes, R.J.; Croley, T.R.; March, R.E.; Potter, T. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2001, 20, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Zillioux, E.J.; Johnson, I.C.; Kiparissis, Y.; Metcalfe, C.D.; Wheat, J.V.; Ward, S.G.; Liu, H. The sheepshead minnow as an in vivo model for endocrine disruption in marine teleosts: A partial life-cycle test with 17alpha-ethynylestradiol. Environ. Toxicol. Chem. 2001, 20, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Fujimoto, T.; Maegawa, S.; Inoue, K.; Tanaka, M.; Arai, K.; Yamaha, E. Visualization of primordial germ cells in vivo using GFP-nos1 3′UTR mRNA. Int. J. Dev. Biol. 2006, 50, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Tzung, K.W.; Goto, R.; Saju, J.M.; Sreenivasan, R.; Saito, T.; Arai, K.; Yamaha, E.; Hossain, M.S.; Calvert, M.E.K.; Orbán, L. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Rep. 2015, 4, 61–73. [Google Scholar] [CrossRef]
- Matsui, Y.; Mochizuki, K. A current view of the epigenome in mouse primordial germ cells. Mol. Reprod. Dev. 2014, 81, 160–170. [Google Scholar] [CrossRef]
- Ribas, L.; Vanezis, K.; Imués, M.A.; Piferrer, F. Treatment with a DNA methyltransferase inhibitor feminizes zebrafish and induces long-term expression changes in the gonads. Epigenet. Chromatin 2017, 10, 59. [Google Scholar] [CrossRef]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B.; et al. A Role for Piwi and piRNAs in Germ Cell Maintenance and Transposon Silencing in Zebrafish. Cell 2007, 129, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Drastichová, J.; Svobodová, Z.; Groenland, M.; Dobšíková, R.; Žlábek, V.; Weissová, D.; Szotkowská, M. Effect of Exposure to Bisphenol A on the Sex Differentiation in Zebrafish (Danio rerio). Acta Vet. Brno 2005, 74, 287–291. [Google Scholar] [CrossRef]
- McCarrey, J.R. Distinctions between transgenerational and non-transgenerational epimutations. Mol. Cell. Endocrinol. 2014, 398, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Labbé, C.; Robles, V.; Herráez, M.P. Epigenetics in fish gametes and early embryo. Aquaculture 2017, 472, 93–106. [Google Scholar] [CrossRef]
- Aluru, N. Epigenetic effects of environmental chemicals: Insights from zebrafish. Curr. Opin. Toxicol. 2017, 6, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Robles, V. Cryopreservation Causes Genetic and Epigenetic Changes in Zebrafish Genital Ridges. PLoS ONE 2013, 8, e67614. [Google Scholar] [CrossRef]
- Steilmann, C.; Paradowska, A.; Bartkuhn, M.; Vieweg, M.; Schuppe, H.C.; Bergmann, M.; Kliesch, S.; Weidner, W.; Steger, K. Presence of histone H3 acetylated at lysine 9 in male germ cells and its distribution pattern in the genome of human spermatozoa. Reprod. Fertil. Dev. 2011, 23, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zuo, X.; He, D.; Ding, S.; Xu, F.; Yang, H.; Jin, X.; Fan, Y.; Ying, L.; Tian, C.; et al. Long-term exposure to a “safe” dose of bisphenol A reduced protein acetylation in adult rat testes. Sci. Rep. 2017, 7, 40337. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays 2014, 36, 359–371. [Google Scholar] [CrossRef]
- Marandel, L.; Labbé, C.; Bobe, J.; Le Bail, P.-Y. Nanog 5′-upstream sequence, DNA methylation, and expression in gametes and early embryo reveal striking differences between teleosts and mammals. Gene 2012, 492, 130–137. [Google Scholar] [CrossRef]
- Le, T.; Kim, K.-P.; Fan, G.; Faull, K.F. A sensitive mass spectrometry method for simultaneous quantification of DNA methylation and hydroxymethylation levels in biological samples. Anal. Biochem. 2011, 412, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Johansson, S.; Stach, D.; Corcoran, M.; Grandér, D.; Schalling, M.; Bakalkin, G.; Lyko, F.; Larsson, C.; Ekström, T.J. LUMA (LUminometric Methylation Assay)—A high throughput method to the analysis of genomic DNA methylation. Exp. Cell Res. 2006, 312, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- González-Rojo, S.; Fernández-Díez, C.; Lombó, M.; Herráez, M.P. Distribution of DNA damage in the sperm nucleus: A study of zebrafish as a model of histone-packaged chromatin. Theriogenology 2018, 122, 109–115. [Google Scholar] [CrossRef] [PubMed]




| Sex Ratio | Male Fertility (Fertilized Eggs ± SE) | ||
|---|---|---|---|
| (Males/Females) × 100 | |||
| Control | Replicate 1 | 161.90 | 429.75 |
| Replicate 2 | 47.83 | 442.77 | |
| Replicate 3 | 253.33 | 82.62 | |
| Mean | 351.71 ± 146.58 | ||
| 4000 µg/L BPA | Replicate 1 | 189.47 | 282.33 |
| Replicate 2 | 23.81 | 274.83 | |
| Replicate 3 | 205.56 | 289.83 | |
| Mean | 282.33 ± 7.5 |
| Transcript Name | Primer Set | Amplicon Size | Annealing Temperature | Accession Number |
|---|---|---|---|---|
| sdf1a | F: ATTCGCGAGCTCAAGTTCCT R: ATATCTGTGACGGTGGGCTG | 214 | 62 | NM_178307.2 |
| cxcr4b | F: GGCGCTGGCATATTTCCA R: ACGCCTAGGAAAGCATAAAGGA | 56 | 60 | AY057094.1 |
| cxcr7b | F: CGCCAGCATCTTCTTCCTGA R: GCGAATAAAGCAAGCAGCCA | 138 | 64 | EF467375.1 |
| 18S rRNA | F: GCCGTTCTTAGTTGTGGAG R: CCGGAGTCTCGTTCGTTATC | 60 | 58 | FJ915075.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombó, M.; Getino-Álvarez, L.; Depincé, A.; Labbé, C.; Herráez, M.P. Embryonic Exposure to Bisphenol A Impairs Primordial Germ Cell Migration without Jeopardizing Male Breeding Capacity. Biomolecules 2019, 9, 307. https://doi.org/10.3390/biom9080307
Lombó M, Getino-Álvarez L, Depincé A, Labbé C, Herráez MP. Embryonic Exposure to Bisphenol A Impairs Primordial Germ Cell Migration without Jeopardizing Male Breeding Capacity. Biomolecules. 2019; 9(8):307. https://doi.org/10.3390/biom9080307
Chicago/Turabian StyleLombó, Marta, Lidia Getino-Álvarez, Alexandra Depincé, Catherine Labbé, and María Paz Herráez. 2019. "Embryonic Exposure to Bisphenol A Impairs Primordial Germ Cell Migration without Jeopardizing Male Breeding Capacity" Biomolecules 9, no. 8: 307. https://doi.org/10.3390/biom9080307
APA StyleLombó, M., Getino-Álvarez, L., Depincé, A., Labbé, C., & Herráez, M. P. (2019). Embryonic Exposure to Bisphenol A Impairs Primordial Germ Cell Migration without Jeopardizing Male Breeding Capacity. Biomolecules, 9(8), 307. https://doi.org/10.3390/biom9080307